Abstract
The definition of papillary microcarcinoma (PMC) by the WHO is papillary thyroid carcinoma (PTC) measuring 10 mm or less regardless of extension to adjacent organs (Ex), clinical lymph node metastasis (N), and distant metastasis (M). Low-risk PMC is defined as PMC without Ex, N, or M. Recent development of imaging techniques, especially ultrasound examination, has enabled us to detect small nodules measuring 3 mm or less. Additionally, ultrasound-guided fine needle aspiration cytology (FNAC) facilitated diagnosis of small nodules detected by ultrasound. Because of the prevalence of these techniques, PMC has been very frequently detected and diagnosed. Largely due to these developments, the incidence of thyroid cancer, especially of small PTC, is increasing globally without an increase in thyroid cancer mortality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
10.1 Background
The definition of papillary microcarcinoma (PMC) by the WHO is papillary thyroid carcinoma (PTC) measuring 10 mm or less regardless of extension to adjacent organs (Ex), clinical lymph node metastasis (N), and distant metastasis (M). Low-risk PMC is defined as PMC without Ex, N, or M. Recent development of imaging techniques, especially ultrasound examination, has enabled us to detect small nodules measuring 3 mm or less. Additionally, ultrasound-guided fine needle aspiration cytology (FNAC) facilitated diagnosis of small nodules detected by ultrasound. Because of the prevalence of these techniques, PMC has been very frequently detected and diagnosed. Largely due to these developments, the incidence of thyroid cancer, especially of small PTC, is increasing globally without an increase in thyroid cancer mortality.
In the past, PMC was frequently found in autopsy studies as latent carcinoma, and previous reports demonstrated that latent PMCs measuring 3–10 mm, which would be detectable by ultrasound examinations, were found in as much as 0.5–5.2% of study subjects [1]. Takebe et al. detected and diagnosed thyroid carcinoma by ultrasound screening and FNAC in 3.5% of women aged 30 years or older, and most were PMC [2]. This incidence was 1000 times higher than the prevalence of clinical thyroid carcinoma reported at that time and was not divergent from the incidence of latent thyroid carcinoma in previous autopsy studies. These facts strongly suggest that a very small proportion of PMCs grow and become a clinical disease over a long time period.
10.2 History of Active Surveillance for PMC
Based on the findings detailed above, in 1993, Dr. Miyauchi hypothesized that most PMCs stay small and are harmless to patients. He doubted the belief common at that time that PMCs are an early stage of clinical disease and should immediately be treated surgically. Although no data about the natural history of PMCs was available, he hypothesized that most PMCs do not grow or grow very slowly and that surgical treatment for all PMCs might result in more harm than good.
Of course, not all PMCs remain stable and latent. All advanced thyroid cancers were small cancers in the beginning. However, there were no markers predicting the future progression of PMCs at that time, a situation that continues to this day. As a result, active surveillance (AS) is the only method available for discriminating PMCs showing disease progression from others. In 1993, Dr. Miyauchi proposed AS without immediate surgery for low-risk PMC at a doctors’ meeting at Kuma Hospital. He considered that surgical treatment after the appearance of signs of progression such as enlargement and novel appearance of N detected on ultrasound would not be too late. A clinical study of AS for low-risk PMCs was initiated after the approval of the trial by doctors at Kuma Hospital. Two years later, in 1995, the Cancer Institute Hospital (Tokyo, Japan) also started AS for low-risk PMC based on concepts similar to ours.
10.3 Recent Increase in the Incidence of Thyroid Carcinoma But Not in Mortality
A great difference has been reported between trends in the incidence and mortality of thyroid carcinoma in various countries [3,4,5,6]. In the United States, the incidence of thyroid carcinoma increased by 2.4-fold between 1973 and 2002 and 2.9-fold between 1975 and 2009 [3, 4]. In South Korea, the incidence of thyroid carcinoma increased as much as 15-fold between 1993 and 2011 [5]. Increased incidence of thyroid carcinoma was also reported in Italy, France, England, Scotland, Australia, and Nordic countries [6].
