Abstract
In the present study, feasibility of recovering the coagulant from water treatment plant sludge with sulphuric acid and reusing it in post-treatment of upflow anaerobic sludge blanket (UASB) reactor effluent treating municipal wastewater were studied. The optimum conditions for coagulant recovery from water treatment plant sludge were investigated using response surface methodology (RSM). Sludge obtained from plants that use polyaluminium chloride (PACl) and alum coagulant was utilised for the study. Effect of three variables, pH, solid content and mixing time was studied using a Box–Behnken statistical experimental design. RSM model was developed based on the experimental aluminium recovery, and the response plots were developed. Results of the study showed significant effects of all the three variables and their interactions in the recovery process. The optimum aluminium recovery of 73.26 and 62.73 % from PACl sludge and alum sludge, respectively, was obtained at pH of 2.0, solid content of 0.5 % and mixing time of 30 min. The recovered coagulant solution had elevated concentrations of certain metals and chemical oxygen demand (COD) which raised concern about its reuse potential in water treatment. Hence, the coagulant recovered from PACl sludge was reused as coagulant for post-treatment of UASB reactor effluent treating municipal wastewater. The recovered coagulant gave 71 % COD, 80 % turbidity, 89 % phosphate, 77 % suspended solids and 99.5 % total coliform removal at 25 mg Al/L. Fresh PACl also gave similar performance but at higher dose of 40 mg Al/L. The results suggest that coagulant can be recovered from water treatment plant sludge and can be used to treat UASB reactor effluent treating municipal wastewater which can reduce the consumption of fresh coagulant in wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coagulation is the most common process used to remove suspended particles, colloids, organics, colour and microorganisms from the drinking water supplies (Duan and Gregory 2003; Ghafari et al. 2009). Enormous quantities of water treatment plant sludge (WTS) or waterworks sludge are produced when coagulants (generally aluminium or iron salts) are added to raw water. For example, water treatment plants produce annually 18,000 t as dry solids from Ireland, 34,000 t as dry solids from The Netherlands and 182,000 t as dry solids from UK (Babatunde and Zhao 2007). Globally, available literature estimates that 10,000 t of waterworks sludge is produced daily (Dharmappa et al. 1997). Due to regulatory changes in the recent past, WTS now has to be disposed of into landfills or through land application in developed countries. However, in developing countries, it is disposed into water bodies or sanitary sewers (Nair and Ahammed 2013). WTS disposed of into water bodies is reported to be toxic to aquatic life (Muisa et al. 2011), while that disposed into landfill increases the land requirement of the landfill site. Since the levels of pollutants in WTS are relatively low, as the best quality raw water sources are generally selected for drinking water production (Ishikawa et al. 2007), the reuse of WTS may be a feasible option.
A number of research efforts have been made particularly in recent years to reuse waterworks sludge in many beneficial ways. These include its use in building and construction materials (Monteiro et al. 2008; Pan et al. 2004), in wastewater treatment (Babatunde et al. 2010; Moghaddam et al. 2010; Nair and Ahammed 2013) and for soil improvement (Hovsepyan and Bonzongo 2009). Two different approaches have been tried for the reuse of WTS in water and wastewater treatment. In the first approach, coagulant is first recovered from the sludge and is reused as a coagulant for the treatment of water/wastewater. In the second approach, wet/dry sludge itself is used as a coagulant or adsorbent for removal of different contaminants. Water treatment sludge has recently been used as an adsorbent for removal of anions and cations such as phosphate (Belyaeva and Haynes 2012; Gibbons et al. 2009; Makris et al. 2010; Wang and Pei 2013), arsenic (Makris et al. 2006), lead (Zhou and Haynes 2011), boron (Irawan et al. 2011), selenium (Ippolito et al. 2009), fluoride (Sujana et al. 1998), mercury (Hovsepyan and Bonzongo 2009) and chromium (Zhou and Haynes 2011). Since aluminium salts are the most commonly used coagulants in drinking water supplies, most of these studies focussed on aluminium-based WTS (Babatunde et al. 2010; Ippolito et al. 2009; Makris et al. 2010; Nair and Ahammed 2013).
