Abstract
Reverse osmosis (RO) membrane technology is considered to be the premier process used for the purpose of seawater and brackish water desalination and water treatment of municipal and industrial wastewater for water reclamation and reuse. Membrane biofouling is a significant challenge in RO processes due to the interference of biofilm formed on the membrane surface on membrane performance. Thus, diverse areas of research are geared towards the understanding, prevention, and control of biofouling. Diagnosis of biofouling is difficult since no single microbial assay on the source water can accurately predict biofouling during the RO process. Biofouling evaluation methods of fouled membranes and collected biofoulants from the treatment processes are counterproductive when biofouling prevention is warranted. It is therefore important for the detection tests to be predictive enough taking into consideration the water quality characteristics of the source feed water, the properties of the RO membrane used for the water treatment, and the hydrodynamic properties during the RO process. This chapter provides an overview of biofouling tests most commonly used for detection of biofouling in the source feed water, and in the foulants and fouled membranes. It has a brief section on the use of flow cell units that can simulate hydrodynamic conditions in the RO plant with the ability to predict biofouling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Reverse Osmosis Membrane Technology and Biofouling
1.1 The Global Water Demand and the Role of RO Membrane Technology
Water is a very essential part of human life: for consumption and daily living use as well as for non-potable services like recreation, agriculture, and industrial applications. Due to the differences in seasons and location, freshwater distribution and availability through the natural water cycle like precipitation and runoff are very irregular worldwide, resulting in significant variations in per capita water availability between countries and with some countries experiencing absolute scarcity of fresh water (WWAP 2015). The availability, demand, and use of water have also suffered huge impacts due to climate change, socioeconomic development, and population growth (Arnell 2004; Alcamo et al. 2007; Vörösmarty et al. 2010). Aside from population growth and urbanization, food and energy security policies, as well as macro-economic processes, i.e. globalization in trade and changing consumption patterns have influenced the global water demand. And due to the expected growing demand from major sectors such as domestic use, manufacturing, and thermal electricity, the demand is projected to increase by 55% by the year 2050 (WWAP 2015).
Research studies and recent technologies are geared towards the management of existing water resources as well as the development of alternative ones such as water reclamation, recycling, water reuse, desalination of seawater and inland saline aquifers, and others (Miller 2006; Shannon et al. 2008). On a global scale, desalination, despite the limits posed due to energy concerns associated with the technology, has great development potential due to the following reasons (Ghaffour et al. 2013): firstly, large cities lacking fresh water sources are located along the coastal regions and thus these areas have ready access to seawater. And then secondly, desalination has a secure and unlimited source of water supply since feed water supply is unaffected by climatic conditions. Desalination can be categorized based on the mechanism of separation: (1) thermal processes (multi-stage flash, multiple effect distillation, vapor compression distillation) and (2) membrane-based processes [electrodialysis, nanofiltration (NF), reverse osmosis (RO)] (Greenlee et al. 2009). Compared to the thermal-based processes, membrane desalination is increasing due to substantial improvements and innovations towards reducing the costs of desalinated water compared to other water resources, mainly in the RO process (Ghaffour et al. 2013), with a reported 55% growth rate per year in desalination capacity (Global Water Intelligence (GWI/IDA DesalData) 2013). As of 2015, there are about 18,426 desalination plants worldwide and 150 countries practicing desalination (IDA (International Desalination Association) 2015). The four major membrane suppliers, DOW, Hydranautics, Toray, and Toyobo are responsible for some of the state-of-the-art seawater reverse osmosis (SWRO) large-scale desalination plants which are found in Perth in Australia, Llobregat in Spain, Tuas in Singapore, and Fukuoka in Japan, respectively (Lee et al. 2011). Compared to conventional thermal desalination, the RO membrane system does not suffer from corrosion, has the ability to use low-grade heat, and has been shown to reduce energy consumption owing to the efficient energy recovery systems and the development of more robust membranes (Shannon et al. 2008). Usage of the RO process has evolved, originally for the desalination of seawater and brackish water (Greenlee et al. 2009; Afonso et al. 2004; Fritzmann et al. 2007; Khan et al. 2014) to the treatment of different water types containing varying inorganic and organic contaminants (Ang et al. 2011; Malaeb and Ayoub 2011), and more specifically in the treatment of municipal and industrial wastewater (Khan et al. 2014; Ang et al. 2011; Ridgway et al. 1983; Wilf and Alt 2000; Bódalo-Santoyo et al. 2003). In the past, wastewater treatment is focused on pollution abatement, but in the last two decades, there is an increased amount of municipal wastewater recovered for reuse (Levine and Asano 2004). Global interests and efforts are also made in utilizing reclaimed wastewater for both potable and non-potable purposes (Levine and Asano 2004; Toze 2006). Although conventional treatment can produce water to meet existing regulations, information about its effectiveness to control harmful trace contaminants are limited such that advanced treatment technologies such as membrane bioreactors, microfiltration (MF), ultrafiltration (UF), NF, and RO proved to be more effective for wastewater reuse (Levine and Asano 2004). The RO membrane process plays a vital part in this goal due to its higher rejection of impurities (Fig. 18.1). It essentially produces clean water only at a lower cost compared to other membrane-based technology (Pandey et al. 2012). The presence of large-scale commercial RO membrane plants is proof of the use and capability of the RO technology for wastewater reclamation purposes. These 6 large-scale plants are found in West Basin and Orange County in California, Kranji, Bedok, and Ulu in Singapore, and in Sulabaiya, Kuwait (Bartels 2006). The range and scope of studies in RO membrane technology encompass the whole process, from its application to treating various types of water until post-treatment of the clean water, despite the challenges posed during operation (Greenlee et al. 2009; Malaeb and Ayoub 2011). For both desalination and water reclamation and reuse, the application of RO membranes faces a significant challenge because of membrane performance decline due to membrane fouling.
1.2 Membrane Biofouling and RO Membrane Performance
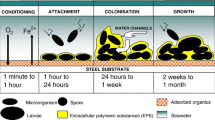
Major challenges in RO desalination include relatively low recovery for seawater desalination thereby producing large volumes of concentrated brine, fairly low removal of low-molecular-weight contaminants, specifically, boron in seawater, and membrane fouling (Shannon et al. 2008). Membrane fouling is the accumulation of unwanted substances on the membrane surface and remains a critical issue to the desalination industry worldwide since it decreases the performance efficiency of the RO process. The main types of membrane fouling are crystalline fouling/scaling, organic fouling, particulate and colloidal fouling, and microbiological fouling or biofouling, which are categorized based on the materials responsible for the fouling (Flemming 1997), such as sparingly soluble inorganic compounds, dissolved and macromolecular organic substances, suspended and colloidal particles, and microorganisms, respectively (Pandey et al. 2012). Membrane fouling is a complex and dynamic process involving various steps in the development of the fouling layer. During organic, inorganic, and colloidal fouling, a rapid initial step involves foulant-membrane interactions and then a gradual long term-step involving foulant–foulant interactions (Tang et al. 2009). Biofouling, on the other hand, involves conditioning, cell attachment, cell growth, and then cell dispersion (Khan et al. 2013a).
Microorganisms are found everywhere in the environment and can grow to create biofilms in the presence of nutrients and under favorable conditions. However, its adverse effect on the RO process is determined by the extent to which biofilm grows and proliferates (Flemming 2002). All nutrients in the water, inactive bacterial biomass after disinfection, biofilm fragments, and dead cells can serve as potential energy sources to the remaining microorganisms when disinfection has been incomplete resulting in biomass production and biofilm formation (Baker and Dudley 1998; Mansouri et al. 2010). These substances should be completely removed or at the very least kept to a minimum, making the pretreatment of the RO feed water an important part in the RO process.
