Abstract
Arsenic (As) is a toxic metalloid of global concern derived from natural, geothermal, and anthropogenic sources. Arsenic has deleterious effects in all forms of life including plants. Between the two inorganic forms, the highly oxidized pentavalent arsenate (AsV) is prevalent in the aerobic environment, while the highly reduced trivalent arsenite (AsIII) is the predominant form in an anaerobic environment. The main route of AsV uptake in plants is through the phosphate transporters, while AsIII and methylated As species enter through nodulin 26–like intrinsic protein (NIP) or aquaglyceroporins. After entering into the plant cell As can severely impede plant metabolism which leads to various physiological disorder. Subsequently, growth of the plants is subdued, and it results in delaying or restraining accrual of biomass and induces loss of fertility, yield, and fruit production. Exposure to inorganic As in plants promotes oxidative stress by generating reactive oxygen species (ROS) during their conversion from AsV to AsIII. Plants have a well-organized antioxidant defense system to combat As stress. In plants, As intoxication triggers the activation of enzymatic antioxidants like superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), glutathione S-transferase (GST), and glutathione peroxidase (GPX); synthesis of nonenzymatic antioxidants, such as ascorbate and γ-Glu-Cys-Gly-tripeptide glutathione (GSH); and accumulation of anthocyanin in the leaves. As tolerance in plants is achieved by the production of phytochelatin following As exposure which is derived from GSH. This chapter aims to provide current updates about the molecular mechanism involved in uptake of the inorganic and organic species of As, their translocation, and the As-induced stress in plants with a special emphasis on oxidative stress.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Arsenic (As) is a toxic metalloid. Naturally, it exists in four oxidation states (-III), (0), (+III), and (+V) (Rathinasabapathi et al. 2006). Mostly available chemical forms of As having diverse physicochemical properties are: arsenite (AsIII), arsenate (AsV), trimethylarsine (TMA), dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), arsenosugars, arsenocholine (AsC), arsenobetaine (AsB) (Panda et al. 2010). Due to various factors including dumping of industrial wastes and dust from smelters, As contamination in soils is indiscriminate in many parts of the world (Chatterjee et al. 2017a). Depending on the redox status, inorganic arsenite or arsenate is primarily present in soil solution, which is the most phytoavailable form (Meharg and Hartley-Whitaker 2002). Among the inorganic As species, trivalent state of As is most toxic in comparison to the pentavalent state (Gupta and Chatterjee 2017), whereas the organic As has less toxicity than inorganic species (Chung et al. 2014). AsV is present in aqueous solution in the form of H3AsO4, H2AsO4 −, HAsO4 2−, and AsO4 3, whereas AsIII exists in reducing form, for example, H3AsO3 in anaerobic groundwater (Panda et al. 2010). Arsenic may also associate in nature with several other metals like copper, cobalt, nickel, silver, and lead (Gupta et al. 2017).
Arsenic concentration usually varies from noncontaminated soil to contaminated soils from 10 mg kg−1 to 30,000 mg kg−1, respectively (Adriano 1986; Vaughan 1993). Terrestrial plants grown in noncontaminated soil show less than 10 mg As kg−1 in tissues, but a typical threshold of 40 mg kg−1 of As has been reported from different tissues of crop plants (Matschullat 2000). High-affinity phosphate transporters help plants to readily take up arsenate (being an analogue of phosphate) from the soil (Meharg and Macnair 1992). Incorporation of As to the food chain via the groundwater-soil-plant system due to the use of high As contaminated groundwater in agriculture and bioaccumulation of As in crop plants are potentially hazardous to public health (Rahman et al. 2008; Patra et al. 2004).
Arsenic has no known biological function in plants. The exposure of plants to a higher level of AsIII and AsV induces the production of reactive oxygen species (ROS) (Gupta et al. 2013a). Transformation of arsenate to arsenite within plant cell produces ROS directly through Haber–Weiss reactions (Mascher et al. 2002, Mithöfer et al. 2004). Heavy metal interaction with the antioxidant system generates oxidative stress in plants (Srivastava et al. 2004), either indirectly through disruption of electron transport chain (Qadir et al. 2004), creating disorders in the essential elemental metabolisms, or directly through ROS-mediated cellular damages, enhanced lipid peroxidation, and membrane leakage (Dong et al. 2006). Arsenic-induced negative effect in plant development is a well-known fact (Islam et al. 2015), where significant interspecific variation and also among cultivars within the same species (like, Oryza sativa) are reported (Lei et al. 2013; Lemos Batista et al. 2014; Begum et al. 2016). Shorter length and lower biomass, mainly in roots, accompanied by oxidative stress of a plant, signify arsenic triggered stress symptoms (Abercrombie et al. 2008; Shri et al. 2009; Talukdar 2011; Upadhyay 2014).
Plants have evolved several mechanisms to combat As-induced stress such as suppression of high-affinity phosphate/arsenate transporter and to bind the metal to extracellular exudates and cell wall constituents thereby reducing uptake, sequestration of metals in the vacuole, complexation of metalloids by different substances, activation or modification of plant metabolism, and synthesis of antioxidant enzymes (Duquesnoy et al. 2010). Antioxidative defense is achieved either by nonenzymatic antioxidants with low molecular mass (like GSH, glutathione, and ascorbate (AsA)) and enzymatic antioxidants like ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione reductase (GR), and catalase (CAT) (Finnegan and Chen 2012; Sharma 2012; Talukdar 2013a).
Although a number of reports are available on the morphological and physiological mechanism of As uptake and accumulation in plants, however, oxidative information on stress induced by As and related defense mechanisms are still poorly recognized. The present chapter is an attempt to focus on oxidative stress in plants induced by As and antioxidant defense mechanisms relating to As uptake, translocation, and phytochelatin (PC)-mediated As detoxification mechanism.
5.2 Uptake of Different Arsenic Species by Plant
5.2.1 Arsenate Uptake
The pathways of As uptake in plants have been extensively investigated by several authors (Tripathi et al. 2007; Zhao et al. 2009, 2010; Mitra et al. 2017a). Physiological and electrophysiological studies revealed that as the oxyanion structure of arsenate (AsV) is analogous to inorganic phosphate (Pi), both are transported through shared transporter in higher plants (Meharg et al. 1994; Gupta et al. 2011). During uptake of each phosphate (H2PO4 −)/arsenate (H2AsO4 −) molecule, two protons (2H+) are co-transported across the membrane (Ullrich-Eberius et al. 1989). Although hundreds of phosphate transporters are recognized in higher plants, the PHT1 family of Pi transporter present in the roots is likely to be involved in AsV transport (Ullrich-Eberius et al. 1989; Wu et al. 2011). Studies reported that Pht protein transports AsV in As hyperaccumulators (Wang et al. 2002; Tu and Ma 2003; Cesaro et al. 2015), As-tolerant non-hyperaccumulators (Meharg and Macnair 1992; Bleeker et al. 2003), and also in As-sensitive non-accumulators (Esteban et al. 2003). However, different phosphate transporters present in hyperaccumulator plants show greater affinity for AsV than non-accumulator species of plants (Wang et al. 2002; Poynton et al. 2004). Double mutant Arabidopsis thaliana, for two high-affinity Pht1 isoform Pht 1;1 and Pht 1;4, was found to be resistant for arsenate than wild-type plants, which strongly supports the role of Pht 1;1 and Pht 1;4 in arsenate transport (Shin et al. 2004). Magnitude of phytotoxicity was greater following increasing uptake in soil with low levels of Pi as PHT transporters have higher affinity for phosphate than arsenate; therefore, AsV may outcompete Pi for entry through the root (Meharg et al. 1994). This can be overcome by applying larger amounts of phosphates that compete with arsenate at root surfaces to decrease uptake and phytotoxicity (Tu and Ma 2003; Titah et al. 2013). Some of the As-tolerant plants species such as Holcus lanatus and Cytisus striatus can grow in soil with higher As concentration without exhibiting any toxicity, which can be achieved by restricting the inflow of As by constitutive suppression of high-affinity phosphate/AsV transporter (Meharg and Macnair 1992; Bleeker et al. 2003).
