Abstract
Nowadays, aquaculture activities play a major role in increasing the organic waste and toxic compound in the aquatic water bodies. Along with the development of aquaculture, there is an ever-increasing aquaculture waste both on productivity inside the aquaculture system and on the ambient aquatic ecosystem. Therefore, it is apparent that appropriate wastewater recycling processes are needed for sustainable aquaculture development. Hence, the best environmentally acceptable “biofloc technology” (BFT) has been developed. The present study aims the application of BFT to recycle the wastewater nutrients. Pacific white shrimp, Litopenaeus vannamei, was selected to be culture. Three cement tanks C1, C2, and C3 with a stocking density of 120 PL/m2 were assigned for the study. C1 is taken as control and C2, C3 as test. In BFT, excess of nutrients in aquaculture systems is converted into microbial biomass, which can be consumed by the cultured animals as a food source and the remaining are used for agriculture purpose. This technology has also the capacity to control harmful pathogens in aquaculture. The microorganisms of biofloc successfully converted the toxic ammonia and nitrite into nutritious diet and maintained the water quality in the safety levels for the shrimp. This study has been made to summarize the features and management aspects of the environmental friendly BFT to achieve sustainable aquaculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biofloc technology
- Sustainable aquaculture
- Recycling
- Wastewater
- International society of waste management
- Air and water
1 Introduction
Globally, India occupies the second position after China, with sharing 10.0% of world’s aquaculture production (FAO 2016). The vast resources in terms of water bodies and species of fish and shellfish in different regions of the country provide a wide array of culture systems and practices. The shrimp, Litopenaeus vannamei, is the major contributor in the aquaculture production. However, intensification of the aquaculture activities generates an immense amount of excess organic pollutants that are likely to cause acute toxic effects and long-run environmental risks (Piedrahita 2003). It is reported that effluents released from the aqua farms contain more pollutants than the other industries. A lot of chaos from the public and the environmentalists is due to the discharge of untreated aqua wastages into natural water bodies. Discharge of this untreated water is contaminating the natural water bodies causing outbreak of diseases, imbalancing the biodiversity and ecosystem. To overcome all these challenges and to conserve the ecological sustainability, biofloc technology (BFT) has been developed. Because of economical, environmental, and marketing advantages over the conventional system, BFT has been implemented successfully in shrimp farming. Compared to conventional aquaculture techniques, BFT provides more economical alternative and sustainable technique in terms of minimal water exchange and reduced feed input making it a low-cost sustainable technology for sustainable development (Avnimelech and Kochba 2009; De Schryver et al. 2008). BFT involves the manipulation of C/N ratio to convert toxic nitrogenous wastes into the useful microbial protein and helps in improving water quality under a minimal/zero water exchange system (Ahmad et al. 2017). Moreover, BFT offers a sustainable aquaculture tool by simultaneously addressing its environmental, social, and economical issues. In this context, BFT can also be used in the specific case of maintaining appropriate water quality, and thus, it can reduce the pollution of the pond water. With these positive effects, the present study was undertaken to evaluate the growth and survival of L. Vannamei in BFT.
2 Materials and Methods
2.1 Tank and Floc Preparation
Specific-pathogen-free (SPF) seed, L. vannamei (PL 9), obtained from a local hatchery. The PL was carried in oxygenated double-layered polythene bags to the laboratory in specific fish transportation tanks. Before releasing the PL, all the tanks were cleaned and disinfected using bleaching powder and dried for 2 days. The biofloc was prepared as per the protocol described by Avnimelech (2012). In brief, on the first day ammonium chloride was added to initiate nitrogen source in the system. On third and fifth day carbon sources were added and on day seven double the quantity of carbon source was added. Due to the addition of carbon and nitrogen source, the color of the water changed to light brown indicating the formation of floc. After tank preparation on the day nine, the PL was brought to farm side and were treated with potassium permanganate, and tank water was added slowly into the seed bags to adjust the salinity and pH; subsequently, the PL was released slowly into the tanks C1, C2, C3 (each with 54.6 m2) at a stocking density of 120/m2. Among three tanks (C1, C2, C3), C1 was taken as control, i.e., without application of biofloc, and C2, C3 were taken as test which consist of biofloc. All the tanks were supplied with aeration for sufficient supply of oxygen which is necessary for the formation of biofloc and also for shrimp.
