Abstract
Flavonoids are polyphenolic compounds of very important class of plant secondary metabolites having a broad spectrum of biological activities. Because of their antioxidative, anti-inflammatory, antimutagenic, and anticarcinogenic properties, flavonoids have become an indispensable component in nutraceuticals, pharmaceuticals, medicinal, and cosmetic applications. The bioavailability, metabolism, and biological activities of many flavonoids have drawn the attention of researchers to use them as an alternative source of therapeutics for the treatment of various diseases; flavonoids have been shown to disrupt the initiation, promotion, and progression of cancer by modulating various signaling pathways and their downstream components associated with cellular proliferation, differentiation, inflammation, apoptosis, metastasis, angiogenesis, and reversal of multidrug resistance. Many natural flavonoids and their synthetic analogs are being investigated for their potential applications in anticancer therapies, because of their multi-targeted mechanism of action. Thus, the aim of the present chapter is to highlight the new insights on the recent progress of flavonoids as effective candidates in cancer therapeutics and prevention.
Irfan A. Ansari and Mohd Sayeed Akhtar have equally contributed for this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Plants have been an integral part of our daily diet due to their nutritional properties (Namdeo 2007). Since decades, several chemical and biological studies have well elucidated the role of their primary metabolites, such as carbohydrates, amino acids, and lipids in performing critical functions such as cell division, growth, respiration, storage, and reproduction (Bourgaud et al. 2001). Besides this, the plants also synthesize a broad range of low molecular weight chemical compounds, which may be distinctive from primary metabolites and vary from species to species which are known as “secondary metabolites.” They are responsible for specific tastes, odors, and colors of plant. Secondary metabolites do not have any significant role in maintenance of basic functions of plants, but play an imperative role in the communication of plant with its environment (Dixon 2001; Oksman-Caldentey and Inze 2004).
Plants are the chief sources of secondary metabolites, which have been frequently used in pharmaceutical, agrochemical, flavor, and aroma industries. Secondary metabolites are known as allelochemicals, which function as chemical defense compounds and influence molecular targets in herbivores or microbes. Secondary metabolites can be grouped into the alkaloids, terpenes, phenolics, and flavanoids (Karuppuswamy 2009; Rattan 2010). Alkaloids are the important class of highly diversified group of secondary metabolites containing a ring structure having a nitrogen atom and are often characterized by their bitter taste. Alkaloids are widely distributed in the plant kingdom mainly in higher plants. Moreover, several alkaloids also exhibited significant biological activities like anti-inflammatory, anticancer, antimetastatic, and antiangiogenic properties (Benyhe 1994; Lee 2011; Huang et al. 2007; Chen et al. 2008). Similarly, terpenoids are more numerous and structurally diverse compounds which have been used in the perfumery and cosmetic industries and also possess several biological and pharmacological properties. Terpenoids are classified on the basis of number and carbon skeleton formed by the joining of isoprene units followed by cyclization and rearrangements into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes. The terpenoids such as mono-, di-, and tetraterpenoids are synthesized from 2-C-methyl-D-erythritol 4-phosphate pathway while sesqui- and triterpenoids by the mevalonate pathway (Ludwiczuk et al. 2017). It has been demonstrated that terpenoids exhibit various properties such as antimicrobial activity, anti-inflammatory activity, anticancer activity, etc. (Raut and Karuppayil 2014; Lesgards et al. 2014).
Phenolics, being ubiquitously present in plant organs, are often regarded as integral component of our diet. These are the compounds which possess one or more aromatic rings with one or more hydroxy groups. These are the most abundant plant secondary metabolites. They are prominently involved in plant defense against pathogens, parasites, and predators, and also they are responsible for the colors of the plants. They are the prevalent constituents of plant foods like fruits, vegetables, etc. and beverages such as tea, coffee, beer, wine, etc. Plant phenolics chiefly include phenolics, flavonoids, tannins, and the less common stilbenes and lignans. Phenolics are primarily responsible for the bitterness and astringency of fruits and fruit juices (Nijveldt et al. 2001). The biosynthesis of phenolic compounds initiates with the commitment of glucose to the pentose phosphate pathway (PPP) and transforming glucose-6-phosphate irreversibly to ribulose-5-phosphate. On the other hand, PPP also produces erythrose-4-phosphate along with phosphoenolpyruvate from glycolysis, which is then used through the phenylpropanoid pathway to generate phenolic compounds after being channeled to the shikimic acid pathway to produce phenylalanine (Lin et al. 2010; Vattem et al. 2005). Phenolics can inhibit the absorption of amylase in the treatment of carbohydrate absorption, such as diabetes (Sales et al. 2012). There are many fruits and vegetables that contain phenolic compounds, especially, grapes, berries, and tomatoes. Phenolic compounds, such as phenolic acids and flavonoids, could promote health benefits by reducing the risk of metabolic syndrome and the related complications of type 2 diabetes. However, different groups of phenolic compounds have different biological characteristics, and very little is known about the mechanisms by which they could contribute to the prevention of disease; there still is a need for further studies. Reactive oxygen (ROS) and reactive nitrogen species (RNS) are highly reactive oxidized molecules, which are generated constantly by normal cellular conditions, for instance, the activity of the mitochondrial respiratory chain and inflammation, which could lead to damage in other biological molecules, like proteins and DNA (Halliwell 2002; Urso and Clarkson 2003; Lea et al. 2015).
Flavonoids are widely distributed phenolics in the plant kingdom. More than 5000 different flavonoids have been discovered till date, and on the basis of their chemical structure, they are classified into various groups. Among them, flavones, flavonols, flavanols, flavanones, anthocyanins, and isoflavones are particularly important because of their presence in various fruits and vegetables (Harbone 1993).
Reactive oxygen species (ROS) can damage DNA, and division of cells with unrepaired or misrepaired damage leads to mutations. If these changes appear in critical genes, such as oncogenes or tumor suppressor genes, initiation or progression of carcinogenesis may result. Moreover, ROS can interfere directly with cell signaling and growth (Loft and Poulsen 1997; Pryor 1997). The antitumor activity of flavonoids has recently gained attention because of their potential to quench and neutralize the ROS and ROS-mediated damage in the cellular compartment. Moreover, flavonoids have also been shown to modulate various altered cellular signaling pathways associated with cancer progression and development. Thus, the aim of the present chapter is to highlight the new insights on the recent progress of flavonoids as effective candidates in cancer therapeutics and prevention (Table 13.1 and 13.2).