However the mortality of thyroid carcinoma was stable during the duration of these studies. In the United States, the increased incidence of small papillary carcinoma, including PMC, was also reported [3, 4], which was the reason for the difference in the trends of incidence and mortality. These data indicated that many harmless small carcinomas, including low-risk PMCs, were detected and diagnosed by ultrasound and ultrasound-guided FNAC and surgically treated immediately after diagnosis.
10.4 Exclusion Criteria of AS for PMC
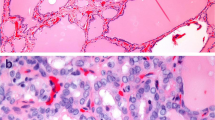
There are two types of contraindication for AS (Table 10.1). One is the presence of clinically high-risk features. The presence of N or, although very rare, M on imaging studies should be immediately treated as advanced PTC. Patients with recurrent laryngeal nerve paralysis because of PMC located on the course of the nerve are also strong candidates for immediate surgery (Fig. 10.1a). Although rare, PMCs with high-grade malignancy on cytology, such as those with suspected tall-cell variant, and poorly differentiated carcinoma should be operated on rather than undergoing AS. It goes without saying that PMCs that show signs of progression during AS should be surgically treated. Finally, observation of the following features renders cases unsuitable for AS, although it is not clear whether such tumors are biologically aggressive. We cautiously include PMCs attaching the trachea or located on the course of the recurrent laryngeal nerve on imaging studies in the contraindication category (Fig. 10.1b, c).
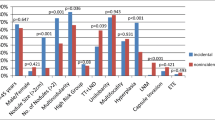
Multiplicity of PMCs and the presence of family history of differentiated thyroid carcinoma are not included in the contraindications. It is known that the incidence of multiplicity is higher in PTC with family history than in PTC without. We also know that multiple PMCs without clinical node metastasis are more likely to show microscopic N than solitary PMCs [7]. Thus, positive family history or multiple foci might be mild risk factors. However, if these were included in the category recommending immediate surgery, the incidence of total thyroidectomy would be substantially higher, resulting in higher incidence of surgical complication such as hypoparathyroidism and recurrent laryngeal nerve paralysis. We speculated that this drawback would outweigh the merit of immediate surgery. With these considerations, we did not include these features as contraindications for AS category. Our recent study showed these features were not significant factors of PMC progression [8].
10.5 Practice of AS for Low-Risk PMC
The algorithm in our practice of AS for low-risk PMC is summarized in Fig. 10.2. The newest American Thyroid Association (ATA) guidelines (2015) do not require FNAC for nodules suspected of PMC unless they have features indicative of high risk, such as N and/or M [9]. In our institutions, we diagnose PMC measuring 5 mm or larger with FNAC and notify patients of the diagnosis.
There are two reasons for this. The size cutoff on performing FNAB for thyroid nodules with suspicious sonographic features is 5 mm in the guidelines published by the Japan Association of Breast and Thyroid Sonology. At present, this is the only guideline on performing FNAC on thyroid nodules in Japan. If we do not diagnose PMC with cytology, patients might see other doctors in other hospitals. The doctors might perform FNAC and tell the patients that Kuma Hospital missed the diagnosis and that they should undergo surgery. Such a situation would be unhappy for both Kuma Hospital and the patients. The other reason is that the diagnosis of PMC should encourage patients to visit the hospital for regular checkups. Without the diagnosis of malignancy, it could be difficult to convince patients to have regular checkups. We think that regular follow-up is necessary, since some PMCs do show disease progression.
Before starting AS, accurate evaluation of PMC is mandatory to determine whether it is suitable for AS based on ultrasound and, if necessary, on CT scan. The most important issue is the location of tumor. Careful evaluation is needed especially when the tumor is located on the dorsal side and near to the recurrent laryngeal nerve or it touches the trachea. For PMC with coarse strong echoes causing strong acoustic shadows, evaluation by CT scan is more useful than that by ultrasound. Also, evaluation of regional lymph nodes is important. FNAC for suspicious nodes and thyroglobulin (Tg) measurement of the washout of the needle [10] is very useful to confirm node metastasis. If patients are diagnosed as positive for node metastasis, they should undergo immediate surgery.
