Abstract
Sarcopenia is a common clinical symptom in aging and patients with wasting diseases, characterized by a decreased skeletal muscle mass. As a consequence of lifestyle change, the nonalcoholic fatty liver disease (NAFLD) presents a rising trend. In the past three decades, increasing evidence has proved that sarcopenia is related to NAFLD. In this chapter, we will summarize the emerging evidence of the predictive role of sarcopenia in NAFLD and review the diagnosis value, feasible mechanism, and therapy strategies of sarcopenia in NAFLD. Sarcopenia is a potential risk factor for NAFLD, and targeting sarcopenia can benefit NAFLD to some extent.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
Skeletal muscle is the major component of the mammalian motor system, with the function of secretory, mechanical, and supporting activities [1]. Similar to bone, the weight of muscle peaks at about 45–50 years old and then gradually decreases at a rate of 1–2% per year [2,3,4]. This kind of typical changes in human body composition related to aging is a progressive loss of muscle mass and strength, called sarcopenia [5, 6]. Sarcopenia is one of the most common types of muscle atrophy in aging population, strongly associated with senescence and malnutrition [7,8,9,10,11,12].
Sarcopenia is defined as reduced skeletal muscle mass, which is a common complication of most liver disease patients. It is observed in up to 60% of patients with end-stage liver disease (ESLD) [13, 14]. Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease, which refers to hepatic steatosis that is not caused by significant alcohol consumption or other causes of liver disease. In Western Europe and the United States, about 64 and 52 million people suffered from NAFLD, respectively [15, 16]. NAFLD is classified into different degrees, from the “benign” called simple steatosis (overall 20–30% prevalence) to steatohepatitis (NASH, 2–5% prevalence) and fibrosis [17]. Regarded as a metabolism disease, NAFLD shares amounts of pathophysiology process with sarcopenia. For example, both the liver and muscle are target organs for insulin action, and insulin resistance is known as a key factor in the pathophysiology for both NAFLD and sarcopenia.
During the last four decades, researchers have made a lot of efforts to investigate the relationship between sarcopenia and NAFLD [18,19,20,21,22]. In this chapter, we will give an introduction of involvement of sarcopenia in liver disease, including the pathology, diagnosis, and management of NAFLD associated with sarcopenia [23].
2 Current Proof in the Relationship Between Sarcopenia and Chronic Liver Disease
2.1 Sarcopenia as an Independent Predictor of NAFLD
Compelling evidence have shown the connection of sarcopenia and NAFLD [24]. To confirm the relationship between sarcopenia and NAFLD, the Korean Sarcopenic Obesity Study (KSOS) was conducted. The researchers built a cohort including 452 apparently healthy adults to perform a prospective observational cohort study and explore the correlation of sarcopenia and NAFLD with cardiometabolic risk factors. They found that after adjusting for confounding factors (insulin resistance and inflammation), the risk of NAFLD increased in patients with low muscle weight [25]. The next study showed that all these relationships happened among people of different sexes, although age group and menopausal status have an effect on it; and further confirmation of this relationship was required [26].
Another research group carried out a cross-sectional study in representative samples of the Korean population in 2015. In addition, based on the existence of liver fibrosis in patients with NAFLD, further stratification was carried out to preliminarily study the connection between sarcopenia and the progression of NAFLD. The data showed that regardless of condition of obesity or metabolic control, sarcopenia was associated with increased risks of NAFLD and advanced fibrosis [27]. They further stratified the sample according to the grade of liver fibrosis and continuously studied the relationship between sarcopenia and NAFLD-related cirrhosis. Interestingly, they found that sarcopenia was associated with significant liver fibrosis in subjects with NAFLD, and the association is independent of obesity and insulin resistance when comparing patients with fibrosis and NAFLD patients without fibrosis [28]. A rough analysis based on another NAFLD cohort showed that sarcopenia was related to NAFLD, with an OR of 3.82 (95% CI, 1.58–9.25), which was confirmed by biological systems.
In practice, NAFLD patients are often associated with other metabolic diseases. Yoshitaka Hashimoto et al. focused on the patient with type 2 diabetes mellitus and assessed the correlation between skeletal muscle mass index and hepatic steatosis. They draw a conclusion that mass of skeletal muscle was negatively related to hepatic steatosis in patient with type 2 diabetes mellitus which was consistent with previous results [29]. Similarly, worsening fibrosis was found related to increased prevalence of sarcopenia, independent of IR and obesity. Furthermore, the presence of fibrosis was 22% in nonsarcopenic patients compared to 60% in those with sarcopenia.
2.2 Sarcopenia in Prediction of Chronic Liver Disease and Its Complication
Liver cirrhosis is the end stage of liver disease characterized by the destruction of hepatic lobules. Among the multitudinous etiologies of cirrhosis, nonalcoholic steatohepatitis (NASH) is the most familiar one with increasing incidence year by year. Liver cirrhosis accompanied with sarcopenia is very common; the estimated prevalence of sarcopenia in subject with liver fibrosis is 40–70% [30]. The incidence is 50–70% in men slightly higher than that in women [31, 32]. A Canadian study showed that sarcopenia was associated with both visceral obesity and IR [33]. The median survival time of the patients with sarcopenia (19 ± 6 months) was shorter than that of nonsarcopenia patients (34 ± 11 months) (P = 0.005). They also observed L3 skeletal muscle index was not relative to Child–Pugh scores (r = −0.14; P = 0.1) and Model for End-Stage Liver Disease (MELD) (r = −0.07; P = 0.5) [33]. Another study revealed the median survival was 16 ± 6 months and 28 ± 3 months, respectively, in patients suffering from concurrent cirrhosis and HCC with or without sarcopenia [34]. The 1-year probability of survival in patients with sarcopenia was significantly lower compared to that of patients without sarcopenia as a conclusion of multiple results from different groups (85% vs 97%, P = 0.01 [35]; 52% vs 82%, P = 0.003 [34]; 53% vs 83%, P = 0.005 [33]; 63% vs 79%, P = 0.04) [36].
Sarcopenia is not only associated with the survival of patients with cirrhosis but also has a suggestive role on the complications of cirrhosis. Sepsis is one of the leading causes of death in cirrhosis patients. In patients with sarcopenia, the death rate associated with sepsis is 22%, higher than that of nonsarcopenia (P = 0.02). In earlier studies, however, no difference was found in the frequency of sepsis-related deaths in patients with or without sarcopenia. Hormones and biochemical changes and circulating endotoxins and other factors leading to sarcopenia in patients also impaired immune function and increased the risk of infection [37, 38]. In addition, patients with refractory ascites are particularly prone to malnutrition and sarcopenia, as increased ascites increases the static energy consumption, while the food intake is reduced by increased abdominal pressure. The treatment of refractory ascites by transjugular intrahepatic portosystemic shunt (TIPS) has been proven to improve refractory ascites of patients with dystrophic liver cirrhosis, which will ameliorate the sepsis recurrence. Other complications including hepatic encephalopathy are also related to sarcopenia. Previous study has confirmed a higher incidence of hepatic encephalopathy in patients with reduced muscle mass and muscle contraction force [39]. The increase of ammonia content in peripheral blood of patients with sarcopenia may be one of the reasons [40]. Therefore, it is recommended to include sarcopenia into the evaluation system for prediction and prognosis of the patients with cirrhosis. Sarcopenia alone or in combination with conventional prognostic systems has shown promise for cirrhosis prognosis. How to include an objective assessment of sarcopenia with conventional scores to optimize the prediction outcome for patients with cirrhosis requires further researches [41, 42].
