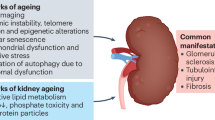
Abstract
With the development of society and improvement of health care, the life span is much longer than before, which brings serious aging problems. Among all the aging problems, renal aging grows to be nonnegligible issue. The aging process of kidney is always accompanied with structural and functional changes. Molecular changes, including Klotho and Sirtuins, are the basic causes of phenotypical changes. Cell senescence and cell autophagy play fundamental roles in the process of renal aging. To effectively intervene in the process of renal aging, different methods have been tried separately, which could produce different effects. Effective intervention of renal aging could be meaningful for healthy state of the whole body.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
While life expectancy in most countries has constantly increased in the last century, resulting from the improvements in sanitation and health care, the proportion of older people in the general population is continuously increasing. It is predicted that there will be 72 million 65-year-old or older Americans, which account for around 20% of the population by 2030 (O’Sullivan et al. 2017). In China, the number of 60-year-old or older people is estimated to be 374 million by 2040, comprising 24.8% of the Chinese population (Zhou et al. 2008a). By 2050, the population that is older than 60 years old will likely outnumber those younger than 14 years and constitute two billion people in the world (Zhou et al. 2008b). The rapid increase of geriatric population would bring a series of medial, social, and economic problems. A wise response to the increase aging population would require finding out the origin of this phenomenon, catching on the opportunities, as well as solving the problems effectively.
An essential geriatric medical issue is that of aging-related renal disease (Buemi et al. 2005). Although the aging process does not cause renal disease directly, the kidney goes through obvious physiologic change during the aging process, which predispose kidney to kinds of pathology. Although those diseases are not specific to the elderly, some certain disorders are more prevalent in the elder population, for example, renal disease secondary to type 2 diabetes mellitus (Silva 2005a). The impact of aging on the whole body showed impaired maintenance of physiological homeostasis and increasing burden of “wear and tear.” Therefore, aging kidney is not an independent disease but a complicated state owing to accumulative stress. Renal aging is normally mixed with a series of diseases, which makes it hard to distinguish normal features of the aging process from age-related dysfunction. This phenomenon is pretty common in a range of morbidities and is particularly relevant in the context of chronic kidney disease (CDK). CDK is an important public health problem that is characterized by poor health outcomes and very high health-care costs. CDK is also a major risk multiplier in patients with diabetes, hypertension, heart disease, and stroke (Couser et al. 2011). The incidence of CKD is about 10–12% in the developed world, and it is still increasing (Levey et al. 2015).
Aging in most species associates with impaired adaptive and homeostatic mechanisms, which induce an organism susceptible to inner or outer stresses (Anderson et al. 2009).
There is no gold standard for determining what constitutes normal aging. Although a loss of physical capability and function is induced by increasing chronological age, solid differences exist among people of the same chronological aging, which owes to genetic and epigenetic factors. However, over the past 50 years, a series of candidate biomarkers of aging have been found, even though they are not sufficient to prove aging (Baker and Sprott 1988). There are nine hallmarks of aging, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (Lopez-Otin et al. 2013). As for renal senescence, the typical histologic features are decreasing cortical mass, increases in glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis (Zhou et al. 2008b). In terms of renal function, elderly individuals usually exhibit increased renal vascular resistance, reduced renal plasma flow, and increased filtration fraction (Fliser et al. 1993). There are several reviews talking about the role of cell senescence in renal aging. Some features of cell senescence, such as the appearance of senescence-associated β-galactosidase (SA-β-gal) and P16INK4a, appear before showing morphologic changes, which suggests cell senescence may be part of the reason for renal diseases. To discover efficient method attenuating renal senescence, it is necessary to understand the factors driving age-associated changes in kidney.
11.2 Features of Renal Senescence
11.2.1 Structural Changes of the Aging Kidney
The structural changes of the aging kidney mainly include a decline in total nephron size and number, tubulointerstitial changes, glomerular basement membrane thickening, and increased glomerulosclerosis and so on (Nyengaard and Bendtsen 1992), as shown in Fig. 11.1. The average kidney weight progressively declines after 50 years old, and the renal cortex is more vulnerable to the aging-related stress than the medulla (Zhou et al. 2008b). Decreasing glomeruli are always accompanied by decreasing kidney weight and thinning of the cortical ribbon during aging (Nyengaard and Bendtsen 1992; Hughson et al. 2006; Newbold et al. 1992). Many morphologic changes have been noted before, including progressive decline in the number of intact or normal glomeruli with age, increase in the number of globally sclerotic glomeruli, abnormal glomeruli with shunts between the afferent and efferent arterioles bypassing the glomeruli, progressive decrease in the size of intact glomeruli, focal or diffuse thickening of the glomerular basement membranes, and increased mesangial volume/matrix-sclerosis (Silva 2005b). The pathogenesis of aging-associated global glomerulosclerosis is multifactorial. Dysregulation of the afferent and efferent arterioles of the glomerulus may lead to increased glomerular plasma flow, increased glomerular intercapillary pressures, and subsequent “hyperperfusion” glomerular injury with mesangial matrix accumulation (Brenner 1983).
Structural changes of the aging kidney. The structural changes of the kidney can be classified into two ways. Firstly, at the microscopic level, nephron number decreases significantly and some of tubules atrophy, while some of them hypertrophy. Arteriosclerosis and interstitial fibrosis are common in the aging kidney. Sclerosis and obsolescence appear in the glomerulus. Secondly, at the macroscopic level, medullary volume, kidney surface roughness, and number and size of cysts are all increased in the aging kidney, while cortical volume decreased. Besides, renal artery atherosclerosis and fibromuscular dysplasia are both typical in the aging kidney
It is reported that the vascular changes seen in aging kidneys first cause cortical glomerulosclerosis and consequent juxtamedullary glomerular hypertrophy, followed by juxtamedullary glomerulosclerosis (Newbold et al. 1992). Cadaver studies estimate that the upper limit of normal glomerulosclerosis in aging exceeds 10% (Chan et al. 1990). Normally, severe glomerular inflammation and crescent formation always incur a series of abnormities, including sclerosed glomeruli with breaks in the glomerular basement membrane, which should be paid attention to the possibility of underlying IgA nephropathy, antineutrophil cytoplasmic antibody-associated vasculitis, or lupus nephritis (Glassock and Rule 2012).
