Abstract
Conventionally, activated sludge process (ASP) is most commonly employed for wastewater treatment. However, the conventional treatment system is inefficient for the removal of biological nutrient to greater extent. Nutrients such as nitrogen (N) and phosphorus (P) has high influence on the receiving water body, cause eutrophication and algal bloom. It leads to reduce of dissolved oxygen (DO) level and in turn high risk to aquatic life. Eutrophication is a major problem in developing countries and is responsible for water pollution. Removal of biological nutrient in the wastewater is an essential task to lead the positive impacts on solving the environmental pollution issues. Developing the biological nutrient removal approach in the wastewater treatment to balance the biogeochemical system of the receiving aquatic environment. Biological nutrient removal is a challenging task and number of operational parameters governs its efficiency. This chapter focusses on recent development in the biological nutrient removal and governing factors of the process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Water scarcity and shortage of clean water supply threaten developed, developing, and undeveloped countries (Subramaniam 2018). According to the United Nations world population prospects 2017 reports, the global population was expected to be 7.6 billion in 2018, it may be increased to 8.6 billion in 2030. On seeing the population growth rate, the supply of clean water demands high in the upcoming years. In order to minimize the water scarcity, treating wastewater is a beneficial option to save the ground and surface water quality. In worldwide, 80% of wastewater is directly discharged into the global waterway which causes eutrophication and depleting the dissolved oxygen (DO) level. Domestic is the major source of wastewater generation and direct discharge with a high concentration of nutrient (Powley et al. 2016). Domestic wastewater contains a high concentration of inorganic matter such as nitrogen and phosphorus in the dissolved form (Mekonnen and Hoekstra 2018).
In worldwide, the municipal wastewater treatment plant (MWWT) with nutrient removal system was successful in progress, which upgrade the wastewater treatment efficiency, and to control eutrophication issue in the receiving water bodies. Several researchers have suggested physical, chemical, biological, and combined physiochemical methods for nutrient removal (Sen 2015; Bunce et al. 2018). Among these, biological nutrient removal method is effective in removing toxic and harmful compounds in an inexpensive wat (Rajasulochana and Preethy 2016). Biological nutrient removal (BNR) is the process of removing of total nitrogen (TN) and total phosphorus (TP) from wastewater with the help of microorganism. Discharge of wastewater without removal of nutrients cause an adverse effect on the aquatic environment such as excess growth of phytoplankton, reduction in dissolved oxygen (DO) level, odor occurrences and increasing the pollution load.
Most commonly, municipal wastewater contains a high amount of nutrients residues when compared with industrial wastewater. In municipal wastewater stream TN occurs in the dissolved form and the concentration range between 25 and 45 mg/L. TN composed of 60% organic nitrogen and 40% ammonia. Similarly, P is also considered as an essential nutrient that presents in the wastewater. P enters into the environment through sedimentation, rock formation, and degradation process. In the agricultural sector, phosphorus plays a vital role and it acts as fertilizer and feed for crop production (Egle et al. 2016). In municipal wastewater, the concentration of phosphorus varied from 6 to 20 mg/L in the dissolved form.
Many researchers have suggested a cost-effective biological method to degrade the inorganic nitrogen compounds. The following processes are successfully established and executed for biological nutrient removal they are Anoxic/Oxic (AO) (Rajesh Banu et al. 2009a; Do et al. 2012) anaerobic/anoxic/aerobic (A2/O) (Rajesh Banu et al. 2009b; Uan et al. 2013), Sequencing batch reactor (SBR) (Guo et al. 2007; Yuan et al. 2016), Attached growth (Al-Zreiqat et al. 2018), Modified Ludzack–Ettinger (Liu and Wang 2017), Modified Bardenpho (Emara et al. 2014), Step feed (Guo et al. 2007), University of Cape town (Mannina et al. 2016), Phostrip (Salehi et al. 2018) and Bio-Denitro (Irizar et al. 2004; Tabassum et al. 2018). Developing a proper treatment system for removal of excess nutrients from wastewater will control the environmental issues. Essential nutrient such as N and P is present in wastewater, which has market value when it can be recovered in the form of fertilizer. This chapter provides knowledge about recent updates in biological nutrient removal, different source and composition of nutrient in the wastewater. Meanwhile, the chapter offers information regarding treatment process involved in both biological nitrogen and phosphorus removal.
2 Biological Nitrogen Removal
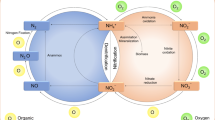
Removal of nitrogen derivatives from treated wastewater is often essential before discharging into the sensitive environment. This will prevent eutrophication on receiving water body and accumulation toxic contamination in the groundwater. Removal of nitrogen can be adopted as fundamental part of treatment system or an additional part to an existing treatment system. Biological nitrogen removal is the process of removing toxic ammonia with the help of enzyme. The toxic nitrogen substances such as ammonia, nitrate, and nitrite are easily biodegradable with the help microorganism. Total nitrogen removal can be achieved by simultaneous biological nitrification and denitrification process. Some example reactors frequently suggested for biological nitrogen removal single sludge system, two sludge system, anoxic/aerobic, step feed anoxic/aerobic, intermittent aeration, postanoxic denitrification and sequencing batch reactor (Metcalf and Eddy 2014). Figure 11.1 show biological nitrogen removal.
2.1 Nitrogen as Pollution Compound
Nitrogen compound frequently occurs in food, air, water, and soil. If the concentration of nitrogen exceeds the limit cause unfavorable health condition to all living organism. Since nitrogen compound management in water and wastewater becomes mandatory due to their toxic effect. In drinking water, present nitrogen residue in the form nitrite and nitrate cause harmful effect to infants and adults. Excess intake of these compounds results in anemia, methemoglobinemia, and cause damage to liver by reducing the content of vitamin A. Especially for infants, excess ingestion of nitrate reduces the oxygen carrying capacity of hemoglobin and leads to death (Garcia-Segura et al. 2018). Wastewater contains varies forms of nitrogen such as organic nitrogen, ammonia nitrogen, nitrite (NO2−) and nitrate (NO3−). The direct discharge of untreated wastewater into the nearby water bodies results in eutrophication, deplete dissolved oxygen level and toxic to aquatic life (Zhang et al. 2018).
2.2 Nitrification and Denitrification Process
Nitrification is the process of converting ammonia into nitrate with the help of aerobic microbes. The biological conversion of ammonium nitrogen to nitrate takes place in two-phase. In the first-phase autotrophic microbe, Nitrosomonas convert ammonia into nitrite and in the second phase, Nitrobacter converts nitrite into nitrate. Figure 11.1 represents biological nitrification process. The following equation shows the two-phase of ammonia conversion :
Denitrification is the process of converting nitrate into nitric oxide, nitrous o xide and nitrogen gas with the help of facultative heterotrophic microbes. Microbes involved in this process consumes nitrate as a final electron acceptor instead of oxygen. During conversion, the additional supply of methanol is required as carbon source. The degradation of carbonaceous organic substrate in this process is same as aerobic but only difference it takes place under strict anaerobic condition. Figure 11.1 represents nitrogen removal pathway. The following equation show the denitrification process.
