Abstract
Immune checkpoints are molecules in the immune system that either turn-on a signal (co-stimulatory molecules) or turn-off a signal for maintaining self-tolerance and modulating the duration and amplitude of physiological immune responses. Many cancers protect themselves from the immune system by inhibiting the T cell signal Inhibitory receptors such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1), expressed on tumor-specific T cells, lead to compromised activation and suppressed effector functions such as proliferation, cytokine secretion, and tumour cell lysis. Modulating these receptors using monoclonal antibodies, an approach termed “immune checkpoint blockade,” has gained momentum as a new approach in cancer immunotherapy. This treatment concept was first introduced in patients with advanced melanoma: In this patient population, the anti-CTLA-4 antibody Ipilimumab was the first drug ever to show improved overall survival in phase III trials. Antibodies directed against PD-1 and its ligand, PD-L1, have shown much promise in the treatment of melanoma, renal cell cancer, non-small cell lung cancer, and other tumours, as evident by encouraging rates and durability of tumour responses. Because of the successes with immune checkpoint inhibitors in cancer immunotherapy, many new agents and strategies, including combination approaches, are being developed at a fast pace.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Immune checkpoints are regulators of the immune system, play the critical role in the maintenance of the self-tolerance, prevention of autoimmune and protection of tumor tissue from the immune collateral damages [1]. The above immune checkpoints are often appropriated by the tumors, to contain the capacity of the immune system to post an efficient anti-tumor reaction.
To reboot anti-tumor immunity, stalling immune checkpoints is therefore an encouraging tactic [2]. Lately, the antibody-based medications delaying the immune checkpoints CTLA4 and PD-1 appeared as “game changers” in cancer treatment, running to strong clinical reactions even in patients with progressive malignancy. The discovery achievements in melanoma, lung and kidney cancers offered a remedial confirmation for this method for a novel age of cancer management [3] (Table 1).
2 Immune’s Checkpoints Proteins in Immune System
Notwithstanding the achievement of CTLA4 and PD-1 blockers, numerous patients do not react to these managements. Additionally, clinical value is still restricted to a small subset of cancer indications, and only a minority achieves the promise of ongoing survival [5, 6]. It is consequently obvious there is added immune avoidance means facilitated by extra immune checkpoint proteins. In this appraisal, we discuss separately about two utmost reviewed immune checkpoints.
3 Cytotoxic T-Lymphocyte-Related Proteins 4 (CTLA4)
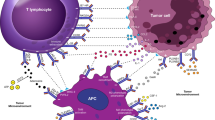
This protein, correspondingly identified as cluster of differentiation 152 (CD152), is a receptor, which works as an immune checkpoint and down synchronizes the immune reactions. CTLA4 is presently conveyed in regulatory T cell once initiation of a occurrence, which is specifically noted in cancers. It behaves as an “off” switch when attached to CD80 or CD86 on the exterior of antigen exhibiting cell (APC). The ctla4 gene in mice and CTLA4 gene in human encrypt the CTLA4 protein.
3.1 Function
Generally, CTLA4 is a representative of the immunoglobulin superfamily that is conveyed by stimulated T cells and conducts an inhibitory wave to T cell. The stimulation of T cell, across the TCR and CD28, results in increasing manifestation of CTLA-4.
CTLA4 is similar to the T-cell co-stimulatory protein, CD28, and the two molecules join to CD80 (B7-1) and CD86 (B7-2), localizing on the cell exterior of antigen-presenting cells (APC), permitting to contest CD28 from its ligands. CTLA4 diffuses an inhibitory sign to T cells, while CD28 conveys a stimulatory signal. CTLA4 is similarly located in Treg cells and provides to its inhibitory role.
The method by which CTLA-4 works in T cells remnants being described by biochemical proof that implied CTLA-4 employs a phosphatase to the TCR, hence weakening the sign. This work stays unverified in the literature. More contemporary research has implied that CTLA-4 may work in vivo by seizing and eliminating B7-1 and B7-2 from the membranes of antigen-presenting cells, consequently creating these inaccessible for activating of CD28. Furthermore, it has been discovered that dendritic cell (DC)—Treg collaboration produces repossession of Fascin-1, an actin-bundling protein vital for immunological synapse establishment, and twists Fascin-1–dependent actin polarity in antigen exhibiting DCs to the T reg cell connection region [6]. Though it is rescindable on T regulatory cell detachment, this requisitioning of crucial cytoskeletal constituents triggers an exhausted condition of DCs, resulting in lessened T cell preparing. This indicates Treg-mediated immune clampdown is a many-step development. Also the CTLA-4 CD80/CD86 communication, the fascin related polarity of cytoskeleton concerning DC-Treg immune synapse performs a key function.