5.2.2 Arsenite Uptake
In reducing environment, like swampy areas, arsenite (AsIII) is the predominant As species (Marin et al. 1993; Chatterjee et al. 2017b). In plants, members of nodulin 26–like intrinsic proteins (NIPs) commonly known as aquaporins are known to involve in AsIII transport through the root cells (Isayenkov and Maathuis 2008; Ma et al. 2008; Mitra et al. 2014). Additionally, NIPs also facilitate the transport of multiple uncharged solutes including glycerol, urea, ammonia, boric acid, and silicic acid, hence called aquaglyceroporins (Wallace et al. 2006) but impermeable to water (Bienert et al. 2008). The other three plant aquaporins comprise tonoplast intrinsic protein (TIPs), plasma membrane intrinsic protein PIP), and small basic intrinsic protein (SIPs) (Chaumont et al. 2005; Maurel et al. 2008). In contrast to arsenate, arsenite uptake is repressed by glycerol and antimonite instead of phosphate (Zhao et al. 2009). Aquatic macrophytes take up As either through phosphate transporter by active transport or passively through aquaglyceroporins and/or physicochemically adsorb in the root (Rahman and Hasegawa 2011; Mitra and Chatterjee 2016).
Ma et al. (2008) have isolated an arsenite transporter OsNIP 2;1, also known as Lsi1 in the rice root, which primarily transports silicon. Efflux of arsenite directed from the root toward xylem is mediated by another arsenite transporter Lsi2 also described by Ma et al. (2008). Role of Lsi2 gene was confirmed from the observation of Lsi2 mutant rice species in which AsIII accumulation was found much lower in the shoots or xylem sap in comparison to those xylem sap of wild species (Ma et al. 2008). Recently, two transporters OSNIP 3;3 and HvNIP1;2 have been reported to involve in AsIII transport in the yeast cell (Katsuhara et al. 2014).
5.2.3 Uptake of Organic Species of Arsenic
Organic forms of As such as MMAV and DMAV are in very small proportion in soil and may derive from the previous application of arsenical pesticides and herbicides or may be synthesized by the microorganism. The organic As compounds are less efficiently taken up by plants than that of inorganic As species (Carbonell-Barrachina et al. 1998; Raab et al. 2007). Very little information is available about the mechanism involved in the uptake and transport of methylated As species by plants. In aquatic plants, AsIII is transported passively through aquaglyceroporin channel in the form of dimethylarsinic acid (DMAA) and monomethyl arsinic acid (MMAA) (Rahman and Hasegawa 2011). The aquaporin OsLsi1 is involved in the uptake of MMAV, and the loss of function in rice OsLsi1led to an 80% reduction in MMAV uptake and 50% for DMAV compared to wild species (Li et al. 2009). Although rate of uptake of MMAV and DMAV by plant roots occurs very slowly than that of arsenate or arsenite (Abbas and Meharg 2008; Li et al. 2016), greater mobility of MMAV and DMAV was found within the plant tissue than that of inorganic As species (Li et al. 2009; Carey et al. 2010,2011). Involvement of OsLsi1 was confirmed in the uptake of organic As species, but no role is played by OsLsi2 in plants in the efflux of the MMAV and DMAV (Li et al. 2009).
5.3 Translocation of Arsenic
Arsenic hyperaccumulators have greater mobility of As relating to translocation from roots toward shoots in comparison to non-hyperaccumulator. The less efficient translocation of As directing toward shoot from root tissue in non-hyperaccumulators is indicative of the low ratios of shoot As to root As concentrations (Burlo et al. 1999) and thereby justifying the phenomenon that the reduction of arsenate to arsenite occurs rapidly in roots, following complexation with thiols and insulation within the root vacuoles. In A. thaliana knocked out AtACR2 gene (arsenate reductase) using RNAi leads to increased accumulation of As in the shoots (Dhankher et al. 2006). Blocking AtACR2 leads to more arsenate available for xylem transport to the shoots from root via the phosphate transport pathway. Among all As species, DMA is translocated more proficiently from roots to shoots, although root uptake is less efficient compared to other As species (Raab et al. 2007). The inorganic form in which As is transported from root to shoot is questionable. Some authors reported that arsenite prevalently exists in the xylem sap, accounting for 60–100% of the total As (Zhao et al. 2009). A. thaliana mutant for phosphate transporter, defective in xylem loading of phosphate but showed no effect on As distribution to the shoots (Quaghebeur and Rengel 2004), suggests that As is not loaded into the xylem as phosphate analogue arsenate. Duan et al. (2005) also support that majority of the transported As is in arsenite form as AR activity was solely confined within the roots. In contrary, a number of reports showed that arsenate is present in the xylem as it is being loaded by PHT protein, into the xylem vessels (Catarecha et al. 2007; Zhao et al. 2010; Mendoza-Cózat et al. 2011; Wu et al. 2011). However, methylated As is detected very meager amount in xylem sap as DMA was found in xylem sap of cucumber (Cucumis sativus) and tomato plants only at <4% of the total As (Mihucz et al. 2005; Xu et al. 2007).
5.4 Arsenic-Induced Oxidative Stress in Plants
Arsenic exposure leads to abiotic stress which emanates to oxidative stress at cellular level by producing reactive oxygen species (ROS) (i.e., singlet oxygen, 1O2; superoxide, O2 •−; hydrogen peroxide, H2O2; hydroxyl radical, OH•) that surpass the pace of their metabolism (Gill and Tuteja 2010; Mallick et al. 2011). Arsenic induces ROS production by blocking the activity of key enzyme system along with electron drainage during AsV to AsIII conversion (Sharma 2012). The reduction of AsV is succeeded by methylation of inorganic As, a redox-directed process that may also give rise to ROS (Zaman and Pardini 1996). Methylated As species such as dimethylarsinic acid (DMA) causes iron-dependent oxidative stress which is based on iron released from ferritin. DNA damage takes place by reactive oxygen species which are generated directly from DMA3+ (Shi et al. 2004). ROS induces chain like peroxidation of polyunsaturated fatty acid in membrane lipids, damaging the proteins, amino acids, nucleotides, and nucleic acids (Noctor et al. 2016; Moller et al. 2007). Malondialdehyde (MDA), a product of lipid peroxidation resulting from membrane damage, is considered as an indicator of oxidative stress (Shri et al. 2009). Lipid peroxidation also increases thiobarbituric acid-reacting substances (TBARS) and H2O2 content in H. lanatus (Hartley-Whitaker et al. 2001), Trifolium pratense (red clover) (Mascher et al. 2002), Vigna radiata (mung bean) (Singh et al. 2007), and Oryza sativa (rice) (Shri et al. 2009). In Pteris vittata and Sphagnum nemoreum, As exposure leads to alteration of chloroplast membrane structure and subsequent rupture and enlargement of thylakoid membranes (Simola 1997; Li et al. 2006). Elevated and nonmetabolized cellular H2O2 is responsible for severe damages to biomolecules such as cellular lipids and proteins and consequent interruption of key cellular functions (Gill and Tuteja 2010; del Río 2015).
Differential modulation in the antioxidant system occurs in the plant under As stress as reported from several studies (Dwivedi et al. 2010; Tripathi et al. 2012). Activated antioxidant system and increased levels of PC production in different plants like Hydrilla verticillata and C. demersum suggest that specific proteins are responsive to As stress (Srivastava et al. 2007; Mishra et al. 2008; Dave et al. 2013a). Similarly, enhanced activities of antioxidative enzymes such as superoxide dismutase, APX, peroxidase (POD), and GR indicate As exposure generates oxidative stress (Shri et al. 2009; Dave et al. 2013b). The first line of defense in higher plants includes activation of CAT, SOD isozymes, and the AsA-GSH cycle in response to As stress. To mitigate the negative effects of excess ROS, the plant defense system functions in a coordinated manner under adverse environmental circumstances in the different cell compartments and organs (Airaki et al. 2015). However, following exposure to higher As level, ROS production reaches too high that the antioxidant defense mechanisms may be devastated, leading to cellular damage which ultimately leads to cell death (Van Breusegem and Dat 2006).
