Abstract
The transient receptor potential ankyrin subtype 1 protein (TRPA1) is a nonselective cation channel permeable to Ca2+, Na+, and K+. TRPA1 is a promiscuous chemical nocisensor that is also involved in noxious cold and mechanical sensation. It is present in a subpopulation of Aδ- and C-fiber nociceptive sensory neurons as well as in other sensory cells including epithelial cells. In primary sensory neurons, Ca2+ and Na+ flowing through TRPA1 into the cell cause membrane depolarization, action potential discharge, and neurotransmitter release both at peripheral and central neural projections. In addition to being activated by cysteine and lysine reactive electrophiles and oxidants, TRPA1 is indirectly activated by pro-inflammatory agents via the phospholipase C signaling pathway, in which cytosolic Ca2+ is an important regulator of channel gating. The finding that non-electrophilic compounds, including menthol and cannabinoids, activate TRPA1 may provide templates for the design of non-tissue damaging activators to fine-tune the activity of TRPA1 and raises the possibility that endogenous ligands sharing binding sites with such non-electrophiles exist and regulate TRPA1 channel activity. TRPA1 is promising as a drug target for novel treatments of pain, itch, and sensory hyperreactivity in visceral organs including the airways, bladder, and gastrointestinal tract.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nociceptor
- Sensory transduction
- Pain
- Hydrogen sulfide
- Hyperalgesia
- Chemosensitivity
- Mechanotransduction
- Thermosensitive
- Irritants
- Allyl isothiocyanate
- Menthol
- Cannabinoid
- Mustard oil
- TRPA1
- TRPV1
1 Gene
In 1999, Jaquemar and coworkers described an mRNA transcript encoding a TRP-like protein (~1,100 amino acids) with 18 putative N-terminal ankyrin repeats in human fibroblasts (Jaquemar et al. 1999). The corresponding human gene, denoted trpa1, contains 27 exons and is located on chromosome 8 (8q13) (Nilius and Owsianik 2011; Story et al. 2003). Several TRPA1 homologues exist in the animal kingdom, and the ability of TRPA1 to sense potentially harmful electrophilic compounds has been conserved for ~500 millions of years, whereas the thermosensitive properties of TRPA1 have diverged later (Kang et al. 2010; Panzano et al. 2010). The sequence homology of TRPA1 in mammals is only 79 % between primates and rodents, which is important to consider when screening for TRPA1 active drugs for treatment of human diseases (Bianchi et al. 2012; Chen et al. 2013; Chen and Kym 2009).
2 Expression
In sensory neurons from trigeminal, dorsal root, and nodose ganglia, TRPA1 is expressed in both peptidergic and non-peptidergic neurons classified as Aδ- and C-fiber primary afferents (Figs. 1 and 2) (Andrade et al. 2012; Hjerling-Leffler et al. 2007; Kim et al. 2010). TRPA1 is mostly found in a subpopulation of TRPV1-positive neurons, but non-TRPV1-containing neurons expressing TRPA1 exist, including a small population of myelinated Aβ-fibers, which are activated by innocuous mechanical force (Fig. 2) (Hjerling-Leffler et al. 2007; Kim et al. 2010; La et al. 2011). TRPA1 as well as TRPV1 is also present along the axon of primary afferents, the physiological significance of which is less obvious (Brenneis et al. 2011; Weller et al. 2011). Outside sensory neurons, TRPA1 is found in epithelial cells, melanocytes, mast cells, fibroblasts, odontoblasts, and enterochromaffin cells and β-cells of the Langerhans islets (Fig. 1) (Andrade et al. 2012; Baraldi et al. 2010; Bellono et al. 2013; Bellono and Oancea 2013; Buch et al. 2013; Cao et al. 2012; Earley 2012; Nilius et al. 2012; Oh et al. 2013; Prasad et al. 2008). Many of these cells have sensory properties and communicate with nearby nociceptors (Kwan et al. 2009; Lumpkin and Caterina 2007). TRPA1 may even exist in the central nervous system (Vennekens et al. 2012). Importantly, the TRPA1 developmental expression pattern in mouse sensory neurons is highly dynamic before adult age (Fig. 2) and can change dramatically in pathological conditions including inflammatory and neuropathic diseases (Dai et al. 2007; Diogenes et al. 2007; Hjerling-Leffler et al. 2007; Ji et al. 2008; Katsura et al. 2006; Malin et al. 2011; Obata et al. 2005; Oh et al. 2013; Schwartz et al. 2011; Wang et al. 2008a).
The human body receives information about the external and internal environment through the somatic senses (thermo-, mechano-, and chemosensation), consisting of different types of primary sensory neurons with myelinated (Aβ), thinly myelinated (Aδ), and unmyelinated (C) nerve fibers. TRPA1 is present on Aδ- and C-fibers and on epithelial cells in the airways, gastrointestinal tract, bladder, and skin. Mucosal enterochromaffin cells (ECC), epidermal melanocytes (MeC) and mast cells (MC) also express TRPA1, which may play an important role in the regulation of gastrointestinal motility, UV radiation (UVR)-induced skin pigmentation and the innate immune system, respectively. In addition to pain signaling, primary sensory neurons participate in visceral reflexes, involving the release of acetylcholine (ACh) and noradrenaline (NA), as well as in local responses to tissue injury. Activation of TRPA1 on primary sensory neurons results in both afferent and efferent signaling. An influx of Na+ and Ca2+ through TRPA1 triggers an action potential and a local release of sensory neuropeptides such as calcitonin gene-related peptide (CGRP), substance P (SP), and neurokinin A (NKA). In blood vessels these neuropeptides cause vasodilation and vascular leakage, signs of inflammation, and TRPA1 activation of the trigeminovascular system, including neurogenic CGRP-mediated vasodilation of cerebral arteries, may contribute to migraine and cluster headache (Benemei et al. 2013; Messlinger et al. 2012; Nassini et al. 2012). In airways, activation of sensory neurons can cause bronchospasm, cough, sneezing, congestion, rhinorrhea and itch (Alenmyr et al. 2009, 2011; Bautista et al. 2013; Taylor-Clark and Undem 2011). In the bladder and gastrointestinal tract, changed properties of sensory signaling are also believed to play an important role in diseases associated with sensory hyperreactivity (Andersson et al. 2010; Bautista et al. 2013; Birder 2013; Holzer 2011)
In adult mouse sensory neurons from dorsal root ganglia, TRPA1 is expressed alone and together with TRPV1 both in peptidergic (IB4−) and non-peptidergic (IB4+) neurons. From a methodological point of view, it is important to consider that before adult age, the TRP expression pattern in sensory neurons is dynamic. From Hjerling-Leffler et al. (2007)
3 The Channel Protein Including Structural Aspects
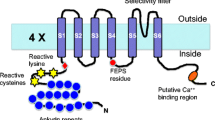
The mammalian TRPA1 belongs to the transient receptor potential (TRP) channel superfamily that consists of 28 different ion channels divided into six subgroups, of which TRPA1 constitutes its own subgroup (Nilius et al. 2012; Nilius and Owsianik 2011; Venkatachalam and Montell 2007). TRPA1 is a nonselective cation channel with six putative transmembrane segments (S1–S6), intracellular N- and C-termini, and a pore loop between S5 and S6 (Fig. 3). The N-terminus (half the size of the protein) contains between 14 and 18 ankyrin repeats that probably are important for protein–protein interactions and insertion of the channel into the plasma membrane (Gaudet 2008; Nilius et al. 2011). Because of the unusually large N-terminal ankyrin repeat domain, it is also possible that TRPA1 is involved in mechanosensation, in which the N-terminal could act as a link between mechanical stimuli and channel gating (Gaudet 2008; Howard and Bechstedt 2004). The N-terminal region contains a large number of cysteines, some of which can form a complex network of protein disulfide bridges within and between monomers (Fig. 4) (Cvetkov et al. 2011; Eberhardt et al. 2012; Gaudet 2008; Wang et al. 2012). N-terminal cysteine and lysine residues are key targets for electrophilic TRPA1 activators, but cysteines outside the N-terminal region may also contribute to channel gating as such cysteines also bind electrophiles (Fig. 4) (Macpherson et al. 2007b; Takahashi et al. 2011; Wang 2012). Furthermore, the potent TRPA1 activator Zn2+ may bind to cysteine and histidine residues in the C-terminus (Andersson et al. 2009; Hu et al. 2009). The N- and C-termini have been suggested to contain binding sites for Ca2+ that can both sensitize and desensitize TRPA1 (Doerner et al. 2007; Jordt et al. 2004; Sura et al. 2012; Wang et al. 2008b; Zurborg et al. 2007).
The functional ion channel TRPA1 is a putative homotetrameric protein complex, where each monomer contains six transmembrane segments (S1–S6) with cytoplasmic N- and C-termini. Between S5 and S6 is the pore region, allowing Ca2+ and Na+ to enter the cell and K+ to flow in the outward direction at physiological membrane potentials. The pore loop Asp918 (D) is supposed to be critical for Ca2+ selectivity. The N-terminus with its many ankyrin repeats (blue ovals) contains cysteine (C) and lysine (K) residues that are targets for electrophiles and oxidants. Cysteines outside the N-terminus, such as Cys856 (C, S4), as well as other amino acid residues may also contribute to the complex regulation of TRPA1 gating. Hydroxylation of Pro394 (P) in the N-terminus reduces human TRPA1 activity at normoxia. A gain-of-function mutation at Asn855 (N) in S4 of human TRPA1 causes familial episodic pain syndrome. Menthol is a non-electrophilic compound that activates TRPA1 possibly by binding to the amino acid residues Ser873/Ser 877 and Thr874/Thr 878 (S and T) in S5 of the human/mouse TRPA1. Phosphatidylinositol-4,5-bisphosphate (PIP2), which is cleaved by phospholipase C into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), may regulate TRPA1 activity by interacting with charged residues in the C-terminus, as suggested for TRPV1. Cysteine (C) and histidine (H) residues on the N- and C-termini of mouse TRPA1 are putative binding sites for Zn2+, which potently activates TRPA1. The N- and C-termini contain putative binding sites for Ca2+ (D and E), which can sensitize or desensitize human TRPA1. Human and mouse amino acid residues are shown as yellow and red, respectively, and both colors for common residues
As shown for the mouse TRPA1, N-terminal cysteines can form a dynamic network of disulfide bonds (orange lines), having a substantial impact on the global protein configuration and function. Blue circles show cysteine residues, and those identified as critical for activation by electrophilic compounds are highlighted in yellow. Notably, the mutation of C622 will affect several disulfide bonds and most likely have drastic effects on the overall protein function also affecting pharmacological interventions not targeting cysteine residues. Modified from Wang et al. (2012)
4 Interacting Proteins
Noxious heat sensitization evoked by bradykinin, which like other endogenous proalgesic or pro-inflammatory agents stimulates various Gq protein-coupled phospholipase C signaling pathways, was shown to be dependent on both TRPA1 and TRPV1, demonstrating a mutual relationship between these two ion channels (Bautista et al. 2006). It was suggested that a release of intracellular Ca2+ and an influx of Ca2+ via TRPV1 could lead to activation of TRPA1 (Fig. 5). Acute noxious heat responses with an activation threshold around 42 °C were initially expected to be entirely mediated by TRPV1, but studies of TRPV1 knockout mice indicated a contribution of other mechanisms within the temperature range of TRPV1 activation (Caterina et al. 1997, 2000; Davis et al. 2000). Indeed, a recent study provided evidence that TRPA1 contributes to the control of this heat threshold for acute nociceptive thermosensation (Hoffmann et al. 2013). Thus, because of its Ca2+ sensitivity, TRPA1 may act as an amplifier to TRPV1 under both normal and pathophysiological conditions (Bautista et al. 2006; Hoffmann et al. 2013). The opposite may also occur as TRPA1 can sensitize TRPV1 in a Ca2+ and protein kinase A-dependent manner (Anand et al. 2008; Spahn et al. 2013). In addition to Ca2+, other potential mediators or mechanisms of TRPA1 activation recruited by the phospholipase C signaling pathways include direct channel phosphorylation and the recruitment of TRPA1 to the cytoplasmic membrane by protein kinase A, possibly by interacting with the A-kinase anchor protein 5 (AKAP-5) and other proteins (e.g., CYLD) controlling cytoplasmic levels, trafficking, and turnover of TRPA1 (Fig. 5) (Dai et al. 2007; Schmidt et al. 2009; Stokes et al. 2006; Takahashi et al. 2012; Wang et al. 2008a; Zhang et al. 2008a). Notably, activation of TRPA1 or TRPV1 increased cytoplasmic surface expression of TRPA1, but not TRPV1 (Schmidt et al. 2009). The formation of functional TRPA1 and TRPV1 heterotetramers is another interesting interaction between these proteins (Fig. 5) (Akopian et al. 2007; Salas et al. 2009; Staruschenko et al. 2010).
