Abstract
We report the design and hydrodynamic performance of a pulsatile blood pump and a pneumatic driver system to treat pediatric patients in need of circulatory support while waiting for a heart transplant. The blood pump consists of a pumping chamber with 15 ml stroke volume separated by a flexible diaphragm from a pneumatic chamber. The blood chamber has two orifices fitted with rings in which tri-leaflet tissue valves are placed to control the inflow and outflow of blood. Blood contacting surfaces are heparin coated. The driving unit allows operation of two pumps to assist the left and right side of the heart, independently or simultaneously in three different modes of operation: full-to-empty, ECG synchronized and asynchronous. The flow generated by the pump increases with preload and application of auxiliary negative pressure during the filling phase reaching approximately 1,6 L/min when the pump is operating in full-to-empty mode. The results suggest the performance of the pediatric VAD designed is compatible with the needs of pediatric patients up to 15 kg body weight.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Pediatric Ventricular Assist Devices
The use of ventricular assist devices (VADs) in children as a bridge to transplantation improves survival of patients with congenital or acquired heart disease [1, 2]. Technical challenges still remain for the construction of a pump with reduced size having low risk for thromboembolism, non-hemolytic properties and closely matching the cardiac output of infants and small. The most common form of mechanical support available to pediatric patients is extracorporeal membrane oxygenation (ECMO) [3]. However, ECMO is mainly recommended for short-term use due to the high incidence of complications during medium and long-term use [4]. Improvements in VAD technology and the FDA approval of the Berlin heart EXCOR device for long-term support for small children in the United States of America resulted in a large increase in the use of VADs [5]. Clinical results from centers in Europe and USA show that the rates of survival of children bridged to transplantation with VADs is estimated to vary from 75 to 96% [6]. Costs associated with this technology, however, are a barrier to its use and dissemination in developing countries. In Brazil, there is no relevant clinical experience with pediatric VADs. Therefore, there is an urgent need for development of devices specific for children. This paper presents the design and hydrodynamic performance of a pulsatile pump for paracorporeal implantation as a bridge to heart transplantation in children.
2 Methods
2.1 Pump Design and Materials
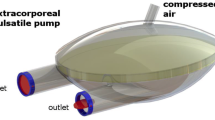
The circulatory assist system developed comprises a pneumatically-actuated VAD and a bedside driver. The VAD is a pulsatile displacement pump for paracorporeal placement for temporary cardiac support. The pump is made by biocompatible polymer chambers separated by a diaphragm with heparin-coated blood contacting surfaces. Tri-leaflet bovine pericardium valves are mounted on the inlet and outlet titanium connectors of the blood chamber. Figure 1 shows a schematic of the pump (left) and a sagittal section showing the diaphragm separating the chambers (right).
Illustration of the pump (left) showing the blood chamber with an orifice for de-airing, connectors for directing the flow into and out of the blood chambers and the pneumatic chamber with driveline port. The figure also illustrates a sagittal section of the blood chamber showing the diaphragm separating both chambers (right)
The driving unit allows operation of one or two pumps to assist the left and/or the right ventricle simultaneously and independently in two modes of operation: (a) Full to Empty mode with full filling and emptying of the pump, usually referred to as automatic; (b) Asynchronous mode or fixed pumping rate. In this mode of operation outflow values are adjusted independently. The system allows application of auxiliary negative pressure on the pneumatic chamber in situations of low filling pressures. The system has a console with a monitor which displays the flow generated by the one or two VADs in univentricular or biventricular assistance, respectively.
2.2 VAD Hydrodynamic Performance
A hydraulic simulator of the arterial circulation was developed to simulate hemodynamic conditions considering flow and pressure found in pediatric patients during circulatory support. The simulator was based on the compartment model usually referred in the literature as arterial mock loop [7]. For testing the pediatric device, the simulator was filled with a blood analogue (35% glycerin in 0.9% NaCl salt solution) with a dynamic viscosity of about 3.7 × 10−3 Pa. s at a temperature of 37 ℃, maintained with the use of a heat exchanger inserted in the circuit. The tests were performed with initial compliance and resistance values set at 1 ml mm Hg−1 and 1 mm Hg s ml−1, respectively. The simulator was equipped with pressure transducers (TruWave PXMK2051, Edwards Lifescience, USA) and ultrasonic flowmeters (TS-410, ME6PXN219 probes, Transonic Systems, Inc., USA). Signals were recorded for off line analysis (WinDaq DI-720, Dataq Instruments, USA).
3 Results
3.1 Flow and Pressure Curves for the Mock Loop Simulator
Figure 2 shows the pediatric VAD connected to the mock loop simulator (top). In this figure, aortic and atrial pressures are provided by compartments identified by aortic and A, respectively. Aortic compliance is set by adjusting the ratio of air pressure and fluid volume in the Aortic compartment. Hydrostatic pressure in atrial (A) compartment simulates filling preloads between 0 and 20 mm Hg. Systemic resistance is set by a clamp on the tubing connecting the aortic compartment and the atrial compartment, not shown for the sake of simplicity. Representative waveforms of arterial pressure and flow generated by the device are shown (bottom).
Top: VAD connected to the mock loop of the circulation. Aortic and atrial (A) compartments, pressure (P) and flow (F) transducers are connected to record the inflow and the outflow of blood analog. Bottom: Representative waveforms of pressure (dotted line) and flow generated by the device (continuous line) recorded in the loop. Systolic interval (pumping) is indicated by the bold traces above waveforms
3.2 VAD Flow Generation
The performance of the pediatric VAD operating in full-to-empty mode at a constant afterload pressure of 100 mmHg is shown in Fig. 3. The figure shows mean flows (L/min) generated by the pump versus preloads (mmHg) with and without application of auxiliary negative pressure during filling. Flow increases with preload and application of auxiliary negative pressure and the pump is capable of generating up to 1.6 L/min.