Recovery of coagulant from WTS is an attractive proposition and has been reported by many researchers (Abdo et al. 1993; Chen et al. 2012; Ishikawa et al. 2007; Parsons and Daniels 1999; Wang and Yang 1975; Xu et al. 2009). Generally, four ways of coagulant recovery are employed for the water treatment sludge which includes acidification, basification, ion exchanging, and membrane processes (Xu et al. 2009). Most of the studies reported recently used acidification for coagulant recovery due to high efficiency and low cost compared to other methods (Chen et al. 2012; Huang et al. 2010; Ishikawa et al. 2007; Xu et al. 2009).
Several factors are known to affect coagulant recovery from WTS by acidification. These include pH of the solution, mixing speed and intensity, mixing time, temperature and sludge content in the mixture (Chen et al. 2012; Ishikawa et al. 2007; Xu et al. 2009). Cost-effective methods are needed for successful recovery of coagulant from sludge and its subsequent reuse. Since a large number of factors are involved in the recovery process, simulation studies based on limited laboratory data would be a better option to predict and optimise the coagulant recovery. The studies reported in the literature used one-factor-at-a-time approach which estimates the influence of a single variable while keeping all other variables at fixed conditions. This traditional approach is time-consuming and cannot estimate the interactions among the variables. It also requires large number of tests to be conducted. On the other hand, statistically designed experiments are economical, and valid conclusions can be drawn with a small number of experiments. Response surface methodology (RSM) is one such statistical technique and is used for designing experiments, building models, evaluating the effects of several variables and obtaining the optimum conditions for responses with a limited number of planned experiments (Montgomery 2010; Anderson and Whitcomb 2005).
Recently, RSM has been used for studying and optimising different processes used in water and wastewater treatment (Bashir et al. 2009; Ghafari et al. 2009; Moghaddam et al. 2010; Nair et al. 2014; Zhang et al. 2012). Different types of RSM designs such as three-level factorial design, central composite design, Box–Behnken design and D-optimal design have been used in these studies. No studies, however, have been reported on the use of RSM as a tool for modelling coagulant recovery process from WTS.
One of the concerns on the reuse of recovered coagulant is the high content of heavy metals and natural organic matter in the recovered coagulant (Chen et al. 2012; Xu et al. 2009) and the possible enrichment of these in treated water. Hence, the recovered coagulant cannot be reused in water treatment. However, it can be used for wastewater treatment since higher concentration of metals is generally permitted in treated effluent. Coagulants recovered from WTS have been used in sewage treatment (Ishikawa et al. 2007; Parson and Daniels 1999), wastewater from a coastal landfill site (Ishikawa et al. 2007), and in chemically enhanced primary treatment (Xu et al. 2009).
The use of upflow anaerobic sludge blanket (UASB) reactor has become popular for the treatment of municipal wastewater in tropical countries due to its low capital investment, less land and energy requirements, low maintenance cost and the clean energy produced in the form of biogas (Chong et al. 2012). However, UASB reactor effluent cannot meet the discharge standards without any post-treatment (Chong et al. 2012). The post-treatment options studied not sustainable for developing countries due to their high mechanisation level, high construction and operational costs and large land and energy requirements (Chong et al. 2012; Nair and Ahammed 2013). Hence, an attempt was made in this study to reuse the coagulant recovered from WTS in post-treatment of UASB reactor effluent.
In the present study, coagulant recovery from WTS using aluminium salts as coagulant was carried using acidification. The aluminium recovery process was modelled using RSM to obtain optimum conditions for aluminium recovery. The feasibility of using the recovered coagulant in post-treatment of UASB reactor effluent treating municipal wastewater was also investigated.
