Abstract
Sugar is the most important food supplement of our daily diet. During the production of sugar, large volume of water is used by sugar mills for processing, and produces large amount of wastewater. The sugar mill wastewater have color, organic compounds, low pH, high temperature, BOD, COD, total dissolve solid (TDS), sludge, press mud and bagasse etc. If this wastewater is released in the environment before the treatment, it will cause harmful effect on aquatic life, animals, plants, human being and also change the soil properties. Therefore, it is necessary to treat the wastewater before their disposal. Three important treatment methods i.e. physical, chemical and biological are employed to treat the wastewater. Biological treatment of sugar mill wastewater has several significant advantages over other available methods. Treatment of sugar mill wastewater mainly affected by pH and temperature of effluents, biomass during the reaction, reaction time, type and speed of reactions, aerobic or anaerobic conditions, presence of catalyst, inhibitor, nutrients and concentration of the sulfide and its other compound in the wastewater. The treated wastewater can be reused in the industry for processing and may also be used for ferti-irrigation for agriculture or other purposes like compost and biofertilizers within the limit prescribed by the Central Pollution Control Board. Reuse of treated effluent can reduce the fresh water demand in various sectors. Treated effluent contains well balanced chemicals with low toxic metal ion. The diluted treated effluent have shown significant increase in chlorophyll, carotenoids, total sugar, amino acids, protein contents and suitable for seed germination and seedling growth over the bore well water and undiluted treated effluent.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
As world population growing fast, requires increased demand for food leading to massive increase in both agricultural and industrial practices. Rapid industrialization without sufficient environmental safety measures lead to pollution of water, which results in lack of good quality water both for irrigation and drinking purposes. Every human civilization, whether urban or rural require technological advancement resulting the releases of some kind of waste products which ultimately affects the normal functioning of the ecosystem and has undesirable effect on plants, animals and humans. Sugar is obtained from sugarcane crops and it is one of the most important parts of human diet and it is vital product of the human life. Bagasse, a byproduct of sugarcane used to provides energy in the form of fuel for the generation of steam and electricity (Renouf et al. 2008). Worldwide bagasse is used as energy resource in 80 sugarcane producing countries (Botha and Blottnitz 2006). Initially, sugar mills produced only sugar but presently they are also involved in the production of electricity and ethanol. Hence, sugar industry is now called as the cane industry (Ramjeawon 2008). Globally, the top 5 countries as India, Brazil, Thailand, Australia, and China (Table 7.1) accounted for nearly 40% of the total world sugar production. Sugar crops are cultivated in approximately 115 countries of the world. The sources of sugar produced by these countries are different, for example, out of 115 countries, 67 countries produced sugar from sugarcane, 39 countries from sugar beets and 9 countries from sugarcane as well as sugar beets (Lichts 2007). It is better to say that the sugar is produced 70% from sugarcane and 30% from sugar beet and cassava etc. (Contreras et al. 2009). Worldwide, Brazil and India are positioned at first and second rank in sugarcane production countries, respectively. Both are producing 275 million tons of sugarcane annually (Prakash et al. 1998). Sugar mills are basically seasonal in nature and operate only for 150–210 days in a year (November to May) (Kolhe et al. 2000). Considerably large amount of wastewater is produced during the production of sugar. Wastewater from these mills contains a moderate amount of pollution load including suspended solids, dissolved solids, organic matter etc. A number of chemicals that are used in sugar industries mainly for removal of impurities and refining of end products are also present in wastewater. The waste water discharged from sugar mill not only offers potent threat to the environmental quality but also possess energy value which is not fully utilized despite the fact that they are cheap and abundant in most parts of the world.
Water is essential for all living organisms because it plays a most valuable and important role in the natural metabolic cycles. It is believed that among the whole water availability, only 3% fresh water is available on the earth for drinking and irrigation purposes. Industries and domestic or anthropogenic activities facilitate the entry of significant amount of pollutants in the available fresh water sources. In the present scenario of conservation policies plays a significant role in the conservation of fresh water bodies as well as water quality. Large amount of fresh water is used for the production purposes which is stored in the industry for continuous supply of water. It is estimated that the amount of consumption of fresh water is equal to the amount of discharge of wastewater as effluent (Kumar and Srikantaswamy 2015). Rapid urbanization and industrialization leads to severe problems in collection, treatment and disposal of effluents in the many developing countries. Untreated organic waste portions from industries, municipalities and agricultural area are naturally decomposed in the environment ensuing large scale contamination of water, air and land imposing serious public health problems and environmental degradation. Most of sugar mills in developing countries release their wastewater without proper treatment due to lack of knowledge, financial support and sometimes unwillingness to spend on treatment of wastewater. However awareness of environmental problems and the potential hazards caused by sugar mill wastewater has endorsed by many countries to prevent the discharge of wastewater.
7.2 Physico-chemical Properties of Sugar Mill Wastewater
The physico-chemical analysis showed that the sugar mill wastewater is acidic in nature and have dark yellowish color. It is very rich in total suspended and dissolved solids with large amount of Biological oxygen demand (BOD) and Chemical oxygen demand (COD) with low dissolved oxygen (DO). Higher concentration of chloride, sodium, potassium, calcium, magnesium, iron, sulfur and their oxidized and reduced compound is also present in the released sugar mill wastewater. These effluents severely affected the plants, soil microbes, soil properties and texture when used for irrigation purposes. The wastewater or spillage products from such mills include massive quantity of dissolved organic matter, heavy metals along with other hazardous pollutants. Their discharge into fresh water bodies affects the aquatic life severely with decrease in the quality of water and irrigation land. The unmanaged sugar mill wastewater could possibly lead to soil deterioration and low productivity. The pollution standards specify that BOD of wastewater should be less than 30 mg/l for disposal into inland surface waters and less than 100 mg/l for disposal on land. Whereas, the sugar mill wastewater has a BOD of 1000–1500 mg/l (Amin et al. 2010).
In the developing countries sugar mill wastewater is used as fertilizer and has gained much importance, considered as a good source of organic matter along with plant nutrients, and also serves as good fertilizer. Sugar mill wastewater holds considerable amount of potentially harmful stuffs including soluble salts and heavy metals such as iron, copper, zinc, manganese and lead etc. (Vermeulen and Vawada 2008). The continuous application of sugar mill wastewater for irrigation objectives contaminates soil to such an extent that it becomes toxic to plants and soil (Fakayode 2005). The effects vary from crop to crop because each plant species has its own tolerance of the different wastewater concentrations. According to the permissible limit suggested by Bureau of Indian Standards (BIS), the physico-chemical parameters of sugar mill wastewater are temperature (30 °C), turbidity (84.7 NTU), pH (8.1), electrical conductivity (5530 dSm), chloride (1894 mg/l), total alkalinity (254 mg/l), total hardness (342 mg/l), BOD (6856 mg/l), COD (7432 mg/l), total dissolved solids (2516 mg/l), sulphate (540 mg/l), phosphate (224 mg/l), total acidity (45 mg/l), calcium (364 mg/l) and magnesium (151 mg/l), but almost all the water quality parameters in the sugar wastewater have been found to be very high and well above the permissible limits (Shivappa et al. 2007).
The physico-chemical characteristic (Table 7.2) such as silt, clay, water holding capacity, electrical conductivity, organic matter, total nitrogen contents and microbial population were significantly higher in the samples collected from sugar industry wastewater dump sites (Nagaraju et al. 2009). Analysis of sugar mill effluents and soil samples had shown high metal content than the permissible limits except lead. Further, analysis of plant samples have indicated the maximum accumulation of iron followed by manganese and zinc in root, shoot, leaves and seeds of mustard and wheat. The above mentioned physical and chemical characteristics of sugar mill wastewater make it useless for drinking and other purposes thus it is necessary to treat this wastewater before release into environment to minimize its harmful effects. In view of this, scientist searches the treatment methods which are cost effective and efficient.