2.2 Analysis of Water Quality Parameters
Water samples for the analysis were carried throughout the study period. Surface water temperature was recorded between 6:00 and 9:00 during the morning hours and between 5:00 and 6:00 during the evening hours with the help of Mercury thermometer. Samples were collected in separate reagent bottles and analyzed by standard methods of (APHA 2005). pH of the water was measured by using the laboratory model ELICO pH meter. Transparency of the water column was assessed with the help of Secchi disk. In case of dissolved oxygen, the collected water samples were fixed with modified/Winkler’s titration method (APHA 2005). The cones Imhoff were used to measure the quantity of biofloc which have marked graduations on the outside that can be used to measure the volume of solids that settle from 1 L of system water.
2.3 Microbiological Analysis of Water
Microbiological analysis of water was done regularly thrice a week. To observe the Vibrio count, spread plate technique was used. One ml of cultured water was serial diluted and 108, 109 ml of serially diluted culture water was spreaded on Thiosulfate citrate bile salts sucrose (TCBS) agar and incubated overnight at 37 °C to find out the presence of Vibrio spp.
2.4 Feed Management
L. vannamei fed with commercial diet, Blanca feed pellets (CP Aquaculture, India private limited) was used for four times daily at 6 am, 10 am, 2 pm, 6 pm. The feed quantity was estimated depending on the floc volume. No water exchange was done during the culture period except addition of water to refill the water levels after siphoning out the wastes. Sludge was removed regularly in order to control Nitrite and TSS in the system.
3 Results and Discussions
Water quality parameters, i.e., salinity, temperature, pH, dissolved oxygen, and transparency ranges, are presented in Table 1. Dissolved oxygen (DO) concentration and temperature were similar between treatments throughout the trial. The oxygen dissolved in the system is an important factor not only for the respiration of aquatic organisms but also to maintain favorable chemical and hygienic environment. Muthu (1980) stated that the DO should not be lower than 3.5 ml/l in shrimp culture pond. But in the biofloc system, the aeration should be more than 5.0 mg/l (Avnimelech 2012). In the present investigation, the dissolved oxygen content was ranged from 6.0 to 7.0 mg/l in the system. Continuous aeration was done during the culture period, and therefore, the oxygen level exceeds the limit. The temperature is an ecological factor which influences the hydrological parameters, which in turn influences the metabolism, growth, and other biochemical processes. The optimum range for warm water species is 24–30 °C (Ramanathan et al. 2005). In the present study, the water temperature range varied from 27 to 29 °C. Shrimps are poikilothermic that can modify their body temperature to the environment in normal conditions. The continuous aeration will circulate the equal temperature in the biofloc system. pH is one of the important environmental parameters which decides physiological process of shrimps. The optimum range of pH is from 6.8 to 8.7 and should be maintained for maximum growth and production (Ramanathan et al. 2005). The best condition is from 7.8 to 8.5. If pH changes significantly, it makes shrimp shocked, weakened, and stops eating. If high or low pH extends for a long time, it will make shrimp grow slowly and susceptible to diseases. In the present study, the pH concentration was ranged from 8.0 to 8.5 which is the best condition for shrimp growth. Salinity is the most important factor influencing many functional responses of the organism such as metabolism, growth, migration, osmotic behavior, reproduction. Sudden fluctuation in salinity will affect the osmoregulatory functions of growing organism and lead to mortality. In the present study, the salinity was maintained at 6 to 7 ppt. During the culture period, the salinity was maintained at 6 ppt, but sometimes, it reached 7 ppt. The transparency of water was 15 cm.
