Abstract
Biofouling is referred to as the unwanted deposition and growth of biofilms, which leads to increase of operating pressure, more frequent chemical cleaning, and shorter membrane lifespan. This chapter aims to provide a comprehensive overview of reverse osmosis (RO) membrane biofouling in desalination applications. Firstly, the necessity of implementation of RO technique for water treatment and reclamation to combat water scarcity is briefly introduced along with the basic transport mechanisms, membrane types, and major challenges (fouling) hindering practical application. Afterwards, the phenomenon of biofouling is discussed describing the formation of biofilms, the role of extracellular polymeric substances (EPS), and crucial factors affecting biofilms. This is followed by a section related to the impact of biofouling on membrane processes with emphasises on permeate water flux and salt rejection. The performance degradation mechanism and enhanced energy consumption are also discussed in this part. Finally, the chapter concludes with a summary with the significance of better understanding of membrane biofouling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The global freshwater crisis is considered as one of the most critical challenges currently faced by the international community. Wastewater reuse and seawater desalination have been considered as highly feasible ways to alleviate global water scarcity. In desalination processes, the cost of water production by seawater reverse osmosis (SWRO) is reported to be one-half to one-third of the cost of thermal distillation [1]. Reverse osmosis (RO) has proven to be a competitive technology for various types of wastewater reclamation and brackish/seawater desalination. RO is preferred for its superior efficiency in the removal of small-sized contaminants (salt, metal ions, pharmaceuticals, organic colloid, etc.), smaller footprints [2] as well as for lower capital and operating costs when compared to traditional treatment methods e.g., thermal distillation [3].
RO is a high-pressure membrane-based process which utilizes a dense membrane to separate water from molecular-sized contaminants such as dissolved organic compounds, colloids, and monovalent ions (e.g., Na+, Cl−) [4]. In microfiltration (MF ) and ultrafiltration (UF) processes, membrane pore structures are designed to remove contaminants based upon their size (size exclusion mechanism) [5], and only the contaminants which are larger than membrane pore sizes are retained as shown in Fig. 13.1 (a). The pore size of RO membrane is about 0.1 nm where “solution-diffusion” is the main mechanism [6, 7] for water transport as shown in Fig. 13.1 (b).
The semi-permeable membrane is the core component of the RO process. The first generation of commercially available RO membranes were developed in the 1960s using cellulose acetate (CA) [8]. In the early 1980s, a polyamide (PA) casted membrane with a thin film composite structure (TFC) was introduced by the Film Tec corporation [9]. TFC membranes displayed higher water permeability, operated at higher temperatures , and operated at higher pressures than CA membranes, while also using wider range of pH values. TFC is still regarded as a “state-of-art” material in RO process [8]. PA is a widely used material for the fabrication of commercial TFC membranes. PA active layers can be formed through cross-linking between trimesoyl chloride (TMC) and m-phenylene diamine (MPD) [10].
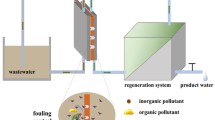
Despite having a high-quality permeate product, one of the major limitations that hinders the widespread application of RO is membrane fouling . Feed water in RO systems generally contains four main types of contaminants: inorganic compounds (salts, metal hydroxide, metal carbonate, etc.), natural organic matter (NOM), gel-colloids, and microorganisms that can cause four different categories of membrane fouling: inorganic fouling /scaling, organic fouling , particulate fouling, and biofouling respectively. During long operational periods, these contaminants may reside on the membrane surface and form an additional fouling layer, which jeopardizes membrane performance (Fig. 13.2). Periodic physical/chemical cleaning is necessary [11] to maintain the desired flux of the RO membrane. The cleaning actions, especially by chemicals, shortens the membranes life. The membrane fouling has significant economic impact on RO plant operation as it accounts for about 50% of the total costs [12] via cleaning, loss of operation due to cleaning, and increasing pressures to maintain constant flux through the clogged membrane. RO desalination is extensively used in Middle East, and around 70% of these desalination plants experience biofouling [12, 13] due to the water in the Gulf region having high organic content, considerable amount of microorganisms, and total dissolved solids (TDS) content. As biofouling account up to 35–45% of all fouling in the RO process [14], the comprehensive understanding of biofouling of RO membranes is crucial for effective biofouling management. This chapter will provide an insight into the mechanism of biofouling, formation of biofilms , role of EPS , and critical factors affecting the biofilms. Also, biofouling impact on permeate water flux and salt rejection is further discussed along with performance degradation mechanism and energy consumption .