13.2 Flavonoids
Flavonoids are the most ever-present plant-specific secondary metabolites having a benzo-ǖFE;-pyrone in their variable phenolic structures and are predominantly found in fruits, vegetables, grains, bark, roots, stems, flowers, tea, wine, etc. A large number of flavonoids have been recognized, many of which are responsible for the attractive colors of flowers, fruit, and leaves (Nijveldt et al. 2001). These compounds are chiefly involved in various essential functions such as reproduction by recruiting pollinators and seed dispersers. It has been suggested that flavonoids are also responsible for the beautiful display of fall color which as a result protect leaf cells from photo-oxidative damage, thereby enhancing the efficiency of nutrient retrieval during senescence (Winkel-Shirley 2002). The chemical nature of flavonoids depends on their structural class, degree of hydroxylation, other substitutions and conjugations, and degree of polymerization (Kumar and Pandey 2013).
13.2.1 Structure and Classification of Flavonoids
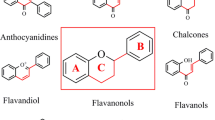
Chemically, flavonoids consist of 15 carbons and two phenyl rings (A and B) and a heterocyclic ring C. Flavonoids are chemically diverse group of secondary metabolites which can be divided into subgroups including anthocyanidins, flavonols, flavones, flavanols, flavanones, chalcones, dihydrochalcones, and dihydroflavonols (Treutter 2006). Flavonoids are classified on the basis of degree of oxidation, annularity of ring C, and connection position of ring B. Flavones and flavonols contain the largest number of compounds, representing the narrow sense flavonoids, namely, 2-benzo-γ-pyrone category. Quercetin is the most extensively studied flavonoids which belong to the flavonol class. The classification of flavonoids is shown in the figure (Fig. 13.1). The class flavanones and flavanonols possess saturated C2 = C3 bonds and frequently coexist with relevant flavones and flavonols in plants. Isoflavones, such as daidzein, are 3-phenyl-chromone substances. Chalcones being as the key precursors of flavonoid biosynthesis are ring C-opening isomers of dihydroflavones which are responsible for color appearance of plants. Although aurones are five-membered ring C benzofuran derivatives, they lack the typical structure of flavonoids. Anthocyanidins are primarily responsible for characteristic color of plants because they belong to a group of important chromene pigments and thus exist in the form of ions. Flavanols are reduction products of dihydroflavonols, particularly with flavan-3-ols widely distributed in the plant kingdom, also known as catechins. Nevertheless, there are still other flavonoids without C6—C3—C6 skeleton, for instance, biflavones, furan chromones, and xanthones (Fig. 13.2).
13.2.2 Biosynthesis of Flavonoids
Biosynthesis of flavonoids takes place through the phenylpropanoid pathway in which phenylalanine is converted into 4-coumaroyl-CoA, the ultimate precursor of the flavonoid biosynthesis pathway as shown in Fig. 13.3. Chalcone synthase is the first enzyme specific for the flavonoid pathway which produces chalcones. All the flavonoids are derivatives of these chalcones. Although the central pathway for flavonoids biosynthesis is conserved in plants, depending on the species, a group of enzymes, such as isomerases, reductases, hydroxylases, and several Fe2+/2-oxoglutarate-dependent dioxygenases, modify the basic flavonoid skeleton, leading to the different flavonoid subclasses (Martens et al. 2010). Lastly, transferases modify the flavonoid backbone with sugars, methyl groups, and/or acyl moieties, modulating the physiological activity of the resulting flavonoid by altering their solubility, reactivity, and interaction with cellular targets (Bowles et al. 2005; Ferrer et al. 2008).
Biosynthesis of flavonoids begins with the condensation of one molecule of 4-coumaroyl-CoA and three molecules of malonyl-CoA yielding naringenin chalcone, and the reaction is catalyzed by the enzyme chalcone synthase (CHS) (Fig. 13.1). The two immediate precursors of the chalcone, coumaroyl-CoA and malonyl-CoA, originate from two different pathways of primary metabolism. Coumaroyl-CoA is derived from the amino acid phenylalanine by phenylpropanoid pathway and is common to the biosynthesis of a variety of compounds such as lignin, coumarins, stilbenes, and flavonoids. Malonyl-CoA is produced by the carboxylation of acetyl-CoA, a central intermediate in the Krebs cycle. Chalcone is subsequently isomerized by the enzyme chalcone flavanone isomerase (CHI) to yield a flavanone. From these central intermediates, the pathway diverges into several side branches, each yielding a different class of flavonoids (Heller and Forkmann 1988).
13.2.3 Biological Properties of Flavonoids
The biological properties of flavonoids have been attributed to their potential cytotoxic property and their capacity to interact with various enzymes. The cumulative effects of flavonoid are shown in Fig. 13.4. A large number of flavonoids are accountable for providing stress protection via acting as scavengers of free radicals such as reactive oxygen species (ROS), as well as chelating metals that generate ROS through the Fenton reaction (Williams et al. 2004). Flavonoids are also involved in developing resistance to metal toxicity in plants (Kidd et al. 2001). Flavonoids could inhibit polar auxin transport and enhance consequent localized auxin accumulation in plants (Peer and Murphy 2007; Kuhn et al. 2011; Lewis et al. 2011). The various biological properties of flavonoids are as follows:
13.2.3.1 Role of Flavonoids in Root Nodulation
Flavonoids have been shown to be predominantly involved in the initiation of nodulation process. This phenomenon has been observed in transgenic plants having flavonoid-deficient roots produced by knockdown of chalcone synthase enzyme by RNA interference. The flavonoid-deficient roots were not able to initiate formation of nodules (Wasson et al. 2006).
13.2.3.2 Role of Flavonoids in Plant Defense
In several studies, flavonoids have been shown to protect plants from UV rays, and it is attributed to their UV-absorbing property. Moreover, biosynthesis of flavonols has been shown to induce by the UV light in various studies (Ryan et al. 2002; Berli et al. 2010; Stracke et al. 2007; Agati et al. 2011; Kusano et al. 2011). The presence of the hydroxy group in the third position of the flavonoid skeleton is accountable for chelating metal ions such as aluminum, zinc, iron, and copper and, consequently, inhibiting the formation of free radicals as well as to reduce ROS (Verdan et al. 2011). Flavonoids also provide protection to plants against pathogen and herbivores (Cornell and Hawkins 2003; Kliebenstein 2004; Bidart-Bouzat and Imeh-Nathaniel 2008).