Tubular changes in the aging kidney are obvious, including a decrease in the tubular volume and number; a decrease in the tubular length and increased numbers of tubular diverticula, especially of the distal convoluted tubules; tubular atrophy, often with implication of tubular epithelium with thickening of the tubular basement membranes; and increase in the interstitial volume with interstitial fibrosis and sometimes even inflammatory cells (Silva 2005b). Tubular atrophy and interstitial fibrosis may be aging-related or may occur due to chronic inflammation or vascular disease (Nadasdy et al. 1994). Normally, there are three types of tubular atrophy, including the classic form with wrinkling and thickening of the tubular basement membranes and simplification of the tubular epithelium, thyroidization form with hyaline cast-filled dilated tubules, and the endocrine form with simplified tubular epithelium, thin basement membranes, and numerous mitochondria (Glassock and Rule 2012). These three types of tubular atrophy can be taken as a result of incomplete healing due to any type of renal injury. Among people over 40 years, it is common to see simple renal cysts, which might derive from the diverticula in distal and collecting tubules, probably promoting bacterial growth and contributing to the frequent renal infections in the old individuals (Tada et al. 1983).
As for renal vasculature, there are also a number of changes noted in the aging kidney. Those changes include tortuous interlobar arteries with thickening of the medial muscle cell basement membranes, “fibroelastic hyperplasia” of the arcuate and subarcuate arteries, myointimal fibrosis of the interlobular arteries, and hyaline change insudation of the afferent and the efferent arterioles (Gibson et al. 1996). “Arterial sclerosis” denotes thickening of the arterial wall and narrowing of the vascular lumen produced by thickening of the medial muscle cell basement membrane, fibrosis of the media, or intimal thickening, and may be seen with hypertension, diabetes mellitus, and aging, with the frequency of the arterial sclerosis increasing with advancing aging (Silva 2005b).
11.2.2 Function Changes of the Aging Kidney
It is reported that the glomerular filtration rate (GFR) begins to decline at a rate of 8–10 ml/min/decade after 30 years old (Morrissey and Yango 2006). However, there are still one-third of elderly individuals showing no change in GFR. This variability suggests that there should be other factors besides aging responsible for apparent reduction in renal function. Increased mean arterial blood pressure correlated directly with more aggressive decline in the aging process (Lindeman et al. 1985). Besides, low high-density lipoprotein cholesterol is also associated with accelerated age-related loss of renal function (Sesso et al. 2008). However, it is not clinically significant of the decline in renal function until some diseases further impaired the renal structure or function in the aging individuals. Besides traditional clearance of infused exogenous filtration markers, including insulin and iothalamate, creatinine clearance in a timed urinary sample is used as an estimate of GFR (Maher et al. 1971). However, creatinine clearance is affected by the nutritional status, protein intake, and muscle mass, which makes it not accurate to estimate the GFR in the elderly (Kimmel et al. 1996). The urinary creatinine output and creatinine production decline gradually with the aging-related decrease in muscle mass and body weight proportionately (Musch et al. 2006). Therefore, the serum creatinine does not increase with time in the old individuals, even in those with mild azotemia (Silva 2005a). Coresh has suggested: “Methods to estimate risks of CrCl for older subjects as a function of age and race in large epidemiologic studies are not properly standardized or of sufficient accuracy raising the question of validity in estimating renal function and therefore better methods to estimate CrCl are strongly encouraged.”
Under normal conditions, renal vasodilation leads to a significant increase in the renal blood flow and GFR, representing renal hemodynamic and functional reserves. The increase in the renal plasma flow (RPF) and GFR in response to maximum renal vasodilation induced by concurrent infusion of amino acids and dopamine is markedly reduced in healthy elderly individuals (Fuiano et al. 2001). A study involving 207 healthy kidney donors showed an explicit progressive reduction in mean blood flow per unit mass with aging, which suggests that the decrease in RBF does not simply reflect decrease in the renal mass with aging (Hollenberg et al. 1974). The reduction in RPF induced by intravenous administration of the nitric oxide (NO) synthase (NOS) inhibitor increased markedly with aging in men but not in women. Increasing plasma levels of endogenous NOS inhibitor and associated NO deficiency are shown to contribute to the aging-related decline in RPF (Kielstein et al. 2003a). In aging, there is a tendency for the response to vasodilators, for example, endothelial-derived hyperpolarizing factor (EDHF) to be attenuated, while the responsiveness to vasoconstrictors like angiotensin II (A-II) is enhanced, which might lead to enhanced vaso-constructive responses in aging, potentially causing renal damage and ultimately a fall in GFR (Long et al. 2005).
Due to fatigue and fracture of the medial elastin, most of the elastic arteries showed changes with aging but little changes in distal muscular arteries (Virmani et al. 1991). Increased arterial stiffening results in an increase in pulse wave velocity (PWV). Aortic PWV is the speed with which pulse wave travels along the wall of the artery, and there is a 2.5-fold increase of the value comparing 80-year-old and 20-year-old individuals (Zhou et al. 2008b).