2 Biofouling
The unwanted deposition /growth of microorganisms on or within the membrane surface results in biofouling of the membrane. Generally, any fouling resulting from microbial colonization and biofilm formation is considered biofouling. Very few organisms present in the feed water can lead to significant biofouling of the membrane. Even membranes using pre-treated influent with substantial removal of microbe is susceptible to significant biofouling [14, 15]. Biofouling causes membrane flux decline, membrane biodegradation, enhanced salt passage, increased differential and feed pressure, permeate quality degradation, the necessity of frequent cleaning which eventually would result in high treatment cost, and often process failure. Bacteria, fungi, and yeasts are the primary microorganisms causing biofouling. The bacteria can tolerate a wide range of pH (0.5–13) and temperature (-12–110 °C) while being able to colonize on all membrane surfaces in RO plants in varying conditions [16]. Table 13.1 represents frequently observed microorganism on membranes in RO plants.
2.1 Mechanism of Biofilm Formation
Accumulation of microbial cells on/within the membrane surface along with a matrix of extracellular polymeric substances (EPS) is known as biofilm. In general, microbes are abundant in all water systems and can colonize rapidly on favourable surfaces. The microbes get attached on the membrane surface and grow due to the presence of nutrients in the feed/influent. Eventually, the microbes excrete EPS, in which they are embedded, and form further biofilm.
Biofilm formations are usually a complex multistage process that can be reversible or irreversible. The development of biofilms on membrane surfaces usually follows the following steps [18], as illustrated in Fig. 13.3:
-
(1)
Adsorption and attachment of cells on the membrane surface altering the membrane properties and forming the conditioning film.
-
(2)
Aggregation and growth of new cells which are controlled by different chemical and physical factors with hydrophobic and non-polar surfaces enhancing the irreversible attachment along with excretion of EPS.
-
(3)
Formation of microbial colonies and biofilm development and maturation with the continuous production of EPS .
-
(4)
Three-dimensional growth and further maturation of the biofilm, and
-
(5)
Detachment or release of cells from the matrix of biofilm to form new colonies on new locations.
Different stages of biofilm development. Adapted with permission from [19]; Copyright 2007 © Don Monroe, Creative Commons CC BY
During the initial induction phase, the attachment can occur on membranes in as early as 2 h. A logarithmic microbial growth phase occurs after the adhesion and primary colonization of microbes from the initial induction phase. The growth phase is subsequently followed by the nutrient controlled plateau phase where the membrane is covered by biofilm [12]. The plateau phase attains a balance between biofilm growth and cell detachment. Biofilm growth and cell detachment are governed by nutrient concentration, the resultant growth rate, the mechanical stability of the biofilm, and the effective shear force on the biofilm.
Biological substances are unavoidable in any water treatment environment. Even if 99.9% of the bacteria are destroyed in the pre-treatment process [20], those entering the RO system still deposit on the membrane surface and start the formation of a biofilm. Due to the non-porous layer, almost all organic molecules (organic acids, proteins, polysaccharides, etc.) can be retained on the membrane surface during the filtration process. The adhered microbes may utilize these organic compounds as a source of nutrients to multiply further and form more microbial colonies.