5.5 Arsenic-Induced Metabolic Alterations in Plants
The potential of AsV to substitute for Pi and the aptness to bind and alter the activities of fundamental enzymes and hazardous effects of ROS have a direct and significant effect on plant metabolism. Arsenic vulnerability leads to changes in the levels of various compounds like starch and sugars and modulates the activities of the key enzymes of interrelated metabolic pathways like RNase, protease, and leucine aminopeptidase in plants (Mishra and Dubey 2006; Choudhury et al. 2010). Productivity was severely hindered due to significant disruption of carbohydrate metabolism in plants growing in As-contaminated soil and may be due to the rise in the level of soluble sugars in the tissues, especially sucrose and hexoses, the end products of the photosynthesis (Mishra and Dubey 2013). A comparative transcriptomic analysis revealed variation in the lipid metabolism and phytohormone signaling in plants under As(III) stress (Yu et al. 2012).
To encounter the ROS generated by the As exposure, plants need to produce sufficient metabolites, and such response predominantly impacts on carbon, nitrogen, and sulfur metabolism of plants (Finnegan and Chen 2012). Promoting accumulation of AsA is the main effect of AsV on plant carbon metabolism to reinforce protection against ROS (Srivastava et al. 2005; Singh et al. 2006; Khan et al. 2009). However, genomic analysis on carbon metabolism proved no changes in transcriptional profiles as observed both in Arabidopsis and Oryza sativa (Abercrombie et al. 2008; Norton et al. 2008; Chakrabarty et al. 2009). Exposure to AsV, AsIII, and MMAIII are able to interfere the photosynthetic process in different ways like decrease in chlorophyll content (Duman et al. 2010; Gupta et al. 2013b) or Photosystem II activity (Stoeva and Bineva 2003) which may perturb photosynthetic electron flow across the membrane of thylakoid sinking the efficiency to produce ATP and NADPH, both of which are essential to fuel the carbon fixation reactions.
Arsenic exposure has the potential to strongly reduce the nitrogen fixation in alfalfa roots as observed when alfalfa growing in As-contaminated soil had less than half of the total number of root nodules formed in the absence of As (Carrasco et al. 2005; Pajuelo et al. 2008). Transcriptomic analysis by Lafuente et al. (2010) reported that AsIII exposure prevents the gene expression required for early nodule development. As a result, soil contaminated with As shows lower potential for N2 fixation in ecosystem involving legume-rhizobium symbiosis as evidenced from alfalfa. Considerable changes in the amino acid pool have been reported to occur after As exposure (Dwivedi et al. 2010; Pavlík et al. 2010). A number of the study reported that the RuBisCo, an abundant protein having the capacity to store nitrogen, can be a target for disruption in AsV treated plants (Duquesnoy et al. 2009; Ahsan et al. 2010; Bona et al. 2010). Thus, As exposure that accompanies lower protein content in plants may be due to As-induced diminution in carbohydrate metabolism that would deter the biosynthesis of amino acids (Finnegan and Chen 2012).
The major As detoxification pathway, that is, binding of As with the thiol group of GSH and PC, indicates the crucial importance of sulfur metabolism regulating plant survival in As-contaminated soils. Adequate supplies of the GSH building blocks Glu, Cys, and Gly are required immediately after As exposure. According to Munoz-Bertomeu et al. (2009), cysteine is the limiting substrate for GSH biosynthesis in Arabidopsis. Decreasing cysteine pool following As exposure (Sung et al. 2009) signifies that higher Cys biosynthesis is needed to support GSH and PC generation that is also steered from sulfur metabolism (Finnegan and Chen 2012). Plants that overproduce the enzymes mediating GSH and PC biosynthesis were found to maintain higher levels of nonprotein thiols than wild species (Guo et al. 2008). Sulfur is acquired from the soil in the form of sulfate to sustain biosynthesis of GSH and PC at high rate. Both species of As induces the expression of sulfur transporter genes. Norton et al. (2008) observed that in rice subsequent to AsV treatment upregulation of five sulfate transporter genes, but Sung et al. (2009) reported that in Arabidopsis at least one gene is upregulated. Similarly, AsIII treatment in B. juncea and rice seedlings at least one sulfate transporter gene was found to be upregulated (Chakrabarty et al. 2009; Srivastava et al. 2009). However, Takahashi et al. (2011) suggested that small number of transporters may be adequate to direct the mobility of sulfate from the soil toward the plants root.
5.6 Enzymatic Antioxidative System
5.6.1 Superoxide Dismutase
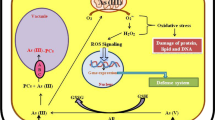
Superoxide dismutases or SODs are metalloenzymes that play key roles in protecting cells from oxidative stress by catalyzing the dismutation of O2•− to H2O2 (Li et al. 2017). Superoxide dismutase enzyme requires metals as cofactors. SOD associated with Cu/Zn is found in the cytosol, plastid, peroxisomes, and root nodules. Mn-SOD is confined in the mitochondria, and Fe-SOD is localized in the plastids. In maize root, the proteomic analysis reveals Cu/Zn SOD as one of the highly responsive enzymes to As which is involved in cellular homeostasis during redox disturbance (Requejo and Tena 2005). SOD activity was found to significantly increase in response to As toxicity as evidenced from As hyperaccumulator and sensitive fern species (Srivastava et al. 2005), in maize (Mylona et al. 1998) and in the grass H. lanatus (Hartley-Whitaker et al. 2001); in contrast, high concentration of As inhibits the accumulation of SOD mRNA, thus reducing its activity (Gong et al. 2005; Gunes et al. 2009). The inhibition of SOD activity in response to high As exposure could be attributed to inactivation of the enzyme by H2O2 produced in different cellular compartments where SOD neutralizes O2 •- (Khan et al. 2009). ROS- detoxifying enzymes are induced during abiotic stress but are also susceptible to oxidative damage (Dietz et al. 1999). Hydrogen peroxide itself is a highly reactive oxidizing agent that undergoes detoxification by CAT and the AsA–GSH cycle, both regulates H2O2 level (Shigeoka et al. 2002; Fig. 5.1). The equilibrium between the activity level of SOD and enzymes involved in AsA–GSH cycle and sequestration of metal ions promotes to maintain the steady-state level of O2 and H2O2 and play crucial role by inhibiting formation of the ROS via the metal-dependent Haber–Weiss or Fenton reactions (Mittler 2002).
Antioxidant defense system in plants after As exposure including enzymatic and nonenzymatic antioxidants (modified from Hasanuzzaman et al. 2012). As, arsenic; SOD, superoxide dismutase (in peroxisomes and plastids); CAT catalase (in mitochondria, peroxisomes, cytosol), APX peroxidase (in mitochondria, peroxisomes, cytosol, chloroplast), MDHA monodehydroascorbate, DHA dehydroascorbate, DHAR DHA reductase, GSH glutathione, GSSG glutathione disulfide, GR glutathione reductase (in chloroplast, mitochondria, cytosol), GPX glutathione peroxidase (in cytosol, chloroplasts, mitochondria, peroxisome, apoplast), GST glutathione sulfo-transferases (in cytosol)
5.6.2 Catalase
Catalase is another H2O2 scavenger, located mainly in peroxisomes, glyoxisome, cytosol, mitochondria, and root nodules (Shugaev et al. 2011; Sharma et al. 2012; Su et al. 2014). This tetrameric, heme-containing enzyme degrades hydrogen peroxide promptly into water and molecular oxygen without utilizing cellular reducing supplements, thereby, protecting the cell by removing hydrogen peroxide by saving energy (Sharma 2012). Following As exposure, an upsurge of CAT activities was found in Zea mays (Mylona et al. 1998). As-tolerant Chinese brake fern (P. vittata) displays higher degree of CAT activity than As-susceptible slender brake fern (P. ensiformis) and Boston fern (Nephrolepis exaltata) (Srivastava et al. 2005). In contrast, As-induced deterioration of CAT has also been reported by Singh et al. (2007) in Vigna radiata (mung bean) and in moss, Taxithelium nepalense. The association of a heme prosthetic group with CAT has been established by the irreversible inhibition of CAT by cyanide, azide, and hydroxylamine, all of which are hemeprotein inhibitors (Anjum et al. 2016). In addition, existence of a thiol group close to the active center of CAT contributing in the CAT-mediated reactions has been proven from the inhibition of CAT by thiol inhibitors like aminotriazole and mercaptoethanol.
