Abstract
Primary sensory neurons detect potentially dangerous environmental situations via many “sensor” proteins located on the plasma membrane. Although receptor-type cation channels are thought to be the major sensors in sensory neurons, anion channels are also important players in the peripheral nervous system. Recently, we showed that transient receptor potential vanilloid 1 (TRPV1) interacts with anoctamin 1 (ANO1, also called TMEM16A) in primary sensory neurons and that this interaction enhanced TRPV1-mediated pain sensation. In that study, we induced ANO1 currents by application of capsaicin to small DRG neurons and showed that ANO1-dependent depolarization following TRPV1 activation could evoke more action potentials. Furthermore, capsaicin-evoked pain-related behaviors in mice were strongly inhibited by a selective ANO1 blocker. Together these findings indicate that selective ANO1 inhibition can reduce pain sensation. We also investigated non-specific inhibitory effects on ion channel activities to control ion dynamics via the TRPV1-ANO1 complex. We found that 4-isopropylcyclohexanol (4-iPr-CyH-OH) had an analgesic effect on burning pain sensations through its inhibition of TRPV1 and ANO1 together. Additionally, 4-iPr-CyH-OH did not have clear agonistic effects on TRPV1, TRPA1, and ANO1 activity individually. These results indicate that 4-iPr-CyH-OH could function globally to mediate TRP-ANO1 complex functions to reduce skin hypersensitivity and could form the basis for novel analgesic agents.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Transient receptor potential (TRP) channels are involved in a diverse range of physiological functions, including pain sensation. TRP channels expressed in primary sensory neurons can be activated by several different physical and chemical stimuli, including temperature changes and irritants [34]. However, the output phenotypes produced by these stimuli are not solely dependent on TRP channel activities. As is well known, almost all TRP channels have high calcium permeability [9]. This calcium influx could affect other calcium-dependent proteins located within a micrometer range of the channel pore [22]. Anoctamin (ANO) is one of the calcium-dependent proteins [1, 26, 37]. We recently showed that the calcium-activated chloride channel ANO1 (also known as TMEM16A) can be strongly activated by calcium influx through TRPV1 activation and that TRPV1-ANO1 interaction is involved in pain enhancement [30]. This chapter reviews the recent findings concerning TRP interactions in sensory systems and potential strategies for pharmacological control of the ion dynamics.
3.2 TRPV1: ANO1 Interaction
Both TRPV1 and ANO1 are expressed in primary sensory neurons and are involved in acute pain sensation [30]. TRPV1 is activated by various natural ligands, including capsaicin, resiniferatoxin, bivalent tarantula toxin, acid, and noxious heat [13]. Rat TRPV1 is phosphorylated at Ser502 and Ser800 by protein kinase C epsilon (PKCε) activated in response to signaling by G protein-coupled receptors (GPCR), including the bradykinin receptor and P2Y receptor [32, 38]. This PKCε phosphorylation is mediated by A-kinase anchoring proteins [38]. Because phosphorylation reduces the threshold for TRPV1 activation, phosphorylated TRPV1 can be activated at temperatures lower than core body temperature [32]. This characteristic is thought to be involved in molecular mechanisms that cause inflammatory pain. Therefore, TRPV1 is a primary target for pain therapy. However, the chloride channel ANO1 is also thought to play a major role in generating pain signals in primary sensory neurons due to its heat sensitivity and immediate activation following GPCR activation [3, 18]. ANO1 directly interacts with the IP3 receptor on the endoplasmic reticulum (ER) membrane [11]. Interestingly, TRPV1 and ANO1 are also co-expressed in small dorsal root ganglia (DRG) neurons [2]. We previously demonstrated an interaction between TRPV4 and ANO1 in choroid plexus epithelial cells [29]. Similar to TRPV1, TRPV4 has high calcium permeability (Na+:Ca2+ = 1:10). Therefore, calcium entering the cell rapidly induces ANO1 activation followed by secretion of fluids such as cerebrospinal fluid, saliva, and tears [6, 29]. We thus investigated whether TRPV1-ANO1 interaction occurs in DRG neurons and the physiological relevance of this interaction.