13.2.3.3 Role of Flavonoids in Plant Reproduction and Fertility
The role of flavonoids in pollination has been established through the revelation of their role in providing characteristic colors to the pollen grains in different species of plants which can be detected by insects, facilitating successful pollination (Zerback et al. 1989; Van Der Meer et al. 1992). The role of flavonoids in pollen germination and pollen tube formation is elucidated by producing flavonoid-deficient mutants lacking chalcone synthase in maize and petunia (Pollak et al. 1993). Moreover, the silencing of chalcone synthase gene results in parthenocarpy in tomato (Schijlen et al. 2007). The silencing of FLS in tobacco causes production of less-seeded fruits, and silenced lines had lower flavonol and anthocyanidins levels. In addition, the pollen of these silenced lines was unable to produce functional pollen tubes. These experiments revealed that flavonoids have essential roles in pollen germination and consequently in plant fertility (Mahajan et al. 2011).
13.3 Pharmacological Effects of Flavonoids
Various studies have now established the protective role of flavonoids in various human ailments. The polyphenolic structure of flavonoids is attributed to their various pharmacological activities. Flavonoids are considered to be good source of natural antioxidants in human diets, and this property is accredited to the hydroxy (OH-) group present in the flavonoids via scavenging free radicals or by chelating metal ions. Thus, flavonoids could prevent free radical generation that leads to oxidative stress and consequently, many diseases. Flavonoids have been shown to play a protective role against many diseases such as cancer, cardiovascular and respiratory disorders, arthritis, and early aging. They have been accredited to boost the antioxidant defense system via inducing the expression of many antioxidant enzymes. In addition to all these properties, flavonoids also possess diverse biological activities which are important for various health aspects in human, for instance, anti-inflammatory, anti-ulcer, antiviral, anticancer, anti-diabetic, and cytotoxic properties (Nijveldt et al. 2001). The mechanism of action of flavonoids is shown in Fig. 13.5. In the next section, we have explained these biological properties individually in detail.
13.3.1 Antioxidative Property of Flavonoids
Antioxidant property of almost every group of flavonoids is the most studied property. In this regard, flavones and catechins have been recognized as the most powerful flavonoids having free radical scavenging property against reactive oxygen species. Human cells and tissues are in a continuous threat to be damaged by indigenous free radicals and reactive oxygen species produced during normal metabolism or by exogenous radicals generated due to environmental factors (Groot 1994; Grace 1994). The most important event by which free radicals interfere with cellular functions is lipid peroxidation resulting in cellular membrane damage. This membrane damage causes a shift in the net charge of the cell and osmotic pressure, leading to swelling and eventually cell death. Moreover, free radicals can induce pro-inflammatory mediators and initiate an inflammatory cascade, contributing to a general inflammatory response and tissue damage. To protect themselves from the damage caused by reactive oxygen species, living organisms have evolved an effective antioxidant defense system that includes enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, as well as nonenzymatic counterparts such as glutathione, ascorbic acid, and alpha-tocopherol (Halliwell 1995). The increased production of reactive oxygen species during injury results in consumption and depletion of the endogenous scavenging compounds.
Antioxidants are specific compounds that protect human, animal, and plant cells against the damaging effects of free radicals. Flavonoids are best known phytochemicals that act as antioxidants and may have an additive effect to the endogenous scavenging compounds (Kelly et al. 2002; Kukic et al. 2006). The antioxidant action of flavonoids includes suppression of ROS formation via inhibition of certain enzymes like NADPH oxidases, by scavenging free radicals, and regulation of antioxidant defense system (Mishra et al. 2013). Flavonoids have also been shown to protect the lipid membranes which are damaged due to lipid peroxidation. Thus, the flavonoids act as antioxidants and could play protective role in the onset and prevention of many diseases caused due to oxidative stress (Ramchoun et al. 2009).
13.3.2 Antiviral Property of Flavonoids
Naturally occurring flavonoids have been shown to exhibit significant antiviral property. They have been found to inhibit various enzymes associated with the life cycle of viruses. Flavon-3-ol was found to be more effective than flavones and flavonones in selective inhibition of HIV-1 and HIV-2. Baicalin, another flavonoid, isolated from Scutellaria baicalensis, also is known to inhibit immune-deficiency virus infections (Gerdin & Srensso 1983). Anti-dengue virus properties of quercetin, hesperetin, and naringin have also been reported, recently (Zandi et al. 2011).
13.3.3 Free Radical Scavenging Property of Flavonoids
Flavonoids play protective role in preventing injuries caused by free radicals. Flavonoids neutralize the highly reactive oxygen radicals via their hydroxy groups (Korkina and Alfanas’ev 1997). Epicatechin and rutin are powerful radical scavengers (Hanasaki et al. 1994). Moreover, the scavenging property of rutin has been attributed to its inhibitory activity on the enzyme xanthine oxidase. Furthermore, flavonoids can also inhibit LDL oxidation in vitro by neutralizing free radicals. Thus, flavonoids may exert protective effect against atherosclerosis (Kerry and Abbey 1997).
13.3.4 Anti-Inflammatory Effect
Eicosanoids such as prostaglandins, thromboxanes, and leukotrienes are known to be important lipid-derived inflammatory mediators (Moroney et al. 1988). They are produced by the action of cyclooxygenases and lipoxygenases on arachidonic acid released from lipid membrane under the influence of certain inflammatory signals. Flavonoids have been shown to inhibit eicosanoid biosynthesis, thus acting as potent anti-inflammatory agents. Different flavonoids like quercetin have been demonstrated to inhibit both cyclooxygenase and lipoxygenase activities (Kim et al. 1998).
13.3.5 Hepatoprotective Activity
A number of flavonoids such as quercetin, rutin, catechin, apigenin, and naringenin have been previously described for their hepatoprotective properties (Tapas et al. 2008). Zhu et al. demonstrated that anthocyanin cyanidin-3-O-𝛽- glucoside (C3G) increases hepatic glutamate-cysteine ligase (GCLC) expression by increasing cAMP levels to activate protein kinase A (PKA), which in turn upregulates cAMP response element binding protein (CREB) phosphorylation to promote CREB-DNA binding and increase GCLC transcription. Increased GCLC expression results in a decrease in hepatic ROS levels and proapoptotic signaling. Furthermore, C3G treatment lowers hepatic lipid peroxidation, inhibits the release of pro-inflammatory cytokines, and protects against the development of hepatic steatosis (Zhu et al. 2012).
13.3.6 Anticancer Property
The anticancer property of flavonoids has garnered the attention of researchers to use them as anticancer therapeutics. Flavonoids, having antioxidant activity, have been accredited to exert anticarcinogenic activity (Stefani et al. 1999). Some flavonoids such as luteolin, apigenin, and fisetin have been demonstrated to be potent inhibitors of cell proliferation (Fotsis et al. 1997). An inverse association between flavonoid intake and the subsequent incidence of lung cancer has been established in a large clinical study (Knekt et al. 1997). This effect was mainly ascribed to quercetin, which provided >95% of the total flavonoid intake in that particular study. Moreover, quercetin and apigenin inhibited melanoma growth and influenced its invasiveness and metastatic potential in mice (Caltagirone et al. 2000). Furthermore, flavonoids have also been speculated to inhibit angiogenesis (Fotsis et al. 1997).