2.2 Role of Extracellular Polymeric Substances (EPS)
EPS are the metabolites generated during the cell growth process, consisting mainly of polysaccharides, proteins, lipids, humic substances, and DNA [21]. It is reported that EPS accounts for 50–90% of the organic compounds in a biofilm [22]. The EPS encases cells into its polymeric structure and changes the physical-chemical properties (hydrophilicity, zeta potential, surface energy, roughness, etc.) of a membrane surface, which in turn may cause more settlement and deposition of organic contaminants. Accumulated bacteria may be further released from the colony and relocate onto other parts of the membrane surface, starting a new bacterial colony and further spreading the biofilm.
The gradual growth of bacterial colonies within the EPS polymer eventually forms an intact and stable bio-layer across the membrane surface. EPS not only enhances the adhesion of the biofilm but also shields the microorganism from the biocidal components of the cleaning process [23]. Long-term growth of the biofilm can degrade membrane materials and cause irreversible fouling on the RO membrane [13]. It has also been observed that the commonly used disinfectant sodium hypochlorite is only effective against free bacterial cells and exhibited only slight inactivation ability against biofilm capsuled cells [23]. Biofouling not only affects the membrane’s lifespan but also adds an energy burden which consequently impedes the widespread application of RO technique [18].
2.3 Crucial Factors
The biofouling of reverse osmosis membranes results in the performance degradation of the RO plants. The structure and composition of the biofilm on the membrane surface have considerable effect on the RO desalting system performance. The crucial factors that need to be considered to better understand the biofilm formations are: the membrane surface type, the microbial driving force, interactions between surfaces and microorganisms , and the factors affecting microbial adhesion.
-
(a)
Surface type
The microbial adhesion can occur on two different surfaces through two different mechanisms, either the pristine membrane surfaces (macroscopic adhesion) or a surface covered with a conditioned film like protein layer covering the membrane surface (microscopic adhesion) [24]. Macroscopic adhesion is due to the macroscopic properties of the pristine membranes, such as surface charge , hydrophilicity, etc., that governs the microbial adhesion on the pristine membrane surface [25, 26]. Microscopic interaction controls the interaction between the conditioning film and microorganisms [24]. This specific interaction is known as “ligand-receptor bond” where the receptor is the protein molecules present on the conditioning film of the membrane [27] and ligand is the substance that binds with the receptor. The interaction of microorganisms with membrane properties, such as surface charge becoming altered by the presence of conditioning films on the membrane surface [28], often can result in enhanced cell attachment to the surfaces [29].
-
(b)
Driving force
Different types of driving forces (Fig. 13.4), control the microbial adhesion on the membrane surfaces:
-
Hydrodynamic force
-
Physicochemical interactions between the microbes and membrane surface
-
Ligand-receptor interactions
-
Adhesive interaction
Driving forces that control the microbial adhesion on the membrane surface with (1) Hydrodynamic force (2) Physico-chemical interactions between the microbes and membrane surface, (3) Ligand-receptor interactions, and (4) Adhesive interaction; Reproduced with permission from [30]; Copyright 2012 @ Elsevier
The hydrodynamic forces of convection and diffusion transports the microbes from the wastewater towards the membrane surface. Once the microorganisms reach the vicinity of the membrane, the physicochemical interactions play a crucial role in microbial adhesion on the membrane surface [25]. Initial adhesion and secondary adhesion are the two steps of microbial adhesion on the RO membrane surface. The initial adhesion occurs due to the physicochemical interactions between the membrane surface and the microbes, whereas the secondary adhesion results from the interaction between the adhered (on the membrane surface) microbes and the suspended (in solution) microbes [31]. The conditioning film layers have binding sites where the ligand-receptor interaction between the receptors of the cell membranes and the binding sites (e.g., polarized bonds, charged groups or OH groups) occurs [32]. Moreover, the adhesive nature of the EPS and the appendages of some microbes cause the adhesive interactions which can facilitate the cell attachment on the membrane surface [32]. The interaction of microbial adhesion on the membrane surface occurs when there is an attraction between microorganisms and the membrane surface (negative total free energy of the interaction exists) [31].