Biofouling refers to the deposition and growth of biofilms to the point that the presence of biofilms on the membrane systems leads to performance decline (Flemming 1997). A biofilm is a complex assembly of sessile microbial communities permanently attached to the membrane due to the presence of self-produced extracellular polymeric substances (EPS), which are mainly polysaccharides and proteins (Flemming and Wingender 2010). For membrane systems like the RO system, the biofilms interfere in the separation process and give rise to enhanced concentration polarization, increased hydraulic resistance, decreased membrane permeability, and decreased salt rejection, but in terms of process efficiency, permeate flux decline and the decrease in salt rejection are considered to be the main concerns (Matin et al. 2011). The kinetics of the flux decline usually shows an initial rapid decline typically correlated with the early attachment and growth of microorganisms on the membrane surface and a slow decline (plateau) phase whereby an equilibrium condition between biofilm growth and EPS production and biofilm loss is taking place (Flemming and Geesey 1991). Rather than a result of changes in the inherent properties of the membrane, the flux decline is most likely a consequence of the biofilm acting as a transport barrier leading to increased hydraulic resistance against water transport (Matin et al. 2011). Studies related to fouling mechanisms have reported that bacterial cells and EPS play major roles in the flux decline (Herzberg and Elimelech 2007; Chong et al. 2008). An increase in the transmembrane pressure (TMP) is a result of a hindered back diffusion of salts, elevating the osmotic pressure on the membrane surface due to the presence of biofilm, resulting in permeate flux decline (Herzberg and Elimelech 2007; Chong et al. 2008; Herzberg et al. 2009). The associated EPS surrounding the biofilm, on the other hand, increases the hydraulic resistance to permeate flow (Herzberg and Elimelech 2007; Herzberg et al. 2009), contributing to the decline in flux.
A similar mechanism has been reported to be responsible for the decrease in salt rejection, wherein EPS and biofilm have significant contributions (Matin et al. 2011; Herzberg and Elimelech 2007). The presence of bacterial cells in the EPS matrix hinders the back diffusion of salts thereby increasing the salt concentration near the membrane surface (Herzberg and Elimelech 2007). This phenomenon results in an enhanced solute transport through the membrane due to the increased solute concentration in the boundary layer (Matin et al. 2011), leading to a decrease in salt rejection. In addition, adverse effects of biofilm growth on boron rejection were attributed to both an increase in hydraulic resistance to permeate flow due to the bacterial EPS and a biofilm-enhanced osmotic pressure near the membrane surface (Huertas et al. 2008).
2 Methods for Biofouling Detection
Biofouling, unlike the other types of membrane fouling that can generally be controlled by reducing the foulant concentration, is difficult to manage and control by simple reduction of microorganisms in the source feed water (Vrouwenvelder et al. 2008). Thus, biofouling can only be prevented and controlled with the proper selection of anti-fouling strategies (Pandey et al. 2012). A vital part in designing cost-effective strategies is biofouling detection. Since biofouling is a consequence of a severe biofilm formation in the membrane units resulting in membrane performance decline, specifically operational problems during the RO process, a mixture of methods of quantification of biofilm amount and the associated operational problem encountered, such as pressure drop, flux decline, or salt rejection, are determined to have a reasonable diagnosis of biofouling. This section describes various methods of detecting biofilm amount in the source water, in foulants, and in fouled membranes. Biofouling detection units employing hydrodynamic conditions simulating real filtration conditions in the RO process is also briefly presented.
2.1 Water Quality Parameters of RO Feed
Regardless of the fouling type, evaluation of the quality of the feed water is essential in determining the proper pretreatment and RO design for water treatment. Research studies have shown that the differences in fouling layer composition are influenced by the quality of the source water (Khan et al. 2013b, 2014). Different source waters treated by the RO process can be roughly categorized based on two fundamental water quality parameters (DOW 2010), total dissolved solids (TDS) content, which can be referred to as salinity, and the organic load in terms of total organic carbon (TOC). Brackish water has medium TOC content with medium to high salinity and seawater has low to high TOC and high salinity, while municipal wastewater has a high organic load with medium salinity. Silt density index (SDI) is a water quality parameter used to describe the fouling potential of water and is regarded as an important feed water quality guideline for pretreatment and RO system design (Teng et al. 2003; Mosset et al. 2008). It is the most common indicator used in practice and the feed SDI should be consistently <5 or <3, depending on the RO system design, as recommended by membrane manufacturers (DOW 2010). It measures the rate at which a 0.45 μm membrane filter is plugged when the water is passed through the membrane filter at a constant applied gauge pressure (DOW 2010). However, SDI is not always reliable and accurate and its sensitivity to other factors such as pH, membrane characteristics, and turbidity of water poses a huge drawback in its use as a fouling index (Mosset et al. 2008; Alhadidi et al. 2012; Rachman et al. 2013). It also has limited capability in measuring fouling potential due to other foulants such as colloidal deposition and organic adsorption (Choi et al. 2009). Studies have also shown that despite the SDI values of feed water within limits prescribed by membrane suppliers, biofilm formation and biofouling were not prevented (Schneider et al. 2005; Huang et al. 2013), confirming the need of additional or other water quality parameters to predict biofouling or at the very least, indicate biofouling potential. Thus, as a measure of biofouling potential, quantification of organic matter, biomass of microorganisms, and substances promoting bacterial adhesion and growth are also determined.
The starting point of biofilm growth is the rapid accumulation of organics (humic substances and polysaccharides) on the membrane surface (Subramani et al. 2009), which are also accompanied by bacterial cell adhesion and cell adsorption (Khan et al. 2013a). In the second stage of adhesion, the adhesion process is strengthened by loosely bound organisms when EPS are released (Dunne 2002). Thus, feed water quality parameters for biofouling propensity include the determination of organics in the feed water. The usual tests for determination of organics are TOC content and dissolved organic carbon (DOC) content. The organic carbon in the water sample is oxidized to carbon dioxide, by combustion in an oxidizing gas or by UV-promoted or heat-catalyzed chemical oxidation, and the carbon dioxide released from the oxidized sample is detected by a conductivity detector or a nondispersive infrared detector (Potter and Wimsatt 2005). These tests are used to determine the water quality of feed water in order to evaluate pretreatment efficiency, establish operating conditions, and maintain the quality of discharged effluents (Khan et al. 2013a, b, 2014; Schneider et al. 2005; Huang et al. 2013; Norberg et al. 2007; Xu et al. 2010; Zhao et al. 2010; Jeong et al. 2013a; Miyoshi et al. 2016). Based on membrane manufacturers, pretreatment should be considered when TOC exceeds 3 mg/L (DOW 2010). UV254, like TOC and DOC, is a bulk parameter used to determine organic amounts in feed water (Khan et al. 2013b; Huang et al. 2013; Norberg et al. 2007; Zhao et al. 2010). It is mainly used to quantify the aromatic compounds and unsaturated bonds in a compound that strongly absorb UV radiation and is related to the humic component of the organic matter (Choi et al. 2009; Norberg et al. 2007; Sim et al. 2018). For this determination, the water sample is passed through a 0.45 μm filter, transferred to a quartz cell, and then analyzed using a spectrophotometer to measure the UV absorbance at 254 nm (Potter and Wimsatt 2005). Most recently, it has been used as a monitoring and control parameter for organic micropollutants in wastewater (Altmann et al. 2016). However, monitoring the changes in bulk parameters like TOC, DOC, and UV254 is not suitable for the evaluation of the changes in the concentration of specific organic components (Miyoshi et al. 2016) that will be helpful in assessing the cause of biofouling in the RO units.