2.3 Factors Influence the Biological Nitrogen Removal
The following factors are an influence on the growth of nitrifying and denitrifying microbes for effective biological nitrogen removal in the wastewater.
2.3.1 Dissolved Oxygen (DO)
DO is an essential parameter for the growth of nitrifier in the aerobic basin. For effective nitrogen removal, the maximum DO concentrations maintained in the reactor around 2.0–4.0 mg/L. Presences of DO concentrations less than 0.5 mg/L in anoxic zone will enhance the denitrifier activity.
2.3.2 Temperature
Temperature directly impact on the growth of nitrifier and denitrifier in the aerobic and anaerobic basin, respectively. The best temperature for growth of nitrifier was 22–37 °C and similarly for denitrifier was 2–50 °C (Fontenot et al. 2007; Sudarno et al. 2011).
2.3.3 pH and Alkalinity
During biological nitrogen removal, the pH and alkalinity of wastewater play an essential role. During the nitrification process, the nitrifier consumes alkalinity and it leads to pH drop. Low alkalinity will affect the growth of nitrifying microbes. Typically, 60 to 100 mg/L of alkalinity need to be maintained in the system for effective nitrification process (Breisha and Winter 2010). The desired alkaline condition in the system can be controlled by adding of the following alkaline agents for example hydrated lime, soda ash, or caustic soda.
2.3.4 Food to Microorganism Ratio
The term food to microorganism ratio refers to the amount of biodegradable substrate available for microorganism in the aerobic basin. Most commonly, 0.2–0.4 ratio was followed for activated sludge system (Metcalf and Eddy 2014). For biological nitrogen removal system, it depends on the growth of nitrifier and denitrifier microbes. During the design of F/M ratio for nitrogen removal, F value is based secondary influent BOD concentration. If the nitrogen removal reactors were designed in series with three compartments, then the M value may vary from one to another compartment. M value for the first compartment is based on reactor volume and solids concentration (microorganism). Similarly, for the second compartment, based on the combined volume of reactor (first and second compartment) and combined solid concentration. The same condition was followed for the third compartment M value.
2.3.5 Sludge Retention Time (SRT)
SRT is the essential parameter that influences in biological nitrogen removal. However, SRT is otherwise known as sludge age or mean cell residence time. SRT can express in terms of average time length of microorganism active in the system. If SRT is too low then the system may get affected due to insufficient growth of microorganism. Usually, many treatment systems were designed to remove carbonaceous biological oxygen demand (CBOD) within 2–4 days of SRT. However, the SRT for biological nitrogen removal may vary with respect to the following parameters temperature, reaction size and solid concentration.
2.4 Suspended Growth System
In this system, microbes are responsible for nutrient removal and upholding the substrate in suspension. Most of the industrial and municipal wastewater treatments were successfully operated by adopting suspended growth system. In aerobic suspended growth system, the dissolved oxygen level was maintained and it helps the microbes to maintain the substrate in suspension. For high organic biomass concentration, anaerobic suspended growth system was adopted to treat the industrial wastewater followed by removal of nutrients. For the effective removal of nitrogen, the following suspended growth systems were commonly adopted such as Ludzack–Ettinger, Modified Ludzack–Ettinger, aerobic granular activated sludge sequencing batch reactor (Hafez et al. 2010; Ekama 2015; Liu and Wang 2017).
2.4.1 Ludzack–Ettinger (LE) Process
In the year 1960–1970s, wastewater engineers had made many attempts to improve the efficiency of conventional activated sludge system for effective nitrogen removal. Ludzack–Ettinger have modified the system by introducing anoxic zone before aeration and clarifier. In this system, the thickened mixed liquor from the bottom of the clarifier was recycled to the influent line. This action could improve the activity of facultative microbes to metabolize the inorganic nitrate. If the recycling performance is get reduced then the rate of denitrification affected, due to insufficient supply of nitrate. Figure 11.2 represents Ludzack–Ettinger (LE) process.
Ludzack–Ettinger (LE) Process (Metcalf and Eddy 2014)
2.4.2 Modified Ludzack–Ettinger (MLE) Process
In this process, the original design of Ludzack–Ettinger process was upgraded to target the nitrogen compounds and to improve the removal efficiency. MLE process was similar to LE process, but slight modification was done to enhance the process efficiency. In MLE process, recycling of mixed liquor was between oxic to anoxic zone. The nitrogen removal efficiency was accelerated due to the internal recycling of nitrified wastewater. Excess recycling increases the dissolved oxygen concentration in anoxic zone. DO concentration above 0.5 mg/L in anoxic zone affect denitrification efficiency of the system and subsequent nitrogen removal efficiency (Hocaoglu et al. 2011).
2.4.3 Aerobic Granular Activated Sludge Sequencing Batch Reactors
Sequencing batch reactors (SBR) treatment system is opposite to conventional activated sludge process (ASP). SBR has more advantages than ASP, whereas the area required for treatment of wastewater is less for SBR when compared with ASP. In SBR, a single tank involves in four phases of the following process, fill, react, settle and decant. For denitrification, during fill phase mixing is provided in place of aeration. After completion of fill phase, methanol (carbon source) was supplied to enhance the nitrogen removal efficiency. In AGAS-SBR, only three phases are involved namely fill, react and settle.
-
i.
Fill/draw
Due to rapid settling velocity of granules in the reactor, wastewater can be introduced into the reactor at bottom of sludge bed. The concentration of sludge bed becomes denser after settling phase and it creates strict anaerobic environment. This favorable condition will reduce the concentration of nitrate in the wastewater and enhances denitrification process.
-
ii.
Aeration
In this phase, microbes play a vital role in simultaneous biological nutrient removal. This action is possible due to the granular structure; it comprises of both nitrifying and denitrifying microbes. The outer layer of granule contains nitrifying microbes and the inner core contains of denitrifying microbes. High concentration of dissolved oxygen (DO) was maintained in the reactor during aeration. Therefore, the outer layer of granular was closely contacted with DO, which enhances the growth of nitrifying microbes and ends up with nitrification process. The heterotrophic and autotrophic microbes present in the inner core of the granular denitrify the nitrate/nitrite into nitrogen gas.
-
iii.
Settling
In this phase, the granular where settled very faster and it requires short settling time. In addition to this, the idle time of the reactor was neglected. Then the treated wastewater can be easily separated from the granule.