13.4 Flavonoids as Prospective Anticancer Agents
Flavonoids are a large group of heterogenous polyphenols ubiquitously present in fruits and vegetables having several health benefits. Dietary factors play an imperative role in the prevention of cancers. It has been reported that fruits and vegetables having flavonoids are promising source of cancer chemopreventive agents. The critical relationship of fruit and vegetable intake and cancer prevention has been extensively documented. In one such study, an inverse relationship between consumption of onions and/or apples, two major sources of the flavonol quercetin with the incidence of cancer of the prostate, lung, stomach, and breast, has been observed. Several mechanisms have been proposed for the effect of flavonoids on the initiation and progression of carcinogenesis including regulation of developmental and hormonal actions. Major molecular mechanisms of action of flavonoids are (i) inhibition of mutant p53 protein expression, (ii) abrogation of cell cycle progression, (iii) inhibition of tyrosine kinase signaling, (iv) suppression of heat shock proteins, (v) affinity with estrogen receptor, and (vi) suppression of Ras family protein expression.
Mutations of p53 are among the most common genetic abnormalities in human cancers (Nigro et al. 1989). The inhibition of p53 expression could lead to arrest the cancer cells in the G2/M phase of the cell cycle. Flavonoids have been found to suppress the expression of mutant p53 protein to nearly undetectable levels in human breast cancer cell lines (Avila et al. 1994). Tyrosine kinases are a family of proteins located in or near the cell membrane involved in the transduction of growth factor signals to the nucleus. Their expression is thought to be involved in oncogenesis through an ability to override normal regulatory growth control (Boutin 1994). Drugs inhibiting tyrosine kinase activity are thought to be possible antitumor agents without the cytotoxic side effects seen with conventional chemotherapy. Quercetin was the first tyrosine kinase inhibiting compound tested in a human phase I trial. Heat shock proteins form a complex with mutant p53, which allows tumor cells to bypass normal mechanisms of cell cycle arrest. Heat shock proteins also allow for improved cancer cell survival under different bodily stresses. Flavonoids are known to inhibit production of heat shock proteins in several malignant cell lines, including breast cancer, leukemia, and colon cancer. Recent studies have shown that the flavanol epigallocatechin-3-gallate inhibited fatty acid synthase (FAS) activity and lipogenesis in prostate cancer cells, an effect that is strongly associated with growth arrest and cell death. Contrastingly, expression of FAS is markedly increased as compared to normal tissues in various human cancers. Upregulation of FAS occurs early in tumor development and is further enhanced in more advanced tumors. Quercetin is known to produce cell cycle arrest in proliferating lymphoid cells. It has been reported that in addition to its antineoplastic activity, quercetin exerted growth inhibitory effects on several malignant tumor cell lines in vitro. These included P-388 leukemia cells, gastric cancer cells (HGC-27, NUGC-2, NKN-7, and MKN-28), colon cancer cells (COLON320DM), human breast cancer cells, human squamous and gliosarcoma cells, and ovarian cancer cells (Zhao 2003). Markaverich et al. proposed that tumor cell growth inhibition by quercetin may be due to its interaction with nuclear type II estrogen binding sites (EBS). It has been experimentally proved that increased signal transduction in human breast cancer cells is markedly reduced by quercetin acting as an antiproliferative agent. Barnes has extensively reviewed the anticancer effects of genistein on in vitro and in vivo models. In a study to determine the effects of isoflavones genistein, daidzein, and biochanin A on mammary carcinogenesis, genistein was found to suppress the development of chemically induced mammary cancer without reproductive or endocrinological toxicities. Neonatal administration of genistein (a flavonoid) exhibited a protective effect against the subsequent development of induced mammary cancer in rats (Chung 1995). Hesperidin, a flavanone glycoside, is known to inhibit azoxymethane-induced colon and mammary cancers in rats. The anticancer properties of flavonoids contained in citrus fruits have been reviewed by Carroll et al. (2003). Several flavonols, flavones, flavanones, and the isoflavone biochanin A are reported to have potent antimutagenic activity. A carbonyl function at C-4 of the flavone nucleus was found to be essential for their activity. Flavone-8-acetic acid has also been shown to have antitumor effects. In earlier studies, ellagic acid, robinetin, quercetin, and myricetin have been shown to inhibit the tumorigenicity of BP-7, 8-diol-9, and 10-epoxide-2 on mouse skin (Urso and Clarkson 2003). Higher consumption of phytoestrogens, including isoflavones and other flavonoids, has been shown to provide protection against prostate cancer risk. It is well known that due to oxidative stress, cancer initiation may take place, and thus, potent antioxidants show potential to combat progression of carcinogenesis. Potential of antioxidant as an anticancer agent depends on its competence as an oxygen radical inactivator and inhibitor. Therefore diets rich in radical scavengers would diminish the cancer-promoting action of some radicals (Treutter 2006).
13.5 Anticancer Activities of Some Bio-active Flavonoids
The essential feature of bio-active flavonoids is their free radical scavenging activity. These antioxidant properties are solely responsible for their antitumor effects (Nijveldt et al. 2001). They reportedly prevent cell damage caused by reactive oxygen formed via normal metabolic processes and induced by exogenous factors (e.g., UV radiation, xenobiotics) that can modify transcriptional factor and protein kinase activities and lead to DNA damage that increases mutation probability and mismatch repair. Proto-oncogene activation and changes in suppressor genes can initiate cancer transformation (Nijveldt et al. 2001). These bio-active flavonoids have antiproliferative effects and induce apoptosis in diverse cancer cell lines. As free radical scavengers, flavonoids inhibit invasion and metastasis (Kuntz et al. 1999). Nijveldt et al. (2001) reported that these bio-active flavonoid compounds were cytotoxic for cancer but not for normal cells. Flavones also regulate macrophage function in cancer cell elimination and are potential inhibitors of cell proliferation (e.g., apigenin and luteolin). An inverse relationship exists between bio-active flavonoids in the diet and the occurrence of lung cancer (Nijveldt et al. 2001). The anticancer properties of some bio-active flavonoids have been thoroughly described below.
13.5.1 Quercetin
Quercetin (3, 3′,4′,5,7-pentahydroxyflavone) is a polyphenolic flavonoid widely found in plants. Frequently, quercetin exists as glycosides (sugar derivatives), e.g., rutin, in which the hydrogen of the R-4 hydroxy groups is replaced by a disaccharide. Quercetin is termed as aglycone, or sugarless form of rutin (Cody 1988). Several in vitro and in vivo studies have suggested that quercetin plays an imperative role in protecting cells from oxidative stress induced by reactive oxygen species. Oxidative stress is one of the chief hallmarks of cancer development as shown in Fig. 13.6. It is widely demonstrated that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play key role in cancer development (Wiseman and Halliwell 1996) (Fig. 13.7).