Liver transplantation (LT) is considered as the only cure for current end-stage liver disease, and the occurrence of sarcopenia is also closely related to its therapeutic effect [43,44,45]. By observing a cohort from the United States, researchers found that 59% patients have sarcopenia during LT evaluation. CT scan was performed on 59 patients with pre-transplant sarcopenia at 6 months posttransplant, and 56 (95%) remained sarcopenic, and a large proportion of patients would continue to remain sarcopenic in 1 year. Meanwhile they found that obesity was an independent predictor of pre-transplant sarcopenia (P = 0.00001, odds ratio [OR] 0.22) in cirrhotic patients [43, 46, 47].
3 Emerging Mechanism in Sarcopenia with NAFLD
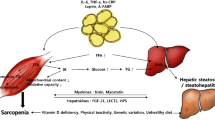
Finding the common pathological process between sarcopenia and NAFLD is a key strategy to analyze their correlation in the mechanism. The current study mostly focuses on the insulin resistance, inflammation response, vitamin D, oxidative stress, decreased physical activity, and other possible mechanisms.
Insulin Resistance
Insulin resistance (IR) is a common pathophysiological mechanism between sarcopenia and NAFLD [48,49,50]. In NAFLD patients with insulin resistance, the liver and adipose tissue are less sensitive to insulin. When adipose tissue becomes resistant to the antilipolytic effect of insulin, fat decomposition increases and free fatty acids (FFA) are released [23, 51]. The increased levels of triglycerides in the liver caused by IR are the main factors leading to liver steatosis. First, insulin cannot inhibit the lipolysis of adipose tissue by hormone-sensitive lipase, leading to FFA influx and subsequent absorption by the liver. Second, IR-associated hyperinsulinemia and hyperglycemia are upregulated by membrane-associated transcription factors sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate response element-binding protein (ChREBP). Third, hyperinsulinemia directly inhibits β-oxidation. These phenomena together promote the FFA accumulation in the liver and the hepatic triglyceride accumulation and steatosis through esterification [52, 53].
Study showed that even NAFLD patients without obesity have increased concentration of FFA and Adipo-IR compared to the control group [54, 55]. FFA enriched in the liver inhibits growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis, which has protective effect in age-related muscle loss and muscle regeneration [56]. In addition, IR is accompanied with compensatory hyperinsulinemia, which leads to glucose production disruption, decreased glycogen synthesis, increased lipolysis, and/or increased fat intake. Triglyceride (TG) transfer changes and inhibits β-oxidation, which caused TG accumulation in muscle tissue.
The gluconeogenesis is caused by IR-aggravated muscle protein loss and muscle degradation. IR itself can be a contributing factor to age-related muscle mass loss and leads to sarcopenia directly [57]. As an important mean of maintaining muscle mass and muscle metabolism, autophagy or lysosomal degradation is inactivated by IR through mammalian target of rapamycin (mTOR) pathway [53]. Collectively, these are how IR reduces muscle mass and leads to sarcopenia. Interestingly, the study found a negative correlation between IR and muscle mass, while IR was directly related to hepatic fat accumulation. These results support the common understanding of pathophysiological basis underlying the IR-mediated pathogenesis. Consistent with this view, the metabolic syndrome (MS) associated with IR should also be associated with NAFLD and sarcopenia [25].
Sarcopenia is associated with adverse glucose metabolism disorder, and the evidences indicate that low muscle mass can predict diabetes susceptibility. Given the increase in the prevalence of obesity, there is an urgent need for further research in developing control strategy of obesity and metabolic effects of sarcopenic disorders. Similarly, myosteatosis has also been shown to be related to IR. Synergistic effects of sarcopenia and obesity can lead to severer IR and metabolic disorders. In this regard, sarcopenia is also a factor that contributes to the onset of NAFLD by promoting IR.
Inflammation
Chronic inflammation and oxidative stress are essential processes in the development of and liver fibrosis followed directly. NASH is accompanied with an inflammatory reaction that occurs in the absence of pathogens or external antigens belonging to sterile inflammation. Lipid-induced hepatocyte stress, damage, and cell death could be the reason of sterile inflammation. Fatty acid oxidation (FAO) in the liver enhances the production of oxygen free radicals, causing lipid peroxidation and inducing pro-inflammatory cytokine synthesis. For example, transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α) are the most common factors of NAFLD. Furthermore, these cytokines stimulate protein degradation and metabolism, resulting in muscle mass loss and sarcopenia. These cytokines support both the recruitment of T cells and development of specific immune response against antigens. They activate synthesis of each other and stimulate IL-6 secretion. Although these cytokines show highest levels and activities in acute diseases like sepsis and are upregulated in trauma or after surgery, they also play key roles in NAFLD and infections which lead to loss of muscle cells and acceleration of muscle protein breakdown, contributing to sarcopenia. Inflammation markers in circulation, including CRP, TNF-α, and interleukins (IL-6 in particular), are closely related to the occurrence of sarcopenia.
Vitamin D
Low vitamin D levels have been reported to be involved in the pathogenesis of both sarcopenia and NAFLD [58]. NAFLD and vitamin D deficiency are associated with insulin resistance, obesity, type 2 diabetes mellitus, and cardiovascular disease. Many studies exploring the relationship have emerged over the past few years. Recent animal studies have shown that vitamin D is of critical importance in the production of pro-inflammatory cytokines and consequently regulates oxidative stress, hepatocyte apoptosis, and even hepatic fibrosis, although the mechanism of the association between vitamin D and NAFLD is not fully understood. The insulin receptor in pancreatic β-cells and in peripheral target organ (including the liver) is induced by vitamin D by activating vitamin D response elements (VDREs) in the human insulin receptor (hIR) gene promoter [59]. VDR is a receptor for 1α, 25-dihydroxy-vitamin D3 (1α, 25-(OH)2-VD3), activated from vitamin D3, and has a significant effect on calcium–phosphate homeostasis and bone metabolism but also on other physiological functions, including immunomodulation, cell growth, and differentiation. The effect of vitamin D on insulin sensitivity changes was mediated by vitamin D receptor (VDR) by improving systemic inflammation [60,61,62]. VDR in skeletal muscle can also be activated by vitamin D, which mediates muscle genesis, skeletal muscle growth, and inflammation. Results from animal studies prove vitamin D deficiency myofibrinolysis is increased with vitamin D deficiency. Lower levels of vitamin D were associated with lower muscle strength, poor muscle function, and increased muscle loss. People with muscular dystrophy have significantly lower levels of vitamin D. Vitamin D supplements may improve muscle strength and function in muscular dystrophy patients.