Changes of renal function during aging represent in many ways. Firstly, abnormal sodium balance and slower homeostatic responsiveness that result from the inability to conserve and excrete sodium chloride are common in the elderly as compared with young individuals (Kielstein et al. 2003b). Inability to conserve sodium may predispose the elderly to hemodynamic instability in the setting of sodium loss (Shannon et al. 1986). Due to a decline in the countercurrent maintenance of the medullary hypertonicity, there is a significant decline of the ability to maximally dilute or concentrate urine, which might cause volume depletion and dehydration as well as abnormal osmolar states. Compared with healthy young subjects, the mean lithium clearance that is an indicator of proximal tubular function was much lower in the elderly. Besides, the fractional proximal sodium reabsorption was much higher in the elderly (Fliser et al. 1997). The ability of the kidney to both maximally concentrate and dilute the urine deteriorates with age, which makes older individuals more susceptible to the development of delusional hyponatremia in the setting of excess water load (Rowe et al. 1976). Reduced renal diluting capacity also predisposes the elder to stress situations such as surgery, fever acute illness, and administration of drugs like diuretics or others that enhance vasopressin action. The susceptibility to drug toxicity is partly due to the altered drug pharmacokinetics that stems from a decline in the functional capacity of the kidney, as well as altered the body composition, which is popular among the elderly. Furthermore, aging affects pharmacodynamics of many drugs by modulating the sensitivity and physiological response to their actions. The homeostasis of acid is also regulated by the kidney through conserving bicarbonate and by net acid secretion as ammonium and titratable acid in the renal tubular lumen. Normally, the kidneys of the elderly could function similar to those in young subjects. However, once challenged with acute acid load, the senescent kidneys do not increase acid excretion and lower urinary pH efficiently as those of younger kidneys (Luckey and Parsa 2003). Age-related mild metabolic acidosis develops apparently partly due to normal age-related decline of renal function, although the blood pH and serum bicarbonate of the geriatric population without renal disease do not differ significantly from the young under basal conditions (Frassetto et al. 1996).
Decreasing renal function causes prevalence of anemia, which is related to the reduced erythropoietin (EPO) production by the kidney (Eisenstaedt et al. 2006). The prevalence of anemia increases with age. It has been reported that there is a trend toward an increase in prevalence of anemia with decreasing renal function (Ble et al. 2005). Serum EPO levels rise with age in healthy subjects, perhaps a compensation for aging-related subclinical blood loss, increased red blood cell turnover, or increased erythropoietin resistance of red cell precursors (Ershler et al. 2005). The kidney could remove around 50% of insulin from the systemic circulation, which is accomplished by glomerular filtration and proximal tubular uptake and degradation. Whereby, decreased kidney function leads to reduced insulin clearance ability. Some research suggested that the impaired insulin secretion of aging rats might not result from old age but might be associated with chronic renal insufficiency and excess of parathyroid hormone (Massry et al. 1991). Insulin secretion was actually impaired in the elderly individuals, and total body insulin clearance is lower in the elderly than in the young subjects. In a study, elderly women with osteoporosis and low creatinine clearance had lower calcium absorption, lower serum 1,25-dihydroxyvitimin D, and normal serum 25-hydroxyvitamin D, suggesting decreased conversion between them by the kidney (Gallagher et al. 2007). Besides reduced activation of vitamin D, elderly individuals also demonstrate increased renal calcium loss from reduced calcium reabsorption in the distal convoluted and connecting tubules (Arnaud and Sanchez 1990).
11.2.3 Autophagy and Cell Senescence in the Aging Kidney
Autophagy has been proved to be essential for many fundamental biological activities, such as cellular stress response and maintenance of cellular homeostasis (Wang and Klionsky 2011). Dysregulation of autophagy is involved in the pathogenesis of a variety of metabolic and age-related diseases (Shibata et al. 2006). The role of autophagy in the diseased kidney has been studied mainly in proximal tubular cells and podocytes. The most fragile cells in glomerulus have been identified to be podocytes, and a decreased number of podocytes is directly correlated with disease progression in several renal diseases (Ziyadeh and Wolf 2008). Glomerular endothelial cells are also altered in several renal diseases. During aging, it is common to see accumulation of damaged organelles and mitochondria, which will cause organ dysfunction. The kidney is particularly susceptible to age-related renal damage, such as tubular atrophy, interstitial fibrosis, and glomerulosclerosis. The elderly are more sensitive to ischemia and toxic stress and show high rates of end-stage renal disease and chronic kidney disease (Nitta et al. 2013; Bolignano et al. 2014). It is reported that autophagy is the mechanism for kidney clearing those damaged components in podocyte, which could maintain their capacities for regeneration. Furthermore, podocytes have a high level of basal autophagy, which suggests autophagy is essential for this kind of cells.
There are a series of causes for acute kidney injury (AKI), including sepsis, xenobiotic agents, drugs, and ischemia-reperfusion. Autophagy has been proved as a renoprotective cellular response in AKI (Huber et al. 2012). The renal tubulointerstitial compartment is not spared from aging-induced damage. Tubular atrophy, apoptosis, and fibrosis are associated with a decline in the filtration function in the aged kidney. Some research found that mice with a specific autophagy deficiency in renal proximal tubular cells have increased accumulation of damaged mitochondria and ubiquitinated proteins, which suggests that autophagy is protective in proximal tubules cells during aging and autophagy deficiency in renal tubular cells leads to premature aging (Liu et al. 2012; Kimura et al. 2011). According to these results, autophagy activation in the aging kidney would protect from age-related renal dysfunction.
