Abstract
Sulfur (S) is an essential macronutrient in plants that serves numerous plant functions and is vital for the metabolic processes. Moreover, it is the constituent of some essential amino acids and metabolites. Recent studies have provided the notion that S not only improves the productivity of plants under normal condition but also protects them from abiotic stresses like salinity, drought, and toxic metals/metalloids. Different S compounds directly act as antioxidants or modulate antioxidant defense system. Among them, glutathione (GSH) is regarded as one of the powerful antioxidants and stress protectors. Interactions of S with other biological molecules afford stress signaling to provide defense against environmental stresses. However, the S uptake, translocation, and mechanisms of action in plants under stressful conditions are still under research. The recent progress on the roles of S in conferring abiotic stresses and related literature is presented in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Water stress, temperature stress, salinity stress, metal stress, nutrient deficiency, UV radiation, and ozone stress are being the most common abiotic stresses throughout the world. Growth and phenological pattern and reproductive development are hampered due to obstruction in water and nutrient uptake, photosynthetic activity, mitochondrial reaction, and plasma membrane transportation of cell organelles in plants grown under abiotic stress condition (Hasanuzzaman et al. 2012, 2017a). The intensity of these stresses is increasing day by day at an alarming rate, and because of that, the abiotic stresses become a matter of immense anxiety to plant productivity. That is why research on abiotic stress effects and how to decrease abiotic stress effects on plants have been increased noticeably previously. Inherent struggling capacity for survival in the era of abiotic/biotic stresses determines the healthy growth of any organisms including plants. Naturally, like any other organisms, plants’ genetic potential determines the ability to struggle and survive against abiotic stresses.
Keen observation of plant processes and biomolecules within plants has recognized sulfur (S) as one of the most abundant elements in organic structures. Sulfur is the fourth most important plant nutrient after N, P, and K. Sulfur after taken up by the root in the form of sulfate (SO4 2−) integrated into cysteine (Cys). Cysteine acts as a precursor or donor of key S compounds such as methionine (Met), S-adenosylmethionine, glutathione (GSH), homo-GSH (h-GSH), phytochelatins (PCs), sulfolipids, iron-sulfur clusters, allyl Cys, and glucosinolates, which play role in plant developmental processes and/or stress adaptation processes (Rausch and Wachter 2005; Khan et al. 2014; Anjum et al. 2015). Glutathione and h-GSH are involved in stress signal transmission. Several key stress metabolites such as ethylene (C2H2) are controlled by S-adenosylmethionine. Sulfur compound-mediated function of ATP-S has been reported for stress tolerance response (Anjum et al. 2015). Cysteine improved growth and lessened oxidative stress by modulating cellular redox status and antioxidant defense in barley (Genisel et al. 2014). Sulfur-induced GSH synthesis decreased reactive oxygen species (ROS) and improved photosynthetic efficiency and growth in salt-affected barley (Astolfi and Zuchi 2013). Higher content of S uptake increased accumulation of proteinogenic and non-proteinogenic thiols which improved cadmium (Cd) tolerance (Sun et al. 2007; Lancilli et al. 2014). The S deficiency reduces chl (chl) content, photosystem (PS) II efficiency, and performance of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) (Lunde et al. 2008). Sulfur moiety of Cys can regulate disulfide bond of proteins, which affect the structure of the Fe-S cluster and function of photosynthetic apparatus and electron transport chain (Rochaix 2011). Glyceraldehyde-3-P-dehydrogenase (GAPDH), malate dehydrogenase (MDH), and elongation factor-thermo unstable (EF-TU) are some Cys-bearing protein having oxidative thiol modification functions (Leichert et al. 2008). Ethephon and N jointly augmented S-mediated ethylene and diminished glucose sensitivity, thus improving photosynthesis and growth (Iqbal et al. 2011).
Sulfur has been reported to improve antioxidant defense and metal chelation under Cd stress. In the presence of S, the GSH content, ratio of GSH and glutathione disulfide, GSSG (GSH/GSSG), nonprotein thiols (NPTs) and PCs, ascorbate (AsA) content, ratio of AsA and dehydroascorbate, DHA (AsA/DHA), activities of ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT) improved in Cd-affected Indian mustard plant. Moreover, superoxide dismutase (SOD) expression was upregulated that decreased O2 •− (Bashir et al. 2015). Sulfur addition decreased the As uptake and content in the shoot and modulated thiol metabolism, glycolysis, and amino acid in rice (Dixit et al. 2015a). Thus, S is proved as a potent plant nutrient for plant developmental processes.
There are some sporadic researches on the effect of S on abiotic stress alleviation in plants. Few research findings said about signaling function of S and its derivatives. Based on available research findings, it is obvious that S is a potent molecule that functions in plant developmental and abiotic stress adaptation processes. It is necessary to extend research with S to exploit its function thoroughly. So, a comprehensive review presenting available information and research updates of S will be a base for filling up gaps of previous or existing researchers and exploring new research areas. Therefore, this review concentrates and gathers information on various aspects of S in plants including S metabolism, biological roles and roles in abiotic stress condition of S and its derivatives in plants, and molecular approaches in regulating S status.