The molecular mechanism of action of quercetin has been reported in the downregulation of mutant p53 protein expression in various breast cancer cell lines (Avila et al. 1994). The inhibition of expression of p53 has been found to arrest the cells in the G2/M phase of the cell cycle. This downregulation is found to be much less in cells with an intact p53 gene (Avila et al. 1996). The G1 checkpoint controlled by the p53 gene is a major site for the control of cellular proliferation. It has been reported that quercetin arrests the human leukemic T-cells in the late G1 phase of the cell cycle (Yoshida et al. 1992). This G1 arrest is also observed in gastric cancer cells when treated with quercetin (Yoshida et al. 1990).
Various studies have confirmed that the intravenous administration of quercetin (dosages 60–1700 mg/m2) led to the inhibition of lymphocyte tyrosine kinase at 1 h in 9 of 11 cases of human cancer patients (Ferry et al. 1996). In vitro experiments have confirmed these results, both in nonmalignant cells (Yokoo and Kitamura 1997) and in rat mammary tumor cells (Levy et al. 1984). Quercetin has been reported to inhibit the production of heat shock proteins in several malignant cell lines, including breast cancer, leukemia, and colon cancer (Elia et al. 1996). Heat shock proteins form a complex with mutant p53, which allows tumor cells to bypass normal mechanisms of cell cycle arrest. Heat shock proteins also allow for improved cancer cell survival under different bodily stresses (low circulation, fever, etc.) and are associated with shorter disease-free survival and chemotherapy drug resistance in breast cancer (Ciocca et al. 1993). In addition to this, quercetin also inhibits the expression of the p21-ras oncogene in cultured colon cancer cell lines (Oesterreich et al. 1993; Ranelletti et al. 1999; DeVita et al. 1997).
13.5.2 Rutin
Rutin is also known as rutoside or quercetin-3-O-rutoside. It is a glycoside of the flavonoid quercetin. Rutin is present in many typical plants such as buckwheat, apples, black tea, apples, and vegetables. Various researches on natural compounds have explored the beneficial effects of rutin, including inhibition of platelet aggregation, being anti-inflammatory, antioxidant, and reduction of blood fat and cholesterol (Chan et al. 2007). The anticancer research has demonstrated that rutin could exert significant potential effect in decreasing the amount of precancerous lesions and inducing apoptosis in the large intestine cancer (Volate et al. 2005), but for the treatment of neuroblastoma, this effect has not been reported. Furthermore, it has been previously described that rutin induced in vitro cytotoxic effects on various cancer cell lines including human colon cancer cells (Kuntz et al. 1999; Guon and Sook Chung 2016). Rutin and their analogs, such as EGCG and quercetin, act as efficient radical inhibitors and have been shown to have chemopreventive activity in both variety of colonic cancer cell lines and in murine models (Deschner et al. 1993; Mahmoud et al. 2000). Nevertheless, rutin has shown antitumor effects in some in vivo models such as NK/Ly ascites and B16F10 cells (Molnar et al. 1981; Menon et al. 1995). Therefore, it has also been illustrated that rutin exerted significant beneficial effects on decreasing the amount of precancerous lesions and inducing apoptosis in the large intestine cancer and human neuroblastoma LAN-5 cells (Chen et al. 2013).
13.5.3 Hesperitin and Naringenin
Major citrus flavanone naringenin and hesperetin possess antioxidant activities, although lower in comparison to other polyphenols (van Acker et al. 2000; Jeon et al. 2002). Hesperetin has been shown to inhibit chemically induced colon, urinary bladder, and mammary carcinogenesis in in vivo animal models. They have been reported to regulate apolipoprotein B secretion by HepG2 cells, possibly through inhibition of cholesterol ester synthesis, and to inhibit 3-hydroxy-3-methylglutaryl-coenzyme A reductase and acyl coenzyme A:cholesterol O-acyltransferase in rats (Lee et al. 1999; Kurowska et al. 2000a, b). Naringenin has been attributed to possess anti-inflammatory actions and different types of effects on sex hormone metabolism (Ruh et al. 1995; Rosenberg et al. 1998; Dechaud et al. 1999; Yoon et al. 2001). It has been shown to bind to estrogen receptors (Kuiper et al. 1998). Naringenin has been shown to increase the intestinal cytochrome P-450 IIIA (CYP3A4) enzyme expression (Bailey et al. 2000; Dresser et al. 2000).
13.5.4 Apigenin and Scutellarin
Apigenin (4-, 5, 7-trihydroxyflavone) is a member of the flavone class and possesses free radical scavenging, anticarcinogenic, and anti-inflammatory effects (Siddiqui et al. 2008). As a prospective anticancer agent, apigenin is capable of inhibiting cell growth and inducing apoptosis in cancer cells without incurring cytotoxic effects on normal cells (Liu 2004). It has been demonstrated that apigenin possessed growth inhibitory properties in breast cancer by the regulation of the p14ARF-Mdm2-p53 pathway, in colon cancer by increasing the expression of UDP-glucuronosyltransferase, and in pancreatic cancer through the downregulation of NF-κB activity with the suppression of Akt (Lee et al. 2008). In addition, apigenin can also increase the effect of cancer drugs when combined with other therapeutic reagents such as doxorubicin and taxol. Apigenin has also been shown to inhibit in vitro angiogenesis (Jiang and Fang 2006). The flavones derived from Scutellaria possess cytostatic and cytotoxic activities against many human cancer cells. They show no toxicity to normal epithelial and peripheral blood and myeloid cells. The combination use of baicalin and scutellarin could exhibit a synergistic effect and significantly improve their antitumor activity (Wu 2007). A new safe natural drug composed by berberine and baicalin has the property of inhibiting carcinogenesis and lowering tyrosine kinase activity (Zhao 2003).
13.5.5 Genistein
Genistein has been shown to exhibit both chemopreventive and chemotherapeutic potentials in multiple tumor types (Dixon and Ferreira 2002; Empie and Gugger 2005). Several in vitro and in vivo studies have established its anticancer activity in colon, prostate, breast, skin, urinary, and bladder cancer (Empie and Gugger 2005). Genistein has shown protective effect against UVR-induced skin sunburns, premature aging, and skin cancer. It has also shown to reduce bone loss in patients with osteoporosis and metastatic bone cancers. Moreover, a pharmaceutical product containing genistein has been shown to prevent epithelial ovarian cancer. The human epidermal growth factor (EGF) exerts its biological effect by binding to a specific 170 kDa cell membrane receptor (EGF-Rc). Conjugates of EGF-genistein have been shown to inhibit the EGF-Rc tyrosine kinase in breast cancer cells and trigger cell apoptosis. In addition, the conjugates had potent antitumor activity against breast cancer xenografts both in SCID mice and monkeys. Furthermore, EGF-genistein conjugates were also used as chemopreventive agent for the development or recurrence of EGF-Rc expressing breast cancer in mammal.