Decreased Physical Activity
The decrease in physical activity and the atrophy of muscles cross-promote each other. In addition, the decrease of physical activity is one of the main reasons which lead to IR and metabolic diseases. Patients with muscle atrophy, due to limited mobility, tend to live sedentary lifestyles and lack exercise [63, 64]. A sedentary lifestyle can increase the risks of obesity, metabolism diseases, and NAFLD, which has been well proven. It is speculated that this sedentary lifestyle will lead to a decrease in energy expenditure, which consequently leads to obesity and liver fat. In fact, studies have shown that in patients with sarcopenia, the amount of fat increases, as well as the body composition and the level of CRP, which further increased the risk of NAFLD [65].
Myokines and Myostatins
Skeletal muscle is considered as an endocrine organ. Myokines are defined as the peptides that are produced, expressed, and released by muscle fibers, including cytokines and other peptides with autocrine, paracrine, or endocrine effects. Muscle-derived hormones provide a new thought to build the communication between skeletal muscle and other organs, such as the adipose tissue, liver, pancreas, bones, and brain [66]. IL-6, one of the many myokines, appears to have systemic effects on the liver mediating crosstalk between intestinal L cells and pancreatic islets. Activation of IL-6/STAT3 pathway subsequently downregulates lipogenic genes but upregulates fatty acid oxidation-associated genes in the liver of interleukin-10-deficient mice [67]. Moreover, increased muscle peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 alpha (PGC-1α) expression protects mice from sarcopenia and metabolic disease and prolongs their lifespan [68]. The PGC-1α-dependent myokine irisin drives brown-fat-like development and causes a significant increase in total body energy expenditure whereby reducing body weight and thus obesity and IR [69]. Serum irisin concentrations were downregulated in the patient with NAFLD and inversely associated with the triglyceride contents in the liver and liver enzymes in obese adult [70]. The downstream signal transduction pathway activated by irisin involves the peroxisome proliferator-activated receptors α (PPARα), which are of vital importance in fatty acid β-oxidation in the liver [71]. FGF21 regulated by PPARα reduces hepatic steatosis and leads to reduced lipogenic gene expression and possibly the rate of fatty acid and triglyceride synthesis [72]. Myokines also significantly blunt insulin-stimulated glucose uptake and may participate in the occurrence of IR in the liver [73]. Therefore, it is determined that the protective effect of muscle on NAFLD will disappear mediated by hormone secretion when sarcopenia occurred.
Myostatin (also known as growth differentiation factor 8, GDF-8) is a member of TGF-β superfamily, with an inhibiting effect in protein synthesis and regeneration [74, 75]. In skeletal muscle, myostatin can activate mediated autophagy proteolysis and ubiquitin proteasome pathway which are two main pathways of skeletal muscle protein hydrolysis [76]. Myostatin also increases the quality of adipose tissue, leading to decreased adiponectin production [77,78,79]. The receptor of myostatin expressed on hepatic stellate cells. In hepatocytes, myostatin inhibits hepatocyte proliferation and insulin-stimulated glucose uptake [73]. Increased serum myostatin level is related to poor prognosis in liver cirrhosis patients [80]. This may be a potential link between sarcopenia and liver disease. But there is still doubt about which one is the consequence.
Other
Daniel Cabrera et al. found that the American Lifestyle-Induced Obesity Syndrome (ALIOS) diet-induced NAFLD mouse showed decreased muscle fiber diameter and myosin heavy chain (MHC) protein levels. Serum insulin-like growth factor-1 (IGF-1) was detected decreased, which is an anabolic hormone essential for muscle homeostasis without increase of inflammatory mediators. Since leptin in the brain can stimulate the production of IGF-1 in the liver, a later study explored the relationship between sarcopenia and NAFLD based on this regulatory mechanism [81].
Metabolic disturbances, inadequate dietary intake, and malabsorption are also involved in the pathogenesis in the end stage of NAFLD. After entering the stage of cirrhosis of the liver, because of glycogen synthesis and storage damage in the cirrhotic liver tissue, fat and muscle catabolism glycosylation of noncarbohydrate sources are promoted [82]. About 15% to 30% of patients with cirrhosis are in a highly catabolic state. Only if ensured adequate protein intake, it usually causes muscle atrophy [83]. The cause of highly catabolic state is unknown. The cause may include the activation of the sympathetic nervous system through the hypermetabolic pathway, the displacement of the gut bacteria, or systemic inflammation. At the same time, sepsis will exacerbate energy consumption in patients with cirrhosis and accelerate protein degradation. As a consequence of portosystemic shunt, the lack of cholestasis, and intestinal bacterial overgrowth, malabsorption of nutrients is a possible cause of muscle loss [36].
4 Diagnosis and Management of Sarcopenia in Liver Disease
The Asian Working Group for Sarcopenia (AWGS) (AWGS/grip criteria) and European Working Group on Sarcopenia in Older People (EWGSOP) (EWGSOP/grip criteria) are always used in the diagnosis of sarcopenia in patient with chronic liver disease [84,85,86,87]. EWGSOP/grip criteria found that age-related muscle volume reduction is related to low muscle strength and/or physical performance [88,89,90]. Low muscle mass and decreased muscle function (muscle strength or properties) are used as a screening test, according to the diagnostic criteria of EWGSOP in 2010, it means establishing a diagnosis requires meeting criteria 1 and criteria 2 or criteria 3 at the same time [91, 92]. Different from EWGSOP/grip criteria, the patients are also diagnosed as sarcopenic by both muscle strength (handgrip strength) and physical performance (usual gait speed) as the instruction of AWGS/grip criteria [85]. Due to differences in body size, lifestyles, ethnicities, and cultural backgrounds, each criterion describes the cutoff value used for Asian and European populations by detail. The cutoff threshold for calf circumference is 33 cm, and that of hand grip strength in male and female are 32 kg and 22 kg, respectively [87]. Psoas muscle thickness and total muscle and adipose tissue cross-sectional area at the level of the third lumbar vertebra (L3) transverse processes are always commonly used for measuring muscle mass imaging with computed tomography (CT) or magnetic resonance imaging [93,94,95]. In the diagnosis and screening of sarcopenia in patients with chronic liver disease (CHD), scientists have made many attempts and explorations. Some scholars have found that serum BCAA and albumin levels are significantly associated with handgrip strength and PSI (psoas index) in patients without BCAA granule supplement, though the contact strength is weak [96, 97]. The reduction of BCAA level as a manifestation of CHL progress may play a role in the muscle atrophy associated with primary disease. Researchers are still looking for highly sensitive and noninvasive markers to improve diagnostic efficiency.
5 Method of Reversing Sarcopenia of Cirrhosis
Because muscle reduction is associated with adverse outcomes of liver cirrhosis, limited data has shown that increased muscle mass can improve survival of patients with liver cirrhosis after transplantation. Therefore, reversing muscle mass reduction is a key measure for patients with cirrhosis [98]. According to the physiopathological mechanism of sarcopenia, the method of managing sarcopenia was built by considering nutritional status, physical activity, ammonia, and hormones [99]. Guidelines and consensus statement put forward basic concept. The present therapeutic strategies for sarcopenia in cirrhosis include exercise and nutrition therapy, supplemental hormone therapy, and mechanistic targeted treatments.