Different physical and chemical factors affect the transport and attachment of microorganisms , such as the mass transport condition, pH and ionic strength of the solution, surface charges , surface hydrophobicity /hydrophilicity, surface roughness , nutrient and EPS concentrations, the amount of the microorganisms, etc.
Mass transport condition affects microorganism growth and build-up on the membrane surface as well as the shear force generation. Enhanced shear force hinders microbe adhesion and restrains microbial growth on the membrane surface, thus reducing biofouling [30]. The electrostatic double layer interaction between the membrane and microorganisms is influenced by solution pH, which has significant impact on the colloids’ charge [31]. In addition to solution pH, ionic strength of the solution is another key parameter affecting the electrostatic double layer interaction between the membrane and the microorganisms . As substantial amount of microbes contains negative charges which would repel the microbes from the negatively charged membrane [30]. Hydrophobicity , hydrophilicity, and surface roughness has a profound impact on biofilm formation as they influence the interaction of microbes with the membrane surfaces. In general, hydrophilic membranes interact more with water whereas hydrophobic membranes interact more with microbial matter. Rough surfaces of the membranes contain a greater quantity of sites as well as more surface area available for microbial attachment and adhesion. Moreover, the rough surfaces lead towards a reduction of the van der Waals and electrostatic double layer interactions of the membrane [31]. As the nutrients facilitate the growth of microorganisms, the decreasing nutrient concentrations in the feed stream/influent will hinder biofouling development. Studies have found that enhanced carbon concentrations in the feed cause lower microbial mass as it decreases the time of the initial growth of the microorganisms [31]. Increased amounts of microorganisms play a crucial role in biofouling as the probability of adhesion and growth of microbes is higher along with enhanced EPS concentration [31]. The development of biofilm is also dependent on the redox potential and carbon, nitrogen, and phosphorous (C:N:P) ratio. In addition, the growth rate of microorganisms depends on the following factors [13, 33]:
-
(1)
feed water quality
-
(2)
temperature
-
(3)
pH
-
(4)
dissolved oxygen content
-
(5)
the presence of organic and inorganic nutrients
-
(6)
pollution
-
(7)
depth and location of the intake
3 Biofouling Impact on RO Membranes Performance
Biofouling will have many negative impacts on RO membrane system. Biofilm formed on an RO membrane surface can act as an extra thin layer on the membrane that increases the concentration polarization on the membrane and reduces the efficiency of the conventional transport processes (Fig. 13.5) [12, 34].
Schematic representation of the fouled RO membrane; Reproduced with permission from [34]; Copyright 1997 @ Elsevier
The adverse consequences of the biofilm formed on membrane surface are as follows [12,13,14]:
-
(a)
Permeability declines on the RO membrane due to the formation of gel-like biofilm on RO membrane surface.
-
(b)
Reduces the salt rejection and quality of water production due to the accumulation of dissolved ions on RO membrane surface.
-
(c)
Degrades the RO membrane materials and causes irreversible fouling on the RO membrane, and,
-
(d)
Increases energy consumption due to the higher-pressure requirement after the formation of biofilm.
Although biofouling has many adverse consequences on the RO membranes performance, the main concerns are the permeability decline, reduced salt rejection , and increased energy consumption .
3.1 Permeability Decline
Permeability decline is attributed to the formation of biofilm layer, which increases the hydraulic resistance and transmembrane osmotic pressure of the fouled membrane [35]. The decline rate depends on the physicochemical properties of the biofilm and microbiological properties of the feed water [36].