TRPA1 is part of a complex protein signaling network. (a) Pro-inflammatory and pain-producing agents, such as bradykinin, histamine, prostaglandins (PG), and trypsin, acting on G protein-coupled receptors, stimulate via phospholipase C (PLC) or adenylate cyclase (AC) the production of intracellular mediators that directly or indirectly activate TRPV1 and TRPA1. TRPV1 is activated by protein kinase C (PKC), protein kinase A (PKA), and monoacylglycerols, such as 2-arachidonoyl glycerol (2-AG). An increased intracellular Ca2+ concentration via release from intracellular Ca2+ stores by the action of 1,4,5-triphosphate (IP3) and influx through TRPV1 can stimulate the Ca2+-sensitive TRPA1, which is also desensitized by Ca2+. PLC stimulation also generates H+ that can activate TRPA1. PKA may phosphorylate TRPA1 and increase its cell surface expression. The hydroxylation of Pro394 in the N-terminus by the oxygen-dependent prolyl hydroxylase (PHD) suppresses TRPA1 activity at normoxia. Importantly, this break is removed by hypoxia. There is also evidence that TRPA1 and TRPV1 physically interact and create unique hybrid channels. (b) Sodium and potassium channels are key in setting the resting membrane potential and the threshold of excitation in neurons. The increased activity in voltage-gated sodium channels (Na V ) by, e.g., ciguatoxins increases the excitability, so that also mild cold temperatures via activation of TRPA1 can evoke action potential discharge, causing cold allodynia, which is a major pain symptom in human chronic pain. Also, potassium channels, such as voltage-sensitive delayed rectifiers (K V ) and two-pore domain channels (K 2P ), act as excitatory breaks in sensory neurons, and any change in their activity can dramatically affect the membrane excitability and TRPA1 responses
Voltage-gated sodium and potassium channels are key players controlling cell excitability and intracellular Ca2+ levels (Fig. 5). Of particular importance is the expression of certain voltage-gated sodium channels (NaV1.7, NaV1.8, and NaV1.9), and voltage-gated (KV) and two-pore domain (K2P) potassium channels in sensory neurons associated with perception of thermal, mechanical, and chemical stimuli (Belmonte et al. 2009; Momin and Wood 2008; Noel et al. 2011). As shown by the use of ciguatoxins, activation of NaV and closure of KV in sensory neurons induced TRPA1-dependent cold allodynia (Vetter et al. 2012). Also, the chemotherapeutic agent oxaliplatin downregulates the “excitability breaks” K2P and KV, and upregulates the pro-excitatory channels NaV1.8, hyperpolarization-activated cyclic nucleotide-gated subtype 1 channel (HCN1), and TRPA1 in mouse dorsal root ganglia, whereas NaV1.9 and TRPA1 are upregulated in rat dorsal root ganglia in diabetic animals explaining the lowered thresholds for cold and mechanical responses (Barriere et al. 2012; Descoeur et al. 2011).
TRPA1 activity may be controlled by secretogranin III, a protein present in various endocrine and neuroendocrine cells, as shown in mast cells and HEK293 cells overexpressing these proteins (Prasad et al. 2008). The hydroxylation of a proline residue in the TRPA1 N-terminus by the oxygen-dependent prolyl hydroxylase is believed to depress channel activity at normoxia (Takahashi et al. 2011). This “break” is removed by hypoxia and the prolyl hydroxylase inhibitor dimethyloxalylglycine, providing a potential role for TRPA1 in autonomous tissue adaptation to mild hypoxic conditions by controlling perivascular sensory neurons and tissue blood flow (Zygmunt 2011).
Taken together, a number of proteins that regulate TRPA1 either directly or indirectly have been proposed. The threshold for TRPA1-mediated responses is dependent on the cellular context, including the expression levels of TRPA1 and other ion channels regulating membrane excitability, the phosphorylation and hydroxylation state of TRPA1, and the cytoplasmic Ca2+ level (Fig. 5). This is in line with the view that the combinatorial expression of different ion channels, and not a single sensory channel transducer, determines the sensory output (Belmonte and Viana 2008).
5 A Biophysical Description of the Channel Function, Permeation, and Gating
The TRPA1 protein is a nonselective cation channel permeable to both monovalent and divalent ions including Ca2+, Na+, and K+. TRPA1 has a high Ca2+ permeability compared to most other TRP ion channels and a unitary conductance of ~70 pS and ~110 pS in the inward and outward directions, respectively, under physiological conditions (cell-attached patch configuration and the presence of extracellular Ca2+ and Mg2+) when the channel is constitutively open (Nilius et al. 2011, 2012). The presence of Ca2+ and Mg2+ in the extracellular solution has a major impact on channel activity, and the single-channel conductance is generally smaller at negative than at positive membrane potentials. However, this voltage dependency is lost in the absence of divalent ions (Nilius et al. 2011). Measurements of the single-channel conductance of wild-type human, rat, and mouse TRPA1 have generated values between 40 and 180 pS depending on the experimental conditions and stimuli used (Table 1). Whether differences in channel conductance related to species exist is yet to be determined.
Interestingly, the TRPA1 pore, the size of which has been estimated as 11.0 Å, can undergo dilation, as shown in the presence of TRPA1 activators, thereby increasing the Ca2+ permeability and allowing larger charged molecules to pass through the channel (Banke et al. 2010; Chen et al. 2009; Karashima et al. 2010). This feature of TRPA1 as well as TRPV1 has been explored as an entrance for cell membrane impermeable local anesthetics into sensory neurons to achieve selective nociceptor fiber block (Brenneis et al. 2014). The amino acid residue Asp918 in the putative ion selectivity filter of the pore seems important in determining Ca2+ as well as Zn2+ permeation through the channel (Fig. 3) (Hu et al. 2009; Karashima et al. 2010; Wang et al. 2008b). This is further supported by the dramatic decrease of TRPA1 single-channel conductance from 111 ± 7 pS to 37 ± 5 pS when Asp918 is replaced by glutamine (D918Q) (Nilius et al. 2011). The pore vestibule is a target not only for the nonselective TRP channel blocker ruthenium red but also for newly developed TRPA1 antagonists such as AZ465, AZ868, and A-967079, whereas HC030031 most likely binds to channel structures outside the pore (Klement et al. 2013; Nyman et al. 2013).
Although there is no apparent voltage sensor in transmembrane segment 4, as shown for voltage-gated potassium channels, TRPA1 displays some voltage dependency, although less pronounced compared to TRPV1 and TRPM8 (Karashima et al. 2009; Samad et al. 2011; Voets et al. 2004). A single point mutation in transmembrane segment 4 of the human TRPA1 causes a shift in channel gating properties with a dramatic increase of inward currents and activation at normal resting potentials (Kremeyer et al. 2010). It has been suggested that a putative voltage-sensing domain of positively charged amino acid residues within the C-terminus could act as a sensor for changes in transmembrane voltage (Samad et al. 2011).
Both inorganic polyphosphates (e.g., PPPi) and Mg-ATP, which stimulates phosphatidylinositol 4-kinase activity and the generation of membrane bound phosphatidylinositol-4,5-bisphosphate (PIP2), have been shown to preserve TRPA1 single-channel activity in excised inside-out cell membrane patches, suggesting that intracellular modulators mimicking the action of polyphosphates exist and that TRPA1 function is modulated by PIP2 (Cavanaugh et al. 2008; Karashima et al. 2008; Kim and Cavanaugh 2007). The sites at which polyphosphates and PIP2 interact with TRPA1 are not resolved, but could include the N-terminal ankyrin repeat domain and the channel pore (polyphosphates) and both the N- and C-termini (PIP2) (Nilius et al. 2011; Sura et al. 2012). However, the role of PIP2 in TRPA1 channel gating is debated (Dai et al. 2007; Karashima et al. 2008; Kim et al. 2008; Wang et al. 2008b). In contrast, there is no doubt that one of the most important cytosolic regulators of TRPA1 is Ca2+, which has been suggested to gate TRPA1 directly by binding to sites on the N- and C-termini (Doerner et al. 2007; Jordt et al. 2004; Sura et al. 2012; Wang et al. 2008b; Zurborg et al. 2007). The bimodal effect of Ca2+ on TRPA1, potentiation or activation at low and inhibition at high intracellular concentrations, most likely reflects independent processes (Wang et al. 2008b). Interestingly, whereas Ca2+ and electrophilic compounds require the presence of polyphosphates to activate TRPA1 in excised inside-out cell membrane patches, Δ9-tetrahydrocannabinol does not, further adding to the intriguing complexity of TRPA1 channel gating (Cavanaugh et al. 2008; Kim and Cavanaugh 2007).
6 Physiological Functions in Native Cells, Organs, and Organ Systems
In addition to its clear function as a chemosensor, there is growing evidence that TRPA1 is involved in thermo- and mechanosensation. Whether TRPA1 is intrinsically thermo- or mechanosensitive remains, however, to be determined.
6.1 TRPA1 and Chemosensation
The proposal of an ionotropic cannabinoid receptor belonging to the TRP ion channel family (Zygmunt et al. 2002), also being sensitive to irritants (Fig. 6), and the frequent use of mustard oil in animal models of acute pain and hyperalgesia prompted a search for Δ9-tetrahydrocannabinol and mustard oil-sensitive clones upon expression of a rat trigeminal ganglia cDNA library (Jordt et al. 2004). This led us to the identification of the rat and human TRPA1 as ionotropic cannabinoid receptors and targets for mustard oil (Jordt et al. 2004). Another study identified mouse TRPA1 as a target for both electrophilic and non-electrophilic compounds (Bandell et al. 2004), and this also provided a molecular target by which cinnamaldehyde and related spicy compounds induced sensory nerve-mediated vasodilation (Fig. 6). Shortly after, we and others showed that certain thiol-reactive garlic-derived compounds activated TRPA1 (Bautista et al. 2005; Macpherson et al. 2005). Targeted gene mutations identified three cysteines present in the N-terminal region of the human (Cys621, Cys641, and Cys665) and mouse (Cys415, Cys422, and Cys622) TRPA1 as important for electrophilic TRPA1 channel activation (Figs. 3 and 4) (Hinman et al. 2006; Macpherson et al. 2007a). Together, these studies revealed TRPA1 as a detector of thiol-reactive electrophiles and oxidants in addition to non-electrophilic compounds as well as being indirectly regulated by G protein-coupled receptor signaling including the bimodal action of Ca2+.