7.3 Eco-toxicological Concerns of Sugar Mill Wastewater
In the present scenario immense concern has been given throughout the globe regarding the environmental pollution. Sugar mills are the backbone of rural, agricultural and socio-economic progress in many countries. Many industries are directly or indirectly rely on sugar mills which in turn are responsible for overall development of particular country. In view of this sugarcane production has vital significance for its products and by-products. It has been reported that an average of 30,000–40,000 l of wastewater was generated per tons of sugar processed (Belliappa 1991). The sugar mill wastewater, as it released has a relatively clear appearance. However, after stagnating for sometime it turns black and starts emitting foul odor (Baskaran et al. 2009). The waste water entering the water bodies from sugar mills are one of the key sources of environmental toxicity. Internationally, water pollution is the serious and significant threat mainly due to the contamination of aquatic bodies like rivers, canals, lakes, oceans and ground water resources (Richardson and Temes 2011). Contamination of water usually begins when wastewater from industries, agriculture and domestic regions are released into the surrounding water bodies without adequate treatment to remove hazardous chemicals constituents (Dougherty et al. 2010).
Sugar mill wastewater not only affects the value of drinking water but also has harmful effect on the soil micro flora and aquatic ecosystems. Soil is the most favorable habitat for a broad range of microorganisms such as bacteria, fungi, algae and protozoa. Industries continuously release wastewater which is quite toxic for the soil micro flora whether it is from sugar mill or other industries. The organic, inorganic and non biodegradable material such as toxic chemicals of wastewater adversely affects the soil parameters and soil fertility up to large extent (Kisku et al. 2000). Sugar mill wastewater has an unbearable odor and unlikable color when poured into the environment without appropriate treatment. Farmers have been using this wastewater for crop irrigation found that the soil health was compromised. Such harmful wastewater is injurious to plants, animals and human beings in many aspects. The effects of various sugar mill wastewaters on seed germination, growth and yield of crops have captivated the attention of many workers. In addition, sugar mill effluent released in the environment leads serious health hazard to the rural and semi-urban populations that use water stream and river water for domestic purposes. Sugar mill wastewaters entering in agricultural land contribute largely to fish mortality and spoils paddy crops leading to agro-economic losses (Baruah et al. 1993).
Several anomalous changes were found in the physico-chemical properties, for example, pH, temperature, odor, color, TDS, DO, COD, BOD, conductivity and turbidity etc. of natural aquatic bodies due to the release of the wastewater mainly from the sugar and allied industries (Kolhe et al. 2009). Several studies are available for the physico-chemical characteristic features of sugar mill wastewater and their undesirable effects on aquatic life and the influence of sugar mill wastewater on the seed germination of various commercially important crops such as maize, pine, green gram, rice, wheat and Jowar etc. (Siva and Suja 2012). The growth of certain aquatic plants such as water hyacinth and water lettuce was also greatly influenced by the effluent discharged from the sugar industry (Ayyasamy et al. 2008). Untreated wastewater from sugar mills contains total dissolved solid (TDS) and total suspended solid (TSS) up to significant amount. The high value of TSS in wastewater offers salt deposition in land which decreases soil porosity. The presence of high concentration of different solids in the wastewater reduces the growth and development of the new seedlings. High TDS value in wastewater may also have undesirable effect on agricultural crop plants. A TDS of 500–1000 ppm may have negative effect on susceptible crops.
However, various types of metallic and nonmetallic elements of wastewater act as important nutrients but at the higher concentration they may show toxic effect on seed germination and seedling growth which ultimately adversely affecting plant growth and yield in agricultural field. Therefore, the polluted wastewater directly or indirectly affects the living organisms which are found nearby the water sources not only in the industrial area but also in agricultural fields, river and river beds (Nath et al. 2005). Although in some of the industries, the wastewater may have high level of nutrients, heavy metals and hazardous chemical compounds which may help to the microorganism’s growth during the biological treatment of the wastewater (Malaviya and Rathore 2007). Thus it is well established fact that the sugar mill wastewater has direct and indirect adverse effect on living organisms of different habitats by diminishing their growth, reducing energy supply and photosynthetic efficiency.
7.4 Treatment of Sugar Mill Wastewater
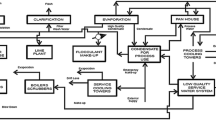
The large amount of sugar mill wastewater is released in environment per day throughout the world which has harmful effect on all living organism. Globally several treatment methods are used to treat this wastewater before their release into water bodies. The physico-chemical and biological methods are employed for removal of pollutant from sugar mill wastewater. Several physico-chemical methods such as coagulation, flocculation, ultrafiltration, electrodialysis and reverse osmosis are used to treat sugar mill wastewater. The biological methods are frequently used in bioremediation of sugar wastewater which is very effective and offer some advantages over physico-chemical methods (Fig. 7.1).
7.4.1 Physico–chemical Methods
7.4.1.1 Chemical Oxidation Method
Chemical oxidation method is one of the physico-chemical methods used for chemical oxidant (H2O2, O3, KMnO4, etc.) to oxidize the hazardous pollutant in slightly toxic, harmless substances or transform it into convenient form. Though, chemical oxidation methods comprise the use of oxidizing agents such as ozone and hydrogen peroxide, exhibit lower rates of degradation. Ozone is a gas at normal pressure and temperature. Its solubility in water is function of its partial pressure and temperature. Ozone is produced by high voltage discharge in air and oxygen. Ozone is unstable and tends to react to form:
Ozone is a very strong oxidizing agent and much effective for decolorization.
7.4.1.2 Coagulation and Flocculation
Coagulation-flocculation process is employed for removal of suspended solids materials from sugar mill wastewater. The process operates in steps which break down forces, which stabilize charged particles present in the waste allowing inter particle collision to occur, hence, generating flocs. The purpose of coagulation/flocculation process is to destabilize the charged particles of suspended solids. Addition of coagulants with opposite charges of the suspended solids destabilizes the particles charge. Coagulants are mixed in wastewater to neutralize the negative charge of suspended particles. Upon neutralization, the suspended particles fix together to form slightly larger particles.
7.4.1.3 Reverse Osmosis
It is a process in which heavy metals are separated by a semi permeable membrane at a pressure greater than osmotic pressure caused by the dissolved solids in wastewater. The disadvantage of this method is that it is highly expensive.
7.4.1.4 Electrodialysis
In this process, the ionic components are separated through the use of semi-permeable ion selective membranes. Application of an electrical potential between the two electrodes causes a migration of cations and anions towards respective electrodes. Because of the alternate spacing of cation and anion permeable membranes, cells of concentrated and dilute salts are formed. The disadvantage of this technique is the formation of metal hydroxides which clog the membrane.
7.4.1.5 Chemical Precipitation
Precipitation of pollutants is achieved by the addition of coagulation such as alum, lime, iron salts and other organic polymers. The main drawback of this method is the production of large amount of sludge during the process which contains toxic compounds.