CTLA-4 may also function via modulation of cell motility and/or signal through PI3 kinase. Early multi-photon microscopy studies observing T-cell motility in intact lymph nodes appeared to give evidence for the so-called “reverse-stop signaling model”. In this model CTLA-4 reverses the TCR-induced ‘stop signal’ needed for firm contact between T cells and antigen-presenting cells (APCs). The purpose of these reports is to equate CTLA-4 positive cells, which are primarily supervisory cells and are at least partly stimulated, with CTLA-4 negative naive T cells whilst other clusters, having investigated the influence of antibodies to CTLA-4 in vivo, have established slight or no consequence on motility in the framework of anergic T-cells [7].
3.2 Structure
The protein comprises an extracellular V area, a trans-membrane field, and a cytoplasmic tail. Different link variations, encrypting diverse isoforms, have been depicted. The membrane-bound isoform works as a homodimer interlocked by a disulfide bond, whilst the resolvable isoform behaves as a monomer. The intracellular area is analogous to that of CD28, in that it has no inherent catalytic action and includes one YVKM motif able to fix PI3K, PP2A and SHP-2 and one proline-rich motif capable to fix SH3 comprising proteins. The principal function of CTLA-4 in impeding T cell reactions appear to be candidly through SHP-2 and PP2A dephosphorylation of TCR-proximal indicating proteins for example CD3 and LAT. CTLA-4 can additionally influence signing subtly thru opposing with CD28 for CD80/86 attachment [8]. CTLA-4 can similarly attach PI3 K, though the significance and outcomes of this interface are undefined.
3.3 Clinical Significance
The variations of this gene have been linked with various conditions such as insulin-dependent diabetes mellitus, Hashimoto’s thyroiditis, Graves’ disease, celiac disease, thyroid-associated orbitopathy, systemic lupus erythematosus primary biliary cirrhosis and further autoimmune disorders. Polymorphisms of the CTLA-4 gene are coupled with autoimmune syndromes for example autoimmune thyroid disorder and numerous scleroses; nevertheless this relationship is frequently feeble. In Systemic Lupus Erythematosus (SLE), the link alternative sCTLA-4 found to be abnormally formed and located in the serum of patients with vigorous SLE.
3.4 Germline Haploinsufficiency
Generally, germline haploinsufficiency of CTLA4 results in CTLA4 deficit or CHAI and LATAIE disorder (CTLA4 haploinsufficiency with autoimmune penetration), an uncommon genetic syndrome of the immune structure. Patients with CHAI and LATAIE, present with autoantibody-mediated cytopenias, lymphadenopathy/splenomegaly, hypogammaglobulinemia, organ-specific autoimmunity, and lymphocytic infiltration of nonlymphoid organs. Although features of CHAI and LATAIE are similar, a notable difference is the typically earlier age of onset with LATAIE, where disease onset is often apparent in preschool-age children, whereas CHAI presents in older children or young adults. The autoantibody-mediated cytopenias (i.e., autoimmune hemolytic anemia, autoimmune thrombocytopenia, and neutropenia), lymphadenopathy, and splenomegaly resemble the autoimmune lymphoproliferative syndrome [9].
3.5 Clinical and Laboratory Indications
For any symptomatic patients with CTLA4 mutations categorized by an immune dysregulation condition involving widespread T cell penetration in some organs, involving the gut, lungs, bone marrow, central nervous system, and kidneys [2]. Most patients have diarrhea or enteropathy. Lymphadenopathy and hepatosplenomegaly are likewise as autoimmunity. The tissues disturbed by autoimmunity differ nonetheless involve thrombocytopenia, and thyroiditis, psoriasis, type I diabetes, and arthritis. Furthermore, respiratory contagions are very usual. Notably, the clinical appearances and illness progressions are alterable with certain persons relentlessly affected, while others display minor symptom of complaint. This disorder is defined to have partial penetrance of complaint. Penetrance is thought to be partial when particular persons stop to carry the characteristic and appear fully asymptomatic, though they pass the allele. The penetrance is expected to be approximately 60%.
The clinical signs are initiated by anomalies of the immune arrangement. Majority of patients acquire reduced quantities of at least one immunoglobulin isotype, and have trivial CTLA4 protein manifestation in T regulatory cells, hyperactivation of effector T cells, muted switched memory B cells, and gradual damage of travelling B cells [10].