5.1 Exercise and Nutrition Therapy
Vast solid evidences have identified the positive effects of exercise, whereas, unfortunately, this “panacea” has not been applied properly. Smart selection of exercise type is important to ensure maximum benefit to the patients [100]. Resistance exercise (RE) can stimulate muscle protein synthesis (MPS) which has the potential to modulate muscle mass gain [101]. Different from RE, endurance exercise (EE) may improve the exercise capacity and muscle strength. Only few studies have been conducted to assess the benefit of patients undergoing exercise training in combination with RE and EE by far, so the benefits still remain unclarified. It is still not possible to predict whether a synthetic metabolic nutrient resistance will be observed during exercise. The mechanical stimuli activate mTOR signaling in muscle through a PLD-dependent increase of phosphatidic acid (PA) [102]. The current exercise guidelines for patients with chronic diseases recommend that individuals perform 150 min of moderate physical activity per week, and two times a week for endurance and flexibility training. Due to the limitations of exercise capacity, these guidelines may not be feasible in most patients with cirrhosis. It is still advocated that the exercise experts should assess the patient’s motor ability and clinical status and formulate the individualized exercise prescription [64, 103, 104]. But all the studies were carried out in the patients or animal models without cirrhosis, and it was not clear whether the responses were tested in patients with cirrhosis or not. For example, studies have shown that hyperammonemia leads to decreased muscle function without affecting the muscle mass and that hyperammonemia impairs skeletal muscle strength and increases muscle fatigue. These suggest that blood ammonia may also affect the therapeutic value of exercise for muscle atrophy in the liver disease model, different from that in the simple sarcopenia model [105].
Because the lack of nutrition in patients is an important cause of sarcopenia, which is mainly due to insufficient intake of total calories and protein, thus guidelines and consensus statements recommend frequent feeding. Oral rehydration is the best way to supplement, and enteral or parenteral nutrition is applied if necessary [106,107,108]. There are numerous strategies for extra nutrition through high-calorie feeding and/or enteral feeding provided by different studies [109,110,111].
In terms of nutrition, the two main problems are the plan and time of nutrition supply. Study indicated that giving patients late-night food is a feasible intervention to reverse the reduction of synthetic metabolism and muscle atrophy in patients with cirrhosis and can improve the life quality of patients with cirrhosis. The long-term benefits and the value on lifespan were critically evaluated. The subsequent meta-analysis was disappointing, and nutritional supplements for patients with alcoholic hepatitis and liver cirrhosis demonstrated no improvement in survival rate. The exact mechanism of the protective effect of supplemental nutrition on muscle loss is unclear, which allows us to consider other factors that contribute to such uncertainty. As a form of resistance to synthetic metabolism, the nutritional problem of cirrhosis may not be compromised by supplementing energy alone. We need to consider the effects of impaired mitochondrial function on nutrition management. Other clinical symptoms, including encephalopathy and septicemia, and how to improve the life quality are also needed to be considered in future studies.
Protein supplementation is another way to improve the supply of essential amino acids. However, liver cirrhosis and high blood ammonia may accelerate the decomposition of amino acid. This results in ammonia accumulated in skeletal muscle, which damages the protein synthesis and further increases the autophagy. These are not benefits to reverse sarcopenia. In the selection of protein sources, plant proteins have an advantage over animal protein, which are rich in branched-chain amino acid (BCAA) rather than aromatic amino acids [112,113,114]. For example, leucine is particularly an important activator of mTORC1 via the Rag small GTPases and a plethora of regulatory proteins, leading to decreased autophagy and protein synthesis, which is the protection mechanism against loss of muscle. Confirmed results have provided direct evidence on interference of the molecules in skeletal muscle during cirrhosis [115, 116]. A single oral BCAA mixture enriched with leucine (BCAA/LEU) can impair mTOR1 signaling, autophagy, and GCN2 activation in cirrhotic patients without altering myostatin expression [117]. Combined with in vivo and in vitro data of, hyperammonemia is considered as the mediator of hepato-muscular axis and BCAA supplement is beneficial for cirrhosis [118, 119].
5.2 Supplemental Hormone Therapy
Both sarcopenia and low testosterone have been found associated with poor prognosis in men with cirrhosis, independent of the Model for End-Stage Liver Disease (MELD) score. Testosterone and growth hormones are used to improve nutritional status and muscle mass in cirrhosis patients, but the clinical benefits remain to be verified [120,121,122,123]. Anabolic androgenic steroid oxandrolone shows an improvement in nutritional status, body composition, and muscle function, as well as the non-muscle beneficial effects such as the ameliorating condition of the original disease in men with cirrhosis. But unfortunately, testosterone treatment can significantly reduce the mortality of patients (16% vs. 25.5%, p = 0.352). Even though research suggests that low testosterone has its advantages in predicting mortality in men with advanced liver disease than sarcopenia [124], it still needs to be addressed whether testosterone is continuously effective in improving the prognosis in liver cirrhosis patients with sarcopenia.
5.3 Other Potential Strategies
According to the documented mechanism mentioned above, the scientists propose treatment strategies for the corresponding targets, which require preclinical trials to clarify the effect. Myostatin antagonists, antioxidants, mitochondrial protectants, and direct mTORC1 activators may benefit skeletal muscle protein turnover but are not adequately evaluated [117, 118, 125].
Hyperammonemia could be another common concern in both sarcopenia and end-stage liver disease. Current methods for decreasing plasma ammonia include nonabsorbable disaccharides and antibiotics by preventing the production of ammonia. In the treatment of patients with liver cirrhosis, the main purpose of lowering blood ammonia originally is to cure hepatic encephalopathy; however, the latest views suggest that blood concentration of ammonia is completely not associated with the severity of hepatic encephalopathy [126]. Since it takes a long time for serum ammonia to affect the muscles, lowering blood ammonia in the short term does not reduce muscle blood ammonia concentration. The changes of high blood ammonia on signal pathway activation and metabolism cannot be reversed. Loss of muscle mass and function can be saved only by long-term, continuous ammonia-lowering therapies, or by targeting lower levels of ammonia in the skeletal muscle. Supplemental BCAA are used as a therapy in patients with cirrhosis, especially in the patients with hepatic encephalopathy (HE) [127,128,129]. The oral dosage of BCAA can enhance the metabolism of muscle ammonia, reducing the ammonia content in muscle. However, this method may also temporarily increase the concentration of arterial ammonia, which may be due to the external metabolism of glutamine (GLN). The contents of GLN in skeletal muscle can be maintained by parenteral α-KG supplemental after surgery. GLN synthesis may exert adverse effects of catabolism stimulation by BCAA in skeletal muscle. Thus, reducing the use of α-KG and other drugs that promote GLN synthesis should be considered [130,131,132].