Cell senescence is not only a marker of renal aging but also a participant, as shown in Fig. 11.2. Development senescence is thought to be induced as part of a physiological program that promotes cell replicative senescence. Besides, some other pathologic stresses, such as mitochondrial injury and oxidative stress, could activate p53 or p16 pathway. The growth of senescent cells has arrested, and the balance between apoptosis and proliferation has also broken down. Senescent cells obtain distinct phenotypes, including chromatin modifications and profound changes in protein secretion, which are referred to as the senescence-associated secretory phenotype (SASP). The SASP is a critical characteristic of senescence programs. SASP factors with developmental functions remain to be identified. Chronic senescent cells acquired with aging or following treatment are highly variable depending on the type of stressor, tissue, and species; however, they consistently induce IL-6 and plasminogen activator inhibitor I in vivo. So far, IL-6, IL-1a, plasminogen activator inhibitor 1, and MCP-1 are taken as SASP factors induced by senescent cells (Yang and Fogo 2010).
The relationship between renal aging and cell senescence. Both shorter telomeres and DNA damage are main causes of cellular senescence. Compared to normal cells, senescent cells have arrested growth, which manifests disordered proportion of apoptosis and proliferation. The kidneys containing senescent cells are more sensitive to inner or outer injury and recover more slowly after injury, which results in many complications. Senescence-associated secretory phenotype (SASP) is secreted by senescent cells, which affect negatively other surrounding cells, inducing aging of the whole kidney
11.2.4 Molecular Changes in the Aging Kidney
The Klotho gene was originally identified as a gene mutated in a mouse strain, which exhibited a syndrome resembling premature aging. In contrast, overexpression of the Klotho gene extended life span in the mouse, which suggests Klotho gene may function as an aging-suppressor gene (Kuro-o et al. 1997). Other research has found Klotho gene is related to osteoporosis, stroke, and coronary artery diseases (Arking et al. 2002). The Klotho gene is expressed notably in the distal convoluted tubules in the kidney and the choroid plexus in the brain. The multiple aging phenotypes caused by mutation of Klotho are mediated by its hormonal action via its binding to a cell-surface receptor and repressing the intracellular signals of insulin and insulin-like growth factor 1. The inhibition of insulin/IGF-1 signaling by Klotho is associated with increased resistance to oxidative stress (Kurosu et al. 2005). It has been demonstrated that renal Klotho mRNA is downregulated under sustained circulatory or metabolic kidney disease (Koh et al. 2001). Angiotensin-II might be the reason of decrease of renal Klotho gene expression during aging, which may be mediated through promoting intrarenal iron deposition and inducing oxidative stress (Saito et al. 2003).
Telomeres in somatic cells shorten with each cell division, and this progressive attrition leads to critically short telomeres and cellular senescence (Jiang et al. 2007).
It is commonly believed that telomeres act as a mitotic clock, initiating replicative senescence when telomeres become short enough after a certain number of divisions. The enzyme telomerase is required for the maintenance of the size and stability of the length of telomeres. Senescent cells express p16 and p21, inhibiting cellular proliferation by inhibiting cyclin-dependent kinases (Hara et al. 1996). There is an overall increase in p16 expression in the elderly compared with young individuals, particularly in the renal cortex, although this expression varies among different individuals (Chkhotua et al. 2003).
Sirtuins are a series of NAD+-dependent deacetylase associated with numerous aspects of health and physiology. Among the seven members, Sirt1, 3, and 6 have been reported to function positively in the renal aging. The renal protective effects of Sirtuin are found in various models of renal disorders with metabolic impairment, such as diabetic nephropathy. Sirt1 could exhibit histone deacetylation activity and renal protective effects through regulation of various factors such as p53, NF-κB p65 subunit, STAT, FoxO1, and FoxO3, which are transcriptional factors related to apoptosis, cellular aging, and inflammation (Wakino et al. 2015). The expression of Sirt6 decreases significantly during aging process in mice. High-fat diet, an aging-promoting factor, could reduce expression of Sirt6 in the kidney (Zhang et al. 2016). Recently, it has been proved overexpression of Sirt6 could protect the kidney from cisplatin-induced acute kidney injury, and absence of Sirt6 would aggravate the injury caused by cisplatin (Li et al. 2018).
Increased oxidative stress and accumulation of free radicals are believed to play an essential role in the process of aging. Lipofuscin is free radical damaged proteins and fat, and it is insoluble and not degradable by either lysosomal enzymes or the proteasomal system. The release of lipofuscin and lysosomal contents cause cell damage and dysfunction (Jung et al. 2007). The increase in oxidative stress and lipid peroxidation in the aging kidney is related to an increase in the advanced glycosylation end products and their receptors, which might degrade hypoxia inducible factor-1a (HIF-1a) and limit the capacity of the aging cells to form hypoxia inducible factor-1-DNA hypoxia-responsive recognition element complexes. In the kidney, the consequent decrease in the ability of the cells to respond to hypoxia could explain the attenuated anemia-induced secretion of erythropoietin (Frenkel-Denkberg et al. 1999).
To elucidate the intricate relationship among diverse pathways, which are involved in the aging process, researchers performed a whole-genome analysis of gene expression in kidney samples from 74 patients ranging from 27 to 92 years old. In the results, more than 900 aging-related genes are found to change, and there are small changes of transcriptional differences of many genes, rather than huge changes of several essential genes, which suggests aging is a complex process involving a large number of intimately related molecular pathways (Rodwell et al. 2004).