5.6.3 Ascorbate Peroxidase
An alternative mechanism to detoxify H2O2 by peroxidase through AsA-GSH pathway is found in higher plants that require AsA as a reductant to reduce hydrogen peroxide into water (Fig. 5.1; Mehlhorn 1990). APX are class I heme-peroxidases, which function as active scavengers of H2O2 in higher plants and prevail as cAPX or cytosolic isoforms, mit APX or mitochondrial isoforms, and also in microbodies as mAPX, including peroxisomal and glyoxysomal isoforms, and ch APX or chloroplastic isoforms (Miyake and Asada 1996; Yadav et al. 2014; Anjum et al. 2016). Isoforms are unlike in their molecular weight, stability, and substrate specificity optimal pH and have been refined and characterized from several plant species including Pisum sativum (Caverzan et al. 2012), Camellia sinensis (Chen and Asada 1989), Gossypium hirsutum (Bunkelmann and Trelease 1996), Cucumis sativus (Battistuzzi et al. 2001), Nicotiana tabacum (Madhusudhan et al. 2003), Oryza sativa (Sharma and Dubey 2004), Olea europaea (Lopez-Huertas and del Rio 2014), and Ziziphus mauritiana (Yadav et al. 2014). APX catalyzes the reduction of hydrogen peroxide into water and two molecules of monodehydroascorbate (MDHA; Noctor and Foyer 1998). APX activity has been upregulated after As exposure as observed in maize (Miteva and Peycheva 1999), beans (Stoeva et al. 2005), mung bean (Singh et al. 2007), and rice seedling (Shri et al. 2009).
5.6.4 Glutathione Reductase
Glutathione reductase (GR, NADPH: oxidized glutathione oxidoreductase) is another key component of ROS scavenging system, located predominantly in chloroplast but also in mitochondria and cytosol in a small amount (Gill and Tuteja 2010). Glutathione reductase reduces glutathione disulfide (GSSG) to GSH using NADPH as reducing equivalent (Fig. 5.1), and thus conserves the cellular redox levels by retaining a high ratio of intracellular GSH/GSSG and AsA/dehydroascorbate (AsA/DHA) during oxidative stress (Anjum et al. 2012). Two genes, namely GR1 and GR2, have been distinguished to encode GR in plants; both are expressed in plastids and mitochondria (Jozefczak et al. 2012). A range of biotic and abiotic stress factors such as heavy metals and metalloids affect the activity of GR in plants (Anjum et al. 2010, 2011a, b; Gill and Tuteja 2010). Unfortunately, there is paucity of reports about the active role of GR in higher plants during oxidative stress induced by As. It is found in rice seedlings that higher level of GSH required during As-induced oxidative stress is achieved by the activation of GR (Shri et al. 2009). Similar reports, that is, elevated level of GR activity has been observed in roots of P. vittata, P. ensiformis, and Nephrolepis exaltata after As exposure (Srivastava et al. 2005).
5.6.5 Glutathione Peroxidases
Glutathione peroxidase (GPX) belongs to large peroxidase family with broad substrate specificity, localized in cytosol, chloroplasts, mitochondria, peroxisome, and apoplast of plant cell, and catalyzes the reduction of H2O2, organic and lipid hydroperoxides consuming GSH pool as a reducing substrate, thereby protecting the cells from ROS (Anjum et al. 2010, 2011b). Some authors opined that, plant GPXs are actually peroxiredoxins (Prx) as they can use both GSH and thioredoxin (Trx) as electron donor, but Trxs act as more efficient reductants (Herbette et al. 2002; Iqbal et al. 2006; Navrot et al. 2006; Noctor et al. 2011). Millar et al. (2003) identified a family of protein isoforms called AtGPX1–AtGPX7 in Arabidopsis among which AtGPX1and AtGPX7 are present in chloroplast providing antioxidant protection and synchronizes salicylate, and ROS triggered plant immune responses (Chang et al. 2009). The other GPXs isoforms are found in the cytosol, mitochondria, and the endoplasmatic reticulum (Milla et al. 2003). Arsenate stress induced to increase the GPX activity in dose-dependent manner as observed in mung bean and in rice (Singh et al. 2007; Singh et al. 2015). A study carried out in P. vittata reported that a rise in GPX activity has occurred up to 20 mg kg−1 As and then declined with the increasing As concentration (Cao et al. 2004).
5.6.6 Glutathione S-transferase
Glutathione S-transferases (GSTs) found in plant cytosol are major phase II, ROS-detoxifying enzymes (Sheehan et al. 2001) and dependent on GSH for catalyzing the conjugation of GSH via the sulfhydryl group to diversified electrophilic centers of hydrophobic compounds (Marrs 1996; Fig. 5.1). This reaction renders the compound more polar and facilitates its transport to vacuole or apoplast (Mylona et al. 1998). GSTs perform versatile roles where GSH serves as a co-substrate or coenzyme (Ghelfi et al. 2011). Like other antioxidant enzyme GST activity increases in plants after As exposure (Mylona et al. 1998; Srivastava et al. 2005; Norton et al. 2008; Mokgalaka-Matlala et al. 2009; Chakrabarty et al. 2009). As for example, upregulation of at least 10 GST genes has been observed in rice in response to AsV exposure, while not more than two GST genes are downregulated (Norton et al. 2008; Chakrabarty et al. 2009). However, no noticeable changes in GST transcript were found in response to AsIII (Chakrabarty et al. 2009), focusing that two inorganic As forms have differential effects on cellular metabolism.
5.7 Nonenzymatic Antioxidants
5.7.1 Ascorbate
Ascorbate (AsA) is the most abundant antioxidant in plants, present in cytosol, apoplast, and in the stroma of chloroplast. Synthesis of AsA occurs in the cytosolic region chiefly from the transformation of d-glucose (Hasanuzzaman et al. 2012). AsA reacts with a range of ROS such as H2O2, O2• −, and 1O2 and is the most important reducing substrate for the removal of H2O2 (Singh et al. 2006) and restore membrane-bound carotenoids and α-tocopherol via the AsA-GSH cycle in plant cells (Sharma 2012). In the AsA-GSH cycle, two molecules of AsA (reduced) are utilized by APX to reduce H2O2 to water with the concomitant generation of oxidized form MDHA that immediately disproportionates into DHA and AsA (Gapper and Dolan 2006) by MDHAR or ferredoxin with the electron donor NADPH in the chloroplasts (Gapper and Dolan 2006). Recycling of AsA (reduced) from dehydroascorbate (DHA) is a GSH-dependent pathway catalyzed by dehydroascorbate reductase (DHAR) that consumes NADPH as a reducing agent. A report from the study by Singh et al. (2006) showed that following As exposure an upsurge of AsA (reduced) concentration and the ratio of AsA/DHA occurs in the fronds of As-hyperaccumulator P. vittata and As-sensitive P. ensiformis.
5.7.2 Glutathione
The potential detoxification mechanism found in plants for combating heavy metal induced phytotoxicity is by synthesizing low molecular weight thiols having high affinity for the toxic metals (Bricker et al. 2001). GSH is one of the vital low molecular weight tripeptide thiol associated with sulfur and found as reduced (GSH) and oxidized (GSSG) forms. GSH takes part in a slew of cellular processes including defense against ROS, sequestration and complexation of heavy metals, control of cell division, in budding, and in transport and storage of reduced sulfur (Vernoux et al. 2000; Cobbett and Goldsbrough 2002; Freeman et al. 2004; Ogawa et al. 2004; Foyer and Noctor 2005; Mullineaux and Rausch 2005). Formation of GSH involves two ATP-dependent enzymes namely γ-glutamylcysteine synthetase (GSH1) and GSH synthetase (GSH2). In the first reaction, synthesis of γ-glutamylcysteine (γ-EC) occurs through a peptide bonding between the carboxyl group of glutamate and the amino group of cysteine by the catalytic action of GSH1. In the second reaction, ligation between glycine residue and γ-EC is catalyzed by GSH2 to form GSH. GSH1 plays major role in the regulation of GSH biosynthesis (Yadav 2010).