13.5.6 Epigallocatechin Gallate
Green tea mainly contain catechins such as (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin gallate (EGCG). Tea catechins are potent inhibitors of cancer cell proliferation and metastasis against various cancer cell lines like prostate, lung, colon, bladder, and cervical cancer cell lines. Epigallocatechin gallate (EGCG), the most abundantly found catechin in green tea, has been the focus of many investigations. EGCG could inhibit the growth of human lung cancer, particularly for 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone (NNK) lung tumorigenesis (Chung 1995). In addition, EGCG is an anti-folate agent, which can inhibit the activity of dihydrofolate reductase (DHFR). EGCG acts by disturbing the folic acid metabolism in cells, causing the inhibition of DNA and RNA synthesis, alteration of DNA methylation, and modulation of cell signaling pathways (Navarro-Perán et al. 2007). Thus, the anti-folate compounds based on EGCG may be useful in the treatment of a range of disorders including cancer (Rodriguez-Lopez et al. 2007). It has been found that EGCG and other tea catechins might exert a synergistic effect in inhibiting tumor cell growth when combined with some active ingredients, such as A3 adenosine receptor agonists or thymidylate synthase inhibitors (Rodriguez-Lopez et al. 2008). Vanillylamine, the head group of capsaicin, combination with the tea catechins, displays unexpected potential utility for the treatment of cancer (Morre and Morre 2007). Ascorbic acid, L-proline, and L-lysine could effectively enhance the activity of tea catechins in blocking cancer cell proliferation and metastasis (Netke et al. 2006; Rath et al. 2006).
13.6 Conclusions and Future Prospects
Flavonoids have fascinated the researchers due to its nontoxic nature and wide range of biological activities. These flavonoids as natural compounds have received great advantage as therapeutic agent because these polyphenolic compounds are consumed daily and their half-life is also long and they are easily absorbed by the intestine. Consequently, the role of dietary flavonoids in cancer prevention is immensely studied. Epidemiological studies have established the association between high dietary intake of flavonoids with low cancer incidence in humans. These studies are supported by large number of in vitro and in vivo studies, which show that flavonoids may inhibit various stages in the carcinogenesis process, namely, tumor initiation, promotion, and progression. Mechanisms behind the anticarcinogenic activity of flavonoids include carcinogen inactivation, antiproliferation, cell cycle arrest, induction of apoptosis and differentiation, inhibition of angiogenesis, antioxidation, and reversal of multidrug resistance or a combination of these mechanisms. Furthermore, the intriguing results from various laboratories have encouraged the development of flavonoids as chemotherapeutic agents in human clinical trials. While these experiences strengthen the notion that flavonoids could be useful anticancer agents, to date, only few clinical studies have demonstrated the anticancer property of flavonoids in vivo. Therefore, more focused clinical studies are required to establish whether the dietary effects of these compounds can be exploited to achieve cancer preventive or therapeutic effects in human. This book chapter can be concluded by considering that many chemotherapeutic agents against tumor cells exhibit cytotoxicity against normal cells which remain a major obstacle in successful chemotherapy. Moreover, development of multidrug resistance further limits chemotherapy in cancer. Thus, the promising results will stimulate the development of flavonoids for cancer chemoprevention and chemotherapy.
References
Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M (2011) The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol 168:204–212
Avila MA, Velasco JA, Cansado J, Notario V (1994) Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res 54:2424–2428
Avila MA, Velasco JA, Harter KW, Velasco JA, Notario V (1996) Quercetin as a modulator of the cellular neoplastic phenotype. Adv Expl Med Biol 401:101–110
Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR (2000) Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther 68:468–477
Benyhe S (1994) Morphine: new aspects in the study of an ancient compound. Life Sci 55:969–979
Berli FJ, Moreno D, Piccoli P, Hespanhol-Viana L, Silva MF, Bressan-Smith R, Cavagnaro JB, Bottini R (2010) Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ 33:1–10
Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global change effects on plant chemical defenses against insect herbivores. J Integr Plant Biol 50:1339–1354
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Boutin JA (1994) Tyrosine protein kinase inhibition and cancer. Int J Biochem 26:1203–1226
Bowles D, Isayenkov J, Lim EK, Poppenberger B (2005) Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol 8:254–263
Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M (2000) Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer 87:595–600
Chan HJ, Ji YL, Chul HC, Chang JK (2007) Anti-asthmatic action of quercetin and rutin in conscious Guinea-pigs challenged with aerosolized ovalbumin. Arch Pharma Res 30:1599–1607
Chen J, Zhao H, Wang X, Lee FSC, Yang H, Zheng L (2008) Analysis of major alkaloids in Rhizoma coptidis by capillary electrophoresis-electrospray-time of flight mass spectrometry with different background electrolytes. Electrophoresis 29:2135–2147
Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian L, Pan J, Yang Y (2013) Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci World J 2013:269165
Chung FL (1995) Inhibition of lung tumorigenesis by administration of a polyphenol. US Patent No. US5391568
Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL (1993) Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst 85:570–574
Cody V (1988) Plant flavonoids in biology and medicine part II. Prog Clin Biol Res 280:461
Cornell HV, Hawkins BA (2003) Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am Nat 161:507–522
de Groot H (1994) Reactive oxygen species in tissue injury. Hepatogastroenterol 41:328–332
Dechaud H, Ravard C, Claustrat F, de la Perriere AB, Pugeat M (1999) Xenoestrogen interaction with human sex hormone–binding globulin (hSHBG). Steroids 64:328–334
Deschner EE, Ruperto JF, Wong GY, Newmark HL (1993) The effect of dietary quercetin and rutin on AOM-induced acute colonic epithelial abnormalities in mice fed a high-fat diet. Nutr Cancer 20:199–204
DeVita NT, Hellman S, Rosenberg SA (1997) Cancer: principles and practice of oncology, 5th edn. Lippencott-Raven, Philadelphia
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847
Dixon RA, Ferreira D (2002) Genistein. Phytochemistry 60:205–211
Dresser GK, Spence JD, Bailey DG (2000) Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 38:41–57
