Abstract
Recently, the quickly growing population living in urban location has caused numerous conflicts related to increase in water demand and water pollution. In urban areas, the surface water bodies allow runoffs and storms and in addition act as wastewater drainage pathways. Mostly, the imperfect separation of rainwater and clean wastewater has made large quantities of wastewater discharged into the surface water, resulting in serious pollution. There are many treatment methods for the polluted water bodies such as coagulation, filtration, and ecological floating bed which are related to nutrient removal. The above listed methods are usually capable in reducing pollution load. Wastewaters generated from two sources such as point source (domestic and industries) and non-point source (agricultural and storm water runoff). Finally it reaches nearby water bodies and the abovementioned methods are to be frequently employed in a wastewater treatment plant to remove nutrients. Most of the pollutants in the vastly polluted water are in dissolved forms; hence, an appropriate treatment method relevant to the design and development of the integrated multistage reactor with extended wastewater treatment is reviewed in this paper. Evaluating the accumulation, precipitation, retention, and removal of phosphorus, along with removal of nitrogen, is discussed in brief.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The poor quality of the surface water is often interlinked to eutrophication (a condition occurring due to excessive growth of aquatic plants and algae) [1,2,3]. It not only disturbs the food chain of the aquatic organisms but also creates problems in areas which solely depend on the supply of surface water. In recent years, eutrophication is known to appear in coastal regions too. Nutrients such as nitrogen and phosphorus are found to be the limiting factor for the growth of algal bloom and bacteria in the eutrophic surface waters [4,5,6,7,8,9,10]. It results in the degradation of the quality of water and it also impairs the ecosystem of the freshwater. These harmful algal blooms pose serious threats such as production of toxins in anoxic conditions, killing of fish and thereby altering the biodiversity [1]. Due to the production of toxic secondary metabolites, it is also considered as risk for public health [2]. Table 1 shows the sources, properties, and fate of low strength nitrogen wastewater.
Nutrient removal during the treatment of wastewater is considered as an apt method to limit these nutrients. During wastewater treatment, nitrogen and phosphorus are consumed by microbes and hence there is reduction of these nutrients during biological treatment. This strategy is used to control eutrophication in water bodies, either in designing a new wastewater treatment facility or to upgrade an existing facility. Organic pollutants such as polycyclic aromatic carbons, polychlorinated biphenyls (released from chemical industries), insecticides, and pesticides (released from agriculture fields) pollute the wastewater making the water unsafe. Wastewater can be classified as high, medium, or low strength based on the carbon/nitrogen ratio or the chemical oxygen demand/total Kjeldahl nitrogen ratios [16]. A characteristic biological nutrient removal (BNR) system is a variation of a basic activated sludge system that includes recycling of the suspended solids, inoculum of microbes, varied HRT, and inactive residues [17]. To remove the nutrients in significant levels, these systems entail additional reactors. Thus, a bioreactor of BNR is divided into three different zones such as anaerobic zone, anoxic zone, and aerobic zone with the characteristic recirculation of suspended solids. These redox zones are classified based on the electron acceptor which is utilized. Electron acceptors are absent in the anaerobic zone, oxygen is the electron acceptor in the aerobic zone, and nitrate is the electron acceptor in the anoxic zone.
Various methods have been developed for treatment of organic wastewater which includes reverse osmosis [18], ion exchange [19] gravity [20], and adsorption [21]. Implementation of strict nutrient discharge standards requires effective wastewater treatment technologies. Membrane bioreactor (MBR) is one of the recently developed reactors to treat, recover, and recycle wastewater effectively [22]. It has a vast range of applications and it is more flexible to operate [23], discharges effluent of good quality [24] occupies less space due to the smaller size of the plant [25], its ability to remove microbes due to small pore size of the membrane, and reduced production of sludge [26] are the important advantages when compared to the standard biological treatment processes. Besides the MBR, another promiscuous technology that can effectively remove nutrients from the wastewater streams is the sequencing batch reactor (SBR). They have been used to remove carbonaceous material and also to remove nutrients [27]. It is vital that existing bench-scale SBRs can be modified to be operated easily in a way that guarantees production of effluent with the desired nutrient concentrations through incorporating sequential anaerobic, anoxic, and oxic zones. This paper reviews the process of BNR along with the recent trends used in BNR such as MBR, SSPR, and constructed wetlands, the factors which limit the removal of nutrients and the cost incurred to remove nutrients from low strength organic wastewater. Thus, this review mainly focuses on the identification of the cost efficient systems in terms of treatment and construction which can intensify BNR process.
Biological Nutrient Removal
During biological treatment, significant amount of nutrients is removed. After biological treatment of the wastewater, the sludge comprises of 12% of nitrogen and 3% of phosphorus by weight. BNR is designed in such a way to remove the most amount of nutrients compared to these metabolic ranges. BNR consists of two important steps, namely the biological nitrogen removal which in turn comprises of nitrification and denitrification processes and biological phosphorus removal [28]. The nutrient removal usually occurs through (i) denitrification of nitrates by denitrifiers along with nitrification of ammonia to nitrate by nitrifiers and (ii) increased consumption of phosphorus by phosphate-accumulating organisms.
Biological Nitrogen Removal
The nitrogen removal process via conventional wastewater treatment systems demands excess energy for creating aerobic environment during bacterial nitrification. By utilizing organic carbon, the produced nitrate can be removed via bacterial denitrification. The major reaction mechanisms involved in biological nitrogen removal process are described below. The conventional method includes nitrification, denitrification, ammonification, and uptake of nitrogen essential for cell growth. The process of nitrification is performed in two steps. In each step, two different obligate anaerobic nitrifying bacterial consortia oxidize particular form of nitrogen biologically. In the initial step, ammonia is oxidized to hydroxylamine, catalyzed by an enzyme mono-oxidase. The resulting hydroxylamine is further oxidized to nitrite catalyzed by an enzyme named hydroxylamine oxidoreductase. These steps occur in the presence of ammonia oxidizing bacteria (Nitrosomonas sp.). The second step is the further oxidation of nitrite to nitrate biologically, performed by nitrite-oxidizing bacteria (Nitrobacter sp.) under oxic conditions [17]. The overall process of nitrification and denitrification is shown in Fig. 1.
Nitrification
During the nitrification process, oxygen acts as the electron acceptor, ammonia acts as the electron donor, and carbon dioxide is the main source of carbon. Nitrification is the rate limiting step of the BNR process as the ammonia oxidizing bacteria constitute only 2% of the total microbial biomass and hence there is a lack of functional heterogeneity. Moreover, these bacteria are very sensitive to their surrounding conditions and have very strict growth requirements. Thus, through nitrification nitrogen is converted from its reduced form to its oxidized form. But this step exclusively is not a nitrogen removal process; thus, denitrification is also employed for effective nitrogen removal. The chemical reaction involved during nitrification is given in Eq. (1)
Denitrification
Denitrification involves the consumption of readily biodegradable organic matter by a particular heterotrophic nitrite-oxidizing bacteria. The process takes place in the presence of combined oxygen and in the absence of dissolved or free oxygen and it reduces nitrate which acts as a terminal electron acceptor and converts it into insoluble nitrogen. This reduction reaction involves few steps such as initial conversion of nitrate to nitrite, which is converted into nitric oxide and this in turn is converted into nitrous oxide and finally into nitrogen. The equation representing the reduction reaction is shown in Eq. (1). Microbes that are able to denitrify are Bacillus sp., Alcaligenes sp., Aerobacter sp., Pseudomonas sp., Micrococcus sp., Achromobacter sp., Brevibacterium sp., etc. The chemical reaction involved during denitrification is given in Eq. (2)
Denitrification decreases the pH of the media. Still, addition of chemicals to control pH is not required as the entire nitrification and denitrification process stabilizes the pH of the media. Nitrification decreases the pH and thus the process balances the pH level.
Ammonification
Ammonification is the process of conversion of nitrite and/or nitrate into ammonium ions (NH4+). During this process, a series of decomposers are involved in the formation of ammonium ions such as Bacillus, Clostridium, Flavobacter, and Pseudomonas. Otherwise, the ammonification process is quite reverse of nitrification process. It the last step involved in nitrogen cycle. The chemical reaction involved during ammonification is given in Eq. (3)
Simultaneous Nitrification and Denitrification (Aerobic Denitrification)
In an aerobic reactor, both the nitrification and denitrification processes happen simultaneously within the microbial flocs (Fig. 1). A higher nitrogen removal efficiency of 80 to 96% can be achieved through this process without requiring any extra carbon and alkalinity. During this process, a carbon to nitrogen ratio of 10 and a dissolved oxygen concentration ranging from 0.3 to 0.7 mg/L were maintained to achieve higher removal efficiency. This process mainly relies on dissolved oxygen concentration, size of sludge, and diffusion blockades.
Short Cut Biological Nitrogen Removal and Nitrite Shunt
The short cut biological nitrogen removal is the process involving removal of nitrogen from through non-conventional means by employing two approaches—nitrite shunt and deammonification [29]. The “Nitrite Shunt” process is also known as nitritation-denitritation. In this process, the oxidation step of ammonia stops at nitrite production stage and this is called as partial nitrification and the produced nitrite is reduced to dinitrogen via heterotrophic denitrification. This process involves accumulation of ammonia oxidizing bacteria and inhibition of nitrite-oxidizing bacteria. This may lead to total nitrogen removal efficiency of 40% besides 25% of cost associated with aeration could be reduced by eliminating nitrite oxidation [30]. Zhuang et al. [31] have reported about nitrite shunt process in single-stage bioreactor treating synthetic wastewater. The authors operated the bioreactor with low dissolved oxygen and this can be a good alternative on the basis of energy recovery and less carbon to nitrogen ratio demand. Single-stage nitrite shunt denitrification (through nitrite rather than nitrate) with low dissolved oxygen (DO) supply is a better alternative in terms of energy efficiency, short-footprint, and low C/N ratio requirement. They obtained higher nitrogen and organic removal efficiency of 60.7% and 97.9% under dissolved oxygen level of 0.3 mg/L at 20 °C, respectively, reporting that such operational circumstances obtained efficient nitrite-oxidizing microbe inhibition and effective denitrification.
Nitritation—ANAMMOX
ANAMMOX process which is also known as anaerobic ammonium oxidizing process is the promising strategy for treating high strength nitrogen-rich effluent. During this process, partial oxidation of ammonia to nitrite takes place by aerobic ammonia oxidizers. The produced nitrite is used as electron acceptor by anaerobic ammonia oxidizing microbe (Candidatus Brocadia fulgida). This bacterium oxidizes the residual ammonia to dinitrogen. Ge et al. [32] have investigated the removal of nitrogen from saline and hypersaline wastewater through nitritation-anammox process and reported the impact of salt stress to the microbial action during this process. The authors reported that with the aid of improved aeration, ammonia oxidizing bacteria were able to tolerate 3% salinity via ammonia monooxygenase enzyme which could promote and mediate the ammonium to nitrite conversion. However, the hypersaline condition (5% salinity) inhibits the activity of anammox especially the genus Nitrospira bacteria which were salt sensitive and deteriorate the whole process under such conditions.
Designed Biological Nitrogen Removal Processes
Many advanced nitrogen removal systems have been designed based on the known reactions mechanisms for treatment of wastewater. Based on the microbial growth process, the existing BNR methods are two types: (1) Suspended sludge growth process and (2) attached sludge growth process. The attached sludge growth processes are classified into two major types: (a) biofilm and (b) granulated sludge process. Current research progress in biological wastewater treatment leads to the development of hybrid or integrated process that involves biofilm as well as granular sludge strategies to treat wastewater.
Suspended Sludge Processes
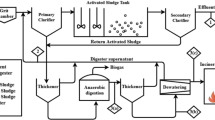
Suspended sludge process is the conventional approach used mainly for nutrient removal. The traditional nitrogen removal approaches for example, Ludzack–Ettinger and its modified form, Bardenpho, and anoxic processes work on the basis of activated sludge systems but these processes are pliable to modified already prevailing activated sludge process (ASP). The main drawback of suspended sludge process is the lack of good regulation on inner recycling flow to obtain effective nitrogen removal. The examples of suspended sludge process such as sequential batch reactor systems and Cannon process are explained below. Figure 2 shows a different type of suspended growth reactor.
Sequential Batch Reactor
Sequential batch reactor is a recent alternative to ASP and is acquiring fame because of its promising working condition and broad probable applications. There are six phases in sequential batch reactors which include fill, mixing, areation, settle, draw, and idle. For continuous treatment system, several sequential batch reactors are employed concurrently and as a minimum a single system is nourished every time. Sequential batch reactor is a significant implement for biological nutrient reduction, proficient in obtaining effluent after treatment with much less nitrogen and phosphorus level from high strength influent. During startup phase, the reactor possesses extreme biological oxygen demand and nil dissolved oxygen and it is appropriate for nitrogen removal and turns anaerobic phase for phosphate removal. During aeration phase, uptake of phosphate takes place. Then, oxidation of organics takes place in these reactors [33].
CANON Process
CANON (completely autotrophic nitrogen removal over nitrite) process depends on a steady association among the two groups of autotrophs in which both the reaction such as nitritation and ANAMMOX take place in inadequate oxygen environments. In sequential batch reactors, Sliekers et al. [34] have performed CANON process treating ammonium-rich influent and obtained 0.3 kg N/m3 day of nitrogen removal efficiency. The main limitations of this process are very slow operation rate, regulation of biomass, and stringent dissolved oxygen level (< 0.5 mg/L). Xiao et al. [35] have showed that partial nitrification, ANNAMOX, and nitrogen removal process take place simultaneously at effective condition in sequential batch reactors.
Attached Growth Process
Attached growth process is another well-developed approach employed for BNR process. Pedros et al. [36] have investigated about the submerged attached growth reactor treating centrate form wastewater and the study revealed nearly 85% nitrogen removal. This process is of two types—biofilm-based and granular-based processes. Examples of biofilm-based processes are aerobic fixed-film bioreactor (submerged form), trickling filters, and biofilters. Trickling filters and biofilters are fixed-film bioreactors. Figure 3 shows a different type of attached growth reactor.
Trickling Filters and Biofilters
The trickling filters and biofilters are fixed-film-based bioreactors employed for biological nitrogen removal. Sánchez Guillén et al. [37] have reported 52–54% of nitrogen removal via partial nitritation in sponge-bed trickling filter reactors. A conventional trickling filter reactor showed improved nitrogen reduction efficiency of more than 60% [38]. A study reported that biofilter reactor showed 59.8–82.1% of nitrogen reduction. The study showed that the reactors were accumulated with ammonia oxidizers and nitrite oxidizers [39]. On the other hand, the application of fixed-film reactors is limited due to its complexity in sustaining anoxic condition.