10.2 Biological Role of Sulfur in Plants
Plants require a right combination of nutrients to survive, grow up, and reproduce. Sulfur is considered as an indispensable plant macronutrient required by all crops for their normal growth and development (Fig. 10.1). Sulfur is uptaken from the soil solution by the plant principally in the form of SO4 2− (Davidian and Kopriva 2010; Capaldi et al. 2015). Moreover, different S-containing amino acids provide S to the plants. Very little amount of S is supplied from the atmospheric SO2 and hydrogen sulfide (H2S) where SO2 is absorbed by leaf and fruit of plant (Mazid et al. 2011), and H2S is absorbed through stomata of the leaf (Riemenschneider et al. 2005). Sulfur has an immense function in fundamental processes of plants such as electron transport, cellular structure, and regulation of different metabolic pathways (Capaldi et al. 2015). Sufficient S nourishment to the plants improves photosynthesis by increasing chl formation and contributes to growth and development of plants (Scherer 2008). Furthermore, it has insightful relation with N assimilation. Carciochi et al. (2017) reported that optimum S in the growing media of wheat (Z51) increased nitrogen uptake and improved the root growth, which played a central role in improving yield (Salvagiotti et al. 2009). Sulfur is also a component of various amino acids (Cys, Met etc.), antioxidants, sulfolipids, proteins, and enzymes that regulate photosynthesis and biological nitrogen fixation and assimilation by the plant (Abdallah et al. 2010; Capaldi et al. 2015). Also, S is an important constituent of Fe-S clusters, lipids, polysaccharides, and a wide range of biomolecules, for instance, vitamins, cofactors, peptides, and different secondary products (allyl cysteine sulfoxides, glucosinolates, etc.) (Nocito et al. 2007; Iqbal et al. 2012). Sulfur is essential for the vegetative development and production of oil and proteins of the plant especially in oilseed crops (D’Hooghe et al. 2013; Mária et al. 2017). Many reports demonstrated the influential effect of S on the yield and total oil content of oilseed crops (Jankowski et al. 2008; Egesel et al. 2009). Sulfur is also responsible for the production of glucosinolates in both the vegetative parts and the seed of oilseed crops, which determine the pungency of plants (Walker and Booth 2003). Sulfur is known to interact with almost all essential macro- and micronutrients by influencing their uptake and utilization (Abdin et al. 2003).
Besides playing an imperative role in growth, development, and productivity of higher plants, S has an immense role to develop stress tolerance in plants (Nazar et al. 2011; Osman and Rady 2012). Elemental sulfur, H2S, GSH, PC, S-rich proteins, and various secondary metabolites are important S-containing defense compounds that are very important for plant survival during biotic and abiotic stresses. The development of these compounds in the plant is intimately linked to the supply, demand, uptake, and assimilation of S (Capaldi et al. 2015).
10.3 Sulfur Metabolism in Plants
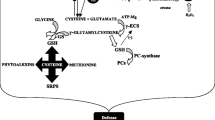
Plants produce many S-containing metabolites of diverse groups: amino acids (Cys, Met), antioxidants (GSH), vitamins (biotin, thiamine), and secondary metabolites (glucosinolates, alliinase). Although these metabolites contain S, their roles in a biological system are functionally distinct. Thus, S metabolism in plants is a topic of interest to explain how S is assimilated into a living system from the environment (Fig. 10.2). Like other macronutrients, S is taken up by the plant through the root as sulfate (SO4 2−). To be incorporated in the metabolic pathways, sulfate is first activated by ATP sulfurylase to yield adenosine-5′-phosphosulfate (APS) which is then reduced to sulfite (SO3 2−) by APS reductase. Finally, sulfite reductase converts the sulfite into sulfide that reacts with O-acetylserine in the presence of O-acetylserine lyase (OAS-TL) to produce Cys (Fig. 10.2). From Cys, GSH is produced by two-step ATP-dependent reactions, where Cys is converted to γ-glutamylcysteine by γ-glutamylcysteine synthetase (also known as glutamate-cysteine ligase, GCL), and the subsequent reaction is catalyzed by glutathione synthetase. Cysteine also serves as a precursor of Met. Homocysteine is produced from cysteine and O-phosphohomoserine by the action of cystathionine γ-synthase (CGS) and cystathionine β-lyase (CBL) (Fig. 10.2). Homocysteine is then converted into Met by methionine synthase (MS). Methionine is considered as the main precursor of glucosinolate synthesis pathway by initiating the side chain elongation reaction to Met (Hirani et al. 2012). Cysteine conjugated with the tryptophan-derived complex is involved in camalexin biosynthesis (Bottcher et al. 2009; Romero et al. 2014). Glutathione is a key regulator of redox signaling pathway and the antioxidant required for abiotic stress tolerance (Noctor et al. 2012). Glutathione is oxidized by reactive oxygen species (Noctor et al. 2012). The oxidized glutathione, GSSG, can be regenerated into GSH by GR. In addition, GSH is involved in two-step methylglyoxal (MG) detoxification: in the first step, MG is converted in S-d-lactoylglutathione (SLG) by glyoxalase I (Gly I) using GSH, and in the second step, d-lactate is produced from SLG by glyoxalase II (Gly II) releasing GSH (Hasanuzzaman et al. 2017b). The red circle marked in Fig. 10.2 is very responsive to different abiotic stresses.
