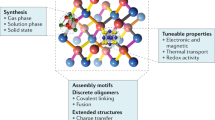
Abstract
This chapter describes two superatoms, each comprising a central atom and a silicon or aluminum cage. Binary nanoclusters (NCs) at optimized mixing ratios are key components in designing the functionalities relevant to their electronic properties. To form chemically robust functional NCs, it is important to design the cooperatively synergistic effects between the electronic and geometric structures because these stabilize the individual NCs not only against charge transfer into the corresponding cations or anions but also against structural perturbations in their assemblies. Among binary NCs, synergistic effects are particularly expected when one central atom encapsulating cage structure completes a specific electron shell because electronic and geometric factors can operate simultaneously. Although the term “superatom” is widely used when the valence electrons in NCs complete an electron shell, more synergistic effects appear when the superatom adopts a close-packed structure, such as a highly symmetric cage as a binary cage superatom. Representative examples are given for one central atom encapsulated by silicon and aluminum cages, M@Si16 and X@Al12, their formation and characterization are described, and a large-scale synthetic approach is established for M@Si16. The perspectives for binary cage superatom assembly are discussed in terms of theoretical calculations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanocluster
- Superatom
- Binary cage superatom
- Silicon cage
- Aluminum cage, superatom salt
- Dimeric superatom
- Superatom assembly

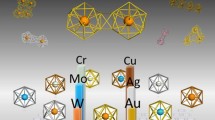
Periodic table for binary cage superatoms of X@Al12 and M@Si16
1 Introduction
Aluminum (Al) and silicon (Si) are elements adjacent to each other in the periodic table. However, their electrical characteristics are distinctly different; Al is highly conductive, while Si is semiconductive. Both are found abundant on the earth, and they are indispensable elements for technological civilizations in modern society.
Si element has greatly contributed to modern society as an excellent semiconductor electronic material from the mid-twentieth century, in terms of innovative progress in informationalization. Particularly, photolithographic cycles for patterning circuits on Si substrates have allowed the development of highly integrated electronic devices by large-scale integration (LSI). Moreover, LSI has been continuously downsized with shorter wavelength lithography until the twenty-first century according to Moore’s law [1]. Even though the miniaturization is finally approaching a physical limit of a scale of several nanometers, Si element still plays a central role as the main electronic material in modern society. In contrast to the top-down approach such as fine photolithography toward bulk Si, it is also crucial to explore electronic materials by a bottom-up approach using Si atoms.
Beyond the physical limit of several nanometers, creating nanoscale Si compounds can provide novel functionalities, including luminescence and thermoelectric properties [2,3,4,5]. Above all, nanoclusters (NCs) of several to hundreds of atomic aggregates allow us to develop the potential for promising Si compounds for exploring the bottom-up Si nanotechnology. When fullerene C60 was discovered in a NC beam from carbon vapor [6], much attention was also focused on creating nanoscale Si species having the same cage structure as elemental Si [7, 8], because they belong to the same group in the periodic table. Although further development was required to establish caged Si NCs compared to the progress with C60, recently these showed rich chemistry between the gas phase and the condensed phase [9,10,11,12,13].
A study on the cage structure of Si atoms was reported in 1987 [7]. Based on the mass spectrum, Dr. Beck reported that when one molybdenum (Mo) atom was mixed with Si vapor, MoSi15+ and MoSi16+ cations were generated. He deduced that a Mo atom was contained in a Si cage, which was inferred from analogous metallofullerenes in which metal atoms are contained inside the hollow cage of a carbon fullerene [14]. Although experimental and theoretical studies were extensively performed [15,16,17,18,19,20,21,22,23,24,25], it was not easy to distinguish new nanoscale Si compounds, including a Si cage.
On the other hand, Al element is a low-density metal that is resistant to corrosion, so it is widely used as a building material in the aerospace industry. Al element forms alloys with various elements, widening the diversity of physical properties in various materials. When miniaturizing Al to form NCs, an emerging feature is an electron shell structure [26,27,28], which was found in NCs consisting of alkali or coinage metals. Particularly, Al13− affords a 40 electron-completing 2P shell together with an icosahedral close-packed structure, and this is known as a “superatom”, mimicking the atomic configuration in the periodic table [29,30,31,32,33]. The novel electronic properties of Al superatoms are intriguing with respect to fabricating nanostructured functional materials.
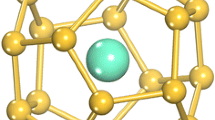
This chapter describes two binary cage superatoms (BCSs) of metal-atom-encapsulating Si (M@Si16) [9,10,11,12,13] and heteroatom-encapsulating Al (X@Al12) [34,35,36,37,38,39,40] (Fig. 7.1); M = group 3–5 transition metals and X = boron (B), Si, phosphorus (P), scandium (Sc), and titanium (Ti). To form chemically robust functional NCs, it is important to design the cooperatively synergistic effects between electronic and geometric structures because these stabilize the NCs individually not only against charge transfer into the corresponding cations or anions but also against structural perturbations in their assemblies. The synergistic effect is particularly expected for BCSs in which one central atom encapsulating cage structure completes a specific electron counting because electronic and geometric factors can then work simultaneously, retaining their structural symmetry. Although the term “superatom” is widely used when the valence electrons in NCs complete an electron shell [29,30,31,32,33], more synergistic effects appear when the superatom takes a close-packed structure such as a highly symmetric cage as a BCS [13, 38, 39]. For representative examples of M@Si16 and X@Al12, which have Td and Ih symmetries, respectively, the formation and characterization are described and a large-scale synthetic approach is established for M@Si16 [12, 13]. The perspectives for the binary cage superatom assembly are discussed in terms of theoretical calculations [39, 41,42,43].

Reprinted with permission from Ref. [13]. Copyright 2017 American Chemical Society
Binary cage superatoms (BCSs) of M@Si16 (M = Ti and Ta) and Si@Al12.
2 Experimental Methods for Gas Phase Nanoclusters
2.1 Dual-Laser Vaporization Nanocluster Source
Figure 7.2 shows a schematic of the NC source of face-to-face laser plasma mixed with pulsed helium (He) carrier gas [44, 45]. An Even-Lavie pulsed valve with a high stagnation pressure of 60–100 atm. [46] was used at a repetition rate of 10 Hz, which was suitable for the effective formation of MSi +/0/– n and XAl +/0/– n . Intense supersonic He gas pulses were operated to recombine and cool the laser-ablated metal vapor. The optimum laser fluence required to maximize the NC intensities depends on the properties of the target under investigation; specifically, the dual-laser vaporization method allows control of the elemental mixing ratio.