2.5 Attached Growth System
In this system, microbes are responsible for converting the organic and inorganic substances. In the attached growth system, microbe forms a layer over surface of packing material and it is termed as biofilm. Recently, researchers have suggested natural and artificial packing material for the suspended growth system. In the nineteenth century, most commonly used attached growth reactor for biological nitrogen removal such as trickling filter and rotating biological contactors. Currently, researchers have developed integrated suspended growth system such as integrated fixed film activated sludge (IFAS) (Ekama 2015), integrated anoxic fixed bed and oxic moving bed biofilm (Gong et al. 2012) and hybrid upflow carrier suspended growth reactor (Le et al. 2018) for effective nitrogen removal.
-
Advantages
-
Operated at high biomass concentration
-
No phase separation in nitrogen removal
-
Withstand shock loading
-
Withstand in fluctuating operational condition.
2.5.1 Integrated Fixed Film Activated Sludge (IFAS)
In this system, conventional activated sludge system merged with fixed film media to enhance the nitrogen removal. Fixed film system is commonly employed in the cold regions, for effective oxidization of ammonia in the wastewater. The fixed media retained the biomass in the system for prolonged time and it is not removed from the system by conventional sludge waste method. In IFAS, ammonia-oxidizing microbes present on the media carry out effective nitrification than conventional activated sludge system. This could reduce system SRT, as it was based on nitrifier population. The static or moving fixed media, suspended rope or moving bed carrier is introduced to the aerobic reactor. Due to aeration, the nitrifier grows on the surface of fixed media and increases their population density in the aerobic reactor. Figure 11.3 shows the schematic diagram of integrated fixed-film activated sludge reactor.
Schematic diagram of integrated fixed-film activated sludge reactor (Ekama 2015)
2.5.2 Integrated Anoxic Fixed Bed and Oxic Moving Bed Biofilm
In this system, the conventional A/O reactor incorporated with anoxic fixed bed and oxic moving bed biofilm reactor for effective biological nitrogen removal (Gong et al. 2012). The organics substrate in influent could be employed for anoxic pre-denitrification, which causes more advantage in reduction of heterotrophic biomass production in oxic zones. The reactor was divided into seven-phase, the first two are anoxic phase (fixed bed biofilm) then next four where oxic phase (moving bed biofilm), and the settler placed at last. Heterotrophic biomass was recycled from settler to oxic phase for improving the microbial activity. The DO level was maintained in the range of 2–4 mg/L, in order to uphold the carrier in suspension. In anoxic zone, the DO level is maintained in the range of 0.5–0.7 mg/L. This could create a favorable environment for denitrifying microbes to oxidize the nitrite/nitrate into nitrogen gas. Figure 11.4 shows the schematic diagram of integrated anoxic fixed bed and oxic moving bed biofilm reactor.
Schematic diagram of integrated anoxic fixed bed and oxic moving bed biofilm reactor (Gong et al. 2012)
2.6 Anammox Process
Anammox is the process of oxidizing ammonium with the help of microbes under favorable anaerobic condition (Chi et al. 2018). Figure 11.5 show steps involved in anammox process. This process is an eco-friendly and economically feasible to treat nitrate and ammonia enriched wastewater. In this process ammonium oxidizing microbes plays a vital role in conversion of ammonium (NH4+) and nitrite (NO2−) into nitrogen (N2) and nitrate (NO3−) without additional supply of carbon source under suitable anaerobic condition (Suneethi and Joseph 2011). The growth rate of ammonium oxidizing microbes was more effectively in high concentrated ammonium-rich wastewater with low COD/N ratio at long SRT (Tsushima et al. 2007; Bae et al. 2010; Shen et al. 2012). The following below equation explains the anammox process.
Anammox process has following advantages (1) reducing the oxygen requirement for nitrification (2) ignoring the additional supply of carbon source for denitrification (3) significant reduction in generation of excess sludge (4) achieving higher COD (chemical oxygen demand) removal rate (5) eradicating the release of greenhouse gas (CO2) during conventional denitrification. It has certain disadvantages (1) adding significant quantity of nitrogen to atmosphere (2) slow growth rate (3) biomass washout. In order to overcome the following issues, many researchers have developed a new process to oxidize ammonia. They are (1) completely autotrophic nitrogen removal over nitrite—CANON (Yue et al. 2018) (2) single reactor system for high activity ammonia removal over nitrite—SHARON (Sri Shalini and Joseph 2018) (3) Deammonification (DEMON) (Gonzalez-Martinez et al. 2015), and (4) Oxygen Limited Autotrophic Nitrification/Denitrification—OLAND (Nhu Hien et al. 2017).
-
Advantages
-
Compared with nitrification–denitrification processes a small amount of excess sludge was produced.
-
No need of additional supply of carbon source
-
Aeration cost saves due to partial ammonia converted to nitrite.
3 Biological Phosphorous Removal
Phosphorous exist in different forms in water bodies. Various methods such as chemical precipitation, sedimentation, membrane filtration, and biological methods are implemented to remove phosphorous from water. Among these methods, biological is economically viable (Banu et al. 2008) and produces less sludge when compared with other methods. In the conventional treatment process soluble phosphorous present in the wastewater is getting integrated with microbial biomass. Later it was removed via sludge wasting (Banu et al. 2007). In general, microbes present in activated sludge process (ASP) accounts for uptaking phosphorus in the range of 1.5 to 2.5% of its biomass weight (Raj et al. 2013). In, enhanced biological phosphorus removal (EBPR) processes a group of specialized microbes called phosphorous accumulating organisms (PAO) can accumulate phosphorus into its biomass in the range of 5–12% (Rajesh Banu et al. 2009b). PAO communities consist of Acinetobacter, Rhodocyclus, and some coccus shaped bacteria (Bond et al. 1995; Wong et al. 2005). PAO is facultative microorganism and of capable of living in anaerobic and aerobic condition. EBPR process facilitates aerobic and anaerobic conditions for PAO to remove phosphorous from the wastewater.
3.1 Factor Influence the Biological Phosphorus Removal
EBPR is an economical process and by which comparatively higher amount of phosphorus removal is possible. The stability and reliability of EBPR were affected by various factors. It includes environmental and operational conditions such as carbon source, COD/P ratio, temperature, pH, cations, dissolved oxygen, solid retention time, and secondary phosphorus release.
3.1.1 Carbon Source
The efficiency of EBPR depends on the availability of carbon in the form of readily biodegradable carbonaceous oxygen demand or VFA. The economics of EBPR is mainly influenced by the choice of substrate and cost of the carbon source (Puig et al. 2008). Earlier study reveals that acetate is widely utilized as a carbon sole. Recently, the utilization of many other substrates was performed in EBPR. Table 11.1 list various substrate used as carbon source in EBPR.
