Abstract
Aging, which is associated with several undesirable processes, is a complex phenomenon. Aging mechanisms are not fully comprehended yet, however, diseased and aged cells are correlated with accumulation of senescent cells. Various studies have revealed that aging leads to structural and functional changes in cardiac cells. During aging, stresses such as telomere shortening and reactive oxygen species (ROS) induce cellular senescence which is characterized by permanent cell cycle arrest and secretion of inflammatory proteins that affect tissue environment. Especially, the effect of oxidative stress and ROS generation on macromolecules have been considered important in the modulation of various age-associated chronic disorders and the lifespan. Recent studies have shown the presence senescent phenotype in endothelial cells and smooth muscle cells of patients with heart failure, diabetes, and atherosclerosis. Existence of senescent vascular cells indicates that cellular senescence has a causative role in the pathology of cardiovascular diseases. There is also accumulating evidence that impairment in systemic metabolism is connected by cellular senescence. Therefore, clearance of senescent cells or suppression of cellular senescence is suggested as an important field for future investigation and the discovery of senolytics is seen as a promising therapy of vascular diseases. This chapter describes the phenomenon of cellular senescence with emphasis to its link to oxidative stress during aging and its essential role on the pathology of the vascular system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As life-expectancy increases, healthy aging is becoming an important problem [1]. Chronological age is the significant risk factor for the cardiovascular diseases (CVDs) and CVDs remain to be the major health problem in the elderly people [2]. CVDs such as heart failure, diabetes, and atherosclerosis are also termed as age-associated diseases [3]. The vascular system deteriorates within aging process shows the powerful link between age and vascular disorders [4]. Aging is also associated with oxidative stress, endoplasmic reticulum (ER) stress, inflammation, apoptosis and mitochondrial dysfunction, all of which are associated with CVD pathogenesis [5]. Among these aging etiologies, low-grade chronic inflammation and elevated oxidative stress are suggested to be most significant mechanisms to contribute disease pathology [6].
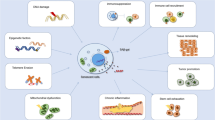
Aging process includes several complex mechanisms that are interconnected. Among these mechanisms, cellular senescence recently gains interest having a pivotal role in the progression of cardiovascular pathology [7, 8]. Over a half century ago, cellular senescence was originally described by Hayflick et al. in human lung fibroblasts [9]. They showed the limited replication capacity of human primary fibroblast cells which then, enter to permanent cell cycle arrest. In accordance with cell cycle arrest, alterations of gene expression changes lead to secretion of pro-inflammatory proteins which is called as the senescence-associated secretory phenotype (SASP) [10]. SASP is especially essential in CVDs for leading to chronic inflammation and therefore, tissue remodeling [4]. Increasing evidence also have demonstrated that cellular senescence is an essential step in the development of vascular aging [11]. It has been shown that the vasculature and the myocardium undergo changes with aging and cellular senescence termed as “vascular senescence”. Vascular senescence leads to cardiac morbidity and mortality that eventually promote atherosclerosis [12], systolic cardiac dysfunction [13] and systemic metabolic dysfunction [14].
With aging, large arteries go into progressive alterations in the mechanical properties such as endothelial dysfunction [15]. Vascular endothelial cells (ECs) are identified as an essential part of the vascular wall and therefore, are important for the maintenance of cardiovascular homeostasis [16]. Impairments in endothelial function significantly contribute to arterial inflammation, lesion formation, deterioration in vasodilation, and loss of compliance. Endothelial dysfunction is also accepted as the biomarker for future cardiac pathology since it is an early event in the development of atherosclerotic plaque [17]. Well-known clinical risk factors for CVDs, such as high blood pressure and oxidative stress lead to endothelial dysfunction [18]. Therefore, identification of mechanisms that underlies EC senescence and vascular aging may develop next-generation therapy strategies for cardiovascular diseases. Aging process is associated with changes in the vasculature at the cellular and molecular levels and microvascular changes may be seen through the onset of vascular remodeling. Specifically, increased oxidative stress and low-grade chronic inflammation are possible underlying mechanisms that may trigger physiological and early vascular aging [19, 20].
This review addresses the cellular senescence in aging-related diseases exploring in-depth role of oxidative stress with the focus on vascular system. It is reviewed the mechanisms underlying vascular senescence and the context of senescent cells in the vessels and usefulness of clearance of these senescent cells as next generation therapies for cardiovascular diseases. It is discussed the importance of fully understanding of aging mechanisms could emerge new strategies against vascular pathologies.
2 Cellular Senescence
Cellular senescence is described as an irreversible process in which cells enter permanent cell cycle arrest and typically, followed by a severe damage [9, 10]. Cellular senescence of cells is suggested to play a protective role in cancer process since cell cycle arrest occurs as a response to DNA damage could avoid tumor progression [21]. Therefore, in cancer process, cellular senescence functions as a vital safeguard against hyperproliferative states of the cells. On the other hand, it is known that cellular senescence can be also detected in other cases than cancer, like aging and aging-associated disorders. Therefore, whether the senescence would be beneficial or detrimental, depends on the status and the age of the organism.
As described by Hayflick and Moorehead, cultured cells have a finite lifespan [9] and it was also shown that critical loss of telomeres causes cellular senescence when somatic cells undergo many cell divisions [22]. The discovery of senescence during serial passaging of human diploid fibroblasts started the speculation that senescence is a natural process during aging. In addition to fibroblast cells, cellular senescence has been observed in various cell types including epithelial cells, endothelial cells, lymphocytes and chondrocytes and more on [23]. The cells enter a state of cell cycle arrest following serial passaging of cells, termed as “replicative senescence” or “Hayflick limit”, has been characterized by the telomere shortening [4]. Many studies have also reported the increase of senescent cells with age and aging pathologies and have also shown the relationship of senescence to other biological processes including cardiovascular pathologies [24].
However, the cellular senescence could be induced by other non-telomeric signals which include various types of stressors, mainly, oxidative stress [25]. When there is more stress than the physiological stress range, a different type of cellular senescence can also occur which is referred as “stress-induced premature senescence” (SIPS). SIPS also includes the other candidate drivers of senescence including oncogene signaling such as Ras, disrupted chromatin, DNA damage, intense mitogenic signals, oncogene activation, metabolic stress, and stress owing to cell culture conditions [26]. It is worth noting that telomere damage occurs at SISP as well, however, only to some extent [27].
Cellular senescence is also defined by another phenomenon which is termed as SASP [28]. This phenomenon is characterized by the secretion of many biologically active proteins that affect both the senescent cell itself and adjacent cells. The SASP includes proinflammatory cytokines that are particularly crucial in driving aging and the pathogenesis of age-associated diseases. The SASP develops consequence of extensive chromatin remodeling during senescence and which in turn suppresses the nuclear lamina protein, lamin B1 transcription [29]. Inhibition of lamin B1 leads to increase in inflammatory cytokines and chemokines, alterations in the production of growth factors, proteases secretion, and as a result, leads to reactive oxygen species (ROS) generation [29]. Activation of inflammation by chronic SASP causes disruption in normal tissue structure and function [28], and further leads to death of cells around them, tissue remodeling, and attraction of immune elements. Innate immunity activation leads to the removal of component cells which is increases during aging, potentially contributing to senescent cell accumulation in old age [30]. The fact that activation of inflammation by SASP underlies many age-associated pathologies and even may drive cancer.
Recent studies reported the effect of cellular senescence by its growth arrest phenotype and SASP factors as an important contributor in the pathology of age-associated disorders. In addition, it is also claimed that age-associated disease development by positive feedback mechanisms is maintained mainly by SASP factors. The overview of oxidative stress related mechanisms and its effect on the cardiovascular diseases by the cellular senescence induction will be discussed in the following sections [31].