11.3 Intervention Strategies of Renal Senescence
11.3.1 Clearance of Senescent Cells
The goal of any therapeutic intervention improving the features of kidney aging is the preservation of long-term kidney health and function. It emerges a new field in aging research intending to reduce the number of senescent cells. To clear the senescent cells efficiently and avoid potential off-target effects, identifying the differences between senescent cells and non-senescent cells is critically important. Senolysis could be best achieved with strategies similar to those used to kill cancer cells, such as activation of the immune system, inhibition of pro-survival pathways, or activation of pro-apoptotic pathways. In most organs, senescent cells should be cleared by immune system, whereas some chronic senescent cells could resist clearance in different contexts (Hoenicke and Zender 2012). The adaptive immune system is essential in recognizing premalignant hepatocytes, and the innate immune response is particularly important for the physiological removal of senescent cells. The SASP of senescent liver cells activates macrophages and induces their polarization toward the secretory M1 type, which ultimately leads to senescent cells clearance (Lujambio et al. 2013). On the contrast, it will cause increased liver fibrosis when the physiological natural killer cells are impaired, which leads to low efficiency of clearance of activated stellate cells in livers (Sagiv et al. 2016). Therefore, we have the reason to conclude that pharmacological agent increasing the susceptibility of senescent cells to immune cell-mediated clearance could be an efficient way improving aging-associated diseases.
Although it seems to be beneficial to remove renal senescent cells in many kidney diseases and renal aging, the effect has not been confirmed yet. It is found that during the healing period after implantation, senescent cells would promote cutaneous wound healing (Demaria et al. 2014).
Inducing autophagy could be an effective way to clear senescent cells, which might be a promising therapeutic strategy for the treatment of renal diseases. However, the protective effect of autophagy in renal diseases is still lacking for human diseases. Autophagy inducers commonly used in human medicine are mTOR inhibitors, such as sirolimus, everolimus and rapamycin. They are normally used as immunosuppressive agents, but they have strong adverse effects on compensatory renal epithelial cell hypertrophy and recovery from ischemia following renal transplantation (Pallet and Legendre 2013; McTaggart et al. 2003). Therefore, it seems not to be a feasible therapy to use mTOR inhibitors as autophagy inducers for renal diseases. It is promising to discover a specific approach that enables pathway-specific and kidney-selective regulation of autophagy, which could minimize the side effects.
11.3.2 Modifying the Epigenome
More and more evidences have shown up from model organisms to humans, indicating that psychosocial interventions, nutrition, and even exercise can interfere aging process (Simpson et al. 2010; Epel et al. 2004; Blackburn et al. 2015; Fontana and Partridge 2015). Furthermore, those interventions could target pathways that are essential in the epigenetic regulations, such as nutrient sensing 5-AMP-activated protein kinase (AMPK) and mTOR pathways. Metformin targeting AMPK could attenuate the production of proinflammatory SASP, and rapamycin could inhibit mTOR pathway slowing the aging process both in vitro and in vivo (Imai and Guarente 2014; Mitchell et al. 2014; Mercken et al. 2014). Sirtuins play an essential role in many processes, especially Sirt1 and Sirt6 that are closely related to life span and health span (Fontana and Partridge 2015; Mitchell et al. 2014; Mercken et al. 2014; Field and Adams 2017; Selman et al. 2009). Animal studies showed individual sirtuin family members have dramatic effects on chromatin regulation, and the loss of function would cause progeria and a series of aging-related diseases (McGuinness et al. 2011).
It is also a promising strategy to target the cellular methylome. It has been proved by several studies that there is a functional relationship between differential DNA methylation, transcriptomic effects, and the development of aging-related renal diseases. The synergistic removal of calciprotein particles may be a good strategy to attenuate the adverse effects of hyperphosphatemia on the epigenome (Painter et al. 2008; Au et al. 2013). As mentioned before, Klotho promoter methylation is a feature of chronic kidney disease, which is usually found in elderly individuals. Treatment with Rhein reverses Klotho methylation efficiently in murine models (Selman et al. 2009).
More interventions deserve further investigation, like activation of telomerase and reduction of p16INK4a, which is potentially functional but also with adverse effects. Stains delay senescence of endothelial cells through reducing overproduction of intracellular OFRs and inhibiting nuclear export of telomerase reverse transcriptase (Haendeler et al. 2004). PPAR-g agonists protect against renal injury in aging by reducing proteinuria, improving GFR, and decreasing sclerosis. On one hand, PPAR-g agonists could regulate p66SHC phosphorylation, which is an integration point for many signaling pathways that influence mitochondrial function and longevity. On the other hand, PPAR agonists could also increase expression of Klotho and decrease systemic and renal oxidative stress and regulate pathways that are associated with cell senescence in the kidney (Yang et al. 2009).
It is deniable that the epigenetics of aging is clearly important for health span. However, it still needs to be clarified whether the effect is uniform among different organisms. Furthermore, regulation of epigenetics of aging has different meanings for life course in the early stage and late stage, which suggests that aging-associated epigenetic processes might be subject to context-dependent intervention.
11.3.3 Caloric Restriction
Long time ago, McCay and colleagues have demonstrated caloric restriction to be able to extend life span of rats. Since then, calorie restriction has consistently shown beneficial effects on longevity, age-associated diseases, tumorigenesis, and attenuation of functional decline across many kinds of organisms (Gross and Dreyfuss 1984; Kritchevsky 2002; Weindruch and Sohal 1997). However, long-time caloric restriction is not feasible for humans, but short-time calorie restriction could produce significant reductions in body weight, blood pressure, blood cholesterol, and blood sugar, as well as reduced the development of atherosclerosis and ameliorated the decline in diastolic function (Walford et al. 2002). The exact mechanism of calorie restriction is still unclear. There are several extensive mechanisms that have been found, including reducing insulin and insulin-like growth factor 1 (IGF-1) signaling, reduction in oxidative stress, increase of antioxidant (De Cabo et al. 2004), enhanced mitochondrial function (Nisoli et al. 2005), improved proteostasis and autophagy (Cuervo 2008; Hansen et al. 2008), and a reduction in reproductive investment (Mitchell et al. 2015).