In most cases, permeability decline tends to exhibit two phases. Sharp permeability declines at the initial RO membrane separation stage followed by a smooth decline. The initial sharp permeability decline is attributed to the early deposited bacterial cells, which leads to increased trans-membrane osmotic pressure [35]. The biofilm layer is formed gradually at this initial stage. After that, the formation of biofilm and EPS production will reach a balanced state, which means there is an equilibrium in the loss of and growth of biofilm and EPS at the feed solution-membrane interface. In general, increased pressure is applied to compensate for the permeability decline and maintain constant water production.
In order to elucidate the mechanisms governing the decline in RO membrane performance caused by cell deposition and biofilm growth, a bench-scale investigation of RO biofouling with Pseudomonas aeruginosa PA01 was conducted by Herzberg [35]. The contribution of bacterial cells and EPS that impacts the permeability decline of RO membrane was evaluated by comparing the permeability decline of dead cell deposition in different solution mediums to the growth of biofilm on RO membrane (Fig. 13.6).
Normalized permeability decline upon deposition of formaldehyde fixed PA01 dead cells, PA01 biofilm growth (initial concentration of 107 cells/mL) on the RO membrane in wastewater medium (ionic strength of 14.6 mM and pH 7.4), and PA01 dead cells (initial concentration of 109 cells/mL) in wastewater medium (−1 and − 2 represent two replicates of fouling experiments conducted with the same synthetic wastewater used in the previous runs); Adapted with permission Ref. [35]; Copyright 2007 @ Elsevier
The decrease in flux (production of clean water) is higher for dead cells in the wastewater medium (ionic strength of 14.6 mM and pH 7.4) when compared with dead cells in deionized water with 0.01 mM LaCl3 at pH 5.8. This rapid decline was attributed to the high ionic strength of the wastewater medium. The sharp permeability declines of PA01 biofilm in the wastewater medium should be attributed to the growth of biofilm and production of EPS . The proposed mechanism of permeability decline caused by growth of biofilm and EPS was further confirmed by Scanning Electron Microscopy (SEM) images of the fouling layer formed from cells and EPS layer (Fig. 13.7b) produced from PA01 cells can be easily observed by comparing with dead cells (Fig. 13.7a).
SEM images of PA01 biofouling layers: (a) Dead cells fixed in formaldehyde and deposited on the RO membrane in DI water supplemented with 0.01 mM LaCl3 after 38 h of deposition . (b) Live cells with their PES (biofilm ) growth for 19 h on the RO membrane in a synthetic wastewater medium; Reproduced Adapted with permission from Ref. [35]; Copyright 2007 @ Elsevier
3.2 Salt Rejection Decline
Desalination using the RO membrane process is a pressure-driven transport of water through a membrane medium, which will lead to accumulation of solutes retained by the membrane on the feed side. Biofilm formed during the separation processes will inevitably increase the trans-membrane pressure (TMP ) as well as the concentration polarization (CP) [37]. TMP is the pressure that is needed for the transport of water through the membrane. CP refers to the concentration gradient of solutes at the membrane surface resulted from the accumulation of solutes retained by the membrane, which is one of the most important factors influencing the performance of RO membrane separation processes. Upon the formation of a secondary biofilm membrane on RO membrane surface, the back diffusion of salt ions from membrane to feed solution is hindered. The concentration of solutes (Cw) at the membrane surface can be significantly elevated (Cw ∗) due to the development of biofilm, where CP∗ > CP as illustrated in Fig. 13.8 [38]. Therefore, with an increase in CP caused by the formation of fouling layer on the membrane surface, the salt passage through the RO membrane can be significantly increased.
The biofilm consisting of an EPS matrix and bacterial cells increases the TMP , which leads to a decreased salt rejection ability. Their roles in decreased salt rejection were also investigated by Herzberg [35]. A drastic increase in salt passage was observed for two experiments with the deposition of dead cells on the membrane in wastewater medium 1 and 2. This increase indicates that not only the EPS matrix, but also the deposition of dead bacterial cells on the RO membrane surface can decrease salt rejection (Fig. 13.9) [35]. Salt rejection by biofilm is due to the increased CP, whereas dead bacterial cells cause salt rejection due to causing decreased back diffusion of salt ions [27].