6 Challenges in Study on Sarcopenia in Liver Disease
Sarcopenia is a common manifestation of chronic liver diseases. On one hand liver disease accompanied with sarcopenia adds the burden of the disease; on the other hand, sarcopenia can become a potential monitor of liver diseases and its complications. Although the researches have drawn a similar conclusion of correlation between sarcopenia and NAFLD and put forward the possible mechanism, there remain questions to be addressed. Firstly, some researchers have pointed out that it needs to pay attention to the diagnostic criteria of NAFLD used in studies. Skeletal muscle index (SMI) is the most commonly used index for assessing sarcopenia (SMI = total appendicular skeletal muscle mass [kg]/body mass index [kg/m2]). NAFLD is diagnosed by noninvasive evaluation methods, such as NAFLD liver fat score and liver attenuation index (LAI). NAFLD patients are likely to be more obese, which affects the score of SMI. Moreover, there is no uniform standard to the choice of cutoff point in NAFLD diagnosis [133]. Hence, it is indispensable to build a research based on biopsy-proven or imaging-defined fatty liver. Secondly, the analysis results of the above data used adjustment variable in the logistic model. Some exposed factors such as IR, obesity, and low vitamin D, which will affect the results, are not included, though the researchers adjusted for other variables. The effect of these moderators should be considered deliberately. Meanwhile it is clear that lifestyles, ethnicities, and cultural background have a great influence on IR, which is the important component in the formation of either NAFLD or sarcopenia. Multicenter large-scale trials need to put into practice for formulating feasible and effective primary intervention strategies. Thirdly, the evidence shows a significant correlation between sex and the occurrence of sarcopenia in patient with NAFLD, which maybe a consequence of sex hormone. But there are no individualized treatment options for male and female. Lastly, we are still not certain about whether NAFLD is a cause or a consequence of IR. In conclusion, sarcopenia is a promising early warning factor for chronic liver disease, especially NAFLD, whereas lots of issues will need to be discussed in future studies.
References
Kim TN, Choi KM (2013) Sarcopenia: definition, epidemiology, and pathophysiology. Journal of bone metabolism 20(1):1–10. https://doi.org/10.11005/jbm.2013.20.1.1
Tsekoura M, Kastrinis A, Katsoulaki M, Billis E, Gliatis J (2017) Sarcopenia and its impact on quality of life. Adv Exp Med Biol 987:213–218. https://doi.org/10.1007/978-3-319-57379-3_19
Afilalo J (2016) Conceptual models of frailty: the sarcopenia phenotype. Can J Cardiol 32(9):1051–1055. https://doi.org/10.1016/j.cjca.2016.05.017
Aguiar R, Sequeira J, Meirinhos T, Ambrosio C, Barcelos A (2014) SARCOSPA – sarcopenia in spondyloarthritis patients. Acta Reumatol Port 39(4):322–326
Poggiogalle E, Lubrano C, Sergi G, Coin A, Gnessi L, Mariani S, Lenzi A, Donini LM (2016) Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. J Nutr Health Aging 20(9):958–963. https://doi.org/10.1007/s12603-015-0638-1
Chung JH, Hwang HJ, Shin HY, Han CH (2016) Association between Sarcopenic obesity and bone mineral density in middle-aged and elderly Korean. Ann Nutr Metab 68(2):77–84. https://doi.org/10.1159/000442004
Baracos V, Kazemi-Bajestani SM (2013) Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol 45(10):2302–2308. https://doi.org/10.1016/j.biocel.2013.06.016
Holecek M (2012) Muscle wasting in animal models of severe illness. Int J Exp Pathol 93(3):157–171. https://doi.org/10.1111/j.1365-2613.2012.00812.x
Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ (2013) Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr 56(1):270–278. https://doi.org/10.1016/j.archger.2012.09.007
Seo JA, Cho H, Eun CR, Yoo HJ, Kim SG, Choi KM, Baik SH, Choi DS, Park MH, Han C, Kim NH (2012) Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: the Ansan geriatric study. J Am Geriatr Soc 60(4):700–706. https://doi.org/10.1111/j.1532-5415.2012.03887.x
Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC (2010) Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean longitudinal study on health and aging (KLoSHA). Diabetes Care 33(7):1652–1654. https://doi.org/10.2337/dc10-0107
Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM (2009) Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (2005) 33(8):885–892. https://doi.org/10.1038/ijo.2009.130
Dasarathy S (2016) Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol 32(3):159–165. https://doi.org/10.1097/MOG.0000000000000261
Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K (2010) Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology (Baltimore, Md) 51(6):2040–2048. https://doi.org/10.1002/hep.23588
Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R (2016) The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology (Baltimore, Md) 64(5):1577–1586. https://doi.org/10.1002/hep.28785
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md) 64(1):73–84. https://doi.org/10.1002/hep.28431
Beier JI, Banales JM (2018) Pyroptosis: an inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol 68:643. https://doi.org/10.1016/j.jhep.2018.01.017
Zoli M, Marchesini G, Dondi C, Bianchi GP, Pisi E (1982) Myofibrillar protein catabolic rates in cirrhotic patients with and without muscle wasting. Clin Sci (Lond) 62(6):683–686
Martin F, Ward K, Slavin G, Levi J, Peters TJ (1985) Alcoholic skeletal myopathy, a clinical and pathological study. Q J Med 55(218):233–251
de Sousa C, Leung NW, Chalmers RA, Peters TJ (1988) Free and total carnitine and acylcarnitine content of plasma, urine, liver and muscle of alcoholics. Clin Sci (London, England : 1979) 75(4):437–440
Weber FL Jr, Macechko PT, Kelson SR, Karajiannis E, Hassan MO (1992) Increased muscle protein catabolism caused by carbon tetrachloride hepatic injury in rats. Gastroenterology 102(5):1700–1706
Gayan-Ramirez G, van de Casteele M, Rollier H, Fevery J, Vanderhoydonc F, Verhoeven G, Decramer M (1998) Biliary cirrhosis induces type IIx/b fiber atrophy in rat diaphragm and skeletal muscle, and decreases IGF-I mRNA in the liver but not in muscle. J Hepatol 29(2):241–249
Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA (2002) Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76(2):473–481
Gowda C, Compher C, Amorosa VK, Lo Re V 3rd (2014) Association between chronic hepatitis C virus infection and low muscle mass in US adults. J Viral Hepat 21(12):938–943. https://doi.org/10.3748/wjg.v20.i25.806110.1111/jvh.12273
Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM (2014) Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic obesity study. Hepatology (Baltimore, Md) 59(5):1772–1778. https://doi.org/10.1002/hep.26716
Kim HY, Kim CW, Park CH, Choi JY, Han K, Merchant AT, Park YM (2016) Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: the fifth Korea National Health and nutrition examination survey. Hepatobiliary Pancreat Dis Int: HBPD INT 15(1):39–47
Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS (2015) Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol 63(2):486–493. https://doi.org/10.1016/j.jhep.2015.02.051
Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH (2016) Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology (Baltimore, Md) 63(3):776–786. https://doi.org/10.1002/hep.28376