It has been shown that caloric restriction in animals modulates the aging-related physiological processes in the kidney. Caloric restriction could increase the resistance of the rat kidney to ischemic injury, since kidneys are usually susceptible in elderly individuals. The mechanism might rely on the molecular alterations such as the attenuation of aging-related changes in expression of genes such as claudin-7, kidney injury molecule-1, and matrix metalloproteinase-7 (Chen et al. 2007). Furthermore, it has been found that calorie restriction could reduce glomerulosclerosis, tubular atrophy, interstitial fibrosis, vascular wall thickening, and the expression of cytochrome c oxidase-deficient tubular epithelial cells (McKiernan et al. 2007). Calorie restriction could also prevent or delay the development of structural and functional changes, such as glomerulosclerosis and tubulointerstitial damage (Keenan et al. 2000). As for 24-month-old rats, calorie restriction could reduce the aging-related proteinuria, extracellular matrix accumulation, and the renal expression of connective tissue growth factor, vascular endothelial growth factor, and plasminogen activator inhibitor-1. The authors concluded caloric restriction affects aging-related renal physiology by altering renal SREBP expression and renal lipid accumulation, since those changes of the kidney are associated with a decline in the renal expression of sterol regulatory element-binding proteins, SREBP-1 and SREBP-2, and a decline in renal triglyceride and cholesterol content (Jiang et al. 2005). The markers of oxidative stress, malondialdehyde and 4-hydroxynonenal, and markers of apoptosis, Bax and procaspase 3, were significantly lower in aged rats under calorie restriction compared to the rats fed ad libitum (Lee et al. 2004). Calorie restriction has been found to be an effective way to activate Sirt1, which has been proved as a protective factor of the kidney as mentioned before.
11.3.4 Renal Transplantation
Studies have clearly shown that the elderly patients with end-stage renal failure could benefit from kidney transplantation (Silva 2005b; Macrae et al. 2005). However, the survival of renal transplant recipients is still lower than that of the general population. It is not surprising that among the more than 300,000 US patients currently on dialysis, the patients with end-stage kidney disease are popular, especially around 70–80 years old (Silva 2005b). Questions come like whether a donor kidney should be transplanted to an elderly patient with comorbidities and an average life expectancy of 10–15 years over a younger patient. Studies showed the relative risk of renal graft failure, adjusted for comorbidities, is statistically similar for renal transplant recipients older than 65 years and their younger counterparts (Fabrizii and Horl 2001). The most common cause of graft loss in the elderly transplant recipient is the comorbidity-related demise of the patient. Thus, careful screening of elderly disease, peripheral vascular disease, diabetes mellitus, and chronic obstructive pulmonary disease may help minimize early posttransplant morbidity and mortality (Zhou et al. 2008a). Older renal allografts do have an increased risk of graft failure, mainly because of aging-related decline in renal function, predisposition to ischemia and drug toxicity, reduced capacity for repair, and a higher degree of immunogenicity (Nyberg et al. 2005; Bunnapradist et al. 2003).
Renal transplantation seems to halt but not reverse vascular calcification (Cianciolo et al. 2014). Exercise capacity and muscle strength were lower in renal transplant recipients than in healthy controls, but improved significantly following a rehabilitation program, indicating an important reversible component (van den Ham et al. 2005, 2007). With regard to the underlying mechanisms of premature aging, oxidative stress generally improves or even normalizes after renal transplantation. Levels of advanced glycation and products, which are associated with aging, decrease after kidney transplantation (Jin 2010; Crowley et al. 2013). Immunosuppressive treatment was reported to affect renal transplantation. Treatment of rapamycin could lower serum phosphate levels and increase insulin resistance in renal transplant recipients (Tataranni et al. 2011). Rapamycin treatment also increased Klotho expression in immortalized proximal tubular cell lines, consistent with the supposed antiaging properties (Tataranni et al. 2011). Another example of the complex effects of immunosuppressive agents concerns the reduced telomerase activity associated with use of azathioprine (Getliffe et al. 2005). Corticosteroid treatment after transplantation might accelerate senescent processes (Bauer et al. 2009).
11.4 Conclusion
Aging is accompanied with decreased function of all organs, including the kidney. Old kidneys are functional but fragile. Although much progress has been made in the understanding of the renal aging process and the associated decline in renal function, there are still some questions remaining unanswered and deserving future investigation. Improved understanding of renal aging may help to optimize management of renal allografts obtained from older donors. More research should be done on the distinction between the decline in renal function and alteration in renal structure because of aging-associated diseases such as hypertension and diabetes mellitus and that solely because of aging.
As mentioned before, there are several ways to interfere the process of renal aging, but they are not working efficiently enough separately. Combination of different ways could be a potential method to prevent renal aging or attenuate aging and aging-related diseases. The final goal of any intervention battling features of kidney aging is to preserve long-term kidney health and function.
The epigenetics offers the promise of providing a context-dependent understanding of human health span. Improved knowledge of how epigenetic process regulates aging and how they interact with disease process will enable the identification and stratification of patients at increased risk of age-related morbidities. How to identify and track several of environmental factors influencing health and interacting with individual cellular and molecular processes is critical to the identification and curing for renal diseases. Long-term calorie restriction with optimal nutrition is pretty hard to achieve in daily life, which makes it unlikely to become clinically relevant in the near future. Some researches come up with several nutritional factors and small compounds mimicking the calorie restriction effects by regulating nutrient sensing pathways or by inducing autophagy are in different phases of preclinical and clinical testing.
All in all, epidemiologic, biochemical, and molecular evidence suggest that aging of the kidney is a complex interplay of different molecular mechanisms going far beyond a simple “wear and tear” process. There are many pathways found to be useful targets for slowing the aging process. Because combatting aging will require a long-term strategy, the route to clinical translation requires further insight from preclinical studies.