Percent salt passage. PA01 biofilm in wastewater medium (initial cell concentration: 107 cells/mL); PA01 dead cells in wastewater medium 1 and 2 (initial cell concentration: 109 cells/mL); Adapted with permission from Ref. [35]; Copyright 2007 @ Elsevier
Another possible mechanism for salt rejection decline is the biodeterioration from the growth of biofilms. Biofilms formed on RO membrane surface can attack membranes by excreting acids and/or exoenzymes that attack the membrane materials. This process is called “biodeterioration” [39, 40]. Some reports show that cellulose acetate RO membranes can be biodegraded by microorganisms [41, 42]. Reports indicate that common membrane materials, such as polyamide and polyethersulfone, appear to not be attacked by microorganisms [34].
3.3 Increased Energy Consumption
Desalination using RO membrane system is a pressure-driven process. This method inevitably leads to the accumulation of bacterial cells, salt ions, and other materials on or within the RO membrane. Once fouling occurs, the membrane permeability decreases. To combat this, an increased applied pressure is required in order to offset the loss of water production from the development of biofilm [43].
Theoretically, 0.7 kWh/m3 is the minimum energy required for seawater desalination [44]. In reality, the energy consumption of seawater desalination ranges from 2 to 5 kWh/m3 with modern materials, modules, and technologies [45, 46]. With the formation of secondary biofilm membrane on RO membrane surface, about 150% of the initial operating pressure (200 psi) is required to compensate the flux loss [34, 44].
4 Conclusions and Outlook
RO technique plays an irreplaceable role in seawater desalination industries. However, biofouling caused by bacterial adhesion and propagation on the RO membrane surface hinders the widespread application of RO. Biofouling in RO desalination plant is inevitable as seawater contains substantial amount of organics, nutrients, and microorganisms, especially bacteria, fungi, and yeasts. The contact between the membrane surface and contaminant containing seawater in the RO desalination plants causes the biofouling through adsorption , transport, attachment, growth, multiplication, and detachment of microorganisms that eventually lead towards biofilm formation. Continuous production of EPS facilitates the biofouling of the membrane through enhanced adhesion of the biofilm along with shielding the microbes from the cleaning agents. Biofouling increases the concentration polarization as well as the transmembrane pressure due to the formation of biofilm on the RO membrane surface. Enhanced hydraulic resistance and reduced permeability of RO membranes due to deposition of dead cells, the growth of biofilm, and production of EPS can significantly affect the RO desalination plant performance. Increased concentration polarization results in decreased salt rejection while energy consumption increases due to increased applied pressure to offset the loss of water production. The RO plant performance, as well as the efficiency, degrades due to biofouling which eventually affects the plant expenditures by requiring frequent membrane cleaning and membrane replacement. Advancement in research has adapted different strategies for biofouling mitigation through minimizing microbial concentration, e.g., feed pretreatment, biocide application, etc. and preventing microbial adhesion and/or inactivation of bacteria adhered to membrane surface through development of antibiofouling membrane through surface modification .
Although biofouling of the RO membrane is a huge challenge, RO membrane separation technology is still a promising way for desalination. With the development of diverse fouling-resistance materials and new cleaning procedures, fouling-resistance performance of RO membranes has been improved significantly. A better understanding of the fundamental of the biofouling of RO membrane will contribute towards efficient biofouling management, and hence, will enhance the RO desalination application combating the global water crisis.