2.1 Signaling Pathways of Cellular Senescence
Deterioration of various organ functions during cellular senescence is accompanied by several molecular mechanisms [7]. Although the molecular mechanism of the cellular senescence is not fully clarified yet, recent studies underlined several signaling pathways. Lessons from what happens during senescence show the important role of p53 pathway [32]. The p53 protein which is known to protect the genome by the inhibition of tumorigenesis, also have pivotal roles in apoptosis, cell cycle control and DNA repair [33]. Additionally, it is well known that p53 is effective through many other biological processes including, autophagy, antioxidant defenses and angiogenesis [34, 35]. However, studies have shown that in aging process, p53 pathway leads to increase in the levels of ROS and some other stresses as well, such as DNA damage and oncogenic stress [32] and therefore, is one of the well-studied mechanism related to cellular senescence. As previously discussed, during replicative senescence incomplete replication of telomeres leads to shortening of telomeres as a result of cell cycle arrest. Accordingly, when telomere shortening is extensive, chromosomal stability and DNA replication is disturbed leading to DNA damage. At this point, DNA damage induces cellular senescence via p53/p16 signaling pathway is suggested to play the main roles during replicative senescence [34].
However, stress induced SIPS is triggered independent of cell cycle arrest and telomere shortening which critically erodes telomeres. Signals from various stresses including oxidative stress, UV, oncogenic stress, metabolic stress (Fig. 10.1) results in DNA damage leading to senescence is also regulated by the p53 or p16 pathways. Among stress factors, especially ROS is known to have crucial role for the induction of senescence in vascular cells since ROS can induce senescence either dependent or independent of telomere shortening by driving DNA lesions [36]. Other less characterized stressors for vascular cells is the components of SASP itself which function via transforming growth factor β (TGFβ) signaling. As mentioned before, SASP takes crucial role during the pathology of the disorders since it affects nearby cells in the vessels [31]. The decision to activate p53 or p16 pathway depends either on the cell type or the stress type. p16 pathway is mainly activated almost in all cells during senescence progress [37]. On the other hand, when there is DNA damage and telomere dysfunction, p53 pathway is the preferred pathway. However, general cellular stresses such as mitogenic stress lead to activation of p16 signaling pathway [38, 39]. This type of senescence is typically linked to vascular aging since vessel wall-resident cells such as ECs, smooth muscle cells (SMCs), fibroblasts might not replicate as much as replicative senescence. On the other hand, in some cases such as when endothelial cell repopulation is occurred following angioplasty, both replicative and nonreplicative senescence can be observed [40, 41].
Consequences of aging on the vascular system. Chronological aging induces cellular senescence via the increase of stresses such as oxidative stress, oncogenic stress, elevated telomere attrition and inflammation. Cellular senescence is associated with an increase of ROS, p53/p21, p16 and SA-β-gal that results in pathological changes in the vascular system
The role of p53 signaling in cardiovascular/heart diseases has been shown with several studies. Increased level of p53 protein in old vessels, failing hearts and in the visceral fat of obese patients has been reported. The role of cellular stress triggered by p53 pathway has also been elicited in the pathology of aging and age-associated diseases such as atherosclerosis, diabetes, obesity and heart failure [13, 14, 35, 42]. On the other hand, some other studies have shown its beneficial effect during aging. A study with Trp53/Cdkn2a transgenic mice showed resistance to carcinogenesis with an increased lifespan [43]. Additionally, “Super p53” mice was also shown to be resistant to carcinogenesis and displayed normal glucose tolerance on a standard diet [44, 45]. In addition, another study showed deletion of p53 or p21 increased cellular senescence in the progeroid mice [46]. Depending on these studies, p53/p21 signaling pathway plays a crucial role in the cellular senescence process, however, the response depends on the cell or stress type.
2.2 Biological Markers of Cellular Senescence
Although, there is no direct biological marker reflecting cellular senescence, identification of cellular senescence can be accomplished by using combination of several markers. Of them, senescence-associated beta-galactosidase (SA-β-gal) activity is the most widely used biological marker that is used for the detection cellular senescence [47]. SA-β-gal activity is mainly detected by immunohistochemical method in cell culture and less preferably in tissue sections. In senescent cells, lysosomal beta-galactosidase activity can be detected only in pH 6 as a result of marked expansion of the lysosomal compartment [47]. Other common used markers depend on the signaling status of the cell such as increased levels of p53, p16Ink4a, p21, p38 mitogen-activated protein kinase. In some cases, epigenetic markers such as high mobility group A proteins or heterochromatin markers have been used as biological markers of cellular senescence [48]. Senescence-associated heterochromatin foci (SAHF) are heterochromatin markers which have DNA domains stained by 4′,6′- diamidino-2-phenylindole (DAPI) and have enriched histone methylations (H3K9me). Additionally, the modified histone, γH2AX contributes to DNA repair and stabilization and can be used as a proof of DNA damage linked by telomere-induced foci [24, 48]. In addition, the presence of senescence can be supported by the appearance of the cells which display flat morphology and vacuolated cells with enlarged nucleoli, by the microscopy. The senescent cells also exhibit stable growth arrest although they are metabolically active [37].
SASP factors including interleukin-6 (IL-6), growth factors, proteases and other pro-inflammatory factors can be detected as well in the presence of cellular senescence [49]. SASP factors can be easily detected form the media of cell culture by using ELISA method or expression analysis of the factors by RNA and protein expressions of the tissues. The levels of ROS are yet, another biological marker of senescent cells. The detection of these markers could be performed in days to weeks depending on the development process of cellular senescence [36].
3 Oxidative Stress and Cellular Senescence
The effect of ROS which is explained by the “Free radical theory of ageing” has been speculated for a long time as one of the main contributor of aging pathology in mammals. Many studies on various species have reported the effects of oxidative stress suggesting ROS has a central role on age-associated diseases and possibly on lifespan. Elevated oxidative stress levels, which is higher than normal cellular levels, damage macromolecules and promote cellular senescence. Extensive ROS production results in the oxidative modification of biomolecules including proteins, DNA and lipids which in turn leads to cellular and vascular dysfunction [50]. The consequences of ROS includes other mechanisms such as apoptosis or autophagy, however, the fate of oxidative stress depends on the duration and level of the oxidative stress [51].
Oxidative stress caused by factors such as exposure to oxygen, hydrogen peroxide or tert-butylhydroperoxide can induce SIPS in cells [52, 53]. Other stressors such as oncogenes (H-RasV12) can also result in oxidative stress induction [54] and lead to oncogene induced SIPS by DNA damage response (DDR) [55]. The cellular senescence induced by oxidative stress has two main pathways as mitochondrial and non-mitochondrial pathways. These two pathways are likely to merge at some point by several molecular factors such as p53, pRB, p16 and p21 [56, 57]. p53/p21 pathway has been suggested to act as the main molecular player when the stress is related to DDR [55], however, p16 pathway also has been shown to be activated during DDR damage (Fig. 10.1). Several studies also showed that cellular senescence that is induced by ROS also involves a positive feedback pathway that results in the amplification of senescence factors. In this case, when senescence is induced by ROS, SASP factors would lead to even more increased oxidative stress and, thus, to increased senescence [58], and in turn, generated ROS would lead to more mitochondrial mutations and ROS that finally would result in ultimate senescent phenotype [56, 57].
Although some mechanisms have been proposed to link oxidative stress and senescence, the exact mechanisms haven’t fully cleared yet. A study conducted with a senescent mice model [59], reported that the nuclear factor erythroid 2–related factor 2 (Nrf2) pathway could be the responsible molecular factor in the generation of oxidative stress in senescent animals. Nrf2 is a transcription factor which binds to cytosolic Kelch-like ECH associated protein 1 (Keap1) in its inactive form in normal cell conditions and activated under oxidative stress conditions. Activated Nrf2 translocates into the nucleus where it binds antioxidant response element and induces the gene expression of antioxidant and phase II enzymes. Studies have shown that the effect of Nrf2 signaling on the inhibition of oxidative stress in adverse cardiac remodeling via its antioxidant role and Nrf2 silencing has also shown to lead to the activation of proinflammatory genes such as IL-1β and tumor necrosis factor (TNFα). Therefore, any impairment in Nrf2 signaling is suggested to lead to an increase in the cardiac disease severity by the activation of inflammation factors (Fig. 10.2) [60].