Fluidized Bed or Moving Bed Bioreactors
Moving bed bioreactor (MBBR) processes are frequently used bioreactors for nitrogen removal in A2O (anoxic–oxic–oxic) mode. To obtain strict nitrate reduction and to get efficient nitrogen removal, pre-anoxic bioreactor having internal recycle can be used following with post-anoxic bioreactor involving external carbon supplementation. Anoxic MBBR can be employed as post nitrogen removing bioreactor with external carbon supplementation next to activated sludge units (nitrification).
Granular Sludge Based
The granular sludge–based processes have wide applications in simultaneous nitrification–denitrification reactions and generate possibility for various explorations in many fields for example attaining granulation of sludge in definite treatment unit conditions, governing size of granules, upholding less dissolved oxygen, recycling of sludge, and dropping the entire trail. Kagawa et al. [40] have studied about the biological nitrogen removal reaction in sequential batch reactor possessing anaerobic–aerobic–anoxic series. The authors reported that the constancy of nutrient reduction with granular biomass is liable mainly on dissolved oxygen level at the aerobic condition.
Hybrid Process
ENBNRAS
The external nitrification (EN) BNRAS system (ENBNRAS) resembles biological anoxic phosphorus removal process (DEPHANOX). The major variation is the presence of pre-anoxic reactor to remove nitrate in BNRAS. The influent flow is exit to the anaerobic region in ENBNRAS. The treated effluent rich in ammonia in the anaerobic zone flows into an interior settlement chamber to segregate the solids rich in organics. The easily biodegradable organics are anaerobically fermented to volatile fatty acids (VFA) in the anaerobic reactor. These VFAs are utilized by the phosphorus-accumulating organisms and accumulated as intracellular granules such as polyhydroxyalkanoates. The easily biodegradable and particulate organics are trapped within the sludge liquor in the anaerobic bioreactor. The external nitrified wastewater is exit to the anoxic region for nitrogen removal. The denitrified wastewater exits to the last reactor from the anoxic reactor in which stripping of nitrogen gas, oxidation of residual organics, and completion of phosphorus uptake take place.
Integrated Fixed-Film Activated Sludge Process
In integrated fixed-film activated sludge (IFAS) tanks, interior fixed media such as ringlace and free-floating media have been used in aerobic reactor to reduce sludge age. The fixed media can be used for the development of nitrifying bacterial populations fixed media and this population develops a permanent residing place in the reactor. These reactors are specifically useful for treating low-temperature wastewaters (10 to 15 °C) due to reduction in sludge age. Moreover, these IFAS reactors can be combined with traditional biological nitrogen removal process (A2O TYPE). The main drawback of this process is the existence of nitrification inside the biofilm which may require adequate amount of oxygen; due to dense biofilm growth and the boundary line, the penetration of dissolved oxygen will be limited and therefore, IFAS needs dissolved oxygen (DO) in the range of 4 to 6 mg/L in the liquid in contrast to 2 mg/L in the case of traditional suspended sludge process [41].
Modified UCT Type
Modified UCT type is a modified configuration of membrane bioreactor system and the main use of MBR BNR technology is the decreased footprint and the capability to make advance the prevailing process without any additional reactor configuration. An important drawback of modified UCT system is the elevated energy spent for aeration. This could be due to lack of oxygen transfer when the reactors are functioned with highly concentrated wastewaters. In addition, this modified process can meet the strict nutrient levels with complete solids reduction. On the other hand, this benefit mainly depends on characteristics of influent and it is not possible in all cases. However, achieving this still depends on having favorable influent wastewater characteristics and may not be feasible in all cases. In those critical conditions, biological nutrient reduction can be supplied with few chemicals (ferric or alum) for precipitation of nutrients. Biological P removal can be supplemented with limited addition of chemicals for precipitation of P in order to make the biological process to achieve maximal nutrient removal efficiency. Figure 4 shows different types of hybrid reactor: (a) Integrated fixed-film activated sludge (IFAS) and (b) modified UCT type reactor.
Biological Phosphorus Removal
Phosphorus plays an important role in encouraging the growth of photosynthetic microbes and algal blooms and thus they should be removed to avoid eutrophication of the aquation ecosystem [42]. It is also required for the cellular metabolic activities in providing energy and in biosynthesis. Enhanced biological phosphorus removal (EBPR) is based on the greater uptake of phosphorus more than the requirement of the cell, which halts the metabolism of other nutrients within the cells. The group of specialized hetertrophic aerobes which are principally in charge for EBPR are phosphorus-accumulating organisms (PAOs) [43]. These organisms store the phosphorus as polyphosphate [42]. Acinetobacter such as Accumulibacter phosphatis is an important PAO which is known extensively.
EBPR is achieved by recirculation of mixed liquor solids between the aerobic and anaerobic conditions thus providing a suitable environment for the cultivation of PAOs. In the absence of oxygen, nitrite, or nitrate (anaerobic condition), these organisms are able to degrade the VFA (acetate or propionate) accumulated after fermentation by anaerobic bacteria and they are stored as biodegradable polymers such as polyhydroxyacetones or polyhyroxybutyrates. These polymers are utilized in the subsequent aerobic/anoxic zones. Liu et al. [44] have reported that VFA plays an essential part in the BNR process as it is the best, cheap carbon source for phosphorus uptake, and VFA is an energy organic material which influences the efficiency of phosphorus-accumulating organisms. The PAO bacteria grow well by utilizing VFA as an organic-rich carbon source. Greater the availability of food sources (VFAs), better the activity and development of PAOs. During biological phosphorus removal, nearly 7 to 10 mg of VFAs is utilized to remove 1 mg of phosphorus. IF adequate VFAs are not synthesized naturally during biological phosphorus removal process, then artificial chemical supplementation is essential to enhance the PAOs accumulation and enhanced biological phosphorus removal efficiency [45]. Glycolysis of the intracellular stored glycogen provides the necessary reducing energy for this reaction which conforms with the Mino Model [46]. Polymerization of the short-chain fatty acids by PAOs requires energy which is obtained by the cleavage of intracellular stored granules of inorganic polyphosphates (poly-P). Orthophosphate is released into the bulk fluid of the anaerobic zone. Thus, the source of phosphorus in the anaerobic tank is through the influent feed and through the phosphorus released by the organisms. The capacity of the PAOs to store the anaerobic substrate makes it advantageous over the other microbes in the system. Consequently only PAOs survive in the anaerobic tank though their specific growth is less compared to the other heterotrophic bacteria [47].
When oxygen and nitrate are available as electron acceptors in the second phase of aerobic zone, the PAOs utilize the already stored polyhydroxyacetones or polyhyroxybutyrates to acquire energy for their growth and nourishment. As the organisms are exposed to both aerobic and anaerobic environments, they are strained and accumulate excess of phosphorus. The energy received is more than sufficient to absorb both the phosphorus released as well as the feed phosphorus. These organic polymers help the organisms to replenish the polyphosphates and glycogen by absorbing the orthophosphates from the media. PAOs store the inorganic polyphosphates and energy within the cells as volutins or metachromatic granules. Polyhydroxyacetones or polyhyroxybutyrates along with the inorganic polyphosphates help the PAOs to grow even in the absence of oxygen (anaerobic condition). Removal of phosphorus both in aerobic condition and anaerobic condition by PAOs is depicted in Fig. 5. Effective enhanced biological phosphorus removal performance depends on the activity of PAOs, and the polyphosphate granules or polymers impart an important role in enhanced biological phosphorus removal performance. The polyphosphate granules accumulated within the cells are tetrahedral phosphate residues with linear chains polymers connected through common oxygen atom linkages with the help of phosphoanhydride bonds. The counterions of polyphosphate granules include many monovalent/or divalent metals such as Mg2+, K+, Ca2+, and Na+. These counter ions form complexes with phosphate residues which are negatively charged. It has been reported in literature that there are probable relationships among metals of polyphosphates composition, enhanced biological removal performance, and long-standing stable performance of the process [48]. On the other hand, the mechanism behind that is largely unknown. Limited concentration of magnesium and potassium ions in the influent wastewater during biological phosphorus removal process may affect the performance of the reactors. Schönborn et al. [49] reported that increase in influent magnesium level and the subsequent raised magnesium concentration (i.e., increment of Mg/P molar ratio from about 0.13 to 0.23) in polyphosphate granules enhanced the extended and stable phosphorus removal performance by 12% in enhanced biological phosphorus removal system. Metal contents of polyphosphate and its compositions were reported to rely on enhanced biological phosphorus removal operational conditions such as influent composition, and stable process performance.
Enhanced Biological Phosphorus Removal
In recent years, enhanced biological phosphorus removal (EPBR) along with activated sludge process has been investigated extensively. It is recognized to be a cheap and ecofriendly alternative to chemical-based process [50]. On the other hand, the main limitation of this process is the fluctuation in function and more need on accomplished operators and this results in process control limitations. Therefore, it lacks potential to be applicable at decentralized plants [51]. However, it has wide range of applications and frequent progress in proficiency and dependability, and therefore it can be implemented at small operational units.
Granular Sludge Reactors
These bioreactors can be progressed to function aerobically or anaerobically. Granulated sludge process, biofilm-based approach depends on granular biomass growth. These reactors are gaining attention [52] for the removal of nitrogen, phosphorus and organics removal [53]. This process in commercial form (NeredaR process) obtained 87% of phosphorus removal efficiency in large-scale units [53].
Sequencing Batch Biofilm Reactors
These bioreactors are still regarded as a novel promising one [54]. On the other hand, they are reputable. The carrier used for biofilm growth may be immobile, a mobile bed, or in suspension. These three processes provide benefits of greater biomass retention nearly 50% than activated sludge–based process. The advantages of these process are the less requirement for sludge settling, and the less space requirement [55]. More than 90% of total phosphorus was achieved by Yin et al. [56] using fixed-carrier sequencing batch biofilm reactors at lab-scale level. In some cases additional supplementation of carbon source or chemicals are required to lower the phosphorus level in the treated wastewater.
Novel EBPR Systems
Sequencing Batch Moving Bed Membrane Bioreactor
Novel membrane bioreactors have been designed to obtain optimized nutrient removal. For example, Yang et al. [57] have designed and employed sequencing batch moving bed membrane bioreactor and this reactor attained a phosphorus removal efficiency of nearly 84%. Even though these reactors have potential applications and advancements, even at lab-scale, the consistency and steadiness of those bioreactors for phosphorus removal are very less. Additional investigation is needed to develop the consistency of these, bioreactors specifically employing influent wastewater concentration. In addition, the costs related with capital, operations, and environment must be evaluated with these bioreactors.
ANOX-an Bioreactors
ANOX-an bioreactors are the novel advancement of granulation single systems and it works at anaerobic–anoxic environment for phosphorus reduction. These bioreactors are employed for removal of numerous nutrients simultaneously [58]. These bioreactors in upflow form offer savings in cost and space on the basis of energy input. These benefits make it probable for biological phosphorus removal efficiency. Using these reactors, nearly 89% of phosphorus removal efficiency can be achieved but higher energy is required with the aid of mechanical mixers [58].
Various Methods in BNR
Reactors in Wastewater Treatment
BNR is carried out conventionally in various reactors operating either with suspended growth of microbes or with attached growth [17]. Microbes which are free floating in the wastewater are used to remove the nutrients in suspended growth operations. Generally this requires aerobic treatment. However, to enhance the nutrient removal, anoxic treatment can be combined with the aerobic treatment. Usually activated sludge process is employed to treat wastewater. Air diffusers aerate the reactor and the infused oxygen allows the microbes to remove nutrients [59]. They also provide uniform mixing inside the reactor. Nitrification takes place at higher hydraulic retention time and sludge retention time. Whereas denitrification occurs when there is insufficient oxygen supply. In a study by Kim et al. [60], wastewater was treated using aerobic–anoxic methane oxidation bioreactor. After continuous operation for three months, the total nitrogen removal rate was 76.3% and the total phosphorus removal rate was 83.7%. Microbes are attached to an inert surface in attached growth operations [61]. The wastewater is passed over the surface and the bacteria remove the nutrients. Development of bacteria on a fixed surface is usually termed as biofilm. Moving bed bioreactors with nutrient removal efficiency of 68.17% [62]. Circulating fluidized bed bioreactor with removal efficiency of 82% [63, 64]. Membrane bioreactor with a removal efficiency of 87.79% [65] and granular anammox reactor with nitrogen removal efficiency of 2.12 kg N m−3 day−1 [66] are few examples of attached growth reactors. Wang et al. [67] have employed SBR to treat municipal wastewater, where the microorganisms are suspended. The mixed liquor suspended solid (MLSS) in the reactor is maintained in the range of 3.0 ± 0.5 g/L with dissolved oxygen level of 0.5–1.5 mg/L. As a result, they have achieved higher nitrogen and phosphorus removal of 75.3 and 92.3% respectively. Baddour et al. [68] have employed moving bed biofilm reactor (MBBR) to remove nutrients accounts in poultry slaughterhouses. The maximum nitrogen and phosphorus removal is achieved as 50.8% and 33.7%. Table 2 shows the various types of suspended and attached growth reactor used for nutrient removal.
MBR in BNR
Advantages of MBR for Nutrient Removal
Membrane bioreactor (MBR) technology has been used for various specialty treatment applications for nearly 30 years [81]. More than thousands of these reactors are installed in Asia, Europe, and North America. The wastewater discharged from the reactor after treatment is of very high quality and thus it can be reused rather than releasing it into the blue waters. Thus, MBRs can be a feasible application to treat wastewater in developed and developing countries [82].
Moreover, MBR is an emerging technology to treat both municipal and industrial wastewater as it is very efficient and compact [83]. The market growth of MBR is increasing at a tremendous pace and it is certain that it will be maintained over the next consecutive decade [84].
MBR occupies little space and demands lesser reactor specifications; it also discharges high-quality effluent with minimum production of sludge. In addition, there are several other advantages such as control over the solids residence time, dependability and steadiness of the operational parameters, mechanical control, and solidity of the entire system [85]. Thus, it can be noticed that MBR is more advantageous over the usual activated sludge system as the former produce effluent without any particles and without the input of the organics such as phosphorus and nitrogen from the suspended solids. In a research carried out by Galil et al. [85], it was observed that the total phosphorus removal efficiency of the MBR was 97% with respect to approximately 0.4 mg/L of total phosphorus in the effluent. The efficiency of the reactor did not decrease even when the concentration of phosphorus in the influent was higher.
As there is absolute nitrification and denitrification in the reactor, the effluent is free of particles. Thus, the total nitrogen removal is 10% higher than the predicted nitrogen removal in the system with similar recycle ratios [85]. Generally in a wastewater treatment plant, nitrification is an important step. The nitrifiers are more prone to inhibition and their growth rate is very slow compared to the heterotrophs [86]. As mentioned earlier, the solid retention time can be controlled by the operator in MBR and consequently the wash-out of the nitrifying bacteria and the other slow-growing organisms can be prevented. Thus, the diversity of the microorganisms can be increased in the system [83]. The high mixed liquor suspended solids in the MBR assist the growth of the nitrifying bacteria thereby resulting in reduced Food to Mass ratio [86]. But, there is a decreased phosphorus removal at higher solids retention time. Phosphorus can be removed when it is accumulated within the cell of the organism and consecutively wasted from the reactor. Thus, phosphorus removal in a MBR solely depends on the PAOs. These organisms can survive under starvation when there is a low food to mass ratio in the reactor whereas the non-polyphosphate-accumulating organisms are subsequently washed out [87]. Those bacteria which have accumulated polyphosphate can remain active for a longer time due to the energy stored intracellularly. Thus, the effluent generated from MBR has a lower level of total phosphorus when compared to the traditional phosphorus removal method which is due to the higher solids retention time [87].