Sulfate metabolism in plant. APS, adenosine-5′-phosphosulfate, Cys cysteine, Cyst cystathionine, γ-GluCys γ-glutamylcysteine, GSH glutathione, GSSG oxidized glutathione, Hcy homocysteine, MG methylglyoxal, OAS O-acetylserine, OPH O-phosphohomoserine, PAPS 3-phosphoadenosine-5-phosphosulfate, SLG S-d-lactoylglutathione, Ser serine, APK APS kinase, APR APS reductase, ATPS ATP sulfurylase, CBL cystathionine β-lyase, CGS cystathionine γ-synthase, γ -ECS γ-glutamylcysteine synthetase, Gly I glyoxalase I, Gly II glyoxalase II, GR glutathione reductase, GSHS glutathione synthetase, MS methionine synthase, OAS-TL OAS(thiol)lyase, SAT serine acetyltransferase, SiR sulfite reductase
Sulfur metabolism in the plant is greatly affected by environmental factors including biotic and abiotic factor. ATP sulfurylase activity or expression increases under sulfate starvation, salt stress, and light, Cd, and cold stress condition, and it contributes to abiotic stress tolerance (Khan et al. 2014; Anjum et al. 2015; Dixit et al. 2015b). Drought-induced alteration in S metabolic pathway in the plant is organ dependent. For example, drought stress decreased the Cys and GSH generation due to the lower assimilation of S in maize leaf. However, Cys, total glutathione, and SO4 2− content were higher in the root (Ahmad et al. 2016). Salinity induced threefold higher expression of APS reductase (APR) in Arabidopsis roots (Koprivova and Kopriva 2008). However, salt stress induced by Na2SO4 decreases the expression of APR in roots of Brassica rapa but not affected by NaCl, indicating APR expression depends on sulfate availability (Reich et al. 2017). Cadmium stress (10 mg kg−1) enhanced the activity of enzymes (ATPS, OASTAL, γ-ECS) of S assimilatory pathway in both root and shoot of Brassica chinensis L. (Lou et al. 2017). Above mentioned reports suggest that environmental stresses can alter the metabolic pathway of S. Therefore, this pathway could be the potential target for enhancing abiotic stress tolerance since there are few reports on this topic.
10.4 Sulfur Transporters in S Metabolism
As an inorganic molecule, S first needs to get into the cell. There are many membrane-bound transport proteins that facilitate the entry of nutrient inside the root from the outer environment. A motive force generated due to proton gradient mediates the sulfate influx using H+/SO4 2− cotransport system. To be metabolized into different metabolites, both inorganic forms of S and an organic molecule containing S must pass the membrane of cellular compartments through transport proteins. Smith et al. (1995) isolated three cDNAs from Stylosanthes hamata encoding sulfate transporters, which were highly conserved in other biological organisms: fungi, yeast, plants, and mammals. The two high-affinity H+/SO4 2− cotransporters, shst1 and shst2, facilitate sulfate uptake from sulfur-deficient media, whereas the low-affinity H+/SO4 2− cotransporter, shst3, transports sulfate to the cellular compartments. All the above mentioned sulfate transporters (SULTRs) contain characteristic 12 putative membrane-spanning domains and STAS (sulfate transporter and anti-sigma factor antagonist) domain playing a role in protein folding and regulating protein activity, respectively (Takahashi 2010; Takahashi et al. 2011). With time some sulfate transporter has been reported in Brassica oleracea, potato, and tomato (Buchner et al. 2004; Hopkins et al. 2005; Howarth et al. 2003). Also, 12 sulfate transporters gene were identified in Arabidopsis using modern genomic tools and techniques. These transporter proteins are classified into four groups, which have distinct functions; group 1 includes high-affinity SULTRs, while group 2 consists of low-affinity transporters. Both group 1 and group 2 SULTRs mediate SO4 2− uptake from nutrient media. Unlike groups 1 and 2, group 3 includes the transporter localized in plastid and symbiosome membrane playing specific function, sometimes unknown function, whereas group 4 mediates the SO4 2− transport from the vacuole to other cell organelles (Takahashi 2010; Gigolashvili and Kopriva 2014). Localization and expression of sulfate transporters indicate their function in the sulfate metabolism (Table 10.1).
High-affinity sulfate transporters (SULTR1;1, SULTR1;2) categorized in group 1 play a role in the initial uptake of sulfate from the growing media (Takahashi 2010; Takahashi et al. 2011). Yeast mutant lacking Sultr1;1 showed lower sulfate uptake, while mutant containing overexpressed Sultr1;1 vector improved sulfate uptake confirming SULTR1;1 is a sulfate transporter (Takahashi et al. 2000). Based on phylogenetic tree relationship and sequence information, it was predicted that other sulfate transporters might be present in the root having similarity in functions and affinity to sulfate (Takahashi et al. 2000). Selenate-resistant mutant showed lower uptake of sulfate both in sulfur-sufficient and sulfur-deficient medium. By yeast complementation technique, it is proved that the mutant was lacking Sultr1;2 gene, which is responsible for sulfate uptake in the root (Shibagaki et al. 2002). Interestingly, either SULTR1;1 or SULTR1;2 is not involved in root to shoot transport which indicates the involvement of other sulfate transporters to facilitate sulfate transport from the root to shoot. Getting entry through root hairs, sulfate reaches the endodermis to epidermis and cortex followed by xylem parenchyma through the symplastic pathway. However, sulfate can get entry to xylem parenchyma from the apoplast by SULTR2;1 (Maruyama-Nakashita et al. 2003). Mutant in Sultr3;5 restricted the transport of sulfate root to shoot. However, Sultr2;1 coexpressed with Sultr3;5 increased the root to shoot transport compared to Sultr2;1 expressed alone (Kataoka et al. 2004b; Takahashi et al. 2011). Furthermore, Sultr2;1 is found to be expressed in the leaf phloem suggesting its role in the reduction of excess sulfate from above ground part (Takahashi et al. 2000). SULTR2;2 transports sulfate to the mesophyll and palisade cell of leaf unloading from the xylem vessel (Takahashi et al. 2000). Under sulfate-deprived condition, overexpressed Sultr1;3 facilitates sulfate uptake in the phloem companion cell as Sultr2;1 repressed under sulfate-deficient condition (Yoshimoto et al. 2003; Takahashi 2010). When excess in supply, sulfate is reserved in the vacuole, but sulfate influx mechanism into the vacuole has not been well studied. However, the efflux of sulfate from the vacuole into the cytoplasm is mediated by SULTR4;1 and SULTR4;2 transporter protein, categorized in group 4 (Kataoka et al. 2004a). With these transporters, sulfur is taken up by roots and subsequently transported to different organs. Finally, sulfate gets into chloroplast where sulfur assimilatory pathway takes place. Using green fluorescent protein (GFP), SULTR3;1 transporter localization in the chloroplast was confirmed. Furthermore, mutant that lack this transporter showed lower sulfate uptake in the chloroplast, indicating SULTR3;1 is associated with sulfate transport in the chloroplast (Cao et al. 2013). Like SULTR3;1, other sulfate transporters like SULTR3;2, SULTR3;3, and SULTR3;4 are also involved in sulfate transport in the chloroplast (Cao et al. 2013). Among the four groups of sulfate transporters, transporters categorized in group 3 are less investigated compared to the others. For example, SULTR3;2-, SULTR3;3-, and SULTR3;4-mediated sulfate uptake mechanisms are still not clear.