Development of cardiovascular diseases by the effect of oxidative stress. Aged vessels are characterized by elevated reactive oxygen species (ROS) levels and decreased nitric oxide (NO) levels modulated by nitric oxide synthase NADPH oxidases (NOX). Decreased antioxidant capacity induces cellular senescence that promotes senescence-associated secretory phenotype (SASP) factors that lead to pathogenesis of heart failure, atherosclerosis, diabetes and hypertension
4 The Mitochondrial Pathway of Oxidative Stress and Cellular Senescence
Mitochondria has the central role on the production of ROS by the escape of electrons through electron transport chain (ETC), therefore, mitochondrial dysfunction has been associated to senescence and related disease pathologies [61]. ROS produced by mitochondria results in the mutations of mitochondrial DNA leading to mitochondrial function defects. As a result, especially in aging process, mitochondrial dysfunction results in biological function decline and leads to age-associated heart disorders [62].
Recent findings reported the fact that oxidative stress can result in DDR by various mechanisms such as telomeric and non-telomeric mechanisms. Oxidative stress can also induce ROS production by the positive feedback mechanism [63] and the molecular players that is known to responsible for the positive feedback mechanism includes p53-dependent signaling pathway such as p21, GADD45A, p38 and TGFβ [57]. On the other, as it is previously discussed, oxidative stress can be generated also by non-mitochondrial mechanisms which leads to cellular senescence in collaboration with the mitochondrial ROS. Recently, it was reported that the presence of mitochondrial ROS is required for the cellular senescence generation which is shown by typical markers of senescence such as SA-β-gal and SASP factors [51]. In addition, uncoupling of mitochondria suggested to lead to proteasomal degradation and autophagy however, no common markers of senescent phenotype were detected [51]. The study also suggested mTOR could be the responsible pathway to drive DDR for the induction of cellular senescence. Recent findings also suggested the role of mitochondria on senescent phenotype not only by ROS production but also other factors including mitochondrial dynamics, altered redox state and metabolism and impaired ETC [64]. It is also worth noting that mitochondrial dysfunction has a regulative role on SASP as well which induces growth arrest as the senescent phenotype [57].
It has been reported that a proper model to study age-associated cardiovascular diseases is the accelerated senescent model of mice named as “senescence accelerated mice prone 8” (SAMP8). A study of SAMP8 showed increased mitochondrial dysfunction and cardiac ROS in the mitochondria [61]. Various studies have also reported impairment of mitochondria is related to apoptosis [65] and it was shown that hearts of SAMP8 mice is affected by apoptosis significantly by caspase3 pathway during aging process [66]. The reports of senescent mice model also confirm that aging-associated diseases and typically cardiac remodeling are related ROS, mitochondrial dysfunction, and apoptosis.
Sirtuins (SIRTs) which are also known as Nicotinamide adenine dinucleotide (NAD+)-dependent histone/protein deacetylases are controlled by NAD+ physiological levels in the cell. It has been reported that NAD+ and SIRT activity is decreased by aging, and this decrease is responsible for mitochondrial dysfunction. Reduced NAD+ levels result in ROS production either via PGC-1α (PPAR-γ coactivator 1 alpha) dependent and independent pathways [67]. On the other hand, AMPK increases SIRT1 activity via affecting NAD+/NADH ratio [68]. Another study reported elevated NAD+ levels inhibit senescent phenotype in muscle, neural and other adult stem cells [69]. Overall, these reports demonstrate the importance of NAD+ and SIRTs as molecular players in mitochondrial dysfunction and oxidative stress production for healthspan. All information above suggest that mitochondrial ROS is crucial in process of cellular senescence and related aging pathologies.
5 Oxidative Stress, Senescence and Cardiovascular Pathologies
Previous sections explain common processes of ROS and oxidative stress levels, their mechanisms and responses against various conditions that result in dysfunction of the cellular and tissue physiology. Many studies have suggested the role of cellular senescence in the development of age-associated pathologies [21, 46] that has been linked with oxidative stress, mitochondrial dysfunction and telomere shortening [70] all of which are considered as the markers of aging [71]. Changes in the level of ROS has been suggested to be important in the association of oxidative stress with aging and aging-associated heart and cardiovascular diseases [72]. In the following sections, the effects of oxidative stress and cellular senescence on aging-associated pathologies including heart failure, atherosclerosis, hypertension and diabetes will be described (Fig. 10.2).
Processes of oxidative stress are identified in ageing vessels [73, 74] including elevated vascular ROS levels and decreased nitric oxide levels leading to the formation of injurious peroxynitrite in aorta of aged rodents [50]. Oxidative stress typically promotes several molecular events of vascular aging such as vascular dysfunction, fibrosis and calcification, altered calcium homeostasis, activation of redox-sensitive or pro-inflammatory factors, and activation of cellular senescence and autophagy in ECs and VSMCs. Increased ROS levels could be reversed by superoxide dismutase (SOD) mimetics, such as tempol, leading to decrease in endothelial impairment in old rodents suggest the essential role of oxidative stress in age-associated endothelial dysfunction [75]. The alterations in cellular anti-oxidant systems by aging such as the decrease of SOD as an antioxidant enzyme, also take crucial role. Nrf2, which is previously described as the master transcription factor regulating anti-oxidant genes, is also downregulated by the reduced anti-oxidant capacity leading to extensive dysfunction of the cells [76]. Finally, these processes are followed by low-grade chronic inflammation via NFκB molecular pathway in aged vessels (Fig. 10.2) [63].
Telomere shortening and dysfunction which have crucial role during replicative senescence also related with CVDs [77]. Particularly, telomere dysfunction of senescent cells has been associated with chronic ROS production. Relatively, it is suggested that the senescence phenotype SASP leads to degenerative and proliferative activities in the cells and their component cells which is also important in the pathology of cardiovascular disorders [78]. It has been shown that senescent human umbilical venous endothelial cells (HUVECs) contributed to endothelial dysfunction that resulted in atherosclerosis development [79]. Senescence of HUVECs is further proposed to elevate the expression of pro-inflammatory cytokines that could lead to a progressive development in the pathogenesis of CVDs. It has been also demonstrated that pro-inflammatory molecules including TNF-α and IL-6 levels are elevated during aging process [79].
p66Shc, which is an adaptor protein, has been suggested to control oxidative stress and be involved in CVD pathogenesis [80]. It was shown in fibroblast cells that p66Shc controls various cellular fates including apoptosis and senescence [81]. On the other hand, several studies have shown that p66Shc silencing decreased the levels of ROS under stress conditions. In addition, knockout mice model of p66Shc was shown to extend lifespan. Elevated levels of oxidative stress and decreased NO were shown to lead to vessel impairment [82] and elevated expression of p66Shc was shown in coronary artery disease patients [83]. Another study in knockdown mice model of p66Shc showed that myocardial injury was decreased, and resistance to endothelial dysfunction was increased due to low oxidative stress [49]. Therefore, it is suggested that the regulators of cellular senescence such as p66Shc are essential to develop therapeutic interventions for CVDs.
EC senescence which can be induced by several factors has important role on cellular homeostasis and relatively, on vascular aging and diseases. Although the exact mechanism of EC senescence is not known, it is suggested that ROS levels are crucial for the generation of senescent ECs and vascular aging [84]. It is well known that senescence-induced vascular aging is crucial in disorders such as atherosclerosis, diabetes, and hypertension [3]. p53 which is the target of SIRT1 is also regulated by oxidative stress levels and, therefore, antiaging mechanisms are controlled by ROS at some level [85]. Particularly, the p53-p21-Rb molecular pathway is suggested to have an important role for the generation of senescent cells in various cellular stimuli. When activated, p53 protein induces the expression of p21 that leads to cell cycle arrest and activation of cell cycle repressor retinoblastoma (Rb) [86]. A very recent study also explained the relation of EC senescence and vascular aging with excess ROS levels and decreased SIRT1 levels by the activation of p53-p21-Rb pathway [87]. Another recent study on SAMP8 as a vascular aging model showed the increased levels of ROS and inflammation in perivascular adipose tissue which resulted in the vascular dysfunction [88]. These studies suggested that association of increased oxidative stress levels and senescence have big impact on the vascular aging and dysfunction through the senescence of vascular cells and, therefore, on the development of CVDs.