Typically, we began by conducting an electrophysiological analysis using whole-cell patch-clamp recording in HEK293T cells expressing TRP channels and ANO1. The main composition of the bath and pipette solutions in these assays is N-methyl-D-glutamine chloride (NMDG-Cl), and the free calcium in the pipette solution was maintained at 100 nM using 5 mM O,O′-Bis (2-aminophenyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid (BAPTA). To study TRPV1-ANO1 interactions, we activated TRPV1 by applying 300 nM capsaicin, which is approximately the half effective concentration, although in DRG neurons the concentration is 1 μM [15]. Under these conditions, large chloride currents that could induce cell shrinkage at −60 mV holding potential were observed in cells expressing TRPV1 and ANO1, but not cells expressing TRPV1 or ANO1 alone. Moreover, these currents were abolished in a calcium-free bath solution and a reversal potential shift occurred in NMDG-aspartate bath solution. These results clearly suggest that calcium influx through TRPV1 activation strongly induces ANO1 activation. Furthermore, immunoprecipitation results indicated that TRPV1 and ANO1 directly interact. Thus, TRPV1 directly and functionally interacts with ANO1 although ANO1 alone could be activated by global calcium increases depending on ER calcium stores and voltage-gated calcium channels on plasma membrane [12].
3.3 Pain-Enhancing Mechanisms in DRG Neurons
The physiological activity of ANO1 is dependent on concentration differences in extracellular and intracellular chloride. Interestingly, in many DRG neurons, the intracellular chloride concentration is reportedly higher than in other neurons, such as those in the central nervous system [20]. The equivalent potential in DRG neurons containing high chloride can reach −20 mV, and the resting potential is approximately −60 mV. Therefore, ANO1 activation should induce depolarization due to chloride efflux and neuronal excitations. To examine this possibility, we performed the same experiments as those for HEK293T cells using isolated small DRG neurons. In whole-cell patch-clamp recordings, capsaicin-induced currents decreased by half following application of the selective ANO1 inhibitor T16Ainh-A01 with a physiological ion concentration in the bath solution (NaCl base solution containing 2 mM CaCl2). The capsaicin-induced current is composed of cations and chloride movements, even though capsaicin-mediated neuronal excitation in DRG neurons was thought to depend only on TRPV1 function. Moreover, action potentials evoked by capsaicin applications were almost completely inhibited by T16Ainh-A01. Together, these results indicate that a TRPV1 and ANO1 interaction should also occur in DRG neurons in the presence of high intracellular chloride concentrations.
However, the efficacy of this interaction remained unclear because some DRG neurons have low concentrations of intracellular chloride. In these neurons, ANO1 could induce hyperpolarization with TRPV1 activation. To clarify whether ANO1 activation following TRPV1 activation is involved in pain generation but not pain reduction, we analyzed the effect of T16Ainh-A01 on capsaicin-induced pain-related behaviors in mice. We found that pain-related behaviors were significantly ameliorated by concomitant administration of T16Ainh-A01. Thus, the TRPV1 and ANO1 interaction appears to be involved in pain enhancement, and TRPV1 and ANO1 behave as irritant detector and signal amplifier, respectively, although ANO1 could act as a suppressor in some DRG neurons (Fig. 3.1).
Schematic model of interactions between TRPV1 and ANO1. TRPV1 interacts with ANO1 on both free nerve endings and synapses of DRG neurons. TRPV1 is initially activated and ANO1 is also immediately activated in calcium nano-domains. The ANO1 activation enhances action potential generation (ΔΨ). TRPV1 also interacts with ANO1 on the central side, and the depolarization activates voltage-gated calcium channels. These two pathways are involved in neurotransmitter release from presynaptic regions in secondary neurons of the spinal cord
3.4 Analgesic Agents to Target TRPV1-ANO1 Interactions
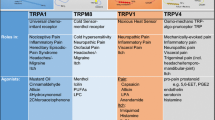