10.5 Sulfate Transporter and Abiotic Stress
Different environmental factors including soil salinity, drought, waterlogging, high temperature, chilling, and heavy metal (HM) stresses affect nutrient metabolism from transport to assimilation. Under such extreme conditions, transporter proteins fail to function properly. As a result, ion homeostasis or nutrient balance is disrupted. Glutathione, a sulfur derivative compound, plays a diverse role under abiotic stress condition. In the presence of selenium (Se), reduction in endogenous GSH has a regulatory role in the expression of primary sulfate uptake transporter Sultr1;1 (Takahashi et al. 2000; Fig. 10.3). Abscisic acid is a key factor to regulate abiotic stress tolerance. Surprisingly, a mutant lacking sulfate transporter Sultr3;1 showed lower abscisic acid (ABA) content both in control and salt stress (200 mM NaCl) condition compared to wild type (Cao et al. 2014). This result indicates the possibility of sulfate transporters to be involved in abiotic stress tolerance. In Medicago truncatula, almost all sulfate transporters are affected by either drought or salinity (Gallardo et al. 2014). Under HM stresses in particular Cd stress, sulfate uptake increases due to the expression of ZmST1;1, very similar to high-affinity sulfate transporter which belongs to group 1 (Table 10.1). Higher sulfate uptake is associated with higher production of PC to detoxify toxic Cd (Nocito et al. 2006). Another high-affinity sulfate transporter Sultr1;2 is proved to be associated with As stress tolerance as Arabidopsis mutant lacking Sultr1;2 showed sensitivity to As in response to growth parameters (Nishida et al. 2016). The expression of sulfate transporters may vary from the shoot to root under unfavorable condition. For example, under prolonged drought for 10 and 12 days, Sultr1;2 expression reduced in the leaf and not affected in the root. However, Sultr4;1 overexpressed in leaf but repressed in roots. This result suggests that with the help of this transporter maize seedling maintained S homeostasis in the root. As a result root growth increased in search of water (Ahmad et al. 2016). As SULTRs are distinguishable in function, their responses also vary depending on the type of stresses, indicating a particular transporter may play a role in tolerance of a particular type of stress. Though there are many indirect pieces of evidence that explain the involvement of sulfate transporter in abiotic stress tolerance, still this hypothesis needs further confirmation with rigorous experimental evidence.
Role of sulfate transporters in sulfate uptake and distribution (Adapted from Takahashi et al. (2011))
10.6 Exogenous Use of Sulfur in Improving Plant Performances Under Abiotic Stress
Environmental stressors affect cell homeostasis inside plant tissue, and increasing ROS production leads to oxidative damage (Boaretto et al. 2014; Hasanuzzaman et al. 2017). Sulfur is an indispensable macronutrient for plant growth, development, and survival, and at the same time, many S-containing compounds play defensive roles in abiotic stress response, as well as cellular acclimation and adaptation in adverse condition (Cao et al. 2014). Plants respond to abiotic stresses in many ways, but the S assimilation pathway, the important source of reduced S for a variety of cellular mechanisms including Cys synthesis, which further used for Met biosynthesis or GSH production (Siddiqui et al. 2012). Thus the exogenous supply of S helps plants to survive in stress condition by maintaining their usual metabolic process (Table 10.2).