Fluorescence measurements given as 3D excitation-emission matrices (EEMs) have found favor in the analysis of dissolved organic matter based on characteristic regions of excitation-emission wavelength corresponding to distinct organic components (Chen et al. 2003) and have been used to characterize different water systems (Miyoshi et al. 2016; Hudson et al. 2007; Henderson et al. 2009; Pype et al. 2013; Carstea et al. 2016). A study showed the capability of F-EEM coupled with fluorescence regional technique in assessing RO membrane integrity by analyzing feed and permeate samples from pressure vessels, complete stages, and RO trains of two full-scale plants (Pype et al. 2013). Another advanced method of characterizing humic, non-humic, and other fractions of organic compounds based on molecular size is through the use of the liquid chromatography-organic carbon detector (LC-OCD) or size exclusion chromatography-organic carbon detector (SEC-OCD) (Huber et al. 2011; Chen et al. 2016). It has been extensively used for determining the efficiency of pretreatments for water treatment through analysis of raw and treated water (Miyoshi et al. 2016; Jeong et al. 2012, 2016; Kimura and Oki 2017; Monnot et al. 2017). Although these methods are promising, they are mainly used in research studies and are not used online or on-site (Sim et al. 2018) but permeate water samples can be collected in strategic locations to evaluate the performance of the RO units (Pype et al. 2013).
Commonly used biological tests for quantifying microorganisms in the feed water are heterotrophic plate count (HPC) (Reasoner and Geldreich 1985) and total direct cell count (TDC) (Hobbie et al. 1977). HPC is determined by culturing the microorganisms from the sample water in an appropriate nutrient medium to develop colonies, which are then counted and reported as the number of colony-forming units (CFU) as a measure of culturable microorganisms (Reasoner and Geldreich 1985). Direct count methods are preferred instead of culture techniques because they are much faster by simply counting the stained microorganisms directly under a microscope (DOW 2010). TDC requires filtration of the water sample through a membrane filter and the microorganisms retained on the filter are stained with a dye, usually acridine orange, and then observed and counted under an epifluorescence microscope (Hobbie et al. 1977). As expected, feed water with low and high concentrations of bacterial content based on these tests showed low and high biomass concentrations, respectively after membrane autopsies (Vrouwenvelder and Van der Kooij 2001). Biomass can also be determined by measuring adenosine triphosphate (ATP), a biomolecule found in all cells, whether plant or animal (Holm-Hansen and Booth 1966). The accumulation of ATP on the surface of glass rings in an online operated biofilm monitor is determined as a function of time and is reported as the biofilm formation rate (BFR) (Vrouwenvelder and Van der Kooij 2001). ATP concentrations are obtained by an enzymatic reaction using the luciferine-luciferase assay (Holm-Hansen and Booth 1966). The amount of light produced is determined by a luminometer and the ATP concentration is derived from the linear relationship between light production and reference ATP concentrations (Vrouwenvelder et al. 2008). Studies have shown a good correlation between ATP and the biomass content in feed water (Vrouwenvelder et al. 2003; Veza et al. 2008). Thus, ATP is considered as a suitable water quality parameter for biofouling potential. However, HPC and ATP values were found to be correlated in water only when organic loads are low, but not when organic loads are high (Veza et al. 2008). Furthermore, even low concentrations of biodegradable components could still lead to biofouling based on assimilable organic carbon (AOC) test and BFR test (Vrouwenvelder and Van der Kooij 2001). And although lessening bacterial concentration in the feed water is the primary countermeasure to avoid biofouling, it has been reported that biofouling reduction was largely dependent on decreasing the concentrations of AOC (Schneider et al. 2005). This indicates that despite being direct methods for bacterial content, tests like HPC and TDC are not suitable as biofouling parameters. In addition, HPC values are generally a small fraction of the total number of cells present, as little as 1–10% or less than TDC values (DOW 2010), an extreme underestimation when considering a single assay as a measure of biofouling potential.
Thus, biofouling potential of water has also been determined through quantification of substances that promote bacterial adhesion and growth. Microorganisms are surrounded by self-produced EPS, which are composed of polysaccharides, proteins, DNAs, nucleic acids, and other biopolymers rendering the structure and architecture of biofilms (Flemming and Wingender 2010). Therefore, these substances are crucial in initial bacterial adhesion and attachment, and biofilm growth. As previously mentioned, according to several studies, EPS and biofilms are instrumental to the decline in permeate flux and salt rejection (Matin et al. 2011; Herzberg and Elimelech 2007; Chong et al. 2008). Feed and permeate waters are usually analyzed for EPS components, typically polysaccharides and proteins, to characterize the components of the water samples associated with biofilm formation as well as to ascertain their removal or production during pretreatment or during the RO process itself (Chong et al. 2008; Herzberg et al. 2009; Zhao et al. 2010; Jeong et al. 2012; Lee et al. 2010; Baek et al. 2011). Polysaccharide content is usually analyzed by the phenol-sulfuric acid method wherein carbohydrates in the sample are stained yellow–orange when treated with phenol and sulfuric acid, which later can then be spectrophotometrically measured, and the concentrations are determined using a carbohydrate standard like glucose (DuBois et al. 1956). Protein concentrations can be determined through various means but are typically determined through the traditional dye-based absorbance measurements like Bicinchoninic acid (BCA) assay (Chong et al. 2008; Jeong et al. 2012) or the Lowry (Alkaline Copper Reduction) Assay (Herzberg et al. 2009; Lee et al. 2010). As a subclass of EPS, transparent exopolymer particles (TEPs) are individual, free-floating particles and have been found to be an important agent in biofilm formation and knowledge and understanding of their dynamics in seawater, surface water, and wastewater would be beneficial in pretreatment and fouling/biofouling control strategies (Miyoshi et al. 2016; Berman et al. 2011; Meng et al. 2013; Bar-Zeev et al. 2015). Similar to the polysaccharides and protein measurements, TEP concentrations are determined through dye-absorbance measurements. The water sample is filtered through a polycarbonate filter and the accumulated TEP on the filter is stained with the Alcian blue dye, which is subsequently eluted with sulfuric acid and the absorbance of the resulting solution measured spectrophotometrically with xanthan gum as the calibration standard used to quantify TEP (Passow and Alldredge 1995). Although the quantitative analysis of each component is relatively straightforward, an important aspect of characterizing EPS is the extraction of the component from the biofilm. A good extraction method should keep the characteristics of the component intact, should not cause cell lysis, and should be able to collect all EPS components (Nielsen and Jahn 1999). AOC is the fraction of the TOC used by microorganisms that result in an increased biomass concentration (Hobbie et al. 1977) and is used to evaluate bacterial regrowth potential (BRP) (Van der Kooij 1992) in the feed water of the RO unit (Jeong et al. 2012, 2013a; Vrouwenvelder and Van der Kooij 2001; Dixon et al. 2012a, b). High amounts of AOC may also cause severe biofouling, especially when there is a high bacterial load in the feed water (Schneider et al. 2005). The principle of the AOC test is based on the measurement of the growth of an inoculum in a water sample until the stationary phase, with the assumption that the growing bacteria have assimilated all of the AOC in the water (Van der Kooij 1992). The net growth of the bacteria is measured by various methods and then converted to an AOC or AOC-equivalent concentration (Van der Kooij 1992). It has been shown that AOC values in seawater were strongly correlated to RO biofouling in a full-scale plant, showing its predictive capability for biofouling (Weinrich et al. 2016). However, since the test is not yet standardized for seawater, in one study, the organism used for the inoculum was isolated first from the fouled RO membrane in the experimental site (Carstea et al. 2016) Thus, existing AOC tests are continuously developed and improved to increase its sensitivity, decrease the turnaround time to have a faster analysis, and make it applicable to highly saline marine water samples (Jeong et al. 2013b). These water quality tests are still lacking in terms of reliable predictive capabilities for biofouling (Sim et al. 2018) since low values of AOC, ATP, and BFR do not essentially signify the absence of biofouling (Vrouwenvelder et al. 2008). Table 18.1 shows a summary of the advantages and disadvantages offered by the detection methods discussed in this section and some methods used for the extracted foulant. Unless otherwise specified, the methods are applicable for both seawater desalination and wastewater reclamation.