3.6 Treatment
After a diagnosis is completed, the management is established on an person’s clinical circumstance and may involve typical treatment for autoimmunity and immunoglobulin defects. A new report cured a Korean CHAI ailment patient with CTLA4 mimetic, CTLA4-Ig (e.g.. abatacept) and was capable to manage immune action and recuperate patient signs [10].
3.7 Agonists to Diminish Immune Action
The moderately greater attraction of CTLA4 has created it an impending treatment for autoimmune disorders. Synthesis proteins of CTLA4 and antibodies (CTLA4-Ig) have been employed in clinical experiments for rheumatoid arthritis. The mixture protein CTLA4-Ig is commercially obtainable as Orencia (abatacept). A succeeding group form of CTLA4-Ig recognized as belatacept lately ratified by the FDA established on encouraging outcomes from the randomized Phase III BENEFIT report. It was accepted for renal transplantation in patients that are exposed to Epstein–Barr virus (EBV) [11].
3.8 Antagonists to Surge Immune Endeavor
Equally, there is growing attention in the conceivable remedial aids of hindering CTLA4 (exploiting antagonistic antibodies alongside CTLA such as ipilimumab (FDA accepted for melanoma in 2011) as a ways of stopping immune system acceptance to tumors and thus offering a hypothetically valuable immunotherapy scheme for patients with malignance [5]. This is the initial permitted immune checkpoint deter treatment. Alternative is tremelimumab, however it is still not ratified [12].
4 Programmed Cell Death Protein 1 (PD-1)
Planned cell death protein 1, similarly recognized as PD-1 and cluster of differentiation 279 (CD279), is a cell exterior receptor participating an vital function in down-regulating the immune structure and stimulates self-tolerance by overpowering T cell inflammatory endeavor.
PD-1 is an immune checkpoint and protects alongside autoimmunity over a twofold mechanism of encouraging apoptosis (programmed cell mortality) in antigen-specific T-cells in lymph nodes whilst concurrently decreasing apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells). PD-1 impedes the immune system, inhibits autoimmune disorders, although it can similarly block the immune system from extermination of tumor cells.
A latest group of medications that impede PD-1, the PD-1 inhibitors, trigger the immune system to assault cancers and are thus employed with variable realization to handle specific sorts of malignancy. The PD-1 protein in humans programmed via the PDCD1 gene. PD-1 is a cell superficial receptor that fits to the immunoglobulin superfamily and is conveyed on T cells and pro-B cells. PD-1 attaches dual ligands, PD-L1 and PD-L2 [2, 3].
4.1 Structure
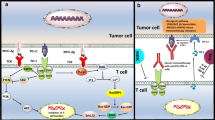
Predetermined death 1 is considered a type I membrane protein of 268 amino acids. PD-1 is a affiliate of the expanded CD28/CTLA-4 family of T cell regulators. The protein’s configuration embraces an extracellular IgV field pursued by a transmembrane area and an intracellular end. The intracellular end includes double phosphorylation locations situated in an immunoreceptor tyrosine-based inhibitory motif and an immunoreceptor tyrosine-based control motif, which indicates that PD-1 depressingly regulates T-cell receptor TCR signals. This is coherent with fastening of SHP-1 and SHP-2 phosphatases to the cytoplasmic end of PD-1 on ligand attachment. Additionally, PD-1 ligation up-regulates E3-ubiquitin ligases CBL-b and c-CBL that trigger T cell receptor down-modulation. PD-1 is conveyed on the exterior of stimulated T cells, B cells, and macrophages, proposing that contrasted to CTLA-4, PD-1 extra mostly negatively controls immune reactions [12].
4.2 Ligand
PD-1 has dual ligands, PD-L1 and PD-L2, which are partners of the B7 family. Normally, PD-L1 protein is upregulated on macrophages and dendritic cells (DC) in reply to LPS and GM-CSF therapy, and on T cells and B cells on TCR and B cell receptor marking, while in quiescent rats, PD-L1 mRNA can be identified in the heart, lung, thymus, spleen, and kidney. PD-L1 is conveyed on nearly entirely murine cancer cell lines, involving PA1 myeloma, P815 mastocytoma, and B16 melanoma on therapy with IFN-γ. The PD-L2 manifestation is further constrained and is communicated chiefly by DCs and a limited cancer cell lines [6, 13].