6 Heart Failure
Among age-associated diseases, heart failure has a high prevalence in old people [89] and, therefore, well-established therapies for severe heart failure is urgent. Age-associated heart failure is also observed without known risk factors, such as hypertension, obesity, diabetes, or atherosclerotic pathologies [3, 90]. %50 of heart failure patients develop the disease without systolic dysfunction and this type is named as “heart failure with a preserved ejection fraction” (HFpEF). HFpEF is the common type of heart failure in aging populations and a major clinical problem since its mechanism is still not fully known. HFpEF pathology is suggested to be related with cardiac endothelial cell remodeling [91] and endothelial inflammation [92]. In addition, several studies have suggested the pathological influence of senescent phenotype in the development of heart failure. A recent study showed the important role of EC senescence in SAMP8 model (46) and another recent study showed the increase of oxidative stress biomarkers in the hearts of senescent mice model [93]. Therefore, the physiological aging and correlatively cellular senescence process are suggested to elevate the risk of heart failure.
EC senescence has been proposed to have a critical role in the failing heart although the mechanism has not been fully explained yet. It was shown that p53 level in cardiac aging is elevated in mouse model of left ventricular (LV) pressure overload. In addition, LV pressure overload leads to capillary rarefaction, tissue hypoxia, and cardiac dysfunction [35]. Another recent study by LV pressure overload model also showed the inflammation and remodeling in vascular ECs induced via p53 molecular pathway [13]. Increased p53 expression induces inflammation and exacerbates the intercellular adhesion molecule (ICAM)−1 expression leading to cardiac dysfunction in endothelial cells. It has been suggested that sympathetic nervous system is activated in heart failure [94] and the sympathetic nervous system/ROS axis leads to elevated p53 expression in LV pressure overload [13]. Additionally, it has been suggested that accumulation of p53 in ECs lead to deterioration of cardiac function, resulting in angiogenesis and failing heart [95]. These studies suggested that senescent ECs could potentially become therapeutic target for cardiac dysfunction of failing heart.
In addition to elevated p53 and p21 expression, telomere shortening as another characteristic of cellular senescence plays a critical role in heart pathologies [96]. Correlatively, decreased levels of telomerase activity was reported in ECs of people with coronary heart disease [97] and in circulating leukocytes of chronic heart failure [98]. It is also suggested that telomere shortening is related to cardiovascular diseases independent of known vascular risk factors [99, 100].
As half of the HFpEF patients are diagnosed with a preserved ejection fraction, the rest develops the disease with other well-known risk factors such as obesity, hypertension, diabetes, and aging. Coronary microvascular inflammation is another most established risk factor for the development of HFpEF [91], and a recent study showed that senescent ECs have an influence on HFpEF. A study performed by SAMP8 showed that when accelerated senescent mice fed with high-fat diet, cardiac cellular senescence and inflammation are significantly elevated along with HFpEF physiological alterations [101]. It is not surprising that cardiac cellular senescence leads to vascular dysfunction and inflammation and accordingly, to the pathology of HFpEF. Thus, inhibition of senescent EC generation is suggested as a possible therapeutic intervention for the treatment of HFpEF.
7 Structural Changes of Arteries with Aging and Atherosclerosis
Although aging is still not considered as a pathological condition, aging-related arterial remodeling is suggested to be one of the pathological determinant of cardiovascular disease. During cardiovascular pathology, the arterial walls are damaged by the increased oxidative stress levels that leads to the generation of oxidized low-density lipoproteins (oxLDL). The initial and pivotal step of atherosclerosis progress is the infiltration of oxLDL to the subendothelial space of the arterial wall [102]. Meanwhile, monocytes are attracted, which in turn, transform into lipid-loaded foam cell macrophages. More monocytes are attracted with the help of proinflammatory factors that leads to the accumulation of more inflammatory cells and results in the formation of lesions and plaques. The plaque stability is determined by the content of the plaques and aged arteries have thicker intima/media by two- to three-fold than young arteries [2, 3].
Aging modifies SMCs structure to a more synthetic phenotype that contributes to the development of atherosclerosis. Both intima and media of the artery get thickened through aging is accompanied by increased collagen and decreased elastin generation which lead to impairment in the integrity of the arteries [103, 104]. In addition, calcification is yet another phenotype of aging arteries and, therefore, plaques become more severe by aging. A study of aged rabbits showed that high fat diet resulted in more developed plaques when compared to the young rabbits [105]. All these characteristics of vascular dysfunction by old age increase the risk for atherosclerosis and recently, cellular senescence has been proposed to have the pivotal role in the pathology.
Growing evidence have showed the presence of senescent cells in the vessel content and vascular senescence is linked by disorders such as atherosclerosis, intimal hyperplasia, hypertensive arteries, aneurysms and diabetic arteries [4]. Although the mechanisms of cellular senescence in the vascular dysfunction and development of atherogenesis are not fully understood, it is known that oxidative stress is one of the major contributor. A study suggested that increased oxidative stress levels are caused by the elevated TNF-α levels which also modulates the inflammation process by NFκB activation. NFκB is a redox sensitive transcription factor which regulates many inflammation processes in the arteries and the main controller of SASP [106, 107]. Moreover, in a recent study that compared the young and elderly people, NFκB expression was found to be significantly increased in elderly people [108]. In addition, a protein named as Klotho inhibits cellular senescence and prolongs lifespan of mice and its suppression results in the development of the atherogenesis [109, 110].
It is a well known fact that cellular senescence increases over time in the presence or absence of atherosclerotic process [79, 111]. Particularly, advanced plaques show senescent cell phenotype SASP and the presence of common senescence markers such as SA-β-Gal, p16Ink4a, p53, and p21 expression [112]. Several studies have also suggested the emergence of cellular senescence mechanisms in the pathology of atherosclerosis among other cellular fates [113]. Both ECs and SMCs were reported in patients with abdominal aortic aneurysm (AAA) [111]. In addition, both ECs and SMCs were also reported to be induced by well-known stressors such ROS and angiotensin II [77, 114]. The presence of cellular senescence in ECs has been shown in atherosclerotic human coronary arteries [115] and thoracic aorta [116] by the SA-β-gal activity. Senescent ECs are associated to be induced by aortic flow impairment in the atherosclerotic mouse model and the molecular pathway responsible for senescence is suggested to be p53 signaling [117]. Typically, ECs are suggested to take a critical role for several vascular functions such as angiogenesis and coagulation and ECs show loss of function by aging process. Most importantly, decreased nitric oxide synthase (NOS) activity results in reduced nitric oxide (NO) generation leading to deterioration in vasodilation and cardiovascular pathology. Additionally, the impairments in ECs function result in oxidative stress and inflammation phenotypes and, therefore, it is suggested that cellular senescence contributes these properties [105]. During replicative senescence, progressive generation of senescent ECs is typically important since it initiates SASP phenotype resulting in tissue remodeling and elevates pro-inflammatory cytokines [28]. Senescence of ECs are, therefore, essential to have causal role in chronic inflammation and tissue remodeling. It was also demonstrated that aging-related loss of function in ECs is linked to telomere shortening [115]. In aortic aneurysm samples, elevated oxidative stress and telomere attrition were also shown in ECs as well [111]. Since replicative senescence is proven to be a common characteristic of aging, increased ROS levels and decreased NO in ECs cells contribute to the occurrence of vascular senescence [105].
In atherosclerosis patients, cellular senescence of VSMCs was also shown in atherosclerotic lesions of patients with coronary artery disease, AAA, and peripheral artery disease [115]. Another study demonstrated the SA-β Gal activity, p16, and p21 expression and IL-6 as SASP phenotype in SMCs of carotid artery plaques [118]. Additionally, angiotensin II treatment resulted in senescent vascular SMC generation in ApoE −/− mice suggests the relation of well-known stressors with senescence [119]. As articulated above, the switch of SMCs to a more synthetic form is partly explained by the impairment of TGF-β pathway. In stress conditions, SMCs was shown to have increased inducible NOS, ICAM-1 and angiotensinogen in aging process [105, 120]. Smooth muscle 22α protein is suggested to be marker for senescent SMCs and a recent study demonstrated that it contributes to senescent cell generation by the inhibition of p53 degradation [121]. Moreover, it was previously demonstrated that VSMCs from the aneurysms display oxidative DNA damage [111]. As expected, senescence of VSMCs has common senescent phenotype such as increased of pro-inflammatory cytokines, growth factors, and matrix metalloproteases all of which contribute to the vascular dysfunction.