Early screening of known irritants, using the rat mesenteric artery as a bioassay for sensory neuronal signaling, identified benzyl isothiocyanate (BITC) and similar mustard oil compounds, cinnamaldehyde (CA), and safranal (SF) as activators of a putative TRP channel (TRPA1) on TRPV1-containing primary sensory neurons (authors’ unpublished data, 2003 and 2004). Data are expressed as mean ± SEM of five to eight independent experiments (animals) performed as previously described (Zygmunt et al. 2002). This bioassay has also been useful for identifying anandamide (AEA) and similar endogenous N-acyl ethanolamines as well as monoacylglycerols, including 2-arachidonoyl glycerol (2-AG), as endovanilloids acting on the capsaicin (CAP) receptor TRPV1 (Movahed et al. 2005; Zygmunt et al. 1999, 2013). Influx of Ca2+ through TRPV1 and TRPA1 causes release from sensory neurons (SN) of the calcitonin gene-related peptide (CGRP), which through activation of its receptor on the vascular smooth muscle cell (SMC) stimulates cyclic adenosine monophosphate (cAMP) production, leading to vasodilation. BITC, CA, and SF displayed a similar pharmacological vasodilator profile as the cannabinoids Δ9-tetrahydrocannabinol (Δ9-THC) and cannabinol (Zygmunt et al. 2002); reduced vasodilation after depletion of CGRP-containing perivascular sensory neurons by capsaicin (CAP) or in the presence of the CGRP receptor antagonist (8–37 CGRP) or the nonselective TRP channel blocker ruthenium red (RR), but not in the presence of capsazepine that blocks the vasodilation by TRPV1 agonists (CAP, AEA, and 2-AG) (Zygmunt et al. 1999, 2002, 2013)
Today, a large number of compounds are known to activate mammalian TRPA1 (Fig. 7, Table 2). The ability to activate TRPA1 may explain why the use of apomorphine, disulfiram, glibenclamide, and in the past clioquinol in drug treatment is associated with pain and nausea (Andersson et al. 2009; Babes et al. 2013; Maher et al. 2008; Schulze et al. 2013). Many endogenous and exogenous irritants, including those in spices, mainly bind to cysteine residues in the N-terminus (Andrade et al. 2012; Baraldi et al. 2010; Holzer 2011; Nilius and Appendino 2013; Nilius et al. 2012; Takahashi and Mori 2011). However, other amino acids such as lysine in the N-terminus and cysteines outside the N-terminal region are potential targets for electrophiles and oxidants and may contribute to the regulation of TRPA1 (Eberhardt et al. 2012; Escalera et al. 2008; Ibarra and Blair 2013; Macpherson et al. 2007a; Nilius et al. 2012; Takahashi et al. 2011; Wang et al. 2012). In this context, it is important to remember that several of the N-terminal cysteines may form a complex network of protein disulfide bridges within and between monomers (Fig. 4), and thus, it is not unlikely that mutations of such amino acids could lead to various conformational changes of the N-terminal region affecting the functional integrity of TRPA1 (Chen et al. 2008; Cvetkov et al. 2011; Eberhardt et al. 2012; Ibarra and Blair 2013; Wang et al. 2012). Indeed, a general defect of TRPA1 channel function caused by cysteine and lysine mutations or the creation of xenogeneic (artificial) channels has been reported (Andersson et al. 2013; Chen et al. 2008; Hu et al. 2009; Ibarra and Blair 2013; Miyamoto et al. 2009; Takahashi et al. 2008, 2011; Xiao et al. 2008), and the use of non-electrophilic activators at single supramaximal agonist concentrations may not always be enough to rule out a defect in channel function.
Chemical structures of key compounds initially used to define TRPA1 as a chemosensor, responding to both exogenous and endogenous cysteine-reactive electrophiles and oxidants as well non-electrophilic compounds. Red compounds (left panel) bind to cysteines by strong covalent mechanisms, whereas some weaker electrophiles and oxidants, shown as blue (middle panel), promote disulfide formations within the TRPA1 protein that can be rectified by thiol-reducing agents, such as dithiothreitol. Importantly, the ability of noncovalent compounds (shown as green, right panel), including the analgesic plant cannabinoids Δ9-THC and C16 (not shown), to activate TRPA1 may provide opportunities to treat human pain using a TRPA1 agonistic approach (Andersson et al. 2011). This also raises the possibility that chemically similar endogenous compounds may exist to control TRPA1 activity. AITC allyl isothiocyanate, CA cinnamaldehyde, NMM N-methylmaleimide, 4-HNE 4-hydroxy-2-nonenal, DADS diallyl disulfide, MTSEA 2-aminoethyl methanethiosulfonate, Δ 9 -THC Δ9-tetrahydrocannabinol, MSC methyl salicylate
Importantly, the chemical mechanism by which covalent modification of cysteines occurs differs between electrophilic TRPA1 activators also when very close in chemical structure (Bessac et al. 2009; Gijsen et al. 2010; Ibarra and Blair 2013; Sadofsky et al. 2011). The electrophilic paracetamol (acetaminophen) metabolite p-benzoquinone and the noxious fungal sesquiterpenes isovelleral and polygodial, containing α,β-unsaturated dialdehyde moieties, all produced intact TRPA1 responses in the heterologously expressed triple mutant hTRPA1-3C (Escalera et al. 2008; Ibarra and Blair 2013) that was initially used to identify certain N-terminal cysteine residues as key targets for electrophiles (Hinman et al. 2006). The electrophilic ketoaldehyde methylglyoxal displays a dual mechanism of activation of TRPA1 by binding irreversibly to N-terminal lysine 710 and also promoting disulfide formation of N-terminal cysteines (Eberhardt et al. 2012), and the headache-inducing monoterpene ketone umbellulone activates TRPA1 by a non-covalent interaction in addition to covalently reacting with N-terminal cysteines (Zhong et al. 2011a). An interesting example is the cyclopentenone prostaglandin 15-deoxy-δ(12,14)-prostaglandin J2 (Fig. 7), an endogenous electrophilic TRPA1 activator (Andersson et al. 2008; Cruz-Orengo et al. 2008; Maher et al. 2008; Materazzi et al. 2008; Takahashi et al. 2008; Taylor-Clark et al. 2008b), that in contrast to allyl isothiocyanate is antinociceptive when injected into the mouse paw (Weng et al. 2012). Thus, differences in the chemical properties between TRPA1 activators may contribute to unique ligand-specific pharmacology of TRPA1 in terms of sensitization, desensitization, channel trafficking, pungency, and duration of action.
Agents such as complete Freund’s adjuvant and carrageenan are widely used to evoke inflammation, hyperalgesia, and allodynia in animals. They all increase the production of numerous inflammatory mediators, some of which act directly (e.g., 4-hydroxynonenal, hydrogen peroxide, 15-deoxy-δ(12,14)-prostaglandin J2) or indirectly (e.g., prostaglandins, bradykinin, histamine, and trypsin) on TRPA1 (Andrade et al. 2012; Mogil 2009; Moilanen et al. 2012). Many of these indirectly acting mediators bind to G protein-coupled receptors that regulate TRPA1 via the phospholipase C signaling pathway including PIP2 depletion, generation of H+, Ca2+ release, and protein kinase A-mediated phosphorylation (Fig. 5) (Andrade et al. 2012; Bautista et al. 2013; Bessac and Jordt 2008; Cavanaugh et al. 2008; Huang et al. 2010; Nilius et al. 2012). The nociceptive responses triggered by intraplantar injection of complete Freund’s adjuvant and carrageenan are sensitive to TRPA1 antagonists (Andrade et al. 2012; Gregus et al. 2012), and TRPA1 plays a key role in the development of carrageenan-induced inflammation (Moilanen et al. 2012). The rat and mouse formalin test is also a widely used pain model for evaluating analgesic compounds on acute pain (first phase) and inflammatory pain (second phase). This biphasic nociceptive response is driven by a direct action of formaldehyde on TRPA1 (Macpherson et al. 2007b; McNamara et al. 2007).
The gasotransmitter H2S is an endogenous signaling molecule (also produced by bacteria) that can contribute to inflammation and the associated sensitization of nociceptors (Li et al. 2011). This gaseous molecule activates ATP-sensitive potassium channels (KATP) and T-type voltage-gated calcium channels (CaV3.2). Our original finding that TRPA1 is activated by H2S (Streng et al. 2008) together with other studies suggests that H2S may act in concert with T-type voltage-gated calcium channels to excite nociceptive sensory neurons in the skin, airways, bladder, and colon (Fig. 8) (Andersson et al. 2012; Hsu et al. 2013; Ogawa et al. 2012; Okubo et al. 2012; Tsubota-Matsunami et al. 2012). In the vasculature, both endothelium and sensory neurons contain powerful vasodilator mediators (Deanfield et al. 2007; Maggi 1995). Stimulation of endothelial cells releases endothelium-derived relaxing factors (EDRFs), such as nitric oxide and the endothelium-derived hyperpolarizing factor (EDHF) (Furchgott and Zawadzki 1980; Petersson et al. 1995, 1997, 1998; Zygmunt and Hogestatt 1996; Zygmunt et al. 1994a, b, 1998), and the activation of TRPV1 and TRPA1 on sensory neurons releases calcitonin gene-related peptide (Figs. 1 and 6) (Bautista et al. 2005; Graepel et al. 2011; Högestätt et al. 2000; Zygmunt et al. 1999, 2000, 2002). With regard to vascular signaling, a multicellular communication seems to exist between capsaicin-sensitive neuronal, endothelial, and smooth muscle cells (Högestätt et al. 2000); H2S can produce sensory nerve-mediated vasodilation and may share properties with the proposed EDHF (Pozsgai et al. 2012; Tang et al. 2013). However, future studies are needed to explore a possible physiological role of H2S as an EDHF and TRPA1 activator within the cardiovascular system. The exact mechanism by which H2S activates TRPA1 is not obvious as H2S is a mild reducing agent (Zhang et al. 2014). However, it has been suggested that direct or indirect sulfhydration of thiols by H2S leads to protein activation (Li et al. 2011; Zhang et al. 2014), which is supported by the finding that H2S can activate TRPA1 in inside-out membrane patches (Fig. 8) (Andersson et al. 2012). Nevertheless, other indirect mechanisms for H2S-dependent TRPA1 activation cannot be excluded.
Hydrogen sulfide (H2S) is a recently discovered gasotransmitter in mammalians shown to activate the ATP-sensitive potassium channel (KATP) and the T-type voltage-gated calcium channel (CaV3.2). H2S can be produced enzymatically by, e.g., cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), using l-cysteine as a substrate. The production of H2S is also stimulated by lipopolysaccharide (LPS) derived from bacteria. The H2S donor NaHS activates both human and mouse TRPA1, as shown by calcium imaging (Streng et al. 2008), and evokes TRPA1 currents in excised inside-out membrane patches, as measured by the patch-clamp technique (Andersson et al. 2012)
It has been shown that TRPA1 is part of the O2 homeostatic system, being activated directly by hyperoxia and indirectly by mild hypoxia (Takahashi et al. 2011), of which the latter may indicate an important role for TRPA1 present on perivascular sensory neurons in rectifying hypoxic conditions via release of potent vasodilator peptides.
Extracellular acidification accompanies ischemia, inflammation, and cancer growth, conditions associated with pain and sensitization of nociceptive primary afferents. A recent study disclosed important species differences in the extracellular proton sensitivity of TRPA1 (de la Roche et al. 2013). Interestingly, the human TRPA1 seems to have acquired the ability to sense extracellular acidosis, a property that is absent in rodent and rhesus monkey TRPA1 orthologs (de la Roche et al. 2013). Activation of human TRPA1 is observed at pH levels below 7, and the ensuing Ca2+ or current responses are graded, sustained, and reproducible, as shown by calcium imaging and patch-clamp recordings (de la Roche et al. 2013; Takahashi et al. 2008). Amino acids in both the N-terminus (C621) and transmembrane segment 6 (V942 and S943) were identified as crucial for responses to extracellular acidosis (de la Roche et al. 2013; Takahashi et al. 2008). However, the role of C621 in proton sensing is enigmatic, as this cysteine is also present in the rodent and rhesus TRPA1, which are insensitive to extracellular acidosis (de la Roche et al. 2013). These findings also raise concerns about the use of rhesus monkey TRPA1 as a surrogate species in human TRPA1 drug development (Bianchi et al. 2012; Chen et al. 2013).
Rodent TRPA1 is also sensitive to the intracellular acid–base environment, displaying a U-shaped pH response relationship, and mutations of two N-terminal cysteines (C422 and C622) disrupted channel activation evoked by intracellular alkalization (Fujita et al. 2008; Wang et al. 2010, 2011). In this respect, TRPA1 differs from TRPV1, which is inhibited by intracellular acidosis (Chung et al. 2011), although both ion channels may be activated by intracellular alkalosis (Dhaka et al. 2009; Fujita et al. 2008). Notably, the intracellular pH sensitivity of TRPA1 was retained in inside-out patches, ruling out a second messenger role of Ca2+ or any other cytosolic components in the pH responses (Dhaka et al. 2009; Fujita et al. 2008; Wang et al. 2010). Its role in both pH and oxygen sensation makes TRPA1 particularly suitable as a mediator of ischemic pain and other ischemia-related protective or adaptive responses, such as hypoxic vasodilation and ischemic preconditioning.