4.3 Function
Numerous literatures indicate that PD-1 and its ligands adversely control immune reactions. PD-1 knockout mice have been revealed to grow lupus-like glomerulonephritis and enlarged cardiomyopathy on the C57BL/6 and BALB/c settings, correspondingly. In vitro, management of anti-CD3 encouraged T cells with PD-L1-Ig leads to reduced T cell fabrication and IFN-γproduction. IFN-γ is an important pro-inflammatory cytokine that encourages T cell inflammatory commotion. Downgraded T cell production was likewise associated with reduced IL-2 production. Mutually these facts imply that PD-1 deleteriously controls T cell reactions.
Trials employing PD-L1 transfected DCs and PD-1 conveying transgenic (Tg) CD4+ and CD8+ T cells indicate that CD8+ T cells are further vulnerable to inhibition by PD-L1, though this could be reliant on the potency of TCR signaling. Coherent with a function in adversely controlling CD8+ T cell reactions, exploiting an LCMV viral vector standard of chronic disease, Rafi Ahmed’s group revealed that the PD-1-PD-L1 collaboration deters stimulation, growth, and attainment of effector roles of virus-specific CD8+ T cells, which can be overturned by impeding the PD-1-PD-L1 communication [2, 14].
Since CTLA-4 undesirably controls anti-tumor immune reactions, the diligently associated molecule PD-1 has been freely discovered as a goal for immunotherapy. Manifestation of PD-L1 on tumor cells constrains anti-tumor action within arrangement of PD-1 on effector T cells. Manifestation of PD-L1 on cancers is linked with reduced survival in esophageal, pancreatic and other types of cancers, highlighting this pathway as a target for immunotherapy. Triggering PD-1, conveyed on monocytes and up-regulated upon monocytes initiation, via its ligand PD-L1 stimulates IL-10 manufacture, which reduces CD4 T-cell task [15].
In mice, manifestation of this gene is produced in the thymus when anti-CD3 antibodies are introduced, and great amounts of thymocytes experience apoptosis. Mice lacking for this gene raised on a BALB/c setting acquired enlarged cardiomyopathy and perished from congestive heart failure. These reports indicate that this gene outcome may also be essential in T cell role and add to the inhibition of autoimmune disorders.
Overexpression of PD1 on CD8+ T cells is only sign of T-cell fatigue (e.g., in prolonged infection or malignancy).
4.4 Clinical Significance
-
Cancer
PD-L1, the ligand for PD1, is highly expressed in several cancers and hence the role of PD1 in cancer immune evasion is well established. Monoclonal antibodies targeting PD-1 that boost the immune system are being developed for the treatment of cancer. Numerous tumor cells express PD-L1, an immunosuppressive PD-1 ligand; inhibition of the interaction between PD-1 and PD-L1 can enhance T-cell responses in vitro and mediate preclinical antitumor activity. This is known as immune checkpoint blockade.
Assortment therapy employ dual anti-PD1 and anti-CTLA4 therapeutics have seemed as significant cancer managements in the arena of checkpoint inhibition.
A mixture of PD1 and CTLA4 antibodies has been revealed to be additional efficient than any antibody only in the management of various malignancies. The outcomes of the double antibodies do not emerge to be superfluous. Anti-CTLA4 therapy results in a superior antigen-specific T cell-dependent immune response whilst anti-PD-1 seems to resurrect CD8+ T cells capability to destroy tumor cells.
In clinical experiments, mixture remedy has been revealed to be successful in lessening cancer magnitude in patients that are insensitive to solo co-inhibitory barricade, notwithstanding growing quantities of noxiousness owing to anti-CTLA4 management [8]. A mixture of PD1 and CTLA4 produced up to a ten-times greater amount of CD8+ T cells that are dynamically penetrating the cancer mass. The writers postulated that the greater concentrations of CD8+ T cell insinuation were owing to anti-CTLA-4 subdued the transformation of CD4 T cells to T regulator cells and supplementary decreased T regulatory destruction with anti-PD-1. This amalgamation encouraged a further strong inflammatory reaction to the cancer that condensed the extent of tumor. Recently, the FDA has accepted a mixture treatment with dual anti-CTLA4 (Ipilimumab) and anti-PD1 (Nivolumab) in October 2015.
The molecular influences and receptors are crucial creation a cancer responsive to anti-PD1 therapy stay undetermined. The PDL1 manifestation on the exterior of tumor cells shows a major function. PDL1 positive cancers were twofold as probable to react to mixture therapy. Nonetheless, patients with PDL1 negative growths additionally have restricted reaction to anti-PD1, signifying that PDL1 manifestation is not an utter factor of the efficiency of treatment [8].