7.4.1.6 Electro-oxidation (EO)
Wastewater treatment by electro-oxidation goes back to the nineteenth century, when electrochemical decomposition of cyanide was examined. Extensive study of this method initiate since the late 1970s. During the last two decades, research works have been focused on the competence in oxidizing the various pollutants on different electrodes, improvement of the electrocatalytic activity and electrochemical stability of electrode materials, investigation of factors affecting the process performance, and exploration of the mechanisms and kinetics of pollutant degradation. The electrochemical oxidation can be achieved by the application of electricity both in direct and/or indirect form. Moreover its effectiveness strongly depends upon several factors i.e. the treatment condition, waste composition, the nature of the electrode materials used and mode of operation both in batch or continuous process.
7.4.2 Biological Methods
Industrial pollution is one of the major factors causing the degradation of the environment around us. Also, increasing industrialization is not only consuming large areas of agriculture lands, but also simultaneously causing serious environmental threats (Saranraj and Stella 2014). In India, sugar industries have an important position in economic development. However, the wastewater released from these industries bear a high extent of pollution. Sugar industries generate nearly 1000 L of wastewater for 1 ton of sugar cane crushed (Kushwaha 2013). If wastewater is discharged without proper treatment, it poses pollution problems in environment. The generation of organic compounds as liquid effluents is one of the important environmental problems in sugarcane processing industry. The inadequate and indiscriminate disposal of such wastewater in soils and water bodies has gained much attention because of their environmental toxicity. There are several conventional technologies involves physical and chemical methods that have been used for treating sugarcane industry waste. However, these methods are expensive and ecologically not fit as chemical methods may cause secondary pollution. Thus, nowadays eco-friendly and cost effective biological methods are used for treating such waste generated from sugar mill. The present technology has initiated the zero discharge by using wastewater in press mud, bagasse burring and in biofertilizer production unit of the industry.
Biological treatment is a better alternative for treating sugar industry wastewater. The biological treatment over other physical and chemical treatment processes is better due to economic advantage, both in terms of capital investment and operating costs. Major biological methods involve aerobic, anaerobic and combined biological treatments that can be suitably adopted in different processing steps of sugar mill wastewater treatment process. The aerobic biological methods for sugar mill wastewater treatment occurs through sequential batch reactor (SBR), activated sludge processes (ASP), wetlands or stabilization pond. Among the different anaerobic methods, anaerobic digester (AD), anaerobic filter (AF) and Upflow anaerobic sludge blanket (UASB) are mainly used for the treatment of wastewater.
Aerobic treatment processes occurs in the presence of oxygen using aerobic microbes to assimilate organic impurities i.e. convert them in to carbon dioxide, water and biomass. The anaerobic treatment processes, on other hand take place in the absence of oxygen by anaerobic microorganisms to assimilate organic impurities into methane and carbon dioxide gases and biomass (Fig. 7.2a, b).
7.4.2.1 Anaerobic Biological Treatment
The high organic content of wastewater from sugar cane industry makes anaerobic treatment attractive in comparison to direct aerobic treatment. Therefore, biomethanation is the primary treatment step and is usually followed by two-steps aerobic treatment before discharge into a water bodies. Anaerobic treatment method converts half of the effluent COD into biogas and it can be successfully operated at high organic loading rates; also, the biogas thus generated can be utilized for steam generation in the boilers thereby meeting the energy demands of the unit. Further, low nutrient requirements and stabilized sludge production are other associated benefits (Rais and Sheoran 2015).
Anaerobic lagoons are the simplest way for the anaerobic treatment of wastewater (Wilkie et al. 2000). It was reported that employing two anaerobic lagoons in series resulted in final BOD levels up to 600 mg/l. However, large area requirement, odor problem and chances of ground water pollution restrict its usage (Rais and Sheoran 2015). These reactors offer the advantage of separating the hydraulic retention time (HRT) from solids retention time (SRT) so that slow growing anaerobic microbes can remain in the reactor independent of wastewater stream (Mall and Kumar 1997).
Anaerobic treatment converts the wastewater organic pollutants into small amount of sludge and large amount of biogas as source of energy whereas aerobic treatment method needs external input of energy for aeration (Ayati and Ganjidoust 2006).
Anaerobic processes could treat the effluent with high loads of easy to degrade organic materials (effluent from the sugar industry) efficiently. Anaerobic method can be categorized into anaerobic activated sludge and anaerobic biological membrane processes. The anaerobic activated sludge method involves conventional stirred anaerobic reactor, upflow anaerobic sludge blanket reactor (USAB), anaerobic contact tank, etc.
7.4.2.1.1 Upflow Anaerobic Sludge Blanket Reactor (UASB)
The upflow anaerobic sludge blanket (UASB) reactor is by far the most widely used high rate anaerobic treatment system for variety of wastewater (Van Haandel and Lettinga 1994). The most characteristic device of UASB reactor is the three phase separator or settler. The presence of the settler on the top of the digestion zone enables the system to maintain a large sludge mass in the UASB reactor, while effluent essentially free of suspended solids is discharged. In this process, mixing of sludge and waste water occurs through the production of methane within the blanket as well as by hydraulic flow and the triphase (gas, liquid, sludge biomass) separator which could restrict the loss of biomass sludge via the gas emission and release of water. The benefits of USAB system are as follows:
-
1.
It has a high population of naturally immobilized bacteria with good settling characteristic, and also remediates the organic contaminants from wastewater of sugar industry.
-
2.
In USAB system, increased concentrations of cells biomass can be achieved without support materials that reduces the cost that make the process feasible and efficient.
Hampannavar and Shivayogimath (2010) studied the treatment of sugar industry wastewater in a UASB reactor seeded with nongranular anaerobicallly digested sewage sludge. They also reported a maximum COD removal efficiency of 89.4% was achieved at ambient temperature and Successful reactor startup with granulation was achieved within 95 days of operation.
7.4.2.1.2 Anaerobic Biofilter
Anaerobic biofilter is a type of efficient anaerobic treatment equipment which was developed in 1960s for treatment of wastewater. These reactors use inert support materials to make available a surface for the development of anaerobic bacteria and to decrease disorder to allow free bacterial populations to be retained in the anaerobic biofilter.
7.4.2.2 Aerobic Biological Treatment
After post-anaerobic treatment step, the anaerobically treated waste has still high organic load and is dark brown in color, hence it is generally followed by a secondary, aerobic treatment. Solar drying of bio-methanated sugar mill waste water is one way but the large land area need restricts this practice (Rais and Sheoran 2015). Other techniques that have been demonstrated for bio-methanated sugar mill effluent are aquaculture, constructed wetlands (CWs), biocomposting, and microbial (bacteria, fungi and algae) treatments. Biological techniques employing aerobic processes such as activated sludge, biocomposting, etc. are presently used by many sugar industries. Only a part of the wastewater gets consumed in biocomposting due to the large volumes generated. Biocomposting utilizes sugarcane press mud as the filler material; thus it is typically employed by sugar mills. Since sugar manufacturing is a seasonal operation, press mud availability is often a constraint. Further, biocomposting requires large amount of land. Also, it cannot be carried out during the rainy season (Rais and Sheoran 2015).