3.1.2 COD/P Ratio
The influent COD or BOD to total phosphorus ratio (influent COD:P or influent C:P) plays a significant role in phosphorus removal from wastewater. According to Oehmen et al. (2007), the COD:P ratios should be 10–20 mg-COD/mg-P so that PAO tend to dominate the EBPR.
3.1.3 Cations
The stability of EBPR mainly depends on cation concentration of the influent wastewater (Schönborn et al. 2001). Water-soluble orthophosphate (\( {\text{PO}}_{4}^{3 - } \)) is an anion having three negative charges. Phosphorus on its own cannot move across the membrane as it was negatively charged. Its transportation across the microbial membrane need charge neutralization and was facilitated by cations (Mg+2, P+). The charge of orthophosphate is neutralized, when it binds with cations. The neutralized ion can be transported across the cell membrane. Therefore, the presence of these cations increases P removal, while other cations such as calcium are not essential (Esakki Raj et al. 2012).
3.1.4 Dissolved Oxygen
Oxygen is required for PAO to metabolize the storage products and uptake of phosphorus. An extreme aeration leads to negatively impact on EBPR process as termination of P-uptake happens due to exhaustion of poly-hydroxy-butyrate (PHB). DO concentrations around 2.5–3.0 mg/L appeared to associate with a plenty of PAOs. Presences of DO greater than 5 mg/L in return and internal recycle line between aerobic to anaerobic and aerobic, anoxic to anaerobic relatively affect EPBR performance (Raj et al. 2013).
3.1.5 Solid Retention Time (SRT)
SRT has been specified as one of the most essential factors that affects the performance of EBPR. It was revealed that SRT of 30 days resulted in increases P elimination, reduction of biomass growth and sludge wastage (Lee et al. 2007). SRT above 30 days causes domination of GAO over PAO. This results in a decline of phosphorus removal efficiency in EBPR system (Seviour et al. 2003). Hence, shorter SRT are favorable for PAO in EPBR.
3.1.6 Secondary Phosphorus Release
In anaerobic zone the release of phosphorus occur by the uptake of acetate, and it was stored as PHB, which is known as primary Release (Barnard 1984). Yet, phosphorus was also discharged from deposited polyphosphate under anaerobic circumstances when there was an insufficient availability of VFA. The phosphorous discharges during these actions are known as secondary release of phosphorus. The release of secondary phosphorous is much lower than the primary release and it depletes stored PHB. Hence, secondary release must be avoided as much as possible because it unfavorably affects the performance of an EBPR process.
4 Combined Biological Nutrient Removal
In combined nutrient removal, both nitrogen and phosphorus removal occurs in a single set of reactor. This kind of arrangement will reduce area requirement as well as energy demand for the process. The process is having an advantage of using single clarifier i.e. responsible for maintaining heterogeneous biomass inside the reactor. Most of the full-scale wastewater treatment systems are based on combined process. Some of the widely used reactor configurations for the combined removal of nutrients are discussed below.
4.1 Reactor Configurations
Classical example for biological P removal process is anaerobic/oxic system. It is a suspended growth biological treatment system where the microbes are kept in suspension for treating the wastewater are known as suspended growth system. In suspended growth system microbes has the advantage of getting substrate in radial direction. This leads to greater performance efficiency. Under optimal circumstances, the microbes break down organic matter in the wastewater and reduce pollutant load.
4.1.1 In the Anaerobic Zone
PAOs are usually aerobic bacteria and it cannot multiply in anaerobic environment. Anaerobic zone was provided with an adequate source of biodegradable carbonaceous oxygen demand. In anaerobic zone, organic matters are fermented to form volatile fatty acids (VFA). It was utilized by PAO organism under anaerobic condition and was stored in the form of polyhydroxybutyrate (PHB). The formation of PHB need energy and it comes from breakdown of poly-P. This resulted in net rise in the quantity of phosphate across the anaerobic zone. The anaerobic zone in EBPR process act as a conditioning tank, where PAOs are being trained for their work in the consequent aerobic zone (Metcalf and Eddy 2014) for P-uptake.
4.1.2 In the Aerobic Zone
In the aerobic zone stored PHB is metabolized, which in turn provides energy and carbon for cell proliferation. PHB metabolism leads to production of glycogen. During this process, luxury uptake of orthophosphate by PAO happens. This leads to the accumulation of phosphate in the form of poly-P inside the PAO and energy discharge by PHB was used to form poly phosphate bond. PHB utilization also enhances cell proliferation. In EBPR process, P removal was achieved by periodical removal of P rich sludge.
4.1.3 Anaerobic/Anoxic/Oxic (A2O) Process
In this process, anaerobic zone earlier to the anoxic and aerobic zone was facilitated. In between the zones mixed liquors are internally recirculated to create favorable conditions for phosphorous and nitrogen removal. In case of phosphorous removal, PAO was cyclically exposed to anoxic and aerobic condition to facilitate the luxury uptake of phosphorous. Internal recycling of nitrified wastewater to anoxic zone facilitates conversion of nitrate to nitrogen. (Rajesh Banu et al. 2009b). Based on A2O, several other processes have been developed to enhance nutrient removal by rearranging its zones. Numerous combinations and modifications of the A2O system have been established to meet commercial and regulatory difficulties of wastewater treatment. Figure 11.6 Schematic diagram of Anaerobic/Anoxic/Oxic (A2O) process.
Schematic diagram of Anaerobic/Anoxic/Oxic (A2O) process (Rajesh Banu et al. 2009b)
4.1.4 PhoStrip Process
In PhoStrip process, only microorganisms are involved in uptake and release of phosphate the process is also known as sidestream process. The overview of PhoStrip is shown in Fig. 11.7.
Schematic diagram of Phostrip process (Metcalf and Eddy 2014)
During conventional waste activated sludge process. Thickened biomass is reroute to aeration tank from the secondary clarifier but in the PhoStrip process a thickened biomass is rerouted to an additional tank known as stripping thank. In stripper tank, the sludge endures anaerobic detention so that the sludge release dissolved phosphate. The dissolved phosphates are present in supernatant are separated and chemical such as lime is added in order to precipitate phosphate. The remaining biomass is recycled back into aerobic zone, where it absorbs dissolved phosphate. This resulted in enhanced removal of phosphate from the influent. More than 75% of phosphorous is removed during PhoStrip process and remaining is removed by biological phosphorous removal (Salehi et al. 2018). It has to be noted that the Phostrip process is unsuitable to treat wastewaters with high nitrogen concentrations.
4.1.5 Virginia Initiative Process (UCT-BNR)
It is a modified A2O process with an addition of interior mixed liquor recycling line between anoxic zone to the anaerobic zone. In UCT process, return activated sludge was recycled into anoxic zone rather than the anaerobic zone. This arrangement reduces the adverse effects of the nitrate recycle to the anaerobic zone. In UCT process, PAOs are specified with the selective benefit of full contact to all accessible organic matter. It is good for both total nitrogen and total phosphorus removal. Figure 11.8 Schematic diagram of Virginia Initiative Process.