Recently, there are many research with respect to treatment of wastewater using anaerobic membrane bioreactors (AnMBRs) [88]. The benefits provided by both the anaerobic and aerobic bioreactors are similar. Both can generate high-quality effluent [89]. The only disadvantage of AnMBR is its inability to remove nutrients directly. There is lesser biomass growth leading to reduced nutrient removal. But there is an increase in the concentration of phosphate and ammonia due to ammonification [90]. To increase the efficiency of nutrient removal, the reactor can be coupled with the traditional nutrient removal technologies which require an organic electron donor to increase phosphorus and nitrogen removal. When AnMBR is coupled with anaerobic ammonium oxidation (ANNAMOX), a process in which ammonium and nitrite are oxidized into nitrogen by a specific anammox organism, nitrogen removal can be enhanced in the downstream of AnMBR [91].
In comparison with the values in the literature, the TN removal in the MBR system was 10% higher than the theoretical nitrogen removal for similar recycle ratios due to complete nitrification–denitrification and absence of particles in the effluent [85]. It should be noted that the nitrification is a crucial process in biological wastewater treatment. Nitrification bacteria have generally slow growth rate compared to the heterotrophs and they are more sensitive to inhibition [86]. In MBR system, the membrane separation step could be an absolute barrier for the activated sludge microorganisms, which allows the operator to maintain sludge retention time (SRT) independent of the wastewater flow rate or settling properties of the biomass. Higher sludge retention time is necessary to prevent the wash-out of nitrifiers and other slow-growing microorganisms from the system, and to increase the biodiversity of the microorganisms in activated sludge [83]. In addition, nitrifiers can benefit from the high MLSS concentration in the MBR and resulting low F/M ratios [86]. However, MBR operation at high SRT is usually characterized by a reduced biological phosphorus removal as ultimately phosphate removal is the result of phosphate incorporation into new cell material and its wastage from the reactor. Due to the complete retention of suspended solids, an MBR is capable of generating an effluent with total phosphorus levels lower than a conventional enhanced biological phosphorus removal process [87].
Recent Configurations of MBR in BNR
The BNR efficiency of MBR can be enhanced by coupling it with a different type of conventional or hybrid reactor for example activated sludge process, sequencing batch, MBBR, and IFAS [92, 93]. da Costa et al. [93] have compared the nutrient removal efficiency of MBR coupled with sequencing batch reactor. In their study, two MBRs were used to treat the municipal wastewater such as sequencing batch coupled with conventional MBR (SB-CMBR) and sequencing batch coupled with hybrid MBR (SB-HMBR). Both reactors were operated to treat 18.3 L/day with two different HRTs 12 h and 6 h with a membrane flux of 15.2 L/m2 h. The COD removal efficiency of both reactor achieves > 98% during HRT reduced from 12 to 6 h. During reduction of HRT, the total nitrogen and phosphorus removal of SB-CMBR is increased from 65.1 to 66% and 81.9 to 84.2% respectively. Similarly for SB-HMBR reactor, the total nitrogen removal is increased from 73 to 73.3%, but the total phosphorus removal is gradually decreased from 84.2 to 78.3%. The decrease trend of total phosphorus removal in SB-HMBR indicates the lower phosphorus uptake in shorter HRT. Adoonsook et al. [65] have studied the effect nutrient removal using two MBRs such as anoxic–oxic membrane bioreactor (AO-MBR) and biofilm anoxic–oxic–membrane bioreactors (BAO-MBR). Both reactors were operated to treat 4.5 L/day at HRT of 9 h with a membrane flux of 20 L/m2 h. The total phosphorus removal shows higher removal efficiency of 95.30% for BAO-MBR when compared with AO-MBR (24.02%). Similarly the total and ammonia nitrogen removal shows higher removal efficiency of 87.79% and 99.93% for BAO-MBR when compared with AO-MBR (81.48% and 98.92% respectively). In contrast, the higher COD removal of 98.32% is achieved for AO-MBR when compared with BAO-MBR (96.98%). The highest of uptake of phosphorus is due to stimulating the active growth of actinobacteria growth in AO-MBR.
Strategies to Improve Biological Nutrient Removal
Addition of External Carbon Sources
In conventional BNR systems, inadequate supply of easily biodegradable organics in substrate (influent) has been considered as the major limitation in implementing at large scale and in particular to maintain the strict total phosphorus and total nitrogen levels in the effluent wastewater. Generally two methods are practiced to add extra organics; one method involves using commercial chemicals such as methanol or using appropriate wastage materials from industrial units and other method involves using VFA and easily hydolyzable organics generated via anaerobic fermentation of sludge. The method one resulted in increased operational cost and carbon footprint. This leads to initiation of wide exploration and research on anaerobic fermentation of sludge to generate VFA and easily hydrolysable organics. Fermentative solids of sludge sources such as primary sludge and suspended solids from anaerobic zone of return activated sludge are usually used. Generation of VFA or easily hydrolysable organics (as additional organics) from anaerobic fermentation of primary sludge is considered as a developed strategy employed for the improvement of biological nutrient removal. Nearly 0.15 g VFA/g volatile suspended solids (signifying 10 to 30% range of the influent organics) have been reported to be generated in anaerobic fermentation of primary sludge. Generation of addition carbon through waste activated sludge fermentation has gained focus recently to enhance BNR strategy and many literature have reported about the research and investigations on generating easily hydrolysable organics from waste activated sludge fermentation [94, 95]. Based on cost, VFA attained via anaerobic fermentation are considered as the economical source of carbon when compared to carbon sources that are supplemented externally [96]. Fermented mixed liquor of waste activated sludge is more complicated carbon source that fermented primary sludge as decomposition of biomass happens within mixed samples along with nitrogen and phosphorus release. Another feasible and economical carbon source is the fermentation liquor of food waste. Feng et al. [97] have investigated the viability of using fermentation liquor of food waste as additional source of carbon that was added externally to improve phosphorus and nitrogen removal in sequencing batch reactors. The authors explored that the ammoniacal nitrogen in fermented food liquor is required to be removed prior it was added to the reaction system and the liquor that was added externally does not leads to deterioration of nitrogen and polyphosphate removal externally. The impact of fermented food liquor to influent wastewater (FFL/IWW) ratio on BNR was explored by the same authors. It was suggested that the optimal FFL/IWW was found to be 1/90. When compared to control, the experiment reactor with 1/90 of FFL/IWW ratio showed greater nitrogen and phosphorus removal and they were observed to be 92.70% and 92.38%, respectively. The enhanced phosphorus and nitrogen removal were mostly due to additional VFA in the fermented food liquor and the accumulation of granular substances (polyhydroxyalkanoates) during anaerobic phase. Both the accumulated VFA and polyhydroxyalkanoate granules are considered to be the major sources of energy for nitrogen and phosphorus removal.
Pretreatment
Pretreatment is an enhancement strategy employed to enhance phosphorus and nitrogen removal. Kim and Chung [98] have employed chemical pretreatments to enhance nutrient removal from membrane bioreactor based municipal wastewater. The authors employed two chemicals (ferric chloride and polyaluminum chloride) for pretreating influent wastewater. They observed that higher total phosphorus removal was obtained from wastewater pretreated with ferric chloride than polyaluminum chloride.
Constructed Wetland
Constructed wetland (CW) is an engineered system. In recent years, it has widely been considered as an ecological technology attracted to alternative solutions for wastewater treatment with the advantages of functional efficiency and low operating and maintenance cost. In CW, the natural method of purifying wastewater is carried out on the surface using plants, substrate, and microbes. Plants take up nitrogen and phosphorus, substrates attract inorganic phosphorus ions, and microbes utilize organic carbon for conversion of nitrogen into nitrogen gas (N2) along with absorption of phosphorus ion. Plant roots absorb 25% of nitrogen and 50% of the nitrogen is removed through microbial activity. The strategy followed is that the oxidation of ammonia is processed through available atmospheric oxygen, where denitrification is progressed through the organic carbon source. Reasonable increases in dissolved oxygen through atmospheric oxygen increase the growth of heterotrophic microbes that benefit nitrification, but need more effective steps for the rate of removal of total nitrogen. In this case in the nitrification process if ammonia is completely oxidized into nitrate with oxygen, this nitrate may further enhance denitrification with the supply of C/N through organic carbon source. In CW, the oxygen can be supplied chiefly from plants, atmosphere, and via artificial aeration [99]. The obtained oxygen from photosynthesis and atmosphere can be transported to the roots of the plants through diffusion and pressure gradient. This process not only is beneficial to the root respiration but also discharges some oxygen into the rhizosphere. This process is also called as radial oxygen loss (ROL). ROL provides suitable environments for the different microbes in the rhizosphere [100]. Light intensity is an important environmental factor that influences the release of atmospheric oxygen from roots of the plants. Wang et al. [100] have noticed gradual increase in oxygen release in the day time (morning) with increment in light intensity. On the other hand, a decrement in the release rate of oxygen was noticed and occurred with respect to decreased light intensity at night and the oxygen release rate was observed to be zero at night with no light intensity.
Limiting Factors of Nutrient Removal
In recent decades, water scarcity has continuously been increased due to the increasing population, urbanization, industrialization, and environmental change. Around 80% of wastewater has been released into the close by waterbodies without nutrient evacuation [101], which could enrich the concentration of nutrients in the water bodies and leads eutrophication and deterioration of water quality [9]. According to Hu et al. [102], the effluent discharge nutrient management from wastewater treatment plant is a vital duty to protect the freshwater resource. Over other methods such as physical, chemical, or combined physicochemical valorizes excess nutrients in the wastewater. BNR from wastewater gained more attention due to less energy demand. Besides, high removal efficiency is of low operating cost and reduced sludge formation [98, 103]. Generally, the BNR process performs in various controlled environments, namely anaerobic, anoxic, and aerobic. However, it has some limiting factors such as wastewater characteristics and carbon ratio, which could be causing the hindrance in the nutrient recovery process.
Characteristics of Wastewater
The volume and concentration of wastewater effluent from processing plants depend mainly on the raw material composition, which treated, additive used, processing water source, and the unit process [63]. The primary aim of wastewater treatment is to decrease the concentration of a pollutant in the effluent below the tolerable level in order to reduce the risk of public health and atmosphere. Wastewater treatment technologies depend on different characteristics of effluent wastewater, operational and management practices, reliability of equipment, and flexibility of the process [104]. A review of wastewater characteristics from different sources, various treatment methods, and its nutrient removal efficiencies is indicated in Table 3.
Industrial wastewater treatment can be done in part or as a whole by biological nutrient removal. To reduce the effects created by the characteristics of wastewater, chemical and biological treatments can be applied to make this water source more compatible. The features of wastewater differ even in 24 h and thus the rate of discharge cannot be maintained with uniformity and consistency. To produce effluent with uniformity, the wastewater should be equalized before treatment [114]. Prior to the treatment or nutrient recovery process, the suspended particles should be removed by physical treatment [107]. Certain wastewater requires to be subjected to chemical pretreatment prior to biological treatment and certain wastewater requires exclusive chemical treatment [104]. Secondary wastewater treatment standards are more stringent with regard to the removal of pathogens and solids. BOD and COD determine the treatment process when the effluent is blended with wastewater. Most of the water body mutilation is due to the nutrients including eutrophication and algal bloom, and thus it is essential to remove nutrients in the wastewater effluent before discharging in water bodies. BNR with the help of microbes can remove nutrients such as nitrogen and phosphorus under various environmental conditions [124]. The individual nutrient content in the wastewater should match with the requirements of the bacteria in the sludge. Also, there should be an unbiased relationship between organics such as carbon, nitrogen, and phosphorus, which is vital to the efficiency of the nutrient recovery method [107]. The ratio of carbon, nitrogen, and phosphorus should be in between 100:10:1 and 100:5:1. The ratio of COD and BOD is a measure of the biodegradability of the wastewater pollution load. If the COD:BOD ratio does not exceed 2:1, the biodegradability is said to be good and high values indicate the existence of inadequate biodegradable substances [9].
Fate of Recalcitrant Organics
The presence of residual organic nitrogen in the treated effluent is the major limitation in wastewater treatment units obtaining less than 3 mg N/L [125]. Mostly, inorganic nitrogen is significantly removed by biological nitrogen removal plants, however a considerable portion of organic nitrogen (ON) yet left over in the treated wastewater. The transformation of nitrogen species in the treatment process unit is the key parameter to understand the fate and existence of dissolved organic nitrogen which is observed as the major portion. The organic nitrogen in the effluent exists in three forms: particulate form, colloidal form, and dissolved form. Most of the particulate form in the treated influent is removed in the primary clarifier itself and left over in the biological process. The colloidal and dissolved form has been reported to be the considerable portion of the total nitrogen in the treated wastewater of some treatment units. Pagilla et al. [126] have reported that the colloidal form contributes 62% of the treated wastewater and the dissolved form contributes 56 to 95% of the total nitrogen. The fate of organics in a wastewater treatment plant is particularly the existence of organic nitrogen which is obtained as a result of the treatment process and functions employed in treatment units. Removal of both particulate and colloidal solids, hydrolysis of solids, utilization, and oxidoreduction of inorganic nitrogen in the treatment operations aid in removal of nitrogen in wastewater treatment plants. On the other hand, the existence of nitrogen in organic form could not be removed via the treatment process and the presence of organic form of nitrogen in the treatment units are cumulatively accountable for effluent organic nitrogen. The effluent organic nitrogen can be removed via prevailing treatments in wastewater treatment plants. For instance, effluents which are added to the water bodies or reutilized can be characteristically treated via tertiary treatment approaches for removal of phosphorus, biomass, and residual organics [125].