Hashimoto Y, Osaka T, Fukuda T, Tanaka M, Yamazaki M, Fukui M (2016) The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr J 63(10):877–884. https://doi.org/10.1507/endocrj.EJ16-0124
Dasarathy S (2012) Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 3(4):225–237. https://doi.org/10.1007/s13539-012-0069-3
Montano-Loza AJ (2014) Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol 20(25):8061–8071. https://doi.org/10.1002/lt.2397810.3748/wjg.v20.i25.8061
Kalafateli M, Konstantakis C, Thomopoulos K, Triantos C (2015) Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol 21(24):7357–7361. https://doi.org/10.3748/wjg.v21.i24.7357
Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB (2012) Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10(2):166–173. https://doi.org/10.1016/j.cgh.2011.08.028
Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB (2013) Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 47(10):861–870. https://doi.org/10.1097/MCG.0b013e318293a825
Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H (2015) Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition (Burbank, Los Angeles County, Calif) 31(1):193–199. https://doi.org/10.1016/j.nut.2014.07.005
Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP (2012) Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 18(10):1209–1216. https://doi.org/10.1002/lt.23495
Toshima T, Shirabe K, Kurihara T, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, Kawanaka H, Ikeda T, Maehara Y (2015) Profile of plasma amino acids values as a predictor of sepsis in patients following living donor liver transplantation: special reference to sarcopenia and postoperative early nutrition. Hepatol Res 45(12):1170–1177. https://doi.org/10.1111/hepr.12484
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M (2015) Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 261(2):345–352. https://doi.org/10.1097/SLA.0000000000000628
Lucero C, Verna EC (2015) The role of sarcopenia and frailty in hepatic encephalopathy management. Clin Liver Dis 19(3):507–528. https://doi.org/10.1016/j.cld.2015.04.003
Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Bjornsson E (2007) Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int 27(9):1194–1201. https://doi.org/10.1111/j.1478-3231.2007.01562.x
Kim HY, Jang JW (2015) Sarcopenia in the prognosis of cirrhosis: going beyond the MELD score. World J Gastroenterol 21(25):7637–7647. https://doi.org/10.3748/wjg.v21.i25.7637
Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, Hattori A, Ishidome M, Kobayashi Y, Hasegawa H, Iwata K, Takei Y (2016) Sarcopenia and Sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med (Tokyo, Japan) 55(8):863–870. https://doi.org/10.2169/internalmedicine.55.5676
Bergerson JT, Lee JG, Furlan A, Sourianarayanane A, Fetzer DT, Tevar AD, Landsittel DP, DiMartini AF, Dunn MA (2015) Liver transplantation arrests and reverses muscle wasting. Clin Transpl 29(3):216–221. https://doi.org/10.1111/ctr.12506
Mizuno Y, Ito S, Hattori K, Nagaya M, Inoue T, Nishida Y, Onishi Y, Kamei H, Kurata N, Hasegawa Y, Ogura Y (2016) Changes in muscle strength and six-minute walk distance before and after living donor liver transplantation. Transplant Proc 48(10):3348–3355. https://doi.org/10.1016/j.transproceed.2016.08.042
Montano-Loza AJ (2014) Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 20(11):1424. https://doi.org/10.1002/lt.2395910.1002/lt.23978
Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, Barrett T, Trushar P, Esser K, Gedaly R (2016) Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 31(3):628–633. https://doi.org/10.1111/jgh.13166
Clark K, Cross T (2014) Sarcopenia and survival after liver transplantation. J Korean Med Sci 20(11):1423. https://doi.org/10.3346/jkms.2014.29.9.125310.1002/lt.23959
Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE (2014) Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 63(8):2702–2713. https://doi.org/10.2337/db14-0276
Bambha K, Wilson LA, Unalp A, Loomba R, Neuschwander-Tetri BA, Brunt EM, Bass NM, Nonalcoholic Steatohepatitis Clinical Research Network (2014) Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int 34(8):1250–1258. https://doi.org/10.1111/liv.12379
Chang CY (2011) Understanding the relationship between PNPLA3, NAFLD and insulin resistance: do ethnic differences bring more questions or more answers? Liver Int 31(9):1246–1249. https://doi.org/10.1111/j.1478-3231.2011.02612.x
Bril F, Sninsky JJ, Baca AM, Superko HR, Portillo Sanchez P, Biernacki D, Maximos M, Lomonaco R, Orsak B, Suman A, Weber MH, McPhaul MJ, Cusi K (2016) Hepatic steatosis and insulin resistance, but not steatohepatitis, promote Atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab 101(2):644–652. https://doi.org/10.1210/jc.2015-3111
Oh C, Jeon BH, Reid Storm SN, Jho S, No JK (2017) The most effective factors to offset sarcopenia and obesity in the older Korean: physical activity, vitamin D, and protein intake. Nutrition (Burbank, Los Angeles County, Calif) 33:169–173. https://doi.org/10.1016/j.nut.2016.06.004
Li H, Liu S, Yuan H, Niu Y, Fu L (2017) Sestrin 2 induces autophagy and attenuates insulin resistance by regulating AMPK signaling in C2C12 myotubes. Exp Cell Res 354(1):18–24. https://doi.org/10.1016/j.yexcr.2017.03.023
Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K (2009) Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md) 50(4):1087–1093. https://doi.org/10.1002/hep.23116
Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M (2005) Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48(4):634–642. https://doi.org/10.1007/s00125-005-1682-x
Kalyani RR, Corriere M, Ferrucci L (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2(10):819–829. https://doi.org/10.1016/S2213-8587(14)70034-8
Guillet C, Boirie Y (2005) Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab 31 Spec No 2:5S20–25S26
Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R (2013) Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 38(3):246–254. https://doi.org/10.1111/apt.12377
Maestro B, Davila N, Carranza MC, Calle C (2003) Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84(2–3):223–230
Abdalla M, Khairy E, Louka ML, Ali-Labib R, Ibrahim EA (2018) Vitamin D receptor gene methylation in hepatocellular carcinoma. Gene 653:65. https://doi.org/10.1016/j.gene.2018.02.024
Del Pinto R, Ferri C, Cominelli F (2017) Vitamin D Axis in inflammatory bowel diseases: role, current uses and future perspectives. Int J Mol Sci 18(11). https://doi.org/10.3390/ijms18112360
Camperi A, Pin F, Costamagna D, Penna F, Menduina ML, Aversa Z, Zimmers T, Verzaro R, Fittipaldi R, Caretti G, Baccino FM, Muscaritoli M, Costelli P (2017) Vitamin D and VDR in cancer cachexia and muscle regeneration. Oncotarget 8(13):21778–21793. https://doi.org/10.18632/oncotarget.15583
Beauregard ME, Provost S, Pineault R, Grimard D, Perez J, Fournier M (2018) Effects on patients of variations in the implementation of a cardiometabolic risk intervention program in Montreal. Health Promotion Chronic Dis Prev Can Res Pol Pract 38(2):64–77. https://doi.org/10.24095/hpcdp.38.2.03
Jones JC, Coombes JS, Macdonald GA (2012.) Exercise capacity and muscle strength in patients with cirrhosis) Liver Transpl 18(2):146–151. https://doi.org/10.1002/lt.22472