References
Anderson S et al (2009) Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20:1199–1209
Arking DE et al (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A 99:856–861
Arnaud CD, Sanchez SD (1990) The role of calcium in osteoporosis. Annu Rev Nutr 10:397–414
Au CP, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery H (2013) Fetal and maternal factors associated with neonatal adiposity as measured by air displacement plethysmography: a large cross-sectional study. Early Hum Dev 89:839–843
Baker GT 3rd, Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:223–239
Bauer ME, Jeckel CM, Luz C (2009) The role of stress factors during aging of the immune system. Ann N Y Acad Sci 1153:139–152
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–1198
Ble A et al (2005) Renal function, erythropoietin, and anemia of older persons: the InCHIANTI study. Arch Intern Med 165:2222–2227
Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C (2014) The aging kidney revisited: a systematic review. Ageing Res Rev 14:65–80
Brenner BM (1983) Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23:647–655
Buemi M et al (2005) Kidney aging: from phenotype to genetics. Rejuvenation Res 8:101–109
Bunnapradist S, Daswani A, Takemoto SK (2003) Graft survival following living-donor renal transplantation: a comparison of tacrolimus and cyclosporine microemulsion with mycophenolate mofetil and steroids. Transplantation 76:10–15
Chan KW, Leung CY, Chan CW (1990) Age-related glomerular sclerosis: baseline values in Hong Kong. Pathology 22:177–180
Chen G et al (2007) Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol 293:F1272–F1281
Chkhotua AB et al (2003) Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am J Kidney Dis 41:1303–1313
Cianciolo G et al (2014) Importance of vascular calcification in kidney transplant recipients. Am J Nephrol 39:418–426
Couser WG, Remuzzi G, Mendis S, Tonelli M (2011) The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80:1258–1270
Crowley LE et al (2013) Tissue advanced glycation end product deposition after kidney transplantation. Nephron Clin Pract 124:54–59
Cuervo AM (2008) Autophagy and aging: keeping that old broom working. Trends Genet 24:604–612
De Cabo R et al (2004) Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol 39:297–304
Demaria M et al (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31:722–733
Eisenstaedt R, Penninx BW, Woodman RC (2006) Anemia in the elderly: current understanding and emerging concepts. Blood Rev 20:213–226
Epel ES et al (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 101:17312–17315
Ershler WB et al (2005) Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc 53:1360–1365
Fabrizii V, Horl WH (2001) Renal transplantation in the elderly. Curr Opin Urol 11:159–163
Field AE, Adams PD (2017) Targeting chromatin aging – the epigenetic impact of longevity-associated interventions. Exp Gerontol 94:29–33
Fliser D, Zeier M, Nowack R, Ritz E (1993) Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3:1371–1377
Fliser D et al (1997) Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int 51:1196–1204
Fontana L, Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161:106–118
Frassetto LA, Morris RC Jr, Sebastian A (1996) Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Phys 271:F1114–F1122
Frenkel-Denkberg G, Gershon D, Levy AP (1999) The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett 462:341–344
Fuiano G et al (2001) Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int 59:1052–1058
Gallagher JC, Rapuri P, Smith L (2007) Falls are associated with decreased renal function and insufficient calcitriol production by the kidney. J Steroid Biochem Mol Biol 103:610–613
Getliffe KM et al (2005) Lymphocyte telomere dynamics and telomerase activity in inflammatory bowel disease: effect of drugs and smoking. Aliment Pharmacol Ther 21:121–131
Gibson IW, Downie TT, More IA, Lindop GB (1996) Atubular glomeruli and glomerular cysts – a possible pathway for nephron loss in the human kidney? J Pathol 179:421–426
Glassock RJ, Rule AD (2012) The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 82:270–277
Gross L, Dreyfuss Y (1984) Reduction in the incidence of radiation-induced tumors in rats after restriction of food intake. Proc Natl Acad Sci U S A 81:7596–7598
Haendeler J et al (2004) Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res 94:768–775
Hansen M et al (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet e24:4
Hara E et al (1996) Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16:859–867
Hoenicke L, Zender L (2012) Immune surveillance of senescent cells – biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33:1123–1126
Hollenberg NK et al (1974) Senescence and the renal vasculature in normal man. Circ Res 34:309–316
Huber TB et al (2012) Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8:1009–1031
Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE (2006) Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69:671–678
Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol 24:464–471
Jiang T, Liebman SE, Lucia MS, Phillips CL, Levi M (2005) Calorie restriction modulates renal expression of sterol regulatory element binding proteins, lipid accumulation, and age-related renal disease. J Am Soc Nephrol 16:2385–2394
Jiang H, Ju Z, Rudolph KL (2007) Telomere shortening and ageing. Z Gerontol Geriatr 40:314–324
Jin K (2010) Modern biological theories of aging. Aging Dis 1:72–74
Jung T, Bader N, Grune T (2007) Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 1119:97–111
Keenan KP et al (2000) Chronic nephropathy in ad libitum overfed Sprague-Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol 28:788–798
Kielstein JT et al (2003a) Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 107:1891–1895
Kielstein JT, Bode-Boger SM, Haller H, Fliser D (2003b) Functional changes in the ageing kidney: is there a role for asymmetric dimethylarginine? Nephrol Dial Transplant 18:1245–1248
Kimmel PL, Lew SQ, Bosch JP (1996) Nutrition, ageing and GFR: is age-associated decline inevitable? Nephrol Dial Transplant 11(Suppl 9):85–88
Kimura T et al (2011) Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22:902–913
Koh N et al (2001) Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280:1015–1020
Kritchevsky D (2002) Caloric restriction and experimental carcinogenesis. Hybrid Hybridomics 21:147–151
Kuro-o M et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Lee JH et al (2004) Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol 39:1361–1368
Levey AS, Inker LA, Coresh J (2015) Chronic kidney disease in older people. JAMA 314:557–558