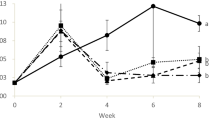
The values of ammonia, nitrite, and nitrate were presented in Fig. 1. The results revealed that the ammonia was 0.05 mg/l in the system initially due to the addition of ammonium chloride. After that, it increased to 0.08 mg/l and then gradually decreased. The heterotrophic bacteria present in the biofloc can absorb ammonia 40 times faster than nitrifying bacteria. Thus, biofloc has the capability of balancing ammonia concentration in the culture system (Ebeling et al. 2006). In addition, De Schryver and Verstraete (2009) stated that biofloc grown in the bioreactor can convert 98% ammonia into nitrate at a concentration of 110 mg L−1 day−1. The ability of heterotrophic bacteria to absorb the ammonia is influenced by the ratio of carbon and nitrogen (C/N ratio) in water where the ammonia will be absorbed quickly when C/N was higher (Schneider et al. 2006). The nitrite level was 0.01 mg/l, and then it increased to 2.0 mg/l after 15 days of culture. By increasing the carbon source, removing sludge, and decreasing the feed quantity controlled the nitrite level to 0.5 mg/l. The low value of nitrite in the biofloc system is probably due to the conversion of ammonia to nitrite by heterotrophic bacteria which in turn utilized by micro-algae for growth (Avnimelech 1999; Ebeling et al. 2006). This is an indication that the nitrification process occurred in the culture medium (Stickney 2005). Nitrate, which is the final product from the nitrification of ammonia, is the least harmful nitrogenous compound for shrimp (Van Wyk and Scarpa 1999). In the present study, nitrate ranged between 100 and 150 mg/l. Lower nitrite and higher nitrate concentrations were recorded, due to the formation of biofloc. The total hardness of the water was 1800–1850 mg/l. When alkalinities are maintained from 40 to 160 mg L−1 in the BFT system, no significant effect on the TSS, ammonium, nitrites, and nitrates has been determined (Piérri 2012). pH increases when the alkalinity increases above 160 mg L−1, as well as the tendency of increase of heterotrophic and ammonium oxidant bacteria increases with a decrease in nitrites oxidant bacteria (Piérri 2012). Thrice during the culture period alkalinity was reduced to 100 mg/l, in order to increase it sodium bicarbonate was added to maintain the C:N ratio at 15:1. Calcium and magnesium were maintained at a rate of 1:2.5. No H2S was observed during the entire culture period. In the initial days, the color of the water was light brown and gradually changed to brown color and then to green color. In the day one, the floc volume was 0.1 ml/l, and it increased to 15 ml/l. The quantity and quality of floc were monitored regularly.
The floc volume was presented in Fig. 2. The microscopic observation of floc shows rotifers, copepods, algae, bacteria, etc. But sometimes protozoans like zoothamnium and vorticella were also observed in the floc. Few yellow colonies of Vibrio spp were seen in microbiological analysis; however, there is no disease outbreak throughout the culture period. In aquaculture, the principal source of waste is ultimately the manufactured feeds that are necessary to increase production beyond natural levels (Iwama 1991). According to Avnimelech (2012), reducing the feed quantity and increasing the floc volume reduces the aquaculture waste and also the feed cost. Hence, the adoption of this technology increases the efficiency of feed utilization, because organic and inorganic metabolites, as well as unused or partially used food, are recycled by microorganisms into microalgae and bacterial biomass, which tend to coalesce into flocculated material (bioflocs).
In the present study, biofloc tanks achieved a survival rate of 77% and the growth rate was 27 g with a total production of 135 kgs. Large amounts of manure are required to meet the nitrogen requirements of agricultural crops. For regional agricultural field crops, aquaculture manure is also used as principal nitrogen source for better effects. On-site, smaller-scale agricultural ventures or non-commercial gardening seem more appropriately scaled for using aquaculture wastes (Yeo et al. 2004). The post-harvested water was used to grow vegetables present near the pond or else the same water can be reused to culture the next crop.
4 Conclusion
Biofloc technology is an environmental and sustainable technology used in aquaculture to maintain water quality through converting nitrogenous waste into bacterial proteinaceous biomass after the addition of carbohydrate sources and also can be subsequently consumed by the cultivated aquatic organisms. In the present study, the biofloc system can maintain the good water quality parameters and recycles the wastewater in shrimp culture. Hence, the BFT is an eco-friendly approach reducing pollution; as there is zero or minimal water exchange to eradicate the environmental pollution and economic problems associated with aquaculture.