Some of atherosclerotic plaques are also characterized by telomere shortening which is a hallmark of senescent phenotype [12]. VSMCs that are shown to be senescent by elevated p16 and p21 and SA-β-gal activity are reported to have telomere shortening in the atherosclerotic plaques. Shorter telomeres are suggested to be result of oxidative stress induced by DNA damage and leading senescence in VSMCs [122]. Since cellular senescence functions to initiate atherosclerosis process, it is not surprising that the decreased levels of telomeric repeat-binding factor-2 (Trf2) in VSMCs contributes to the plaque development in ApoE −/− mice. However, increased plaque growth by the knockout of senescence associated genes such as p53, p21, or p19Arf suggests the anti-atherosclerotic role of senescence [105, 123]. Relatively, it was demonstrated that decreased p16Ink4a and p14Arf expressions in human and mouse studies elevated the atherosclerosis development [86]. On the other hand, a very recent study of Childs et al. showed the deleterious effect of senescent cells in the progression of atherosclerosis by using both transgenic and pharmacological models [124]. In the study, the clearance of senescent cells in Ldlr−/− mice demonstrated the pivotal role of senescence during atherogenesis. It was suggested that even early stages of the plaques contain senescent cells and accumulate in the subendothelial space. Accumulation of senescent macrophages in the early atheroma leads to elevated inflammation factors and, therefore, contributes to the development of the atherogenesis. On the other hand, clearance of senescent foam cell macrophages was shown to reduce plaque formation. It was also suggested that in the severe plaques, senescent phenotype results in the plaque instability not only by inflammation factors and chemokines but also contributes to the plaque development by the increased expression of matrix metalloproteases and clearance of the senescent cells resulted in the regression of plaque growth and remodeling [124].
In addition to ECs and SMCs, immune cells show senescence phenotypic properties as well. A report conducted in old people who have the higher risk of heart diseases were shown to be characterized by the telomere attrition [125]. Another study showed elevated oxidative stress and inflammation factors in the monocytes of atherosclerotic patients [126]. A recent study also established the important role of cellular senescence for the pathogenesis of atherosclerosis in the macrophages by the deletion of senescent cells [124]. Additionally, it was previously shown that senescent macrophages are driven by p16 signaling pathway and cellular senescence has the central role in the occurrence of senescent phenotypes in the macrophages [127]. Collectively, studies have suggested the central role of cellular senescence in the atherosclerosis and therefore, senolytic agents as promising interventions for combating the disease.
8 Hypertension
Another well-known risk factor for cardiovascular diseases is hypertension, therefore, the association of vascular senescence and hypertension have gained interest in the recent years. The reports have suggested that typically increased blood pressure over time has the most significant effect on the development of hypertension within aging [128]. Other characteristics of hypertension such as vascular dysfunction, inflammation, extracellular matrix deposition are also common features of aging as well [129]. The presence of premature vascular aging was also shown in the young hypertensive individuals and it is suggested that hypertension fastens the vascular aging process similar to accelerated aging syndrome, progeria [130]. Therefore, it is proposed that understanding of the relationship of vascular deterioration with hypertension during aging is crucial.
The presence of senescent cells were demonstrated in vessels of the patients with hypertension. The study suggested the role of p53/p21 signaling in the telomere attrition by the uncapping of telomeres [131]. There is also growing evidence of animal studies that support the role of cellular senescence in hypertension. In a transgenic mouse model of aging produced by the defect of nucleotide excision repair genes, senescent ECs and VSMCs formation were shown to be increased along with elevated hypertension and vascular dysfunction [132]. Another report showed that hypertension resulted in the inhibition of cell cycle and activation of aortic p16 signaling both in the transgenic rat model and in humans [133]. Furthermore, the inhibition of NOS resulted in higher p16 levels in the arteries and in the development of hypertension suggesting the relation of cellular senescence with hypertension [134]. NO which acts as vasodilator contributes to modulation of blood pressure and accordingly has anti-hypertensive features. Several studies have shown that NO donor decreases the levels of senescent ECs and activates telomerase activity in aging process [135]. In normal conditions, endothelium releases NO that modulates vasodilation, however, in case of aging related hypertension, other molecules such as endothelin-1, angiotensin II and superoxide anions are secreted as well. These substances are known to lead to defects in the vasodilation exacerbating vascular remodeling through aging [136].
The common role of oxidative stress and inflammation has been explained in various cardiovascular pathology. Several inflammation factors including C-reactive protein, IL-6, TNF-α, and IL-1β are shown to be increased in hypertension patients [137]. On the other hand, since ROS affects vascular physiology, it is proposed to have essential effects on hypertension during aging by promoting cellular senescence of vascular cells [73, 74]. Clinical reports have revealed that vascular O2 − generation supports hypertension development by the increased blood pressure [138]. In addition, other human studies have suggested the increased oxidative damage in plasma and urine samples [139] and increased levels of O2 − and H2O2 in VSMCs in hypertension [140]. Various studies have demonstrated the role of ROS generated by NADPH oxidases (NOX) in vascular dysfunction through aging process (Fig. 10.2). Particularly, NOX1 and NOX2 were found to be elevated in old and hypertensive rat vessels and vascular dysfunction was reversed by NOX inhibition [141]. Therefore, among other oxidases that also generate ROS, NOXs are suggested to play key role for the hypertension related cardiovascular pathology [142]. These findings suggest that cellular senescence is driven by various factors takes a critical role in the development of hypertension and vascular remodeling.
9 Diabetes
Similar to other cardiovascular diseases, chronic inflammation is also related to obesity and suggested as a critical contributor to insulin resistance [143]. Clinical studies have demonstrated elevated SASP factors including IL-6, IL-8 and MCP-1 in obese patients [144, 145] and IL-6, IL-1 as the major markers of diabetes [146]. Another study demonstrated elevated SASP factors both in blood and in the vessels of diabetic patients [147]. The SASP factor MCP-1 is suggested to lead to macrophage infiltration related insulin resistance and responsible mechanism of this process is proposed to be autophagy [148].
A recent study also demonstrated that cellular senescence induced by p53 molecular pathway drives inflammation in diabetes [149]. In addition, animal studies have shown the presence of senescent vascular cells in the diabetes models and it is speculated that accumulated senescent cells in obesity could be the key drivers of diabetes [150]. In diabetic rat and mice, the presence of cellular senescence was also demonstrated in the aorta, suggesting that hyperglycemia may lead to senescent EC accumulation [151]. The mechanisms responsible for the vascular senescence are suggested to be increased oxidative stress, decreased NO levels and SIRT1 activation.
Dysfunction of adipose tissue is also proposed as a crucial contributor to cellular senescence linked diabetes. Accumulated senescent preadipose cells were confirmed both in young and old obese patients suggests the key role of senescence in adipogenic function [152]. SASP which is another senescent phenotype also results in the impairment of adipogenesis process and, therefore, results in insulin response [153]. It was also demonstrated that senescent cells lead to lipodystrophy in accelerated aging mouse model, however, consequences of senescent cell removal in diabetes have not been fully explained yet. In addition, adipose dysfunction could lead to fat deposition in various tissues such as liver and heart resulting in the pathogenesis of other diseases including atherosclerosis [154].
Senescent pancreatic β-cells are also suggested to play critical role in the development of type 2 diabetes. It was shown that high fat diet resulted in cellular senescence that induced β-cell function loss in mice [155]. Another study of mice demonstrated the association of type 2 diabetes with cell cycle inhibition which is a common phenotype of cellular senescence [156]. Furthermore, deletion of p53-dependent apoptosis in mice led to senescent cell accumulation and dysfunctional β-cells formation resulting in accelerated development of diabetes [157]. Although more evidence is required to clarify the contribution of cellular senescence on diabetes, several studied already provided information about the increased senescent cells in damaged tissues in diabetes process [155, 158]. It is suggested that accumulated senescent cells during diabetes represent a critical risk factor for the inflammatory state and, therefore, for cardiovascular diseases.