Materials and methods
Sample collection and preparation
PACl sludge was collected from the clariflocculator of the Katargam Water Treatment Plant at Surat, India, which treats water from Tapi River using polyaluminium chloride (PACl) as the coagulant. Alum sludge was collected from Barabangla Water Treatment Plant at Nasik, India, treating water from Godavari River using alum as the coagulant. The sludge samples were collected with polyethylene bottles and were transported to the Environmental Engineering Laboratory of SV National Institute of Technology, Surat, India. The average values of pH, total solids and volatile solids of the collected PACl sludge were 5.62, 383 g/L and 54 g/L respectively while those of alum sludge were 7.28, 23.53 g/L and 3.14 g/L respectively. The same batch of sludge was used in all the tests. The sludge was dried in a hot air oven at 105 °C for 24 h and crushed using a mortar and pestle, and the fraction passing through 2-mm sieve was collected and was used in the tests. Concentrations of relevant elements detected in the sludge are presented in Table 1. Lead and chromium were below the detectable limit. Both the sludge contains high proportion of Al and Fe. The difference in the other heavy metal content can be due to variation in quality of water source and coagulants. The UASB reactor effluent was collected from the municipal wastewater treatment plant located at Bamroli, Surat, India, whose characteristics have been reported in Nair and Ahammed (2013).
Aluminium recovery
Aluminium recovery from the sludge was carried out using a jar test apparatus (DBK Instruments, Mumbai, India) at room temperature (25–27 °C). Certain amount of powdered sludge (designated as solid content (%) from here onwards) was added to 100 mL of deionised water, and the pH of the mixture was adjusted to a desired value by adding 1 N sulphuric acid. The sulphuric acid required for each condition was noted. The solution was then mixed at 100 rpm for various mixing times and then kept quiescent for 15 min. The solution was filtered and the filtrate was analysed for Al. All experiments were carried out in triplicates. The aluminium recovery was calculated as follows:
Experimental design
A Box–Behnken statistical experimental design with the RSM was used to investigate the effects of the three independent variables, namely, pH of the solution, solid content (that is, the gramme of dry sludge taken in 100 mL water), and mixing time on aluminium recovery. Box–Behnken design is an efficient, rotatable and economical design. The total number of runs required in Box–Behnken design is defined as N = 2 k(k − 1) + C o where k is the number of variables studied and C o the number of central points (Bezerra et al. 2008). In the present study, 17 runs were conducted, with five replicates at the centre of the design for estimation of pure error sum of squares. The efficiency of the recovery process was evaluated as percentage aluminium recovery (dependent variable). Experimental data were fitted to a second-order polynomial model:
where y is the predicted response (aluminium recovery in %) used as dependent variable; x i and x j are the independent variables; b 0 is the constant coefficient, b i is the coefficient that determines the influence of variable i in the response, b ij is the coefficient that determines the effect of interaction between variables i and j, b ii is the parameter that determines the shape of the curve (quadratic effect); and k is the number of variables studied (Bashir et al. 2009; Zhang et al. 2012).
Two sets of preliminary tests were conducted to decide the range of independent variables: (i) effect of pH (in the range 1.0–4.0) at a fixed solid content (1.0 %) and mixing time (50 min) and (ii) effect of mixing time (in the range 10–70 min) at a fixed pH (2.0) and solid content (1.0 %), and the results of these tests are presented in “Results and discussion” section.
The experimental design matrix in coded and uncoded units is given in Table 2. The coded values for the process parameters were determined by the following equation:
where x i = dimensionless coded value of independent variable, z i = uncoded value of the ith independent variable, z 0 = uncoded ith independent variable at the centre point and the Δz i = step change value between the low level (−1) and high level (+1).
The regression analysis of the experimental data was prepared using the software Design Expert 8.0 (Stat-Ease Corporation, Minneapolis, USA). The software was also used for obtaining the 3-D surface plots and contour plots of the response models.
Analytical methods
In order to estimate the total metals present in the WTS, acid digestion method by the US EPA 3050B (US EPA 1996) was followed. The resultant solution was analysed for metals using inductively-coupled plasma atomic emission spectrometry (ICP AEP) (ARCOS Spectro, Germany). The chemical oxygen demand (COD) of the samples was analysed using the closed reflux titrimetric method in accordance with the Standard Methods (APHA 1998). pH and turbidity were measured using pH metre (Hanna 209) and turbidimeter (Hach 2100P), respectively. Phosphate was measured with the ammonium molybdate method using a UV–visible spectrophotometer (Varian CARY50).
Results and discussion
Preliminary tests
Preliminary tests were conducted by varying one variable at a time while keeping all other variables at fixed conditions. Preliminary tests were conducted only for PACl sludge, while RSM study was conducted for both PACl and alum with the same experimental conditions. Results of the test to determine the effect of pH on aluminium recovery along with the acid consumption are given in Fig. 1. The aluminium recovery pattern at different pH is similar to that found in the literature (Chen et al. 2012).