Carbon Ratio
As described previously, the rapid urbanization and economy cause the release of enormous quantity of both municipal and industrial wastewater which should be subjected to BNR before it is released into the environment to prevent eutrophication [127]. The ratio of carbon, nitrogen, and phosphorus in the wastewater plays an important role in removing nutrients [114]. Conversely, the presence of carbon sources in wastewater hinders the removal of nutrients such as phosphorus and nitrogen. Hence, research is being carried out to enhance BNR through the external carbon sources [128]. Frison et al. [129] reported that high concentrated organic wastewater with carbon sources has concerned much attention in the BNR process because it can help to increase the efficiency of nutrient removal thereby reducing the environmental pollution. Zhang et al. [130] stated in their study that fermented fluid of the wastes can be used as supplementary source of carbon. Acetic acid is the main ingredient of the fermented liquid and it increases the efficiency of BNR. The conventional BNR process solely depends on the nitrifying and denitrifying bacteria to remove nitrogen. Kumar et al. [131] suggested that to enhance the operation, carbon/nitrogen ratio should be higher than 3 in a joined nitrification and denitrification system. Carrera et al. [132] described that the carbon/nitrogen ration should be higher than 7 during the pre-denitrification process to achieve complete denitrification. In 2009, Fu et al. [133] also specified that when the carbon/nitrogen ratio of the system was maintained greater than 8, the nitrogen removal efficiency reached around 80%, due to the increased competition between nitrifiers and heterotrophic microbes and the use of carbon sources for their metabolic activities. Hence, the carbon/nitrogen ratio has an influence on nitrification in the nutrient removal process.
Operational Parameters in WWT
A BNR system for nitrogen and phosphorus removal generally results in lower effluent concentrations. Hence, to embrace BNR with high effluent concentrations, it is required to know how different parameters such as microbial growth, temperature, amount of sludge recycled, acidity, alkalinity, and dissolved oxygen influence the system [104]. The rate of reaction of depends on temperature. Moreover, nitrification and denitrification rates also elevate with increase of temperature up to a certain operating limit. The necessary quantity of DO must be maintained in the system for nitrification, denitrification, and consumption of phosphorus to take place properly. On the other hand, it is very important not to aerate the effluent beyond the limit thereby maintaining the dissolved oxygen around 1 mg/L [134]. Over-aeration can result in the significant release of phosphorus due to the breakdown of the microbial cell leading to high dissolved oxygen. This may consecutively lead to reduced BNR and finally increases the cost of maintenance and operation [124].
Aeration
Aeration is a significant variable in the BNR process and affects removals of nutrients such as phosphorus and nitrogen. Contradictory results from the literature show that a proper aeration decreased the release of nutrients [135]. The aeration system should be customized to include an anaerobic reactor thereby decreasing the concentration of dissolved oxygen in the return sludge. This can be made possible by just removing the aerator from the flow of recycled sludge to evade introducing oxygen for nitrogen removal but presence of dissolved oxygen is more important in the aerobic zone to facilitate uptake of phosphorus [104]. Jeyanayagam [124] has reported filamentous growth is able to cause deprived sedimentation of particular nutrients in the final filter. Though, various parameters are essential to attain excellent rate of nutrient removal, dissolved oxygen is an essential rate limiting parameter [136]. Lower concentration of dissolved oxygen increases the rate of nitrogen removal [137]. Hence, there is no perfect limitation and standard with regard to the concentration of dissolved oxygen to obtain complete nitrification. There is an inclination of the concentration of dissolved oxygen within the aggregates of microbes in the biofilms is due to their inability to diffuse. Thus to degrade more nutrients and produce lesser sludge is the wastewater treatment plant, DO is considered as an essential parameter.
Internal Recycling
The internal sludge recycling ratio of BNR is a significant parameter that affects both the nutrient removal and the cost of operation. The purpose of returning the sludge from the final clarifier to the influent of the treatment system is to maintain a proper concentration of the activated sludge in the aeration tank such that the desired level of treatment efficiency could effectively be achieved at the appropriate time. However, the recycling of sludge may also strengthen the processes for the removal of organic matter in treatment systems due to increased contact time between wastewater and biotic components equipped with the supply of nutrients. The sludge recycle ratio could be influenced or regulated by factors such as reactor volume, HRT, OLR, aeration, and settling hydraulic loads and the concentration of mixed liquor suspended solids (MLSS) [138]. In a research carried out by Liu et al. [139], elevated sludge recycling ratios inhibit the discharge of phosphorus in the anaerobic zone. However, reduced recycling ratio decreased the performance of BNR due to insufficient microbial biomass. An optimized rate of sludge recycling rate is required to remove nutrients. When the rate of sludge recycling is increased, the removal of nitrogen is deteriorated due to an increase in the concentration of mixed liquor suspended solids in the sequential reactor. Grady et al. [140] clearly indicated that the performance of a wastewater treatment plant can be optimized by various parameters such as flow rate of sludge return and recirculation, and selection of an appropriate reactor volume. The components of the sludge and the amount of sludge recycled have a direct impact on the performance of the system. Anaerobic digestion generates nitrates and hence denitrification and removal of phosphorus will be reduced if the sludge is not recycled properly [139]. However, in order to attain high nitrogen and phosphorus removal efficiencies, the sludge recycle ratio should be retained though it incurs high cost.
HRT, SRT, and OLR of System
Hydraulic retention time and organic loading rate are regarded as among the most influential operating conditions on the functional attributes of biological nutrient removal, which have been often employed as control factors to facilitate the desired nutrient removal process [141]. Kumar et al. [142] described that efficient nutrient removal could be achieved in membrane bioreactor operation through high organic loading rate and short hydraulic retention time for cost-effective operation, and less sludge production. When HRT was reduced, there was an increase in the efficiency of nitrogen removal due to the growth of nitrifiers. However, over reduction of HRT does not result in significant removal of nitrogen [143]. Zhao et al. [144] examined COD removal of a contact-stabilization system with different HRTs in contact phase and observed COD removal efficiency was stable as HRT increased and conventional activated sludge systems are limited in the extent that the HRT can be differed. But, the process allows greater flexibility in selecting the HRT as the constraints imposed by a settling clarifier are removed. The anaerobic HRT is necessary for phosphorus removal as it depends on the influent characteristics. The determined time was too short as 30 min. The phosphorus-accumulating organism (PAO) would not have sufficient energy reserves to enhance phosphorus uptake in the aerobic zone. Therefore, increase in HRT could increase the availability of fatty acids for PAO to perform maximally [139]. Wasting rate is an important factor that affects solids retention time. In a BNR process, SRT should be long enough for nitrification. It is too long, then the proliferation of glycogen accumulating bacteria will takes place and these bacteria outcompete PAO and suppress activity. This in turn results in decreased phosphorus removal performance of the reactor. SRT of 5–12 days are recommended to be favorable for enhanced BNR performance [45].
Operational Parameters in Constructed Wetland
Consistent with the regime of water flow, CWs are classified into surface flow, subsurface flow, and hybrid CW [145]. Free water surface CWs contain a shallow basin or sequence of basins, growing vegetation, and a water surface above the substrate. Therefore, it may be a viable option for the restoration of polluted water resources. Surface flow CW effectively removed 86.07% and 82.07% of TSS and BOD, respectively. Removal of microbes, sedimentation of suspended solids, and colloidal particles through sedimentation and filtration is the most crucial pathway for organic removal in Surface flow CW [146]. Vymazal [147] stated that nutrient removal is inconsistent with respect to hydraulic loading rate and the size of the system used. Hence, surface flow CWs show less capacity for nutrient removal, especially phosphorus whereas, the removal efficiency for nitrogen was typically ranging from 40 to 50%. In addition, the efficiency of the removal of nutrients and the growth of plants is directly or indirectly influenced by temperature, due to its impact on the availability of oxygen that affect redox levels [148]. The characteristics of the wetland media may also be a significant factor that controls the level of redox potential within the porous material. Subsurface flow constructed wetlands (SSFCW) can afford better protection from heat than surface flow constructed wetlands systems (SFCW) because the less saturated exterior layer in cold climate provides the insulation effect. In accordance with flow regime, SSFCW is classified into horizontal subsurface flow constructed wetlands (HSSFCW) and vertical subsurface flow constructed wetlands (VSSFCWs) as described by Wu et al. [145]. HSSFCW showed high removal efficiency for TSS, BOD, and COD of about 81.4, 83.9, and 70.3%, respectively. Liu et al. [149] noted that the NO3-N removal efficiency in the upper and lower horizontal wetland zones was 70–90% with a redox potential of > 200 mV and 50–80% with a redox potential < − 200 mV, respectively. It suggests that there has been a possible co-existence in both regions, such as aerobic and anoxic/anaerobic, which contributes to a successful increase in BNR and improves the quality of water in horizontal flow wetlands. Trein et al. [150] examined the efficiency of vertical flow built wetlands with only the first level of area reduced French system for the removal of organic matter and the conversion of nitrogen in the tropical area. The authors observed a shift in redox potential in the effluent between 150 and 350 mV and claimed that the aerobic conditions were effective in the treatment system and encouraged the degradation of organic matter and the nitrification process. Vymazal [147] observed that VSSFCWs system exhibited more removal percentage of TSS (93.4%), BOD (99.9%), and COD (98.9%) than HSSFCWs. The hybrid systems that consist of various types of wetlands arranged in series and have been mainly used for enhanced removal of nitrogen as these wetland systems could provide different redox conditions suitable for nutrient reduction [151]. When compared to single HSSFCW and SFCW, mostly the hybrid CW systems were known to be more proficient in the removal of total suspended solids, biological and chemical oxygen demand, and nutrients. However, information on the influence of design and operational parameters on nutrient reduction in CW with different configurations and corresponding removal mechanisms needs to be investigated together to adopt the system for efficient recovery of organics in the highly polluted effluents. Table 4 summarizes the essential operational parameters of CW design and its treatment efficiencies. Principal component analyses were achieved for confirming CW influence on the area, depth, size of the system, clogging, pH, organic loading, temperature, hydraulic retention time, and hydraulic loading rate.
Hydraulic Retention Time and High Hydraulic Loading Rate
Zhang et al. [130] reported that batch feeding operation shows better nitrogen and phosphorus removal efficiency when compared to a continuously operating system by supporting more oxidizing situation in treatment wetlands. The variation in the mode of feeding wastewater to the system influences the redox conditions, transfer of oxygen and diffusion in CW. In the VSSCW, excess oxygen produced in the aeration improves nitrification and is a good way to volatilize ammonia in the system, as discussed by Wu et al. [164]. It has been well established that this is a consecutive correlation between the efficiency of the removal of contaminants and hydraulic retention time (HRT). The removal efficiency of the pollutant is high in low HRT, whereas it is lower in high HRT. This may be the reason that there is a shortage of supply of nutritional sources to biotic components at higher HRT levels [165]. Tunçsiper et al. [166] documented that a long HRT provides prolonged time for interaction between the contaminating organisms and wastewater whereas, at lower HRT, the wastewater progresses quickly to the outlet unit without contacting each other appropriately.
High hydraulic loading rate (HLR), on the other hand, is an essential parameter and affects the reduction of nutrients in CWs. Weerakoon et al. [167] have built a small-scale HSSCW planted with a T. angustifolia plant species, which leads to the efficient removal of contaminants under different HLR up to 25 cm/day. It is important to remember that adequate interaction between the roots of planted species and wastewater enhances dissolved contaminants removal and promotes the action of various microbial communities. Wu et al. [168] investigated the removal efficiency of triazophos at different concentrations, such as 0.79 ± 0.29 mg/L and 3.96 ± 1.17 mg/L with an HLR of 100 mm/day. The observed the removal efficiency was 97.8 ± 2.9% and 84.0 ± 13.5%, respectively. In comparison, the removal efficiency was significantly reduced to 96.2 ± 1.7% and 61.7 ± 11.1%, respectively, at 200 mm/day of HLR. The findings show that the efficiencies in the removal of contaminants are correlated with HLR in the treatment of wastewater using CWs.
Organic Loading
Increased organic load in the influent of CWs typically improves the pollutant rates within the tolerable limits [169]. Excessive loading rates can lead to the accumulation of organic matter, reduced empty space, and reduced efficiency in the removal of contaminants in constructed wetlands [170]. Various investigations have confirmed the range of NH4-N from 0.15 to 30.0 g/m2/day for the successful output of CWs. It does, however, have a detrimental impact on the growth of wetland plants when excess ammonia (> 100 mg/L) is present in wastewater [171, 172]. Villasenor et al. [173] examined the treatment process of HSSFCW coupled with the microbial cell with different organic loading concentrations viz., 13.9 g COD/m2 day, 31.1 g COD/m2 day, and 61.1 g COD/m2 day, respectively. It was found that most of the organic matter in the wastewater was oxidized, and the efficiency of COD removal and the output of electricity improved when the organic load was lower. In commercial treatment systems, the concept of step-feeding was introduced to analyze the more productive use of the entire CWs surface area and to avoid clogging by releasing suspended solids and organic loading in the influent [174]. Wastewater effluents from various food industries, dairy industries, and distilleries hold a significant amount of biologically degradable substances and solids matters. For instance, the food processing industry wastewater majorly constitutes of oil and grease substances as well as carbohydrates. Calheiros et al. [175] stated that the organic content of the industrial wastewater was high after being subjected to various treatment process in CW. Wu et al. [145] observed that there is a change in the vegetation and treatment efficiency of CW due to high loading rate. High loading rate will lead to better growth of plants near the outlet than the inlet [176]. So, it is recommended to remove excess organic matters through the preliminary treatment processes before entering into next step treatment.
Clogging
Clogging influences the CWs system performance. The treatment of industrial wastewater effluents mostly depends on the composition of the effluent which has to be processed and also depends on the hydraulic properties of the CWs system. Clogging might happen due to the aggregation of solids on the surface leading to reduced porous surface, reduced hydraulic conductivity, and formation of biofilms [177]. At the initial stage of wastewater treatment in the CWs, the microorganisms might be separated from one another. In the later stage, it could flocculate leading to formation of biofilm on the surface which occupies the pores of gravels and blocks water passage. The continuous growth of biofilm in CWs clogs the system. The extent of clogging usually depends on the solids aggregated which are controlled by the hydraulic loading rate of the CWs. If the elevated clogging takes place at the inlet unit of CWs, there the solids in effluent are filtered and retained [178]. HSSCWs are commonly used for treating the wastewater. However, their efficiency in pollutant removal is severely restricted by clogging problems that are unusually frequent throughout the lifetime of CWs. Shen et al. [179] created a new treatment method by periodically altering the direction of flow and evaluating its efficiency in removing contaminants. The experimental results show that the designed wetland fitted with a new methodology of treatment has achieved more efficiency of removal of pollutants than conventional ones. The microbes analysis shows that the reciprocating direction of the wastewater flow in the CWs retained a more significant amount of microorganism, which successfully prevented the accumulation of organic matter. Finally, it was concluded that there was no apparent issue of clogging or infiltration in CWs with the mode of reciprocating flow direction [179]. Nivala et al. [180] reported that clogging decreases oxygen permeability and reduces the efficiency leading to reduction in the life span of CWs systems. Varga et al. [181] suggested that primary screening is required to avoid clogging in order to make the process more efficient.
pH
pH influences the microbial processes in the CW system for industrial wastewater treatment. The availability of various pollutants in industrial wastewaters determines acidity or alkalinity of water. Changes in pH may significantly alter the surface charge of the substrates present in the wastewater. The range of optimum pH for the degradation of various contaminants is varied. Bailey et al. [182] reported that the degradation of diazinon was very rapid when the treatment process took place under acidic conditions. On the other hand, the degradation rate of chlorpyrifos was lower under acidic conditions and significantly improved with an improvement in the pH of the treatment system [183]. Saeed and Sun observed that ammonification was progressing when the system’s pH was maintained between 6 and 8 [184]. Growth of heterotrophs was higher at neutral pH and the floating organisms were affected if pH was below 3 and above 9 [185]. Research on pH and hydroponic crop proliferation could be better aligned with the conditions defined in CWs. The pH of the aquaponic ecosystems is held at about 7.0 due to the nutrients uptake of plants are higher in pH range of 6 to 7 [186, 187]. It was optimized that pH 8.5 could enhance the microbial nitrification of NH4+ to NO2− and NO2− to NO3− in the wastewater treatment systems. The pH may also have an effect on the supply of phosphorus to biotic components in CWs. The liberation of dihydrogen phosphate (H2PO4−) from phosphoric acid (H3PO4) happens at pH 2.1, while the formation of hydrogen phosphate (HPO42−) from dihydrogen phosphate (H2PO4−) occurs at 7.2 [188]. Becquer et al. [189] stated that plants absorb phosphorus only when it is in the form of orthophosphate ions. It was obvious that the absorption of phosphorus was also regulated by the pH of the treatment system. Awareness of pH effects is, therefore, a crucial factor in the proper management of the removal of nutrients from various wastewaters in CWs.