In the past, we presented two management options, AS and surgery, almost equally to the patients and asked them to choose between them. This is because there was little evidence of the appropriateness of AS. At present, however, we can recommend AS as the first line of management for low-risk PMC because there is now sufficient evidence.
Patients who decided to undergo AS were followed up by ultrasound 6 months after the diagnosis of PMC and at least once per year thereafter to check for signs of progression, such as enlargement or novel appearance of node metastasis. If suspicious nodes are detected by FNAC, Tg measurement of the washout of needles is useful for diagnosis of metastasis, as described above. This technique is useful especially for the nodes in the lateral compartment.
During AS, surgery is recommended and performed if any signs of progression are detected; otherwise, patients undergo continued AS. Surgery is recommended when tumor size increases by 3 mm or more as compared to the initial size. If patients prefer, however, AS can be continued until tumor size reaches 13 mm. PMCs with novel appearance of N are, of course, strong candidates for immediate surgery.
10.6 Data Accumulation of AS
The first report of AS was published in 2003 from Kuma Hospital, demonstrating that more than 70% of PMC did not change or decreased in size compared to the size at the initiation of AS [11]. The second report, published in 2010, had 340 enrolled patients [12]. In that study, we demonstrated that PMC in patients aged 45 years or younger tended to be more progressive, although no statistical difference could be established. Other clinicopathological features such as gender, family history, and multiplicity did not relate to PMC progression.
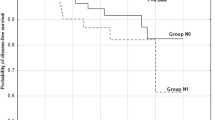
In 2014, we published the third manuscript, which enrolled as many as 1235 patients [8]. Here, we showed that only 8% and 3.8% of patients showed an enlargement of ≥3 mm and novel appearance of node metastasis, respectively, after 10 years of AS for. In that study, we demonstrated the significant relationship between age and PMC progression. Old age was a known important prognostic factor of PTC, and in our previous study for M0 PTC, old age (≥55 years) was the most important predictor of carcinoma death of patients [13]. Later, we also showed that young age (<30 years) and old age (≥60 years) independently affected disease-free survival, but only old age was a predictor of carcinoma death [14]. These suggest that older patients with clinical PTC have a poorer prognosis than younger patients.
In 2013, Miyauchi et al. demonstrated that the percentages of biochemically persistent disease (BPD) of PTC patients who underwent total thyroidectomy were significantly higher among patients <40 years and those ≥60 years compared to patients aged 40–60 years. The incidence of patients with thyroglobulin doubling time (Tg-DT) < 2 years among the BPD patients increased with age [15]. Therefore, we considered that the cutoff ages at 40 and 60 years are appropriate for evaluating the biological characteristics of PTC. Therefore, in a 2014 study about PMC, we divided patient age into three categories: young age (<40 years), middle age (40–59 years), and old age (≥60 years) [8]. We then investigated the relationship between age and PMC progression.
Unlike clinical PTC, the incidence of enlargement and novel appearance of node metastasis was significantly high in young patients and then decreased from young to old patients by the Kaplan-Meier method. Also, in multivariate analysis, young age was an independent predictor of size enlargement and novel appearance of node metastasis, whereas gender, family history, and multiplicity were not. Although clinical PTC in older patients is more likely to progress, low-risk PMCs in older patients are excellent candidates for AS.
The Cancer Institute Hospital published their first study in 2010 showing that only 7% of 300 lesions of low-risk PMCs enlarged and that among 230 patients, only 1% showed a novel appearance of node metastasis during AS [16]. Studies from the two institutions showed that the incidence of PMC progression during AS was low and, most importantly, that none of the patients showed M or died of PMC during AS. Also, studies from both institutions demonstrated that surgery after the detection of progression signs was not too late and that no life-threatening recurrence was detected thereafter. These are important points for adopting an AS policy as a management option for low-risk PMCs. The important findings of active surveillance from two institutions are summarized in Table 10.2.