Microbial treatment has been considered as an eco-friendly and cost competitive technique involving the natural way which results in conversion of hazardous toxic organic and inorganic compounds into nontoxic forms. This technique involves compatible microbes in the polluted water system and the pollutants are bioremediated during the microbial metabolism process. Sugar industries consume large quantities of water for various processes and discharge equally large volumes of waste waters containing variety of pollutants and coloring matter. Microorganism due to their inherent capacity to metabolize a variety of complex compounds have been utilized since long back for biodegradation of complex toxic and recalcitrant compounds present in various industrial wastes for environmental safety (Gupta and Mukarji 2001). Microbial treatment systems have advantage of being simple in design and low in cost (Banat et al. 1996). Several species of fungi, bacteria and algae have been used for the removal of pollutant from sugar mill wastewater. Buvaneswari et al. (2013a) reported the role of Staphylococcus aureus, Bacillus cereus, Klebsiella pneumoniae, Enterobacter aeruginosa and Escherichia coli in bioremediation of Sugar mill waste water. Similarly, Saranraj and Stella (2012) studied the sugar mill effluent bioremediation by immobilized bacterial consortium (Bacillus subtilis + Serratia marcescens + Enterobacter asburiae). They reported that the immobilized bacterial consortium used for bioremediating the effluent showed a sharp decrease in the levels of COD, TSS, TDS, heavy metals and other physical properties after 6 months of treatment. To overcome the contamination caused by polluted soil of sugar industry the addition of some seeds of Moringa oleifera, Acacia nilotica, Tamarindus indicus and cow dung were used by Shinde et al. (2016) in that polluted soil.
Fungi, their biology, economic value and pathogenic capabilities are not new to human society. They have been used from fermentation of foods to production of pharmaceuticals. Fungi thrive well in hospitable habitats with environmental extremes because of their enzyme systems (Cooke 1979). Fungi are involved in the biodegradation of undesirable materials or compounds and convert them into harmless tolerable or useful products. Fungi are recognized for their superior aptitudes to produce a large variety of extracellular proteins, organic acids and other metabolites and for their capacities to adapt to severe environmental constraints. Fungal systems appear to be most appropriate in the treatment of colored and metallic effluent. Fungi not only produce various metabolites like citric acid, homogeneous proteins, heterogenous proteins, peroxides but have shown their effectiveness for removal, reduction and detoxification of industrial effluent ingredients. Several fungi have been used for treating sugarcane industry waste such as Aspergillus niger, Penicillium sp. and Fusarium sp. (Buvaneswari et al. 2013b).
Many biological methods have been investigated for the treatment of wastewater from sugarcane industry. Due to high COD, the anaerobic treatment with biogas recovery is employed mainly as the first treatment step that reduces the organic pollution load and brings down BOD to 80–95% of the original value; however, the bio-digested effluent still contains BOD in the range of 5000–10,000 mg/l (Rais and Sheoran 2015). Further the problem of color associated with sugar mill waste water not only remains unsolved but actually gets aggravated under anaerobic conditions (Patil et al. 2003). Therefore anaerobically treated effluent is darker in color compared to untreated wastewater and needs several-fold dilution by fresh water prior to discharge. Biological treatment using aerobic processes like activated sludge, biocomposting etc. is presently practiced by various sugar mill industries.
Due to the large volumes generated, only a part of the wastewater gets consumed in biocomposting. Biocomposting utilizes sugarcane press mud as the filler material; thus it is typically employed by distilleries attached to sugar mills. Since sugar manufacturing is a seasonal operation, press mud availability is often a constraint. Further, biocomposting requires large amount of land; also, it cannot be carried out during the rainy season. Though aerobic treatment like the conventional activated sludge process leads to significant reduction in COD, the process is energy intensive and the color removal is still inadequate. Thus several pure cultures of fungi, bacteria and algae have been investigated specifically for their ability to decolorize the effluent as discussed earlier. In all instances, supplementation with either nitrogen or carbon source is almost always necessary because the microbial species are not able to utilize the wastewater as the sole carbon source. Further, high dilution (typically up to 1:10-fold for untreated wastewater) is required for optimal microbial activity. In addition, these studies are mostly limited to laboratory scale investigations and no pilot/commercial scale operations are reported as yet.
Kushwaha (2013) reviewed about sugar industry wastewater. The wastewater generated from these industries bear a high level of pollution. Most of the studies on sugar industry wastewater treatments have been carried out by anaerobic treatment processes. However, oil and grease are not easily degraded by anaerobic processes. Anaerobic-aerobic combined systems can remove organics completely. However, more work is needed in use of combined systems (Kushwaha 2013).
The aerobic microbial degradation have significant role in biological treatment. In this process, the oxygenases and peroxidases synthesizing microbes are mainly used. The microbes get the energy, carbon and nutrient elements released during the degradation process. The classic aerobic biodegradation process involves activated sludge reactor and membrane bioreactor.
7.4.2.2.1 Activated Sludge Reactor
It is frequently used technique for treating wastewater generated from industry that uses oxygen and microorganisms to biologically degrade organic pollutants. In the process, there is an aeration tank (Fig. 7.3), a setting tank or clarifier is present to allow the waste sludge to settle. Part of the waste sludge is recycled to the aeration tank and the remaining waste sludge is removed for further treatment and ultimate disposal.
Activated sludge process is used to treat sugar mill wastewater using air and a biological floc composed of microbes. In general, the process occurs in two steps, viz; adsorption and biological oxidation. The technique could effectively remediate the organic pollutants of the wastewater.
7.4.2.2.2 Trickling Filter Method
A trickling filter consists of a bed of rocks, gravel, slag, peat moss, or plastic media over which sugar industry wastewater flows downward and contacts to microbial slime covering the bed. Aerobic conditions are maintained by forced air flowing through the bed. Trickling filter process involves adsorption of organic compounds of the wastewater by the microbial slime layer and diffusion of air into the slime layer to provide oxygen required for the biochemical oxidation of the organic compounds. The end products include carbon dioxide gas, water and other products of the oxidation.
7.4.2.2.3 Membrane Bioreactor
This process is the combination of microfiltration or ultra filtration with a suspended growth bioreactor, and is used for industrial wastewater treatment including sugar mill wastewater. The technique is nearly the same as activated sludge process, except that instead of separating water and sludge via settlement. This processes is capable to treat effluent with higher suspended solids (SS) concentrations compared to activated sludge process, thus reducing the reactor volume to achieve the same loading rate.
7.4.2.3 Combination of the Aerobic and Anaerobic Biological Treatment
The combination of the anaerobic and aerobic reactor is more efficient in sugar mill wastewater treatment compared to the single anaerobic and aerobic reactors. The benefit of using combined process viz., anaerobic process could modify the biochemical property of the wastewater, making the following aerobic process more efficient. The combined aerobic-anaerobic reactors include A/O reactor, A2/O reactor, oxidation ditch, constructed wetland. The two classic aerobic biodegradation reactors such as oxidation ditch and constructed wetland are briefly discussed here.
7.4.2.3.1 Oxidation Ditch
In this method, the effluent treatment occurs in which a circular basin through that the effluent passes. The microorganisms will digest the organic pollutants in the wastewater when activated sludge is added to the oxidation ditch process. Raw wastewater and returned sludge is known as mixed liquor. The addition of oxygen into the flowing mixed liquor occurs through rotating biological contactors. Once the organic waste has been removed from the polluted water, the mixed liquor flows out of the oxidation ditch and sludge is removed in the secondary settling tank, and part of the sludge is pumped to a sludge pumping room where the sludge is thickened with the help of aerator pumps in oxidation ditch. The oxidation ditch is characterized by simple process, low maintain consumption, steady operation, and strong shock resistance.
7.4.2.3.2 Constructed Wetland
It is an artificial wetland which could act as a biofilter, removing sediments and pollutants from wastewater. Constructed wetland is a combination of water, media, plants, microbes. Other physical, chemical, and biological also combine in wetlands to remove pollutants from the effluent. Constructed wetland method is economical and eco-friendly. Thus, it is supposed to be a promising technology to treat the effluent in developing country.