Reproduced from Ref. [45] with permission from The Chemical Society of Japan
Schematic of a dual-laser vaporization NC source with two sample rods, two pulsed valves, and a reaction room.
The NC source was modified to be “face-to-face” so that the NCs could be more effectively generated [44]. In the primary NC source, two target rods were laterally separated by 4–5 mm, and two lasers independently vaporized their front surfaces. The two lasers then irradiated the two rods with a delay of about 5 μs in synchronization with the speed of the He carrier gas. Hence, in addition to binary mixed NCs, individual NCs consisting of each single element were generated, because the time lag between the vaporization and cooling processes significantly reduces the formation efficiency of the binary NCs.
The focusing positions of the two vaporization lasers were then shifted toward each other by 1.5–2 mm and moved off the front surfaces of the target rods. The shift in the focusing positions facilitated vaporization of the curved surface of the sample rods in a face-to-face manner [47]. The effective mixing of the hot sample plasmas allowed efficient formation of the binary NCs when the two rods were almost simultaneously vaporized, within a few hundred nanoseconds (ns). The simultaneous vaporization enabled us to form binary NCs with one pulsed laser to vaporize the two independent rods, although optimization of the laser fluences became more complex. The mixed vapor formed binary NCs, and their reactivity was examined by exposing reactant gas in the downstream reaction room [48], as shown in Fig. 7.2. The binary NCs passed through a source exit and expanded into a differentially pumped chamber through a skimmer.
2.2 Spectroscopic Methods for Nanoclusters
The electronic and geometric structures of the binary NCs were investigated by mass spectrometry, anion photoelectron spectroscopy (PES), and photoionization spectroscopy (PIS). Mass analysis of the neutral binary NCs ionized with an ArF (193 nm; 6.43 eV) or F2 (157 nm; 7.90 eV) laser and of the charged binary NCs was performed using time-of-flight (TOF) mass spectrometry. For cationic and anionic NCs, the beam was directly accelerated with a pulsed voltage approximately 2 keV, while neutral photoionization with the ArF/F2 laser was applied in a static electric field of 2 keV. To achieve the appropriate conditions for one-photon ionization of the NCs with the F2 laser, the laser power dependence was measured by changing the flow rate of He gas toward the laser path tubing between the laser exit and the CaF2 window of the chamber. A laser fluence below 1 mJ/cm2 was used, where the ion intensity was linearly dependent on the laser power. For the PIS measurements of the binary NC neutrals, photoionization efficiency curves were measured with a tunable photoionization laser (5.2–6.4 eV) of an optical parametric oscillator (OPO), and the ionization energy was determined from the threshold energy. The laser fluence (typically around 300 μJ/cm2) was monitored during the measurements to normalize the ion intensities [49].
For the PES measurements of the binary NC anions, a magnetic-bottle-type TOF electron spectrometer was used [50,51,52]. After mass selection in their TOF with pulse acceleration at 900 eV, their kinetic energy was considerably reduced with a pulsed electric decelerator before they entered the photodetachment region. The fifth harmonic (213 nm, 5.83 eV) of a pulsed Nd3+:YAG laser was then used to irradiate the mass-selected NC anions to detach photoelectrons. The electrons were guided by a strong, inhomogeneous magnetic field with an Nd–Fe–B-based permanent magnet [53], and subsequently by a weak guiding magnetic field produced with an electric current (2–3 A), and detected with a microchannel plate (MCP). Their kinetic energy was analyzed from their TOF and calibrated using the Au– (2S1/2 ← 1S0) transition [54, 55]. The photoelectron signal was typically accumulated over 20,000–40,000 laser shots.
3 Silicon (Si)-based Binary Cage Superatoms (BCSs)
3.1 Mass Spectrometry for Si Cage Compounds
In contrast to C60, stable nanoscale compounds comprising only Si atoms have not yet been discovered, but we found that a Si NC containing one metal atom was strongly distributed in the mass spectrum as a magic number by systematically changing the metal atom (M) [9, 44, 45, 56]. To explore the binary M–Si formation, we used the NC source with dual-laser vaporization (Fig. 7.2) to enhance the mixing of hot atomic Si and M vapor, and the NCs produced were mass-analyzed using the TOF spectrometer. The magic number behavior featured with a specific composition coupled with the charge state [9, 45]. Figure 7.3 shows mass spectra for an M–Si NC beam obtained by mixing metal atom M vapor of Sc, Ti, and vanadium (V) of groups 3–5 with Si atoms in the dual-laser vaporization source in cationic, neutral, and anionic charge states. In some TOF mass spectra, the magic number (black arrow) appeared when one M atom is mixed with 16 Si atoms. In nine spectra in Fig. 7.3a–c, the mass spectra in which the magic number appears move from the lower left to the upper right due to the combination of the metal element and charge state [9, 45].

Reproduced from Ref. [45] with permission from The Chemical Society of Japan
Mass spectra of cations, neutrals, and anions for M–Si NC beam (M = scandium (Sc), titanium (Ti), and vanadium (V)), together with those of niobium (Nb) and tantalum (Ta) cations mixed with Si. Black arrows show magic number peaks, while white arrows show non-magic number peaks.
The origin of the magic number is that the NC affords a total valence electron number of 68, and in the Jellium model for NCs with uniform charge distribution [26,27,28], the 68 electrons correspond to the number of closed shell electrons, up to the 2D shell. Furthermore, the most distinct magic number behavior appears with tantalum (Ta) atoms and in their cations (Fig. 7.3e); TaSi16+ ions are predominant compared to their neighbors [44, 45, 56]. This appears attributable to the interplay between electronic and geometric structures; the TaSi16+ cations afford the total valence electron number of the closed shell and are also stabilized by the size of the Ta atom being most suitable for the inner diameter of the Si16 cage. Similarly, TiSi16 neutrals are exclusively produced among neutral forms. Therefore, we structurally evaluated metal-encapsulating Si-based BCSs by further spectroscopic characterization coupled with large-scale synthesis of Ta@Si16 and Ti@Si16.
To quantitatively evaluate the electronic properties of M@Si16, anion PES was used [9]. Figure 7.4 shows the photoelectron spectra for Sc@Si16–, Ti@Si16–, and V@Si16– using 266 nm (4.66 eV) and 213 nm (5.82 eV) detachment lasers. The binding energies of Sc@Si16– and V@Si16– reach 3 eV or more, whereas that of Ti@Si16– is small at around 2 eV. This shows that the electronic stabilization is large due to the pairing energy when the total number of valence electrons in the anion is an even number, while an odd number makes the anion unstable.

Reprinted with permission from Ref. [9]. Copyright 2005 American Chemical Society
Photoelectron spectra of BCS anions of Sc@Si16– a, b, Ti@Si16– c, d, and V@Si16– f, g at 266 nm (4.66 eV; top three spectra) and at 213 nm (5.82 eV; bottom four spectra). Comparing the photoelectron spectrum of Ti@Si16– with that of Ti@Si16F– e enables us to assign the HOMO–LUMO gap.
The threshold binding energy corresponds to the EA of the corresponding neutral, and the EA is shown in Fig. 7.4. Moreover, since Sc@Si16– possesses 68 electrons to complete the 2D shell, Ti@Si16– has one excess electron, and thus the peak labeled X in the Ti@Si16– spectrum appears to be an additional peak to that of Sc@Si16–. To confirm that peak X corresponds to a singly occupied molecular orbital (SOMO), Ti@Si16F– was generated by adding an F atom to Ti@Si16– and the photoelectron spectrum was obtained [57,58,59]. As shown in Fig. 7.4e, peak X disappears for Ti@Si16F– while the spectral features in the higher binding energy region are retained.
This is because the F atom (with one electron deficient) effectively accommodates one electron into a deeper MO. The spectral change enables us to assign peak X as the SOMO and to evaluate the gap between the highest occupied MO (HOMO) and the lowest unoccupied MO (LUMO) to be 1.90 eV, as shown in Fig. 7.4d. The HOMO–LUMO gap is considerably larger than that of C60 (1.57 eV) [60], demonstrating the high stability of the Ti@Si16 BCS. As suggested by theoretical calculations [61], cage “aromaticity” might be an important determinant of the electronic stability of the BCSs.
3.2 Development of Intense Nanocluster Source with Magnetron Sputtering
The dual-laser vaporization source is very powerful to allow investigation of binary NC formation by changing the combinations of two different elements. However, structural analysis methods, such as nuclear magnetic resonance (NMR) and Raman spectroscopy, often require enhancement of the total number of NCs. Furthermore, for well-controlled soft-landing, it is necessary to reduce the kinetic energy distribution of the beam, because a wide energy distribution might cause dissociation of the NCs in collisions with the substrate to which a bias voltage is applied [62]. Based on the production amount and narrow kinetic energy distribution, compared to laser evaporation, it is advantageous to use a magnetron sputtering method in which larger targets are available and NCs are formed in a steady flow of inert cooling gas, as shown in Fig. 1.14 [63, 64]. To form binary NCs, two choices are available: dual magnetron sputtering with two targets or single magnetron sputtering with a mixed target. Since the targeted BCSs of M@Si16 have a specific composition, it is convenient to use a mixed target to produce a stable beam from merging nascent atomic vapor. Considering the sputtering rates of each element, the mixing ratios of the target are optimized for generating M@Si16 BCSs.
Particularly, size-selective soft-landing is performed only for charged NCs excluding neutrals, and then the NC ion density should be enhanced to efficiently accumulate the selected NCs on a substrate. For magnetron sputtering, high-power impulse magnetron sputtering (HiPIMS) [65,66,67] with a pulsed power supply increases the ion density while maintaining the average output. The HiPIMS NC source can provide 2–10 times higher ion density compared to conventional magnetron sputtering using a static DC power supply.
3.3 Immobilization and Characterization of Si-based BCSs on Solid Surface
Using a quadrupole mass filter, only Ta@Si16+ BCS ions formed in the developed NC source were selected, and these were deposited and immobilized on a solid substrate [10, 68]. During the selective deposition, the Ta@Si16 produced is deposited under soft-landing conditions (kinetic energy below 1 eV per atom) to avoid destruction when colliding with the substrate. When the deposited TaSi16 was characterized by X-ray photoelectron spectroscopy (XPS) (Fig. 7.5) [11, 69, 70], both Si 2p and Ta 4f exhibit sharp peaks, showing (1) the preserved 1:16 composition of Ta:Si based on their intensity ratio and (2) a single chemical environment for each element based on their peak envelopes. These results can be explained by the Ta atom being encapsulated by the Si16 cage, because other structures, such as linear, two-dimensional planar ones would have provided broader XPS peaks due to different chemical environments for Si and Ta atoms. When the deposited substrate is heated to 350°C, although TaSi16 reacts slightly with residual oxygen, its oxidation initially occurs at Si atoms (Fig. 7.5, bottom), while Ta is not oxidized [11]. Generally, since naked Ta is more easily oxidized than Si [71, 72], the Ta atom must be encapsulated inside the Si16 cage (hereafter referred to as Ta@Si16). In this soft-landing method, Ta@Si16 can be deposited on the substrate to form several layers, but it is highly desirable to construct a more efficient synthesis methodology for further nanomaterial science as well as developing detailed structural analyses.