Schematic diagram of Virginia Initiative Process (Metcalf and Eddy 2014)
4.2 Simultaneous Precipitation
Simultaneous precipitation of phosphorus is frequently carried out at aerobic basin of biological nutrient removal system (de Haas et al. 2000). Iron salts are broadly used for this process. Two forms of iron salts ferric and ferrous are commonly used by the researcher for simultaneous precipitation. Among the iron salts, ferric chloride is utmost frequently utilized for simultaneous precipitation. pH plays major role in precipitation reaction and optimum pH for ferric and ferrous salts were about to 5 and 8 respectively. From the above, it is noted that the iron salts has an advantage over ferric as its optimum pH lies close to wastewater pH.
Alum it is a hydrated form of aluminum sulfate has been widely utilized for simultaneous precipitation (Rajesh Banu et al. 2009a). Removal of phosphorous occurs in two-step between the formation of aluminum hydroxides (Al(OH)3) and aluminum phosphate (AlPO4). In the first step, alum reacts with water to form aluminum hydroxide. In a second step, it form complex with phosphate and precipitate. The above-mentioned reaction are given in the form of equation below.
Though simultaneous precipitation enhances P removal, it affects nitrifier population in BNR. In aerobic basin ammonia oxidation was carried out by a specific group of autotrophic bacteria called as nitrifier. Their nitrification capacity was calculated by the following equation:
where RN is the specific nitrification rate, expressed in g of N–NH4+ consumed/g VSS/d. Working on simultaneous precipitation in anoxic/oxic Rajesh Banu et al. (2009a) have reported an inhibition of nitrification rate from 0.049 g N–NH4+/g VSS/d to 0.38 g N–NH4+/g VSS/d by alum. In addition, simultaneous precipitation happens to decrease VSS content of sludge if it was added indiscriminately.
The lime-based (calcium derivatives) simultaneous precipitation was also in practice. However, major setback of lime as a simultaneous precipitant was that phosphorus removal happens at pH 9, which was well outside the optimal pH of biological processes. Powdered activated carbon is utilized by researcher in order to remove nutrients from wastewater (Uygur and Kargı 2004). It was used as phosphorus absorbent in three-step anaerobic (An)/anoxic (Ax)/oxic (Ox); the four-step (An/Ox/Ax/Ox), and the five-step (An/Ax/Ox/Ax/Ox). The lowermost discharge nutrient levels were recognized by using the five-step operation which resulted in 75% COD, 44% NH4–N and 44% PO4–P eliminations from effluent after 21 h of operation.
-
Advantages
-
Changing of conditions is flexibility
-
Ease of operation
-
Low investment cost
-
Comparatively lesser solids generation
-
Enhance settling property of sludge.
-
Enhance capillary suction time of sludge.
-
Disadvantages
-
Increased dissolved solids contents on the receiving water
-
Increased sludge generation
-
Inhibitory effects to nitrification
-
Need for pH and alkalinity correction
-
Control P concentration in effluent
-
Polishes effluent quality
-
It prevents sludge bulking.
4.3 Phosphorous Recovery from Wastewater
Phosphorous is the most widely used nutrient in agricultural sector. Its requirement in agricultural sector increases day by day, however, the availability of phosphorus ore is limited. In order to overcome this requirement recovery of phosphate from the natural resources such as wastewater will be the best option. According to Yuan et al. (2012), 15–20% of worldwide demand for phosphorus can be fulfilled by recovering P from waste activated biomass. Phosphorus accumulating organism (PAO) plays a major role in biological phosphorous removal. Accumulibacter phosphatis a kind of dominating PAO and its population accounts for 5–20% of total population. It is capable of accumulating higher amount of P in the form of orthophosphate. This leads to the formation of P enriched sludge (Desmidt et al. 2015). This kind of sludge can be used for recovery of phosphorus.
Membrane technologies integrated with biological P removal process can be used for P recovery. Examples of such process are osmotic membrane bioreactor (OMBR) (Qiu and Ting 2014) and OMBR coupled with reverse osmosis (Luo et al. 2016). In this process, organics and ammonia are removed biologically. The phosphate-enriched supernatant was subjected to precipitation for its recovery. Integration of nanotechnology with wet oxidation proved to be an efficient technology to recover phosphorus from EPBR sludge. During wet oxidation, disintegration of sewage sludge occurs which leads to liquefaction of phosphorous. Subsequent nanofiltration process helps to separate phosphorus from rest of impurities such as heavy metals and other ions, which can be utilized for the production of fertilizer (Blöcher et al. 2012). Wet extraction is done by means of acids (sulfuric, nitric, and hydrochloric acid) and alkalis (oxides of Na, K and Ca). Above 90% of phosphate, recovery occurs in this process and is widely acceptable because it demands low energy and achieves high phosphorous recovery efficiency (Biswas et al. 2009). The recovered phosphorus products is mainly used for the production of fertilizers.
Struvite (MgNH4PO4 · 6H2O) crystallization is frequently used technology for P recovery from sludge produced from EBPR based process. Precovery of about 90% is obtained by struvite crystallization. The precipitates formed were mainly struvite, calcium phosphate and calcite. Anaerobic digestion aid struvite precipitation by releasing PO4–P and Mg2+ from the biomass (Metcalf and Eddy 2014). Metal salt such as Al, Fe can be used to precipitate phosphorous selectively from EBPR sludge. This kind of selective recovery of phosphorous is possible only when phosphorous exist in the form of poly-P or condensed phosphorous. Under such condition, 90% of phosphorous recovery is possible from disintegrated EBPR sludge (Rajesh Banu et al. 2009a).
Phosphorous can also be recovered from ashes, a product of high-temperature incineration process contain good amount of phosphorous. However, the presence of heavy metals concentrate limits its utilization (Petzet and Cornel 2009). European Union developed a method named as SUSAN (Sustainable and safe reuse of municipal sewage sludge for nutrient recovery) to separate heavy metals from ash. In SUSAN, ash was allowed to react with chloride compounds such as magnesium chloride and calcium chloride. The resulting metals chlorides were removed from ash through evaporation at high temperature (850–1000 °C). It removes nearly 90% of heavy metals from ash. Thus, heavy metal devoid ash can be used as an ideal raw material for manufacturing of fertilizers. It also increases the bioavailability of phosphorus in ash from 30 to 50% for fertilizer production.