During As detoxification, coupling of the reduction of arsenate to arsenite and NADPH oxidation occurs where GSH (reduced) is serving as the electron donor for arsenate reductase (Ellis et al. 2006). In plants, As is transported as oxyanion arsenate which is reduced to arsenite and sequestered as thiol–peptide complexes in vacuoles. Transgenic A. thaliana overexpressing both arsenate reductase (arsC) and GSH1 together showed substantially greater As tolerance than GSH1 transgenic and wild-type plants (Dhankher et al. 2002). One protective role of GSH in plants to As exposure is relieving from ROS. Supplementation of exogenous GSH and cysteine to plants under As stress reduced oxidative stress was observed, and the growth of rice seedlings was restored (Shri et al. 2009). Another important role of GSH is to serve as a precursor for the synthesis of phytochelatins a set of novel heavy metal-binding peptides.
5.8 Role of Phytochelatin in Detoxification and Arsenic Tolerance
The most common method of detoxification of heavy metal/metalloid in plants is by synthesis of PC. PC is synthesized by the catalytic action of PC synthase (PCS) from GSH by transpeptidation of (γ-glutamyl-cysteinyl) n-glycine (Gasic and Korban 2007). PC has the capability of binding via sulfhydryl and carboxyl residues to a range of metals like Zn, Cu, Cd, as well as As (Gupta et al. 2013c). Studies support the occurrence of PCs throughout the plant kingdom, in gymnosperms, angiosperms, and bryophytes (Clemens 2006). As tolerance in As-non-hyperaccumulating plants is achieved through considerable increase in the production and procurement of GSH and phytochelatins (PC) following exposure (Schat et al. 2002; Grill et al. 2006). The presence of heavy metal ions and metalloid such as Pb, Cd, Hg, Ag, Cu, Zn, As, etc. is required for the constitutively expression of PCS gene (Vatamaniuk et al. 2004). The presence of AsIII-GSH or AsIII-PC complexes has been recognized in various plants such as Indian snakeroot (Rauvolfia serpentina), in perennial grass commonly known as Yorkshire fog (H. lanatus), sunflower (Helianthus annuus), Indian mustard (B. juncea), and in Cretan brake fern (Pteris cretica) (Pickering et al. 2000; Schmoger et al. 2000; Montes-Bayon et al. 2004; Raab et al. 2004). In sunflower plants (H. annuus), following As exposure, synthesis of 14 different As complexes have been reported including GS-AsIII-PC2, AsIII-PC3, AsIII-(PC2)2, AsIII-GS3, and MMA-PC2 (Raab et al. 2005). Schulz et al. (2008) reported that short chains of PCs instead of long chain dominate in As-tolerant plants. Study of cad1-3 mutant A. thaliana, lacking the functional enzyme for PC synthesis, ascertained the predictable role of PCs in As detoxification; the mutant was unable to produce functional PCs and was found to be more sensitive (10–20 fold) to arsenate than the wild-type plants (Ha et al. 1999). Finally, As is detoxified within root and shoot tissue vacuoles by sequestrating AsIII-PC complexes (Tripathi et al. 2007) thus unable to interfere with the cellular metabolism (Mitra et al. 2017a). In rice leaves, PC-arsenite complexation restricts the mobility of As from leaves to grains (Mitra et al. 2017b). In Arabidopsis, ABC transporter MRP1/ABCC1 and MRP2/ABCC2 are involved in the transport of AsIII-PC conjugates (Song et al. 2010). In rice, transcription-level upregulation of homologous ABCC2 transporter gene was found after As exposure (Chakrabarty et al. 2009). A report of Mendoza-Cózat et al. (2011) has proven the presence of ABCC transporter in different plant species sharing homology with Arabidopsis ABCC1 and ABCC2 transporter. In non-hyperaccumulator plants, phytotoxicity is reduced by rapid formation of As-PC complexes and sequestration within vacuoles of root cells where acidic pH (5.5) is favorable to stabilize the complex following As uptake, thereby restricting the As transport from the root to shoot (Liu et al. 2010; Mendoza-Cózat et al. 2011). The predominating form in which As is transported from root to shoot is controversial. In sporophytes of P. vittata, As is directed to the shoot mainly as AsV form and accumulated in the fronds as AsIII as reported by Zhao et al. (2003). In contrast, Duan et al. (2005) suggested that As is translocated mostly in reduced form (AsIII) and thus supporting the restriction of AR activity within the roots. Dissimilar with non-hyperaccumulators, where most of As is detoxified by the formation of As-PC complexes, hyperaccumulators like P. vittata and P. cretica were found to store 60–90% of arsenic as arsenite (AsIII) form in the vacuole of fronds (Pickering et al. 2006; Su et al. 2008) with little complexation with PC in the roots and fronds (Zhao et al. 2009).
5.9 Conclusion
In recent years, researchers are trying to decipher the As uptake and transport in plants through studying molecular and physiological mechanisms. In plant tissue, oxidative stress produced due to ROS production and disorders of antioxidant defenses have been considered a significant matter in As toxicity. In this chapter, an attempt has been made to compile the updated information about As toxicity specifically on oxidative stress and the antioxidant defense system in plants. Although As is a non-redox active metalloid, excessive ROS is produced during valency conversion and methylation in plant. Common manifestations of As-induced phytotoxicity are growth inhibition, shortening of roots (than shoots), and severe effects on anatomical structures, photosynthetic apparatus, and antioxidant defense activities are found. As a result, agricultural productivity worldwide is hugely affected by As. Therefore, an urgent need is to find As-tolerant plant variety to increase agriculture productivity in affected areas. ROS scavenging are vital for plant defenses, and overexpression of gene coding for ROS-detoxifying enzymes helps to increase tolerance against environmental abiotic stresses. Transgenic plants that overexpress gene coding for ROS-detoxifying enzymes may be a prospective item to grow plants with improved tolerance against As. Another way is to apply exogenous chemical protectants like glycinebetaine, proline, Se, and signaling molecules like NO to alleviate oxidative stress (Hasanuzzaman et al. 2011a, b; Hasanuzzaman and Fujita 2011). Meharg and Meharg (2015) reported that adequate silicon fertilization greatly boosts rice yield by alleviating biotic and abiotic stresses and improving grain quality through lowering the content of inorganic As. Nitric oxide (NO), the gaseous free radical, is a widespread intracellular messenger and has regulatory roles in plant physiological processes (Neill et al. 2002). Though the NO-mediated amelioration against As-induced oxidative stress appeared to be synchronized by modulating antioxidant enzyme activities, NO itself has the capacity to detoxify ROS directly (Talukdar 2013b). Therefore, an integrated approach by producing transgenic plants overexpressing genes related with antioxidant along with exogenous protectants may be implemented in order to achieve greater tolerance to As stress.