Reprinted with permission from Ref. [11]. Copyright 2015 American Chemical Society
XPS spectra of Ta@Si16 BCS deposited on HOPG around a Si 2p and b Ta 4f core levels. The fitted results (red line) and spin-orbit contributions (orange dotted line and green dash-dotted line) are superimposed in (a) and (b). Background-subtracted XPS spectra of the Ta@Si16 BCS film for (c) Si 2p and (d) Ta 4f before and after heating (720 K, 16 h) are shown by blue and red lines, respectively.
3.4 Dispersion Trapping of Si-Based BCSs in Liquid
To identify the structure of the metal-atom-encapsulating Si16 cage, a new apparatus was designed and constructed, as shown in Fig. 7.6, in which species generated in the beam with HiPIMS are dispersed in liquids by a direct liquid embedded trapping (DiLET) [12, 13]. The DiLET is based on the idea that chemical isolation toward liquids trapping all beam species is much more efficient than the size-selective deposition, because (1) neutral NCs can also be captured by liquids and (2) the ion transmittance in the mass selection is as low as 20%. Since the NC source operates under vacuum, a liquid with a low vapor pressure must be used to achieve a vacuum of around 10−2 Pa, and thus NCs are injected into polyethylene glycol dimethyl ether (PEG-DME) liquid. During injection, a fresh liquid surface must be prepared to prevent aggregation of various NCs, and then the liquid is stirred vigorously during beam injection. Liquid trapping was applied to Ti@Si16 as well as Ta@Si16 using a Ti or Ta mixed Si disk target. As the beam was injected into the PEG-DME liquid, the transparent liquid became brown after about 1 h, and after about 3 h, the liquid was removed from the chamber and treated in a glove box under reduced oxygen and moisture. While purifying and isolating the liquid with appropriate solvents, fractionation was repeated three times by changing the mixing ratio of nonpolar hexane and large polar tetrahydrofuran (THF), and finally a fraction soluble in pure THF was obtained.

Reprinted with permission from Ref. [12]. Copyright 2017 American Chemical Society
Schematic of NC synthesis apparatus based on HiPIMS and DiLET.
3.5 Structural Analysis of Si-based BCSs
The resulting fraction was analyzed by XPS, 29Si-NMR, Raman spectroscopy, and quantum chemistry calculations [12]. First, XPS was performed for the obtained Ta–Si fraction, and the Si 2p and Ta 4f spectra were consistent with those in Fig. 7.5. This clearly demonstrates that Ta@Si16 BCSs in the beam can be successfully dispersed in PEG-DME liquid and that Ta@Si16 BCSs can be obtained as stable species even after both solvent extraction and ligation with PEG-DME. In fact, the isolation of Ta@Si16 BCSs was also confirmed by mass spectrometry [12]. Furthermore, in the Raman spectra, broad peaks were observed at around 120, 300, and 450 cm−1 for both Ti–Si and Ta–Si (upper traces in Fig. 7.7a, b). Importantly, the features of these Raman spectra are consistent with those of naked BCSs in the surface-enhanced Raman spectra (SERS) (lower traces in Fig. 7.7a, b), which are obtained by depositing exclusively Ti@Si16 or Ta@Si16 BCS ions on the substrate.

Reprinted with permission from Ref. [12]. Copyright 2017 American Chemical Society
Raman spectra excited at 532 nm for isolated M@Si16:PEG-DME BCS and size-selected naked M@Si16 BCS on the SERS substrate (M@Si16/Ag/SrTiO3); M = (a) Ti and (b) Ta. Stick bars represent the selected Raman active modes calculated by DFT for FK, dist-FK, and f-D4d isomers.
Furthermore, the successful synthesis of M@Si16 BCSs on a 100 mg scale enables us to apply NMR measurements; 29Si–NMR spectra for Ti@Si16 and Ta@Si16 exhibit one and two peaks, respectively, between 100 and −100 ppm (Fig. 7.8). Although these peak positions are inconsistent with the predictions obtained from quantum chemistry calculations, the result suggests (1) the difficulty of calculation-based prediction, (2) the weak coordination effect of PEG-DME, and (3) structural fluctuations of the Si16 cage. Based on these spectroscopic structural evaluations, it was concluded that both Ti@Si16 and Ta@Si16 BCSs are identified as tetrahedral structures of Si16 derived from the Frank-Kasper (FK) structure: a metal-encapsulating tetrahedral Si cage (METS) (Fig. 7.1) [12, 13].

Reprinted with permission from Ref. [12]. Copyright 2017 American Chemical Society
29Si NMR spectra of M@Si16:PEG-DME BCS dispersed in THF for M = Ti (300 K) and for M = Ta (318 K). Stick bars represent chemical shifts (CSs) calculated by ZORA-DFT for FK, dist-FK, and f-D4d isomers at the PBE0/TZ2P level. The CSs averaged over the sites are shown with faint colors.
4 Aluminum (Al)-based Binary Cage Superatoms (BCSs)
4.1 Mass Spectrometry of Al-based BCSs
4.1.1 Aluminum–Silicon
Figure 7.9 shows the mass spectra of the Al–Si NC anions, neutrals, and cations produced [38, 39]. In the photoionization of the AlnSim neutral, the laser power dependence indicated that one-photon ionization occurred with the F2 laser (7.90 eV). Since the mass of Si (28 u) is very close to that of Al (27 u), the mixed Al–Si NCs form bundles of mass peaks for each n + m. Although the most intense mass peaks were observed at n + m = 13 in the mass spectra, high-resolution TOF mass spectrometer [73] revealed that the most abundant peak is systematically changed. The right-hand figures in Fig. 7.9 show intensity distributions at n + m = 13, the most abundant 13-mers being Al13− for anions, Al12Si for neutrals, and Al11Si2+ for cations. The charge state dependence for the most abundant Al13–, Al12Si, and Al11Si2+ shows that the doped Si atoms function as a tetravalent atom to satisfy 2P shell closure(40 e) in the Al to Si substitution, because both Al12Si and Al11Si2+ NCs possess 40 valence electrons in common in addition to Al13−. Furthermore, a charge state dependence is observed in the size-dependent abundance in the mass spectra. Figure 7.10 shows the intensity distributions of the Al − n and AlnSi− anions, AlnSi neutrals, and AlnSi+ and AlnSi2+ cations. Some intensity distributions exhibit magic number behavior at n + m = 13, representing the above-mentioned Al13–, Al12Si, and Al11Si2+. These results clearly confirm that the substitution of Si atoms corresponds to one-electron addition toward the total number of valence electrons in the Al–Si binary NCs.