7.5 Factors Affecting the Treatment of Wastewater from Sugar Mill
India is one of the largest producers and consumers of sugar per annum in the world. In rural economy of India, sugar industry plays an important role by uplifting the livelihood and creation of employment. Byproducts of sugar industry are also used as raw materials in different industries. However, effluents of sugar industries have a great environmental impact upon the surrounding environment due to presence of various pollutants. A significant large amount of waste are generated during the manufacture of sugar, which contains a high amount of pollutants in terms of suspended solids, organic matters, biological and chemical oxygen demand effluent including sludge, press mud and bagasse (Yadav and Daulta 2014). Near about 526 sugars mill are operating in India that produced 33.69 million tons of sugarcane in the year of 2015–2016 (Mane et al. 2015). To crush one tone of sugarcane nearly 2000 l water required, which generated nearly 1000 l of wastewater (Kolhe et al. 2009). If untreated wastewater is discharged on land, decaying organic solids present in the wastewater clog the soil pores and will change soil properties (Reddy et al. 2015). Sugar mill effluent represents an environmental problem due to its high organic load, intense coloration and presence of phenolic compounds. Most of the organic matter present in the effluent can be reduced by conventional biological treatments but the color is hardly removed by such treatments. The remaining color can lead to a reduction of sunlight penetration in rivers and streams which in turn decreases both photosynthetic activity and dissolved oxygen concentrations causing harm to aquatic life. Hence, purification of sugar industry wastewater is a not challenging task due to fewer amounts of pollutants.
The principle factors which mainly affect the ability of sugar mill wastewater treatment are pH, aerobic or anaerobic conditions, temperature, reaction time, type and speed of reactions for the treatment of wastewater, biomass in the aeration tank in each moment during the reaction, catalyst, inhibitor, nutrients and the concentration of the contaminants in the wastewater. Combined sugar mill effluents generally contain high amount of suspended solids, dissolved solids, BOD and COD which have an adverse environmental impact. Wastewater with a high TDS level would have adverse impact on aquatic life, unfit for drinking, and reduce crop yields if used for irrigation. Suspended solids reduce light penetration and, as a result, photosynthetic activity reduces with increasing turbidity and can also clog fish gills. Sugar mill effluents generally change the natural pH level of the receiving water body to some extent. Such changes in pH can disturb the ecological balance of the aquatic system (Roy et al. 2007). The effluents with its high BOD rapidly deplete available oxygen supply when they enter into water ecosystem causes adverse condition for aquatic life. The high BOD also creates septic conditions, generating foul smelling due to hydrogen sulphide, which in turn can precipitate iron and any dissolved salts, turning the water black and highly toxic for aquatic life (Behera and Mishra 1985). A high COD value of effluents indicates the presence of high inorganic and partly organic non-biodegradable content in the effluents. Its effects on the receiving water body are similar to that of a high BOD. Aerobic decomposition of components can decrease oxygen availability while anaerobic decomposition can produce hydrogen sulphide and are unaffected in lack of oxygen (Saranraj and Stella 2014). For the treatment of sugar industry effluent, several biological treatment technologies like aerobic as well as anaerobic methods were used. Wastewater organic pollutants are converted into small amount of sludge and large amount of biogas as source of energy during anaerobic treatment whereas aerobic treatment needs external input of energy for aeration. Anaerobic digestion is ideal for waste treatment, having several significant advantages over other available methods. The advantages become more pronounced when dealing with strong industrial wastes including sugar cane waste which is nutrient deficient. Inspite of that the analyzed parameters of treated wastewater are well within the prescribed by the central pollution control board (CPCB) for the discharge of effluent to on-land standards and discharged wastewater could be used for the agriculture purposes or any other purposes.
7.5.1 pH
pH is one of an important biotic factor that serves as an index for pollution. Any change in the pH value can change the rate of biological and chemical reaction and survival of various microorganisms present in the water. The presence or absence of different anions and cations can have direct relation with pH of the waste water (Doke et al. 2011). From the literature, it has been found that pH of sugar mill effluents varies from 4.8 in the raw waste water and 6.9 in the treated waste water. Khan et al. (2003) observed the pH of sugar industry wastewater was 9.5 however, Maruthi and Rao (2001) observed the pH of the sugar mill effluent discharges from Tummapala sugar factory, Anakapalli (Andhra Pradesh) was 6.5–8.8. Different study showed the difference in pH of sugar mill wastewater (Senthil et al. 2001; Matkar and Gangotri 2002). The reaction between waste water flowing from open drainage system with the soil has direct relevance to the pH of waste water. If the water in a stream is too acidic or basic, the H+ or OH− ion activity may disrupt the biochemical reactions of aquatic organisms. Wastewater pH has been identified as one of the parameters which influence the effective wastewater treatment (Aboulhassan et al. 2006). The lower pH of the reactor contents may be due to acid production by activities of acidogenic bacteria whereas acetogenic and methanogenic bacteria increase the pH by the consumption of the generated acid during methane formation. These bacteria have different growth rates and perform activities at different stages. At pH below 6.2, the growth of methanogens is inhibited; however, fermentative bacteria will continue to function even when pH has dropped to 4.5–5.0. Lower pH values of both treated and untreated effluents may be also due to use of phosphoric acid and Sulphur dioxide during cleaning of sugar cane juice. Low pH of the digester system may be due to inhibition of methanogenic bacterial action. For efficient operation of an anaerobic reactor, pH is an important indicator and a continuous drop in the pH is a sign that all is not right. Hence, before a stable population of each bacterial groups established in an anaerobic digester, external control is done by adding chemicals such as lime and bicarbonates or carbonates of either sodium or potassium. Omol (1997) observed that anaerobic digesters may operate at the satisfactory pH range of 6.2–8.0 while the optimum range is 6.8–7.2. He also noted that low pH of system may be due to accumulation of volatile fatty acid. To see the effect of initial pH on COD and color reduction in waste water treatment, an experiment was carried out from sample pH 2 to 10 at 3 kg/m3 mass loading with treatment temperature 65 °C, reaction time 3 h and in presence of catalyst copper oxide. Maximum 39.7% COD and 43.6% color reduction was found at pH 8 whereas 38.5% COD and 42.5% color reduction was found at pH 4 (Sahu 2016). The COD reductions are due to a combined effect of the active functional groups of effluents which react at certain pH and the catalyst reactivity which varied with pH. Tiwari and Sahu (2017) reported that removal of color 54%, 62%, 66% and COD 51.5%, 59.5%, 63.5% was increased with increased in pH 3.0, 4.5 and 6.0, respectively during the treatment of sugar mill waste water.