10 Future Directions for Therapy of Cardiovascular Diseases
Emerging data have identified the critical role of vascular senescence during aging for the development of cardiovascular diseases. Particularly, studies that have indicated the clearance of senescent cells extends both healthspan and lifespan suggests senolytic agents as a promising candidate for the therapy of age-associated cardiovascular pathology. The recent study by Childs et al. is breakthrough in the senolytic research for the treatment of atherosclerosis. In the study, the presence of senescent cells was shown to be detrimental at all stages of atherosclerosis and selective removal of these cells reversed the plaque formation. Furthermore, various studies have also supported the effect of selective clearance of senescent cells in the vascular homeostasis suggesting that senolytic agents hold a therapeutic paradigm [4].
The term senolytic is first described by Zhu et al. for the compounds that selectively kill senescent cells [159]. It was proposed that accumulation of senescent cells could be resulted from resistance to apoptosis [160] therefore, researchers have focused on the compounds that selectively target apoptosis of senescent cells. Some of the well-known pharmacological agents are already repurposed for senescent clearance. Of them, dasatinib is an approved drug for use in patients with chronic myelogenous leukemia that inhibits tyrosine kinase signaling [161]. The effect of dasantinib was shown in the clearance of senescent cell in preadipocytes cell culture. In addition, quercetin which is a bioflavonoid antioxidant was reported to kill senescent ECs in a selective way [162]. Morever, combination therapy of dasantinib and quercetin was also effective in the clearance of senescent cells in tissues of mice and increased healthspan via reduced cardiac damage in aged mice [159]. Inhibitor of anti-apoptotic proteins (ABT263) was identified as another potential senolytics which cleared senescent hematopoietic stem cells and reversed aging phenotype in mice [163]. However, the molecular pathways underlying the apoptosis of senescent cells has not been cleared yet and further studies are needed to determine the side effects of the therapy.
SASP is the senescent phenotype which transforms cells into a pro-inflammatory status and many reports have identified the damage of SASP to the tissues in aging-associated diseases. Since SASP secretes inflammatory molecules and contributes to the disease pathogenesis, it is suggested to be potential therapeutic target for the treatment of selective cellular senescence. SASP leads to an increase in the metabolic activity of SMCs and inhibitor of glycolysis was reported to clear senescent SMCs. However, the specificity of that treatment and its translation to humans is questioned to be safe and nontoxic [4, 164].
Another potential approach against cellular senescence related cardiovascular diseases would be targeting oxidative stress. Antiaging therapy against inhibition of cellular senescence such as sirtuins was previously studied and it was shown that increase in SIRT1 expression reduced the cellular senescence in SMCs and ECS resulting in lifespan extension [165, 166]. Another antioxidant that was suggested as a potential approach is NAD fueling that regulates metabolic pathways as well [167]. NAD therapy was suggested to lead to SMC translocation in addition to reducing accumulation of senescent SMC and ECs. Especially, migration of SMC in straight-line manner was indicated to be beneficial to suppress vascular damage [168]. Finally, it was stated that statins are also effective in the suppression of cellular senescence and DDR pathways in atherosclerosis [169].
Regarding the critical role of oxidative stress and inflammation in the senescence related cardiovascular diseases, it is important to clarify the underlying mechanisms. As mentioned in the text previously, the transgenic model SAMP8 has the accelerated senescence phenotype. Aged SAMP8 mice display elevated oxidative stress and ER stress, inflammation, vascular dysfunction features, therefore, it is suggested to be convenient model to investigate the vascular homeostasis in aging. Recently, exciting data have been obtained from the SAMP8 studies, though, more work is required to determine potential therapies for age-associated cardiovascular diseases [5, 60, 170].
11 Conclusion
Suggested pathological role of vascular senescence linked to oxidative stress in cardiovascular disorders such as heart failure, diabetes and atherosclerosis is explained throughout this chapter. Particularly, senescence of ECs and VSMCs cells have been shown to be important in the disease pathology however, immune cell senescence and pancreatic β-cells senescence received considerable attention as well. Recently, various studies have also focused on the selective clearance of senescent cells to reverse aging associated cardiac damage and the findings are promising and exciting. Especially, removal of cardiac senescent cells could be an emerging approach since vascular cells are pivotal in the maintenance of vascular homeostasis [105].
Oxidative stress has been suggested to be critical in the aging associated pathologies for a long time, however, recently, its potential effect on cellular senescence to contribute disease development has gained more interest. Various stresses including ROS generation and DDR were shown to have ultimate role on the cellular homeostasis leading to detrimental effects on the cardiac tissues. Therefore, antioxidant molecules such as quercetin have been repurposed to reverse the damage of cellular senescence in the recent years. In addition to antioxidants, various pharmacological agents have been recently studied as senolytic agents, though more evidence is required for the side effects of the senolytics before translation into the clinical studies [171]. On the other hand, approaches to suppress cellular senescence, rather than clearing senescent cells, are suggested to be another potential therapy. There has been no clinical trial for the therapy of senescent cell clearance in cardiovascular diseases, however, cell culture and animal studies have indicated its pivotal potential to hold a next generation therapy for vascular pathology.
Abbreviations
- AAA:
-
Abdominal aortic aneurysm
- CVD:
-
Cardiovascular disease
- DDR:
-
DNA damage response
- DNA:
-
Deoxyribonucleic acid
- EC:
-
Endothelial cells
- ER:
-
Endoplasmic reticulum
- ETC:
-
Electron transport chain
- HFpEF:
-
Heart failure with a preserved ejection fraction
- IL:
-
Interleukin
- Keap1:
-
Kelch-like ECH associated protein 1
- LV:
-
Left ventricular
- MCP:
-
Monocyte chemoattractant protein 1
- mTOR:
-
Mammalian target of rapamycin
- NAD:
-
Nicotinamide adenine dinucleotide
- NFκB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NOX:
-
NADPH oxidases
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2:
- oxLDL:
-
Oxidized low-density lipoproteins:
- PGC-1α:
-
PPAR-γ coactivator 1 alpha
- ROS:
-
Reactive oxygen species
- SAHF:
-
Senescence-associated heterochromatin foci
- SAMP8:
-
Senescence accelerated mice prone 8
- SASP:
-
Senescence-associated secretory phenotype:
- SA-β-gal:
-
Senescence-associated beta-galactosidase
- SIPS:
-
Stress-induced premature senescence
- SIRT:
-
Sirtuin
- SMC:
- SOD:
-
Superoxide dismutase
- TGFβ:
- TNFα:
-
Tumor necrosis factor
- VSMC:
-
Vascular smooth muscle cell
References
Forman DE, Alexander K, Brindis RG, Curtis AB, Maurer M, Rich MW, Sperling L, Wenger NK (2016) Improved cardiovascular disease outcomes in older adults. F1000Res 5(F1000 Faculty Rev):112
Lakatta EG (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 107:490–497
Lakatta EG (1993) Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73(2):413–467
Hao Y, Pickering GP (2016) Cellular senescence and vascular disease: novel routes to better understanding and therapy. Can J Cardiol 32:612–623
Forman K, Vara E, Garcia C, Kireev R, Cuesta S, Escames G, Tresguerres JA (2011) Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci 66:823–834
Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S (2010) Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des 16:609–618
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F (2014) The search for antiaging interventions: from elixirs to fasting regimens. Cell 157(7):1515–1526
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Storer M, Mas A, Robert-Moreno A et al (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155:1119–1130
Childs BG, Durik M, Baker DJ, Deursen JMV (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435
Wang JC, Bennett M (2012) Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 111(2):245–259
Yoshida Y, Shimizu I, Katsuumi G, Jiao S, Suda M, Hayashi Y et al (2015) p53-induced inflammation exacerbates cardiac dysfunction during pressure overload. J Mol Cell Cardiol 85:183–198
Yokoyama M, Okada S, Nakagomi A, Moriya J, Shimizu I, Nojima A et al (2014) Inhibition of endothelial p53 improves metabolic abnormalities related to dietary obesity. Cell Rep 7(5):1691–1703
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110(8):1097–1108
Drummond GR, Sobey CG (2014) Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 25(9):452–463
Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V (2011) Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation 123:1900–1910
Corti R, Fuster V, Badimon JJ (2003) Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol 41:7S–14S
Chen B, Lu Y, Chen Y, Cheng J (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225:R83–R99
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology (Bethesda) 29:325–333