Reprinted with permission from Ref. [38]. Copyright 2006 American Chemical Society
Mass spectra of the Al–Si NC anions, neutrals, and cations. The AlnSim neutrals are photoionized with the F2 laser (7.90 eV). The most intense mass peaks are observed at n + m = 13 in the mass spectra; intensity distributions at n + m = 13 are shown in the right-hand figures, The most abundant 13-mers are the Al13– anion, Al12Si neutral, and Al11Si2+ cation.

Reprinted with permission from Ref. [38]. Copyright 2006 American Chemical Society
Intensity distributions of the AlnSi+ and AlnSi2+ cations, AlnSi neutrals, and Al – n and AlnSi– anions. A prominent peak appears at n + m = 13 for AlnSi2+, AlnSi, and AlnSi–. Solid arrows show the positions for Al12Si+/–.
As reported earlier [15, 38], the chemical stability of the AlnX NCs can be examined using a chemical probe method. When the adsorption reactivity of the Al–Si binary NCs is measured toward O2 reactant gas, Al12Si neutrals show prominent chemical inertness compared to the others [39], as shown in Fig. 7.11. In fact, Al12Si neutrals are favored electronically as well as geometrically. A plausible explanation is that Al12Si adopts a closed electron configuration when a tetravalent Si atom dopant completes the 2P electron shell combined with the valence electrons of sp-hybridized Al atoms [36, 37, 74, 75]. Furthermore, the diameter of a Si atom (1.18 Å) is slightly smaller than that of an Al atom (1.43 Å) [76], leading to geometric stabilization owing to less distorted icosahedral structures. In order to make the icosahedral Al13 more geometrically stable, the Al12 cage favors a smaller central atom, because the distance between adjacent surface atoms is distortedly extended by 5% compared to the distance between the central and surface atoms [34, 77].

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Plots of the relative reactivity of the AlnSi1 NCs against exposure to O2 at n = 7–17 with experimental uncertainties.
4.1.2 Aluminum–Boron
As described in the previous section, when one Al atom is substituted with a Si atom in the Al13– superatoms, the Si atom is located at a central atom in the icosahedron, resulting in: (1) relaxation of structural distortions intrinsically relevant to the icosahedral structure and (2) neutralization of the negative Al13– ion due to one-electron addition with a tetravalent Si atom, forming Si@Al12 BCS. Similar structural relaxation is expected for boron (B), because B is a smaller atom than Al, with both belonging to group 13.
Figure 7.12 shows a mass spectrum of Al NC anions mixed with B atoms [34]. As seen in the distributions for the number of boron atoms (Fig. 7.13; m = 0, 1, 2, and 3), a distribution maximum is observed at n − m = 13–0 and similarly at 12–1, where an Al atom is substituted with a B atom. However, when two B atoms are substituted, no local maximum is observed at 11–2, showing that the second B atom destabilizes 12–1. The results suggest that at 12–1 the B atom is encapsulated in an Al12 cage, and that the second B atom destabilizes the icosahedral cage from Al12 to Al11B due to a vertex-replaced icosahedron (exohedral B@Al11B) with a smaller surface B atom.

Reprinted from Ref. [34] with permission from Elsevier
Mass spectrum of the Al–B nanocluster anions (AlnB – m ). The most intense mass peaks are observed at n + m = 13–0 and 12–1 along with some enhancements around n + m = 23.

Reprinted from Ref. [34] with permission from Elsevier
Intensity distributions of the AlnB – m anions a m = 0, b m = 1, c m = 2, and d m = 3). Magic number behavior appears at n + m = 13 and 23, while it disappears at n − m = 11–2 and 10–3.
In the distributions shown in Fig. 7.13a, larger Al NC anions also exhibit magic number behavior, particularly at n = 23, although this is less prominent compared to n = 13. Al23– electronically satisfies the 3S shell closure by 70 electrons [34]. The behavior of the Al–B distributions in Fig. 7.13b–d suggests that the local maximum at n + m = 23 is retained with substitution of up to two B atoms but the third B atom (m = 3) makes the Al23 derivatives less stable. This suggests that the two B atoms are encapsulated in the Al cage, while the third B atom becomes a surface atom. The structure of Al21B2 is discussed below together with that of Al21Si2.
4.2 Anion Photoelectron Spectroscopy for Al-based BCSs
To quantitatively evaluate the electronic properties, anion PES was used for these two binary NCs. Figure 7.14 shows photoelectron spectra of Al12Si–, Al12SiF–, Al12B–, and Al13Cs– [37, 38, 75]. In the Al12Si– spectrum, a small peak (label X) is observed at 1.5 eV following a large peak at around 3.5 eV, and the EA is as small as about 1.5 eV. The spectral feature is attributed to one-electron addition to the 40 electron closed shell of neutral Al12Si, resulting in the excess electron occupying the orbital above the HOMO of Al12Si. In fact, the F-atom adduct of Al12SiF– anion can be selectively produced when Al–Si NC anions are reacted with fluorine (F2) gas [38, 39], which is seemingly promoted by the excess electron in Al12Si–.

Reprinted with permission from Ref. [38]. Copyright 2006 American Chemical Society
Photoelectron spectra of a Al12Si–, b Al12SiF–, c Al12B–, and d Al13Cs– at 213 nm (5.82 eV).
When the photoelectron spectrum of the Al12SiF– product is obtained, the peak X observed in Fig. 7.14a disappears while maintaining the other features, as shown in Fig. 7.14b. Furthermore, the spectrum of Al12SiF– is almost the same as those of Al13– and Al12B– (Fig. 7.14c). Namely, by adding the F atom [57,58,59], the excess 41st electron is scavenged into a deeper level by the F atom, forming a combination of Al12Si neutral and F–, as discussed for Ti@Si16– and Ti@Si16F– in Sec. 7.3.1. The results show that the peak X of Al12Si– is attributable to a SOMO, with a gap of 1.53 eV between the HOMO and the LUMO for Al12Si from the two peak intervals.
Furthermore, the NC anions of both Al13– and Al12B– satisfy 40 electron shell closure, and they become 41st electron systems when a cesium alkali metal atom (Cs) is added [35, 36, 38, 78]. Interestingly, as shown in Fig. 7.14d, the peak X appears in a low binding energy region, and the photoelectron spectrum is almost the same as that of Al12Si–. Specifically, the Al13Cs neutral is a 40 electron species, forming a superatomic salt of (Al13–)(Cs+) between the halogen-like Al13 superatom and the alkali metal atom [35, 38].
4.3 Photoionization Spectroscopy for Al-based BCSs
The Ei of the neutral NCs can be evaluated by measuring the photoionization efficiency curve with a tunable photoionization laser [38, 39, 49]. Figure 7.15 shows the size-dependent Ei values of Aln, AlnSi, and AlnSi2 [39], and these asymptotically approach 4.08 eV of the Al metal work function [76] as the size increases. Along with the asymptotic Ei decrease, a discontinuous change in Ei is found when crossing the closed shell of a specific electron shell, the 40 electron boundary.