Recovery primarily depends on the solution pH used, with higher recovery reported at lower pH.
Aluminium recovery of 61–100 % has been reported from WTS using acidification for pH range 1–3 (Abdo et al. 1993; Chen et al. 2012; Xu et al. 2009). For example, Xu et al. (2009) reported a rate of 83.6 % at pH of 2.5, a much higher recovery at a relatively higher pH value compared to that in the present study. They, however, used a sludge prepared in the laboratory, while in the present study, sludge from a full-scale plant was used. Further, characteristics of the suspended solids, colloids, and some soluble species present in the source water which would be part of coagulation flocs, type and dose of coagulant used can also affect the recovery (Chen et al. 2012; Wang and Yang 1975).
While the aluminium recovery in this study increased from 62.3 to 80.8 % as the pH was brought down from 2.0 to 1.0 (Fig. 1), the amount of acid consumed increased substantially (from 4.4 to 22.3 mmol), indicating the unsuitability of pH 1.0 for aluminium recovery. At pH 1.5, the aluminium recovery was 73.34 % with a reasonable acid consumption of 14.4 mmol. Thus, a pH range of 1.5–2.0 was chosen in the experimental design with RSM.
Another test was conducted to select the range of mixing time for the recovery process. Results of the test presented in Fig. 2 indicate an increase in recovery of up to 30-min mixing time. Similar pattern for mixing intensity has been reported by Xu et al. (2009). Cheng et al. (2012) studied the effect of mixing time on aluminium recovery at different temperatures and found similar pattern for increase in aluminium recovery with mixing time. However, the optimum mixing time varied for different temperatures. The range of 10–30 min was selected for the mixing time in the experimental design for RSM. For the third variable, i.e. sludge dosage/solid content, a range of 0.5–1.5 % was employed in the experimental design. Mixing intensity was kept constant at 100 rpm, as metal leaching ratio does not increase with mixing speed beyond 80 rpm (Cheng et al. 2012).
RSM model and its validation
Experimental results of the aluminium recovery from PACl and of the alum sludge are presented in Table 2. Based on the experimental data, regression models using a second-order polynomial as represented by Eqs. (4) and (5) were developed for aluminium recovery from PACl and alum sludge respectively.
Al recovery, y 1
Al recovery, y 2
where x 1 = pH, x 2 = solid content, x 3 = mixing time, y 1 = PACl sludge and y 2 = alum sludge.
Analysis of variance (ANOVA) results for the Eqs. (4) and (5) are presented in Tables 3 and 4 respectively. The adequacy and significance of the model are generally checked by model F values, p values (P > F) and adequate precision (Ghafari et al. 2009; Mohajeri et al. 2010). A model and each model terms are significant at 95 % confidence interval if the F test has a p value below 0.05. For the present models, the p value is <0.0001 showing the significance of both the models. The p values of each model terms involved in the aluminium recovery from PACl and alum sludge are presented in Tables 3 and 4 respectively. However, only the statistically significant model terms (p value <0.05) are included in the Eqs. (3) and (4). A large p value for lack of fit, possibly >0.05, is preferred, as it measures the model failure in representing data points in the experimental domain (Mohajeri et al. 2010). In the present case, the values are 0.0592 and 0.5663 for aluminium recovery models for PACl and alum sludge respectively, implying that lack of fit of the model is insignificant. Adequate precision (AP) is the ratio of range of predicted values of the design points to the average standard deviation of all predicted responses and should preferably be greater than 4 (Anderson and Whitcomb 2005). In the present study, AP values of 35.68 and 65.98 were obtained for aluminium recovery for PACl and alum respectively, which confirm that the models can be used for predicting the aluminium recovery.