2.2 Analysis of Foulants and Fouled Membranes
Significant studies have been done on the analysis of foulants and fouled membranes during membrane autopsies in order to establish that biofouling was the cause in the decline of membrane performance. Foulants are detached from membranes, membrane coupons, and spacers, and the collected foulant subjected to tests for bacterial counts or organic content. Plate counts, TDC, EPS concentration, and ATP analyses of foulants are the most typical means of assessing biomass of foulants. Aside from the additional procedure for extraction of the analytes from the foulant, these tests when applied in the characterization of the biofilm extracted from the foulant material offer the same general advantages and disadvantages as when they are used for water quality characterization (Table 18.1). However, additional information gathered by several investigations will be discussed to show these tests’ suitability as biofouling detection methods. Evaluation of EPS on extracted foulants and directly on fouled membranes give information on the biofilm morphology, description of the fouling layers, and most importantly give insights into the biofouling mechanism (Ridgway et al. 1983; Herzberg and Elimelech 2007; Chong et al. 2008; Herzberg et al. 2009; Khan et al. 2013b; Jeong et al. 2012, 2013a; Lee et al. 2010; Baek et al. 2011; Suwarno et al. 2012; Alzahrani et al. 2013; Chen et al. 2013; Kwan et al. 2015). Comparison of EPS in feed solutions and in foulants also showed the preferential adsorption/accumulation of polysaccharide on the membrane surface when forming the fouling layer (Herzberg et al. 2009). ATP analysis of foulants, on the other hand, showed differences in fouling layers extracted from membranes elements from a seawater RO (SWRO) plant and a wastewater RO (WWRO) plant. It was found that generally, lead elements have greater biomass concentration than terminal modules and that the lead elements of the WWRO plant have more developed and mature biofilms than the lead elements of the SWRO plant (Khan et al. 2014). Evaluation of ATP concentration in foulants also showed that higher ATP content leads to higher fouling load, signifying biofilm development as the RO operation progresses (Khan et al. 2013a). ATP was also reported to be a better parameter for biomass concentration than TDC and HPC since ATP values are less scattered upon analysis of biofilm in lead elements from membrane installations (Vrouwenvelder et al. 2008).
The membranes, on the other hand, whether virgin or fouled membranes are usually analyzed for surface characteristics and the observed characteristics correlated to biofilm structure, adhesion, attachment, and growth, as well as biofouling kinetics and mechanism, which then can give insight on designing proper biofouling control strategies (Khan et al. 2010, 2011, 2013a, b, 2014; Herzberg and Elimelech 2007; Herzberg et al. 2009; Norberg et al. 2007; Xu et al. 2010; Jeong et al. 2013a; Lee et al. 2010; Baek et al. 2011; Alzahrani et al. 2013; Subramani and Hoek 2008, 2010). Table 18.2 shows a summary of the advantages and disadvantages of the membrane characterization methods discussed in this section. Hydrophilic/hydrophobic properties are usually determined by measuring contact angles, usually through the sessile drop method using a goniometer (Subramani et al. 2009; Norberg et al. 2007; Xu et al. 2010; Zhao et al. 2010; Jeong et al. 2013a; Lee et al. 2010; Baek et al. 2011; Subramani and Hoek 2008, 2010; Khan et al. 2010, 2011). Multiple probe liquid contact angle measurements were also used to describe membrane surface energies for bacterial cells and membranes (Subramani and Hoek 2008, 2010). A higher contact angle usually represents a higher hydrophobicity of the membrane surface (Jeong et al. 2013a). Studies showed that more hydrophobic membrane surfaces are more favorable to initial bacterial adhesion and attachment, have higher cell deposition rates and hence, have the greater amount of accumulated bacterial cells (Norberg et al. 2007; Xu et al. 2010; Jeong et al. 2013a; Lee et al. 2010; Subramani and Hoek 2008, 2010; Khan et al. 2011). It has also been reported that the exposure to cleaning agents altered membrane surface characteristics, and that only sodium hypochlorite effectively broke the adhesive bonds between bacterial EPS and membrane surfaces, essentially cleaning the membrane (Subramani and Hoek 2010). The typical method used to evaluate membrane surface topography is atomic force microscopy (AFM) and results are usually associated with surface roughness (Khan et al. 2013a; Subramani et al. 2009; Norberg et al. 2007; Xu et al. 2010; Zhao et al. 2010; Jeong et al. 2013a; Lee et al. 2010; Baek et al. 2011; Alzahrani et al. 2013; Subramani and Hoek 2008, 2010; Khan et al. 2010, 2011). A rough membrane surface, in the case of virgin membranes, is assumed to have a greater potential to foul and vice versa (Tang et al. 2011). This assumption has been clearly demonstrated in numerous studies, which showed that the rougher RO surfaces were more prone to bacterial cell and EPS attachment, had higher microbial deposition rates and had a greater accumulation of biofoulants resulting in thicker biofilms (Khan et al. 2013a; Norberg et al. 2007; Zhao et al. 2010; Jeong et al. 2013a; Lee et al. 2010; Baek et al. 2011; Subramani and Hoek 2008, 2010; Khan et al. 2010, 2011). It was reported that organic layers forming on the RO membrane resulted in a rougher surface and had greater bacterial adhesion (Subramani et al. 2009). AFM measurements were also used to provide qualitative and quantitative information on foulant-membrane and foulant-foulant interactions. Herzberg and co-workers (Herzberg et al. 2009) showed an increase in the adhesion forces between a carboxylated-modified latex particle and membranes fouled with EPS upon additions of calcium ions, suggesting the influence of other substances in the biofouling of RO membranes.