Elia G, Amici C, Rossi A, Santoro MG (1996) Modulation of prostaglandin A1-induced thermotolerance by quercetin in human leukemic cells: role of heat shock protein. Cancer Res 56:210–217
Empie M, Gugger E (2005) Method of preparing and using compositions extracted from vegetable matter for the treatment of cancer. US Patent No. US6900240
Ferrer J, Austin M, Stewart CJ, Noel J (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 46:356–370
Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats G (1996) Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 2:659–668
Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L (1997) Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res 57:2916–2921
Gerdin B, Srensso E (1983) Inhibitory effect of the flavonoid on increased microvascular permeability induced by various agent in rat skin. Int J Microcir Clin Exp 2:39–46
Grace PA (1994) Ischaemia-reperfusion injury. Br J Surg 81:637–647
Guon TE, Sook Chung HA (2016) Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol Lett 11:2463–2470
Halliwell B (1995) How to characterize an antioxidant: an update. Biochem Soc Symp 61:73–101
Halliwell B (2002) Effect of diet on cancer development: is oxidative DNA damage a biomarker. Free Rad Biol Med 32:968–974
Hanasaki Y, Ogawa S, Fukui S (1994) The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Rad Biol Med 16:845–850
Harbone JB (1993) The flavonoids. Advance in research since 1986. Chapman and Hall, London
Heller W, Forkmann G (1988) Biosynthesis. In: Harborne JB (ed) The Flavonoids. Chapman and Hall, London, pp 399–425
Huang M, Gao H, Chen Y, Zhu H, Cai Y, Zhang X, Miao Z, Jiang H, Zhang J, Shen H, Lin L, Lu W, Ding J (2007) Chimmitecan, a novel 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin Cancer Res 13:1298–1307
Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT, Jeong TS, Park YB, Choi MS (2002) Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta 317:181–190
Jiang BH, Fang J (2006) Apigenin for chemoprevention, and chemotherapy combined with therapeutic reagents. US Patent No. US20060189680
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Kelly EH, Anthony RT, Dennis JB (2002) Flavonoid antioxidants: chemistry, metabolism and structure-actvity relationships. Nutri Biochem 13:572–584
Kerry NL, Abbey M (1997) Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Atherosclerosis 135:93–102
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Kim HP, Mani I, Iverson L, ZIboh VA (1998) Effects of naturally occurring flavonoids and bioflavonoids on epidermal cyclooxygenase and lipooxygenase in Guinea pigs. Prostaglandins Leukot. Essent Fatty Acids 58:17–24
Kliebenstein DJ (2004) Secondary metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ 27:675–684
Knekt P, Jarvinen R, Seppanen R, Hellövaara M, Teppo L, Pukkala E, Aromaa A (1997) Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol 146:223–230
Korkina LG, Afanasev IB (1997) Antioxidant and chelating properties of flavonoids. Adv Pharmacol 38:151–163
Kuhn BM, Geisler M, Bigler L, Ringli C (2011) Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol 156:585–595
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol 139:4252–4263
Kukic J, Petrovic C, Niketic M (2006) Antioxidant activity of four endemic Stachys taxa. Biol Pharma Bull 29:725–729
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750
Kuntz S, Wenzel U, Daniel H (1999) Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 38:133–142
Kurowska EM, Borradaile NM, Spence JD, Carrol CC (2000a) Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr Res 20:121–129
Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piche LA, Serratore P (2000b) HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr 72:1095–1100
Kusano M, Tohge T, Fukushima A, Kobayashi M, Hayashi N, Otsuki H, Kondou Y, Goto H, Kawashima M, Matsuda F, Niida R, Matsui M, Saito K, Fernie AR (2011) Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. Plant J 67:354–369
Lea AJ, Tung J, Zhou XA (2015) Flexible, efficient binomial mixed model for identifying differential dna methylation in bisulfite sequencing data. PLoS Genet 11:e1005650
Lee MR (2011) The history of Ephedra (ma-huang). J R Coll Physians Edinb 41:78–84
Lee SH, Jeong TS, Park YB, Kwon YK, Choi MS, Bok SH (1999) Hypocholesterolemic effect of hesperetin mediated by inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A: cholesterol acyltransferase in rats fed high-cholesterol diet. Nutr Res 19:1245–1258
Lee SH, Ryu JK, Lee KY, Woo SM, Park JK, Yoo JW, Kim YT, Yoon YB (2008) Enhanced anti-tumor effect of combination therapy with gemcitabine and apigenin in pancreatic cancer. Cancer Lett 259:39–49
Lesgards JF, Baldovini N, Vidal N, Pietri S (2014) Anticancer activities of essential oils constituents and synergy with conventional therapies: a review. Phytother Res 28:1423–1446
Levy J, Teuerstein I, Marbach M, Radian S, Sharoni Y (1984) Tyrosine protein kinase activity in the DMBAinduced rat mammary tumor: inhibition by quercetin. Biochem Biophys Res Commun 123:1227–1233
Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK (2011) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156:144–164
Liu Y (2004) Safe natural pharmaceutical composition for treating cancer. US Patent No. US20040072790A1
Loft S, Poulsen HE (1997) Cancer risk and oxidative DNA damage in man. J Mol Med 74:297–312
Ludwiczuk A, Skalicka-Wozniak K, Georgiev MI (2017) Terpenoids. In: Badal S, Delgoda R (eds) Pharmacognosy: fundamentals, applications and strategied. Elsevier, London, pp 233–266
Mahajan M, Ahuja PS, Yadav SK (2011) Post-transcriptional silencing of flavonol synthase mRNA in tobacco leads to fruits with arrested seed set. PLoS One 6:e28315
Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM (2000) Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis 21:921–927
Martens S, Preuss A, Matern U (2010) Multifunctional flavonoid dioxygenases: flavonols and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 71:1040–1049
Menon LG, Kuttan R, Kuttan G (1995) Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett 95:221–225
Mishra A, Kumar S, Pandey AK (2013) Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci World J 2013:292934. https://doi.org/10.1155/2013/292934
Molnar J, Beladi I, Domonkos K, Földeák S, Boda K, Veckenstedt A (1981) Antitumor activity of flavonoids on NK/Ly ascites tumor cells. Neoplasma 28:11–18
Moroney MA, Alcaraz MJ, Forder RA, Carry F, Hoult JR (1988) Selectivity of 5-neutrophil oxygenase and lipoxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol 40:787–792
Morre DM, Morre DJ (2007) Compositions based on vanilloid catechin synergies for prevention and treatment of cancer. US Patent No. US7192612B2
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites-a review. Pharmacogn Rev 1:69–79