Whereas the sensitivity of TRPA1 to potentially harmful electrophiles and oxidants is in line with its proposed role as a nocisensor, the ability of TRPA1 to respond to many non-electrophilic compounds of diverse chemical structure is intriguing (Fig. 7 and Table 2) (Andrade et al. 2012; Baraldi et al. 2010; Holzer 2011; Nilius et al. 2012). Based on our findings that paracetamol metabolites and non-psychotropic cannabinoids produce spinal antinociception via activation of TRPA1, we have suggested that non-tissue-damaging TRPA1 activators may be developed for treatment of pain (Fig. 9) (Andersson et al. 2011). In this regard, it is interesting that nonsteroidal anti-inflammatory fenamates also activate TRPA1 (Hu et al. 2010; Peyrot des Gachons et al. 2011). Other non-covalent activators, such as dihydropyridines and clotrimazole, may also serve as templates for the development of analgesic TRPA1 activators (Fajardo et al. 2008b; Meseguer et al. 2008). This approach may not be restricted to a spinal site of action, as the electrophilic TRPA1 activator 15-deoxy-δ(12,14)-prostaglandin J2 produced a peripheral antinociceptive effect in mouse models of pain (Weng et al. 2012).
Activation of TRPA1 by electrophilic paracetamol (acetaminophen) metabolites or non-electrophilic cannabinoids, such as Δ9-tetrahydrocannabinol and its plant derivative Δ9-tetrahydrocannabiorcol (C16), in central projections of primary sensory neurons in the spinal cord causes influx of Ca2+ and Na+. This leads to the initial release of the excitatory neurotransmitter glutamate, acting on ionotropic glutamate receptors (iGluR) and the subsequent inhibition of (a and b) voltage-gated sodium channels (NaV; red I–V trace) and the related action potential (flat blue line in the presence and red line after washout of TRPA1 activator) as well as (c) voltage-gated calcium channels (VGCC, red trace). The net result will be the inhibition of incoming pain signals from the periphery. Paracetamol (APAP) is metabolized to p-aminophenol (p-AP) and electrophilic compounds including N-acetyl-p-benzoquinoneimine (NAPQI) and p-benzoquinone (p-BQ) that activate TRPA1 by covalent binding to cysteines (–SH). CYP (cytochrome P450 monooxygenases), COX (cyclooxygenases). Modified from Andersson et al. (2011)
The electrophilic nature of TRPA1 activators has made it possible to use mass spectrometry to show that such compounds interact covalently with TRPA1 and, hence, TRPA1 is an intrinsically chemosensitive protein (Cvetkov et al. 2011; Macpherson et al. 2007a; Wang et al. 2012). For non-electrophilic activators, mutagenesis and chimeric strategies have also indicated a direct interaction with TRPA1; e.g., menthol was suggested to bind to transmembrane segment 5 of TRPA1 (Xiao et al. 2008). Patch-clamp recordings of isolated membrane patches suggest that Δ9-tetrahydrocannabinol also interacts directly with TRPA1 (Cavanaugh et al. 2008; Kim and Cavanaugh 2007). Interestingly, in contrast to Δ9-tetrahydrocannabinol, several other TRPA1 activators of both electrophilic and non-electrophilic nature (allicin, allyl isothiocyanate, cinnamaldehyde, N-ethylmaleimide, 2-aminoethoxydiphenyl borate, methyl salicylate, 2-aminoethyl methanethiosulfonate, and trinitrophenol) as well as Ca2+ require a cytosolic component to activate TRPA1, indicating that cannabinoids activate TRPA1 in a unique way (Cavanaugh et al. 2008). The ability of non-electrophilic compounds to activate TRPA1 also raises the interesting possibility that endogenous ligands sharing binding sites with these non-electrophiles exist and regulate TRPA1 activity. However, it still remains to be shown that menthol and other non-covalent activators indeed interact directly with TRPA1.
Some electrophilic and non-electrophilic TRPA1 activators, including apomorphine, camphor, cinnamaldehyde, ligustilide, menthol, nicotine, umbellulone, and thymol, display a bimodal action (activation at low and inactivation at high concentrations) on heterologously expressed TRPA1 that in some cases is species dependent (Alpizar et al. 2013; Karashima et al. 2007; Schulze et al. 2013; Talavera et al. 2009; Zhong et al. 2011a, b). The list of TRPA1 activators is extensive and rapidly growing (Table 2), whereas only a few natural blockers have been described (Bang et al. 2010, 2011; Park et al. 2011; Takaishi et al. 2013). Among these resolvin D2 is the most potent one, inhibiting both TRPA1 and TRPV1 in the nanomolar range as shown in mouse dorsal root ganglion neurons (Park et al. 2011). However, these effects were pertussis toxin-sensitive and therefore most likely not caused by a direct interaction between resolvin D2 and the channel proteins (Park et al. 2011). Although many TRPA1 activators may interfere with other important TRP channels and membrane receptors (e.g., nicotine) that are involved in TRPA1-sensitive sensory signaling (Holzer 2011; Kichko et al. 2013), further studies of the pharmacology of non-covalent activators and the mechanisms behind the bimodal action of certain drugs should provide a deeper understanding of TRPA1 channel function and help to identify novel activators and antagonists with unique pharmacological properties.
6.2 TRPA1 and Noxious Cold Sensation
Ever since TRPA1 was suggested to respond to noxious cold temperatures (Story et al. 2003), its role in cold sensation has been debated. Nevertheless, several studies have shown that acute noxious cold sensation is impaired in TRPA1 knockout mice and, more importantly, that TRPA1 is involved in cold allodynia induced by inflammation, nerve injury, and peripheral neuropathy (Table 3). Furthermore, as shown at a single-channel level, the open probability increased (due to a change in the rate of channel deactivation) by lowering the temperature just below room temperature (Karashima et al. 2009; Sawada et al. 2007). Interestingly, cold responses were independent on Ca2+ (Karashima et al. 2009; Sawada et al. 2007), but this does not exclude Ca2+ as an important regulator of TRPA1-mediated cold responses in vivo. Thus, there is now substantial evidence from both in vitro and in vivo studies that rat and mouse TRPA1 is involved in noxious cold sensation. However, based on in vitro studies of the expressed human TRPA1, the controversy regarding a similar role for human TRPA1 is still ongoing (Bandell et al. 2004; Chen et al. 2013; Cordero-Morales et al. 2011; Jordt et al. 2004; Klionsky et al. 2007; Kremeyer et al. 2010; May et al. 2012; Wang et al. 2013; Zurborg et al. 2007). A recent study concluded that species differences exist and that in contrast to rodent TRPA1, the human variant is incapable to respond to cold (Chen et al. 2013). However, the cold sensitivity of TRPA1-expressing rat sensory neurons seems to depend on the anatomical origin of the neurons (Fajardo et al. 2008a), indicating that the cellular environment including the oxidation state of the cells can dictate cold sensitivity possibly by influencing the conformation of the N-terminal region. This together with other methodological issues may explain why opposite conclusions exist regarding TRPA1 as a cold sensor (Karashima et al. 2009). Whether or not TRPA1 is gated directly by cold remains, however, to be demonstrated and requires studies on the purified ion channel.
6.3 TRPA1 and Noxious Mechanosensation
The interest in mammalian TRPA1 as a mechanosensor was inspired by the large N-terminal ankyrin repeat domain, potentially acting as a gating spring (Gaudet 2008; Howard and Bechstedt 2004), the expression of TRPA1 in hair cells of the inner ear (Corey et al. 2004), and observations in invertebrates that structurally similar TRP channels play key roles in mechanotransduction (Sidi et al. 2003; Walker et al. 2000). Indeed, CHO cells transfected with a Caenorhabditis elegans ortholog of the mammalian TRPA1 acquired mechanosensory properties (Kindt et al. 2007). Although a role of the mammalian TRPA1 in hearing could not be demonstrated in subsequent studies of gene knockout mice (Bautista et al. 2006; Kwan et al. 2006), the involvement of TRPA1 in noxious mechanotransduction, including mechanical allodynia or hypersensitivity, has received increasing support over time, as shown in naïve animals and various models of inflammatory and neuropathic pain, using both gene silencing and pharmacological strategies to interfere with TRPA1 expression and function (Table 4). In particular, the introduction of selective TRPA1 inhibitors has made it possible to study the effect of locally inhibiting TRPA1 in the receptive field. Such studies have consolidated TRPA1 as an important component of the mechanotransduction pathway in nociceptive somatosensory neurons (Bonet et al. 2013; da Costa et al. 2010; Fernandes et al. 2011; Kerstein et al. 2009; Kwan et al. 2009). Furthermore, direct mechanical or osmotic stimulation of cultured nociceptor-like sensory neurons induced graded current responses that disappeared or were significantly reduced by pharmacological or genetic inactivation of TRPA1, although the possibility that Ca2+ was acting as an upstream mediator of such mechanically induced responses cannot be excluded (Brierley et al. 2011; Vilceanu and Stucky 2010; Zhang et al. 2008b). Except for one study using hypertonic solutions to impose a mechanical force (cell shrinkage) on the cell membrane (Zhang et al. 2008b), heterologous TRPA1 gene expression has so far failed to confer mechanosensitivity to the recipient cells. Functional studies of the purified protein reconstituted in a defined lipid and protein environment will be required to address whether or not TRPA1 is an intrinsically mechanosensitive ion channel.
6.4 TRPA1 and Itch
Itch is a distinct psychophysical sensation associated with a strong urge to scratch. Its sensory and motivational qualities resemble the tickling sensation that may precede cough and sneeze. Itch is mediated by a unique set of primary afferents distinct from those mediating pain (Schmelz et al. 1997). However, painful mechanical stimulation of the skin can inhibit itch at the level of the spinal cord, and this cross-modality interaction may prevent scratch-induced injury (Ross 2011). Itch responses can be evoked by stimulation of two different populations of primary afferent C-fibers: Mechano-insensitive and mechanosensitive fibers selectively activated by histamine and cowhage spicules (containing 5-hydroxytryptamine), respectively (Namer et al. 2008; Roberson et al. 2013). As shown recently, TRPA1 is required for itch-evoked scratching in response to many pruritogens, including cowhage spicules, chloroquine, leukotriene B4, substance P, and reactive oxygen species, acting at least partly through histamine-insensitive primary afferents (Fernandes et al. 2013; Liu et al. 2013; Liu and Ji 2012; Wilson et al. 2011). Intradermal injection of allyl isothiocyanate in the mouse cheek activates pruritogen-responsive second-order neurons in the trigeminal subnucleus caudalis (Akiyama et al. 2010). However, allyl isothiocyanate or cinnamaldehyde produces pain rather than itch sensation when administered to the skin and nasal mucosa in man, possibly reflecting cross-modality inhibition of itch responses (Alenmyr et al. 2009; Namer et al. 2005). A unique pharmacological approach for silencing of capsaicin-sensitive nerve fibers recently disclosed an itch-producing effect of allyl isothiocyanate, providing additional support for a cross-modality interaction between the neural pathways mediating pain and itch (Roberson et al. 2013).
Recent findings have revealed that TRPA1 is an important mediator of non-histaminergic itch and that overactivity in itch-encoding histamine-insensitive primary afferents may be responsible for chronic itch in atopic and allergic contact dermatitis (Cevikbas et al. 2014; Liu et al. 2013; Oh et al. 2013; Wilson et al. 2013). Importantly, the TRPA1 immunoreactivity is dramatically increased in skin biopsies from patients with atopic dermatitis, even in mast cells and keratinocytes that barely express TRPA1 immunostaining in healthy skin (Oh et al. 2013). Thus, TRPA1 is emerging as a promising target for novel anti-pruritic drugs particularly in atopic and allergic contact dermatitis. Pharmacological inhibition of TRPA1 may also break the vicious circle generated by intense scratching in dermatological disease, although disinhibition of itch-encoding neurons may potentially enhance TRPA1-independent itch pathways.