7.5.2 Temperature
Temperature is basically one of the most fundamental parameter which largely affects the chemical and biological reactions of aquatic organisms (Shelavale and Shinde 2016). The temperature of sugar mill waste water depends on season, time of sampling, distance of sampling from sugar mill etc. When effluent is discharged from industries, it has generally higher temperature which affects the aquatic biota and land adversely. In general, the temperature of aquatic condition lies between 20 and 27 °C but the temperature of untreated and treated effluent of sugar industry has 48 °C and 30 °C, respectively. The temperature of the discharge waste water should not exceed 35 °C otherwise it will produce adverse effect on aquatic system, agricultural field and yield of the crop (Beruch et al. 1993). Siddiqui and Waseem (2012) also suggest that the temperature of the discharged wastewater should not exceed 35 °C. The high temperature of the untreated effluent i.e. above 35 °C also affected the germination process. The increased temperature may accelerate the rate of chemical reaction and chemical changes in the aquatic condition (Poddar and Sahu 2017). The activity of various anaerobic bacterial population is depends on temperature. Different bacterial species are functional at different temperature ranges. Omol (1997) observed that anaerobic digestion can occur over a wide range of temperature which may be subdivided into three separate ranges as indicated below. Temperature ranges for bacterial action are:
Type | Range (°C) | Optimum range (°C) |
|---|---|---|
Psycrophillic | 2–30 | 12–18 |
Mesophilic | 20–45 | 25–40 |
Thermophilic | 45–75 | 55–65 |
From above observation, it is expected that any variation in temperature may affect the performance of anaerobic reactors. During anaerobic treatment, acetogenic and methanogenic bacteria are extremely sensitive to temperature variation with even a drop of 2–3 °C affecting the performance of reactors. Souza (1986) observed that an increase in temperature above 15 °C, the rate of anaerobic digestion also increased, but large variations in temperature should be avoided due to adverse effect of it on bacteria. Sahu (2016) showed the effect of reacting temperature (55–95 °C) on COD and color reduction at treatment time 9 h, optimum mass loading and pH. From the experimental data, it has been found that with an increase in temperature (55 °C, 65 °C, 75 °C, 85 °C) decreased the COD (55%, 60%, 65%, 73%) and color (57.5%, 63%, 68% 76%), respectively. It was found that the preheating period up to 85 °C is sufficient for affecting the COD 73% (994.15 mg/l) and color removal 76% (84 PCU), no further increase in COD and color removal was observed even the reactor is maintained at 95 °C temperature. The large molecules of the organic matter present in waste water breaks into smaller molecules and leads to carbon enriched solid residue formation. The decomposition of the large molecules at high temperature resulting in the deposition of the insoluble charred residue, leads to reduction in COD of the treated wastewater (Kumar and Srikantaswamy 2015). Gaseous products formed during decomposition have pungent and foul smell, which escape when the valve is opened after cooling of the reactor. The gases may consist of methane and/or nitrogenous and sulphurous compounds.
7.5.3 Aerobic and Anaerobic Condition
Biological treatment of effluents can be achieved by two processes, aerobic and anaerobic processes. Aerobic processes are occurs in presence of oxygen and usually limited by the waste strength. Aerobic processes require maximum oxygen exchange rate from the gas phase to the liquid phase to treat the waste water aerobically. In compare to aerobic treatment of wastewater, anaerobic treatment process has two distinct advantages (Artsupho et al. 2016). First, due to their low energy yield, the excess biomass is utilized to fulfill the energy requirement. Secondly, the problem of excess sludge disposal is significantly reduced. Because of low sludge generation, the requirements of nutrients are considerably lower in anaerobic treatment as compare to aerobic processes. Many industrial waste water including sugar mill waste water are often nutrient deficient, hence anaerobic treatment has an important advantage over aerobic treatment of sugar mill effluent.
7.5.4 Mass Loading
Sugar mill waste water is characterized by high organic waste load, large volume and high suspended solid content. Anaerobic treatment process was considered as effective treatment procedure for sugar mill effluent when it was compared to aerobic treatment. Anaerobic treatment has several advantages over aerobic treatment. Anaerobic treatment has low nutrient requirement, no limitations of oxygen exchange from gas to liquid phases and can therefore treat stronger organic pollutant of waste water than aerobic process. The effect of mass loading on treatment of waste water, COD and color removal was carried out from 2 to 6 kg/m3 at optimum pH and treatment time 9 h with copper oxide as catalyst. At minimum mass loading of 2 kg/m3, 46.5% COD and 50.5% color removal was observed. However, COD removal 51%, 54%, 60% and color reduction 53.8%, 57%, 63% increased with increase in mass loading 3 kg/m3, 4 kg/m3 and 5 kg/m3, respectively. Further increase in mass loading upto 6 kg/m3, COD (57%) and color removal (63%) decreased. It proves that 5 kg/m3 is the optimum mass loading for maximum COD (60%) and color reduction (63%) in presence of catalyst. Further increase in mass loading decreases the treatment efficiency (Sahu 2016).
7.5.5 Reaction Time
To see the effect of reaction time on COD and color reduction of sugar mill waste water, treatment was carried out with waste water containing 3 kg/m3 mass loading for 0–9 h at temperature 65 °C. Experimental data suggest that the removal efficiency increases with increase in time. Maximum 51% COD and 53.8% color removal were observed in the presence of copper oxide as catalyst at 9 h of treatment time. Without catalyst, marginal effect on COD (37%) and color removal (38%) was observed at same treatment time. Heating and absence of oxygen also increases the COD and color reduction (Sahu 2016). Guimaraes et al. (2005) reported that Phanerocheate chrysosporium can remove color and total phenols from the sugar mill waste water with retention time of 3 days. During the course of operation, color, total phenols and chemical oxygen demand were reduced by 55%, 63% and 48%, respectively. Deshmane et al. (2016) reported that COD reduces up to 91% in 108 h and reduction of COD over 50% was achieved in only 24 h in presence of Spirulina.
7.5.6 Catalyst
The role of catalyst in the treatment of sugar industry wastewater has significant positive impact. In an experiment, maximum 73% chemical oxygen demand and 76% of color removal were obtained at 5 kg/m3 mass loading, reacting temperature 85 °C, treatment time 9 h and optimum pH 8 with copper oxide as catalyst. In presence of catalysts CuSO4 and CuCl, COD decreased 48%, 44.5% and color removal was 51% and 46%, respectively after 9 h of treatment time whereas in absence of catalyst, 37% COD and 38% color removal was observed at same treatment time (Sahu 2016). Similarly, COD removal by 10.5% and color reduction 11% was observed in the presence of poly-aluminum chloride at pH 7.5 (Tiwari and Sahu 2017). The treated wastewater contained more copper compared with pollution control board dischargeable limits. It required further treatment like adsorption or membrane separation of copper before discharge into the receiving stream. The quality of water was found to be suitable for irrigation after the removal of copper. The high removal of chemical oxygen demand and color from wastewater in presence of copper appreciates its use as catalyst in the treatment of sugar mill wastewater.
7.5.7 Nutrients
Along with the substrates, microorganisms require growth factors, trace elements and nutrients for their growth. Nitrogen (N) and phosphorous (P) are two important nutrients that are vital for bacterial growth. It has been found that the biological growth during the anaerobic digestion depends on different types of substrate. The slowest growth was observed with long chain fatty acids and highest growth was observed with carbohydrate as substrate. Growth of microorganism varies considerably and cannot be predicted from knowledge of waste strength alone, but the components of the waste also need to be considered (Omol 1997). Kiestra and Eggers (1986) studied the nutrient requirements i.e. carbon, nitrogen and phosphorous ratios during aerobic and anaerobic processes and obtained the following ratio; for aerobic process, it was 100:5:1, and for anaerobic process, it was 100:1.5:0.3. Therefore, the above ratios indicate that anaerobic organisms have lower nutrient requirements than aerobes. This may be attributed to the slow cellular growth of anaerobes and is an advantage when dealing with wastewaters with relatively inadequate nutrients. It has been found that the optimum BOD:N:P ratio for successful anaerobic ecosystem is 100:0.5:0.1.
7.5.8 Inhibitors
Inhibitors are substances that adversely affect the rate of biochemical or chemical reactions when they are present above certain concentrations. Inhibitory substances mainly affect the microbial activities of methanogenesis phase because methanogenic bacterial group is consists of only a few sensitive species, unlike the diverse hydrolytic and acidogenic bacteria. Hydrolytic and acidogenic bacteria are required during the first phase of decomposition of organic matter and therefore constantly replenished. However, methanogenic bacterial population is required during the second phase of organic decomposition and once the population is depleted, it takes a long time to recover. During decomposition, ammonia and sulphates are the most common inhibitors (Omol 1997). Although, ammonia and ammonium ion are essential as nitrogen sources for bacterial growth during digestion but they are inhibitory at high concentrations. Sulphate concentration greater than 500 mg/l can reduce methane production and lead to excessive sulphide production.