Regarding the findings that 3.8% of our series showed a novel appearance of node metastasis at 10-year AS [8], one may think that this represents a failure of AS. However, if these patients had been surgically treated at their presentation, they would have undergone hemithyroidectomy with or without paratracheal node dissection. This surgical design would have been unlikely to prevent recurrence of N after surgery, and these patients may have required a second surgery, which is complete total thyroidectomy with therapeutic modified radical neck dissection. We think that one surgery, total thyroidectomy with modified neck dissection, is better than two surgeries, because their outcomes would be similarly excellent [17].
10.7 Other Findings Related to AS of PMC
10.7.1 Relationship Between Ultrasound Findings and PMC Progression
Two previous studies focused on carcinoma growth and ultrasound features. Fukuoka et al. showed that initially rich vascularity had a significantly higher rate of tumor enlargement. However, they also demonstrated that the vascularity of the majority of tumors decreased during AS [18]. Strong calcification and poor vascularity at the initial examination were independently related to nonprogressive disease. Meanwhile, Ito et al. showed that PMC with ill-defined edge on ultrasound examination was likely to show recurrence after initial surgery [19]. However, no life-threatening recurrence was detected after surgery (either just after diagnosis or after AS), indicating that these features do not preclude physicians and patients from choosing AS for PMC.
10.7.2 TSH Suppression for PMC Under AS
Regarding TSH suppression, two institutions demonstrated discrepant findings. Sugitani et al. at the Cancer Institute Hospital showed that TSH value was not related to PMC progression [20]. In contrast, we occasionally set TSH value at low normal by l-thyroxine administration for young patients who underwent AS and showed that, although the number of patients who underwent TSH suppression was small, none of them showed PMC progression [8]. No comparative studies have been done, and therefore, evidence is scarce regarding this point. However, mild TSH suppression could be useful when performing AS for young PMC patients. Most of the patients at the Cancer Institute Hospital were older, because of the character of the hospital, with lower possibility of disease progression, which may at least in part explain the discrepancy of findings between the Cancer Institute Hospital and Kuma Hospital.
10.7.3 Lack of Molecular Markers Predicting PMC Progression
Even now, no markers have been identified that predict PMC progression at the time of cytological examination. Hirokawa et al. reported that the Ki-67 labeling index was higher in PMCs surgically removed after enlargement during AS than in those without enlargement on histopathological examinations; however, this was not evaluated in preoperative FNAC specimens [21]. Recently, Xing et al. reported that BRAF mutation and TERT mutation of clinical PTC showed significant prognostic value [22]. We showed that the combination of these mutations and Ki-67 labeling index discriminated the prognosis more clearly in clinical PTC [23]. However, we were not able to detect TERT mutation in any of surgical specimens of PMCs that showed tumor enlargement or novel N or stable disease, indicating that mutation analyses of these genes on fine needle aspiration specimen of PMCs are unlikely to predict progression of the tumors [24].
10.7.4 Relationship Between PMC Progression and Pregnancy
It is an important clinical question how to manage PMC in young women who might be pregnant. The first study from our institution showed that 49.4% of PMCs progressed during pregnancy [25]. However, later, we found that there was a strong selection bias in enrolled patients in this study. We then published a second report about this issue after rechecking the records of all female patients aged 50 or younger. We found that only 4 patients (8%) of the 50 patients with 51 events of pregnancy and delivery showed enlargement ≥3 mm and none showed novel appearance of node metastasis during pregnancy. Only two of the four patients who showed tumor enlargement during pregnancy underwent surgery after delivery [26]. The remaining two were kept on AS, since their tumors did not grow after the delivery. Taken together, low-risk PMCs in young female patients that might be pregnant can still be candidates for AS. Even though some of PMCs might increase in size during pregnancy, surgery after delivery will not be too late.