4.4 Sustainable Nutrient Removal in EBPR Process with Sludge Recycle
Sustainable phosphorous removal is possible for a system with low SRT. Under low SRT condition, phosphorus accumulated in the biomass was wasted periodically and it enables the new biomass to uptake phosphorus. However, systems like AAO-MBR and EBPR with sludge recycling runs with high SRT. Under high SRT condition, sludge wastage is minimum and it causes biomass to reach its saturation level for phosphorous intake. This lead to poor performance of phosphorus removal in such systems in long run.
Following case study was carried out by Rajesh Banu et al. (2009b) to achieve sustainable phosphorus removal in A2O-MBR with sludge recycling system. The operational parameters used for the study are as follows: working volume of the reactor 84 L, flow rate 8.4 L/h, HRT 10 h, sludge recycling 1.5% of Q, Influent TP 5.5 mg/L and TP content of biomass 4.5%. Phosphorus was mass balanced for 100 g (90 days of reactor operation) and details of mass balance were given in Fig. 11.9.
Inlet TP concentration during the study period was calculated by Eq. (11.1)
Effluent TP concentration during the study period was calculated by Eq. (11.2)
The optimum sludge recycling Q for economical operation of EBPR is 1.5% (Raj et al. 2013).
Total amount of sludge recycled for pretreatment at 1.5% was calculated to be 272.16 L. One liter of sludge consist of 7 g of MLSS (biomass) and total amount of SS was calculated to be (272.16 × 7 g = 20412 g) 20.41 kg SS.
From the total SS, TP subjected to pretreatment was calculated by Eq. (11.3)
Even though 85.73 g of TP is exposed to pretreatment (thermochemical) only 48% is being solubilized and it was calculated to be (85.73 * 0.45) 41.15 (g). It is well known that only solubilized phosphorus can alone be removed out of the system. Reaming are bound phosphorus (85.73–41.15) 44.58 g and its removal is not possible. Among the solubilized phosphorus, those present in liquid stream can alone be removed through coagulation. In this study, dissolved air flotation (DF) was used to separate solids from liquid and its solid–liquid separation efficiency was 75%. Hence the amount of solubilized phosphorus in liquid stream was calculated to be (41.15 × 0.75) 30.86 g. Lime was used as a coagulant to remove phosphorus from liquid stream and its efficiency was calculated to be 90% (Rajesh Banu et al. 2009a; Do et al. 2013). Total amount of phosphorus removed via sidestream precipitation was estimated to be (30.86 g × 0.9) = 27.7 g.
Another and major way of TP escape from the system is through biomass wastage. TP removed through biomass wastage can be calculated from substrate consumed and biomass produced. For substrate consumed following equation is used
Biomass produced during the treatment has to be wasted to maintain its balance in EBPR. Hence biomass wasted can be calculated using Yobs (0.19 g SS/gCOD) and was calculated to be (4935.1 g × 0.19) = 937.6 g S. Amount of TP removed from the system was calculated from biomass wastage and was estimated to be = (937.6 g SS × 4.5% TP) = 42.1 g.
The mass balance equation for phosphorous in EBRP can be written as
From the above TP accumulated in the system is calculated by rearranging Eq. 11.7
The accumulated TP (10 g) raises TP content of the biomass to 6.2%. However, if TP is not removed by side stream (27.7 g), it will accumulate in the system and raise TP content of the biomass to 11%. At this value, biomass attains saturation level and its TP uptake from wastewater decrease leading to poor performance of the system. Hence it can be concluded that side stream removal of phosphorous is mandatory for sustainable removal of phosphorus in systems with higher SRT.
5 Conclusion
Excess accumulation of nutrient in the wastewater cause water pollution and create environmental issues. BNR is an essential step before discharging the effluent from treatment plant. However, upgrading the existing treatment plants is recommended to meet the discharge standards and to protect the ground and surface water quality. Numerous integrated technology was developed for BNR to achieve greater nutrient removal. This chapter provides knowledge regarding the recent innovative approaches followed for BNR were discussed. Such as aerobic granular activated sludge sequencing batch reactors, integrated fixed-film activated sludge reactor and integrated anoxic fixed bed and oxic moving bed biofilm reactor. In addition to this, a detailed TP mass balance was also discussed.
References
Al-Zreiqat I, Abbassi B, Headley T, Nivala J, van Afferden M, Müller RA (2018) Influence of septic tank attached growth media on total nitrogen removal in a recirculating vertical flow constructed wetland for treatment of domestic wastewater. Ecol Eng 118:171–178. https://doi.org/10.1016/j.ecoleng.2018.05.013
Bae H, Park K-S, Chung Y-C, Jung J-Y (2010) Distribution of anammox bacteria in domestic WWTPs and their enrichments evaluated by real-time quantitative PCR. Process Biochem 45:323–334. https://doi.org/10.1016/j.procbio.2009.10.004
Banu JR, Kaliappan S, Yeom I-T (2007) Two-stage anaerobic treatment of dairy wastewater using HUASB with PUF and PVC carrier. Biotechnol Bioprocess Eng 12:257–264. https://doi.org/10.1007/BF02931101
Banu JR, Do K-U, Yeom I-T (2008) Effect of ferrous sulphate on nitrification during simultaneous phosphorus removal from domestic wastewater using a laboratory scale anoxic/oxic reactor. World J Microbiol Biotechnol 24:2981–2986. https://doi.org/10.1007/s11274-008-9841-0
Barnard JL (1984) Activated primary tanks for phosphate removal. Water S A 10:121–126
Biswas BK, Inoue K, Harada H, Ohto K, Kawakita H (2009) Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J Environ Sci 21:1753–1760. https://doi.org/10.1016/S1001-0742(08)62484-5
Blöcher C, Niewersch C, Melin T (2012) Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration. Water Res 46:2009–2019. https://doi.org/10.1016/j.watres.2012.01.022
Bond PL, Hugenholtz P, Keller J, Blackall LL (1995) Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl Environ Microbiol 61:1910–1916
Breisha GZ, Winter J (2010) Bio-removal of nitrogen from wastewaters—a review. J of Am Sci 6:508–528. https://doi.org/10.7537/marsjas061210.60