The overall prediction performance of the model is described by coefficient of determination (R 2). A high R 2 value, close to 1, is desirable to ensure a satisfactory adjustment of the model to the experimental data (Anderson and Whitcomb 2005; Nair et al. 2014). The value of R 2 = 0.9869 and 0.9967 for aluminium recovery from PACl and alum sludge respectively. Also, a reasonable agreement of R 2 with adjusted R 2 (R 2 adj) is necessary (Moghaddam et al. 2010; Nair et al. 2014). In the present models, the values of R 2 adj = 0.9790 and 0.9942 were close to R 2 = 0.9869 and 0.9967 for PACl and alum sludge respectively, indicating high significance of the model. If a model contains many terms and the sample size is not large, R 2 adj may be significantly lower than R 2 (Liu et al. 2004; Zhang et al. 2012). The residual plots of the models are presented in Fig. 3. The residuals are normally distributed if the points on the plot follow a straight line. As Fig. 3 illustrates, the assumption of normality is satisfied for the models.
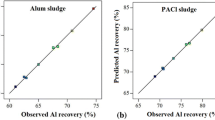
The empirical model developed using RSM study shown in Eqs. (4) and (5) can be used to predict new aluminium recovery values within the experimental domain. For the model to be reliable, the response should be predicted with a reasonable accuracy by the model equation (Moghaddam et al. 2010; Zhang et al. 2012). Figure 4 and Table 2 present the observed and predicted values of aluminium recovery from PACl and alum sludge. The statistical significances of the models are evident from Fig. 4, as observed and predicted values are in good agreement with each other.
Analysis of the coagulant recovery results
In order to explain the effects of variables and their interactive effects, 3-D plots and their corresponding contour plots were generated based on the model developed. These plots are generated as a function two variables at a time, keeping the third variable at a fixed level (centre level). Figures 5 and 6 show the response surfaces for aluminium recovery from PACl and alum sludge respectively. It is evident from Figs. 5a, b and 6a, b that the aluminium recovery continuously increased with decreasing pH from 2.0 to 1.5. Aluminium hydroxide precipitates are the dominant species in the aluminium sludge, and addition of more acid dissolves larger amounts of aluminium from the sludge according to the following equation, resulting in higher recovery at lower pH (Abdo et al. 1993; Li et al. 2005):
Figures 5b, c and 6b, c show that at all conditions of pH and solid content, increase in mixing time increased the sludge recovery. However, the effect of mixing time on recovery was more prominent at higher pH compared to that at lower pH. Figures 5a, c and 6a, c illustrate that aluminium recovery decreased with increase in solid content. Here, also, the effect of solid content on recovery was more significant at higher pH values compared to that at lower values. It should be noted that though higher solid content would result in reduced percentage aluminium recovery, the concentration of aluminium in the recovered solution would be greater at higher solid content. This is due to the higher amount of sludge taken at higher solid content. Thus, if the objective of the recovery process is to obtain a concentrated coagulant solution, then the use of a higher solid content would be preferred. However, if the recovery process is aimed at maximising aluminium recovery from the sludge, then it would be advantageous to use a lower solid content.
In order to determine the optimised conditions for aluminium recovery from the sludge, a cost-based approach is desired by considering the chemical (acid) consumption. The amount of acid consumed for each aluminium recovery experiment for both PACl and alum sludge was also added and analysed as responses (data not shown). The optimisation was performed using the software Design Expert 8.0, which allows choosing the desired goal for each variable and response. Hence, optimisation of the process was carried out to obtain maximum aluminium recovery with minimum acid consumption with the help of the desirability function (Montgomery 2010). In the optimisation process, the desired goals for aluminium recovery and acid consumption were set as “maximum” and “minimum” respectively, while for the independent variables, the goal was chosen as “within range.” The optimum conditions derived from this study are presented in Table 5. In order to confirm the results of the model studies, additional confirmation tests were conducted. The results obtained, as presented in Table 5, show that the measured aluminium recovery was close to the predicted value using the regression model. This further demonstrates the usefulness of RSM in modelling aluminium recovery process from WTS. The RSM models (Figs. 5 and 6) and predicted and experimental values of the aluminium recovery (Table 5) show that aluminium recovery from PACl sludge is greater than that from alum sludge which confirms with the findings reported in literatures (Chen et al. 2012).