6.5 TRPA1 and the Central Nervous System
While noxious cold, mechanical stress, and environmental irritants can trigger TRPA1 activation in the periphery, the physiological role of TRPA1 on central projections of primary afferents is less obvious, as the central nervous system is rarely exposed to noxious cold or mechanical stress. However, TRPA1 can be activated by several endogenous molecules, including reactive oxygen species and 12-lipoxygenas and cytochrome P450 epoxygenase-derived metabolites, which may be formed in the spinal cord during nociceptive stimulation, nerve injury, and neuroinflammation (Due et al. 2013; Gregus et al. 2012; Lee et al. 2007; Sisignano et al. 2012). The outer layers of the dorsal horn are richly innervated by TRPA1-expressing nerve terminals, as shown by immunohistochemistry (Andersson et al. 2011; Kim et al. 2010). However, transcripts encoding TRPA1 cannot be detected in the rat and mouse spinal cord, and dorsal rhizotomy almost completely eliminates spinal TRPA1 immunostaining in the rat, indicating that most if not all TRPA1-containing nerve terminals originate from primary sensory neurons (Andersson et al. 2011; Kim et al. 2010; Story et al. 2003). However, there are indications that TRPA1 is present in the brain both at the transcriptional and translational levels (Stokes et al. 2006; Vennekens et al. 2012). Using whole-cell patch-clamp recordings, presynaptic TRPA1 channels were demonstrated on glutamatergic neurons in the rat supraoptic nucleus (Yokoyama et al. 2011). Furthermore, expression of TRPA1 in astrocytes was recently identified in rodent trigeminal caudal nucleus and hippocampus, potentially regulating interneuron inhibitory synaptic efficacy, long-term potentiation, and neuronal survival (Koch et al. 2011; Lee et al. 2012; Shigetomi et al. 2012, 2013).
The function of TRPA1 on central projections of primary afferents has not been entirely resolved. In substantia gelatinosa neurons from the spinal dorsal horn and trigeminal sensory nuclei, the TRPA1 activators allyl isothiocyanate and cinnamaldehyde increase the frequency of spontaneous excitatory postsynaptic currents and inhibitory postsynaptic currents, the latter effect being sensitive to tetrodotoxin in spinal cord slices and, hence, attributable to recruitment of inhibitory interneurons (Cho et al. 2012; Kosugi et al. 2007; Uta et al. 2010; Wrigley et al. 2009). Intrathecal administration of TRPA1 activators causes tactile allodynia and occasionally also heat hyperalgesia (Due et al. 2013; Gregus et al. 2012; Proudfoot et al. 2006; Raisinghani et al. 2011; Sisignano et al. 2012; Wei et al. 2013a). However, spinal TRPA1 activation by both electrophilic and non-electrophilic activators can also inhibit acute thermal and mechanical pain, as shown in the modified hot and cold plate tests (using lightly restrained animals) and the paw pressure test, and the formation of electrophilic metabolites in the spinal cord may contribute to the analgesic effect of acetaminophen (Fig. 9) (Andersson et al. 2011). This pharmacological effect of intrathecal administration of TRPA1 activators was attributed to a Ca2+-dependent presynaptic inhibition of voltage-gated calcium and sodium channels (Fig. 9) (Andersson et al. 2011), which is in line with Aδ- and C-fiber-evoked excitatory postsynaptic currents being inhibited by TRPA1 activators in spinal cord slices, as also shown for capsaicin (Jeffry et al. 2009; Uta et al. 2010; Yue et al. 2013).
Several studies have addressed the role of spinal TRPA1 in models of inflammatory and neuropathic pain using intrathecal administration of TRPA1 blockers. These studies clearly show that spinal TRPA1 participates in mechanical allodynia or hypersensitivity as well as secondary mechanical hyperalgesia following peripheral capsaicin or allyl isothiocyanate administration (da Costa et al. 2010; Fernandes et al. 2011; Wei et al. 2009, 2010a, b, 2011). Thus, while spinal TRPA1 seems to play a key role in central sensitization, pharmacological activation of the same ion channel can also disrupt spinal nociceptive neurotransmission (Andersson et al. 2011). Further studies are required to understand this intriguing dual action of TRPA1 and its implications for the development of TRPA1-based drug therapies.
7 Lessons from Knockouts
Transgenic mice lacking functional TRPA1 channels have been used to understand the physiological and pathophysiological role of this protein and its potential as a pharmacological target for treatment of pain and sensory dysfunction in man (Patapoutian et al. 2009). Initial studies using TRPA1 knockout mice, generated by different genetic strategies, clearly validated TRPA1 as a chemosensor (Bautista et al. 2006; Kwan et al. 2006; Macpherson et al. 2007b; McNamara et al. 2007). Major concerns when using TRPA1 knockout mice are functional compensation by related channels and receptors and significant differences between mouse strains (Patapoutian et al. 2009). This could explain the lack of consensus regarding the role of TRPA1 in noxious cold and mechanical sensation. Nevertheless, many recent studies using TRPA1 knockout mice suggest an involvement of TRPA1 in both noxious cold and mechanical hypersensitivity (Tables 3 and 4), which is also supported by the use of TRPA1 antagonists, such as HC030031 and the structurally similar compound Chembridge-5861528 (Tables 3 and 4), although HC030031 has off-target effects that must be considered when this and chemically similar pharmacological tools are used to understand the physiological role of TRPA1 (Andersson et al. 2013; Fischer et al. 2010). The recent finding that the skin innervation of TRPA1 knockout mice is substantially less compared to wild-type mice is of concern, as it may explain the reduced responsiveness to cold and mechanical stimuli in such mice (Andersson et al. 2013). The type of nerve fiber affected remains, however, to be determined. The rationale of using nonhuman TRPA1 models in vitro and in vivo to develop drugs for treatment of human pain and sensory discomfort has been questioned (Bianchi et al. 2012; Chen et al. 2013). However, as pointed out in a recent review, “approximately 50 % of the molecular targets of the top 100 selling drugs have been knocked out in mouse transgenic models” and “knockout models are likely, in most cases, to provide a productive source of validated targets for future drug development” (Patapoutian et al. 2009).
8 Role in Hereditary and Acquired Diseases
8.1 Hereditary Disease
It is well known that topical skin application of TRPA1 activators can produce burning pain, mechanical and thermal hypersensitivity, and neurogenic inflammation in man (Koltzenburg et al. 1992; Namer et al. 2005; Olausson 1998). The discovery of an autosomal dominant familial episodic pain syndrome, caused by a gain-of-function mutation in TRPA1, has underscored TRPA1 as a key player in pain perception (Kremeyer et al. 2010). Affected individuals display increased hyperalgesia to punctate stimuli after mustard oil application, but are otherwise healthy except for episodes of mainly upper body pain, triggered by physical stress, such as fasting, exposure to cold, and exercise (Kremeyer et al. 2010). The mutation, which is localized to transmembrane segment 4 (N885S), alters the voltage dependence of TRPA1 and renders the channel hyperresponsive to electrophiles and cold at normal resting membrane potentials (Kremeyer et al. 2010). Paradoxical heat sensation was recently linked to a single nucleotide polymorphism in the N-terminus of TRPA1 (E179K), and HEK293T/17 cells transfected with the mutant failed to display cold sensitivity in contrast to the wild-type channel (Binder et al. 2011; May et al. 2012).
8.2 Inflammatory Disease
Animal studies have shown that TRPA1 is involved in thermal and mechanical sensitization induced by inflammation (Tables 3 and 4) (Andrade et al. 2012; Hoffmann et al. 2013). Many endogenous TRPA1 activators, such as reactive oxygen species, α,β-unsaturated aldehydes, and H2S (Table 2), that are formed during inflammation and bacterial infection may in fact drive or maintain the inflammatory process via activation of TRPA1 on sensory nerve fibers and nonneuronal cells (Andersson et al. 2012; Bautista et al. 2013; Moilanen et al. 2012). Furthermore, inflammatory mediators, such as bradykinin, trypsin, and nerve growth factor, may sensitize TRPA1 or upregulate its expression via different receptor-dependent signaling pathways, leading to hyperalgesia, allodynia, itch, urgency, and other organ-specific symptoms mediated by TRPA1 (Tables 3 and 4) (Dai et al. 2007; Diogenes et al. 2007; Ji et al. 2008; Katsura et al. 2006; Malin et al. 2011; Obata et al. 2005; Schwartz et al. 2011; Wang et al. 2008a). Inflammatory diseases where TRPA1 has been implicated in pain perception, sensory hyperreactivity, or disease progression include arthritis, asthma, dermatitis, inflammatory bowel disease, and pancreatitis (Bautista et al. 2013; Bessac and Jordt 2008; Holzer 2011; Kaneko and Szallasi 2013; Taylor-Clark and Undem 2011).
Cyclophosphamide-induced hemorrhagic cystitis is not only a feared adverse effect, but also a common animal model of interstitial cystitis/bladder pain syndrome, a chronic disease that affects mainly women. The metabolism of both cyclophosphamide and its congener ifosfamide generates the urotoxic TRPA1 activator acrolein, which produces mucosal injury when accumulating in the urine (Bautista et al. 2006). Interestingly, systemic administration of the TRPA1 blocker HC030031 effectively reduced the bladder overactivity induced by cyclophosphamide pretreatment in rats (Meotti et al. 2013).
8.3 Peripheral Neuropathy
Cold and mechanical allodynia are commonly observed in patients with peripheral neuropathy, and these abnormalities can be mimicked in various animal models of nerve injury. Overexpression of TRPA1 is frequently encountered in spared neurons adjacent to the site of injury, possibly reflecting adaptive responses to neuroinflammatory cues (Ji et al. 2008; Katsura et al. 2006; Obata et al. 2005). Interventional downregulation of TRPA1 expression and function reduces behavioral signs of cold and mechanical allodynia (Tables 3 and 4). Methylglyoxal, a potent TRPA1 activator (Table 2) produced under hyperglycemic conditions, has been implicated as a mediator of diabetic neuropathy, although other endogenous electrophilic compounds may also contribute (Moran et al. 2011). Thus, electrophilic activation of TRPA1 may drive the development of peripheral neuropathy and the associated mechanical sensitization (Tables 3 and 4). The complex neurochemistry of cancer, including trophic cues for neuronal sprouting, may create a chemical microenvironment that promotes TRPA1 expression, sensitization, and activation (Lozano-Ondoua et al. 2013; Ye et al. 2011). Many cancer chemotherapies are neurotoxic and can cause thermal and mechanical allodynia. TRPA1 together with TRPM8 and TRPV1 may contribute to such adverse effects, as demonstrated in animals exposed to different chemotherapeutic agents (Tables 3 and 4).
Altered afferent signaling is believed to play an important role in bladder overactivity, a condition that can be mimicked by intravesical administration of TRPA1 activators (Du et al. 2007; Streng et al. 2008). Bladder overactivity, characterized by urgency with or without incontinence, is commonly associated with infections, outflow obstruction (prostate enlargement), diabetes, and various neurological disorders of the central nervous system, including spinal cord injury and multiple sclerosis (Andersson et al. 2010; Birder 2013; Kanai 2011). A large number of people also suffer from overactive bladders without any obvious cause, a condition referred to as overactive bladder syndrome. In a rat model of bladder overactivity following spinal cord injury, featuring detrusor TRPA1 protein overexpression, antisense oligonucleotide-induced downregulation of TRPA1 in sensory neurons and systemic administration of the TRPA1 blocker HC030031 each reduced non-voiding bladder contractions, as demonstrated by cystometry in anesthetized animals (Andrade et al. 2011). Irritable bowel syndrome is another common illness, characterized by abdominal pain, obstipation, and diarrhea, in which TRPA1-dependent sensory dysfunction may play an important role (Hughes et al. 2013; Ryosuke et al. 2014; Yu et al. 2010).