The specificity of channel antagonists might not always be an important property in pain reduction because selective drugs often have strong side effects that discourage their use in vivo. Moreover, complete reduction of pain is not always desirable in clinical applications because pain pathways can have a protective effect in certain situations, such as avoiding bone destruction in Candida infection [21]. An alternative strategy would be to identify an agent that can inhibit several ion channels involved in pain sensation in peripheral regions. For instance, TRPV4 is also thought to be involved in pain sensation, and the weak-specific antagonist, compound 16-8, is more effective at reducing pain than the TRPV4-specific antagonist GSK205 [14]. While investigating the interaction between TRPM8 and ANO1, we fortuitously found that menthol inhibits ANO1 [31]. Although in that study we were unable to characterize the physiological role of the TRPM8-ANO1 interaction, the menthol-related findings were nonetheless interesting because menthol can also inhibit the TRPV1 activation [28]. However, the ability of menthol to inhibit both ANO1 and TRPV1 is puzzling given the differences in the structures of these channels. TRPV1 and ANO1 have six and ten transmembrane regions, respectively, and TRPV1 is a tetramer, whereas ANO1 is a dimer [5, 17, 23]. We first assessed the effects of other menthol analogues, including menthone, 1,4-cineole, and 1,8-cineole, on ANO1 currents. In whole-cell patch-clamp recordings of HEK293T cells expressing ANO1, only 1,8-cineole lacked a strong inhibitory effect on the ANO1 current induced by high free calcium concentration. Because the chemical structure of 1,8-cinaole is the most divergent among the three analogues tested, we surmised that potential menthol-based agents should contain a critical minimum structure. Therefore, we next investigated the separate moieties comprising menthol. From these studies we showed that isopropylcyclohexane is the core structure needed to completely inhibit ANO1 currents. Since the kinetics of current reduction by isopropylcyclohexane were slower than that for menthol, we focused on 4-isopropylcyclohexanol (4-iPr-CyH-OH), which has greater hydrophilicity, which could be valuable if the affinity site lies in the intracellular domain of the ion channel. According to our expectations, 4-iPr-CyH-OH showed rapid inhibition that was similar to that of menthol. Interestingly, 4-iPr-CyH-OH also inhibits TRPV1, TRPA1, TRPV4, and TRPM8 activity. Thus, 4-iPr-CyH-OH could have inhibitory effects on many different irritation pathways. The half inhibition concentration (IC50) of 4-iPr-CyH-OH for mouse TRPA1, TRPV1, and ANO1 was 0.23, 0.73, and 1.09 mM, respectively (Fig. 3.2). IC50 of 4-iPr-CyH-OH in TRPV1 current induced by 100 nM capsaicin was lower than that of ANO1 current. However, the capsaicin at the concentration does not fully activate TRPV1, whereas 500 nM intracellular free calcium strongly activates ANO1 in our experiments. Three hundred micrometer allyl isothiocyanate (AITC) also induces the almost saturated TRPA1 activation. Thus, 4-iPr-CyH-OH could have a lower inhibitory effect toward TRPV1. Furthermore, we investigated the effects of 4-iPr-CyH-OH on capsaicin-evoked action potential in isolated small DRG neurons and capsaicin-induced pain-related behaviors in mice. In these experiments, 4-iPr-CyH-OH completely inhibited capsaicin-evoked action potentials with strong suppression of depolarization, and pain-related behaviors were significantly diminished with concomitant administration of 4-iPr-CyH-OH.
Although 4-iPr-CyH-OH is currently used as a food additive in Japan, the pharmacological understanding of its effects beyond those we found for pain sensation is limited [10, 19]. Thus, 4-iPr-CyH-OH could have potential as a basis for the development of novel drugs that target ion channels, particularly ANO1 and TRP channels.
3.5 Conclusion
TRP-ANO1 interactions are involved in several physiological mechanisms. For instance, TRPC2-ANO1 interaction could be involved in iodide homeostasis in thyroid cells and vomeronasal transduction [7, 33], and TRPC6-ANO1 interaction reportedly enhances vasoconstriction [35]. In addition, our findings indicated that ANO1 activation could generate sufficient depolarization to induce exocytosis in synapses between primary sensory neurons and secondary neurons in the spinal cord (Fig. 3.1). In fact, ANO1-dependent membrane potential changes could accelerate insulin secretion from pancreatic β-cells [4, 36]. Not only ANO1, targeting TRP-ANO interactions could be also a promising approach because ANOs are expressed in the whole body [8, 16, 25, 27], and ANOs have three functions, including chloride channel, scramblase, and internalization [24, 27]. Thus, additional physiological phenomena could be better explained by future investigations that focus on TRP-ANO interactions.