Performance of recovered coagulant
An analysis of the recovered coagulant solution at the optimum conditions was performed for different metals, and the results are presented in Table 6. It is seen that in addition to Al, metals such as iron, manganese, copper, magnesium, sodium, potassium and calcium were also present in significant quantities in the recovered solution. The coagulant recovered at optimum conditions from PACl sludge has higher Al, Ca and Mg content than that from alum sludge, while the Fe content is higher in case of coagulant recovered from alum sludge. This difference is due to their initial concentration in raw sludge itself (Table 1). The concentrations of toxic elements like Cd and Cr are lower in coagulant recovered from PACl sludge than those from alum sludge. Pb is below the detection limit in both cases. The COD of the recovered coagulant was also high, indicating the dissolution of organic matter from the sludge. Dissolved organic carbon (DOC) concentration in the range of 326–1,800 mg/L has been reported in the recovered coagulant from drinking water treatment plant (Prakash and Sengupta 2003). While the presence of iron, calcium and magnesium is beneficial since these elements can act as coagulants, elevated levels of other elements are of concern. This indicates the unsuitability of the recovered coagulant for use in drinking water treatment. However, it can be reused for wastewater treatment (Ishikawa et al. 2007; Xu et al. 2009).
In the present study, the aluminium recovered from PACl sludge was only reused as coagulant for post-treatment of UASB reactor effluent due to its higher Al, Ca and Mg content and lower content of toxic elements like Cr, Cd and Pb. Figure 7 shows the phosphate, COD and turbidity removal of recovered coagulant and fresh PACl. The recovered coagulant gave 89 % phosphate, 71 % COD and 80 % turbidity removal at 25 mg Al/L. Though similar removal efficiency was obtained with recovered coagulant and fresh PACl, a higher dose of 40 mg Al/L was required with fresh PACl. This may be because of the presence of Ca2+, Mg2+ and Fe3+ in the recovered coagulant along with Al3+ which contribute in the coagulation process (Xu et al. 2009), while in fresh PACl, only aluminium contributes to the coagulation process. The presence of organic matter in the recovered coagulant also improves the phosphate removal from the UASB rector effluent (Ishikawa et al. 2007). In addition, the recovered coagulant also gave 77 % suspended solids and 99.5 % total coliform reduction at 25 mg Al/L. The UASB effluent after treated with the coagulant recovered from PACl sludge at 25 mg Al/L had the following mean characteristics: pH = 6.4, turbidity = 41 NTU, SS = 34 mg/L, COD = 68 mg/L, phosphate = 0.5 mg/L and total coliform = 3.2 × 105 MPN/100 mL. The effluent can be discharged safely into water bodies after disinfection process.
Reuse of the coagulants recovered from the WTS could substantially reduce the chemical coagulant requirement for post-treatment of UASB reactor effluent, thereby reducing the cost involved in buying the fresh coagulants and also the consumption of natural resources and fossil fuel utilised for the production of chemical coagulants. Massides et al. (1988) reported 50–60 % reduction in the operating cost of coagulation process due to savings in chemicals to treat wastewater.
Sludge volume reduction and residual sludge disposal
Figure 8 shows that acidification of sludge caused reduction in total volume of the sludge. At the optimum conditions volume reduction of 59.8 % and 45.6 % were obtained with PACl and alum sludge respectively. This reduction in sludge at lower pH could be due to the dissolution of aluminium hydroxide and other aluminium oxides (Huang et al. 2010; Xu et al. 2009). Xu et al. (2009) reported a linear relationship between the reduction of sludge and coagulant recovery. Sengupta and Shi (1992) reported reduction in sludge disposal cost by 28 % due to reduced sludge volume after acidification. However, the acidic sludge is toxic and corrosive in nature and, hence, has to be neutralised before disposal.
Conclusions
In this study, the aluminium recovery from the water treatment sludge using acidification was optimised with the help of RSM. The second-order regression models could simulate well the effects of the different variables and their interactions in the recovery process. The optimum coagulant recovery was obtained at pH 2.0, solid content of 0.5 % and mixing time of 30 min. Confirmation experiment at optimum conditions showed an aluminium recovery of 73.26 and 62.73 % for PACl and alum sludge respectively, which was close to the optimum value predicted by the models. The recovered aluminium could not be reused for drinking water treatment due to the impurities present in it. Hence, the aluminium recovered from PACl sludge was reused as coagulant for post-treatment of UASB reactor effluent. The recovered coagulant at 25 mg Al/L dose gave 89 % phosphate, 71 % COD, 80 % turbidity, 77 % suspended solids and 99.5 % total coliform reduction removal from UASB reactor effluent. The quality of the wastewater treated with recovered coagulant and fresh coagulant was comparable. This shows that the coagulant recovered from the water treatment sludge can be substituted effectively for fresh coagulant in wastewater treatment and reducing the cost of the UASB post-treatment process. Acidification also reduced the sludge volume, thus reducing the sludge handling cost.