Bunce JT, Ndam E, D. Ofiteru I, Moore A, Graham D (2018) A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. https://doi.org/10.3389/fenvs.2018.00008
Chi Y-Z, Zhang Y, Yang M, Tian Z, Liu R-Y, Yan F-Y, Zang Y-N (2018) Start up of anammox process with activated sludge treating high ammonium industrial wastewaters as a favorable seeding sludge source. Int Biodeterior Biodegradation 127:17–25. https://doi.org/10.1016/j.ibiod.2017.11.007
de Haas DW, Wentzel MC, Ekama GA (2000) The use of simultaneous chemical precipitation in modified activated sludge systems exhibiting biological excess phosphate removal-Part 1: Literature review. Water Sa 26:439–452. http://dx.doi.org/10.4314/wsa.v27i2.4987
Desmidt E, Ghyselbrecht K, Zhang Y, Pinoy L, Van der Bruggen B, Verstraete W, Rabaey K, Meesschaert B (2015) Global phosphorus scarcity and full-scale P-recovery techniques: a review. Crit Rev Environ Sci Technol 45:336–384. https://doi.org/10.1080/10643389.2013.866531
Do K-U, Banu RJ, Son D-H, Yeom I-T (2012) Influence of ferrous sulfate on thermochemical sludge disintegration and on performances of wastewater treatment in a new process: anoxic–oxic membrane bioreactor coupled with sludge disintegration step. Biochem Eng J 66:20–26. https://doi.org/10.1016/j.bej.2012.04.013
Do K-U, Rajesh Banu J, Kaliappan S, Yeom I-T (2013) Influence of the thermochemical sludge pretreatment on the nitrification of A/O reactor with the removal of phosphorus by simultaneous precipitation. Biotechnol Bioprocess Eng 18:313–320. https://doi.org/10.1007/s12257-012-0492-5
Egle L, Rechberger H, Krampe J, Zessner M (2016) Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci Total Environ 571:522–542. https://doi.org/10.1016/j.scitotenv.2016.07.019
Ekama GA (2015) Recent developments in biological nutrient removal. Water SA 41:515–524. https://doi.org/10.4314/wsa.v41i4.11
Emara M, Ahmed F, Abdel-Aziz FM, Abdel-Razek A (2014) Biological nutrient removal in Bardenpho process. https://doi.org/10.7537/marsjas100514.07
Esakki Raj S, Kaliappan S, Adish Kumar S, Rajesh Banu J (2012) Combinative treatment (thermal-anaerobic) of EBPR sludge for the enhanced release and recovery of phosphorous. Int J Environ Eng 4:92–104. https://doi.org/10.1504/IJEE.2012.048097
Fontenot Q, Bonvillain C, Kilgen M, Boopathy R, (2007) Effects of temperature, salinity, and carbon: nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater. Bioresour Technol 98:1700–1703. https://doi.org/10.1016/j.biortech.2006.07.031
Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, Westerhoff P (2018) Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl Catal B Environ 236:546–568. https://doi.org/10.1016/j.apcatb.2018.05.041
Gong L, Jun L, Yang Q, Wang S, Ma B, Peng Y (2012) Biomass characteristics and simultaneous nitrification–denitrification under long sludge retention time in an integrated reactor treating rural domestic sewage. Bioresour Technol 119:277–284. https://doi.org/10.1016/j.biortech.2012.05.067
Gonzalez-Martinez A, Rodriguez-Sanchez A, Muñoz-Palazon B, Garcia-Ruiz M-J, Osorio F, van Loosdrecht MCM, Gonzalez–Lopez J (2015) Microbial community analysis of a full-scale DEMON bioreactor. Bioprocess Biosyst Eng 38:499–508. https://doi.org/10.1007/s00449-014-1289-z
Guo J, Yang Q, Peng Y, Yang A, Wang S (2007) Biological nitrogen removal with real-time control using step-feed SBR technology. Enzyme Microb Technol 40:1564–1569. https://doi.org/10.1016/j.enzmictec.2006.11.001
Hafez H, Elbeshbishy E, Chowdhury N, Nakhla G, Fitzgerald J, Van Rossum A, Gauld G (2010) Pushing the hydraulic retention time envelope in modified Ludzack Ettinger systems. Chem Eng J 163:202–211. https://doi.org/10.1016/j.cej.2010.07.033
He Q, Song Q, Zhang S, Zhang W, Wang H (2018) Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: reactor performance, extracellular polymeric substances and microbial successions. Chem Eng J 331:841–849. https://doi.org/10.1016/j.cej.2017.09.060
Hocaoglu SM, Insel G, Cokgor EU, Orhon D (2011) Effect of low dissolved oxygen on simultaneous nitrification and denitrification in a membrane bioreactor treating black water. Bioresour Technol 102:4333–4340. https://doi.org/10.1016/j.biortech.2010.11.096
Irizar I, Suescun J, Plaza F, Larrea L (2004) Optimizing nitrogen removal in the BioDenitro process. Water Sci Technol 48:429–436. https://doi.org/10.2166/wst.2004.0891
Le HT, Jantarat N, Khanitchaidecha W, Ratananikom K, Nakaruk A (2018) Performance of nitrogen removal in attached growth reactors with different carriers. J Water Reuse Desalin 8:331–339. https://doi.org/10.2166/wrd.2017.182
Lee D, Kim M, Chung J (2007) Relationship between solid retention time and phosphorus removal in anaerobic-intermittent aeration process. J Biosci Bioeng 103:338–344. https://doi.org/10.1263/jbb.103.338
Liu G, Wang J (2017) Enhanced removal of total nitrogen and total phosphorus by applying intermittent aeration to the modified Ludzack-Ettinger (MLE) process. J Clean Prod 166:163–171. https://doi.org/10.1016/j.jclepro.2017.08.017
Luo W, Hai FI, Price WE, Guo W, Ngo HH, Yamamoto K, Nghiem LD (2016) Phosphorus and water recovery by a novel osmotic membrane bioreactor–reverse osmosis system. Bioresour Technol 200:297–304. https://doi.org/10.1016/j.biortech.2015.10.029
Mannina G, Capodici M, Cosenza A, Di Trapani D (2016) Carbon and nutrient biological removal in a University of Cape Town membrane bioreactor: analysis of a pilot plant operated under two different C/N ratios. Chem Eng J 296:289–299. https://doi.org/10.1016/j.cej.2016.03.114
Mekonnen MM, Hoekstra AY (2018) Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: a high-resolution global study. Water Resour Res 54:345–358. https://doi.org/10.1002/2017WR020448
Metcalf E, Eddy M (2014) Wastewater engineering: treatment and resource recovery. Mc Graw-Hill, USA
Nhu Hien N, Van Tuan D, Nhat PT, Van Thi Thanh T, Van Tam N, Xuan Que VON, Phuoc Dan N (2017) Application of Oxygen Limited Autotrophic Nitritation/Denitrification (OLAND) for anaerobic latex processing wastewater treatment. Int Biodeterior Biodegradation 124:45–55. https://doi.org/10.1016/j.ibiod.2017.07.009
Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL, Reis MAM (2007) Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res 41:2271–2300. https://doi.org/10.1016/j.watres.2007.02.030