Temperature
Temperature is the crucial factor that may affect the metabolic activity and growth of microbes. Any pollutant removal mechanisms such as oxidation, reduction, precipitation, filtration, adsorption, plant uptake, and volatilization in CWs essentially brought about by microbial action were a cornerstone of the innovation technology [190]. Ouellet-Plamondon et al. [191] reported that low-temperature treatment conditions often do not affect the removal efficiency of organic matter in the processes, namely decantation and sedimentation in the constructed wetlands. Zhang et al. [192] reported that the average degradation efficiency of methamidophos was 60 and 90% at 15 and 24 °C, respectively, while the degradation efficiency reduced by < 50% when the temperature was below 15 °C. Bondarenko et al. [193] specified that the temperature rise in the treatment process from 10 to 21 °C increased by 2 to 4 times the degradation of diazinon. Short-term temperature fluctuations had a lesser impact on removing pollutants due to the adaptive tolerance of the biotic portion to the immediate new environmental conditions. Biological processes, however, are highly dependent on temperature fluctuations and affect wetlands’ overall success in eliminating contaminants. Lower or higher temperatures limit the biological activities and result in the accumulation of organic matter. The maintenance of sufficient temperature could improve the elimination efficiency of contaminants in CWs. Meng et al. [194] documented the efficiencies of pollutant removal in CWs are successful at optimum temperature. Temperature in the range 28–36 °C was found to be optimum and favors nitrification, whereas temperature above 15 °C could be appropriate for NH3 oxidizing bacteria. Moreover, bacteria which can oxidize NO2− are washed out at 25 °C and thus the optimum temperature ranging from 15 to 38 °C is the most suitable for the constructed wetland system.
Cost Analysis
Cost for External Carbon Source
As to make successful BNR processes and accomplish low organic material in the wastewater, the carbon source could be considered as a primary nutrient for the metabolic action of heterotrophic microorganisms. In most cases, external quickly biodegradable carbon sources generally added to the treatment system as a nutritional supplement due to the inadequate availability of internal carbon present in the influent wastewater. Environmental protection agency, USA (2013) stated that the main limiting factor of denitrification (i.e., sequential process of nitrogen removal) in the BNR is being of an insufficient amount of carbon in the influent of wastewater [195]. The addition of external carbon sources limits the process and makes it more expensive. Henceforth, the carbon source utility in wastewater treatment and its expense could be a significant factor in BNR methods to evacuate organic material in a practical manner.
Cost of Additional Energy for Treatment in WWT
The amount of water supply and its need is being expanded with the quick development of urbanization as well as industrialization. At the same time, the quantity of wastewater release has largely been increased because of the enlarged use of water resources all over the globe. Treatment of wastewater or used water requires a significant amount of energy for operating the instruments equipped with wastewater treatment plants, which regularly showed up as a high processing cost. At this juncture, it is an essential factor to investigate and examine the fundamental expense of energy using in wastewater treatment to understand the possible routes to diminish energy consumption and the operating cost of the wastewater treatment system. This could be lead to give a vital aspect for redesigning existing treatment plants and creating new plants in the monetary and proficient manner. In general, the wastewater treatment systems have been working with a number of different technologies, various volumetric capacities, several energy consumption machines, numerous wastewater characteristics, and treatment attributes [196]. The average energy utilization for the UK, Germany, and Italy were respectively to 0.45, 0.67, 0.64, and 0.70 kWh/m3 [197]. These variations in the average energy consumption might be because of various parameters depicted previously and it could ultimately lead to altering the processing cost of wastewater treatment systems. Rothausen and Conway [198] estimated the energy consumption of wastewater treatment plants itself in the USA was around 21 billion kWh per year for about 1.8 million people equivalents. Xu et al. [199] pointed out that approximately 4% of the total electricity generated in the USA was used for wastewater transport and its treatment. Li et al. [200] investigated energy consumption and operating cost of three different wastewater treatment plants based on various parameters (each plant with the treatment capacity of 5.6 lakhs tons/day) located in Shenzhen, China. The outcome of this study showed that the average energy utilization of treatment systems was 0.20 ± 0.06 kWh/t and the estimated average treatment cost was 0.708 yuan (0.10 USD) per tonne of wastewater. In the overall unit cost, 30.1 and 26.3% were spent on labor and electricity consumption, respectively. In recent years, all governments and research scientists have been paying more and more attention to minimize or neutralize the wastewater processing cost in all aspects in order to ensure continuous operational sustainability. It could optimistically be achieved by recovering energy or possible value-added resources from the wastewater as it contains chemical and thermal energy, and by adopting less energy-consuming machines in the wastewater treatment plants. Gu et al. [201] documented that about 52% of the cost was recovered from the total cost of energy consumed in the five WWTPs in Catalonia, Spain, by transforming the sludge into valuable resources, namely biogas. Prateep Na Talang et al. [202] examined the BNR efficiency and cost-effectiveness of eight different centralized municipal WWTPs in Bangkok, Thailand, under five different treatment arrangements. The wastewater treatment plants with the provision of vertical loop reactor activated sludge were significantly reduced the total operating cost from 7.417 (0.218 USD) (without nutrient removal) to 2.754 (0.081 USD) (with nutrient removal) THB2017/m3. It can be seen from the result that about 62.86% of the average total operating cost was reduced by recovering organic materials, including nitrogen and phosphorus. Therefore, current attention to the exploration of nutrient recovery and energy generation from the wastewater, and upgrading wastewater treatment plants with cutting edge machines could assist with diminishing the expense of additional energy consumption in WWTPs. Likewise, it might lead to the sustainable continuous operation of WWTPs to overcome the demand for a massive amount of wastewater treatment in the future without any complications.
Cost Associated with a Constructed Wetland
Currently, CW use, especially in rural areas, has become increasingly preferable for wastewater treatment and it has been accepted as a low cost and secure technology for wastewater treatment. The investment expenses of a CW system for the installation are leveled as land, excavation, stuffing material, piping structure, vegetation, and other activities [203]. CW wastewater treatment comprises capital and operation cost. The capital cost indicates the cost of the land required for the installation, construction cost, and engineer and contractor fees, in contrast, the operation cost refers to the cost of electric energy for pump (wastewater lifting and transferring) and lighting, operator wages, and maintenance [204]. Tyndall and Bowman [205], stated that depending upon design and scale cost for installation and long-term management of a CW may vary significantly and CW located on land with a high Corn Suitability Rating for treating 100 acres of drainage would cost just over 10,000$ for design and installation and 800$ per acre per year over 40 years of lifespan. The replacement cost method could employ for the estimation of the cost of traditional wastewater treatment, an evaluation based on the value of CW. Although CWs comprise some advantages, a few limitations must be considered, such as a large area for wetland construction and designing the same based on the type of wastewater being treated and the location’s climatic conditions. Table 5 shows the comparison on the design and scale cost of different type of constructed wetlands.
Side-Stream Enhanced Biological Phosphorus Removal
Side-stream enhanced biological phosphorus removal (SSEBPR) is a cost-effective technique than other traditional techniques followed for phosphorus removal. In this process, PAOs reduce power generation during hydrolysis of polyphosphate via glycolysis and/or tricarboxylic acid cycle (TCA) cycle to assimilate VFA. The PAOs reverse the assimilated VFA as poly-β-hydroxyalkanoates (PHAs) under anaerobic condition. In subsequent aerobic/anoxic conditions, the PAOs consume the accumulated PHA as energy and carbon sources for their biomass productivity, glycogen renewal, and poly-P production via excess uptake of inorganic orthophosphate. Nguyen et al. [211] have highlighted that the presence of Tetrasphaera bacteria in the reactor significantly improves the relase of phosphorus when compared with Candidatus accumulibacter, which is most commonly found PAO for phosphorus removal and stable operation of SSEBPR. Raj et al. [5] have studied the effect SSEBPR using a lab-scale anaerobic/anoxic/oxic (AAO) system integrated with sludge reduction. In their study, the thermally pretreated sludge was recycled in the reactor. As a result, 99% of phosphorus solubilization efficiency and 0.22 kg MLSS/kg COD of biomass yield were achieved. Wang et al. [212] have operated full-scale SSEBPR and conventional AAO reactor to study the phosphorus removal performance. Comparatively, the highest phosphorus removal of 94% is achieved in SSEBPR and 80% in conventional AAO. As stated earlier, SSEBPR shows higher glycolysis activity than conventional AAO process. In addition to this, they have reported the presence of PAOs in SSEBPR reactor offers more competitive benefits than glycogen-accumulating organisms (GAOs). The high amount of complex VFA is continuously generated and accumulated by PAO in the reactor due to extended anaerobic retention time, which suppress the activity of GAOs. Figure 6 shows different types of side-stream reactor used for removal of phosphorus.
Effect of Sludge Recycling
It should be noted that only one-tenth of the total phosphorus concentration (nearly 0.1 mg/L) is allowed to be discharged from wastewater treatment plants [213]. Phosphorus can be removed either biologically or chemically. Chemical phosphorus removal is achieved by transforming phosphate into hardly soluble and then precipitating the salts. These salts are withdrawn along with the excess sludge. [214]. The efficiency of phosphorus removal in MBR is low due to higher solids retention time. To increase the efficiency, metal salts can be added into the oxic tank. However, precipitation of phosphates with the metal salts would generate inorganic sludge that would be retained within the system thereby leading to excess sludge [76].
A side-stream EBPR process was projected as an emerging alternative to tackle challenges in enhanced biological phosphorus removal (EBPR) associated with the stability and unfavorable C/P ratio in the influent [212]. Aerobic membrane bioreactor achieves sustainable phosphorus removal only when side-stream phosphorus removal or sludge wastage has been carried out [78]. In the case of MBR with high sludge age, the only possibility of achieving sustainable phosphorus removal is through side-stream removal of phosphorus. For instance, working on MBR with high sludge age, Banu et al. [78] have achieved sustainable phosphorus removal through phosphorus precipitation in side stream.
In EBPR processes, microbes accumulate large amounts of polyphosphates. Nearly all of the polyphosphates could be released from activated sludge just by heating at 70 °C for about 1 h [215]. Addition of calcium chloride precipitates the released polyphosphates at room temperature. This could be a good method to recover phosphorus in a reusable form from wastewater [216]. Although, biological phosphorus removal requires principally different operational conditions than carbon and nitrogen removal. Successful biological phosphate removal in an MBR is based on the development of polyphosphate-accumulating organisms (PAOs). The growth of PAOs is favored in an MBR due to their competitive advantages over non-poly-P accumulating microorganisms to survive starvation periods, characteristic of an MBR operating at low F/M ratios (Monclus). Bacteria containing poly-P maintain for a longer time a high activity as a consequence of the accumulated energy.
Methods Used to Control Nutrient Load in SSPR
When using phosphorus precipitating chemicals, it is important to understand that removal of all dissolved phosphorus is not necessary. The key is to remove phosphorus in solution becoming lower than the phosphorus required in regulation. Due to this, no significant amounts of struvite will form downstream of the chemical addition point [214]. As a general rule, 20 to 30% removal efficiency would be sufficient for struvite control downstream of the anaerobic digester discharge [85]. Because of its effect on secondary treatment, minimizing the recycle phosphorus load by maximizing phosphorus precipitation from the solids stream could be beneficial and cost-effective.
Future Prospects and Recommendations
BNR is a well-developed and existing approach and this process has been extensively employed and considerably implemented in practice. On the other hand, the existence and functioning of the BNR process is a major concern in wastewater treatment plants due to its complication and higher cost. To make ease the operation of the BNR process, optimization of both capital and operational cost is essential. Further to understand the process and to progress the more effective and consistent BNR process, modeling of process is essential. Modeling of process has been considered to be an essential tool for optimal design and functioning of wastewater treatment process specifically to meet the treated wastewater standards and to manage the requirement of costs. Modeling of process is considered as an imperative tool for plan engineers to meet the challenges associated with BNR process [102]. Though numerous efforts have been made for the nutrient removal process, much effort must be to targeted towards introducing, instructing, and implementing developed approaches in wastewater treatment plants to overcome the problems such as contamination of surface water and shortage of water sources [217]. Developed approaches for nutrient removal have many benefits and that can be assessed using life cycle analysis approaches for instance in aiding water reutilization or removal of recalcitrant that have gained very low insights in life cycle analysis reports. Considering numerous life cycle analyses of the nutrient removal process may deliver extra beneficial info in assessing different treatment choices to see future regulations of treated effluent [218]. Future treatment process should be designed and planned from a circular economy point of view, and prior to that useful resource recovery route must compensate the overall need of suitable resources and to invest potentially to reduce the traditional resource exploitation considerably. Implementation of resource recovery routes positively will need waste management utilities to increase their engineering expertise and to become market participants with high attention to meet the treated effluent standards [219].
Conclusions
Technological advancement in the fields of BNR is updating day by day following the long-established nitrification–denitrification process for nitrogen removal within the natural environment. The biological treatment process is dependable on internal and external climatic conditions favoring microbial transformation. The purpose of this treatment method is to develop the betterment, support the living condition of human and its health, to cut short, and minimize the wastage of resources. The action of reducing the severity caused by nutrient release to the environment is to be preserved based on adapting the logical methods discussed in this area.
References
Pham TL, Bui MH. Removal of nutrients from fertilizer plant wastewater using Scenedesmus sp.: formation of bioflocculation and enhancement of removal efficiency. J Chemother Hindawi Limited. 2020;2020:8094272.
Pham TL, Utsumi M. An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manag. 2018;213:520–9.