References
Abbas MHH, Meharg AA (2008) Arsenate, arsenite and dimethyl arsenic acid (DMA) uptake and tolerance in maize (Zea mays L.). Plant Soil 304:277–289
Abercrombie JM, Halfhill MD, Ranjan P, Rao MR, Saxton AM, Yuan JS, Stewart CN (2008) Transcriptional responses of Arabidopsis thaliana plants to As (V) stress. BMC Plant Biol 8:87. https://doi.org/10.1186/1471-2229-8-87
Adriano DC (1986) Trace element in terrestrial environment. Springer, New York
Ahsan N, Lee DG, Kim KH, Alam I, Lee SH, Lee KW, Lee H, Lee BH (2010) Analysis of arsenic stress-induced differentially expressed proteins in rice leaves by two-dimensional gel electrophoresis coupled with mass spectrometry. Chemosphere 78:224–231
Airaki M, Leterrier M, Valderrama R, Chaki M, Begara-Morales JC, Barroso JB, del Río LA, Palma JM, Corpas FJ (2015) Spatial and temporal regulation of the metabolism of reactive oxygen and nitrogen species during the early development of pepper (Capsicum annuum) seedlings. Ann Bot 116:679–693
Anjum NA, Umar S, Chan MT (2010) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht
Anjum NA, Umar S, Ahmad A (2011a) Oxidative stress in plants: causes, consequences and tolerance. IK International Publishing House Pvt. Ltd, New Delhi
Anjum NA, Umar S, Iqbal M, Khan NA (2011b) Cadmium causes oxidative stress in moongbean [Vigna radiata (L.) Wilczek] by affecting antioxidant enzyme systems and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MN (2012) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K, Mohamed AA, Thangavel P, Devi GD, Vasudhevan P, Sofo A (2016) Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Battistuzzi G, D’Onofrio M, Loschi L, Sola M (2001) Isolation and characterization of two peroxidases from Cucumis sativus. Arch Biochem Biophys 388:100–112
Begum MC, Islam MS, Islam M, Amin R, Parvez MS, Kabir AH (2016) Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem 104:266–277
Bienert GP, Schuessler MD, Jahn TP (2008) Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci 33:20–26
Bleeker PM, Schat H, Vooijs R, Verkleij JAC, Ernst WHO (2003) Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol 157:33–38
Bona E, Cattaneo C, Cesaro P, Marsano F, Lingua G, Cavaletto M, Berta G (2010) Proteomic analysis of Pteris vittata fronds: two arbuscular mycorrhizal fungi differentially modulate protein expression under arsenic contamination. Proteomics 10:3811–3834
Bricker TJ, Pichtel J, Brown HJ, Simmons M (2001) Phytoextraction of Pb and Cd from a superfund soil: effects of amendments and croppings. J Environ Sci Health 36:1597–1610
Bunkelmann JR, Trelease RN (1996) Ascorbate peroxidase, a prominent membrane protein in oilseed glyoxysomes. Plant Physiol 110:589–598
Burlo F, Guijarro I, Carbonell-Barrachina AA, Valero D, Martinez-Sánchez F (1999) Arsenic species: effects on and accumulation by tomato plants. J Agric Food Chem 7:1247–1253
Cao X, Ma LQ, Tu C (2004) Antioxidant responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ Pollut 128:317–325
Carbonell-Barrachina AA, Aarabi MA, DeLaune RD, Gambrell RP, Patrick WH (1998) The influence of arsenic chemical form and concentration on Spartina patens and Spartina alterniflora growth and tissue arsenic concentration. Plant Soil 198:33–43
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice (Oryza sativa L.). Plant Physiol 152:309–319
Carey AM, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98
Carrasco JA, Armario P, Pajuelo E, Burgos A, Caviedes MA, López R, Chamber MA, Palomares AJ (2005) Isolation and characterisation of symbiotically effective Rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcollar pyrite mine. Soil Biol Biochem 37:1131–1140
Catarecha P, Segura MD, Franco-Zorrilla JM, García-Ponce B, Lanza M, Solano R, Paz-Ares J, Leyva A (2007) A mutant of the Arabidopsis phosphate trans-porter PHT1;1displays enhanced arsenic accumulation. Plant Cell 19:1123–1133
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Gen Mol Biol 35:1011–1019
Cesaro P, Cattaneo C, Bona E, Berta G, Cavaletto M (2015) The arsenic hyperaccumulating Pteris vittata expresses two arsenate reductases. Sci Rep 5:14525. https://doi.org/10.1038/srep14525
Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, Tripathi RD (2009) Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 74:688–702
Chang CC, Ślesak I, Jordá L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpińska B, Karpiński S (2009) Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol 150:670–683
Chatterjee S, Moogoui R, Gupta DK (2017a) Arsenic: source, occurrence, cycle and detection. In: Gupta DK, Chatterjee S (eds) Arsenic contamination in the environment. Springer, New York, pp 13–35
Chatterjee S, Sharma S, Gupta DK (2017b) Arsenic and its effect on major crop plants: stationary awareness to paradigm with special reference to rice crop. In: Gupta DK, Chatterjee S, (eds) Arsenic contamination in the environment. Springer New York, pp 123–143
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Chen GX, Asada K (1989) APX in tea leaves, occurrence of two isoenzymes, the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Choudhury B, Mitra S, Biswas AK (2010) Regulation of sugar metabolism in rice (Oryza sativa L.) seedlings under arsenate toxicity and its improvement by phosphate. Physiol Mol Biol Plants 16:59–68
Chung JY, Yu SD, Hong YS (2014) Environmental source of arsenic exposure. J Pre Med Pub Health 47:253–257
Clemens S (2006) Evolution and function of phytochelatin synthases. J Plant Physiol 163:319–332
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann Rev Plant Biol 53:159–182
Dave R, Singh PK, Tripathi P, Shri M, Dixit G, Dwivedi S, Chakrabarty D, Trivedi PK, Sharma YK, Dhankher OP, Corpas FJ (2013a) Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch Environ Contam Toxicol 64:235–242
Dave R, Tripathi RD, Dwivedi S, Tripathi P, Dixit G, Sharma YK, Trivedi PK, Corpas FJ, Barroso JB, Chakrabarty D (2013b) Arsenate and arsenite exposure modulate antioxidants and amino acids in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes. J Hazard Mater 262:1123–1131
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat Biotechnol 20:1140. https://doi.org/10.1038/nbt747
Dhankher OP, Rosen BP, McKinney EC, Meagher RB (2006) Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc Natl Acad Sci USA 103:5413–5418
Dietz KJ, Baier M, Krämer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants. Springer, Berlin, pp 73–97
Dong J, Wu F, Zhang G (2006) Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64:1659–1666
Duan GL, Zhu YG, Tong YP, Cai C, Kneer R (2005) Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol 138:461–469
Duman F, Ozturk F, Aydin Z (2010) Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As (III) and As (V): effects of concentration and duration of exposure. Ecotoxicology 19:983–993
Duquesnoy I, Goupil P, Nadaud I, Branlard G, Piquet-Pissaloux A, Ledoigt G (2009) Identification of Agrostis tenuis leaf proteins in response to As (V) and As (III) induced stress using a proteomics approach. Plant Sci 176:206–213
Duquesnoy I, Champeau GM, Evray G, Ledoigt G, Piquet-Pissaloux A (2010) Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. Com Ren Biol 333:814–824
Dwivedi S, Tripathi RD, Tripathi P, Kumar A, Dave R, Mishra S, Singh R, Sharma D, Rai UN, Chakrabarty D, Trivedi PK (2010) Arsenate exposure affects amino acids, mineral nutrient status and antioxidants in rice (Oryza sativa L.) genotypes. Environ Sci Tech 44:9542–9549
Ellis DR, Gumaelius L, Indriolo E, Pickering IJ, Banks JA, Salt DE (2006) A novel arsenate reductase from the arsenic hyperaccumulating fern Pteris vittata. Plant Physiol 141:1544–1554
Esteban E, Carpena RO, Meharg AA (2003) High-affinity phosphate/arsenate transport in white lupin (Lupinus albus) is relatively in sensitive to phosphate status. New Phytol 158:165–173
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182. https://doi.org/10.3389/fphys.2012.00182
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17:1866–1875
Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. The Plant Cell 16:2176–2191
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Gasic K, Korban SS (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64:361–369
Ghelfi A, Gaziola SA, Cia MC, Chabregas SM, Falco MC, Kuser-Falcão PR, Azevedo RA (2011) Cloning, expression, molecular modelling and docking analysis of glutathione transferase from Saccharum officinarum. Ann Appl Biol 159:267–280
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Grill E, Mishra S, Srivastava S, Tripathi RD (2006) Role of phytochelatins in phytoremediation of heavy metals. In: Singh SN, Tripathi RD (eds) Environmental bioremediation technologies. Springer, Berlin, pp 101–146
Gunes A, Pilbeam DJ, Inal A (2009) Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 314:211–220
Guo J, Dai X, Xu W, Ma M (2008) Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 72:1020–1026
Gupta DK, Chatterjee S (2017) Arsenic contamination in the environment: the issues and solutions. Springer, New York