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Ionization energies of neutral AlnSim NCs in eV; a m = 0, b m = 1, and c m = 2. The open squares around 6.4 eV show that Ei value is between 6.42 and 7.90 eV, where they can be ionized not by ArF laser (193 nm; 6.42 eV) but by F2 laser (157 nm; 7.90 eV). Calculated ionization energies for some NCs are shown along with their values, where open and solid circles show adiabatic and vertical ionization energies, respectively. The compositions of local maxima and minima are shown with the total valence electrons in parentheses.
The Ei values of the Al13 and Al12Si neutrals reach 6.42 eV or more. When one Al atom is added to these to form Al14 and Al13Si, their Ei values drop drastically to around 5.6 eV. A similar Ei drop is observed when one Al atom in Al12Si is substituted with a Si atom to form Al11Si2. These discontinuous drops in Ei values can be explained in terms of electron occupation above the 40 electron shell closure. A local maximum/minimum in Ei attributable to a similar electron shell is also observed around 70 electrons (3S shell), the local Ei maximum for Al22Si and the local Ei minimum for Al21Si2, which are treated as a closed shell of 70 electrons and a one-electron excess of 71 electrons, respectively.
For another Al-based binary NC doped with phosphorus (P) atoms, the substitution of an Al atom with P adds two valence electrons because of the pentavalent P atom [38]. The Ei of P@Al12 is as low as 5.37 eV, showing a one-electron excess against the 40 electron shell closure, and the Ei is the lowest value compared with those of similarly sized NCs. This feature is consistent with the total valence electron number of P@Al12 of 41, causing P@Al12 to act as an alkali metal-like BCS.
4.4 Theoretical Calculations for Al-Based BCSs
These quantitative experimental evaluations allow us to clarify the electronic and geometric properties of Al12X and Al21X2 NCs in detail using quantum chemistry calculations [39, 40, 42, 43]. Figure 7.16 shows optimized structures of cations, neutrals, and anions of Al12Si, where two isomeric forms are shown; endohedral Si in an icosahedral Al12 cage and a vertex-replaced icosahedron (exohedral Si atom) [39]. In any charge state, Si@Al12 with a central Si atom is calculated to be more stable than the vertex-replaced one.

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Calculated equilibrium structures and energy differences between isomers with central Si and surface Si; a Al12Si1+, b Al12Si1, and c Al12Si1–. Al and Si atoms are shown as red and blue, respectively.
When one electron is depleted or in excess to form Al12Si+ or Al12Si–, the encapsulated Al12 cage structure is unchanged, but the structural symmetry is lowered from Ih symmetry. The lowering of symmetry is caused by the Jahn–Teller effect, in which the NCs are electronically stabilized by structural distortion associated with the undegeneration of electronic states [76]. Retaining the cage structure against charge exchange among Al12Si+/0/– implies that the Si@Al12 superatoms are stable against charge transfer, indicating that these are promising BCSs for fabricating BSC assemblies for an electronic device.
Additionally, molecular orbital (MO) diagrams are obtained from the optimized Al13–, Al12Si–, and Al12SiF–, as shown in Fig. 7.17 [38, 39]. In Al13– and Al12SiF–, the HOMO–LUMO gap is large, which is consistent with the experimental results shown in Fig. 7.14. Furthermore, in Al12Si–, the SOMO level appears below the LUMO level, represented by a small bump in the photoelectron spectrum in Fig. 7.14.

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Molecular orbital diagrams of Al13– (Si@Al12), Si@Al12–, and Si@Al12F–. Both Al13– (Si@Al12) and Si@Al12F– have large HOMO–LUMO gap, whereas Si@Al12– has SOMO.
X@Al12 (X = B, Si, and P) BCSs behave as halogen-like, rare gas-like, and alkali metal-like superatoms, respectively [38, 39, 79,80,81], where smaller atoms, from trivalent to pentavalent atoms of main group elements, are preferred as the central atom. To verify whether electron shell closure occurs by doping transition metals, the electronic properties were examined for Al12M doped with trivalent Sc and tetravalent Ti [40]. It was found that neither Sc nor Ti atoms were encapsulated in the Al12 cage due to their large atomic radius; instead, they formed a vertex-replaced structure, exohedral Al12M. In addition, their electronic states are described not by 40 electron shell closure, but according to the Wade-Mingos rule, in which Al@Al11 is bonded to a transition metal atom, as shown in Fig. 7.18.

Reproduced from Ref. [40] with permission from IOP Publishing
Equilibrium structure of Al12Sc– NCs calculated at the PBE0/def-SV(P) level (Al; red and Sc; blue).
4.5 Size Evolution of Al-Based Nanoclusters for Assembled Materials
In the size dependence of Ei shown in Fig. 7.15, features based on electron shell closure appear not only in the vicinity of Al13 but also around Al23; for Al21Si2, the local Ei minimum is due to 3S shell closure (70 e). It appears reasonable that both B and Si atoms tend to be encapsulated in Al NCs as demonstrated for B@Al12 and Si@Al12. Based on the icosahedral Al12 cage, a face-sharing bi-icosahedral structure is conceivable for Al21B2 and Al21Si2 [39, 43], and then the lowest-energy isomer can be calculated.
For Al21B2, optimization from the initial structure of the face-sharing bi-icosahedral structure affords a triangular rice-ball structure containing two B atoms [43]. For Al21Si2, however, it was calculated that a face-sharing bi-icosahedron is more stable than a triangular structure for cations and neutrals [39], as shown in Fig. 7.19. The structure shares an Al trimer on the cage surface, with a partial overlap of the two icosahedral Si@Al12.

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Calculated equilibrium structures and energy differences between two isomers of a bi-icosahedron and b triangular form for cationic and neutral Al21Si2 with PBE1PBE/6-311+G*. The experimental ionization energy is reproduced by the bi-icosahedrons.
More interestingly, it was found that the superatomic orbital (SAO) of Al21Si2 can be represented by superposing two Si@Al12 superatoms. Figure 7.20 shows the energy diagrams of the Al21Si2 bi-icosahedron, which shares an Al trimer between two Si@Al12. Using a linear combination of SAOs (LCSAO) [39], a MO picture for dimeric superatoms (di-SAs) is obtained similar to linear combinations of atomic orbitals (LCAOs); the 2Pσ* LCSAO-MOs for Al21Si2+ cations and Al21Si2 neutrals are vacant and SOMO, respectively, while up to the 1Fδ* LCSAO-MO the MOs are occupied. For di-SA, the orbital shapes of the SAOs allow wavefunction overlap at closer distances between the two superatoms compared to that between the atomic orbitals of homonuclear diatomics. This feature gives an orbital stability of di-SA in the order of σ < π < δ < ϕ, because the nature of the LCSAO-MOs is closer to the united (super)atom limit than that of the homonuclear diatomics (LCAO-MOs).