References
Abdo MSE, Ewida KT, Youssef YM (1993) Recovery of alum from wasted sludge produced from water treatment plants. J Environ Sci Health Part A: Environ Sci Eng Toxicol: Toxic/Hazard Subst Environ Eng 28:1205–1216
American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environmental Federation (WEF) (1998) Standard methods for the examination of water and wastewater. 20th Ed., Clescerl L, Greenberg A. and Eaton A. eds., American Public Health Association, Washington, DC
Anderson MJ, Whitcomb PJ (2005) RSM simplified. Productivity Press, New York
Babatunde AO, Zhao YQ (2007) Constructive approaches toward water treatment works sludge management: an international review of beneficial re-uses. Crit Rev Environ Sci Technol 37:129–164
Babatunde AO, Zhao YQ (2010) Leachability and leaching patterns from aluminium-based water treatment residual used as media in laboratory-scale engineered wetlands. Environ Sci Pollut Res 17:1314–1322
Bashir MJK, Isa MH, Kutty SRM, Awang ZB, Aziz HA, Mohajeri S, Farooqi IH (2009) Landfill leachate treatment by electrochemical oxidation. Waste Manag 29:2534–2541
Belyaeva ON, Haynes RJ (2012) Use of inorganic wastes as immobilizing agents for soluble P in green waste-based composts. Environ Sci Pollut Res 19:2138–2150
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Chen YJ, Wang WM, Wei MJ, Chen JL, He JL, Chiang KY, Wu CC (2012) Effect of Al-coagulant sludge characteristics on the efficiency of coagulant recovery by acidification. Environ Tech 33:2525–2530
Cheng WP, Fu CH, Chen PH, Yu RF (2012) Dynamics of aluminum leaching from water purification sludge. J Hazard Mater 217–218: 149–155
Chong S, Sen TK, Kayaalp A, Ang HM (2012) The performance enhancements of upflow anaerobic sludge blanket (UASB) reactors for domestic sludge treatment—a state-of-the-art review. Water Res 46:3434–3470
Dharmappa HB, Hasia A, Hagare P (1997) Water treatment plant residuals management. Water Sci Technol 35:45–56
Duan J, Gregory J (2003) Coagulation by hydrolysing metal salts. Adv Colloid Interface Sci 100–102:475–502
Ghafari S, Aziz HA, Hasnain MI, Zinatizadeh AK (2009) Application of response surface methodology (RSM) to optimize coagulation flocculation treatment of leachate using poly aluminum chloride (PAC) and alum. J Hazard Mater 163:650–656
Gibbons MK, Mortula MM, Gagnon GA (2009) Phosphorus adsorption on water treatment residual solids. J Water Supply Res Technol–AQUA 58:1–10
Hovsepyan A, Bonzongo JCJ (2009) Aluminium drinking water treatment residuals (Al-WTR) as sorbent for mercury: implications for soil remediation. J Hazard Mater 164:73–80
Huang S, Chen J-L, Chiang K-Y, Wu C-C (2010) Effects of acidification on dewaterability and aluminum concentration of alum sludge. Sep Sci Tech 45:1165–1169
Ippolito JA, Scheckel KG, Barbarick KA (2009) Selenium adsorption to aluminum-based water treatment residuals. J Colloid Interface Sci 338:48–55
Irawan C, Liu JC, Wu CC (2011) Removal of boron using aluminum-based water treatment residuals (Al-WTRs). Desalination 276:322–327
Ishikawa S, Ueda N, Okumura Y, Lida Y, Baba K (2007) Recovery of coagulant from water supply plant sludge and its effect on clarification. J Mater Cycles Waste Manag 9:167–172
Li C-W, Lin J-L, Kang S-F, Liang C-L (2005) Acidification and alkalization of textile chemical sludge: volume/solid reduction, dewaterability, and Al(III) recovery. Sep Purif Technol 42:31–37
Liu H-L, Lan Y-W, Cheng Y-C (2004) Optimal production of sulphuric acid by Thiobacillus thiooxidans using response surface methodology. Process Biochem 39:1953–1961