It seems that the HM/metalloid stress increases the demand of reduced S, hence activating the expression of SO4 2− transporters and enzymes in the S assimilatory pathway (Takahashi et al. 2011; Hawkesford 2012). Therefore, many research reports have shown the effect of exogenous S in mitigating various abiotic stresses. Sulfur has a vital role in nutrient homeostasis; thus, it can decrease the uptake of the HMs/metalloids and at the same time increase the absorption of essential plant nutrients. On the other hand, exogenous S may increase the uptake of few HMs/metalloids, thus may be further useful for phytoremediation. In As-stressed plants increased S supply increases thiol metabolism and antioxidant activity, As accumulation, as well as tolerance of plants (Srivastava and D’Souza 2010). Sulfate nutrition plays an important role in regulating As translocation from roots to shoots, possibly through the complexation of AsIII-PCs in rice (Zhang et al. 2011). Conclusively S supplementation reduced the As accumulation in a shoot by increasing thiol metabolism and glycolysis toward amino acid accumulation under AsIII stress in rice (Dixit et al. 2015a). Sheng et al. (2016) found a moderate level of S application, beneficial to wheat against Mn toxicity, via upregulating the antioxidant defense, and the translocation and distribution of Mn from roots to shoots, excess Mn sequestering in vacuoles in the form of PCs, and increased production of GSH, where GSH played a very important role. Applying S in guinea grass may be a vital tool for phytoremediation of Cu under Cu toxicity in Cu-polluted areas, by increasing Cu uptake as well as increasing (30–40% higher) shoot and root dry mass and decreasing of lipid peroxidation (Gilabel et al. 2014).
Application of S to Cd-stressed pak choi (Brassica chinensis L.) plants enhanced Cd tolerance by promoting the AsA-GSH cycle and PC biosynthesis. After application of S exogenously, its assimilation increased by the activity of ATP sulfurylase (ATPS) and O-acetylserine (thiol) lyase (OAS-TL) and decreased Cd translocation from the roots to the shoots (Liang et al. 2016; Lou et al. 2017). Indian mustard (Brassica juncea) plant supplemented with S under Cd stress accumulated the higher amount of AsA and GSH content as well as improved AsA/DHA, and GSH/GSSG ratio indicated the role of exogenous S in producing GSH, NPTs and PCs, and tolerance against Cd stress (Bashir et al. 2015). A similar result was also found by other researchers in B. juncea which suggested that Cd stress overproduced ethylene, which can be alleviated with S by antioxidant (GSH) metabolism (Asgher et al. 2014). Under Cd stress, seed priming with the SO2 donor moderately increased the amylase and esterase activities and increased the levels of reducing sugars and soluble protein in germinating wheat seeds. Pretreatment with the SO2 donor also reduced the MDA and and O2 •– and restrain the plasma membrane integrity of the wheat seedlings radical tips as well as increase the activity of POD, APX, SOD, and CAT while reducing the activity of LOX (Hu et al. 2014). Zhu et al. (2015) reported a higher level of H2S in wheat seeds primed with the SO2 donor (NaHSO3/Na2SO3). Wheat seed pretreated with 1.2 mM NaHSO3/Na2SO3 reduced overaccumulation of O2 •–, H2O2, and MDA, with lower lipoxygenase (LOX) activity, while the activity of guaiacol peroxidase (POD), CAT, and APX increased to enhance tolerance against Al toxicity (Zhu et al. 2015).
Tobacco cell culture pretreated with the H2S donor, sodium hydrosulfide (NaHS), KHSO3, and precursor Cys significantly increased the survival percentage of tobacco cells under high heat (Li et al. 2015). They also found an elevated level of sulfhydryl compounds such as H2S, sulfhydryl proteins, Cys, and GSH as well as higher antioxidant enzyme activity, for instance, SOD, CAT, cell wall peroxidases (POX), and GR, by pretreating with NaHS. Meanwhile, NaHS-pretreated wheat seedlings were found with higher antioxidant defense and H2S and soluble sugar contents, while lesser H2O2 and MDA contents are induced by heat stress. But the little effect was found regarding antioxidant enzyme activities and other soluble substance levels compared to control (Min et al. 2016). In cluster beans under heat stress, S supplementation helps to mitigate the oxidative stress with higher AsA-GSH content and to lower the H2O2, MDA, and electrolyte leakage. Sulfur also increased dry weight and chl content under heat stress (Mobin et al. 2016). The study explored that pretreatment of strawberry roots with NaHS (100 μM for 48 h) could induce long-lasting tolerance to salinity (100 mM NaCl) or drought (10% PEG-6000) for 7 days. Furthermore, NaHS pretreatment resulted in lower NO and H2O2 content in leaves along with high AsA and GSH content, following salt and drought stresses. Thus, H2S pretreatment managed the plants to overcome the salt and drought stress through upregulating antioxidant defense mechanisms and the coordination of the salt overly sensitive (SOS) pathway in strawberry (Christou et al. 2013). Salt stress drastically reduces the yield of crops by ionic toxicity and physiological drought. But exogenous S application from a foliar spray can mitigate the salinity stress by increasing photosynthetic rate, stomatal conductance, chl content, Pro, and GB content. The result suggested that S at both 5 and 10 g L−1 of spray solution improved the morphophysiological and biochemical traits in chili plants subjected to salinity (Mukhter et al. 2016). While working with mustard, Fatma et al. (2014) reported that excess S in the soil more rapidly alleviated the negative effects of salinity and improved photosynthesis and growth by increased GSH production. To support their statement, they again applied 1 mM GSH exogenously and found similar results of that excess S supplementation and confirmed excess S/GSH as a potential protectant against salt stress. At the time of salt stress, Cys is required to produce ABA to combat stress, while S supplementation increased the Cys production for ABA biosynthesis (Cao et al. 2014).