10.7.5 Unfavorable Events of Surgery
One may think that surgery of low-risk PMC would be an easy procedure with no significant complications. However, Oda et al. showed that severe unfavorable events, such as permanent vocal cord paralysis and permanent hypoparathyroidism, occurred in 0.2% and 1.6% of patients, respectively, who underwent immediate surgery for low-risk PMC at Kuma Hospital, a center for thyroid disease [27]. All surgeons at Kuma Hospital are thyroid surgery experts, but even so such unfavorable events occurred. Patients receiving treatment by surgeons who do not specialize in thyroid surgery are likely to have a much higher incidence of such unfavorable events.
10.7.6 Medical Costs
Medical costs for AS and immediate surgery vary by country. In Japan, total cost of immediate surgery and postoperative care for 10 years (including prescription of medicine such as L-thyroxine) was US$8437/person, which was 4.1 times higher than the total cost of 10-year management for AS ($2052/person) [28]. Medical cost is an important issue for both society and patients. Costs for examinations, surgeries, and medical prescriptions differ substantially based on country. Insurance coverage also varies greatly according to country. To estimate the costs in one’s location, the flow model and cost table provided here can be used to calculate expenses [28].
10.7.7 Estimation of Lifetime Probability of Disease Progression of PMC on AS
As mentioned previously, at 10 years of AS, 8% and 3.8% of our patients showed tumor enlargement and appearance of nodal metastasis, respectively. With these data, one might argue that AS is a merely procrastinating surgery.
We also showed that the disease progression rates were significantly lower in older patients than in younger patients. After 10 years of surveillance, patients get 10 years older; thus, the risk of progression would decrease over time. We estimated the lifetime probabilities of disease progression with age decade-specific progression rates. The estimates were 48.9% in patients in their 20s at presentation, and 26.7% for those in their 30s; values continued to decrease markedly with age at presentation. These findings indicate that more than half of patients in their 20s and about 75% of those in their 30s will not require surgery in their lifetimes. Older patients are less likely to require surgery [29].
10.8 AS as a First-Line Management for Low-Risk PMC
In the past, we presented patients with low-risk PMC with two options, AS and immediate surgery, and asked them to choose one. This was because, at the initiation of AS, we had no available evidence on its outcomes. At present, however, adequate data have been accumulated, and, thus, we recommend AS as a first-line management. The most important issue is that while surgery was performed after the detection of progression, none of the patients showed significant recurrence or died of thyroid carcinoma.
One important issue is the diagnosis of PMC for suspicious nodules on cytology. Although ATA guidelines recommend not diagnosing suspicious nodules by FNAC, it is, at least in our opinion, mandatory to encourage patients to visit the hospital regularly. Otherwise, patients may quit coming to hospital and if their carcinomas progress, the outcome is likely to be poor. At our institution, we send letters to patients who have not visited the hospital for more than 18 months. AS is a safe procedure only when patients visit the hospital consistently at least once a year.
AS for low-risk PMC is safe and much more beneficial for patients than immediate surgery. AS should be the first-line of management if PMC is diagnosed on cytology at the beginning and patients understand their own disease and unfailingly visit the hospital for checkups.
Short Column: Management of Tumors Diagnosed as Follicular Neoplasm on Cytology
The issue of how best to manage nodules diagnosed as follicular neoplasm on cytology remains a subject of debate. It is very difficult to distinguish between benign follicular adenoma and follicular carcinoma by cytological examination. Usually, the final diagnosis has been based on a pathological examination after surgery. The previous edition of American Thyroid Association (ATA) guidelines published in 2009 [1] recommend that a lobectomy or total thyroidectomy should be considered if the cytology results indicate a follicular neoplasm in a tumor that is not functioning autonomously. In addition, if the cytology reading indicates a Hürthle cell tumor, either a lobectomy or total thyroidectomy is recommended, depending on the lesion’s size and other factors.