The greater mutational problem in the cancer is associated with a superior influence of the anti-PD-1 usage. In clinical experiments, patients who gained from anti-PD1 management had malignances, for example melanoma, bladder cancer, and gastric cancer, that had a usual greater than normal number of mutations than the patients who do did not counter to the treatment. Yet, the relationship amid advanced cancer problem and the clinical efficiency of PD-1 immune blockade is remained unclear.
-
Anti-PD-1 therapeutics
Certain malignancy immunotherapy vehicles that focus the PD-1 receptor have been established.
An example of such anti-PD-1 antibody medicine, Nivolumab, (Opdivo—Bristol Myers Squibb), made far-reaching or fractional reactions in non-small-cell lung cancer, melanoma, and renal-cell cancer, in a clinical trial with a overall of 296 patients 38 Colon and pancreatic cancer did produce a reaction. Nivolumab (Opdivo, Bristol-Myers Squibb) ratified in Japan in July 2014 and by the US FDA in December 2014 to cure metastatic melanoma [16].
A typical agent such as Pembrolizumab (Keytruda, MK-3475, Merck) aims at PD-1 receptors accepted by the FDA, in Sept 2014 to use for metastatic melanoma. Pembrolizumab has been made available to radical melanoma patients in the UK via UK Early Access to Medicines Scheme (EAMS) in March 2015. It has been employed in clinical studies in the US for patients with lung cancer, lymphoma, and mesothelioma. It has had reasonable achievement, with a few reported complications. It is up to the producer of the medication to ask to the FDA for endorsement for application in these illnesses. On October 2, 2015, FDA approved Pembrolizumab for radical (metastatic) non-small cell lung cancer (NSCLC) patients who are exhausted with othertherapies [17].
Further drugs in early-phase of development directing PD-1 receptors (checkpoint inhibitors) are Pidilizumab (CT-011, Cure Tech) and BMS-936559 (Bristol Myers Squibb). Both Atezolizumab (MPDL3280A, Roche) and Avelumab (Merck KGaA, Darmstadt, Germany & Pfizer) aim the analogous PD-L1 receptor.
4.5 Animal Illness
HIV
Medications aiming PD-1 in amalgamation with further negative immune checkpoint receptors, for example (TIGIT), may supplement immune reactions and/or accelerate HIV abolition. T lymphocytes display higher manifestation of PD-1 in situation of long-lasting HIV infection. Increased occurrence of the PD-1 receptors relates to collapse of the HIV specific CD8+ cytotoxic and CD4+ helper T cell residents that are fundamental in fighting the virus. Immune barricade of PD-1 leaded to the renewal of T cell inflammatory phenotype indispensable to battle the development of the illness.
Alzheimer’s disease
Impeding of PD-1 tips to a decrease in cerebral amyloid-β plaques and develops intellectual functioning in mice. Immune block of PD-1 induced an IFN-γ dependent immune reaction that enlisted monocyte-derived macrophages to the brain that were then proficient of clearance the amyloid-β plaques from the mass. Continual admins with anti-PD-1 were discovered to be essential to uphold the remedial outcomes of the therapy. Amyloid plaques are immunosuppressive, and this discovery has been independently established by inspecting the properties of the fibrils in neuroinflammatory disorders. PD-1 offsets the results of the fibrils by encouraging immune activity and activating an immune route that permits for brain restoration.
4.6 Future Consideration
The clinical realizations have unlocked a renewed range of remedial: FDA approval of anti-CTLA4, rapidly pursued by anti-PD-1 therapy [18]. A recently discovered understanding of prospective antitumor endeavor in cancer patients have helped to clarify the role of ICs in immune reactions in different levels. The investigation of ICS yields two challenges: Is the classification of possible biomarkers that can control which IC foremost pathway in specific cancer therapy a difficult decision? Is it conceivable that particular oncogenic pathway such as PI3k-AKT or STAT3 may provoke and can be employed as a replacement biomarker? The clinical development of combined therapy: vaccine associated with anti-ICs? The above-mentioned questions allow the following conclusions to be reached: Clinical effects of CTLA4 and PD-1/PD-L1 inhibitors indicate that B7-H1/PD-1 passageway is a significant objective for the successful antitumor immune reaction. Encouragement and toxicity synopsis of PD-1/B7-H1 inhibitors confirms that they are very useful for the treatment of advanced cancers.