Change history
26 April 2021
The original version of the book was inadvertently published without the copyright information for Fig. 13.2 in Chap. 13. The correct figure caption has been updated in the chapter as given below:
References
L.F. Greenlee, D.F. Lawler, B.D. Freeman, B. Marrot, P. Moulin, Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 43, 2317–2348 (2009)
P.K. Cornejo, M.V.E. Santana, D.R. Hokanson, J.R. Mihelcic, Q. Zhang, Carbon footprint of water reuse and desalination: A review of greenhouse gas emissions and estimation tools. J. Water Reuse Desalin. 4, 238–251 (2014)
Almar Water Solutions, (2016) Desalination technologies and economics: CAPEX, OPEX & technological game changers to come. Mediterranean Regional Technical Meeting Marseille CMI, December 12–14
C. Fritzmann, J. Löwenberg, T. Wintgens, T. Melin, State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007)
R. Perry, D. Green, J. Maloney, Perry’s Chemical Engineers’ Handbook (McGraw-Hill Companies Inc., New York, 1997)
H. Lonsdale, U. Merten, R. Riley, Transport properties of cellulose acetate osmotic membranes. J. Appl. Polym. Sci. 9, 341–1362 (1965)
D.R. Paul, Reformulation of the solution-diffusion theory of reverse osmosis. J. Membr. Sci. 241, 371–386 (2004)
K.P. Lee, T.C. Arnot, D. Mattia, A review of reverse osmosis membrane materials for desalination – development to date and future potential. J. Membr. Sci. 370, 1–22 (2011)
M.E. Mattson, M. Lew, Recent advances in reverse osmosis and electrodialysis membrane desalting technology. Desalination 41, 1–24 (1982)
M. Elimelech, W.A. Phillip, The future of seawater desalination: Energy, technology, and the environment. Science 333, 712–717 (2011)
J. Johnson, Membrane cleaning fundamentals: Cleaning criteria and normalization of reverse osmosis systems. WaterWorld, January (2006), https://www.waterworld.com/municipal/technologies/article/16211727/membrane-cleaning-fundamentals-cleaning-criteria-and-normalization-of-reverse-osmosis-systems
A. Matin, Z. Khan, S. Zaidi, M. Boyce, Biofouling in reverse osmosis membranes for seawater desalination: Phenomena and prevention. Desalination 281, 1–16 (2011)
H. Maddah, A. Chogle, Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 7, 2637–2651 (2017)
T. Nguyen, F. Roddick, L. Fan, Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2, 804–840 (2012)
S.R. Pandey, V. Jegatheesan, K. Baskaran, L. Shu, Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review. Rev. Environ. Sci. Bio. 11, 125–145 (2012)
B.A. Qureshi, S.M. Zubair, A.K. Sheikh, A. Bhujle, S. Dubowsky, Design and performance evaluation of reverse osmosis desalination systems: An emphasis on fouling modeling. Appl. Therm. Eng.Appl. Therm. Eng. 60, 208–217 (2013)
J. Baker, L. Dudley, Biofouling in membrane systems: A review. Desalination 118, 81–89 (1998)
O. Rendueles, J.-M. Ghigo, Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 36, 972–989 (2012)
D. Monroe, Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 5, e307 (2007)
H.-C. Flemming, G. Schaule, T. Griebe, J. Schmitt, A. Tamachkiarowa, Biofouling – The Achilles heel of membrane processes. Desalination 113, 215–225 (1997)
M. Herzberg, S. Kang, M. Elimelech, Role of extracellular polymeric substances (EPS) in biofouling of reverse osmosis membranes. Environ. Sci. Technol. 43, 4393–4398 (2009)
A. Karimi, D. Karig, A. Kumar, A. Ardekani, Interplay of physical mechanisms and biofilm processes: Review of microfluidic methods. Lab Chip 15, 23–42 (2015)
J. Mansouri, S. Harrisson, V. Chen, Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 20, 4567–4586 (2010)