Gupta DK, Srivastava S, Huang H, Romero-Puertas MC, Sandalio LM (2011) Arsenic tolerance and detoxification mechanisms in plants. In: Sherameti I, Varma A (eds) Detoxification of heavy metals (Book series: Soil biology). Springer, Berlin, pp 169–180
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puerta MC, Sandalio LM (2013a) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gupta DK, Huang HG, Nicoloso FT, Schetinger MR, Farias JG, Li TQ, Razafindrabe BH, Aryal N, Inouhe M (2013b) Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology 22:1403–1412
Gupta DK, Vandenhove H, Inouhe M (2013c) Role of phytochelatin in heavy metal stress and detoxification mechanisms in plants. In: Gupta DK, Corpas FJ, Palma JM (eds) Heavy metal stress in plants. Springer, Berlin, pp 73–94
Gupta DK, Tiwari S, Razafindrabe BHN, Chatterjee S (2017) Arsenic contamination from historical aspects till present situation. In: Gupta DK, Chatterjee S (eds) Arsenic contamination in the environment: the issues and solutions. Springer, New York, pp 1–12
Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. The Plant Cell 11:1153–1163
Hartley-Whitaker J, Ainsworth G, Meharg A (2001) Copper and- arsenic induced oxidative stress in Holcus lanatus L. Cloned with differential sensitivity. Plant Cell Environ 24:713–722
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Hossain MA, Fujita M (2011a) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxifi cation system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143:1704–1721
Hasanuzzaman M, Hossain MA, Fujita M (2011b) Nitric oxide modulates antioxidant defense and the methylglyoxal detoxifi cation system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep 5:353. https://doi.org/10.1007/s11816-011-0189-9
Hasanuzzaman M, Hossain MA, Teixeira da Silva JA, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateswarlu B, Shanker A, Shanker C, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–317
Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269:2414–2420
Iqbal A, Yabuta Y, Takeda T, Nakano Y, Shigeoka S (2006) Hydroperoxide reduction by thioredoxin-specific glutathione peroxidase isoenzymes of Arabidopsis thaliana. The FEBS J 273:5589–5597
Isayenkov SV, Maathuis FJM (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582:1625–1628
Islam E, Khan MT, Irem S (2015) Biochemical mechanisms of signaling: perspectives in plants under arsenic stress. Ecotoxicol Environ Safe 114:126–133
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Katsuhara M, Sasano S, Horie T, Matsumoto T, Rhee J, Shibasaka M (2014) Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol 31:213–219
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Safe 72:626–634
del Río LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66:2827–2837
Lafuente A, Pajuelo E, Caviedes MA, Rodríguez-Llorente ID (2010) Reduced nodulation in alfalfa induced by arsenic correlates with altered expression of early nodulins. J Plant Physiol 167:286–291
Lei M, Tie B, Zeng M, Qing P, Song Z, Williams PN, Huang Y (2013) An arsenic-contaminated field trial to assess the uptake and translocation of arsenic by genotypes of rice. Environ Geochem Health 35:379–390
Lemos Batista B, Nigar M, Mestrot A, Alves Rocha B, Barbosa Junior F, Price AH, Raab A, Feldmann J (2014) Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot 65:1467–1479
Li WX, Chen TB, Huang ZC, Lei M, Liao XY (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, Mc Grath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13
Li Z, Han X, Song X, Zhang Y, Jiang J, Han Q, Liu M, Qiao G, Zhuo R (2017) Overexpressing the Sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Front Plant Sci 8:1010. https://doi.org/10.3389/fpls.2017.01010
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152:2211–2221
Lopez-Huertas E, del Rio LA (2014) Characterization of antioxidant enzymes and peroxisomes of olive (Olea europaea L) fruits. J Plant Physiol 171:1463–1471
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Pro Nat Acad Sci USA 105:9931–9935
Madhusudhan R, Ishikawa T, Sawa Y, Shigeoka S, Shibata H (2003) Characterization of an ascorbate peroxidase in plastids of tobacco BY–2 cells. Physiol Plant 117:550–557
Mallick S, Sinam G, Sinha S (2011) Study on arsenate tolerant and sensitive cultivars of Zea mays L. Differential detoxification mechanism and effect on nutrients status. Ecotoxicol Environ Saf 74:1316–1324
Marin AR, Masscheleyn PH, Patrick WH (1993) Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 152:245–253
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. l. Annu Rev Plant Biol 47:127–158
Mascher R, Lippman B, Holiinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Matschullat J (2000) Arsenic in the geosphere – a review. Sci Total Environ 249:297–312
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Meharg AA, Macnair MR (1992) Suppression of the high-affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Meharg C, Meharg AA (2015) Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ Exp Bot 120:8–17
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual 23:234–238
Mehlhorn H (1990) Ethylene-promoted ascorbate peroxidase activity protects plants against hydrogen peroxide, ozone and paraquat. Plant Cell Environ 13:971–976
Mendoza-Cózat DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Mihucz VG, Tatar E, Virag I, Cseh E, Fodor F, Zaray G (2005) Arsenic speciation in xylem sap of cucumber (Cucumis sativus L.). Anal Bio Chem 383:461–466
Milla MA, Maurer A, Huete AR, Gustafson JP (2003) Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. The Plant J 36:602–615
Millar AH, Mittova V, Kiddle G (2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol 133:443–447
Mishra S, Dubey RS (2006) Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol 163:927–936
Mishra P, Dubey RS (2013) Excess nickel modulates activities of carbohydrate metabolizing enzymes and induces accumulation of sugars by upregulating acid invertase and sucrose synthase in rice seedlings. Biometals 26:97–111
Mishra S, Wellenreuther G, Mattusch J, Stark HJ, Kupper H (2008) Speciation and Distribution of arsenic in the nonhyperaccumulator macrophyte Ceratophyllum demersum. Plant Physiol 163:1396–1408
Miteva E, Peycheva S (1999) Arsenic accumulation and effect on peroxidase activity in green bean and tomatoes. Bulg J Agric Sci 5:737–740
Mithöfer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5
Mitra A, Chatterjee S (2016) Environmental amelioration using aquatic macrophytes: emphasizing removal of heavy metals from waste water. South Asian J Exp Biol 5:244–250
Mitra A, Chatterjee S, Datta S, Sharma S, Veer V, Razafindrabe BHM, Walther C, Gupta DK (2014) Mechanism of metal transporter in plants. In: Gupta DK, Chatterjee S (eds) Heavy metal remediation transport and accumulation in plants. Nova Science Publishers, New York, pp 1–27
Mitra A, Chatterjee S, Gupta DK (2017a) Uptake, transport, and remediation of arsenic by Algae and higher plants. In: Gupta DK, Chatterjee S (eds) Arsenic contamination in the environment: the issues and solution. Springer, New York, pp 145–169
Mitra A, Chatterjee S, Moogouei R, Gupta DK (2017b) Arsenic accumulation in rice and probable mitigation approaches: a review. Agronomy 7:67. https://doi.org/10.3390/agronomy7040067
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Miyake C, Asada K (1996) Inactivation of mechanism of ascorbate peroxidase at low concentrations of ascorbate, hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37:423–430
Mokgalaka-Matlala NS, Flores-Tavizon E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2009) Arsenic tolerance in mesquite (Prosopis sp.): low molecular weight thiols synthesis and glutathione activity in response to arsenic. Plant Physiol Biochem 47:822–826
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Montes-Bayon M, Meija J, LeDuc DL, Terry N, Caruso JA, Sanz-Medel A (2004) HPLC–ICP-MS and ESI-Q-TOF analysis of biomolecules induced in Brassica juncea during arsenic accumulation. J Anal At Spectrom 19:153–158
Mullineaux PM, Rausch T (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res 86:459–474
Munoz-Bertomeu J, Cascales-Minana B, Mulet JM, Baroja-Fernández E, Pozueta-Romero J, Kuhn JM, Segura J, Ros R (2009) Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol 151:541–558
Mylona PV, Polidoros AN, Scandalios JG (1998) Modulation of antioxidant responses by arsenic in maize. Free Radic Biol Med 25:576–585
Navrot N, Collin V, Gualberto J, Gelhaye E, Hirasawa M, Rey P, Knaff DB, Issakidis E, Jacquot JP, Rouhier N (2006) Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol 142:1364–1379
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot 53:1237–1247
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol 49:249–279
Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. In: The Arabidopsis Book. The American Society of Plant Biologists, Rockville