Kim TY, Kim MY, Sohn JH, Kim SM, Ryu JA, Lim S, Kim Y (2014) Sarcopenia as a useful predictor for long-term mortality in cirrhotic patients with ascites. J Korean Med Sci 29(9):1253–1259. https://doi.org/10.3346/jkms.2014.29.9.1253
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8(8):457–465. https://doi.org/10.1038/nrendo.2012.49
Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B (2011) Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology (Baltimore, Md) 54(3):846–856. https://doi.org/10.1002/hep.24517
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 106(48):20405–20410. https://doi.org/10.1073/pnas.0911570106
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481(7382):463–468. https://doi.org/10.1038/nature10777
Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, Han CK, Zhuang XJ, Lu Y, Li XJ, Yang SY, Li XY (2013) Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol 59(3):557–562. https://doi.org/10.1016/j.jhep.2013.04.030
Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Muller M (2010) Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology (Baltimore, Md) 51(2):511–522. https://doi.org/10.1002/hep.23337
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM (2009) Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58(1):250–259. https://doi.org/10.2337/db08-0392
Watts R, Ghozlan M, Hughey CC, Johnsen VL, Shearer J, Hittel DS (2014) Myostatin inhibits proliferation and insulin-stimulated glucose uptake in mouse liver cells. Biochemistry and cell biology = Biochimie et biologie cellulaire 92(3):226–234. https://doi.org/10.1139/bcb-2014-0004
Hu SL, Chang AC, Huang CC, Tsai CH, Lin CC, Tang CH (2017) Myostatin promotes interleukin-1beta expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Front Immunol 8:1747. https://doi.org/10.3389/fimmu.2017.01747
Carvalho LP, Basso-Vanelli RP, Di Thommazo-Luporini L, Mendes RG, Oliveira-Junior MC, Vieira RP, Bonjorno-Junior JC, Oliveira CR, Luporini R, Borghi-Silva A (2017) Myostatin and adipokines: the role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults. Cytokine 107:118. https://doi.org/10.1016/j.cyto.2017.12.008
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209(2):501–514. https://doi.org/10.1002/jcp.20757
Astorino TA, Harness ET, Witzke KA (2015) Chronic activity-based therapy does not improve body composition, insulin-like growth factor-I, adiponectin, or myostatin in persons with spinal cord injury. J Spinal Cord Med 38(5):615–625. https://doi.org/10.1179/2045772314Y.0000000236
Suzuki ST, Zhao B, Yang J (2008) Enhanced muscle by myostatin propeptide increases adipose tissue adiponectin, PPAR-alpha, and PPAR-gamma expressions. Biochem Biophys Res Commun 369(2):767–773. https://doi.org/10.1016/j.bbrc.2008.02.092
Wilkes JJ, Lloyd DJ, Gekakis N (2009) Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes 58(5):1133–1143. https://doi.org/10.2337/db08-0245
Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Takata R, Iijima H, Nishiguchi S (2017) Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle 8(6):915–925. https://doi.org/10.1002/jcsm.12212
Cabrera D, Ruiz A, Cabello-Verrugio C, Brandan E, Estrada L, Pizarro M, Solis N, Torres J, Barrera F, Arrese M (2016) Diet-induced nonalcoholic fatty liver disease is associated with sarcopenia and decreased serum insulin-like growth Factor-1. Dig Dis Sci 61(11):3190–3198. https://doi.org/10.1007/s10620-016-4285-0
Thandassery RB, Montano-Loza AJ (2016) Role of nutrition and muscle in cirrhosis. Curr Treat Options Gastroenterol 14(2):257–273. https://doi.org/10.1007/s11938-016-0093-z
Periyalwar P, Dasarathy S (2012) Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 16(1):95–131. https://doi.org/10.1016/j.cld.2011.12.009
Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M, Asian Working Group for S (2016) Recent advances in sarcopenia research in Asia: 2016 update from the Asian working group for sarcopenia. J Am Med Dir Assoc 17(8):e761–e767. https://doi.org/10.1016/j.jamda.2016.05.016
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15(2):95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Soysal P, Isik AT (2016) Comment on “cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition”. Clin Nutr (Edinburgh, Scotland) 35(6):1586. https://doi.org/10.1016/j.clnu.2016.09.007
Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, Erten N, Karan MA, Cruz-Jentoft AJ (2016) Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr (Edinburgh, Scotland) 35(6):1557–1563. https://doi.org/10.1016/j.clnu.2016.02.002
da Silva Alexandre T, de Oliveira Duarte YA, Ferreira Santos JL, Wong R, Lebrao ML (2014) Sarcopenia according to the European working group on sarcopenia in older people (EWGSOP) versus Dynapenia as a risk factor for disability in the elderly. J Nutr Health Aging 18(5):547–553. https://doi.org/10.1007/s12603-013-0424-x
Lera L, Albala C, Sanchez H, Angel B, Hormazabal MJ, Marquez C, Arroyo P (2017) Prevalence of sarcopenia in community-dwelling Chilean elders according to an adapted version of the European working group on sarcopenia in older people (EWGSOP) criteria. J Frailty Aging 6(1):12–17. https://doi.org/10.14283/jfa.2016.117
Kim YP, Kim S, Joh JY, Hwang HS (2014) Effect of interaction between dynapenic component of the European working group on sarcopenia in older people sarcopenia criteria and obesity on activities of daily living in the elderly. J Am Med Dir Assoc 15(5):371 e371–371 e375. https://doi.org/10.1016/j.jamda.2013.12.010
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Bahat G, Tufan A, Kilic C, Karan MA, Cruz-Jentoft AJ (2017) Methodological issues in determination of low muscle mass reference cut-off values: reply to comment on “cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition”. Clin Nutr (Edinburgh, Scotland) 36(3):903–904. https://doi.org/10.1016/j.clnu.2017.02.023
Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, Lucidi C, Di Martino M, Catalano C, Merli M (2015) Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 27(3):328–334. https://doi.org/10.1097/meg.0000000000000274
Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, Tamaki J (2014) Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European working group on sarcopenia in older people. Arch Gerontol Geriatr 59(2):295–299. https://doi.org/10.1016/j.archger.2014.04.016
Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, Ismond K, Mann S, Alaboudy A, Ma M (2016) A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 14 (10):1473–1480.e1473. doi:https://doi.org/10.1016/j.cgh.2016.04.040
Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y (2016) Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol 28(8):940–947. https://doi.org/10.1097/MEG.0000000000000661
Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, Edwards M, Dennison E, Cooper C, Aihie Sayer A (2013) Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 42(3):378–384. https://doi.org/10.1093/ageing/afs197
Tsien C, Shah SN, McCullough AJ, Dasarathy S (2013) Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol 25(1):85–93. https://doi.org/10.1097/MEG.0b013e328359a759
Sinclair M, Gow PJ, Grossmann M, Angus PW (2016) Review article: sarcopenia in cirrhosis–aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 43(7):765–777. https://doi.org/10.1111/apt.13549
Fyfe JJ, Bishop DJ, Stepto NK (2014) Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44(6):743–762. https://doi.org/10.1007/s40279-014-0162-1