The conceivable perpetuation of response of PD-1 and CTLA4 pathways lasting up to ten years, leading to the conduct of extensive international clinical trials on the their effect on solid tumors. This open up future research opportunities in blockades of ICS associated with new vaccines, angiogenesis, MAPK inhibiting targeted therapy.
5 Conclusions
Immune checkpoint blockade has gained momentum as a new approach in cancer immunotherapy. Due to its successes, many new agents and strategies, including combination approaches, are being developed at a fast pace.
References
Thang, H.Q.: Basic molecular biology of cancers. J. Med. HCM city Vietnam 19(5–2015), 1–7 (2015)
Abbas, A.K., Lichtman, A.H., Pillai, S.: Lymphocyte development and Antigen receptor gen rearrangement. In: Cellular and Molecular Immunology, 9th edn, pp. 179–208. Elsevier (2018)
Hanahan, D.: Weinberg RA. The hallmarks of a cancer cell 144, 646–674 (2011)
Marin-Acevedo, J.A., et al.: Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11, 39 (2018)
Harrington, L., Bristow, R.G., Hill, R.P., Tannock, I.F: Introduction to cancer biology. In: The Basic Science of Oncology, pp. 1–4 (2005)
Franklin, M., Kratzke, R.: Molecular biology for the oncologist: the essentials 2013. http://clinicaloption.com/Practice/Oncology/General_Oncology
Bristow, R.G., Harrington, L.: Genomic stability and DNA repair. In: The Basic Science of Oncology, pp. 77–100 (2005)
Baroja, M.L., Vijayakrishnan, L., Bettelli, E., Darlington, P.J., Chau, T.A., Ling, V., Collins, M., Carreno, B.M., Madrenas, J., Kuchroo, V.K.: Inhibition of CTLA-4 function by the regulatory subunit of serine/threonine phosphatase 2A. J. Immunol. 168(10), 5070–5078 (2002). https://doi.org/10.4049/jimmunol.168.10.5070.PMID11994459
Weinberg, R.A.: Multi-step tumorogenesis. In: The Biology of Cancer, pp. 439–510 (2014)
Enderling, H., Hyatky, L., Hahnfeldt, P.: Immunoediting: evidence of the multifaceted role of the immune system in self-metastatic tumor growth. Theor. Biol. Med. Model. 9, 31 (2012)
Cahill, D.P., Lengauer, C.: Tumor genome instability. In: The Genetic Basis of Human Cancer, pp. 129–130 (2002)
Abbas, A.K., Lichtman, A.H., Pillai, S.: Cell and tissue of the immune system. In: Cellular and Molecular Immunology, 9th edn, pp. 13–38. Elsevier, Amsterdam (2018)
Thang, H.Q.: Biological alterations in lung cancer. J. Med. HCM city Vietnam 19(5–2015), 8–15 (2015)
Abbas, A.K., Lichtman, A.H., Pillai, S.: Antigen presentation to T lymphocytes and the function of major histocompatibility complex molecules. In: Cellular and Molecular Immunology, 9th edn, pp. 117–144, Elsevier (2018)
Abbas, A.K., Lichtman, A.H., Pillai, S.: Property and overview of immune responses. In: Cellular and Molecular Immunology, 9th edn, pp. 01–12. Elsevier (2018)
Alexander, W.: The checkpoint immunotherapy revolution what started as a trickle has become a flood, despite some daunting adverse effects; new drugs, indications, and combinations continue to emerge. Pharm. Ther. 41(3), 185–191 (2016)
Dang, O.T., et al.: Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer Ther. 16(1), 13–20 (2016)
Chae, Y.K., et al.: Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer 6, 39 (2018)
Conflict of Interest The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Huynh, T.Q., Tran, D.N., Chau, T.P., Huynh, T.M., Trinh, C.H., Doan, N. (2020). An Overview of Immune Checkpoints and Immunotherapy in Cancer. In: Van Toi , V., Le, T., Ngo, H., Nguyen, TH. (eds) 7th International Conference on the Development of Biomedical Engineering in Vietnam (BME7). BME 2018. IFMBE Proceedings, vol 69. Springer, Singapore. https://doi.org/10.1007/978-981-13-5859-3_105
Download citation
DOI: https://doi.org/10.1007/978-981-13-5859-3_105
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5858-6
Online ISBN: 978-981-13-5859-3
eBook Packages: EngineeringEngineering (R0)