References
Ahmad, I., Rani, A. B., Verma, A. K., & Maqsood, M. (2017). Biofloc technology: an emerging avenue in aquatic animal healthcare and nutrition. Aquaculture International, 25(3), 1215–1226.
Akpor, O. B., & Muchie, M. (2010). Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. International Journal of Physical Sciences, 5(12), 1807–1817.
APHA. (2005). Standard methods for the examination of the water and wastewater. Washington, D.C.: American Public Health Association.
Avnimelech, Y. (1999). Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture, 176(3–4), 227–235.
Avnimelech, Y. (2012). Biofloc technology, a practical guide book (2nd ed.). Baton Rouge, Louisiana, EUA: The world Aquaculture Society.
Avnimelech, Y., & Kochba, M. (2009). Evaluation of nitrogen uptake and excretion by tilapia in bio floc tanks, using 15 N tracing. Aquaculture, 287(1–2), 163–168.
De Schryver, P., Crab, R., Defoirdt, T., Boon, N., & Verstraete, W. (2008). The basics of bio-flocs technology: The added value for aquaculture. Aquaculture, 277(3–4), 125–137.
De Schryver, P., & Verstraete, W. (2009). Nitrogen removal from aquaculture pond water by heterotrophic nitrogen assimilation in lab-scale sequencing batch reactors. Bioresource Technology, 100(3), 1162–1167.
Ebeling, J. M., Timmons, M. B., & Bisogni, J. J. (2006). Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture, 257(1–4), 346–358.
FAO, I. (2016). WFP (2015), the State of Food Insecurity in the World 2015. Meeting the 2015 international hunger targets: taking stock of uneven progress. Food and Agriculture Organization Publications, Rome.
Iwama, G. K. (1991). Interactions between aquaculture and the environment. Critical Reviews in Environmental Science and Technology, 21(2), 177–216.
Muthu, M. S. (1980, August). Site selection and type of farms for coastal aquaculture of prawns. In Proceedings of the First Mat. Symposium on Shrimp Farming (pp. 97–106). Marine Products Export Development Authority, India.
Piedrahita, R. H. (2003). Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture, 226(1–4), 35–44.
Piérri, V. (2012). The effect of biofloc technology (BFT) on water quality in white shrimp Litopenaeus vannamei culture.
Ramanathan, N., Padmavathy, P., Francis, T., Athithian, S., & Selvaranjitham, N. (2005). Manual on polyculture of tiger shrimp and carps in freshwater (pp. 1–161). Tamil Nadu Veterinary and Animal Sciences University, Fisheries College and Research Institute, Thothukudi.
Schneider, O., Sereti, V., Eding, E. H., & Verreth, J. A. (2006). Molasses as C source for heterotrophic bacteria production on solid fish waste. Aquaculture, 261(4), 1239–1248.
Stickney, R. R. (2005). Aquaculture: An introductory text (p. 265). Massachusetts: CABI Publication.
Van Wyk, P., & Scarpa, J. (1999). Water quality requirements and management. In Farming marine shrimp in recirculating freshwater systems (pp. 128–138).
Yeo, S. E., Binkowski, F. P., & Morris, J. E. (2004). Aquaculture effluents and waste by-products characteristics, potential recovery, and beneficial reuse.
Acknowledgements
The authors would like to thank the Department of Zoology and Aquaculture, Acharya Nagarjuna University, Guntur, Andhra Pradesh. We would also like to convey our thanks to our beloved friends who gave support and their help in organizing the research work.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kavitha, K., Krishna, P.V. (2020). Sustainable Growth and Survival of Litopenaeus Vannamei Through Wastewater Recycling. In: Ghosh, S., Saha, P., Francesco Di, M. (eds) Recent Trends in Waste Water Treatment and Water Resource Management. Springer, Singapore. https://doi.org/10.1007/978-981-15-0706-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-0706-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0705-2
Online ISBN: 978-981-15-0706-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)