10.7 Sulfur Derivatives and Their Roles in Abiotic Stress Tolerance
Overproduction of ROS and MG is an inevitable process that occurs in different tissue types, under a diverse adverse environmental conditions including salinity, drought, toxic metal, high temperature, low temperature, waterlogging, etc., which might be regulated by a number of defensive molecules or systems in plants (Hasanuzzaman et al. 2012, 2017a; Sharma et al. 2012). Sulfur-containing compounds, for example, GSH, H2S, Met, Cys, PC, ATP-S, protein thiols, etc., play an immense role in normal functioning of the plant cell (Fig. 10.4). Also, such S derivatives primarily work in plant stress tolerance (Colville and Kranner 2010; Zagorchev et al. 2013). Under stress condition in most of the cases, the content of endogenous S-containing compounds increased up to a certain extent to protect plants from respective stress (Khan et al. 2013; Zagorchev et al. 2013; Hasanuzzaman et al. 2017b). Recently the exogenous application of different S derivatives (GSH, H2S, etc.) against a variety of abiotic stresses is receiving attention due to their effectiveness in enhancing stress tolerance.
Sulfur-rich Cys is very important for plants not only as an amino acid but also due to its role as a precursor for a huge number of vital biomolecules (Haag et al. 2012). Cysteine possesses a vital position in plant metabolism because it plays an essential role in the biosynthesis of important S-containing cellular compounds, for example, GSH and different proteins (Romero et al. 2014). These compounds directly or indirectly work in the redox signaling pathway and stress tolerance mechanisms. Moreover, Cys triggers metabolism of nitrogen at the biochemical and molecular level which plays an imperative role in enhancing plant stress tolerance (Zagorchev et al. 2013; Erdala and Turk 2016). On the other hand, Cys is a powerful metal chelator, but Cys-metal ion complexes are capable of activating the most devastating Fenton reaction, which may create toxic OH• radical in a plant cell (Bashir et al. 2012). Though endogenous Cys played a great function in a plant cell, the exogenous application of Cys for alleviating abiotic stress from the plant is very rare. Erdala and Turk (2016) demonstrated that Cys application to maize seedlings mitigates Cd-induced oxidative stress. Like Cys, Met can undergo ROS-mediated oxidation through a class of cytosolic and plastidic enzymes that are involved in mitigating the oxidative damage of plant (Dos Santos et al. 2005; Cabreiro et al. 2006). Methionine is also a substrate for the synthesis of a range of polyamines (putrescine, spermidine, spermine, etc.), which has important roles in stress tolerance (Alcázar et al. 2010).
Sulfate is first activated by ATP-S to yield APS which is then reduced to SO3 2− by APS reductase and then further reduced to S2− and finally incorporated into Cys which helps to produce S-rich GSH. So, enhancement of ATP-S in a plant cell is very urgent for developing plant stress tolerance. Glutathione is one of the most important nonenzymatic antioxidants for living systems which plays a noteworthy role in cellular metabolism and works as a vital component in scavenging of toxic ROS in a plant cell (Gill and Tuteja 2010; Hasanuzzaman et al. 2012; Noctor et al. 2012; Nahar et al. 2016). Also, it works in glyoxalase system to detoxify toxic MG (Hasanuzzaman et al. 2017a). By upholding the reduced status of α-tocopherol and zeaxanthin, GSH protects the membrane of the cell, which contributes to the reduction of protein denaturation under stress condition. Moreover, it functions as a substrate of glutathione peroxidase (GPX) and glutathione S-transferase (GST). Both GPX and GST play a direct role in scavenging of toxic ROS and protecting plants from oxidative stress induced by different abiotic stresses (Hasanuzzaman et al. 2012; Asgher et al. 2017). Glutathione S-transferase is also involved in detoxification of xenobiotics (Zagorchev et al. 2013). Glutathione, as a precursor of PC, assists in metal chelation, which transfers the toxic metals or metalloids to the cell vacuole as an inactive form (Hasanuzzaman et al. 2017a, b; Sharma and Dietz 2006). Consequently, it acts an imperative function in detoxification of toxic metals/metalloids (Hasanuzzaman et al. 2017b; Srivalli and Khanna-Chopra 2008). In addition to a variety of functions of GSH reported before, there is a confirmation that GSH is highly associated with controlling different genes (Zagorchev et al. 2013). Such abovementioned biochemical characteristics of GSH make it indispensable for plant growth and development under both usual and adverse growing conditions. Many previous reports confirmed that endogenous or exogenous GSH contributes to increase tolerance level of plants to diverse abiotic stresses, including salinity, drought, high temperature (HT), low temperature, and toxic metal stress (Kumar et al. 2010; Mahmood et al. 2010; Wang et al. 2011; Wu et al. 2011; Chen et al. 2012; Hasanuzzaman et al. 2012; Jozefczak et al. 2012). Here, we discussed few recent findings. Cheng et al. (2015) reported that exogenous application of reduced GSH in Arabidopsis plant could improve abiotic stress tolerance. They also claimed that endogenously enhanced GSH bestowed tolerance on the same plant under salt and drought stress. Furthermore, senescence and flowering are delayed due to both exogenous and endogenous GSH. Their translatome analysis also uncovered that GSH treatment triggered the biosynthesis process of ABA, auxin and jasmonic acid (JA), as well as signaling genes, which might be helpful for increasing plant stress tolerance. Çevik and Ünyayar (2015) checked the function of exogenously applied AsA and GSH on antioxidant system of Cicer arietinum plant under drought stress and observed that both GSH and AsA contribute to enhancing tolerance level against drought