7.6 Recycling and Reuse of Wastewater
India is a developing country where small/large scale industrial units mainly sugar industry discharge their waste water without any treatments in open areas or in running water bodies such as river, lakes etc. The main difficulties in the treatment of wastewater is cost of treatment plant/processing which add extra pressure to smaller units. Hence, the values of pH, TSS, TDS, BOD and COD are above the permissible limits. These effluents have deleterious effects on the soil making them unsuitable for cultivation purpose. Qureshi et al. (2015) reported that sugar industry wastewater has high oxygen demand which leads to the depletion of dissolved oxygen content in the water bodies. If discharged untreated, it will produce adverse effect on aquatic biota and humans. Damodharan and Reddy (2012) showed that the yield of sugarcane increased when sugarcane crop was irrigated with the waste water as compared to bore well water irrigated crop. The results indicated a significant increase in plant height, shoot diameter, growth pattern, number of leaves and nodes, and biomass of the saplings, irrigated with wastewater when compared to bore well water irrigated crop. The reuse of treated wastewater is good options due to increasing water supplies to agriculture and use of nutrients by plants reduce the pollution load (Goel and Kulkarni 1994).
With increasing population, water demand in various sectors is now increasing. Now, researchers are concentrating to resolve this problem by the use of treated wastewater for irrigation. A large quantity of wastewater is produced from mill and cane handling station, boiler house and as by product during various sugar mill processes. This wastewater goes to common wastewater treatment plant for primary and secondary treatment. There are several initiatives taken by industries to minimize their water consumption and recycle the treated wastewater. However, research is going on to fill the existing gaps to provide a comprehensive and cost effective solution for small and large scale industries to become low water consuming and zero discharge units (Rais and Sheoran 2015).
7.7 Advantages of Treatment
Sugar industry untreated effluent has high COD, BOD, TDS and low contents of DO which causes negative effects on biological system. All the industries use large quantity of water and throw back almost an equal quantity of effluent which contains highly toxic materials in dissolved or suspended form. If this water is properly treated then it can be reused or recycled and a part of water shortage will surely be solved. During the treatment of waste water, all harmful chemical constituents including oil and grease should be removed. Although oil and grease has been removed successfully from waste water but some better technique is still required for the removal of oil and grease from the effluents. The hazardous pollutants of wastewater and their harmful effects can be removed/minimize after the treatment. Treated waste water of sugar industry has well balanced of chemicals. It may be suitable for irrigation purposes, if diluted with other fresh water. The treated wastewater can accelerates and improves the crop production (Rana et al. 2011). Mane et al. (2015) reported that use of treated sugar mill wastewater is a prospective source of different plant nutrients and can be used for irrigation purpose in agricultural practices. It would be beneficial alternative resources of water and due to presence of nutrients; it can act as fertilizer for crop production. Presently sugar industries are consuming their wastewater inside the industry through various processes like bagasse moisturing before the use in furnace, absorb for biofertilizers and so on.
References
Aboulhassan MA, Souabi S, Yaacoubi A et al (2006) Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int J Environ Sci Technol 3(4):327–332
Amin A, Ahmad T, Ehsanullah M et al (2010) Evaluation of industrial and city effluent quality using physiochemical and biological parameters. Elect J Env Agric Food Chem 9(5):931–939
Artsupho L, Jutakridsada P, Rodriguez et al (2016) Effect of temperature on increasing biogas production from sugar industrial wastewater treatment by UASB process in pilot scale. Energy Procedia 100:30–33
Ayati B, Ganjidoust (2006) Comparing the efficiency of UAFF and UASB with hybrid reactor in treating wood fiber wastewater. Iran J Env Health Sci Eng 3(1):39–44
Ayyasamy PM, Yasodha R, Rajakumar S et al (2008) Impact of sugar factory effluent on the growth and biochemical characteristics of terrestrial and aquatic plants. Bull Environ Contam Toxicol 81:449
Banat IM, Nigam P, Mcmullan G et al (1996) The isolation of thermophilic bacterial cultures capable of textile dyes decolorization. Env Health 23(4):547–551
Baruah AK, Sharma RN, Borah GC (1993) Impact of sugar mill and distillery effluents on water quality of river Gelabil Assam. Indian J Env Health 35(4):288–293
Baskaran LK, Sankar G, Chidambaram ALA et al (2009) Amelioration of sugar mill effluent polluted soil and its effect of green gram. Bot Res Int 2(2):131–135
Behera BK, Mishra BN (1985) The effect of a sugar mill effluent on enzyme activities of rice seedlings. Environ Res 37(2):390–398
Belliappa N (1991) Physico-chemical properties of sugar mill effluent. J Biol Chem 65:79–82
Beruch AK, Sharma RN, Barach GC (1993) Impact of Sugar mills and distilleries effluents on water quality of river Gelabil, Assam. Indian J Environ Health 35(4):288–293
BIS (1983) Indian standard specification for drinking water: IS: 10500. Bureau of Indian standards, New Delhi
Botha T, Blottnitz HV (2006) A comparison of the environmental benefits of bagasse derived electricity and fuel ethanol on a lifecycle basis. Energy Policy 34:2654–2661
Buvaneswari S, Muthukumaran M, Damodarkumar S et al (2013a) Isolation and identification of predominant bacteria to evaluate the bioremediation in sugar mill effluent. Int J Curr Sci 5:123–132
Buvaneswari S, Damodarkumar S, Murugesan S (2013b) Bioremediation studies on sugar-mill effluent by selected fungal species. Int J Curr Microbiol App Sci 2(1):50–58
Contreras AM, Elena R, Maylier P et al (2009) Comparative life cycle assessment of four alternatives for using by-products of cane sugar production. J Clean Prod 17:772–779
Cooke WB (1979) The ecology of fungi. CRE Press, Boca Raton
Damodharan U, Reddy MV (2012) Impact of sugar industrial treated effluent on the growth factor in sugarcane, Cuddalore, India. J Sustain Bioenergy Syst 2:43–48
Deshmane AB, Ghole VS, Darandale VS et al (2016) Sugar mill effluent treatment using Spirulina for recycling of water, saving energy and producing protein. Int J Environ Sci Technol 13:749–754
Doke KM, Khan EM, Rapolu J et al (2011) Physico-chemical analysis of sugar industry effluent and its effect on seed germination of Vigna angularis, Vigna cylindrica and Sorghum Cerium. Annu Environ Sci 5:7–11
Dougherty JA, Swarzenski PW, Dinicola RS et al (2010) Occurrence of herbicides and pharmaceutical and personal care products in surface water and ground water, around Liberty Bay, Puget Sound, Washington. J Environ Qual 39:1173–1180
Fakayode PK (2005) Alteration in physico-chemical characteristics of soil irrigated with sugar mill effluent. J Env Biol 12:103–109
Goel PK, Kulkarni SM (1994) Effects of sugar factory waste on germination of gram seed (Cicer aeritinum L.). Int J Environ Pollut 1(1):35–53