Epifluorescence microscopy is the typical method used for determining TDC in water samples (Hobbie et al. 1977) but it can also be used for the analysis of extracted foulants from biofilms to give TDC (Vrouwenvelder et al. 2008). Owing to the emergence of a variety of fluorochromes, applications of fluorescence microscopy in biofilm studies have extended beyond total bacterial count. When coupled with a specific fluorescent staining dye, epifluorescence microscopy can provide not only total bacterial counts, but also bacterial activity, viability, and 2D distribution of bacteria (Vrouwenvelder et al. 2008; Lee et al. 2010; Subramani and Hoek 2010; Wolf et al. 2002). The disadvantage of epifluorescence microscopy is its limited application to thin biofilms only (McFeters et al. 1995). Confocal laser scanning microscopy (CLSM) coupled with fluorescence dye staining, on the other hand, can provide 3D spatial arrangement within the biofilms, characterize bacterial groups or strains in the biofilm, and is an important tool in assessing the structure-function relationships in biofilms (Wolf et al. 2002). Similar to epifluorescence microscopy, CLSM measurements were used to provide TDC and viability information from extracted foulants (Jeong et al. 2012, 2013a). Because CLSM can analyze thicker biofilms than epifluorescence microscopy, with a suitable imaging software, cut pieces of fouled membranes or membrane coupons can be analyzed for biofilm amount, components, and viability, as well as biofilm characteristics such as thickness, biovolumes, and percentage coverage, and can give insights into biofilm formation and mechanism (Khan et al. 2013a; Herzberg et al. 2009; Vrouwenvelder et al. 2008; Huang et al. 2013; Lee et al. 2010; Berman et al. 2011; Chen et al. 2013). Using the biofilm characteristics obtained by CLSM measurements, it was revealed that an abrupt rise in TMP coincides with biofilm formation (Chen et al. 2013), indicating performance decline due to biofouling. Limitations of the CLSM technique include the restricted number of available fluorescent filter combinations in contrast to epifluorescence microscopy and is relatively time-consuming due to the slow scanning of the laser for the production of high-quality images (Wolf et al. 2002). And though it can analyze thicker biofilms than epifluorescence microscopy, it is not suited for the analysis of very thick and opaque biofilms (McFeters et al. 1995).
It has been shown that the biofilm morphology and accumulation of biofoulants are dependent on membrane surface properties (Khan et al. 2010, 2011; Subramani and Hoek 2010) and it is established that bacterial cell and membrane surface interactions play a significant role in initial bacterial adhesion and thus biofilm formation (Habimana et al. 2014). With this in mind, biofouling potential tests incorporating the properties of the RO membrane used and the water quality of the feed water are more reliable methods for determining biofouling propensity than a single water quality index (Manalo et al. 2016). NF and RO membranes analyzed by fluorescence microscopy showed accelerated growth rate and formation of viable cells within a period of only 5–10 h, suggesting this as the critical time period for biofilm growth and development (Lutskiy et al. 2015). This signifies that the assessment of the susceptibility of membranes to biofilm formation can be done at a significantly shorter period of time. Fluorescence intensity using the microplate reader as a measure of biofilm formed on the RO membrane surface after soaking cut pieces of the membrane in secondary effluent water, which was enhanced with microorganisms and glucose for accelerated biofilm formation, proved to be a reliable biofouling potential method that incorporates fouling tendencies of both feed water and the RO membrane (Manalo et al. 2016). These studies show that surface membrane analysis of biofilm content like fluorescence analytical methods can be utilized as an indicator of biofouling potential of both RO membranes and feed water. However, as much as these tests can be used as biofouling potential methods, the inclusion of RO system performance analyses in detecting biofouling will give the greater advantage of predicting biofouling in a smaller scale but at an earlier time, such that biofouling in the RO plants can be mitigated or controlled.
2.3 Fouling Simulators and Canary Cells
Biofouling potential methods mentioned in the previous sections are not predictive enough to ascertain that biofouling event during the RO process will occur. Using flow cells is very important in biofouling studies since they can simulate hydrodynamic conditions used in real RO plants, and thereby mimic the fouling events. Specifically applied for biofouling studies, a flow cell unit with a transparent window for microscopic viewing of fouling in real-time was developed [Fig. 18.2; (Subramani and Hoek 2008)]. Images taken by a camera are later translated into percentage of coverage of cells deposited on the membrane surface and results showed that cell deposition rate was influenced by permeate flux, membrane resistance, concentration polarization, and membrane surface characteristics such as roughness and hydrophobicity (Subramani and Hoek 2008). It was further improved by incorporating into the high-pressure membrane flow cell an optical microscope with fluorescence imaging as an external monitoring device for detecting biofouling development and cleaning [Fig. 18.3; (Huang et al. 2010)]. The new optical membrane module proves the importance of flow cells that are able to simulate practical seawater desalination operating conditions for the evaluation of the influence of these operating parameters on bacterial adhesion and removal in small-scale laboratory studies (Huang et al. 2010).
a Direct observation module made from two polycarbonate plates with a 2-mm thick glass window and b flow system operating at applied pressures up to 300 psi. The module is placed on the fixed stage of a microscope equipped with a 10× objective and 4× CCD camera to obtain real-time observations during the experiment through a transparent window on the top plate of the module (Subramani and Hoek 2008)
The improved high-pressure optical module made of (a) stainless steel top plate with an optical window and (b) stainless steel bottom plate, and (c) a schematic diagram of the optical membrane module integrated with pressure, flow, and microscopic observation components. The module withstands pressures up to 1200 psi while allowing high transmission of UV and visible light (Huang et al. 2010)
Biofouling detection, while the RO filtration is in operation, is vital to avoid shutting down the process due to irreversible fouling, which in the end, sacrifices the membrane element for an autopsy. Because indicators of fouling while the RO process is ongoing are operational parameters such as flux decline due to increase in TMP, worsening product quality, usually determined as a decrease in salt rejection, and pressure drop across the module, flow cell devices that can monitor the fouling state of the membranes as well as the performance of the membrane unit are in great need (Fig. 18.4). The flow cell devices, when combined with non-invasive methods for fouling detection has the ability to gather information and monitor the fouling state of a filtration process in real-time. These devices, which should be placed in strategic locations, can be used to determine when membrane cleaning is already warranted by the RO process, thereby saving the membrane systems from irreversible fouling and costly membrane replacement. Costs are also minimized when membrane cleaning is applied at the onset of fouling and not at the later stages, where optimum membrane performance can no longer be restored. These fouling detection devices can be directly employed within the module unit (in situ) or on small cell units attached as a side-stream (ex situ) (Sim et al. 2018).
An example of this device has been developed more than 10 years ago, the membrane fouling simulator (MFS, Fig. 18.5), wherein fouling was monitored by changes in the pressure drop, as well as visual, microscopic, and non-destructive observations through a transparent window (Vrouwenvelder et al. 2006). Sections of the membrane, feed and permeate spacers from the MFS and membrane modules were analyzed to evaluate the accumulated fouling layers. The transparent window allowed in situ visual and microscopic observations during the operation of the MFS and with a digital camera, images were captured showing the effect of a nutrient (acetate) on the accumulation of foulant material on the membrane [Fig. 18.6; (Vrouwenvelder et al. 2006)]. A recent study using the MFS units for comparing two RO systems operated in parallel employing different water types showed that biofouling trends in the MFS are similar to those as the spiral wound modules (SWM) in the RO system (Massons-Gassol et al. 2017), signifying the capability of the MFS unit as a biofouling indicator. Incorporation of non-invasive techniques into the MFS makes it a more lucrative tool for monitoring biofouling during operation. The optical coherence tomography, a relatively new and advanced monitoring technique was incorporated into the MFS to characterize the biomass in situ and non-destructively in UF membranes (Fortunato et al. 2017), in NF membranes (Fortunato and Leiknes 2017), and in RO membranes (West et al. 2016). However, since the MFS operates at a low pressure and usually without a permeation flux, it only gives a partial simulation of the conditions in the RO plant (Sim et al. 2018).