8.4 Pharmacological Intervention
Although inhibition of TRPA1 seems the most logical treatment strategy to achieve pain relief in inflammatory and neuropathic disease, it is interesting that intraplantar, systemic, or spinal injection of TRPA1 activators can also disrupt nociceptive signaling (Andersson et al. 2011; Materazzi et al. 2013; Ryosuke et al. 2014; Weng et al. 2012). Thus, depending on the site of action and the intrinsic chemical properties of the drug, both antagonists and agonists of TRPA1 could be useful to treat various pain conditions, as shown for TRPV1 (Andersson et al. 2011; Barrière et al. 2013; Kaneko and Szallasi 2013; Mallet et al. 2010; Moran et al. 2011; Patapoutian et al. 2009; Zygmunt et al. 2013). Species differences in function and pharmacology of TRPA1 together with the lack of predictive animal models of pain have complicated the validation of TRPA1 as a drug target in man (Andrade et al. 2012; Baraldi et al. 2010; Bianchi et al. 2012; Chen and Kym 2009; Chen et al. 2008, 2013; de la Roche et al. 2013; Klionsky et al. 2007; Nyman et al. 2013; Patapoutian et al. 2009; Viana and Ferrer-Montiel 2009). A pure antagonist or agonist at TRPA1 in one species may in fact act as a partial agonist or a dual agonist and antagonist in another species (Bianchi et al. 2012; Chen et al. 2008; Klionsky et al. 2007; Xiao et al. 2008). It is, however, encouraging that the TRPA1 antagonist A-967079 is able to attenuate cold allodynia in animals without affecting noxious heat sensation and body temperature, as such unwanted adverse effects have hampered the development of TRPV1 antagonists as analgesics (Moran et al. 2011). The thermoregulatory consequences of intragastric administration of TRPA1 and TRPV1 activators are also not identical (Masamoto et al. 2009). Thus, although TRPA1 is mainly expressed together with TRPV1 on primary afferents, pharmacological interventions with TRPA1 and TRPV1 may produce very different adverse effect profiles. In order to appreciate the full potential of TRPA1 as a safe drug target, more information is needed about the role of TRPA1 in nonneuronal cells and the cardiovascular and metabolic consequences of interfering with this ion channel.
References
Akiyama T, Carstens MI, Carstens E (2010) Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol 104:2442–2450
Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583:175–193
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci 28:1064–1075
Alenmyr L, Högestätt ED, Zygmunt PM, Greiff L (2009) TRPV1-mediated itch in seasonal allergic rhinitis. Allergy 64:807–810
Alenmyr L, Herrmann A, Högestätt ED, Greiff L, Zygmunt PM (2011) TRPV1 and TRPA1 stimulation induces MUC5B secretion in the human nasal airway in vivo. Clin Physiol Funct Imaging 31:435–444
Alpizar YA, Gees M, Sanchez A, Apetrei A, Voets T, Nilius B, Talavera K (2013) Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Pflugers Arch 465:853–864
Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P (2008) TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett 438:221–227
Andersson DA, Gentry C, Moss S, Bevan S (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494
Andersson DA, Gentry C, Moss S, Bevan S (2009) Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci USA 106:8374–8379
Andersson KE, Gratzke C, Hedlund P (2010) The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 106:1114–1127
Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Högestätt ED, Zygmunt PM (2011) TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun 2:551
Andersson DA, Gentry C, Bevan S (2012) TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide. PLoS One 7:e46917
Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S (2013) Methylglyoxal evokes pain by stimulating TRPA1. PLoS One 8:e77986
Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, Koepp J, Calixto JB (2011) TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol 300:F1223–F1234
Andrade EL, Meotti FC, Calixto JB (2012) TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther 133:189–204
Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R (2008) Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118:2574–2582
Babes A, Fischer MJ, Filipovic M, Engel MA, Flonta ML, Reeh PW (2013) The anti-diabetic drug glibenclamide is an agonist of the transient receptor potential ankyrin 1 (TRPA1) ion channel. Eur J Pharmacol 704:15–22
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Bang S, Kim KY, Yoo S, Kim YG, Hwang SW (2007) Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci 26:2516–2523
Bang S, Yoo S, Oh U, Hwang SW (2010) Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res 33:1509–1520
Bang S, Yoo S, Yang TJ, Cho H, Hwang SW (2011) Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 152:1156–1164
Banke TG, Chaplan SR, Wickenden AD (2010) Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol 298:C1457–C1468
Baraldi PG, Preti D, Materazzi S, Geppetti P (2010) Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem 53:5085–5107
Barriere DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B (2012) Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain 153:553–561
Barrière DA, Mallet C, Blomgren A, Simonsen C, Daulhac L, Libert F, Chapuy E, Etienne M, Högestätt ED, Zygmunt PM, Eschalier A (2013) Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One 8:e70690
Bassoli A, Borgonovo G, Caimi S, Scaglioni L, Morini G, Moriello AS, Di Marzo V, De Petrocellis L (2009) Taste-guided identification of high potency TRPA1 agonists from Perilla frutescens. Bioorg Med Chem 17:1636–1639
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt S, Zygmunt PM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102:12248–12252
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282
Bautista DM, Pellegrino M, Tsunozaki M (2013) TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 75:181–200
Bellono NW, Oancea E (2013) UV light phototransduction depolarizes human melanocytes. Channels (Austin) 7
Bellono NW, Kammel LG, Zimmerman AL, Oancea E (2013) UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA 110:2383–2388
Belmonte C, Viana F (2008) Molecular and cellular limits to somatosensory specificity. Mol Pain 4:14
Belmonte C, Brock JA, Viana F (2009) Converting cold into pain. Exp Brain Res 196:13–30
Benemei S, Fusi C, Trevisan G, Geppetti P (2013) The TRPA1 Channel in Migraine Mechanism and Treatment. Br J Pharmacol. doi: 10.1111/bph.12512
Bessac BF, Jordt SE (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23:360–370
Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118:1899–1910
Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE (2009) Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23:1102–1114
Bianchi BR, Zhang XF, Reilly RM, Kym PR, Yao BB, Chen J (2012) Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J Pharmacol Exp Ther 341:360–368
Binder A, May D, Baron R, Maier C, Tolle TR, Treede RD, Berthele A, Faltraco F, Flor H, Gierthmuhlen J, Haenisch S, Huge V, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Uceyler N, Ufer M, Wasner G, Zhu J, Cascorbi I (2011) Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 6:e17387
Birder LA (2013) Nervous network for lower urinary tract function. Int J Urol 20:4–12
Bonet IJ, Fischer L, Parada CA, Tambeli CH (2013) The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology 65:206–212
Brenneis C, Sisignano M, Coste O, Altenrath K, Fischer MJ, Angioni C, Fleming I, Brandes RP, Reeh PW, Woolf CJ, Geisslinger G, Scholich K (2011) Soluble epoxide hydrolase limits mechanical hyperalgesia during inflammation. Mol Pain 7:78
Brenneis C, Kistner K, Puopolo M, Jo S, Roberson D, Sisignano M, Segal D, Cobos EJ, Wainger BJ, Labocha S, Ferreiros N, von Hehn C, Tran J, Geisslinger G, Reeh PW, Bean BP, Woolf CJ (2014) Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block. Br J Pharmacol 171(2):438–451
Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA (2009) The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137:2084.e3–2095.e3
Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA (2011) TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 589:3575–3593
Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, Gijsen HJ (2008) Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol 231:150–156
Buch TR, Schafer EA, Demmel MT, Boekhoff I, Thiermann H, Gudermann T, Steinritz D, Schmidt A (2013) Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem Biol Interact 206(3):462–471
Cao DS, Zhong L, Hsieh TH, Abooj M, Bishnoi M, Hughes L, Premkumar LS (2012) Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One 7:e38005
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW (2010) Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol 298:G81–G91
Cavanaugh EJ, Simkin D, Kim D (2008) Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience 154:1467–1476
Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M (2014) A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 133:448–460
Chen J, Kym PR (2009) TRPA1: the species difference. J Gen Physiol 133:623–625
Chen J, Zhang XF, Kort ME, Huth JR, Sun C, Miesbauer LJ, Cassar SC, Neelands T, Scott VE, Moreland RB, Reilly RM, Hajduk PJ, Kym PR, Hutchins CW, Faltynek CR (2008) Molecular determinants of species-specific activation or blockade of TRPA1 channels. J Neurosci 28:5063–5071
Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, Reilly RM (2009) Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain 5:3
Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, Bianchi BR, Baker SJ, Zhong C, Simler GH, McDonald HA, Schmidt RG, McGaraughty SP, Chu KL, Faltynek CR, Kort ME, Reilly RM, Kym PR (2011a) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152:1165–1172
Chen Y, Yang C, Wang ZJ (2011b) Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 193:440–451
Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D (2013) Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4:2501
Cho JH, Jeong MY, Choi IS, Lee HJ, Jang IS (2012) TRPA1-like channels enhance glycinergic transmission in medullary dorsal horn neurons. J Neurochem 122:691–701
Chung S, Kim YH, Koh JY, Nam TS, Ahn DS (2011) Intracellular acidification evoked by moderate extracellular acidosis attenuates transient receptor potential V1 (TRPV1) channel activity in rat dorsal root ganglion neurons. Exp Physiol 96:1270–1281
Cordero-Morales JF, Gracheva EO, Julius D (2011) Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci USA 108:E1184–E1191
Corey D, Garcia-Anoveros J, Holt J, Kwan K, Lin S, Vollrath M, Amalfitano A, Cheung E, Derfler B, Duggan A, Geleoc G, Gray P, Hoffman M, Rehm H, Tamasauskas D, Zhang D (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730
Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, Kim D, Story GM (2008) Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain 4:30
Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY (2011) Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem 286:38168–38176
da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB (2010) The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148:431–437
Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117:1979–1987
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187
de la Roche J, Eberhardt MJ, Klinger AB, Stanslowsky N, Wegner F, Koppert W, Reeh PW, Lampert A, Fischer MJ, Leffler A (2013) The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J Biol Chem 288:20280–20292
De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015
Deanfield JE, Halcox JP, Rabelink TJ (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295
del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM (2010) TRPA1 contributes to cold hypersensitivity. J Neurosci 30:15165–15174
Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 3:266–278
Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29:153–158
Diogenes A, Akopian AN, Hargreaves KM (2007) NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 86:550–555
Doerner JF, Gisselmann G, Hatt H, Wetzel CH (2007) Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282:13180–13189
Du S, Araki I, Yoshiyama M, Nomura T, Takeda M (2007) Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology 70:826–831
Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, Shi R (2013) Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem. doi: 10.1111/jnc.12500
Earley S (2012) TRPA1 channels in the vasculature. Br J Pharmacol 167:13–22
Eberhardt MJ, Filipovic MR, Leffler A, de la Roche J, Kistner K, Fischer MJ, Fleming T, Zimmermann K, Ivanovic-Burmazovic I, Nawroth PP, Bierhaus A, Reeh PW, Sauer SK (2012) Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J Biol Chem 287:28291–28306
Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO (2008) HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain 4:48
Eilers H, Cattaruzza F, Nassini R, Materazzi S, Andre E, Chu C, Cottrell GS, Schumacher M, Geppetti P, Bunnett NW (2010) Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology 112:1452–1463
Escalera J, von Hehn CA, Bessac BF, Sivula M, Jordt SE (2008) TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem 283:24136–24144
Fajardo O, Meseguer V, Belmonte C, Viana F (2008a) TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci 28:7863–7875
Fajardo O, Meseguer V, Belmonte C, Viana F (2008b) TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin) 2:429–438
Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD (2011) A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum 63:819–829
Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, Aubdool AA, Liang L, Bodkin JV, Bevan S, Heads R, Brain SD (2013) Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B(4)-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J 27:1664–1673
Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C (2010) The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem 285:34781–34792
Forster AB, Reeh PW, Messlinger K, Fischer MJ (2009) High concentrations of morphine sensitize and activate mouse dorsal root ganglia via TRPV1 and TRPA1 receptors. Mol Pain 5:17
Fujita F, Moriyama T, Higashi T, Shima A, Tominaga M (2007) Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol 151:153–160
Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M (2008) Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118:4049–4057
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Gaudet R (2008) A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst 4:372–379
Gentry C, Stoakley N, Andersson DA, Bevan S (2010) The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain 6:4
Gijsen HJ, Berthelot D, Zaja M, Brone B, Geuens I, Mercken M (2010) Analogues of morphanthridine and the tear gas dibenz[b, f][1,4]oxazepine (CR) as extremely potent activators of the human transient receptor potential ankyrin 1 (TRPA1) channel. J Med Chem 53:7011–7020