Guimaraes C, Porto P, Oliveira R et al (2005) Continuous decolorization of a sugar refinery wastewater in a modified rotating biological contactor with phanerochaete chrysosporium immobilized on polyurethane foam disks. Process Biochem 40(2):535–540
Gupta R, Mukerji KG (2001) Bioremediation: past, present and future. In: Tewari R, Mukerfi KG, Gupta JK et al (eds) Role of microbes in the management of environmental pollution. APH Publishing Corporation, New Delhi, pp 73–81
Hampannavar US, Shivayogimath CB (2010) Anaerobic treatment of sugar industry wastewater by upflow anaerobic sludge blanket reactor at ambient temperature. Int J Environ Sci 1:631–639
Khan M, Kalsoom U, Mahmood T et al (2003) Characterization and treatment of industrial effluent from sugar industry. J Chem Soc Pak 25(3):242–247
Kiestra H, Eggers E (1986) Treatment of industrial wastewater. Water Sci Technol 13(3):5–16
Kisku GC, Barman SC, Bhargava SK (2000) Contamination of soil and plants potentially toxic elements irrigated with mixed industrial effluent and impact in the environment. J Water Air and Soil Pol 120:121–137
Kolhe MP, Iqbal SA, Pawan KS (2000) Role of sugar industry in development of Indian economy. J Environ Sci 7:23–25
Kolhe AS, Sarode AG, Ingale SR (2009) Study of effluent from sugar cane industry. Sodh Samik Mulyan:303–306
Kumar DS, Srikantaswamy S (2015) Evaluation of effluent quality of a sugar industry by using physico-chemical parameters. Int J Adv Res Eng Appl Sci 4(1):16–25
Kushwaha JP (2013) A review on sugar industry wastewater: sources, treatment technologies, and reuse. Desal Water Treat 53:309–318
Lichts FO (2007) International and sweetener report. Int Sugar J 12:139–145
Malaviya P, Rathore VS (2007) Seasonal variations in different physico-chemical parameters of the effluents of century pulp and paper mill, Laluan, Uttarakhand. J Environ Biol 28:219–224
Mall ID, Kumar V (1997) Removal of organic matter from distillery effluent using low cost adsorbent. Chem Eng World XXXII 7:89–96
Mane PC, Chaudhari RD, Papade SE et al (2015) Study on bioremediated sugar industry effluent for irrigation: an evaluative study on the biochemical attributes of Vigna radiata under laboratory conditions. Ann Biol Res 6(6):26–32
Maruthi YA, Rao SR (2001) Effect of sugar mill effluent on organic resources of fish. Pollut Res 20(2):167–171
Matkar LS, Gangotri MS (2002) Physico-chemical analysis of sugar industrial effluents. J Ind Pollut Control 18(2):139–144
Nagaraju MG, Narasimha, Rangaswamy V (2009) Impact of sugar industry effluents on plant growth activity. Int Biodeterior Biodegrad 63:1068–1092
Nath K, Saini S, Sharma YK (2005) Chromium in tannery industry effluent and its effect on plant metabolism and growth. J Environ Biol 26(2):197–204
Omol SO (1997) Anaerobic treatment of cane sugar effluent from Muhoroni sugar factory using batch reactors. Research thesis, University of Nairobi
Patil PU, Kapadnis BP, Dhamankar VS (2003) Decolorization of synthetic melanoidin and biogas effluent by immobilized fungal isolate of Aspergillus niger UM2. All India Distiller’s Association (AIDA) Newsletter, pp 53–56
Poddar PK, Sahu O (2017) Quality and management of wastewater in sugar industry. Appl Water Sci 7:461–468
Prakash R, Henham A, Bhat I (1998) Net energy and gross pollution from bio-ethanol production in India. Fuel 77(14):1629–1633
Qureshi AL, Mahessar AA, Leghari ME et al (2015) Impact of releasing wastewater of sugar industries into drainage system of LBOD Sindh, Pakistan. Int J Environ Sci Dev 6(5):381–386
Rais M, Sheoran A (2015) Treatment of sugarcane industry effluents: science & technology issues. J Eng Res Appl 5:11–19
Ramjeawon T (2008) Life cycle assessment of electricity generation from bagasse in Mauritius. J Clean Prod 16:1727–1734
Rana S, Bag SK, Golder D et al (2011) Reclamation of municipal domestic wastewater by aquaponics of tomato plants. Ecol Eng 37(6):981–988
Reddy SSG, Raju AJS, Kumar BM (2015) Phytoremediation of sugar industrial water effluent using various hydrophytes. Int J Environ Sci 5(6):1147–1158
Renouf MR, Wegener MK, Nielsen LK (2008) An environmental life cycle assessment comparing Australian sugarcane with US corn and UK sugar beet as producers of sugars for fermentation. Biomater Bioeng 208:1144–1155
Richardson SD, Temes TA (2011) Water analysis: emerging contaminants and current issues. Anal Chem 83:4614–4648
Roy RP, Prasad J, Joshi AP (2007) Effect of sugar factory effluent on some physic-chemical properties of soils-a case study. J Environ Sci Eng 49(4):277–282
Sahu O (2016) Treatment of industry wastewater using thermo-chemical combined processes with copper salt up to recyclable limit. Int J Sustain Built Environ 5:288–300
Saranraj P, Stella D (2012) Bioremediation of sugar mill effluent by immobilized bacterial consortium. Int J Res Pure Appl Microbiol 2(4):43–48
Saranraj P, Stella D (2014) Impact of sugar mill effluent to environment and bioremediation: a review. World Appl Sci J 30(3):299–316
Senthil KRD, Swamy RN, Ramkrishan K (2001) Pollution studies on sugar mill effluent physico-chemical characteristics and toxic metals. Pollut Res 20(1):93–97
Shelavale S, Shinde V (2016) Zero waste sugar industry by using bio-tower. Int J Rec Innov Tren Comp Comm 4(4):255–261
Shinde V, Yede S, Yadav S et al (2016) Bioremediation techniques for polluted soil from sugar industry. Imp J Interdiscip Res 2:1436–1438
Shivappa D, Puttaiaha ET, Kiran BR (2007) Physico-chemical characteristics of sugar mill effluent. J Ind Pol Cont 23(2):217–221
Siddiqui WA, Waseem M (2012) A comparative study of sugar mill treated and untreated effluent – a case study. Orient J Chem 28(4):1899–1904
Siva SK, Suja PR (2012) Effect of sugar mill effluent on seed germination of peanut (Arachis hypogaea) and green gram (Vigna radiata). Int J Pharm Chem Sci 1(2):804–806
Souza ME (1986) Criteria for the utilization, design and operation of UASB Reactors. Water Sci Technol 18(12):55–69
Tiwari A, Sahu O (2017) Treatment of food-agro (sugar) industry wastewater with copper metal and salt: chemical oxidation and electro-oxidation combined study in batch mode. Water Resour Ind 17:19–25
Van Haandel AC, Lettinga G (1994) Anaerobic sewage treatment: a practical guide for regions with a hot climate. Wiley, New York
Vermeulen PLM, Vawada AS (2008) Impact of sugar factory effluent on the growth and biochemical characteristics of green gram and maize. J Environ Sci 81(5):449–454
Wilkie AC, Riedesel KJ, Owens JM (2000) Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 19:63–102
Yadav A, Daulta R (2014) Effect of sugar mill on physico-chemical characteristics of groundwater of surrounding area. Int Res J Environ Sci 3(6):62–66
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, P.K., Tripathi, M., Singh, R.P., Singh, P. (2019). Treatment and Recycling of Wastewater from Sugar Mill. In: Singh, R., Singh, R. (eds) Advances in Biological Treatment of Industrial Waste Water and their Recycling for a Sustainable Future. Applied Environmental Science and Engineering for a Sustainable Future. Springer, Singapore. https://doi.org/10.1007/978-981-13-1468-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-1468-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1467-4
Online ISBN: 978-981-13-1468-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)