Damas F, Phillips S, Vechin FC, Ugrinowitsch C (2015) A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45(6):801–807. https://doi.org/10.1007/s40279-015-0320-0
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A 103(12):4741–4746. https://doi.org/10.1073/pnas.0600678103
Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A (2016) The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 22(10):1324–1332. https://doi.org/10.1002/lt.24506
Nishikawa H, Osaki Y (2015) Liver cirrhosis: evaluation, nutritional status, and prognosis. Mediators Inflamm 2015:872152. https://doi.org/10.1155/2015/872152
McDaniel J, Davuluri G, Hill EA, Moyer M, Runkana A, Prayson R, van Lunteren E, Dasarathy S (2016) Hyperammonemia results in reduced muscle function independent of muscle mass. Am J Physiol Gastrointest Liver Physiol 310(3):G163–G170. https://doi.org/10.1152/ajpgi.00322.2015
Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, Uribe M, Vilstrup H, Morgan MY (2013) The nutritional management of hepatic encephalopathy in patients with cirrhosis: international society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology (Baltimore, Md) 58(1):325–336. https://doi.org/10.1002/hep.26370
Plauth M, Cabre E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Dgem FP, Holm E, Vom Dahl S, Muller MJ, Nolte W, Espen (2006) ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr (Edinburgh, Scotland) 25(2):285–294. https://doi.org/10.1016/j.clnu.2006.01.018
Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Muller MJ, Group EC (1997) ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr (Edinburgh, Scotland) 16(2):43–55
Dasarathy S, Merli M (2016) Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 65(6):1232–1244. https://doi.org/10.1016/j.jhep.2016.07.040
Toshikuni N, Arisawa T, Tsutsumi M (2014) Nutrition and exercise in the management of liver cirrhosis. World J Gastroenterol 20(23):7286–7297. https://doi.org/10.3748/wjg.v20.i23.7286
Juakiem W, Torres DM, Harrison SA (2014) Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis 18(1):179–190. https://doi.org/10.1016/j.cld.2013.09.004
Metcalfe EL, Avenell A, Fraser A (2014) Branched-chain amino acid supplementation in adults with cirrhosis and porto-systemic encephalopathy: systematic review. Clin Nutr (Edinburgh, Scotland) 33(6):958–965. https://doi.org/10.1016/j.clnu.2014.02.011
Alexander WF, Spindel E, Harty RF, Cerda JJ (1989) The usefulness of branched chain amino acids in patients with acute or chronic hepatic encephalopathy. Am J Gastroenterol 84(2):91–96
Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, Aagaard NK, Risum N, Vilstrup H (2013) Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr 143(8):1263–1268. https://doi.org/10.3945/jn.113.174375
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM (2008) The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320(5882):1496–1501. https://doi.org/10.1126/science.1157535
Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10(8):935–945. https://doi.org/10.1038/ncb1753
Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, Deutz NE, Dasarathy S (2015) Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology (Baltimore, Md) 61(6):2018–2029. https://doi.org/10.1002/hep.27717
Carroll B, Korolchuk VI, Sarkar S (2015) Amino acids and autophagy: cross-talk and co-operation to control cellular homeostasis. Amino Acids 47(10):2065–2088. https://doi.org/10.1007/s00726-014-1775-2
Guo F, Cavener DR (2007) The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5(2):103–114. https://doi.org/10.1016/j.cmet.2007.01.001
Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ (2016) Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 65(5):906–913. https://doi.org/10.1016/j.jhep.2016.06.007
Moller S, Becker U, Gronbaek M, Juul A, Winkler K, Skakkebaek NE (1994) Short-term effect of recombinant human growth hormone in patients with alcoholic cirrhosis. J Hepatol 21(5):710–717
Bucuvalas JC, Cutfield W, Horn J, Sperling MA, Heubi JE, Campaigne B, Chernausek SD (1990) Resistance to the growth-promoting and metabolic effects of growth hormone in children with chronic liver disease. J Pediatr 117(3):397–402
Orr R, Fiatarone Singh M (2004) The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs 64(7):725–750
Sinclair M, Grossmann M, Angus PW, Hoermann R, Hey P, Scodellaro T, Gow PJ (2016) Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol 31(3):661–667. https://doi.org/10.1111/jgh.13182
Han HQ, Zhou X, Mitch WE, Goldberg AL (2013) Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol 45(10):2333–2347. https://doi.org/10.1016/j.biocel.2013.05.019
Rose CF (2012) Ammonia-lowering strategies for the treatment of hepatic encephalopathy. Clin Pharmacol Ther 92(3):321–331. https://doi.org/10.1038/clpt.2012.112
Marchesini G, Bianchi G, Zoli M (1991) Oral BCAA in the treatment of chronic hepatic encephalopathy. J Hepatol 12(2):267
Caballeria Rovira E, Arago Lopez JV, Masso Ubeda RM, Vidal Clemente JL, Sanchis Closa A (1987) [Treatment of hepatic encephalopathy with branched-chain amino acids (BCAA) by oral route: II. Chronic hepatic encephalopathy]. Revista espanola de las enfermedades del aparato digestivo 72(3):201–205
Freund HR, Fischer JE (1986) The use of branched chain amino acids (BCAA) in acute hepatic encephalopathy. Clin Nutr (Edinburgh, Scotland) 5(3):135–138
Hadjihambi A, Rose CF, Jalan R (2014) Novel insights into ammonia-mediated neurotoxicity pointing to potential new therapeutic strategies. Hepatology (Baltimore, Md) 60(3):1101–1103. https://doi.org/10.1002/hep.27282
Dam G, Ott P, Aagaard NK, Vilstrup H (2013) Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis 28(2):217–220. https://doi.org/10.1007/s11011-013-9377-3
Holecek M (2014) Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis 29(1):9–17. https://doi.org/10.1007/s11011-013-9428-9
Durand F, Buyse S, Francoz C, Laouenan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D (2014) Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 60(6):1151–1157. https://doi.org/10.1016/j.jhep.2014.02.026
Acknowledgments
This work was supported by the grants from Shanghai Municipal Commission of Health and Family Planning (201540082 to Q. Liu), National Natural Science Foundation of China (81670571 and 81370559 to C. Yang; 81400635 to F. Wang), Joint Projects in Major Diseases funding from Shanghai Municipal Commission of Health and Family Planning (2014ZYJB0201 to C. Yang), Joint Projects for Novel Frontier Technology in Shanghai Municipal Hospital from Shanghai Municipal Commission of Health and Family Planning (SHDC12014122 to C. Yang), Shanghai Medical Guide Project from Shanghai Science and Technology Committee (14411971500 to F. Wang), grants from Chinese Foundation for Hepatitis Prevention and Control (TQGB20140141 to F. Wang), and funds from Shanghai Innovation Program (12431901002 to C. Yang).
Competing Financial Interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Song, M., Xia, L., Liu, Q., Sun, M., Wang, F., Yang, C. (2018). Sarcopenia in Liver Disease: Current Evidence and Issues to Be sResolved. In: Xiao, J. (eds) Muscle Atrophy. Advances in Experimental Medicine and Biology, vol 1088. Springer, Singapore. https://doi.org/10.1007/978-981-13-1435-3_19
Download citation
DOI: https://doi.org/10.1007/978-981-13-1435-3_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1434-6
Online ISBN: 978-981-13-1435-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)