Banu JR, Uan DK, Chung I-J, Kaliappan S, Yeom I-T. A study on the performance of a pilot scale A2/0-MBR system in treating domestic wastewater. Undefined. 2009;30(6):959–63.
Meena RAA, Kannah RY, Sindhu J, Ragavi J, Kumar G, Gunasekaran M, et al. Trends and resource recovery in biological wastewater treatment system. Bioresour Technol Reports. 2019;7:100235.
Raj SE, Banu JR, Kaliappan S, Yeom I-T, Adish KS. Effects of side-stream, low temperature phosphorus recovery on the performance of anaerobic/anoxic/oxic systems integrated with sludge pretreatment. Bioresour Technol. 2013;140:376–84.
Kavitha S, Kannah RY, Banu JR, Kaliappan S, Johnson M. Biological disintegration of microalgae for biomethane recovery-prediction of biodegradability and computation of energy balance. Bioresour Technol. 2017;244:1367–75.
Kavitha S, Jayashree C, Kumar SA, Yeom IT, Banu JR. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour Technol. 2014;168:159–66.
Kavitha S, Subbulakshmi P, Banu JR, Gobi M, Yeom IT. Enhancement of biogas production from microalgal biomass through cellulolytic bacterial pretreatment. Bioresour Technol. 2017;233:34–43.
Kannah RY, Gunasekaran M, Kumar G, Ushani U, Do K-U, Banu JR. Recent developments in biological nutrient removal. In: Bui XT, Chiemchaisri C, Fujioka T, Varjani S, editors. Water and wastewater treatment technologies. Energy, Environment, and Sustainability. Singapore: Springer; 2019. p. 211–36.
Kannah RY, Merrylin J, Preethi, Sivashanmugam P, Gunasekaran M, Kumar G, Banu JR. Valorization of nutrient-rich urinal wastewater by microalgae for biofuel production BT - application of microalgae in wastewater treatment: volume 2: biorefinery approaches of wastewater treatment. In: Gupta SK, Bux F, editors. Cham: Springer International Publishing; 2019;393–426.
Shin H-S, Park M-G, Jung J-Y. Nutrient removal processes for low strength wastewater. Environ Technol Taylor & Francis. 2001;22:889–95.
Liang X, Wang Z, Zhang Y, Zhu C, Lin L, Xu L. No-tillage effects on N and P exports across a rice-planted watershed. Environ Sci Pollut Res. 2016;23:8598–609.
Zhou X, Helmers MJ, Asbjornsen H, Kolka R, Tomer MD, Cruse RM. Nutrient removal by prairie filter strips in agricultural landscapes. J Soil Water Conserv Soc. 2014;69:54–64.
Hathaway JM, Tucker RS, Spooner JM, Hunt WF. A traditional analysis of the first flush effect for nutrients in stormwater runoff from two small urban catchments. Water Air Soil Pollut. 2012;223:5903–15.
Zhang M, Zhu C, Gao J, Fan Y, He L, He C, et al. Deep-level nutrient removal and denitrifying phosphorus removal (DPR) potential assessment in a continuous two-sludge system treating low-strength wastewater: the transition from nitration to nitritation. Sci Total Environ. 2020;744:140940.
Ryu H-D, Lee S-I. Comparison of 4-stage biological aerated filter (BAF) with MLE process in nitrogen removal from low carbon-to-nitrogen wastewater. Environ Eng Sci. Mary Ann Liebert, Inc. 2 Madison Avenue Larchmont, NY 10538 USA ; 2009;26:163–70.
Metcalf & Eddy, George Tchobanoglous. Wastewater energy: treatment and reuse. New York [etc.] McGraw-Hill. McGraw-Hill; 2004.
Yang Y, Pignatello JJ, Ma J, Mitch WA. Effect of matrix components on UV/H2O2 and UV/S2O82- advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016;89:192–200.
Beita-Sandí W, Karanfil T. Removal of both N-nitrosodimethylamine and trihalomethanes precursors in a single treatment using ion exchange resins. Water Res. 2017;124:20–8.
Sun J, Dai X, Wang Q, van Loosdrecht MCM, Ni BJ. Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res. 2019:21–37.
Hatton TA, Su X, Achilleos D, Jamison T. Redox-based electrochemical adsorption technologies for energy-efficient water purification and wastewater treatment. Sep Technol IX New Front Media, Tech Technol 2017; 1.
Ezugbe EO, Rathilal S. Membrane technologies in wastewater treatment: a review. Membranes (Basel). 2020;10.
Tong T, Carlson KH, Robbins CA, Zhang Z, Du X. Membrane-based treatment of shale oil and gas wastewater: the current state of knowledge. Front Environ Sci Eng. Higher Education Press; 2019; 1–17.
Butzen EL, Santos GC, Fortuna SS, Brião VB. Membrane bioreactor for mall wastewater treatment. Rev Ambient e Agua. Institute Environ Res Hydrogr Basins (IPABHi); 2020;15.
Kader AM. A review of membrane bioreactor (MBR) technology and their applications in the wastewater treatment systems. Desalin Water Treat. 2015;32:111–9.
Na JH, Nam DH, Ko BG, Lee CY, Kang KH. Reduced sludge production in a membrane bioreactor by uncoupling metabolism and its effect on phosphorus accumulation in the biomass. Environ Technol (United Kingdom). Taylor and Francis Ltd. 2017;38:3007–15.
Akbarzadeh A, Khodabakhshi A, Arbabi M. Optimization of SBR system for enhanced biological phosphorus and nitrogen removal. Int J Environ Health Eng. 2012;1:49.
Ekama GA, Wentzel MC. 3rd AWWA/IAWQ Regional Conference on Biological Nutrient Removal, Brisbane, Australia, 30 November-4 December 1997: difficulties and developments in biological nutrient removal technology and modelling. Water Sci Technol. No longer published by ; 1999;1–11.
Soliman M, Eldyasti A. Development of partial nitrification as a first step of nitrite shunt process in a sequential batch reactor (SBR) using ammonium oxidizing bacteria (AOB) controlled by mixing regime. Bioresour Technol. 2016;221:85–95.
Magdum S, Kalyanraman V. Existing biological nitrogen removal processes and current scope of advancement. Res J Chem Environ. 2017;21:43–53.
Zhuang H, Wu Z, Xu L, Leu S-Y, Lee P-H. Energy-efficient single-stage nitrite shunt denitrification with saline sewage through concise dissolved oxygen (DO) supply: process performance and microbial communities. Microorganisms. Multidisciplinary Digital Publishing Institute. 2020;8:919.
Ge C-H, Dong Y, Li H, Li Q, Ni S-Q, Gao B. Nitritation-anammox process – a realizable and satisfactory way to remove nitrogen from high saline wastewater. Bioresour Technol. 2019;275:86–93.
Magdum S, Varigala S, Minde G, Bornare J, Kalyanraman V. Evaluation of sequential batch reactor (SBR) cycle design to observe the advantages of selector phase biology to achieve maximum nutrient removal. Int J Sci Res Environ Sci. 2015;3:234–8.
Sliekers AO, Derwort N, Gomez JLC, Strous M, Kuenen JG, Jetten MSM. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res. 2002;36:2475–82.
Xiao Y, Xiao Q, Xiang S. Modeling of simultaneous partial nitrification, anammox and denitrification process in a single reactor. J Environ Anal Toxicol. 2014;04:1000204.
Pedros PB, Onnis-Hayden A, Tyler C. Investigation of nitrification and nitrogen removal from centrate in a submerged attached-growth bioreactor. Water Environ Res. 2008;80:222–8.
Sánchez Guillén JA, Jayawardana LKMCB, Lopez Vazquez CM, de Oliveira Cruz LM, Brdjanovic D, van Lier JB. Autotrophic nitrogen removal over nitrite in a sponge-bed trickling filter. Bioresour Technol. 2015;187:314–25.
Dai Y, Constantinou A, Griffiths P. Enhanced nitrogen removal in trickling filter plants. Water Sci Technol. 2013;67:2273–80.
Wang L, Wang X, Yang F, Kong M, Peng F, Chao J. Nitrogen removal performance and ammonia- and nitrite-oxidizing bacterial community analysis of a novel industrial waste-based biofilter. Chem Eng J. 2016;299:156–66.
Kagawa Y, Tahata J, Kishida N, Matsumoto S, Picioreanu C, van Loosdrecht MCM. Modeling the nutrient removal process in aerobic granular sludge system by coupling the reactor-and granule-scale models. Biotechnol Bioeng Wiley Online Library. 2015;112:53–64.
Steichen M, Kadava A, Shaw A, Scanlan P, Martin M, Kazemi S. A new paradigm: carbon footprint and sustainability assessment for process selection. Proc Water Environ Fed. 2009;2009:5381–98.
Oehmen A, Saunders AM, Vives MT, Yuan Z, Keller J. Competition between polyphosphate and glycogen accumulating organisms in enhanced biological phosphorus removal systems with acetate and propionate as carbon sources. J Biotechnol J Biotechnol. 2006;123:22–32.
Coma M Biological nutrient removal in SBR technology: from floccular to granular sludge. Universitat de Girona; 2011.
Liu H, Han P, Liu H, Zhou G, Fu B, Zheng Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour Technol. 2018;260:105–14.
Curtin K, Duerre S, Fitzpatrick B, Meyer P. Biological nutrient removal. 2011.
Mino T, Arun V, Tsuzuki Y, Matsuo T. Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process. Biol Phosphate Remov from Wastewaters 1987: 27–38.
Dorofeev AG, Nikolaev YA, Mardanov AV, Pimenov NV. Role of phosphate-accumulating bacteria in biological phosphorus removal from wastewater. Appl Biochem Microbiol Pleiades Publishing. 2020;56:1–14.
Li Y, Rahman SM, Li G, Fowle W, Nielsen PH, Gu AZ. The composition and implications of polyphosphate-metal in enhanced biological phosphorus removal systems. Environ Sci Technol American Chemical Society. 2019;53:1536–44.
Schönborn C, Bauer H-D, Röske I. Stability of enhanced biological phosphorus removal and composition of polyphosphate granules. Water Res. 2001;35:3190–6.
Acevedo B, Oehmen A, Carvalho G, Seco A, Borrás L, Barat R. Metabolic shift of polyphosphate-accumulating organisms with different levels of polyphosphate storage. Water Res. 2012;46:1889–900.
Brown N, Shilton A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: current understanding and future direction. Rev Environ Sci Bio/Technology. 2014;13:321–8.
Bindhu BK, Madhu G. Influence of organic loading rates on aerobic granulation process for the treatment of wastewater. J Clean Energy Technol. 2013;1:84–7.
Pronk M, de Kreuk MK, de Bruin B, Kamminga P, Kleerebezem R, van Loosdrecht MCM. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015;84:207–17.
Mielcarek A, Rodziewicz J, Janczukowicz W, Thornton A. The feasibility of citric acid as external carbon source for biological phosphorus removal in a sequencing batch biofilm reactor (SBBR). Biochem Eng J. 2015;93:102–7.
Jabari P, Munz G, Oleszkiewicz JA. Selection of denitrifying phosphorous accumulating organisms in IFAS systems: comparison of nitrite with nitrate as an electron acceptor. Chemosphere. 2014;109:20–7.
Yin J, Zhang P, Li F, Li G, Hai B. Simultaneous biological nitrogen and phosphorus removal with a sequencing batch reactor–biofilm system. Int Biodeterior Biodegradation. 2015;103:221–6.
Yang S, Yang F, Fu Z, Wang T, Lei R. Simultaneous nitrogen and phosphorus removal by a novel sequencing batch moving bed membrane bioreactor for wastewater treatment. J Hazard Mater. 2010;175:551–7.
Díez-Montero R, De Florio L, González-Viar M, Herrero M, Tejero I. Performance evaluation of a novel anaerobic–anoxic sludge blanket reactor for biological nutrient removal treating municipal wastewater. Bioresour Technol. 2016;209:195–204.
Dytczak MA, Londry KL, Oleszkiewicz JA. Activated sludge operational regime has significant impact on the type of nitrifying community and its nitrification rates. Water Res. 2008;42:2320–8.
Kim IT, Lee YE, Yoo YS, Jeong W, Yoon YH, Shin DC. Development of a combined aerobic-anoxic and methane oxidation bioreactor system using mixed methanotrophs and biogas for wastewater denitrification. Water (Switzerland). 2019;11:1377.
Evans LV. Biofilms: recent advances in their study and control. Evans LV, editor. Biofilms recent adbances their study control. Br Library Cataloguing in Publication Data; 2005.
Leyva-Díaz JC, Martín-Pascual J, Poyatos JM. Moving bed biofilm reactor to treat wastewater. Int J Environ Sci Technol:Center for Environmental and Energy Research and Studies. 2017;14:881–910.
Chowdhury N, Zhu J, Nakhla G, Patel A, Islam M. A novel liquid-solid circulating fluidized-bed bioreactor for biological nutrient removal from municipal wastewater. Chem Eng Technol Wiley-VCH Verlag. 2009;32:364–72.
Liu A, Nelson MJ, Wang X, Li H, He X, Zhao Z, Zhu J. Decentralized wastewater treatment in an urban setting: a pilot study of the circulating fluidized bed bioreactor treating septic tank effluent. Environ Technol (United Kingdom). Taylor and Francis Ltd.; 2019;1–31.
Adoonsook D, Chia-Yuan C, Wongrueng A, Pumas C. A simple way to improve a conventional A/O-MBR for high simultaneous carbon and nutrient removal from synthetic municipal wastewater. PLoS One. Public Library of Science San Francisco, CA USA. 2019;14:e0214976.
Chen W, Chen S, Hu F, Liu W, Yang D, Wu J. A novel anammox reactor with a nitrogen gas circulation: performance, granule size, activity, and microbial community. Environ Sci Pollut Res Springer. 2020;27:18661–71.
Wang X, Zhao J, Yu D, Chen G, Du S, Zhen J, et al. Stable nitrite accumulation and phosphorous removal from nitrate and municipal wastewaters in a combined process of endogenous partial denitrification and denitrifying phosphorus removal (EPDPR). Chem Eng J. 2019;355:560–71.
Baddour EM, Farhoud N, Sharholy M, Abdel-Magid IM. Biological treatment of poultry slaughterhouses wastewater by using aerobic moving bed biofilm reactor. Int Res J Public Environ Health. 2016;3:96–106.
De Sotto R, Bae S. Nutrient removal performance and microbiome of an energy-efficient reciprocation MLE-MBR operated under hypoxic conditions. Water Res. 2020;182:115991.
Zhang X, Wang C, Wu P, Yin W, Xu L. New insights on biological nutrient removal by coupling biofilm-based CANON and denitrifying phosphorus removal (CANDPR) process: long-term stability assessment and microbial community evolution. Sci Total Environ. 2020;730:138952.