stress in chickpea. Nahar et al. (2015a) reported that exogenous GSH enhances the activity of most of the enzymes of antioxidant defense system (APX, MDHAR, DHAR, GR, GPX, GST, SOD and CAT) and glyoxalase system of drought-affected mung bean plant either considerably or slightly. Accordingly, GSH reduced the content of over-generated ROS and MG which finally successfully decreased the oxidative damage. But the performance of GSH was day dependent. With the increasing stress duration, GSH showed lower effectivity to counter stress. A similar trend of positive findings was recorded due to exogenous GSH in the same plant under high temperature (HT) and salt stress (Nahar et al. 2015b, c). Compared to only stress supplementation of GSH in HT-stressed cucumber plants, considerably increased soluble protein content, proline level, activities of different antioxidants and their associated gene expression, as well as exogenous GSH reduced the ROS production and decreased the cell lipid peroxidation (Ding et al. 2016). Their results suggest that exogenously applied GSH improve tolerance level of HT-stressed cucumber plants due to its positive action in water status, photosynthetic process, and antioxidant defense system. Khan et al. (2016) demonstrated that exogenous GSH application decreased MDA and H2O2 content of cotton leaves because of increased activity of POD, APX, GR, SOD, and CAT. These findings recommended that exogenous application of GSH decreased the undesirable effects of Pb and enhanced tolerance of cotton plants to oxidative stress. Glutathione enhanced the manufacture of NO and total S-nitrosothiol contents and upheld a reduced cellular redox status and increased antioxidant capacity along with induction of transcripts of transcription factors and antioxidant genes under Cd stress in tomato plants (Hashem et al. 2016). Similarly, Daud et al. (2016) observed the GSH-mediated oxidative stress tolerance in the cotton plant under Cd stress. Therefore, it is very obvious that any source of GSH either endogenous or exogenous develop plant stress tolerance under the adverse growing condition, but success depends on the interaction of it to the stress regarding the proper amount of GSH.
Even though H2S is considered as a phytotoxin, it is documented that plants can themselves produce and liberate this gas, particularly when uncovered to external Cys, sulfate, sulfite, or SO2 (Li 2013; Li et al. 2016; Wei et al. 2017). Maybe for the indulgence of excess S, the plant goes through such mechanism. Nevertheless, under few biotic and abiotic stresses, plants release H2S more than basal, endogenously produced rates (Jin et al. 2011; Wei et al. 2017). Additionally, as a vital sulfur compound, H2S works in various developmental processes of plant and contributes to enhance plant stress tolerance as it can upregulate the transcripts of multiple abiotic and biotic stress-related genes and hinder accumulation of ROS (Shi et al. 2014; Li and Hey 2015; Chen et al. 2017). Endogenous H2S accumulation is a widespread feedback of plants to environmental stress, including salt, drought, HM/metalloid, and heat and cold stress which might be intimately connected with the acquisition of plant stress tolerance (Li 2013; Calderwood and Kopriva 2014; Hancock and Whiteman 2014). Hydrogen sulfide is involved in a complex signaling network consisting of many secondary messengers such as Ca2+, ABA, H2O2, and NO, which protect the plant from stress-induced damage (Li et al. 2016). In recent time, low concentration of exogenous H2S is emerging as a novel gaseous signal molecule which confirmed its positive effect in different plants under normal and adverse growing condition. It has numerous positive functions on plant growth and development. The action of supplemented H2S is also found to enhance plant stress tolerance under environmental difficulties (Li 2013; Li et al. 2016). Interestingly, exogenous application of H2S confirms considerable positive effects on germination of seed (Li and He 2015; Wojtyla et al. 2016), plant growth and development (Fang et al. 2014), and regulation of senescence (Zhang et al. 2011), with the increasing of plant stress tolerance against salt (Christou et al. 2013; Chen et al. 2015; Deng et al. 2016), drought (Christou et al. 2013; Shen et al. 2013; Chen et al. 2016; Ma et al. 2016), toxic metal (Chen et al. 2013, 2017; Cui et al. 2014), high temperature (Li et al. 2013a,b; Li 2015), and cold stress (Fu et al. 2013; Du et al. 2017). The above functions of H2S signify that it might be a promising candidate for signal transduction in plant’s cross-adaptation. Recently, Chen et al. (2017) observed that H2S acted as an antioxidant and scavenged ROS through regulating different antioxidant enzymes, thus preventing metal-induced (Hg) oxidative damages. In contrast to stressed plant, exogenous application of H2S in drought-affected wheat seedlings increased the activity of different antioxidant enzymes and reduced ROS production and lipid peroxidation in both leaves and roots. Moreover, H2S addition in plants enhanced ABA biosynthesis, which participated in developing drought stress tolerance (Ma et al. 2016). In parallel, Chen et al. (2016) found that H2S regulates the polyamines and sugar changes in Spinacia oleracea seedlings under drought stress condition, which provided stress tolerance for the plants. Exogenously applied H2S in salt-affected plants maintained the balance between Na+ and K+ in growing media and cell along with upregulation of different ROS scavenging enzymes, which maintained the cellular balance and protected plant from the salt-induced damages (Christou et al. 2013; Chen et al. 2015; Deng et al. 2016). A similar type of actions of H2S in enhancing stress tolerance was observed under other abiotic stresses including heat and cold stress (Li et al. 2013a, b; Fu et al. 2013; Li 2015; Du et al. 2017).