Reproduced from Ref. [39] with permission from the PCCP Owner Societies
Calculated energy diagram of a icosahedral Si@Al12 and b bi-icosahedral Al21Si2 neutral using the linear combination of SAOs (LCSAO) of the dimeric Si@Al12 superatom. With face sharing of Al3, nine electrons are subtracted in the Al21Si2 neutral compared to two Si@Al12. Solid lines show filled or half-filled states, whereas dotted lines show unoccupied states. The LCSAO-MO of 2P σ* is a SOMO for the Al21Si2 neutral, while the LCSAO-MO of 1F δ* is a HOMO for the Al21Si2 cation.
Furthermore, the electronic properties of the superatom dimers of X@Al12–Y@Al12 (X–Y = Si–Si, B–P, Al–P), hetero-assemblies of endohedral Al–based BCSs, are theoretically predicted, where the optimized dimers are obtained by facing the sides of the monomers in a staggered fashion [42]. When the electronic absorption spectra are calculated for the B@Al12–P@Al12 and Al13–P@Al12 heterodimers (a combination between halogen-like and alkali metal-like superatoms), a CT band from B/Al@Al12 to P@Al12 is found in the visible region. The charge distributions in the heterodimer of B@Al12–P@Al12 are unchanged by inserting Si@Al12 between the two superatoms, and the dipole moment of the heterotrimer (3.89 D) is larger than that of the heterodimer (2.38 D). Similarly, a heterodimer and trimer comprising Si-based BCSs, M@Si16 (M = Sc, Ti, and V), are predicted to exhibit electronic excitation involving CT states; this is characterized as electron transfer from V@Si16 to Sc@Si16 in the heterodimer of V@Si16–Sc@Si16 with a dipole moment of 7.63 D [41]. When the Ti@Si16 BCS is inserted between the V@Si16–Sc@Si16 dimer, the linear heterotrimer of Sc@Si16–Ti@Si16–V@Si16 has a larger dipole moment of 15.6 D and one or more localized frontier orbitals compared to the dimer. These dimers and trimers are the smallest assembled BCSs, and the theoretical insight allows combination of different BCSs having various central atoms to create new nanoscale materials [82, 83], which will establish a new area in the field of NC science.
5 Conclusions
The metal-atom-encapsulating Si16 cage (METS) is a novel nanostructure that was synthesized in the gas phase, and the structural properties were successfully characterized in 2017 after a long research period of over 30 years [12]. The BCS formation features synergistic generation from Si and M atomic vapor. In the highly symmetrical Td structure, in which 16 Si atoms form a spherical outer shell, the total number of valence electrons is controlled by replacing the central metal atom. M@Si16 is regarded as a representative “superatoms” in which 17 atoms in total behave as a new “atom”. For example, Ta@Si16 is a superatom with one excess electron, which is like an alkali-metal atom such as Li and Na. By replacing the central metal atom with various metal atoms, the electronic properties are designed while retaining the structural motif, and then their aggregates and hetero-interface exhibit distinct chemical and physical properties. Furthermore, chemical ligation would modify their properties to form designer nanomaterials. The BCS nanomaterials will show novel optical and conduction properties, and M@Si16 BCS itself exhibits new bonding features. The BCS might enable a paradigm shift in Si-based nanoscale material to overcome the integration limit of current top-down Si electronics together with M@Ge16 BCSs [61, 84].
For Al-based BCS, X@Al12 (X = B, Si, and P) BCSs behave as halogen-like, rare gas-like, and alkali metal-like superatoms, where small atoms are preferred as central atoms from trivalent to pentavalent atoms of main group elements. Since Si@Al +/0/–12 BCSs retain the cage structure by charge exchange from the Si@Al12 neutral in Ih symmetry, the Si@Al12 superatoms are stable against charge transfer, affording promising BCS assemblies for an electronic device. The theoretical analysis showed that the SAO of bi-icosahedral Al21Si2 can be represented by superposing two Si@Al12 BCSs. Using the LCSAO, a MO picture for di-SAs is obtained similar to the LCAO.
Finally, the BCS assembly can apparently create new nanoscale materials; hetero-assembly of an M@Si16 or X@Al12 BCS comprising different central atom BCSs is theoretically predicted to show an electronic transition relevant to charge transfer. Beyond M@Si16 and X@Al12 BCSs themselves, these BCS assemblies will further widen a rich diversity to fabricate nanoscale functional materials, and along with that superatom periodic table would evolve from these BCS family members.
References
Moore, G.E.: Cramming more components onto integrated circuits. Electronics 114–117 (1965)
Canham, L.T.: Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 57, 1046–1048 (1990)
Walters, R.J., Bourianoff, G.I., Atwater, H.A.: Field-effect electroluminescence in silicon nanocrystals. Nat. Mater. 4, 143–146 (2005)
Hochbaum, A.I., Chen, R., Delgado, R.D., Liang, W., Garnett, E.C., Najarian, M., Majumdar, A., Yang, P.: Enhanced thermoelectric performance of rough silicon nanowires. Nature 451, 163–167 (2008)
Boukai, A.I., Bunimovich, Y., Tahir-Kheli, J., Yu, J.-K., Goddard III, W.A., Heath, J.R.: Silicon nanowires as efficient thermoelectric materials. Nature 451, 168–171 (2008)
Kroto, H.W., Heath, J.R., O’Brien, S.C., Curl, R.F., Smalley, R.E.: C60: Buckminsterfullerene. Nature 318, 162–163 (1985)
Beck, S.M.: Studies of silicon cluster-metal atom compound formation in a supersonic molecular beam. J. Chem. Phys. 87, 4233–4234 (1987)
Beck, S.M.: Mixed metal-silicon clusters formed by chemical reaction in a supersonic molecular beam: implications for reactions at the metal/silicon interface. J. Chem. Phys. 90, 6306–6312 (1989)
Koyasu, K., Akutsu, M., Mitsui, M., Nakajima, A.: Selective formation of MSi16 (M = Sc, Ti, and V). J. Am. Chem. Soc. 127, 4998–4999 (2005)
Nakaya, M., Iwasa, T., Tsunoyama, H., Eguchi, T., Nakajima, A.: Formation of a superatom monolayer using gas-phase-synthesized Ta@Si16 nanocluster ions. Nanoscale 6, 14702–14707 (2014)
Shibuta, M., Ohta, T., Nakaya, M., Tsunoyama, H., Eguchi, T., Nakajima, A.: Chemical characterization of an alkali-like superatom consisting of a Ta-encapsulating Si16 Cage. J. Am. Chem. Soc. 137, 14015–14018 (2015)
Tsunoyama, H., Akatsuka, H., Shibuta, M., Iwasa, T., Mizuhata, Y., Tokitoh, N., Nakajima, A.: Development of integrated dry-wet synthesis method for metal encapsulating silicon cage superatoms of M@Si16 (M = Ti and Ta). J. Phys. Chem. C 121, 20507–20516 (2017)
Tsunoyama, H., Shibuta, M., Nakaya, M., Eguchi, T., Nakajima, A.: Synthesis and characterization of metal-encapsulating Si16 cage superatoms. Acc. Chem. Res. 51, 1735–1745 (2018)
Heath, J.R., O’Brien, S.C., Zhang, Q., Liu, Y., Curl, R.F., Kroto, H.W., Tittel, F.K., Smalley, R.E.: Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 107, 7779–7780 (1985)