Li Z et al (2018) Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int 93:881
Lindeman RD, Tobin J, Shock NW (1985) Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33:278–285
Liu S et al (2012) Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8:826–837
Long DA, Mu W, Price KL, Johnson RJ (2005) Blood vessels and the aging kidney. Nephron Exp Nephrol 101:e95–e99
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Luckey AE, Parsa CJ (2003) Fluid and electrolytes in the aged. Arch Surg 138:1055–1060
Lujambio A et al (2013) Non-cell-autonomous tumor suppression by p53. Cell 153:449–460
Macrae J, Friedman AL, Friedman EA, Eggers P (2005) Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States. Int Urol Nephrol 37:641–648
Maher FT, Nolan NG, Elveback LR (1971) Comparison of simultaneous clearances of 125-I-labeled sodium lothalamate (Glofil) and of inulin. Mayo Clin Proc 46:690–691
Massry SG et al (1991) Impaired insulin secretion of aging: role of renal failure and hyperparathyroidism. Kidney Int 40:662–667
McGuinness D, McGuinness DH, McCaul JA, Shiels PG (2011) Sirtuins, bioageing, and cancer. J Aging Res 2011:235754
McKiernan SH et al (2007) Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol 292:F1751–F1760
McTaggart RA et al (2003) Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant 3:416–423
Mercken EM et al (2014) SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13:787–796
Mitchell SJ et al (2014) The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6:836–843
Mitchell SE et al (2015) The effects of graded levels of calorie restriction: II. Impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget 6:23213–23237
Morrissey PE, Yango AF (2006) Renal transplantation: older recipients and donors. Clin Geriatr Med 22:687–707
Musch W, Verfaillie L, Decaux G (2006) Age-related increase in plasma urea level and decrease in fractional urea excretion: clinical application in the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol 1:909–914
Nadasdy T, Laszik Z, Blick KE, Johnson DL, Silva FG (1994) Tubular atrophy in the end-stage kidney: a lectin and immunohistochemical study. Hum Pathol 25:22–28
Newbold KM, Sandison A, Howie AJ (1992) Comparison of size of juxtamedullary and outer cortical glomeruli in normal adult kidney. Virchows Arch A Pathol Anat Histopathol 420:127–129
Nisoli E et al (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310:314–317
Nitta K, Okada K, Yanai M, Takahashi S (2013) Aging and chronic kidney disease. Kidney Blood Press Res 38:109–120
Nyberg SL et al (2005) Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation 80:925–929
Nyengaard JR, Bendtsen TF (1992) Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232:194–201
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol 28:407–420
Painter RC et al (2008) Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 115:1243–1249
Pallet N, Legendre C (2013) Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf 12:177–186
Rodwell GE et al (2004) A transcriptional profile of aging in the human kidney. PLoS Biol e427:2
Rowe JW, Shock NW, DeFronzo RA (1976) The influence of age on the renal response to water deprivation in man. Nephron 17:270–278
Sagiv A et al (2016) NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8:328–344
Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R (2003) Iron chelation and a free radical scavenger suppress angiotensin II-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett 551:58–62
Selman C et al (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144
Sesso R, Prado F, Vicioso B, Ramos LR (2008) Prospective study of progression of kidney dysfunction in community-dwelling older adults. Nephrology (Carlton) 13:99–103
Shannon RP, Wei JY, Rosa RM, Epstein FH, Rowe JW (1986) The effect of age and sodium depletion on cardiovascular response to orthostasis. Hypertension 8:438–443
Shibata M et al (2006) Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem 281:14474–14485
Silva FG (2005a) The aging kidney: a review – part II. Int Urol Nephrol 37:419–432
Silva FG (2005b) The aging kidney: a review – part I. Int Urol Nephrol 37:185–205
Simpson RJ et al (2010) Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc Immunol Rev 16:40–55
Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T (1983) The incidence of simple renal cyst by computed tomography. Clin Radiol 34:437–439
Tataranni T et al (2011) Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant 11:1656–1664
van den Ham EC et al (2005) Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am J Transplant 5:1957–1965
van den Ham EC et al (2007) The functional, metabolic, and anabolic responses to exercise training in renal transplant and hemodialysis patients. Transplantation 83:1059–1068
Virmani R et al (1991) Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol 139:1119–1129
Wakino S, Hasegawa K, Itoh H (2015) Sirtuin and metabolic kidney disease. Kidney Int 88:691–698
Walford RL, Mock D, Verdery R, MacCallum T (2002) Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 57:B211–B224
Wang K, Klionsky DJ (2011) Mitochondria removal by autophagy. Autophagy 7:297–300
Weindruch R, Sohal RS (1997) Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med 337:986–994
Yang H, Fogo AB (2010) Cell senescence in the aging kidney. J Am Soc Nephrol 21:1436–1439
Yang HC et al (2009) The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20:2380–2388
Zhang N et al (2016) Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-kappaB signaling. Cell Cycle 15:1009–1018
Zhou XJ et al (2008a) The aging kidney. Kidney Int 74:710–720
Zhou XJ, Saxena R, Liu Z, Vaziri ND, Silva FG (2008b) Renal senescence in 2008: progress and challenges. Int Urol Nephrol 40:823–839
Ziyadeh FN, Wolf G (2008) Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4:39–45
Acknowledgements
This work was financially supported by grants from the National Key R&D Program of China (2018YFD0400204), the Key International S&T Cooperation Program of China (2016YFE113700), the European Union’s Horizon 2020 Research and Innovation Program (633589) and the National Natural Science Foundation of China (81471396).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, Z., Wang, Z. (2018). Aging Kidney and Aging-Related Disease. In: Wang, Z. (eds) Aging and Aging-Related Diseases. Advances in Experimental Medicine and Biology, vol 1086. Springer, Singapore. https://doi.org/10.1007/978-981-13-1117-8_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-1117-8_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1116-1
Online ISBN: 978-981-13-1117-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)