The developed membrane fouling simulator, MFS (external dimensions of 0.07 m × 0.30 m × 0.04 m) with a transparent window. The MFS was operated at a pressure of 100 kPa (~14.5 psi) under crossflow and no permeation conditions (Vrouwenvelder et al. 2006)
Visual in situ observations of the membrane and feed spacer in the MFS during operation through the transparent window (a) with 0.100 mg acetate-C/L and (b) without acetate dosage (after a run time of 6 days). The flow direction is from left to right. The original scale is 10.7 mm × 6.2 mm. Black arrows indicate darker colored material in the feed spacer crossings in MFS with added nutrient (Vrouwenvelder et al. 2006)
The canary cell, a side-stream RO flow cell, that simulates the SWM typically applied in RO plants was recently developed [Fig. 18.7; (Sim et al. 2015)]. It offers non-invasive monitoring of high-pressure colloidal fouling and cleaning and because of a dimensionless correlation curve for the cell and the SWM, it can function as an early warning device for fouling (Sim et al. 2015). Although it is primarily developed as a colloidal fouling monitor, it was reported to be capable of in situ, in real-time, and non-destructive monitoring of growth of biofilm using ultrasonic time-domain reflectometry (UTDR) by periodic dosing of silica (Sim et al. 2015). UTDR uses sound waves to locate biofilm on the membrane surface and based on the transmission and reflection of an ultrasonic waveform traveling through a particular media, for example, the biofilm, information on the physical characteristics of the biofilm can be gathered (Mairal et al. 2000). Other methods for monitoring the fouling state of the membrane for all fouling types have been reviewed and their strengths and capabilities presented by Sim and co-workers (Sim et al. 2018). They have highlighted the importance of fouling indices and monitoring techniques that can be incorporated into the RO process for a better knowledge of the fouling state of the membrane, even when the operation is ongoing.
Schematic diagram of the spiral wound module (SWM) and the canary cell with ultrasonic time domain reflectometry (UTDR). The canary cell was operated at similar crossflow velocities as the SWM under constant flux. The feed was delivered from a continuously stirred tank kept at 30° with cooling water from a chiller. The permeate fluxes for the SWM and the canary cell were held constant by two separate mass flow controllers (MFC). TMP was measured using pressure transducers and salt concentration of the feed and permeate for both SWM and canary cell was determined using a conductivity meter. The UTDR system consisted of a transducer, externally mounted on the cell, a digitizer, and a pulse-receiver (Sim et al. 2015)
3 Summary
There is no single assay for biofilm amount or membrane performance indicator that can accurately predict biofouling. Existing water quality parameters and surface membrane analysis can be used as biofouling potential tests but the incorporation of the hydrodynamic conditions happening in the RO plant is essential for a method to be able to predict biofouling. As a preventive measure, preempting the biofouling event enables the RO plant to take countermeasures to avoid irreversible biofouling, which once it has occurred necessitates costly membrane replacement instead of the less expensive membrane cleaning. The advantage of fouling simulators and canary cells cannot be ignored since these units give the fouling scenarios for the RO process and can serve as a warning in advance of the fouling event. It is evident that there is still a need for developing biofouling detection methods that are specific and function as sensitive indices for biofouling, are non-destructive and can provide results readily and timely.
References
Afonso MD, Jaber JO, Mohsen MS (2004) Brackish groundwater treatment by reverse osmosis in Jordan. Desalination 164:157–171
Alcamo J, Flörke M, Märker M (2007) Future long-term changes in global water resources driven by socio-economic and climatic changes. Hydrol Sci J 52:247–275
Alhadidi A, Kemperman AJB, Shurer R, Schippers JC, Wessling M, van der Meer WGJ (2012) Using SDI, SDI+ and MFI to evaluate fouling in a UF/RO desalination pilot plant. Desalination 285:153–162
Altmann J, Massa L, Sperlich A, Gnirss R, Jekel M (2016) UV254 absorbance as real- time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res 94:240–245
Alzahrani S, Mohammad AW, Hilal N, Abdullah P, Jaafar O (2013) Identification of foulants, fouling mechanisms and cleaning efficiency for NF and RO treatment of produced water. Sep Purif Technol 118:324–341
Ang WS, Tiraferri A, Chen KL, Elimelech M (2011) Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J Membr Sci 376:196–206
Arnell NW (2004) Climate change and global water resources: SRES emissions and socio-economic scenarios. Glob Environ Change 14:31–52
Baek Y, Yu J, Kim S-H, Lee S, Yoon J (2011) Effect of surface properties of reverse osmosis membranes on biofouling occurrence under filtration conditions. J Membr Sci 382:91–99
Baker JS, Dudley LY (1998) Biofouling in membrane systems – A review. Desalination 118:81–90
Bartels CR (2006) Reverse osmosis membranes for wastewater reclamation. http://www.waterworld.com/articles/wwi/print/volume-21/issue-6/features/reverse-osmosis-membranes-play-key-role-in-wastewater-reclamation.html. Last accessed 23 Dec 2015
Bar-Zeev E, Passow U, Castrillon SR-V, Elimelech M (2015) Transparent exopolymer particles: from aquatic environments and engineered systems to membrane biofouling. Environ. Sci. Technol. 49:691–707
Berman T, Mizrahi R, Dosoretz CG (2011) Transparent exopolymer particles (TEP): a critical factor in aquatic biofilm initiation and fouling on filtration membranes. Desalination 276:184–190
Bódalo-Santoyo A, Gómez-Carrasco JL, Gómez-Gómez E, Máximo-Martin MF, Hidalgo-Montesinos AM (2003) Application of reverse osmosis to reduce pollutants present in industrial wastewater. Desalination 155:101–108
Carstea EM, Bridgeman J, Baker A, Reynolds DM (2016) Fluorescence spectroscopy for wastewater monitoring: a review. Water Res 95:205–219
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence ex-citation—emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Chen X, Suwarno SR, Chong TH, McDougald D, Kjelleberg S, Cohen Y, Fane AG, Rice SA (2013) Dynamics of biofilm formation under different nutrient levels and the effect on biofouling of a reverse osmosis membrane system. Biofoul J Bioad Biofilm Res 29(3):319–330
Chen F, Peldszus S, Elhadidy AM, Legge RL, Van Dyke MI, Huck PM (2016) Kinetics of natural organic matter (NOM) removal during drinking water biofiltration using different NOM characterization approaches. Water Res 104:361–370
Choi J-S, Hwang T-M, Lee S, Hong S (2009) A systematic approach to determine the fouling index for a RO/NF membrane process. Desalination 238:117–127
Chong TH, Wong FS, Fane AG (2008) The effect of imposed flux on biofouling in reverse osmosis: role of concentration polarization and biofilm enhanced osmotic pressure. J Membr Sci 325:840–850
Dixon MB, Qiu T, Blaikie M, Pelekani C (2012a) The application of the bacterial regrowth potential method and flow cytometry for biofouling detection at the Penneshaw Desalination Plant in South Australia. Desalination 284:245–252