Navarro-Perán E, Cabezas-Herrera J, del Campo LS (2007) Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int J Biochem Cell Biol 39:2215–2225
Netke S, Niedzwiecki A, Rath M, Roomi WM (2006) Nutrient pharmaceutical formulation comprising polyphenols and use in treatment of cancer. US Patent No. US-7041699-B2
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, Glover T, Collins FS, Weslon A, Modali R, Harris CC, Vogelstein B (1989) Mutations in the p53 gene occur in diverse human tumour types. Nature 342:705–708
Nijveldt RJ, van Nood E, Danny EC, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PAM (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Oesterreich S, Weng CN, Qui M, Hilsenbeck SG, Osborne CK, Fuqua SAW (1993) The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res 53:4443–4448
Oksman-Caldentey KM, Inze D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators. Trends Plant Sci 12:556–563
Pollak PE, Vogt T, Mo Y, Taylor LP (1993) Chalcone synthase and flavonol accumulation in stigmas and anthers of Petunia hybrida. Plant Physiol 102:925–932
Pryor WA (1997) Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 105:S875–S882
Ramchoun M, Harnafi H, Alem C, Benlys M, Elrhaffari L, Amrani S (2009) Study on antioxidant and hypolipidemic effects of polyphenol rich extract from Thymus vulgaris and Lavandula multifida. Pharm Res 1:106–112
Ranelletti FO, Maggiano N, Serra FG, Ricci R, Larocca LM, Lanza P, Scambia G, Fattorossi A, Capelli A, Piantelli M (1999) Quercetin inhibits p21-ras expression in human colon cancer cell lines and in primary colorectal tumors. Int J Cancer 85:438–445
Rath M, Netke S, Niedzwiecki AA (2006) Nutrient pharmaceutical formulation comprising polyphenols and use in treatment of cancer. US Patent No. US7041699B2
Rattan RR (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Protect 29:913–920
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Indust Crops Prod 62:250–264
Rodriguez-Lopez JN, Navarro-Peran EM, Cabezas-Herrera J (2007) Dihydrofolate reductase inhibition by epigallocatechin gallate compounds. US Patent No. US20070249545A1
Rodriguez-Lopez JN, Cabezas-Herrera J, Navarro-Peran EM, Sanchez DCL (2008) Epigallocatechin-3-gallate compositions for cancer therapy and chemoprotection. Patent No. WO2008075201
Rosenberg RS, Grass L, Jenkins DJ, Kendall CW, Diamandis EP (1998) Modulation of androgen and progesterone receptors by phytochemicals in breast cancer cell lines. Biochem Biophys Res Commun 248:935–939
Ruh MF, Zacharewski T, Connor K, Howell J, Chen I, Safe S (1995) Naringenin: a weakly estrogenic bioflavonoid that exhibits antiestrogenic activity. Biochem Pharmacol 50:1485–1493
Ryan KG, Swinny EE, Markham KR, Winefield C (2002) Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 59:23–32
Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D (2012) Amylase Inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci 15:141–183
Schijlen EG, De Vos CH, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, Van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144:1520–1530
Siddique YH, Beg T, Afzal M (2008) Antigenotoxic effect of apigenin against anti-cancerous drugs. Toxicol In Vitro 22:625–631
Stefani ED, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Carzoglio JC, Ronco A, Olivera L (1999) Dietary antioxidants and lung cancer risk: a case-control study in Uruguay. Nutr Cancer 34:100–110
Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50:660–677
Tapas AR, Sakarkar DM, Kakde RB (2008) Flavonoids as nutraceuticals: a review. Trop J Pharma Res 7:1089–1099
Treutter D (2006) Significance of flavonoids in plant resistance: a review. Environ Chem Lett 4:147–157
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189:41–54
van Acker FA, Tromp MN, Haenen GR, van der Vijgh WJ, Bast A (2000) Flavonoids can replace a-tocopherol as an antioxidant. FEBS Lett 473:145–148
van Der Meer IM, Stam ME, Van Tunen AJ, Mol JN, Stuitje AR (1992) Antisense inhibition of flavonoid biosynthesis in Petunia anthers results in male sterility. Plant Cell 4:253–262
Vattem DA, Randhir R, Shetty K (2005) Cranberry phenolics-mediated antioxidant enzyme response in oxidatively stressed porcine muscle. Process Biochem 40:2225–2238
Verdan AM, Wang HC, García CR, Henry WP, Brumaghim JL (2011) Iron binding of 3-hydroxychromone, 5-hydroxychromone, and sulfonated morin: implications for the antioxidant activity of flavonols with competing metal binding sites. J Inorg Biochem 105:1314–1322
Volate SR, Davenport DM, Muga SJ, Wargovich MJ (2005) Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis 26:1450–1456
Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by Rhizobia. Plant Cell 18:1617–1629
Williams R, Spencer J, Rice-Evans C (2004) Flavonoids: antioxidants or signaling molecules. Free Rad Biol Med 36:838–849
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313:17–29
Wu Y (2007) The synergistically pharmaceutical composition of baicalein and baicalin for inhibiting tumor. Patent No. EP1849467
Yokoo T, Kitamura M (1997) Unexpected protection of glomerular mesangial cells from oxidant triggered apoptosis by bioflavonoid quercetin. Am J Phys 273:206–212
Yoon K, Pallaroni L, Stoner M, Gaido K, Safe S (2001) Differential activation of wild-type and variant forms of estrogen receptor alpha by synthetic and natural estrogenic compounds using a promoter containing three estrogen-responsive elements. J Steroid Biochem Mol Biol 78:25–32
Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H, Aoike A (1990) The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett 260:10–13
Yoshida M, Yamamoto M, Nikaido T (1992) Quercetin arrests human leukemic T-cells in late G1 phase of the cell cycle. Cancer Res 52:6676–6681
Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S (2011) Antiviral activity of four types of bioflavonoid against dengue virus type-2. Vir J 8:560. https://doi.org/10.1186/1743-422X-8-560
Zerback R, Dressler K, Hess D (1989) Flavonoid compounds from pollen and stigma of Petunia hybrida: inducers of the vir region of the Agrobacterium tumefaciens Ti plasmid. Plant Sci 62:83–91
Zhao XX (2003) Plant drug for preventing cancer. US Patent No. US200318039
Zhu W, Jia Q, Wang Y, Zhang Y, Xia M (2012) The anthocyanin cyanidin-3-O-𝛽-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: involvement of a cAMPPKA- dependent signaling pathway. Free Rad Biol Med 2012:314–327
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ansari, I.A., Akhtar, M.S. (2019). Recent Insights on the Anticancer Properties of Flavonoids: Prospective Candidates for Cancer Chemoprevention and Therapy. In: Akhtar, M., Swamy, M., Sinniah, U. (eds) Natural Bio-active Compounds. Springer, Singapore. https://doi.org/10.1007/978-981-13-7154-7_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-7154-7_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7153-0
Online ISBN: 978-981-13-7154-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)