Petzet S, Cornel P (2009) P-recovery from sewage sludge and sewage sludge ashes. Present Balt 21:28–30
Powley HR, Dürr HH, Lima AT, Krom MD, Van Cappellen P (2016) Direct discharges of domestic wastewater are a major source of phosphorus and nitrogen to the Mediterranean sea. Environ Sci Technol 50:8722–8730. https://doi.org/10.1021/acs.est.6b01742
Puig S, Coma M, Monclús H, van Loosdrecht MCM, Colprim J, Balaguer MD (2008) Selection between alcohols and volatile fatty acids as external carbon sources for EBPR. Water Res 42:557–566. https://doi.org/10.1016/j.watres.2007.07.050
Qiu G, Ting Y-P (2014) Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour Technol 170:221–229. https://doi.org/10.1016/j.biortech.2014.07.103
Raj SE, Banu JR, Kaliappan S, Yeom I-T, Adish Kumar S (2013) Effects of side-stream, low temperature phosphorus recovery on the performance of anaerobic/anoxic/oxic systems integrated with sludge pretreatment. Bioresour Technol 140:376–384. https://doi.org/10.1016/j.biortech.2013.04.061
Rajasulochana P, Preethy V (2016) Comparison on efficiency of various techniques in treatment of waste and sewage water—a comprehensive review. Resour Technol 2:175–184. https://doi.org/10.1016/j.reffit.2016.09.004
Rajesh Banu J, Do K-U, Kaliappan S, Yeom I-T (2009a) Effect of alum on nitrification during simultaneous phosphorous removal in anoxic/oxic reactor. Biotechnol Bioprocess Eng 14:543–548. https://doi.org/10.1007/s12257-008-0279-x
Rajesh Banu J, Uan DK, Yeom I-T (2009b) Nutrient removal in an A2O-MBR reactor with sludge reduction. Bioresour Technol 100:3820–3824. https://doi.org/10.1016/j.biortech.2008.12.054
Salehi S, Cheng KY, Heitz A, Ginige MP (2018) Re-visiting the Phostrip process to recover phosphorus from municipal wastewater. Chem Eng J 343:390–398. https://doi.org/10.1016/j.cej.2018.02.074
Schönborn C, Bauer H-D, Röske I (2001) Stability of enhanced biological phosphorus removal and composition of polyphosphate granules. Water Res 35:3190–3196. https://doi.org/10.1016/S0043-1354(01)00025-2
Sen TK (2015) Physical, chemical and biological treatment processes for water and wastewater. Nova science publisher
Seviour RJ, Mino T, Onuki M (2003) The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev 27:99–127. https://doi.org/10.1016/S0168-6445(03)00021-4
Shen L, Hu A, Jin R, Cheng D, Zheng P, Xu X, Hu B (2012) Enrichment of anammox bacteria from three sludge sources for the startup of monosodium glutamate industrial wastewater treatment system. J Hazard Mater 199–200:193–199. https://doi.org/10.1016/j.jhazmat.2011.10.081
Sri Shalini S, Joseph K (2018) Combined SHARON and ANAMMOX processes for ammoniacal nitrogen stabilisation in landfill bioreactors. Bioresour Technol 250:723–732. https://doi.org/10.1016/j.biortech.2017.10.077
Subramaniam M (2018) Introduction: water crisis BT - contesting water rights: local, state, and global struggles. In: Subramaniam M (ed). Springer International Publishing, Cham, pp 1–23
Sudarno U, Winter J, Gallert C (2011) Effect of varying salinity, temperature, ammonia and nitrous acid concentrations on nitrification of saline wastewater in fixed-bed reactors. Bioresour Technol 102: 5665–5673. https://doi.org/10.1016/j.biortech.2011.02.078
Suneethi S, Joseph K (2011) ANAMMOX process start up and stabilization with an anaerobic seed in Anaerobic Membrane Bioreactor (AnMBR). Bioresour Technol 102:8860–8867. https://doi.org/10.1016/j.biortech.2011.06.082
Tabassum S, Li Y, Chi L, Li C, Zhang Z (2018) Efficient nitrification treatment of comprehensive industrial wastewater by using Novel Mass Bio system. J Clean Prod 172:368–384. https://doi.org/10.1016/j.jclepro.2017.10.022
Tsushima I, Ogasawara Y, Kindaichi T, Satoh H, Okabe S (2007) Development of high-rate anaerobic ammonium-oxidizing (anammox) biofilm reactors. Water Res 41:1623–1634. https://doi.org/10.1016/j.watres.2007.01.050
Uan DK, Yeom IT, Arulazhagan P, Rajesh Banu J (2013) Effects of sludge pretreatment on sludge reduction in a lab-scale anaerobic/anoxic/oxic system treating domestic wastewater. Int J Environ Sci Technol 10:495–502. https://doi.org/10.1007/s13762-012-0120-0
Uygur A, Kargı F (2004) Biological nutrient removal from pre-treated landfill leachate in a sequencing batch reactor. J Environ Manag 71:9–14. https://doi.org/10.1016/j.jenvman.2004.01.002
Wong M-T, Mino T, Seviour RJ, Onuki M, Liu W-T (2005) In situ identification and characterization of the microbial community structure of full-scale enhanced biological phosphorous removal plants in Japan. Water Res 39:2901–2914. https://doi.org/10.1016/j.watres.2005.05.015
Xu X, Liu G, Zhu L (2011) Enhanced denitrifying phosphorous removal in a novel anaerobic/aerobic/anoxic (AOA) process with the diversion of internal carbon source. Bioresour Technol 102:10340–10345. https://doi.org/10.1016/j.biortech.2011.08.108
Yuan Y, Liu J, Ma B, Liu Y, Wang B, Peng Y (2016) Improving municipal wastewater nitrogen and phosphorous removal by feeding sludge fermentation products to sequencing batch reactor (SBR). Bioresour Technol 222:326–334. https://doi.org/10.1016/j.biortech.2016.09.103
Yuan Z, Pratt S, Batstone DJ (2012) Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol 23:878–883. https://doi.org/10.1016/j.copbio.2012.08.001
Yue X, Yu G, Liu Z, Tang J, Liu J (2018) Fast start-up of the CANON process with a SABF and the effects of pH and temperature on nitrogen removal and microbial activity. Bioresour Technol 254:157–165. https://doi.org/10.1016/j.biortech.2018.01.019
Zhang Y, Song C, Ji L, Liu Y, Xiao J, Cao X, Zhou Y (2018) Cause and effect of N/P ratio decline with eutrophication aggravation in shallow lakes. Sci Total Environ 627:1294–1302. https://doi.org/10.1016/j.scitotenv.2018.01.327
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yukesh Kannah, R., Gunasekaran, M., Kumar, G., Ushani, U., Do, KU., Rajesh Banu, J. (2019). Recent Developments in Biological Nutrient Removal. In: Bui, XT., Chiemchaisri, C., Fujioka, T., Varjani, S. (eds) Water and Wastewater Treatment Technologies. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3259-3_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-3259-3_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3258-6
Online ISBN: 978-981-13-3259-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)