Campisi J (2013) Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705
Bodnar AG, Ouellette M, Frolkis M et al (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Tan FC, Hutchison ER, Eitan E et al (2014) Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15:643–660
van Deursen JM (2014) The role of senescent cells in ageing. Nature 509:439–446
Dimri GP (2005) What has senescence got to do with cancer? Cancer Cell 7:505–512
Correia-Melo C, Hewitt G, Passos JF (2014) Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Healthspan 3(1):1
Hewitt G, Jurk D, Marques FD et al (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3:708
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123:966–972
Barascu A, Le Chalony C, Pennarun G et al (2012) Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J 31:1080–1094
Sebastian C, Lloberas J, Celada A (2009) Molecular and cellular aspects of macrophage aging. In: Fulop T (ed) Handbook on immunosenescence. Springer, Dordrecht, pp 919–945
Acosta JC, Banito A, Wuestefeld T et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990
Stewart SA, Weinberg RA (2006) Telomeres: cancer to human aging. Annu Rev Cell Dev Biol 22:531–557
Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358(6381):15–16
Green DR, Chipuk JE (2006) p53 and metabolism: inside the TIGAR. Cell 126(1):30–32
Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y et al (2007) p53- induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446(7134):444–448
Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A 92:4337–4341
Jeyapalan JC, Sedivy JM (2008) Cellular senescence and organismal aging. Mech Ageing Dev 129:467–474
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P et al (2003) Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22(16):4212–4222
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120(4):513–522
Voghel G, Thorin-Trescases N, Farhat N et al (2007) Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev 128:662–671
Voghel G, Thorin-Trescases N, Farhat N et al (2008) Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech Ageing Dev 129:261–270
Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM et al (2005) Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437(7058):564–568
Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C et al (2007) Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448(7151):375–379
Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM et al (2002) “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 21(22):6225–6235
Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A et al (2008) Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135(4):609–622
Baker DJ, Weaver RL, van Deursen JM (2013) p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep 3(4):1164–1174
Dimri GP, Lee X, Basile G et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92:9363–9367
Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28(2):99–114
Carpi A, Menabo R, Kaludercic N et al (2009) The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta 1787:774–780
Fan Q, Chen L, Cheng S, Li F, Lau WB, Wang Le F et al (2014) Aging aggravates nitrate-mediated ROS/RNS changes. Oxidative Med Cell Longev 376515:2014
Correia-Melo C, Marques FD, Anderson R et al (2016) Mitochondria are required for proageing features of the senescent phenotype. EMBO J 35:724–742
Chen Q, Fischer A, Reagan JD et al (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A 92:4337–4341
Parrinello S, Samper E, Krtolica A et al (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5:741–747
Colavitti R, Finkel T (2005) Reactive oxygen species as mediators of cellular senescence. IUBMB Life 57:277–281
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
Passos JF, Nelson G, Wang C et al (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6:347
Wiley CD, Velarde MC, Lecot P et al (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303–314
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9:81–94
Bayram B, Ozcelik B, Grimm S, Roeder T, Schrader C, Ernst IM, Wagner AE, Grune T, Frank J, Rimbach G (2012) A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependentgene expression. Rejuvenation Res 15:71–81
Karuppagounder V, Arumugam S, Thandavarayan RA, Sreedhar R, Giridharan VV, Watanabe K (2016) Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov Today 21:632–639
Rodriguez MI, Carretero M, Escames G, Lopez LC, Maldonado MD, Tan DX, Reiter RJ, Acuna-Castroviejo D (2007) Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic Res 41:15–24
Miquel J (1998) An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol 33:113–126
Spencer NF, Poynter ME, Im SY, Daynes RA (1997) Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol 9(10):1581–1588
Ziegler DV, Wiley CD, Velarde MC (2015) Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell 14:1–7
Nakahara H, Kanno T, Inai Y, Utsumi K, Hiramatsu M, Mori A, Packer L (1998) Mitochondrial dysfunction in the senescence accelerated mouse (SAM). Free Radic Biol Med 24:85–92
Sreedhar R, Giridharan VV, Arumugam S, Karuppagounder V, Palaniyandi SS, Krishnamurthy P, Quevedo J, Watanabe K, Konishi T, Thandavarayan RA (2016) Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. Biofactors 42(4):368–375
Gomes AP, Price NL, Ling AJ et al (2013) Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155:1624–1638
Ruderman NB, Xu XJ, Nelson L et al (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298:E751–E760
Zhang H, Ryu D, Wu Y et al (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352(6292):1436–1443
Passos JF, Saretzki G, Ahmed S et al (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol 5:e110
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–1198
Rodriguez MI, Escames G, Lopez LC, Garcia JA, Ortiz F, Lopez A, Acuna-Castroviejo D (2007) Melatonin administration prevents cardiac and diaphragmatic and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J Endocrinol 194:637–643
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Harman D (2006) Free radical theory of aging: an update: increasing the functional lifespan. Ann N Y Acad Sci 1067:10–21
Tatchum-Talom R, Martin DS (2004) Tempol improves vascular function in the mesenteric vascular bed of senescent rats. Can J Physiol Pharmacol 82(3):200–207
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G (2011) Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. AmJ Physiol Heart Circ Physiol 301:H363–H372
Zhao W, Zheng XL, Peng DQ, Zhao SP (2015) Myocyte enhancer factor 2A regulates hydrogen peroxide-induced senescence of vascular smooth muscle cells via microRNA-143. J Cell Physiol 230(9):2202–2211
Fyhrquist F, Saijonmaa O, Strandberg T (2013) The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol 10:274–283
Minamino T, Miyauchi H, Yoshida T et al (2004) The role of vascular cell senescence in atherosclerosis: antisenescence as a novel therapeutic strategy for vascular aging. Curr Vasc Pharmacol 2:141–148
Migliaccio E, Giorgio M, Mele S et al (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313
Suski JM, Karkucinska-Wieckowska A, Lebiedzinska M et al (2011) p66Shc aging protein in control of fibroblasts cell fate. Int J Mol Sci 12:5373–5389
Cosentino F, Francia P, Camici GG et al (2008) Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 28:622–628
Franzeck FC, Hof D, Spescha RD et al (2012) Expression of the aging gene p66Shc is increased in peripheral blood monocytes of patients with acute coronary syndrome but not with stable coronary artery disease. Atherosclerosis 220:282–286
Rodríguez-Mañas L, El-Assar M, Vallejo S, López-Dóriga P, Solís J, Petidier R, Montes M, Nevado J, Castro M, Gómez-Guerrero C (2009) Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8(3):226–238
Mavrogonatou DE, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30:89
Muñozespín D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–496
Ke Y, Li D, Zhao M et al (2018) Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med 116:88–100
Agabiti-Rosei C et al (2017) Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens Res 40:41–50
Borlaug BA (2014) The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11(9):507–515
Shih H, Lee B, Lee RJ, Boyle AJ (2011) The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 57(1):9–17
Mohammed SF, Redfield MM (2015) Response to letters regarding article, “coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction”. Circulation 132(16):e206
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271
Rebrin I, Zicker S, Wedekind KJ, Paetau-Robinson I, Packer L, Sohal RS (2005) Effect of antioxidant-enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radic Biol Med 39:549–557
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS et al (1984) Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311(13):819–823
Gogiraju R, Xu X, Bochenek ML, Steinbrecher JH, Lehnart SE, Wenzel P et al (2015) Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J Am Heart Assoc 4(2):e001770
Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD et al (2013) Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol 305(2):H251–H258
Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M (2008) Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis 198(2):347–353
van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ et al (2007) Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 49(13):1459–1464
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349(3):g4227
Salpea KD, Humphries SE (2010) Telomere length in atherosclerosis and diabetes. Atherosclerosis 209(1):35–38
Gevaert AB, Shakeri H, Leloup AJ, van Hove CE, de Meyer GRY, Vrints CJ et al (2017) Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail 10(6):e003806