Sanekata, M., Koya, T., Nagano, S., Negishi, Y., Nakajima, A., Kaya, K.: Electronic and geometric structures of metal-silicide clusters. Trans. Mater. Res. Soc. Jpn 25, 1003–1006 (2000)
Kumar, V., Kawazoe, Y.: Metal-encapsulated fullerenelike and cubic caged clusters of silicon. Phys. Rev. Lett. 87, 045503 (2001)
Hiura, H., Miyazaki, T., Kanayama, T.: Formation of metal-encapsulating Si cage clusters. Phys. Rev. Lett. 86, 1733–1736 (2001)
Lu, J., Nagase, S.: Structural and electronic properties of metal-encapsulated silicon clusters in a large size range. Phys. Rev. Lett. 90, 115506 (2003)
Ohara, M., Koyasu, K., Nakajima, A., Kaya, K.: Geometric and electronic structures of metal (M)-doped silicon clusters (M = Ti, Hf, Mo and W). Chem. Phys. Lett. 371, 490–497 (2003)
Zheng, W., Nilles, J.M., Radisic, D., Bowen, K.H.: Photoelectron spectroscopy of chromium-doped silicon cluster anions. J. Chem. Phys. 122, 071101 (2005)
Jaeger, J.B., Jaeger, T.D., Duncan, M.A.: Photodissociation of metal-silicon clusters: encapsulated versus surface-bound metal. J. Phys. Chem. A 110, 9310–9314 (2006)
Reveles, J.U., Khanna, S.N.: Electronic counting rules for the stability of metal-silicon clusters. Phys. Rev. B 74, 035435 (2006)
Torres, M.B., Fernández, E.M., Balbás, L.C.: Theoretical study of isoelectronic SinM clusters (M = Sc−, Ti, V+; n = 14–18). Phys. Rev. B 75, 205425 (2007)
Lau, J.T., Hirsch, K., Klar, P., Langenberg, A., Lofink, F., Richter, R., Rittmann, J., Vogel, M., Zamudio-Bayer, V., Möller, T., von Issendorff, B.: X-ray spectroscopy reveals high symmetry and electronic shell structure of transition-metal-doped silicon clusters. Phys. Rev. A 79, 053201 (2009)
Claes, P., Janssens, E., Ngan, V.T., Gruene, P., Lyon, J.T., Harding, D.J., Fielicke, A., Nguyen, M.T., Lievens, P.: Structural identification of caged vanadium doped silicon clusters. Phys. Rev. Lett. 107, 173401 (2011)
Knight, W.D., de Heer, W.A., Clemenger, K., Saunders, W.A., Chou, M.Y., Cohen, M.L.: Electronic shell structure and abundances of sodium clusters. Phys. Rev. Lett. 52, 2141–2143 (1984)
de Heer, W.A.: The physics of simple metal clusters: experimental aspects and simple models. Rev. Mod. Phys. 65, 611–676 (1993)
Brack, M.: The physics of simple metal clusters: self-consistent jellium model and semiclassical approaches. Rev. Mod. Phys. 65, 677–732 (1993)
Jena, P.: Beyond the periodic table of elements: the role of superatoms. J. Phys. Chem. Lett. 4, 1432–1442 (2013)
Luo, Z., Castleman Jr., A.W., Khanna, S.N.: Reactivity of metal clusters. Chem. Rev. 116, 14456–14492 (2016)
Tomalia, D.A., Khanna, S.N.: A systematic framework and nanoperiodic concept for unifying nanoscience: hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive mendeleev-like nanoperiodic tables. Chem. Rev. 116, 2705–2774 (2016)
Reber, A.C., Khanna, S.N.: Superatoms: electronic and geometric effects on reactivity. Acc. Chem. Res. 50, 255–263 (2017)
Jena, P., Sun, Q.: Super atomic clusters: design rules and potential for building blocks of materials. Chem. Rev. 118, 5755–5870 (2018)
Nakajima, A., Kishi, T., Sugioka, T., Kaya, K.: Electronic and geometric structures of aluminum-boron negative cluster ions (AlnB – m ). Chem. Phys. Lett. 187, 239–244 (1991)
Nakajima, A., Hoshino, K., Naganuma, T., Sone, Y., Kaya, K.: Ionization potentials of aluminum-sodium bimetallic clusters (AlnNam). J. Chem. Phys. 95, 7061–7066 (1991)
Hoshino, K., Watanabe, K., Konishi, Y., Taguwa, T., Nakajima, A., Kaya, K.: Ionization potentials of aluminum-cesium bimetallic clusters (AlnCsm). Chem. Phys. Lett. 231, 499–503 (1994)
Kawamata, H., Negishi, Y., Nakajima, A., Kaya, K.: Electronic properties of substituted aluminum clusters by boron and carbon atoms (AlnB – m /AlnC – m ); new insights into s-p hybridization and perturbed shell structures. Chem. Phys. Lett. 337, 255–262 (2001)
Akutsu, M., Koyasu, K., Atobe, J., Hosoya, N., Miyajima, K., Mitsui, M., Nakajima, A.: Experimental and theoretical characterization of aluminum-based binary superatoms of Al12X and their cluster salts. J. Phys. Chem. A 110, 12073–12076 (2006)
Akutsu, M., Koyasu, K., Atobe, J., Miyajima, K., Mitsui, M., Tsunoyama, H., Nakajima, A.: Geometric and electronic properties of Si-atom doped Al clusters: robustness of binary superatoms against charging. Phys. Chem. Chem. Phys. 19, 20401–20411 (2017)
Tsunoyama, H., Akutsu, M., Koyasu, K., Nakajima, A.: Stability for binary Al12X nanoclusters (X = Sc and Ti): superatom or Wade’s polyhedron. J. Phys. Condensed Matter 30, 494004 (2018)
Iwasa, T., Nakajima, A.: Geometric, electronic, and optical properties of a superatomic heterodimer and trimer: Sc@Si16–V@Si16 and Sc@Si16–Ti@Si16–V@Si16. J. Phys. Chem. C 116, 14071–14077 (2012)
Iwasa, T., Nakajima, A.: Geometric, electronic, and optical properties of monomer and assembly of endohedral aluminum superatomic clusters. J. Phys. Chem. C 117, 21551–21557 (2013)
Iwasa, T., Nakajima, A.: Geometric, electronic, and optical properties of a boron-doped aluminum cluster of B2Al21−. Chem. Phys. Lett. 582, 100–104 (2013)
Koyasu, K., Atobe, J., Akutsu, M., Mitsui, M., Nakajima, A.: Electronic and geometric stabilities of clusters with transition metal encapsulated by silicon. J. Phys. Chem. A 111, 42–49 (2007)
Nakajima, A.: Study on electronic properties of composite clusters toward nanoscale functional advanced materials. Bull. Chem. Soc. Jpn 86, 414–437 (2013)
Even, U., Jortner, J., Noy, D., Lavie, N., Cossart-Magos, C.: Cooling of large molecules below 1 K and He clusters formation. J. Chem. Phys. 112, 8068–8071 (2000)
Geohegan, D.B.: Imaging and blackbody emission spectra of particulates generated in the KrF-laser ablation of BN and YBa2Cu3O7−x. Appl. Phys. Lett. 62, 1463–1465 (1993)
Geusic, M.E., Morse, M.D., O’Brien, S.C., Smalley, R.E.: Surface reactions of metal clusters I: the fast flow cluster reactor. Rev. Sci. Instrum. 56, 2123–2130 (1985)
Miyajima, K., Muraoka, K., Hashimoto, M., Yasuike, T., Yabushita, S., Nakajima, A., Kaya, K.: The quasi-band electronic structure of Vn(Benzene)n+1 clusters. J. Phys. Chem. A 106, 10777–10781 (2002)
Cheshnovsky, O., Yang, S.H., Pettiette, C.L., Craycraft, M.J., Smalley, R.E.: Magnetic time-of-flight photoelectron spectrometer for mass-selected negative cluster ions. Rev. Sci. Instrum. 58, 2131–2137 (1987)
Ganteför, G., Meiwes-Broer, K.H., Lutz, H.O.: Photodetachment spectroscopy of cold aluminum cluster anions. Phys. Rev. A 37, 2716–2718 (1988)
Ganteför, G., Gausa, M., Meiwes-Broer, K.H., Lutz, H.O.: Ultraviolet photodetachment spectroscopy on jet-cooled metal-cluster anions. Faraday Discuss. Chem. Soc. 86, 197–208 (1988)