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice–arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59:2267–2276
Ogawa KI, Hatano-Iwasaki A, Yanagida M, Iwabuchi M (2004) Level of glutathione is regulated by ATP-dependent ligation of glutamate and cysteine through photosynthesis in Arabidopsis thaliana: mechanism of strong interaction of light intensity with flowering. Plant Cell Physiol 45:1–8
Pajuelo E, Rodríguez-Llorente ID, Dary M, Palomares AJ (2008) Toxic effects of arsenic on Sinorhizobium–Medicago sativa symbiotic interaction. Environ Poll 154:203–211
Panda SK, Upadhyay RK, Nath S (2010) Arsenic stress in plants. J Agron Crop Sci 196:161–174
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Pavlík M, Pavlíkova D, Staszkova L, Neuberg M, Kaliszova R, Szákova J, Tlustos P (2010) The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotoxicol Environ Saf 73:1309–1313
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122:1171–1177
Pickering IJ, Gumaelius L, Harris HH, Prince RC, Hirsch G, Banks JA, Salt DE, George GN (2006) Localizing the biochemical transformations of arsenate in a hyperaccumulating fern. Environ Sci Technol 40:5010–5014
Poynton CY, Huang JWW, Blaylock MJ, Kochian LV, Elless MP (2004) Mechanisms of arsenic hyperaccumulation in Pteris species: root As influx and translocation. Planta 219:1080–1088
Qadir S, Qureshi MI, Javed S, Abdin MZ (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd stress. Plant Sci 167:1171–1181
Quaghebeur M, Rengel Z (2004) Arsenic uptake, translocation and speciation in pho1 and pho2 mutants of Arabidopsis thaliana. Physiol Plant 120:280–286
Raab A, Feldmann J, Meharg AA (2004) The nature of arsenic–phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134:1113–1122
Raab A, Schat H, Feldmann J, Meharg AA (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168:551–558
Raab A, Williams PN, Meharg A, Feldmann J (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4:197–203
Rahman MA, Hasegawa H (2011) Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83:633–646
Rahman MA, Hasegawa H, Rahman MM, Miah MM, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Rathinasabapathi B, Ma LQ, Srivastava M (2006) Arsenic hyperaccumulating ferns and their application to phytoremediation of arsenic contaminated sites. In: da Silva JAT (ed) Floriculture, ornamental and plant biotechnology, vol III. Global Science Books, Middlesex, pp 304–311
Requejo R, Tena M (2005) Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry 66:1519–1528
Schat H, Llugany M, Vooijs R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and nonhyperaccumulator metallophytes. J Exp Bot 53:2381–2392
Schmoger MEV, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122:793–801
Schulz H, Härtling S, Tanneberg H (2008) The identification and quantification of arsenic-induced phytochelatins—comparison between plants with varying As sensitivities. Plant Soil 303:275–287
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Sharma P, Dubey RS (2004) APX from rice seedlings, properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci 167:541–550
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1. https://doi.org/10.1155/2012/217037
Sheehan D, Meade G, Foley VM (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–6
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255:67–78
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Shugaev AG, Lashtabega DA, Shugaeva NA, Vyskrebentseva EI (2011) Activities of antioxidant enzymes in mitochondria of growing and dormant sugar beet roots. Russ J Plant Physiol 58:387–393
Simola LK (1997) The effect of lead, cadmium, arsenate and fluoride ions on the growth and fine structure of Sphagnum nemoreum in aseptic culture. Can J Bot 90:375–405
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Singh HP, Batish DR, Kohali RK, Arora K (2007) Arsenic induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Pandey V, Trivedi PK, Chakrabarty D, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci 6:340. https://doi.org/10.3389/fpls.2015.00340
Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Nat Acad Sci 107:21187–21192
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa–an angiospermic parasite. J Plant Phsiol 161:665–674
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Srivastava S, Mishra S, Trtpathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Srivastava S, Srivastava AK, Suprasanna P, D’souza SF (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60:3419–3431
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulg J Plant Physiol 29:87–95
Stoeva N, Berova M, Vassilev A, Zlatev Z (2005) Effect of exogenous polyamine diethylenetriamine on oxidative changes and photosynthesis in As-treated maize plants (Zea mays L). J Cent Eur Agric 6:367–374
Su YH, McGrath SP, Zhu YG, Zhao FJ (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180:434–441
Su Y, Guo J, Ling H, Chen S, Wang S, Xu L, Allan AC, Que Y (2014) Isolation of a novel peroxisomal catalase gene from sugarcane, which is responsive to biotic and abiotic stresses. PLoS One 9:e84426. https://doi.org/10.1371/journal.pone.0084426
Sung DY, Kim TH, Komives EA, Mendoza-Cozatl DG, Schroeder JI (2009) ARS5 is a component of the 26S proteasome complex, and negatively regulates thiol biosynthesis and arsenic tolerance in Arabidopsis. The Plant J 59:802–813
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Talukdar D (2011) Effect of arsenic-induced toxicity on morphological traits of Trigonella foenum-graecum L. and Lathyrus sativus L. during germination and early seedling growth. Curr Res J Biol Sci 3:116–123
Talukdar D (2013a) Arsenic-induced changes in growth and antioxidant metabolism of fenugreek. Russ J Plant Physiol 60:652–660
Talukdar D (2013b) Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19:69–79
Titah HS, Abdullah SR, Mushrifah I, Anuar N, Basri H, Mukhlisin M (2013) Effect of applying rhizobacteria and fertilizer on the growth of Ludwigia octovalvis for arsenic uptake and accumulation in phytoremediation. Ecol Engin 58:303–313
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Matthuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Tripathi RD, Tripathi P, Dwivedi S, Dubey S, Chatterjee S, Chakrabarty D, Trivedi PK (2012) Arsenomics: omics of arsenic metabolism in plants. Front Physiol 3:275. https://doi.org/10.3389/fphys.2012.00275
Tu C, Ma LQ (2003) Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyper accumulator Pteris vittata L. under hydroponic conditions. Environ Exp Bot 50:243–251
Ullrich-Eberius CI, Sanz A, Novacky AJ (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J Exp Bot 40:119–128
Upadhyay RK (2014) Metal stress in plants: its detoxification in natural environment. Brazil J Bot 37:377–382
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Vatamaniuk OK, Mari S, Lang A, Chalasani S, Demkiv LO, Rea PA (2004) Phytochelatin Synthase, a dipeptidyltransferase that undergoes multisite acylation with γ- glutamylcysteine during catalysis: stoichiometric and site-directed mutagenic analysis of Arabidopsis thaliana pcs1-catalyzed phytochelatin synthesis. J Biol Chem 279:22449–22460
Vaughan GT (1993) The environmental chemistry and fate of arsenical pesticides in cattle tick dip sites and banana land plantations. CSIRO Division of Coal Industry. Center for Advanced Analytical Chemistry, Melbourne
Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, May MJ (2000) The root meristemless1/cadmium sensitive 2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12:97–109
Wallace IS, Choi WG, Roberts DM (2006) The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta Biomembr 1758:1165–1175
Wang JR, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157:498–508
Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176:590–599
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. SA J Bot 76:167–179
Yadav P, Yadav T, Kumar S, Rani B, Kumar S, Jain V, Malhotra SP (2014) Partial purification and characterization of ascorbate peroxidase from ripening ber (Ziziphus mauritiana L.) fruits. Afr J Biotechnol 13:3323–3331
Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS (2012) Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol 195:97–112
Zaman K, Pardini RS (1996) An overview of the relationship between oxidative stress and mercury and arsenic. Toxic Subst Mech 15:151–181
Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159:403–410
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annul Rev Plant Biol 61:535–559
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mitra, A., Chatterjee, S., Gupta, D.K. (2018). Plants Response and Tolerance to Arsenic-Induced Oxidative Stress. In: Hasanuzzaman, M., Nahar, K., Fujita, M. (eds) Mechanisms of Arsenic Toxicity and Tolerance in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-13-1292-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-1292-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1291-5
Online ISBN: 978-981-13-1292-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)