References
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, Raouf R, Wood JN, Oh U (2012) The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 15:1015–1021
Cho H, Oh U (2013) Anoctamin 1 mediates thermal pain as a heat sensor. Curr Neuropharmacol 11:641–651
Crutzen R, Virreira M, Markadieu N, Shlyonsky V, Sener A, Malaisse WJ, Beauwens R, Boom A, Golstein PE (2016) Anoctamin 1 (Ano1) is required for glucose-induced membrane potential oscillations and insulin secretion by murine beta-cells. Pflugers Arch 468:573–591
Dang S, Feng S, Tien J, Peters CJ, Bulkley D, Lolicato M, Zhao J, Zuberbuhler K, Ye W, Qi L, Chen T, Craik CS, Nung Jan Y, Minor DL Jr, Cheng Y, Yeh Jan L (2017) Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552:426–429
Derouiche S, Takayama Y, Murakami M, Tominaga M (2018) TRPV4 heats up ANO1-dependent exocrine gland fluid secretion. FASEB J 32:1841–1854 fj201700954R
Dibattista M, Amjad A, Maurya DK, Sagheddu C, Montani G, Tirindelli R, Menini A (2012) Calcium-activated chloride channels in the apical region of mouse vomeronasal sensory neurons. J Gen Physiol 140:3–15
Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ (2009) Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284:33360–33368
Gees M, Colsoul B, Nilius B (2010) The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2:a003962
Henningsen GM, Salomon RA, Yu KO, Lopez I, Roberts J, Serve MP (1988) Metabolism of nephrotoxic isopropylcyclohexane in male Fischer 344 rats. J Toxicol Environ Health 24:19–25
Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, Zhang H, Gamper N (2013) Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6:ra73
Jin X, Shah S, Du X, Zhang H, Gamper N (2016) Activation of Ca(2+) -activated Cl(−) channel ANO1 by localized Ca(2+) signals. J Physiol 594:19–30
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384
Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, Shahid R, Fan P, Gooden DM, Simon SA, Spasojevic I, Mook RA, Liddle RA, Guilak F, Liedtke WB (2016) Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep 6:26894
Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, Park MY, Kim E, Kim M, Kim BM, Cho H, Shin Y, Oh U (2006) TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci 26:2403–2412
Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R (2009) Bestrophin and TMEM16-Ca(2+) activated Cl(−) channels with different functions. Cell Calcium 46:233–241
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N (2010) The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+−activated Cl- channels. J Clin Invest 120:1240–1252
Magori N, Fujita T, Kumamoto E (2018) Hinokitiol inhibits compound action potentials in the frog sciatic nerve. Eur J Pharmacol 819:254–260
Mao S, Garzon-Muvdi T, Di Fulvio M, Chen Y, Delpire E, Alvarez FJ, Alvarez-Leefmans FJ (2012) Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J Neurophysiol 108:834–852
Maruyama K, Takayama Y, Kondo T, Ishibashi KI, Sahoo BR, Kanemaru H, Kumagai Y, Martino MM, Tanaka H, Ohno N, Iwakura Y, Takemura N, Tominaga M, Akira S (2017) Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Rep 19:2730–2742
Mulier M, Vriens J, Voets T (2017) TRP channel pores and local calcium signals. Cell Calcium 66:19–24
Paulino C, Kalienkova V, Lam AKM, Neldner Y, Dutzler R (2017) Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552:421–425
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94:419–459
Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K (2010) Expression and function of epithelial anoctamins. J Biol Chem 285:7838–7845
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134:1019–1029
Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem 288:13305–13316
Takaishi M, Uchida K, Suzuki Y, Matsui H, Shimada T, Fujita F, Tominaga M (2016) Reciprocal effects of capsaicin and menthol on thermosensation through regulated activities of TRPV1 and TRPM8. J Physiol Sci 66:143–155
Takayama Y, Shibasaki K, Suzuki Y, Yamanaka A, Tominaga M (2014) Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J 28:2238–2248
Takayama Y, Uta D, Furue H, Tominaga M (2015) Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci U S A 112:5213–5218
Takayama Y, Furue H, Tominaga M (2017) 4-isopropylcyclohexanol has potential analgesic effects through the inhibition of anoctamin 1, TRPV1 and TRPA1 channel activities. Sci Rep 7:43132
Tominaga M, Wada M, Masu M (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A 98:6951–6956
Viitanen TM, Sukumaran P, Lof C, Tornquist K (2013) Functional coupling of TRPC2 cation channels and the calcium-activated anion channels in rat thyroid cells: implications for iodide homeostasis. J Cell Physiol 228:814–823
Vriens J, Nilius B, Voets T (2014) Peripheral thermosensation in mammals. Nat Rev Neurosci 15:573–589
Wang Q, Leo MD, Narayanan D, Kuruvilla KP, Jaggar JH (2016) Local coupling of TRPC6 to ANO1/TMEM16A channels in smooth muscle cells amplifies vasoconstriction in cerebral arteries. Am J Phys Cell Phys 310:C1001–C1009
Xu Z, Lefevre GM, Gavrilova O, Foster St Claire MB, Riddick G, Felsenfeld G (2014) Mapping of long-range INS promoter interactions reveals a role for calcium-activated chloride channel ANO1 in insulin secretion. Proc Natl Acad Sci U S A 111:16760–16765
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Zhang X, Li L, McNaughton PA (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461
Acknowledgments
Our study is supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan, the Kato Memorial Bioscience Foundation, and the Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Takayama, Y., Tominaga, M. (2018). Involvement of TRPV1-ANO1 Interactions in Pain-Enhancing Mechanisms. In: Shyu, BC., Tominaga, M. (eds) Advances in Pain Research: Mechanisms and Modulation of Chronic Pain. Advances in Experimental Medicine and Biology, vol 1099. Springer, Singapore. https://doi.org/10.1007/978-981-13-1756-9_3
Download citation
DOI: https://doi.org/10.1007/978-981-13-1756-9_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1755-2
Online ISBN: 978-981-13-1756-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)