Nakajima, A., Taguwa, T., Hoshino, K., Sugioka, T., Naganuma, T., Ono, F., Watanabe, K., Nakao, K., Konishi, Y., Kishi, R., Kaya, K.: Photoelectron spectroscopy of (C6F6) – n and (Au-C6F6)– clusters. Chem. Phys. Lett. 214, 22–26 (1993)
Ho, J., Ervin, K.M., Lineberger, W.C.: Photoelectron spectroscopy of metal cluster anions: Cu – n , Ag – n , and Au – n . J. Chem. Phys. 93, 6987–7002 (1990)
Moore, C.E.: Atomic energy levels as derived from the analyses of optical spectra. NSRDS-NBS, 35, vol. 3. U.S. National Bureau of Standards: Washington, DC (1971)
Koyasu, K., Atobe, J., Furuse, S., Nakajima, A.: Anion photoelectron spectroscopy of transition metal- and lanthanide metal-silicon clusters: MSi − n (n = 6–20). J. Chem. Phys. 129, 214301 (2008)
Kawamata, H., Negishi, Y., Kishi, R., Iwata, S., Nakajima, A., Kaya, K.: Photoelectron spectroscopy of silicon-fluorine binary cluster anions (SinFm−). J. Chem. Phys. 105, 5369–5376 (1996)
Negishi, Y., Kawamata, H., Hayase, T., Gomei, M., Kishi, R., Hayakawa, F., Nakajima, A., Kaya, K.: Photoelectron spectroscopy of germanium-fluorine binary cluster anions: the HOMO-LUMO gap estimation of gen clusters. Chem. Phys. Lett. 269, 199–207 (1997)
Kishi, R., Negishi, Y., Kawamata, H., Iwata, S., Nakajima, A., Kaya, K.: Geometric and electronic structures of fluorine bound silicon clusters. J. Chem. Phys. 108, 8039–8058 (1998)
Wang, X.B., Ding, C.F., Wang, L.S.: High resolution photoelectron spectroscopy of C60−. J. Chem. Phys. 110, 8217–8220 (1999)
Furuse, S., Koyasu, K., Atobe, J., Nakajima, A.: Experimental and theoretical characterization of MSi16–, MGe –16 , MSn –16 , and MPb16– (M = Ti, Zr, and Hf): the role of cage aromaticity. J. Chem. Phys. 129, 064311 (2008)
Bromann, K., Felix, C., Brune, H., Harbich, W., Monot, R., Buttet, J., Kern, K.: Controlled deposition of size-selected silver nanoclusters. Science 274, 956–958 (1996)
Haberland, H., Karrais, M., Mall, M.: A new type of cluster and cluster ion source. Z. Phys. D 20, 413–415 (1991)
Haberland, H., Karrais, M., Mall, M., Thurner, Y.: Thin films from energetic cluster impact: a feasibility study. J. Vac. Sci. Technol., A 10, 3266–3271 (1992)
Tsunoyama, H., Zhang, C., Akatsuka, H., Sekiya, H., Nagase, T., Nakajima, A.: Development of high-flux ion source for size-selected nanocluster ions based on high-power impulse magnetron sputtering. Chem. Lett. 42, 857–859 (2013)
Zhang, C., Tsunoyama, H., Akatsuka, H., Sekiya, H., Nagase, T., Nakajima, A.: Advanced nanocluster ion source based on high-power impulse magnetron sputtering and time-resolved measurements of nanocluster formation. J. Phys. Chem. A 117, 10211–10217 (2013)
Zhang, C., Tsunoyama, H., Feng, Y., Nakajima, A.: Extended Smoluchowski model for the formation of size-selected silver nanoclusters generated via modulated pulsed power magnetron sputtering. J. Phys. Chem. C 120, 5667–5672 (2016)
Nakaya, M., Iwasa, T., Tsunoyama, H., Eguchi, T., Nakajima, A.: Heterodimerization via the covalent bonding of Ta@Si16 nanoclusters and C60 molecules. J. Phys. Chem. C 119, 10962–10968 (2015)
Ohta, T., Shibuta, M., Tsunoyama, H., Eguchi, T., Nakajima, A.: Charge transfer complexation of Ta-encapsulating Ta@Si16 superatom with C60. J. Phys. Chem. C 120, 15265–15271 (2016)
Shibuta, M., Kamoshida, T., Ohta, T., Tsunoyama, H., Nakajima, A.: Alkali-like superatom chemistry of group-5 metal encapsulating Si16 cage; M@Si16 (M = V, Nb, Ta). Comm. Chem. 1, 50 (2018)
Gomoyunova, M.V., Pronin, I.I., Malygin, D.E., Gall, N.R., Vyalikh, D.V., Molodtsov, S.L.: Photoemission study of cobalt interaction with the oxidized Si(100) 2 × 1 surface. Surf. Sci. 600, 2449–2456 (2006)
van der Veen, J.F., Himpsel, F.J., Eastman, D.E.: Chemisorption-induced 4f-core-electron binding-energy shifts for surface atoms of W(111), W(100), and Ta(111). Phys. Rev. B 25, 7388–7397 (1982)
Even, U., Dick, B.: Computer optimization for high-resolution time-of-flight mass spectrometer. Rev. Sci. Instrum. 71, 4415–4420 (2000)
Li, X., Wu, H., Wang, X.B., Wang, L.S.: s-p hybridization and electron shell structures in aluminum clusters: a photoelectron spectroscopy study. Phys. Rev. Lett. 81, 1909–1912 (1998)
Koyasu, K., Akutsu, M., Atobe, J., Mitsui, M., Nakajima, A.: Electronic properties of Cs-atom doped aluminum and silicon clusters: AlnCsm and SinCsm. Chem. Phys. Lett. 421, 534–539 (2006)
Shriver, D. F.; Atkins, P. W., Inorganic Chemistry. 1999, Oxford Univ. Press, 3rd edn
Mackay, A.L.: A dense non-crystallographic packing of equal spheres. Acta Crystallogr. 15, 916–918 (1962)
Kishi, R., Iwata, S., Nakajima, A., Kaya, K.: Geometric and electronic structures of silicon–sodium binary clusters. I. Ionization energy of SinNam. J. Chem. Phys. 107, 3056–3070 (1997)
Gong, X.G., Kumar, V.: Enhanced stability of magic clusters: a case study of icosahedral Al12X, X = B, Al, Ga, C, Si, Ge, Ti, As. Phys. Rev. Lett. 70, 2078–2081 (1993)
Li, X., Wang, L.-S.: Experimental search and characterization of icosahedral clusters: Al12X– (X = C, Ge, Sn, Pb). Phys. Rev. B 65, 153404 (2002)
Lu, Q.L., Jalbout, A.F., Luo, Q.Q., Wan, J.G., Wang, G.H.: Theoretical study of hydrogenated Mg, Ca@Al12 Clusters. J. Chem. Phys. 128, 224707 (2008)
Khanna, S.N., Jena, P.: Assembling Crystals from Clusters. Phys. Rev. Lett. 69, 1664–1667 (1992)
Claridge, S.A., Castleman Jr., A.W., Khanna, S.N., Murray, C.B., Sen, A., Weiss, P.S.: Cluster-assembled materials. ACS Nano 3, 244–255 (2009)
Atobe, J., Koyasu, K., Furuse, S., Nakajima, A.: Anion photoelectron spectroscopy of germanium and tin clusters containing a transition- or lanthanide-metal atom; MGen− (n = 8–20) and MSnn− (n = 15–17) (M = Sc–V, Y–Nb, and Lu–Ta). Phys. Chem. Chem. Phys. 14, 9403–9410 (2012)
Acknowledgments
This work is partly supported by the program of Exploratory Research for Advanced Technology (ERATO) in Japan Science and Technology Agency (JST) entitled with “Nakajima Designer Nanocluster Assembly Project”, by JSPS KAKENHI of Grant-in-Aids for Scientific Research (A) no. 15H02002, and by JSPS KAKENHI of Challenging Research (Pioneering) no. 17H06226. This research is in collaboration with co-authors of Refs. [9–13], including Dr. Hironori Tsunoyama, Dr. Masahiro Shibuta, Dr. Masato Nakaya, Dr. Toyoaki Eguchi, Dr. Takeshi Iwasa, Dr. Kiichirou Koyasu, Professor Norihiro Tokitoh (Kyoto Univ.), and Associate Professor Yoshiyuki Mizuhata (Kyoto Univ.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nakajima, A. (2019). Superatomic Nanoclusters Comprising Silicon or Aluminum Cages. In: Ebata, T., Fujii, M. (eds) Physical Chemistry of Cold Gas-Phase Functional Molecules and Clusters. Springer, Singapore. https://doi.org/10.1007/978-981-13-9371-6_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-9371-6_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9370-9
Online ISBN: 978-981-13-9371-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)