Xu X, Qiu L, Wang C, Yang F. Achieving mainstream nitrogen and phosphorus removal through simultaneous partial nitrification, anammox, denitrification, and denitrifying phosphorus removal (SNADPR) process in a single-tank integrative reactor. Bioresour Technol. 2019;284:80–9.
Cheng C, Zhou Z, Pang H, Zheng Y, Chen L, Jiang L-M, et al. Correlation of microbial community structure with pollutants removal, sludge reduction and sludge characteristics in micro-aerobic side-stream reactor coupled membrane bioreactors under different hydraulic retention times. Bioresour Technol. 2018;260:177–85.
Eslami H, Ehrampoush MH, Falahzadeh H, Hematabadi PT, Khosravi R, Dalvand A, et al. Biodegradation and nutrients removal from greywater by an integrated fixed-film activated sludge (IFAS) in different organic loadings rates. AMB Express. 2018;8:3.
Bai Y, Zhang Y, Quan X, Chen S. Nutrient removal performance and microbial characteristics of a full-scale IFAS-EBPR process treating municipal wastewater. Water Sci Technol. 2016;73:1261–8.
Abualhail S, Naseer Mohammed R, Xiwu L. Integrated real-time control strategy in multi-tank A2O process for biological nutrient removal treating real domestic wastewater. Arab J Chem. 2017;10:S1041–54.
Do K-U, Banu RJ, Son D-H, Yeom I-T. Influence of ferrous sulfate on thermochemical sludge disintegration and on performances of wastewater treatment in a new process: anoxic–oxic membrane bioreactor coupled with sludge disintegration step. Biochem Eng J. 2012;66:20–6.
Banu JR, Do K-U, Kaliappan S, Yeom I-T. Effect of alum on nitrification during simultaneous phosphorous removal in anoxic/oxic reactor. Biotechnol Bioprocess Eng. 2009;14:543–8.
Banu JR, Uan DK, Yeom I-T. Nutrient removal in an A2O-MBR reactor with sludge reduction. Bioresour Technol. 2009;100:3820–4.
Do K, Banu JR, Chung I, Yeom I. Effect of thermochemical sludge pretreatment on sludge reduction and on performances of anoxic-aerobic membrane bioreactor treating low strength domestic wastewater. J Chem Technol Biotechnol. 2009;84:1350–5.
Banu JR, Do K-U, Yeom I-T. Effect of ferrous sulphate on nitrification during simultaneous phosphorus removal from domestic wastewater using a laboratory scale anoxic/oxic reactor. World J Microbiol Biotechnol. 2008;24:2981–6.
Daigger GT, Rittmann BE, Adham S, Andreottola G. Are membrane bioreactors ready for widespread application? Am Chem Soc. 2005;39:19.
Parco V, du Toit G, Wentzel M, Ekama G. Biological nutrient removal in membrane bioreactors: denitrification and phosphorus removal kinetics. Water Sci Technol. 2007;56:125–34.
Iorhemen OT, Hamza RA, Tay JH. Membrane bioreactor (Mbr) technology for wastewater treatment and reclamation: membrane fouling. Membranes (Basel). 2016;6(2):33.
Chen JC, Uan DK. Low dissolved oxygen membrane bioreactor processes (LDO-MBRs): a review. Int J Environ Eng. 2013;5:129.
Galil NI, Malachi KB-D, Sheindorf C. Biological nutrient removal in membrane biological reactors. Environ Eng Sci. 2009;26:817–24.
Dvořák L, Svojitka J, Wanner J, Wintgens T. Nitrification performance in a membrane bioreactor treating industrial wastewater. Water Res. 2013;47:4412–21.
Monclús H, Sipma J, Ferrero G, Rodriguez-Roda I, Comas J. Biological nutrient removal in an MBR treating municipal wastewater with special focus on biological phosphorus removal. Bioresour Technol. 2010;101:3984–91.
Lin H, Peng W, Zhang M, Chen J, Hong H, Zhang Y. A review on anaerobic membrane bioreactors: applications, membrane fouling and future perspectives. Desalination. 2013:169–88.
Maaz M, Yasin M, Aslam M, Kumar G, Atabani AE, Idrees M. Anaerobic membrane bioreactors for wastewater treatment: novel configurations, fouling control and energy considerations. Bioresour Technol. 2019:358–72.
Ribera-Pi J, Badia-Fabregat M, Calderer M, Polášková M, Svojitka J, Rovira M. Anaerobic membrane bioreactor (AnMBR) for the treatment of cheese whey for the potential recovery of water and energy. Waste Biomass Valorization Springer. 2020;11:1821–35.
Smith AL, Stadler LB, Love NG, Skerlos SJ, Raskin L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: a critical review. Bioresour Technol. 2012;122:149–59.
Itokawa H, Thiemig C, Pinnekamp J. Design and operating experiences of municipal MBRs in Europe. Water Sci Technol. 2008;58:2319–27.
da Costa RE, Lobo-Recio MA, Battistelli AA, Bassin JP, Belli TJ, Lapolli FR. Comparative study on treatment performance, membrane fouling, and microbial community profile between conventional and hybrid sequencing batch membrane bioreactors for municipal wastewater treatment. Environ Sci Pollut Res. 2018;25:32767–82.
Yuan H, Chen Y, Zhang H, Jiang S, Zhou Q, Gu G. Improved bioproduction of short-chain fatty acids (SCFAs) from excess sludge under alkaline conditions. Environ Sci Technol. 2006;40:2025–9.
Tong J, Chen Y. Enhanced biological phosphorus removal driven by short-chain fatty acids produced from waste activated sludge alkaline fermentation. Environ Sci Technol. 2007;41:7126–30.
Kang SJ, Zharaddine KPO, Takacs KM, Collins J, Wheeler J, Zharaddine P. Energy sustainability and nutrient removal from municipal wastewater. Proc Water Environ Fed. 2009;2009:6897–908.
Feng X-C, Bao X, Che L, Wu Q-L. Enhance biological nitrogen and phosphorus removal in wastewater treatment process by adding food waste fermentation liquid as external carbon source. Biochem Eng J. 2021;165:107811.
Kim JO, Chung J. Implementing chemical precipitation as a pretreatment for phosphorus removal in membrane bioreactor-based municipal wastewater treatment plants. KSCE J Civ Eng. 2014;18:956–63.
Dong C, Huang Y-H, Wang S-C, Wang X-H. Oxygen supply and wastewater treatment in subsurface-flow constructed wetland mesocosm: role of plant presence. Pol J Environ Stud. 2016;25:573–9.
Wang Q, Hu Y, Xie H, Yang Z. Constructed wetlands: a review on the role of radial oxygen loss in the rhizosphere by macrophytes. Water. 2018;10:678.
IWA. The reuse opportunity. Wastewater Rep. 2018.
Hu Z, Houweling D, Dold P. Biological nutrient removal in municipal wastewater treatment: new directions in sustainability. J Environ Eng. 2012;138:307–17.
Al-Ghouti MA, Al-Kaabi MA, Ashfaq MY, Da’na DA. Produced water characteristics, treatment and reuse: a review. J Water Process Eng. 2019;28:222–39.
Kõrgmaa V, Kriipsalu M, Tenno T, Lember E, Kuusik A, Lemmiksoo V. Factors affecting SVI in small scale WWTPs. Water Sci Technol. 2019;79:1766–76.
Chowdhury P, Viraraghavan T, Srinivasan A. Biological treatment processes for fish processing wastewater - a review. Bioresour Technol. 2010;101:439–49.
Durai G, Rajasimman M. Biological treatment of tannery wastewater - a review. J Environ Sci Technol. 2011;4:1–17.
Rani B, Maheshwari R, Kumar Yadav R, Pareek D, Sharma A. Resolution to provide safe drinking water for sustainability of future perspectives. Res J Chem Environ Sci. 2013;4:50–4.
Kassab G, Halalsheh M, Klapwijk A, Fayyad M, van Lier JB. Sequential anaerobic-aerobic treatment for domestic wastewater - a review. Bioresour Technol. 2010;101:3299–310.
Yaseen DA, Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol. 2019;16:1193–226.
Bhatia D, Sharma NR, Singh J, Kanwar RS. Biological methods for textile dye removal from wastewater: a review. Crit Rev Environ Sci Technol. Taylor and Francis Inc. 2017;47:1836–76.
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB. A critical review on textile wastewater treatments: possible approaches. J Environ Manag. 2016;182:351–66.
Seow TW, Lim CK. Removal of dye by adsorption : a review. J Bioremediat Biodegrad. 2016;7:371 37775854.
Choudhary S, Parmar N. Hazard assessment of liquid effluent treatment plant in pharmaceutical industry. Int J Technol NonTechnol Res. 2013;4:209–2014.
Adishkumar S, Sivajothi S, Rajesh BJ. Coupled solar photo-Fenton process with aerobic sequential batch reactor for treatment of pharmaceutical wastewater. Desalin Water Treat. 2012;48:89–95.
Upadhye V, Joshi SS, Logy T. Advances in wastewater treatment- a review 2012; 1–5.
Toczyłowska-Mamińska R. Limits and perspectives of pulp and paper industry wastewater treatment – a review. Renew Sust Energ Rev. 2017:764–72.
Ashrafi O, Yerushalmi L, Haghighat F. Greenhouse gas emission by wastewater treatment plants of the pulp and paper industry - modeling and simulation. Int J Greenh Gas Control. 2013;17:462–72.
Rubio-Clemente A, Chica E, Peñuela GA. Petrochemical wastewater treatment by photo-Fenton process. Water Air Soil Pollut. 2015;226:1–18.
Raut-Jadhav S, Badve MP, Pinjari DV, Saini DR, Sonawane SH, Pandit AB. Treatment of the pesticide industry effluent using hydrodynamic cavitation and its combination with process intensifying additives (H2O2 and ozone). Chem Eng J. 2016;295:326–35.
Alalm MG, Tawfik A, Ookawara S. Degradation of four pharmaceuticals by solar photo-Fenton process: kinetics and costs estimation. J Environ Chem Eng. 2015;3:46–51.
Zapata A, Oller I, Sirtori C, Rodríguez A, Sánchez-Pérez JA, López A. Decontamination of industrial wastewater containing pesticides by combining large-scale homogeneous solar photocatalysis and biological treatment. Chem Eng J. 2010;160:447–56.
Maiti D, Ansari I, Rather MA, Deepa A. Comprehensive review on wastewater discharged from the coal-related industries – characteristics and treatment strategies. Water Sci Technol. 2019;79:2023–35.
Vaseem H, Singh VK, Singh MP. Heavy metal pollution due to coal washery effluent and its decontamination using a macrofungus, Pleurotus ostreatus. Ecotoxicol Environ Saf. 2017;145:42–9.
Jeyanayagam S True confessions of the biological nutrient removal process. Florida Water Resour J 2005;37–46.
Sattayatewa C, Pagilla K, Sharp R, Pitt P. Fate of organic nitrogen in four biological nutrient removal wastewater treatment plants. Water Environ Res. 2010;82:2306–15.
Pagilla KR, Urgun-Demirtas M, Czerwionka K, Makinia J. Nitrogen speciation in wastewater treatment plant influents and effluents—the US and Polish case studies. Water Sci Technol. 2008;57:1511–7.
Zheng X, Zhou W, Wan R, Luo J, Su Y, Huang H, et al. Increasing municipal wastewater BNR by using the preferred carbon source derived from kitchen wastewater to enhance phosphorus uptake and short-cut nitrification-denitrification. Chem Eng J. 2018;344:556–64.
Hao X, Liu R, Huang X. Evaluation of the potential for operating carbon neutral WWTPs in China. Water Res. 2015;87:424–31.
Frison N, Katsou E, Malamis S, Bolzonella D, Fatone F. Biological nutrients removal via nitrite from the supernatant of anaerobic co-digestion using a pilot-scale sequencing batch reactor operating under transient conditions. Chem Eng J. 2013;230:595–604.
Zhang DQ, Tan SK, Gersberg RM, Zhu J, Sadreddini S, Li Y. Nutrient removal in tropical subsurface flow constructed wetlands under batch and continuous flow conditions. J Environ Manag Academic Press. 2012;96:1–6.
Kumar M, Lee PY, Fukusihma T, Whang LM, Lin JG. Effect of supplementary carbon addition in the treatment of low C/N high-technology industrial wastewater by MBR. Bioresour Technol. 2012;113:148–53.
Carrera J, Vicent T, Lafuente J. Effect of influent COD/N ratio on biological nitrogen removal (BNR) from high-strength ammonium industrial wastewater. Process Biochem. 2004;39:2035–41.
Fu Z, Yang F, An Y, Xue Y. Simultaneous nitrification and denitrification coupled with phosphorus removal in an modified anoxic/oxic-membrane bioreactor (a/O-MBR). Biochem Eng J. 2009;43:191–6.
Yao H, Liu H, He Y, Zhang S, Sun P, Huang C. Performance of an ANAMMOX reactor treating wastewater generated by antibiotic and starch production processes. Front Environ Sci Eng China Springer. 2012;6:875–83.
Fajri JA, Fujisawa T, Trianda Y, Ishiguro Y, Cui G, Li F, et al. Effect of aeration rates on removals of organic carbon and nitrogen in small onsite wastewater treatment system (Johkasou). MATEC Web Conf EDP Sci. 2018;147:04008.
Zhang M, Lawlor PG, Wu G, Lynch B, Zhan X. Partial nitrification and nutrient removal in intermittently aerated sequencing batch reactors treating separated digestate liquid after anaerobic digestion of pig manure. Bioprocess Biosyst Eng. 2011;34:1049–56.
Song X, Liu R, Chen L, Dong B, Kawagishi T. Advantages of intermittently aerated SBR over conventional SBR on nitrogen removal for the treatment of digested piggery wastewater. Front Environ Sci Eng. Higher Education Press. 2017;11:1–10.
Spellman FR. Treatment plant operators. N Y 2004.
Liu H, Leng F, Chen P, Kueppers S. Pollutant removal characteristics of a two-influent-line BNR process performing denitrifying phosphorus removal: role of sludge recycling ratios. Water Sci Technol. 2016;74:2474–82.
Grady L, Daigger GT, Love NG, Filipe CDM. Biological wastewater treatment. 3rd ed. New York: CRC Press; 2011.
Lee WS, Chua ASM, Yeoh HK, Ngoh GC. A review of the production and applications of waste-derived volatile fatty acids. Chem Eng J. 2014;235:83–99.
Kumar G, Bakonyi P, Sivagurunathan P, Nemestóthy N, Bélafi-Bakó K, Lin CY. Improved microbial conversion of de-oiled Jatropha waste into biohydrogen via inoculum pretreatment: process optimization by experimental design approach. Biofuel Res J. 2015;2:209–14.
Xu S, Wu D, Hu Z. Impact of hydraulic retention time on organic and nutrient removal in a membrane coupled sequencing batch reactor. Water Res. 2014;55:12–20.
Zhao W, Ting YP, Chen JP, Xing CH, Shi SQ. Advanced primary treatment of waste water using a bio-flocculation-adsorption sedimentation process. Acta Biotechnol. 2000;20:53–64.