H.J. Busscher, W. Norde, P.K. Sharma, H.C. Van der Mei, Interfacial re-arrangement in initial microbial adhesion to surfaces. Curr. Opin. Colloid Interface Sci. 15, 510–517 (2010)
H.J. Busscher, A.H. Weerkamp, Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiol. Rev. 46, 165–173 (1987)
M. Hermansson, The DLVO theory in microbial adhesion. Colloids Surf. B. Biointerfaces 14, 105–119 (1999)
P. Cuatrecasas, Membrane receptors. Annu. Rev. Biochem. 43, 169–214 (1974)
R. Neihof, G. Loeb, Dissolved organic-matter in seawater and electric charge of immersed surfaces. J. Mar. Res. 32, 5–12 (1974)
A.-C. Olofsson, M. Hermansson, H. Elwing, N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 69, 4814–4822 (2003)
R.A. Al-Juboori, T. Yusaf, Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 302, 1–23 (2012)
J.A. Brant, A.E. Childress, Assessing short-range membrane–colloid interactions using surface energetics. J. Membr. Sci. 203, 257–273 (2002)
J.T. Staley, Growth rates of algae determined in situ using an immersed microscope. J. Phycol. 7(1), 13–17 (1971)
M.O. Saeed, A. Jamaluddin, I. Tisan, D. Lawrence, M. Al-Amri, K. Chida, Biofouling in a seawater reverse osmosis plant on the Red Sea coast, Saudi Arabia. Desalination 128, 177–190 (2000)
H.-C. Flemming, Reverse osmosis membrane biofouling. Exp. Thermal Fluid Sci. 14, 382–391 (1997)
M. Herzberg, M. Elimelech, Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 295, 11–20 (2007)
P. Goh, W. Lau, M. Othman, A. Ismail, Membrane fouling in desalination and its mitigation strategies. Desalination 425, 130–155 (2018)
S. Kim, E.M.V. Hoek, Modeling concentration polarization in reverse osmosis processes. Desalination 186, 111–128 (2005)
T.H. Chong, F.S. Wong, A.G. Fane, Enhanced concentration polarization by unstirred fouling layers in reverse osmosis: Detection by sodium chloride tracer response technique. J. Membr. Sci. 287, 198–210 (2007)
A.H. Rose, History and scientific basis of microbial biodeterioration of materials. Econ. Microbiol. 6, 1–18 (1981)
H.-C. Flemming, G. Schaule, R. McDonogh, H.F. Ridgway, Effects and extent of biofilm accumulation in membrane systems, in Biofouling and Biocorrosion in Industrial Water Systems, ed. by G. G. Geesey, Z. Lewandoski, H. C. Flemming, (Lewish Publishers, CRC Press, Inc, 1994), pp. 63–89
A.P. Murphy, C.D. Moody, R.L. Riley, S.W. Lin, B. Murugaverl, P. Rusin, Microbiological damage of cellulose acetate RO membranes. J. Membr. Sci. 193, 111–121 (2001)
W. Luo, M. Xie, F.I. Hai, W.E. Price, L.D. Nghiem, Biodegradation of cellulose triacetate and polyamide forward osmosis membranes in an activated sludge bioreactor: Observations and implications. J. Membr. Sci. 510, 284–292 (2016)
L. Malaeb, G.M. Ayoub, Reverse osmosis technology for water treatment: State of the art review. Desalination 267, 1–8 (2011)
M. Schiffler, Perspectives and challenges for desalination in the 21st century. Desalination 165, 1–9 (2004)
S.S. Shenvi, A.M. Isloor, A. Ismail, A review on RO membrane technology: Developments and challenges. Desalination 368, 10–26 (2015)
S.F. Anis, R. Hashaikeh, N. Hilal, Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 452, 159–195 (2019)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Anwar, N., Yang, L., Ma, W., Usman, H.S., Rahaman, M.S. (2020). Biofouling in RO Desalination Membranes. In: Saji, V.S., Meroufel, A.A., Sorour, A.A. (eds) Corrosion and Fouling Control in Desalination Industry. Springer, Cham. https://doi.org/10.1007/978-3-030-34284-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-34284-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34283-8
Online ISBN: 978-3-030-34284-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)