4 Discussion and Conclusion
The Berlin Heart EXCOR VAD (Berlin Heart AG, Berlin, Germany) and the Medos HIA-VAD (MEDOS Medizintechnik AG, Stolberg, Germany) are the only pulsatile VAD systems commercially available for pediatric patients, including neonates. The design of the pediatric VAD here presented utilizes the same principle of volume displacement utilized in the Berlin Heart EXCOR and MEDOS devices and also utilized in a previously device designed for adult patients used as a bridge to heart transplantation in our institution [8]. The flow patterns in small ventricular assist devices with higher frequency of operation are much more complex and not dynamically similar to that of larger devices. For pulsatile VADs, the risk of hemolysis and thrombus formation are usually inversely proportional to their ejection volume due to the synthetic material interface and frequency of operation. This explains, in part, the scarcity of devices for cardiac assistance of pediatric patients. Previous results from fluid motion studies using particle image velocimetry on a 30 mL ejection volume VAD obtained by our group [9] were incorporated in the design here presented. The geometry of the blood chamber of the VAD was modified with the aim of reducing turbulent motions and removing regions prone to stagnation in order to reduce the incidence of thrombus formation and hemolysis.
Matching of patients to devices can be made based on cardiac output (CO, in L/min) based on weight and body surface area (BSA) [10]. Using the same principle for estimating the range of support offered by the VAD designed and considering its hydrodynamic performance it is possible to predict that it is capable of providing support to neonates and infants up to 15 kg body weight and BSA up to approximately 0.4 m2.
Clinical application of pediatric VADs is scarce in Brazil where there is no formal funding for long term VADs. In this scenario, the pediatric VAD under development is expected to provide a low cost solution for mechanical circulatory assistance in children who at present lack any alternative for long term support. The results contribute to the initiative of creating a translational research group for neonatal and pediatric cardiac surgery patients in our institution [11].
References
Davies, R.R., Russo, M.J., Hong, K.N., et al.: The use of mechanical circulatory support as a bridge to transplantation in pediatric patients: an analysis of the united network for organ sharing database. J. Thorac. Cardiovasc. Surg. 135(2), 421–427 (2008)
Blume, E.D., Naftel, D.C., Bastardi, H.J., et al.: Outcomes of children bridged to heart transplantation with ventricular assist devices. A multi-institutional study. Circulation. 113(19), 2313–2319 (2006)
The July 2012 Extracorporeal Life Support Organization (ELSO) Extracorporeal Life Support (ECLS) Registry Report International Summary. Ann Arbor, Michigan, Extracorporeal Life Support Organization (2012)
Imamura, M., Dossey, A.M., Prodhan, P., et al.: Bridge to cardiac transplant in children: Berlin heart versus extracorporeal membrane oxygenation. Ann. Thorac. Surg. 87(6), 1894–1901 (2009)
Morales, D.L., Almond, C.S., Jaquiss, R.D., et al.: Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin heart EXCOR ventricular assist device. J. Heart Lung Transp. 30, 1–8 (2011)
Almond, C.S., Morales, D.L., Blackstone, E.H., et al.: Berlin heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation (127), 1702–119 (2013)
Pantalos, G.M., Ionan, C., et al.: Expanded pediatric cardiovascular simulator for research and training. ASAIO J. 56(1), 67–72 (2010)
Moreira, L.F., Galantier, J., Benício, A., Leirner, A.A., Cestari, I.A., Stolf, N.A.: Left ventricular circulatory support as bridge to heart transplantation in Chagas’ disease cardiomyopathy. Artif. Organs 31(4), 253–258 (2007)
Ferrara, E., Muramatsu, M., Christensen, K.T., Cestari, I.A.: Particle-image velocimetry study of a pediatric ventricular assist device. J. Biomech. Eng. 132(7), 1004 (2010)
Connell, J.A., Khalapyan, T., Myers, J.L., et al.: Anatomic fit assessment for the Penn state pediatric ventricular assist device. ASAIO J. (53), 687–691 (2007)
Wang, S., Caneo, L.F., Jatene, M.B., Jatene, F.B., Cestari, I.A., Kunselman, A.R., Ündar, A.: In vitro evaluation of pediatric hollow‐fiber membrane oxygenators on hemodynamic performance and gaseous microemboli handling: an international multicenter/multidisciplinary approach artif organs 41(9), 865–874 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Research supported by CNPq (National council for Research and Development; Grants 467270/2014-7 and 311191/2017-7) and FAPESP (State of São Paulo Research Foundation Grant 2012/50283-6).
Idágene A. Cestari and the other authors are with the Heart Institute (InCor) of the University of São Paulo Medical School, São Paulo, SP, 05403000, Brazil, (phone: +551126615528; fax: 55 11 26615201; e-mail: cestari@incor.usp.br.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Cestari, I.A. et al. (2019). Design and Hydrodynamic Performance of a Pediatric Pulsatile Pump. In: Costa-Felix, R., Machado, J., Alvarenga, A. (eds) XXVI Brazilian Congress on Biomedical Engineering. IFMBE Proceedings, vol 70/1. Springer, Singapore. https://doi.org/10.1007/978-981-13-2119-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-2119-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2118-4
Online ISBN: 978-981-13-2119-1
eBook Packages: EngineeringEngineering (R0)