Wu H, Fan J, Zhang J, Ngo HH, Guo W, Liang S. Strategies and techniques to enhance constructed wetland performance for sustainable wastewater treatment. Environ Sci Pollut Res Springer Verlag. 2015;22:14637–50.
Kadlec RH, Knight RL, Vymazal J, Brix H, Cooper P, Haber R. Constructed wetlands for pollution control. Denmark: IWA; 2000.
Vymazal J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: a review of a recent development. Water Res. 2013;47:4795–811.
Bachand PAM, Horne AJ. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng. 1999;14:17–32.
Liu S, Yan B, Wang L. The layer effect in nutrient removal by two indigenous plant species in horizontal flow constructed wetlands. Ecol Eng. 2011;37:2101–4.
Trein CM, Zumalacarregui JAG, de Andrade Moraes MA, Von Sperling M. Reduction of area and influence of the deposit layer in the first stage of a full-scale French system of vertical flow constructed wetlands in a tropical area. Water Sci Technol. 2019;80:347–56.
Vymazal J, Kröpfelová L. A three-stage experimental constructed wetland for treatment of domestic sewage: first 2 years of operation. Ecol Eng. 2011;37:90–8.
Smith E, Gordon R, Madani A, Stratton G. Year-round treatment of dairy wastewater by constructed wetlands in Atlantic Canada. Soc Wetland Sci. 2006;26:349–57.
Wu H, Zhang J, Li P, Zhang J, Xie H, Zhang B. Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol Eng. 2011;37:560–8.
Bosak V, VanderZaag A, Crolla A, Kinsley C, Gordon R. Performance of a constructed wetland and pretreatment system receiving potato farm wash water. Water. 2016;8:183.
Wang F, Liu Y, Ma Y, Wu X, Yang H. Characterization of nitrification and microbial community in a shallow moss constructed wetland at cold temperatures. Ecol Eng. 2012;42:124–9.
Gao DW, Hu Q. Bio-contact oxidation and greenhouse-structured wetland system for rural sewage recycling in cold regions: a full-scale study. Ecol Eng. 2012;49:249–53.
Rai UN, Upadhyay AK, Singh NK, Dwivedi S, Tripathi RD. Seasonal applicability of horizontal sub-surface flow constructed wetland for trace elements and nutrient removal from urban wastes to conserve Ganga River water quality at Haridwar. India Ecol Eng. 2015;81:115–22.
Yates CN, Varickanickal J, Cousins S, Wootton B. Testing the ability to enhance nitrogen removal at cold temperatures with C. aquatilis in a horizontal subsurface flow wetland system. Ecol Eng. 2016;94:344–51.
Rozema ER, Rozema LR, Zheng Y. A vertical flow constructed wetland for the treatment of winery process water and domestic sewage in Ontario, Canada: six years of performance data. Ecol Eng. 2016;86:262–8.
Sani A, Scholz M, Bouillon L. Seasonal assessment of experimental vertical-flow constructed wetlands treating domestic wastewater. Bioresour Technol. 2013;147:585–96.
Zhang X, Inoue T, Kato K, Izumoto H, Harada J, Wu D. Multi-stage hybrid subsurface flow constructed wetlands for treating piggery and dairy wastewater in cold climate. Environ Technol (United Kingdom). Taylor and Francis Ltd. 2017;38:183–91.
Sharma PK, Takashi I, Kato K, Ietsugu H, Tomita K, Nagasawa T. Seasonal efficiency of a hybrid sub-surface flow constructed wetland system in treating milking parlor wastewater at northern Hokkaido. Ecol Eng. 2013;53:257–66.
Zhu D, Sun C, Zhang H, Wu Z, Jia B, Zhang Y. Roles of vegetation, flow type and filled depth on livestock wastewater treatment through multi-level mineralized refuse-based constructed wetlands. Ecol Eng. 2012;39:7–15.
Wu Y, Han R, Yang X, Zhang Y, Zhang R. Long-term performance of an integrated constructed wetland for advanced treatment of mixed wastewater. Ecol Eng. 2017;99:91–8.
Rani N, Maheshwari RC, Kumar V, Vijay VK. Purification of pulp and paper mill effluent through Typha and Canna using constructed wetlands technology. J Water Reuse Desalin. 2011;1:237–42.
Tunçsiper B, Drizo A, Twohig E. Constructed wetlands as a potential management practice for cold climate dairy effluent treatment - VT. USA Catena. 2015;135:184–92.
Chen Z, Cuervo DP, Müller JA, Wiessner A, Köser H, Vymazal J. Hydroponic root mats for wastewater treatment—a review. Environ Sci Pollut Res. 2016;23:15911–28.
Wu J, Li Z, Wu L, Zhong F, Cui N, Dai Y. Triazophos (TAP) removal in horizontal subsurface flow constructed wetlands (HSCWs) and its accumulation in plants and substrates. Sci Rep Nature Publishing Group. 2017;7:1–8.
Dan TH, Quang LN, Chiem NH, Brix H. Treatment of high-strength wastewater in tropical constructed wetlands planted with Sesbania sesban: horizontal subsurface flow versus vertical downflow. Ecol Eng. 2011;37:711–20.
Maltais-Landry G, Chazarenc F, Comeau Y, Troesch S, Brisson J. Effects of artificial aeration, macrophyte species, and loading rate on removal efficiency in constructed wetland mesocosms treating fish farm wastewater. J Environ Eng Sci. 2007;6:409–14.
Zachritz WH, Hanson AT, Sauceda JA, Fitzsimmons KM. Evaluation of submerged surface flow (SSF) constructed wetlands for recirculating tilapia production systems. Aquac Eng. 2008;39:16–23.
Saeed T, Sun G. Kinetic modelling of nitrogen and organics removal in vertical and horizontal flow wetlands. Water Res. 2011;45:3137–52.
Villaseñor J, Capilla P, Rodrigo MA, Cañizares P, Fernández FJ. Operation of a horizontal subsurface flow constructed wetland - microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013;47:6731–8.
Stefanakis AI, Akratos CS, Tsihrintzis VA. Effect of wastewater step-feeding on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol Eng. 2011;37:431–43.
Calheiros CSC, Rangel AOSS, Castro PML. Constructed wetlands for tannery wastewater treatment in Portugal: ten years of experience. Int J Phytoremediation. 2014;16:859–70.
Worku A, Tefera N, Kloos H, Benor S. Constructed wetlands for phytoremediation of industrial wastewater in Addis Ababa, Ethiopia. Nanotechnol Environ Eng. 2018;3:1–11.
Knowles P, Dotro G, Nivala J, García J. Clogging in subsurface-flow treatment wetlands: occurrence and contributing factors. Ecol Eng. 2011;37:99–112.
Pedescoll A, Sidrach-Cardona R, Sánchez JC, Carretero J, Garfi M, Bécares E. Design configurations affecting flow pattern and solids accumulation in horizontal free water and subsurface flow constructed wetlands. Water Res. 2013;47:1448–58.
Shen C, Yang D, Dong B. A new operation mode solving clogging problems of horizontal subsurface constructed wetlands. Water Sci Technol. 2010;62:1045–51.
Nivala J, Knowles P, Dotro G, García J, Wallace S. Clogging in subsurface-flow treatment wetlands: measurement, modeling and management. Water Res. 2012;46:1625–40.
De la Varga D, Díaz MA, Ruiz I, Soto M. Avoiding clogging in constructed wetlands by using anaerobic digesters as pre-treatment. Ecol Eng. 2013;52:262–9.
Bailey HC, Digiorgio C, Kroll K, Hinton DE, Miller JL, Starrett G. Development of procedures for identifying pesticide toxicity in ambient waters: carbofuran, diazinon, chlorpyrifos. Environ Toxicol Chem. 1996;15:837–45.
Druzina B, Stegu M. Degradation study of selected organophosphorus insecticides in natural waters. Int J Environ Anal Chem. 2007;87:1079–93.
Saeed T, Sun G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J Environ Manag. 2012;112:429–48.
Hadad HR, Mufarrege MM, Di Luca GAD, Maine MA. Salinity and pH effects on floating and emergent macrophytes in a constructed wetland. Water Sci Technol. 2017;2018:270–5.
Wortman SE. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci Hortic (Amsterdam). 2015;194:34–42.
Zou Y, Hu Z, Zhang J, Xie H, Guimbaud C, Fang Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour Technol. 2016;210:81–7.
Schachtman DP, Reid RJ, Ayling SM. Phosphorus uptake by plants: from soil to cell. Plant Physiol. American Society of Plant Biologists. 1998;116:447–53.
Becquer A, Trap J, Irshad U, Ali MA, Claude P, et al. Front Plant Sci. Frontiers Research Foundation. 2014;5:15.
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J. Microbial processes influencing performance of treatment wetlands: a review. Ecol Eng. 2009;35:987–1004.
Ouellet-Plamondon C, Chazarenc F, Comeau Y, Brisson J. Artificial aeration to increase pollutant removal efficiency of constructed wetlands in cold climate. Ecol Eng. 2006;27:258–64.
Zhang J, Wang X, Li P, Zheng Z, Hu J. Treatment of methamidophos wastewater with constructed wetland. Chin J Environ Sci Technol. 2010;33:154–7.
Bondarenko S, Gan J, Haver DL, Kabashima JN. Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ Toxicol Chem. 2004;23:2649–54.
Meng P, Pei H, Hu W, Shao Y, Li Z. How to increase microbial degradation in constructed wetlands: influencing factors and improvement measures. Bioresour Technol. 2014;157:316–26.
EPA. Wastewater treatment fact sheet: external carbon source for nitrogen removal. 2013.1–5.
Chen J, Wang H. Evaluation and utilization of urban sewage treatmentand recycling process analysis. China Archit Ind Press. 2012 1–10.
Guerrini A, Romano G, Indipendenza A. Energy efficiency drivers in wastewater treatment plants: a double bootstrap DEA analysis. Sustainability. MDPI AG. 2017;9:1126.
Rothausen SGSA, Conway D. Greenhouse-gas emissions from energy use in the water sector. Nat Clim Chang. 2011;1:210–9.
Xu J, Li Y, Wang H, Wu J, Wang X, Li F. Exploring the feasibility of energy self-sufficient wastewater treatment plants: a case study in eastern China. Energy Procedia. 2017:3055–61.
Li W, Li L, Qiu G. Energy consumption and economic cost of typical wastewater treatment systems in Shenzhen, China. J Clean Prod. 2017;163:S374–8.
Gu Y, Li Y, Li X, Luo P, Wang H, Robinson ZP. The feasibility and challenges of energy self-sufficient wastewater treatment plants. Appl Energy. 2017;204:1463–75.
Prateep NA, Talang R, Sirivithayapakorn S, Polruang S. Environmental impacts and cost-effectiveness of Thailand’s centralized municipal wastewater treatment plants with different nutrient removal processes. J Clean Prod. 2020;256:120433.
Harrington R, O’Donovan G, McGrath G. Integrated constructed wetlands (ICW) working at the landscape scale: the Anne Valley project. Ireland Ecol Inform. 2013;14:104–7.
Gkika D, Gikas GD, Tsihrintzis VA. Construction and operation costs of constructed wetlands treating wastewater. Water Sci Technol. 2014;70:803–10.
Tyndall and Bowman. IOWA nutrient reduction strategy best management practice cost overview series: constructed wetlant. 2016.
Freeman AI, Widdowson S, Murphy C, Cooper DJ. Economic assessment of aerated constructed treatment wetlands using whole life costing. Water Sci Technol. 2019;80:75–85.
Firth AEJ, Mac Dowell N, Fennell PS, Hallett JP. Assessing the economic viability of wetland remediation of wastewater, and the potential for parallel biomass valorisation. Environ Sci Water Res Technol. 2020;6:2103–21.
Seguí L, Alfranca O, García J. Techno-economical evaluation of water reuse for wetland restoration: a case study in a natural park in Catalonia, Northeastern Spain. Desalination. 2009;246:179–89.
Mburu N, Tebitendwa SM, van Bruggen JJA, Rousseau DPL, Lens PNL. Performance comparison and economics analysis of waste stabilization ponds and horizontal subsurface flow constructed wetlands treating domestic wastewater: a case study of the Juja sewage treatment works. J Environ Manag. 2013;128:220–5.
Alfranca O, García J, Varela H. Economic valuation of a created wetland fed with treated wastewater located in a peri-urban park in Catalonia. Spain Water Sci Technol. 2011;63:891–8.
Nguyen HTT, Le VQ, Hansen AA, Nielsen JL, Nielsen PH. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol Ecol. Blackwell Publishing Ltd Oxford, UK. 2011;76:256–67.
Wang D, Tooker NB, Srinivasan V, Li G, Fernandez LA, Schauer P. Side-stream enhanced biological phosphorus removal (S2EBPR) process improves system performance - a full-scale comparative study. Water Res. 2019;167.
Kwak D-H, Lee K-C. Effect of floated sludge recycling on phosphorus removal in dissolved air flotation. Proc Inst Civ Eng - Water Manag. Thomas Telford Services. 2015;168:270–9.
Shi J, Lu X, Yu R, Zhu W. Nutrient removal and phosphorus recovery performances of a novel anaerobic-anoxic/nitrifying/induced crystallization process. Bioresour Technol. 2012;121:183–9.
Kuroda A, Takiguchi N, Gotanda T, Nomura K, Kato J, Ikeda T. A simple method to release polyphosphate from activated sludge for phosphorus reuse and recycling. Biotechnol Bioeng. 2002;78:333–8.
Ozdemir B, Yenigun O. A pilot scale study on high biomass systems: energy and cost analysis of sludge production. J Membr Sci. 2013;428:589–97.
Qiu Y, Shi H, He M. Nitrogen and phosphorous removal in municipal wastewater treatment plants in China: a review. Int J Chem Eng. 2010;2010:1–10.
Rahman SM, Eckelman MJ, Onnis-Hayden A, Gu AZ. Life-cycle assessment of advanced nutrient removal technologies for wastewater treatment. Environ Sci Technol. 2016;50:3020–30.
Kehrein P, van Loosdrecht M, Osseweijer P, Garfí M, Dewulf J, Posada J. A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environ Sci Water Res Technol. 2020;6:877–910.
Funding
This work is supported by the Department of Biotechnology, India under its initiative Mission innovation Challenge Scheme (IC4). The grant from the project entitled “A novel integrated biorefinery for conversion of lignocellulosic agro waste into value-added products and bioenergy (BT/PR31054/PBD/26/763/2019)” is utilized for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biology and Pollution
Rights and permissions
About this article
Cite this article
Rajesh Banu, J., Merrylin, J., Kavitha, S. et al. Trends in Biological Nutrient Removal for the Treatment of Low Strength Organic Wastewaters. Curr Pollution Rep 7, 1–30 (2021). https://doi.org/10.1007/s40726-020-00169-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-020-00169-x