Dixon MB, Lasslett S, Pelekani C (2012b) Destructive and non-destructive methods for biofouling analysis investigated at the Adelaide Desalination Pilot Plant. Desalination 296:61–68
DOW (2010) FILMTEC™ reverse osmosis membranes—technical manual. The Dow Chemical Company. https://dowwater.custhelp.com/app/answers/detail/a_id/3428. Last accessed 03 Jan 2018
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Dunne WM (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15:155–166
Flemming H-C (1997) Reverse osmosis membrane biofouling. Exp Therm Fluid Sci 14:382–391
Flemming H-C (2002) Biofouling in water systems–cases, causes and countermeasures. Appl Microbiol Biotechnol 59:629–640
Flemming H-C, Geesey GG (eds) (1991) Biofouling and biocorrosion in industrial water systems. Springer, Berlin
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Fortunato L, Leiknes T (2017) In-situ biofouling assessment in spacer filled channels using optical coherence tomography (OCT): 3D biofilm thickness mapping. Bioresour Technol 229:231–235
Fortunato L, Bucs S, Linares RV, Cali C, Vrouwenvelder JS, Leiknes T (2017) Spatially-resolved in-situ quantification of biofouling using optical coherence tomography (OCT) and 3D image analysis in a spacer filled channel. J Membr Sci 524:673–681
Fritzmann C, Löwenberg J, Wintgens T, Melin T (2007) State-of-the-art of reverse osmosis desalination. Desalination 216:1–76
Ghaffour N, Missimer TM, Amy GL (2013) Technical review and evaluation of the economics of water desalination: current and future challenges for better water supply sustainability. Desalination 309:197–207
Global Water Intelligence (GWI/IDA DesalData) (2013) Market profile and desalination markets, 2009–2012 yearbooks and GWI website. http://www.desaldata.com/. Last accessed 20 June 2015
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43(9):2317–2348
Habimana O, Semião AJC, Casey E (2014) The role of cell-surface interactions in bacterial adhesion and consequent biofilm formation on nanofiltration/reverse osmosis membranes. J Membr Sci 454:82–96
Henderson RK, Baker A, Murphy KR, Hambly A, Stuetz RM, Khan SJ (2009) Fluorescence as a potential monitoring tool for recycled water systems: a review. Water Res 43:863–881
Herzberg M, Elimelech M (2007) Biofouling of reverse osmosis membranes: role of biofilm-enhanced osmotic pressure. J Membr Sci 295:11–20
Herzberg M, Kang S, Elimelech M (2009) Role of extracellular polymeric substances (EPS) in biofouling of reverse osmosis membranes. Environ Sci Technol 43:4394–4398
Hobbie JE, Daley RJ, Jasper S (1977) Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Holm-Hansen O, Booth CR (1966) The measurement of adenosine triphosphate in the ocean and its ecological significance. Limnol Oceanogr 11(4):510–519
Huang X, Guillen GR, Hoek EMV (2010) A new high-pressure optical membrane module for direct observation of seawater RO membrane fouling and cleaning. J Membr Sci 364(1–2):149–156
Huang S, Voutchkov N, Jiang SC (2013) Investigation of environmental influences on membrane biofouling in a Southern California desalination pilot plant. Desalination 319:1–9
Huber SA, Balz A, Abert M, Pronk W (2011) Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—organic carbon detection—organic nitrogen detection (LC-OCD-OND). Water Res 45:879–885
Hudson N, Baker A, Reynolds D (2007) Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—a review. River Res Appl 23:631–649
Huertas E, Herzberg M, Oron G, Elimelech M (2008) Influence of biofouling on boron removal by nanofiltration and reverse osmosis membranes. J Membr Sci 318(1–2):264–270
IDA (International Desalination Association) (2015) Desalination by the numbers. http://idadesal.org/desalination-101/desalination-by-the-numbers/. Accessed online on 22 Apr 2018
Jeong S, Kim L, Kim S-J, Nguyen TV, Vigneswaran S, Kim IS (2012) Biofouling potential reductions using a membrane hybrid system as a pre-treatment to seawater reverse osmosis. Appl Biochem Biotechnol 67(6):1716–1727
Jeong S, Kim S-J, Kim LH, Shin MS, Vigneswaran S, Nguyen TV, Kim IS (2013a) Foulant analysis of a reverse osmosis membrane used pretreated seawater. J Membr Sci 428:434–444
Jeong S, Naidu G, Vigneswaran S, Ma CH, Rice SA (2013b) A rapid bioluminescence-based test of assimilable organic carbon for seawater. Desalination 317:160–165
Jeong S, Naidu G, Vollprecht R, Leiknes T, Vigneswaran S (2016) In-depth analyses of organic matters in a full-scale seawater desalination plant and an autopsy of reverse osmosis membrane. Sep Purif Technol 162:171–179
Khan MMT, Stewart PS, Moll DJ, Mickols WE, Burr MD, Nelson SE, Camper AK (2010) Assessing biofouling on polyamide reverse osmosis (RO) membrane surfaces in a laboratory system. J Membr Sci 349:429–437
Khan MMT, Stewart PS, Moll DJ, Mickols WE, Nelson SE, Camper AK (2011) Characterization and effect of biofouling on polyamide reverse osmosis and nanofiltration membrane surfaces. Biofoul J Bioad Biofilm Res 27:173–183
Khan MT, Manes C, Ld O, Aubry C, Gutierrez L, Croué J-P (2013a) Kinetic study of seawater reverse osmosis membrane fouling. Environ Sci Technol 47:10884–10894
Khan MT, Manes CLO, Aubry C, Croué J-P (2013b) Source water quality shaping different fouling scenarios in a full-scale desalination plant at the Red Sea. Water Res 47(2):558–568
Khan MT, Busch M, Molina VG, Emwas A-H, Aubry C, Croue J-P (2014) How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes? Water Res 59:271–282
Kimura K, Oki Y (2017) Efficient control of membrane fouling in MF by removal of biopolymers: comparison of various pretreatments. Water Res 115:172–179
Kwan SE, Bar-Zeev E, Elimelech M (2015) Biofouling in forward osmosis and reverse osmosis: measurements and mechanisms. J Membr Sci 493:703–708
Lee W, Ahn C, Hong S, Kim S, Lee S, Baek Y, Yoon J (2010) Evaluation of surface properties of reverse osmosis membranes on the initial biofouling stages under no filtration condition. J Membr Sci 351:112–122
Lee KP, Arnot TC, Mattia D (2011) A review of reverse osmosis membrane materials for desalination—development to date and future potential. J Membr Sci 370(1–2):1–22
Levine AD, Asano T (2004) Recovering sustainable water from wastewater. Environ Sci Technol 38(11):201A–208A
Lutskiy M-Y, Avneri-Katz S, Zhu N, Itsko M, Ronen Z, Arnusch CJ, Kasher R (2015) A microbiology-based assay for quantification of bacterial early stage biofilm formation on reverse-osmosis and nanofiltration membranes. Sep Purif Technol 141:214–220
Mairal AP, Greenberg AR, Krantz WB (2000) Investigation of membrane fouling and cleaning using ultrasonic time-domain relectometry. Desalination 130:45–60
Malaeb L, Ayoub GM (2011) Reverse osmosis technology for water treatment: state of the art review. Desalination 267:1–8
Manalo CV, Ohno M, Okuda T, Nakai S, Nishijima W (2016) Rapid novel test for the determination of biofouling potential on reverse osmosis membranes. Water Sci Technol 73(12):2978–2985
Mansouri J, Harrisson S, Chen V (2010) Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem 20:4567–4586
Massons-Gassol G, Gilabert-Oriol G, Johnson J, Arrowood T (2017) Comparing biofouling development in membrane fouling simulators and spiral-wound reverse osmosis elements using river water and municipal wastewater. Ind Eng Chem Res 56(40):11628–11633
Matin A, Khan Z, Zaidi SMJ, Boyce MC (2011) Biofouling in reverse osmosis membranes for seawater desalination: phenomena and prevention. Desalination 281:1–16