Graepel R, Fernandes ES, Aubdool AA, Andersson DA, Bevan S, Brain SD (2011) 4-oxo-2-nonenal (4-ONE): evidence of transient receptor potential ankyrin 1-dependent and -independent nociceptive and vasoactive responses in vivo. J Pharmacol Exp Ther 337:117–124
Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL (2012) Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA 109:6721–6726
Hill K, Schaefer M (2007) TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J Biol Chem 282:7145–7153
Hinman A, Chuang HH, Bautista DM, Julius D (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103:19564–19568
Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M (2007) Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 27:2435–2443
Hoffmann T, Kistner K, Miermeister F, Winkelmann R, Wittmann J, Fischer MJ, Weidner C, Reeh PW (2013) TRPA1 and TRPV1 are differentially involved in heat nociception of mice. Eur J Pain 17:1472–1482
Högestätt ED, Johansson R, Andersson DA, Zygmunt PM (2000) Involvement of sensory nerves in vasodilator responses to acetylcholine and potassium ions in rat hepatic artery. Br J Pharmacol 130:27–32
Holzer P (2011) Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther 131:142–170
Howard J, Bechstedt S (2004) Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol 14:R224–R226
Hsu CC, Lin RL, Lee LY, Lin YS (2013) Hydrogen sulfide induces hypersensitivity of rat capsaicin-sensitive lung vagal neurons: role of TRPA1 receptors. Am J Physiol Regul Integr Comp Physiol 305:R769–R779
Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, Wood JD, Zhu MX (2010) Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch 459:579–592
Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A (2009) Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 5:183–190
Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC (2010) Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol 20:189–197
Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, Andrews JM, Holtmann G, Blackshaw LA, Brierley SM (2013) Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut 62:1456–1465
Ibarra Y, Blair NT (2013) Benzoquinone reveals a cysteine-dependent desensitization mechanism of TRPA1. Mol Pharmacol 83:1120–1132
Jaquemar D, Schenker T, Trueb B (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 274:7325–7333
Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS (2009) Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One 4:e7021
Ji G, Zhou S, Carlton SM (2008) Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience 154:1054–1066
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Kanai AJ (2011) Afferent mechanism in the urinary tract. Handb Exp Pharmacol 202:171–205
Kaneko Y, Szallasi A (2013) Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol. doi: 10.1111/bph.12414
Kang K, Pulver S, Panzano V, Chang E, Griffith L, Theobald D, Garrity P (2010) Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464:597–600
Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B (2007) Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 27:9874–9884
Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B (2008) Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch 457:77–89
Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 106:1273–1278
Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B (2010) Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys J 98:773–783
Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K (2006) Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 200:112–123
Kerstein PC, del Camino D, Moran MM, Stucky CL (2009) Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain 5:19
Kichko TI, Lennerz J, Eberhardt M, Babes RM, Neuhuber W, Kobal G, Reeh PW (2013) Bimodal concentration-response of nicotine involves the nicotinic acetylcholine receptor, transient receptor potential vanilloid type 1, and transient receptor potential ankyrin 1 channels in mouse trachea and sensory neurons. J Pharmacol Exp Ther 347:529–539
Kim D, Cavanaugh EJ (2007) Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci 27:6500–6509
Kim D, Cavanaugh EJ, Simkin D (2008) Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol 295:C92–C99
Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC (2010) Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol 518:687–698
Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR (2007) Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci 10:568–577
Klement G, Eisele L, Malinowsky D, Nolting A, Svensson M, Terp G, Weigelt D, Dabrowski M (2013) Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore. Biophys J 104:798–806
Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR (2007) Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain 3:39
Koch M, Kreutz S, Bottger C, Grabiec U, Ghadban C, Korf HW, Dehghani F (2011) The cannabinoid WIN 55,212-2-mediated protection of dentate gyrus granule cells is driven by CB1 receptors and modulated by TRPA1 and Cav 2.2 channels. Hippocampus 21:554–564
Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Akerman KE, Lindstedt K, Pertovaara A (2012) Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res 65:149–158
Koltzenburg M, Lundberg LE, Torebjörk HE (1992) Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51:207–219
Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, Noguchi K (2009) Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut 58:1342–1352
Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E (2007) Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci 27:4443–4451
Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, Villegas A, Acosta N, Pineda-Trujillo NG, Ramirez JD, Zea J, Burley MW, Bedoya G, Bennett DL, Wood JN, Ruiz-Linares A (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66:671–680
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289
Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL (2009) TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29:4808–4819
La JH, Schwartz ES, Gebhart GF (2011) Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K + channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience 186:179–187
Lanosa MJ, Willis DN, Jordt S, Morris JB (2010) Role of metabolic activation and the TRPA1 receptor in the sensory irritation response to styrene and naphthalene. Toxicol Sci 115:589–595
Lee I, Kim HK, Kim JH, Chung K, Chung JM (2007) The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 133:9–17
Lee SP, Buber MT, Yang Q, Cerne R, Cortes RY, Sprous DG, Bryant RW (2008) Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol 153:1739–1749
Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC (2012) An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45:45–49
Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C (2008) The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest 118:763–776
Lennertz RC, Kossyreva EA, Smith AK, Stucky CL (2012) TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One 7:e43597
Li L, Rose P, Moore PK (2011) Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187
Liu T, Ji RR (2012) Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull 28:145–154
Liu K, Samuel M, Ho M, Harrison RK, Paslay JW (2010) NPPB structure-specifically activates TRPA1 channels. Biochem Pharmacol 80:113–121
Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE (2013) TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27:3549–3563
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557 Pt A:52–59
Lumpkin EA, Caterina MJ (2007) Mechanisms of sensory transduction in the skin. Nature 445:858–865
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A (2007a) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545
Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A (2007b) An ion channel essential for sensing chemical damage. J Neurosci 27:11412–11415
Maggi CA (1995) Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol 45:1–98
Maher M, Ao H, Banke T, Nasser N, Wu NT, Breitenbucher JG, Chaplan SR, Wickenden AD (2008) Activation of TRPA1 by farnesyl thiosalicylic acid. Mol Pharmacol 73:1225–1234
Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM (2011) TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 31:10516–10528
Mallet C, Barriere DA, Ermund A, Jönsson BA, Eschalier A, Zygmunt PM, Högestätt ED (2010) TRPV(1) in brain is involved in acetaminophen-induced antinociception. PLoS One 5:e12748
Masamoto Y, Kawabata F, Fushiki T (2009) Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci Biotechnol Biochem 73:1021–1027
Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P (2008) Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 105:12045–12050
Materazzi S, Fusi C, Benemei S, Pedretti P, Patacchini R, Nilius B, Prenen J, Creminon C, Geppetti P, Nassini R (2012) TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch 463:561–569
Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, Andersson DA, Trevisan G, Moncelli MR, Wei X, Dussor G, Pollastro F, Patacchini R, Appendino G, Geppetti P, Nassini R (2013) Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 154:2750–2758
Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP (2008) General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA 105:8784–8789
May D, Baastrup J, Nientit MR, Binder A, Schunke M, Baron R, Cascorbi I (2012) Differential expression and functionality of TRPA1 protein genetic variants in conditions of thermal stimulation. J Biol Chem 287:27087–27094
McClenaghan C, Zeng F, Verkuyl JM (2012) TRPA1 agonist activity of probenecid desensitizes channel responses: consequences for screening. Assay Drug Dev Technol 10:533–541
McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR (2010) TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain 6:14
McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:13525–13530
Meotti FC, Forner S, Lima-Garcia JF, Viana AF, Calixto JB (2013) Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact 203:440–447
Meseguer V, Karashima Y, Talavera K, D’Hoedt D, Donovan-Rodriguez T, Viana F, Nilius B, Voets T (2008) Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J Neurosci 28:576–586
Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ (2012) CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache 52:1411–1427
Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A (2009) TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 4:e7596
Mogil JS (2009) Animal models of pain: progress and challenges. Nat Rev Neurosci 10:283–294
Moilanen LJ, Laavola M, Kukkonen M, Korhonen R, Leppanen T, Högestätt ED, Zygmunt PM, Nieminen RM, Moilanen E (2012) TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep 2:380
Momin A, Wood JN (2008) Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr Opin Neurobiol 18:383–388
Moran MM, McAlexander MA, Biro T, Szallasi A (2011) Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 10:601–620
Motter AL, Ahern GP (2012) TRPA1 is a polyunsaturated fatty acid sensor in mammals. PLoS One 7:e38439
Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, Sterner O, Zygmunt PM, Högestätt ED (2005) Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem 280:38496–38504
Nagata K, Duggan A, Kumar G, Garcia-Anoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25:4052–4061
Nagatomo K, Kubo Y (2008) Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci USA 105:17373–17378
Namer B, Seifert F, Handwerker HO, Maihofner C (2005) TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport 16:955–959
Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M (2008) Separate peripheral pathways for pruritus in man. J Neurophysiol 100:2062–2069
Narukawa M, Koizumi K, Iwasaki Y, Kubota K, Watanabe T (2010) Galangal pungent component, 1′-acetoxychavicol acetate, activates TRPA1. Biosci Biotechnol Biochem 74:1694–1696
Nassini R (2010) Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J 24:4904–4916
Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P (2011) Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152:1621–1631
Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, la Marca G, Andre E, Preti D, Avonto C, Sadofsky L, Di Marzo V, De Petrocellis L, Dussor G, Porreca F, Taglialatela-Scafati O, Appendino G, Nilius B, Geppetti P (2012) The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 135:376–390
Nativi C, Gualdani R, Dragoni E, Di Cesare Mannelli L, Sostegni S, Norcini M, Gabrielli G, la Marca G, Richichi B, Francesconi O, Moncelli MR, Ghelardini C, Roelens S (2013) A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci Rep 3:2005
Niforatos W, Zhang XF, Lake MR, Walter KA, Neelands T, Holzman TF, Scott VE, Faltynek CR, Moreland RB, Chen J (2007) Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597). Mol Pharmacol 71:1209–1216
Nilius B, Appendino G (2013) Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol 164:1–76
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12:218
Nilius B, Prenen J, Owsianik G (2011) Irritating channels: the case of TRPA1. J Physiol 589:1543–1549
Nilius B, Appendino G, Owsianik G (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 464:425–458
Noel J, Sandoz G, Lesage F (2011) Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 5:402–409
Nyman E, Franzen B, Nolting A, Klement G, Liu G, Nilsson M, Rosen A, Bjork C, Weigelt D, Wollberg P, Karila P, Raboisson P (2013) In vitro pharmacological characterization of a novel TRPA1 antagonist and proof of mechanism in a human dental pulp model. J Pain Res 6:59–70
Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115:2393–2401
Ogawa H, Takahashi K, Miura S, Imagawa T, Saito S, Tominaga M, Ohta T (2012) H(2)S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience 218:335–343
Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T (2013) TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol 191(11):5371–5382
Ohkawara S, Tanaka-Kagawa T, Furukawa Y, Jinno H (2012) Methylglyoxal activates the human transient receptor potential ankyrin 1 channel. J Toxicol Sci 37:831–835
Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, Okawa Y, Sekiguchi F, Kawabata A (2012) Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both Cav3.2 and TRPA1 channels in mice. Br J Pharmacol 166:1738–1743
Okumura Y, Narukawa M, Iwasaki Y, Ishikawa A, Matsuda H, Yoshikawa M, Watanabe T (2010) Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem 74:1068–1072
Olausson B (1998) Recordings of human polymodal single C-fiber afferents following mechanical and argon-laser heat stimulation of inflamed skin. Exp Brain Res 122:55–61
Panzano VC, Kang K, Garrity PA (2010) Infrared snake eyes: TRPA1 and the thermal sensitivity of the snake pit organ. Sci Signal 3:pe22
Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR (2011) Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 31:18433–18438
Patapoutian A, Tate S, Woolf CJ (2009) Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 8:55–68
Pereira LM, Lima-Junior RC, Bem AX, Teixeira CG, Grassi LS, Medeiros RP, Marques-Neto RD, Callado RB, Aragao KS, Wong DV, Vale ML, Brito GA, Ribeiro RA (2013) Blockade of TRPA1 with HC-030031 attenuates visceral nociception by a mechanism independent of inflammatory resident cells, nitric oxide and the opioid system. Eur J Pain 17:223–233