In that era, surgical treatment was recommended rather strongly for both diagnosis and treatment (viz., diagnostic surgical excision). In the newest ATA guidelines, molecular testing, namely, a seven-gen panel of mutations including BRAF, RAS, RET/PTC, and PPARgamma, is recommended for nodules diagnosed as FN/SFN in the Bethesda system [2]. If molecular testing is either not performed or inconclusive, surgical excision may be considered for the removal and definitive diagnosis of the nodules. However, molecular testing is expensive and not commonly available in many countries other than the United States.
We previously investigated the diagnosis and surgical indications of Hürthle cell tumors at our institution [3]. Of 998 patients cytologically diagnosed as having Hürthle cell tumors, 426 underwent surgery, and 66 (15%) of those cases were diagnosed as malignancies. In a univariate analysis, serum thyroglobulin (Tg) levels (>500 ng/dL) for anti-Tg antibody-negative patients, tumor size >4 cm, and US class ≥3 based on our diagnostic criteria (Kuma Hospital US classification indicating risk of malignancy [4]) each significantly predicted a malignant histology. On multivariate analysis, US class and tumor size were independent predictors of malignant histology.
Although the ATA guidelines recommend immediate surgery for Hürthle cell carcinoma, the disease-free and cause-specific survival rates of Hürthle cell carcinoma did not differ from non-Hürthle cell carcinoma, at least in Japan [5, 6]. Therefore, in Japan, the diagnosis of Hürthle cell tumor based on cytology alone is not an indication for surgery.
A study of entire follicular tumors in our series is yet to be performed, but of 2115 patients with a solitary nodule that were cytologically diagnosed at our institution as follicular tumor, 1075 underwent surgery. One hundred and eighty-four (8.7%) of these were pathologically diagnosed as malignancy. Indeed, the surgical indications of tumors diagnosed as follicular neoplasm on cytology vary to some extent among the attending physicians at our institution. However, tumor size is one of the important factors. The incidence of carcinoma increases with tumor size, and if it is follicular carcinoma, large size (>4 cm) predicted a poor prognosis in our study [5].
Age is also an important factor of follicular carcinoma. Old age is a predictor of carcinoma death in follicular carcinoma cases [5]. However, we have no data regarding whether the incidence of carcinoma in follicular neoplasms increases with age. Since it is rare that follicular carcinoma is diagnosed on cytology, it is doubtful whether, without other features such as large size, follicular neoplasms in elderly patients are strong candidates for surgical treatment.
Performing surgery, including diagnostic surgical excision, for all nodules diagnosed with follicular neoplasm on cytology is definitely an overtreatment. In practice, the strongest indicator of surgery for follicular neoplasms is tumor size. At our institution, we normally consider surgery for follicular neoplasms larger than 3 cm. We usually recommend surgery for nodules larger than 4 cm even though it is cytologically suspected as—not diagnosed as—follicular neoplasms. Ultrasound findings are helpful for deciding surgical treatment. Further studies of large numbers of patients are necessary, but at this stage, surgery for follicular neoplasm should be considered based on tumor size and ultrasound features (US class ≥3).
References
-
1.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19: 1167-1214.
-
2.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SH et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26:1-133.
-
3.
Ito Y, Hirokawa M, Miyauchi A, Kihara M, Yabuta T, Masuoka H, Fukushima M, Higashiyama T, Kobayashi K, Miya A. Diagnosis and surgical indications of oxyphilic follicular tumors in Japan: Surgical specimens and cytology. Endocr J 2016; 63: 977-982.
-
4.
Yokozawa T, Fukata S, Kuma K, Matsuzuka F, Hirai K, Miyauchi A, Sugawara M. Thyroid cancer detected by ultrasound-guided fine-needle aspiration biopsy. World J Surg 1996; 20: 848-853.
-
5.