Navab M, Berliner JA, Watson AD et al (1996) The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol 16:831–842
Spina M, Garbisa S, Hinnie J, Hunter JC, Serafini-Fracassini A (1983) Age-related changes in composition and mechanical properties of the tunica media of the upper thoracic human aorta. Arteriosclerosis 3(1):64–76
Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML (1977) Echocardiographic assessment of a normal adult aging population. Circulation 56(2):273–278
Katsuumi G, Shimizu I, Yoshida Y, Minamino T (2018) Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med 5:18
Chien Y, Scuoppo C, Wang X et al (2011) Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev 25:2125–2136
Freund A, Patil CK, Campisi J (2011) p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J 30:1536–1548
Csiszar A, Wang M, Lakatta EG et al (2008) Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol (1985) 105:1333–1341
Eren M, Boe AE, Murphy SB et al (2014) PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A 111:7090–7095
Kuro-o M (2008) Klotho as a regulator of oxidative stress and senescence. Biol Chem 389:233–241
Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C et al (2012) Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One 7(4):e35312
Gorenne I, Kavurma M, Scott S, Bennett M (2006) Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res 72:9–17
Wang J, Uryga AK, Reinhold J et al (2015) Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132:1909–1919
Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B (2008) Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere dependent and independent pathways. Circ Res 102(2):201–208
Minamino T, Miyauchi H, Yoshida T et al (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105:1541–1544
Lin JR, Shen WL, Yan C, Gao PJ (2015) Downregulation of dynamin-related protein 1 contributes to impaired autophagic flux and angiogenic function in senescent endothelial cells. Arterioscler Thromb Vasc Biol 35(6):1413–1422
Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S et al (2014) Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler Thromb Vasc Biol 34(5):985–995
Gardner SE, Humphry M, Bennett MR, Clarke MCH (2016) Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Atherosclerosis 244:e5
Kunieda T, Minamino T, Nishi J et al (2006) Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114:953–960
Faggiotto A, Ross R, Harker L (1984) Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis 4(4):323–340
Miao SB, Xie XL, Yin YJ, Zhao LL, Zhang F, Shu YN et al (2017) Accumulation of smooth muscle 22α protein accelerates senescence of vascular smooth muscle cells via stabilization of p53 in vitro and in vivo. Arterioscler Thromb Vasc Biol 37(10):1849–1859. https://doi.org/10.1161/ATVBAHA.117.309378
Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A et al (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99(2):156–164
Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR (2005) Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ Res 96:667–674
Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354(6311):472–477
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361(9355):393–395
Calvert PA, Liew TV, Gorenne I, Clarke M, Costopoulos C, Obaid DR et al (2011) Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol 31(9):2157–2164
Cudejko C, Wouters K, Fuentes L, Hannou SA, Paquet C, Bantubungi K et al (2011) p16INK4a deficiency promotes IL-4-induced polarization and inhibits proinflammatory signaling in macrophages. Blood 118(9):2556–2566
Wang M, Monticone RE, Lakatta EG (2010) Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens 19(2):201–207
Barja G (2013) Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal 19(12):1420–1445
Kotsis V, Stabouli S, Karafillis I, Nilsson P (2011) Early vascular aging and the role of central blood pressure. J Hypertens 29(10):1847–1853
Morgan RG, Ives SJ, Walker AE, Cawthon RM, Andtbacka RH, Noyes D et al (2014) Role of arterial telomere dysfunction in hypertension: relative contributions of telomere shortening and telomere uncapping. J Hypertens 32(6):1293–1299
Durik M, Kavousi M, van der Pluijm I, Isaacs A, Cheng C, Verdonk K et al (2012) Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation 126(4):468–478. https://doi.org/10.1161/CIRCULATIONAHA.112.104380
Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K et al (2008) Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52(1):123–129
Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D et al (2013) Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nω-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 128(21):2318–2324
Vasa M, Breitschopf K, Zeiher AM, Dimmeler S (2000) Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res 87(7):540–542
Rubio-Ruiz ME, Perez-Torres I, Soto ME, Pastelin G, Guarner-Lans V (2014) Aging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res Rev 18:132–147
Singh T, Newman AB (2011) Inflammatory markers in population studies of aging. Ageing Res Rev 10:319–329
Nabha L, Garbern JC, Buller CL, Charpie JR (2005) Vascular oxidative stress precedes high blood pressure in spontaneously hypertensive rats. Clin Exp Hypertens 27:71–82
Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD (2004) Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic Biol Med 36:226–232
Touyz RM, Schiffrin EL (2001) Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19:1245–1254
Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D et al (2010) Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56(3):490–497
Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM (2011) Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120(4):131–141
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105:141–150
Kim CS, Park HS, Kawada T et al (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30:1347–1355
Utsal L, Tillmann V, Zilmer M et al (2012) Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-g levels in 10- to 11-year-old boys with increased BMI. Horm Res Paediatr 78:31–39
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Schneider DJ, Sobel BE (2012) PAI-1 and diabetes: a journey from the bench to the bedside. Diabetes Care 35:1961–1967
Xu H, Barnes GT, Yang Q et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830
Stern DM, Yan SD, Yan SF, Schmidt AM (2002) Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 1:1–15
Palmer AK et al (2015) Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64:2289–2298
Chen J, Brodsky SV, Goligorsky DM et al (2002) Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res 90:1290–1298
Tchkonia T, Morbeck DE, Von Zglinicki T et al (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9:667–684
Hamm JK, el Jack AK, Pilch PF, Farmer SR (1999) Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann N Y Acad Sci 892:134–145
Tchkonia T, Corkey BE, Kirkland JL (2006) Current views of the fat cell as an endocrine cell: lipotoxicity. In: Bray GA, Ryan DH (eds) Overweight and the metabolic syndrome: from bench to bedside (endocrine updates). Springer, New York, pp 105–118
Sone H, Kagawa Y (2005) Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48:58–67
Uchida T, Nakamura T, Hashimoto N et al (2005) Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182
Tavana O, Puebla-Osorio N, Sang M, Zhu C (2010) Absence of p53-dependent apoptosis combined with nonhomologous end-joining deficiency leads to a severe diabetic phenotype in mice. Diabetes 59:135–142
Verzola D, Gandolfo MT, Gaetani G et al (2008) Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 295:F1563–F1573
Zhu Y, Tchkonia T, Pirtskhalava T et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–658
Wang E (1995) Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 55:2284–2292
Montero JC, Seoane S, Ocana A, Pandiella A (2011) Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res 17:5546–5552
Bruning A (2013) Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anti Cancer Agents Med Chem 13:1025–1031
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22(1):78–83
Raez LE, Papadopoulos K, Ricart AD et al (2013) A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71:523–530
van der Veer E, Ho C, O’Neil C et al (2007) Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem 282:10841–10845
Borradaile NM, Pickering JG (2009) Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell 8:100–112
Canto C, Auwerx J (2012) Targeting sirtuin 1 to improve metabolism: all you need is NAD(ş)? Pharmacol Rev 64:166–187
Yin H, van der Veer E, Frontini MJ et al (2012) Intrinsic directionality of migrating vascular smooth muscle cells is regulated by NAD(ş)biosynthesis. J Cell Sci 125:5770–5780
Mahmoudi M, Gorenne I, Mercer J et al (2008) Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ Res 103:717–725
Dong W, Quo W, Wang F, Li C, Xie Y, Zheng X, Shi H (2015) Electroacupuncture upregulates SIRT1-Dependent PGC-1alpha expression in SAMP8 mice. Med Sci Monit 21:3356–3362
Pole A, Dimri M, Dimri G (2016) Oxidative stress, cellular senescence and aging. AIMS Mol Sci 3(3):300–324
Acknowledgment
This work was supported by The Scientific and Technological Council of Turkey (TUBITAK) 2219 Grant Program.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bozaykut, P. (2019). Aging and Cardiovascular Diseases: The Role of Cellular Senescence. In: Chakraborti, S., Dhalla, N., Ganguly, N., Dikshit, M. (eds) Oxidative Stress in Heart Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-13-8273-4_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-8273-4_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8272-7
Online ISBN: 978-981-13-8273-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)