Petersson J, Zygmunt PM, Brandt L, Högestätt ED (1995) Substance P-induced relaxation and hyperpolarization in human cerebral arteries. Br J Pharmacol 115:889–894
Petersson J, Zygmunt PM, Högestätt ED (1997) Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br J Pharmacol 120:1344–1350
Petersson J, Zygmunt PM, Jönsson P, Högestätt ED (1998) Characterization of endothelium-dependent relaxation in guinea pig basilar artery—effect of hypoxia and role of cytochrome P450 monooxygenase. J Vasc Res 35:285–294
Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 3:40
Peyrot des Gachons C, Uchida K, Bryant B, Shima A, Sperry JB, Dankulich-Nagrudny L, Tominaga M, Smith AB 3rd, Beauchamp GK, Breslin PA (2011) Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci 31:999–1009
Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemeny A, Materazzi S, Nassini R, Helyes Z, Szolcsanyi J, Pinter E (2012) The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol 689:56–64
Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ (2008) Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol 181:5024–5034
Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R (2006) Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol 16:1591–1605
Raisinghani M, Zhong L, Jeffry JA, Bishnoi M, Pabbidi RM, Pimentel F, Cao DS, Evans MS, Premkumar LS (2011) Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am J Physiol Cell Physiol 301:C587–C600
Riera CE, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, Vogel H, Simon SA, le Coutre J (2009) Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol 157:1398–1409
Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ (2013) Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 16:910–918
Ross SE (2011) Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 21:880–887
Ryosuke K, Katsura N, Hitoshi D, Yoshihiro K, Hidetaka K, Toshihide Y, Hiroyuki I (2014) Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur J Pharmacol 723:288–293
Sadofsky LR, Boa AN, Maher SA, Birrell MA, Belvisi MG, Morice AH (2011) TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids. Pharmacol Res 63:30–36
Salas MM, Hargreaves KM, Akopian AN (2009) TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29:1568–1578
Samad A, Sura L, Benedikt J, Ettrich R, Minofar B, Teisinger J, Vlachova V (2011) The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem J 433:197–204
Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S (2007) Cold sensitivity of recombinant TRPA1 channels. Brain Res 1160:39–46
Sawada Y, Hosokawa H, Matsumura K, Kobayashi S (2008) Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci 27:1131–1142
Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE (1997) Specific C-receptors for itch in human skin. J Neurosci 17:8003–8008
Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A (2009) Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64:498–509
Schulze A, Oehler B, Urban N, Schaefer M, Hill K (2013) Apomorphine is a bimodal modulator of TRPA1 channels. Mol Pharmacol 83:542–551
Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF (2011) Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 140:1283–1291
Sculptoreanu A, Kullmann FA, Artim DE, Bazley FA, Schopfer F, Woodcock S, Freeman BA, de Groat WC (2010) Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. J Pharmacol Exp Ther 333:883–895
Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80
Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33:10143–10153
Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, Yazawa S, Tominaga M (2012) Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol 165:1476–1486
Sidi S, Friedrich RW, Nicolson T (2003) NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301:96–99
Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, Cobos EJ, Ghasemlou N, Xu ZZ, Kumaran V, Lu R, Grant A, Fischer MJ, Schmidtko A, Reeh P, Ji RR, Woolf CJ, Geisslinger G, Scholich K, Brenneis C (2012) 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J Neurosci 32:6364–6372
Spahn V, Stein C, Zollner C (2013) Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol Pharmacol 85(2):335–344
Staruschenko A, Jeske NA, Akopian AN (2010) Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285:15167–15177
Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H (2006) TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal 18(10):1584–1594
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE (2008) Citral sensing by transient [corrected] receptor potential channels in dorsal root ganglion neurons. PLoS One 3:e2082
Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Högestätt ED, Zygmunt PM (2008) Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53:391–399
Sura L, Zima V, Marsakova L, Hynkova A, Barvik I, Vlachova V (2012) C-terminal acidic cluster is involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J Biol Chem 287:18067–18077
Takahashi N, Mori Y (2011) TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol 2:58
Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y (2008) Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2:287–298
Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y (2011) TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7:701–711
Takahashi N, Kozai D, Mori Y (2012) TRP channels: sensors and transducers of gasotransmitter signals. Front Physiol 3:324
Takaishi M, Uchida K, Fujita F, Tominaga M (2013) Inhibitory effects of monoterpenes on human TRPA1 and the structural basis of their activity. J Physiol Sci 64(1):47–57
Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T (2009) Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12:1293–1299
Tang G, Yang G, Jiang B, Ju Y, Wu L, Wang R (2013) H2S Is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 19:1634–1646
Taylor-Clark TE, Undem BJ (2010) Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol 588:423–433
Taylor-Clark TE, Undem BJ (2011) Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178:406–413
Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ (2008a) Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586:3447–3459
Taylor-Clark TE, Undem BJ, Macglashan DW Jr, Ghatta S, Carr MJ, McAlexander MA (2008b) Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73:274–281
Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ (2009a) Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol 75:820–829
Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA (2009b) Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol 40:756–762
Trevisan G, Materazzi S, Fusi C, Altomare A, Aldini G, Lodovici M, Patacchini R, Geppetti P, Nassini R (2013) Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res 73:3120–3131
Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104:13519–13524
Tsubota-Matsunami M, Noguchi Y, Okawa Y, Sekiguchi F, Kawabata A (2012) Colonic hydrogen sulfide-induced visceral pain and referred hyperalgesia involve activation of both Ca(v)3.2 and TRPA1 channels in mice. J Pharmacol Sci 119:293–296
Uta D, Furue H, Pickering AE, Rashid MH, Mizuguchi-Takase H, Katafuchi T, Imoto K, Yoshimura M (2010) TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci 31:1960–1973
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Vennekens R, Menigoz A, Nilius B (2012) TRPs in the brain. Rev Physiol Biochem Pharmacol 163:27–64
Vetter I, Touska F, Hess A, Hinsbey R, Sattler S, Lampert A, Sergejeva M, Sharov A, Collins LS, Eberhardt M, Engel M, Cabot PJ, Wood JN, Vlachova V, Reeh PW, Lewis RJ, Zimmermann K (2012) Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J 31:3795–3808
Viana F, Ferrer-Montiel A (2009) TRPA1 modulators in preclinical development. Expert Opin Ther Pat 19:1787–1799
Vilceanu D, Stucky CL (2010) TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One 5:e12177
Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754
Walker RG, Willingham AT, Zuker CS (2000) A Drosophila mechanosensory transduction channel. Science 287:2229–2234
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896
Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K (2008a) Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 131:1241–1251
Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER (2008b) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283:32691–32703
Wang YY, Chang RB, Liman ER (2010) TRPA1 is a component of the nociceptive response to CO2. J Neurosci 30:12958–12963
Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER (2011) A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol 137:493–505
Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY (2012) Identification of in vivo disulfide conformation of TRPA1 ion channel. J Biol Chem 287:6169–6176
Wang H, Schupp M, Zurborg S, Heppenstall PA (2013) Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J Physiol 591:185–201
Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A (2009) Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology 111:147–154
Wei H, Chapman H, Saarnilehto M, Kuokkanen K, Koivisto A, Pertovaara A (2010a) Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology 58:578–584
Wei H, Koivisto A, Pertovaara A (2010b) Spinal TRPA1 ion channels contribute to cutaneous neurogenic inflammation in the rat. Neurosci Lett 479:253–256
Wei H, Koivisto A, Saarnilehto M, Chapman H, Kuokkanen K, Hao B, Huang JL, Wang YX, Pertovaara A (2011) Spinal transient receptor potential ankyrin 1 channel contributes to central pain hypersensitivity in various pathophysiological conditions in the rat. Pain 152:582–591
Wei H, Karimaa M, Korjamo T, Koivisto A, Pertovaara A (2012) Transient receptor potential ankyrin 1 ion channel contributes to guarding pain and mechanical hypersensitivity in a rat model of postoperative pain. Anesthesiology 117:137–148
Wei H, Saarnilehto M, Falck L, Viisanen H, Lasierra M, Koivisto A, Pertovaara A (2013a) Spinal transient receptor potential ankyrin 1 channel induces mechanical hypersensitivity, increases cutaneous blood flow, and mediates the pronociceptive action of dynorphin A. J Physiol Pharmacol 64:331–340
Wei H, Viisanen H, Amorim D, Koivisto A, Pertovaara A (2013b) Dissociated modulation of conditioned place-preference and mechanical hypersensitivity by a TRPA1 channel antagonist in peripheral neuropathy. Pharmacol Biochem Behav 104:90–96
Weller K, Reeh PW, Sauer SK (2011) TRPV1, TRPA1, and CB1 in the isolated vagus nerve–axonal chemosensitivity and control of neuropeptide release. Neuropeptides 45:391–400
Weng Y, Batista PA, Barabas ME, Harris EQ, Dinsmore TB, Kossyreva EA, Foshage AM, Wang MH, Schwab MJ, Wang VM, Stucky CL, Story GM (2012) Prostaglandin metabolite induces inhibition of TRPA1 and channel-dependent nociception. Mol Pain 8:75
Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14:595–602
Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM (2013) The ion channel TRPA1 is required for chronic itch. J Neurosci 33:9283–9294
Wrigley PJ, Jeong HJ, Vaughan CW (2009) Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol 157:371–380
Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A (2008) Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci 28:9640–9651
Xu H, Delling M, Jun JC, Clapham DE (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635
Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L (2008) Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett 440:237–241
Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, Gibbs JL, Schmidt BL (2011) Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther 10:1667–1676
Yokoyama T, Ohbuchi T, Saito T, Sudo Y, Fujihara H, Minami K, Nagatomo T, Uezono Y, Ueta Y (2011) Allyl isothiocyanates and cinnamaldehyde potentiate miniature excitatory postsynaptic inputs in the supraoptic nucleus in rats. Eur J Pharmacol 655:31–37
Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ (2010) Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res 35:797–803
Yue HY, Jiang CY, Fujita T, Kumamoto E (2013) Zingerone enhances glutamatergic spontaneous excitatory transmission by activating TRPA1 but not TRPV1 channels in the adult rat substantia gelatinosa. J Neurophysiol 110:658–671
Zhang X, Li L, McNaughton PA (2008a) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461
Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR (2008b) Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci 27:605–611
Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, Xian M (2014) Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl 53:575–581
Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S (2012) Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain 8:55
Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B (2011a) Umbellulone modulates TRP channels. Pflugers Arch 462:861–870
Zhong J, Pollastro F, Prenen J, Zhu Z, Appendino G, Nilius B (2011b) Ligustilide: a novel TRPA1 modulator. Pflugers Arch 462:841–849
Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279
Zygmunt PM (2011) Channels: a TR(i)P in the air. Nat Chem Biol 7:661–663
Zygmunt PM, Högestätt ED (1996) Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br J Pharmacol 117:1600–1606
Zygmunt PM, Grundemar L, Högestätt ED (1994a) Endothelium-dependent relaxation resistant to N omega-nitro-L-arginine in the rat hepatic artery and aorta. Acta Physiol Scand 152:107–114
Zygmunt PM, Waldeck K, Högestätt ED (1994b) The endothelium mediates a nitric oxide-independent hyperpolarization and relaxation in the rat hepatic artery. Acta Physiol Scand 152:375–384
Zygmunt PM, Plane F, Paulsson M, Garland CJ, Högestätt ED (1998) Interactions between endothelium-derived relaxing factors in the rat hepatic artery: focus on regulation of EDHF. Br J Pharmacol 124:992–1000
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgard M, Di Marzo V, Julius D, Högestätt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457
Zygmunt PM, Chuang H, Movahed P, Julius D, Högestätt ED (2000) The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 396:39–42
Zygmunt PM, Andersson DA, Högestätt ED (2002) Δ9-Tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci 22:4720–4727
Zygmunt PM, Ermund A, Movahed P, Andersson DA, Simonsen C, Jönsson BAG, Blomgren A, Birnir B, Bevan S, Eschlier A, Mallet C, Gomis A, Högestätt ED (2013) Monoacylglycerols activate TRPV1—a link between phospholipase C and TRPV1. PLoS One 8:e81618
Acknowledgment
We would like to thank Swedish Research Council (2010-3347 and 2010-5787) and Lund University for their fundings.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zygmunt, P.M., Högestätt, E.D. (2014). TRPA1. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 222. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54215-2_23
Download citation
DOI: https://doi.org/10.1007/978-3-642-54215-2_23
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54214-5
Online ISBN: 978-3-642-54215-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)