Ito Y, Hirokawa M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prognosis and prognostic factors of follicular carcinoma in Japan: Importance of postoperative pathological examination. World J Surg 2007; 31: 1417-1424.
-
6.
Sugino K, Kameyama K, Ito K, Nahagama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Akaishi J, Suzuki A, Masaki C, Ito K. Does Hürthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma. Ann Surg Oncol 2013; 20: 2944-2950.
References
Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007;3:240–8.
Takebe K, Date M, Yamamoto Y. Mass screening for thyroid cancer with ultrasonography [in Japanese]. KARKINOS. 1994;7:309–17.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the Unites States, 1973–2002. JAMA. 2006;595:2164–7.
Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–22.
Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic” — screening and overdiagnosis. N Engl J Med. 2014;371:1765–7.
Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25:1127–36.
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg. 2004;28:1115–21.
Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;26:1–133.
Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, Miya A, Kobayashi K, Matuzuka F, Amino N, Kuma K. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005;29:483–5.
Ito Y, Uruno R, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Kuma S, Kuma K, Miyauchi A. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–8.
Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. An observation trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35.
Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–8.
Ito Y, Miyauchi A, Tomoda C, Hirokawa M, Kobayashi K, Miya A. Prognostic significance of young age in papillary thyroid carcinoma: analysis of 5,733 patients with 150 months’ follow-up. Endocr J. 2014;61:491–7.
Miyauchi A, Kudo T, Kihara M, Higashiyama T, Ito Y, Kobayashi K, Miya A. Relationship of biochemically persistent disease and thyroglobulin-doubling time to age at surgery in patients with papillary thyroid carcinoma. Endocr J. 2013;60:415–21.
Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–31.
Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40:516–22.
Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural history of asymptomatic papillary thyroid microcarcinoma: time-dependent changes in calcification and vascularity during active surveillance. World J Surg. 2016;40:529–37.
Ito Y, Kobayashi K, Tomoda C, Uruno T, Takamura Y, Miya A, Matsuzuka F, Kuma K, Miyauchi A. Ill-defined edge on ultrasonographic examination can be a marker of aggressive characteristic of papillary thyroid microcarcinoma. World J Surg. 2005;29:1007–11.
Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014;38:673–378.
Hirokawa M, Kudo T, Ota H, Suzuki A, Miyauchi A. Pathological characteristics of low-risk papillary thyroid microcarcinoma with progression during active surveillance. Endocr J. 2016;63:805–10.
Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–25.
Matsuse M, Yabuta T, Saenko V, Hirokawa M, Nishihara E, Suzuki K, Yamashita S, Miyauchi A, Mitsutake N. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci Rep. 2017;7:417–52.
Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid. 2017;27:1206–7.
Shindo H, Amino N, Ito Y, Kihara M, Kobayashi K, Miya A, Hirokawa M, Miyauchi A. Papillary thyroid microcarcinoma might progress during pregnancy. Thyroid. 2014;24:840–4.
Ito Y, Miyauchi A, Kudo T, Ota H, Yoshioka K, Oda H, Sasai H, Nakayma A, Yabuta T, Masuoka H, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Effects of pregnancy on papillary microcarcinoma of the thyroid re-evaluated in the entire patient series at Kuma Hospital. Thyroid. 2016;26:156–60.
Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, Masuoka H, Yabuta T, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Incidence of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–5.
Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64:59–64.
Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, Fukushima M, Masuoka H, Kihara M, Miya A. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. 2018;163:48–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ito, Y., Miyauchi, A., Oda, H. (2019). Management of Papillary Microcarcinoma of the Thyroid with A Short Column (Management of Tumors Diagnosed as Follicular Neoplasm on Cytology). In: Kakudo, K. (eds) Thyroid FNA Cytology. Springer, Singapore. https://doi.org/10.1007/978-981-13-1897-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-1897-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1896-2
Online ISBN: 978-981-13-1897-9
eBook Packages: MedicineMedicine (R0)