Makris KC, Sarkar D, Datta R (2006) Evaluating a drinking water waste by-product as a novel sorbent for arsenic. Chemosphere 64:730–741
Makris KC, Sarkar D, Salazar J, Punamiya P, Datta R (2010) Alternative amendment for soluble phosphorus removal from poultry litter. Environ Sci Pollut Res 17:195–202
Massides J, Soley J, Mata-Alvarez J (1988) A feasibility study of alum recovery in wastewater treatment plants. Water Res 22:399–405
Moghaddam SS, Moghaddam MRA, Arami M (2010) Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 175:651–657
Mohajeri S, Aziz HA, Isa MH, Zahed MA, Adlan MN (2010) Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J Hazard Mater 176:749–758
Monteiro SN, Alexandre J, Margem JI, Sanchez R, Vieira CMF (2008) Incorporation of sludge waste from water treatment plant into red ceramic. Constr Build Mater 22:1281–1287
Montgomery DC (2010) Design and analysis of experiments, 7th edn. Wiley India Pvt, New Delhi
Muisa N, Hoko Z, Chifamb P (2011) Impacts of alum residues from Morton Jaffray Water Works on water quality and fish, Harare, Zimbabwe. Phys Chem Earth Parts A/B/C 36:853–864
Nair AT, Ahammed MM (2013) The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J Clean Prod. doi:10.1016/j.jclepro.2013.12.037
Nair AT, Makwana AR, Ahammed MM (2014) The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: a review. Water Sci Technol 69:464–478
Pan JR, Huang C, Lin S (2004) Reuse of fresh water sludge in cement making. Water Sci Technol 50:183–188
Parsons SA, Daniels SJ (1999) The use of recovered coagulants in wastewater treatment. Environ Technol 20:979–986
Prakash P, Sengupta AK (2003) Selective coagulant recovery from water treatment plant residuals using Donnan membrane process. Environ Sci Technol 37:4468–4474
Sengupta A, Shi B (1992) Selective alum recovery from clarifier sludge. J Am Water Works Assoc 84:96–103
Sujana MG, Thakur RS, Rao SB (1998) Removal of fluoride from aqueous solution using alum sludge. J Colloid Interface Sci 206:94–101
US EPA (1996) Acid digestion of sediment, sludge and soils. Method 3050B, second edition. US Environmental Protection Agency, US Governmental Printing Office, Washington DC
Wang C, Pei Y (2013) Effects of light, microbial activity, and sediment resuspension on the phosphorus immobilization capability of drinking water treatment residuals in lake sediment. Environ Sci Pollut Res 20:8900–8908
Wang LK, Yang JY (1975) Total waste recycle system for water purification plant using alum as primary coagulant. Resour Recover Conserv 1:67–84
Xu GR, Yan ZC, Wang YC, Wang N (2009) Recycle of alum recovered from water treatment sludge in chemically enhanced primary treatment. J Hazard Mater 161:663–669
Zhang H, Li Y, Wu X (2012) Statistical experiment design approach for the treatment of landfill leachate by photoelectro-Fenton process. J Environ Eng 138:278–285
Zhou Y-F, Haynes RJ (2011) Removal of Pb(II), Cr(III) and Cr(VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut 215:631–643
Acknowledgment
The authors would like to thank the Department of Science and Technology (DST), Government of India, for providing the financial support through a grant (Diary No. SERB/F/7425/2013-14) under the Science and Engineering Research Board. Authors would also like to thank DST and SAIF-IITB for providing the analytical services for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Nair, A.T., Ahammed, M.M. Coagulant recovery from water treatment plant sludge and reuse in post-treatment of UASB reactor effluent treating municipal wastewater. Environ Sci Pollut Res 21, 10407–10418 (2014). https://doi.org/10.1007/s11356-014-2900-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2900-1