McFeters GA, Yu FP, Pyle BH, Stewart PS (1995) Physiological methods to study biofilm disinfection. J Ind Microbiol 15:333–338
Meng S, Rzechowicz M, Winters H, Fane AG, Liu Y (2013) Transparent exopolymer particles (TEP) and their potential effect on membrane biofouling. Appl Microbiol Biotechnol 97:5705–5710
Miller GW (2006) Integrated concepts in water reuse: managing global water needs. Desalination 187:65–75
Miyoshi T, Hayashi M, Shimamura K, Matsuyama H (2016) Important fractions of organic matter causing fouling of seawater reverse (SWRO) membranes. Desalination 390:72–80
Monnot M, Nguyên HTK, Laborie S, Cabassud C (2017) Seawater reverse osmosis desalination plant at community-scale: role of an innovative pretreatment on process performances and intensification. Chem Eng Process Process Intensif 113:42–55
Mosset A, Bonnelye V, Petry M, Sanz MA (2008) The sensitivity of SDI analysis: from RO feed water to raw water. Desalination 222:17–23
Nielsen PH, Jahn A (1999) Extraction of EPS. In: Wingender J, Flemming H-C, Neu TR (eds) Microbial extracellular polymeric substances: characterization, structure, and function. Springer, New York, p 49
Norberg D, Seungkwan H, James T, Yu Z (2007) Surface characterization and performance evaluation of commercial fouling resistant low-pressure RO membranes. Desalination 202:45–52
Pandey SR, Jegatheesan V, Baskaran K, Shu L (2012) Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: a review. Rev Environ Sci Biotechnol 11:125–145
Passow U, Alldredge AL (1995) A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol Oceanogr 40:1326–1335
Potter BB, Wimsatt JC (2005) Method 415.3—measurement of total organic carbon, dissolved organic carbon and specific UV absorbance at 254 in source water and drinking water. U.S. Environmental Protection Agency, Washington, DC. https://cfpub.epa.gov/si/si_public_record_report.cfm?direntryid=103917. Last accessed 08 July 2018
Pype M-L, Patureau D, Wery N, Poussade Y, Gernjak W (2013) Monitoring reverse osmosis performance: conductivity versus fluorescence excitation-emission matrix (EEM). J Membr Sci 428:205–211
Rachman RM, Ghaffour N, Wali F, Amy GL (2013) Assessment of silt density index (SDI) as fouling propensity parameter in reverse osmosis (RO) desalination systems. Desalin. Water Treat 51(4–6):1091–1103
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Ridgway HF, Kelly A, Justice C, Olson BH (1983) Microbial fouling of reverse-osmosis membranes used in advanced wastewater treatment technology: chemical, bacteriological, and ultrastructural analyses. Appl Environ Microbiol 45:1066–1084
Schneider RP, Ferreira LM, Binder P, Bejarano EM, Góes KP, Slongo E, Machado CR, Rosa GMZ (2005) Dynamics of organic carbon and of bacterial populations in a conventional pretreatment train of a reverse osmosis unit experiencing severe biofouling. J Membr Sci 266:18–29
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310
Sim STV, Krantz WB, Chong TH, Fane AG (2015) Online monitor for the reverse osmosis spiral wound module—development of the canary cell. Desalination 368:48–59
Sim LN, Chong TH, Taheri AH, Sim STV, Lai L, Krantz WB, Fane AG (2018) A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 434:169–188
Subramani A, Hoek EMV (2008) Direct observation of initial microbial deposition onto reverse osmosis and nanofiltration membranes. J Membr Sci 319:111–125
Subramani A, Hoek EMV (2010) Biofilm formation, cleaning, re-formation on polyamide composite membranes. Desalination 257:73–79
Subramani A, Huang XF, Hoek EMV (2009) Direct observation of bacterial deposition onto clean and organic-fouled polyamide membranes. J Colloid Interface Sci 336:13–20
Suwarno SR, Chen X, Chong TH, Puspitasari VL, McDougald D, Cohen Y, Rice SA, Fane AG (2012) The impact of flux and spacers on biofilm development on reverse osmosis membranes. J Membr Sci 405–406:219–232
Tang CY, Kwon Y-N, Leckie JO (2009) The role of foulant–foulant electrostatic interaction on limiting flux for RO and NF membranes during humic acid fouling—theoretical basis, experimental evidence, and AFM interaction force measurement. J Membr Sci 326(2):526–532
Tang CY, Chong TH, Fane AG (2011) Colloidal interactions and fouling of NF and RO membranes: a review. Adv Colloid Interface Sci 164:126–143
Teng CK, Hawlader MNA, Malek A (2003) An experiment with different pretreatment methods. Desalination 156:51–58
Toze S (2006) Reuse of effluent water—benefits and risks. Agric Water Manag 80(1–3):147–159
Van der Kooij D (1992) Assimilable organic carbon as an indicator of bacterial regrowth. J Am Water Works Assoc 84:57–65
Veza JM, Ortiz M, Sadhwani JJ, Gonzales JE, Santana FJ (2008) Measurement of biofouling in seawater: some practical tests. Desalination 220:326–334
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Vrouwenvelder JS, Van der Kooij D (2001) Diagnosis, prediction and prevention of biofouling of NF and RO membranes. Desalination 139:65–71
Vrouwenvelder JS, Kappelhof JWNM, Heijman SGJ, Schippers JC, Van der Kooij D (2003) Tools for fouling diagnosis of NF and RO membranes and assessment of the fouling potential of feed water. Desalination 157:361–365
Vrouwenvelder JS, van Paassen JAM, Wessels LP, van Dam AF, Bakker SM (2006) The membrane fouling simulator: a tool for fouling prediction and control. J Membr Sci 281:316–324
Vrouwenvelder JS, Manolarakis SA, van der Hoek JP, van Paassen JAM, van der Meer WGJ, van Agtmaal JMC, Prummel HDM, Kruithof JC, van Loosdrecht MCM (2008) Quantitative biofouling diagnosis in full scale nanofiltration and reverse osmosis installations. Water Res 42:4856–4868
Weinrich L, LeChevallier M, Haas CN (2016) Contribution of assimilable organic carbon to biological fouling in seawater reverse osmosis membrane treatment. Water Res 101:203–213
West S, Wagner M, Engelke C, Horn H (2016) Optical coherence tomography for the in situ three-dimensional visualization and quantification of feed spacer channel fouling in reverse osmosis membrane modules. J Membr Sci 498:345–352
Wilf M, Alt S (2000) Application of low fouling RO membrane elements for reclamation of municipal wastewater. Desalination 132:11–119
Wolf G, Crespo JG, Reis MAM (2002) Optical and spectroscopic methods for biofilm examination and activity analysis in water and wastewater treatment. Rev Environ Sci Biotechnol 1:227–251
WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report (2015) Water for a sustainable world. Paris, UNESCO. http://unesdoc.unesco.org/images/0023/002318/231823E.pdf. Last accessed 20 Dec 2015
Xu P, Bellona C, Drewes JE (2010) Fouling of nanofiltration and reverse osmosis membranes during municipal wastewater reclamation: membrane autopsy results from pilot-scale investigations. J Membr Sci 353(1–2):111–121
Zhao Y, Song L, Ong SL (2010) Fouling behavior and foulant characteristics of reverse osmosis membranes for treated secondary effluent reclamation. J Membr Sci 349(1–2):65–74
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Manalo, C.V., Nishijima, W. (2019). Biofouling Detection on Reverse Osmosis Membranes. In: Bui, XT., Chiemchaisri, C., Fujioka, T., Varjani, S. (eds) Water and Wastewater Treatment Technologies. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3259-3_18
Download citation
DOI: https://doi.org/10.1007/978-981-13-3259-3_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3258-6
Online ISBN: 978-981-13-3259-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)