Thioredoxins (TRX), glutaredoxins (GRX), and glutathionylation are considered as protein thiols, which contain sulphydryl groups and are considered as protective and regulatory compounds for plant cell (Zagorchev et al. 2013). Different abiotic stresses raise both TRX and GRX in protein or gene level. Proteomics study of rice seedlings demonstrated the upregulation of responsible genes of TRX and GRX under Cu toxicity (Song et al. 2012). However, their function varies from stress to stress. Fatehi et al. (2012) reported that TRX activity increased by manyfold under salt stress in barley plant. On the other hand, amusingly, cold stress seemed to downregulate most TRX genes, but drought stress upregulated them, as a minimum at the earlier stages of the stress treatment (Nuruzzaman et al. 2012). Like TRX and GPX, the function of glutathionylation in a plant cell regarding abiotic stress tolerance is not so clear to date. Colville and Kranner (2010) reported that protein glutathionylation is a probable provider of defense mechanisms that confer desiccation stress tolerance.
10.8 Molecular Approaches in Regulating S Status in Plants
For growth and development, plants require a certain amount of sulfur. Due to the dynamic nature of the environment, plants may not get the exact amount of S as per requirement. Thus, plants have evolved with some strategies to control the S status in plants under diverse situations including S deficiency and unfavorable environmental condition (Table 10.3). From S uptake to assimilation into a metabolite, different regulatory factors tightly control the pathway to ensure proper concentration of S-containing compounds or inorganic SO4 2− in cellular compartments essential for metabolic functions (Table 10.3). Understanding of molecular mechanism involved in S homeostasis allows us to engineer pathway in a way ensuring proper S level in plant even under environmental stress condition. Therefore, the study of transporter proteins, metabolites, and enzymes of the assimilatory pathway under S-deficient condition could be a pertinent approach to find out the regulatory factors of S metabolism. Under S deficiency, Sultr1;1 expression is greatly enhanced, and Sultr1;1 is more responsive to S status than Sultr1;2 (Yoshimoto et al. 2002). To understand how the transporter proteins are controlled, different enzymes inhibitors were used: phosphatase inhibitors, OKA and CalyA, and kinase inhibitors, K252a. Phosphatase inhibitors, OKA and CalyA, inhibited the expression Sultr1;1 under sulfur deficiency indicating the involvement of protein phosphatase in the regulation of Sultr1;1 expression (Maruyama-Nakashita et al. 2004a). Further investigation revealed that among different phytohormones, only cytokinin could repress the Sultr1;1 and Sultr1;2 expressions in Arabidopsis indicating a role of cytokinin in S homeostasis in the plant (Maruyama-Nakashita et al. 2004b). Again, a sulfur-responsive element (SURE) was identified in 5′ promoter region of Sultr1;1. Furthermore, microarray analysis suggested that SURE is associated with some genes responsive to S deficiency (Maruyama-Nakashita et al. 2005). Another transcription factor, SLIM1, was reported to be involved in regulation of Sultr1;2 expressions under S-limiting condition. Interestingly, SLIM1 itself was not affected by S concentration in the growing media. Also, there was no SLIM1 binding site in the sulfate transporters except Sultr4;2 suggesting the presence of another factor that may connect the signaling between transporter genes and SLIM1 (Maruyama-Nakashita et al. 2006). A binding site for EIL group transcription factors was identified in the promoter region of UP9C gene in tobacco, a gene responsive to the sulfur limitation (Wawryzynska et al. 2010). Kasajima et al. (2007) suggested that ASR1, also known as BIG gene, is involved in sulfate metabolism pathway as the mutant asr1-1 showed upregulation of SULTR2;2 and adenosine-5′-phosphosulfate reductase 1 (APR1) which was not affected by SLIM1. To investigate the transcriptional regulation of sulfate assimilatory genes, long hypocotyl 5 (HY5), a transcription factor, was reported to modulate APR expression. Unlike SURE and SLIM1, HY5 can bind to the promoter region of APR1 and APR2 but not in APR3 (Lee et al. 2011). However, APR expression is also regulated by MYB transcription factors (Yatusevich et al. 2010).
In some cases, gene expression is regulated posttranscriptionally by some RNA, microRNA (miRNA). These miRNAs bind with a protein to form RNA-induced silencing complex (RISC). The RISC then binds with mRNA complementary to miRNA. As a result target mRNA fails to be translated into protein. Sulfur deficiency in growth medium induces miRNA395 which in turn regulates the expression of Sultr2;1 and APS1 genes in the sulfur metabolic pathway. Interestingly, transcription factor SLIM1 can induce miRNA under sulfur limitation (Kawashima et al. 2009). The multienzyme complex formed by SAT and OAS-TL, known as cysteine synthase complex (CSC), is involved in sulfur homeostasis (Takahashi et al. 2011; Koprivova and Kopriva 2014). The CSC regulates Cys biosynthesis in different cell organelles and maintains SAT activity. However, S limitation induces accumulation of OAS which dissociates the complex (Droux et al. 1998; Wirtz et al. 2010). Still, there are not enough reports to elucidate the mechanism completely. Furthermore, regulatory networks of S metabolism could be investigated under abiotic stress condition which will allow us to answer the question how S is metabolized under adverse environment.
10.9 Conclusion and Future Perspectives
Although there are several reports on the role of S in plants under abiotic stress, the exact mechanisms and interactions are not revealed yet. Sulfur-associated amino acids (Met, Cys), iron-S-clusters, lipids, vitamins (biotin and thiamine), cofactors (CoA and S-adenosylmethionine), and peptides (such as GSH and PCs) play a significant role in abiotic stress tolerance. Molecular approaches to manipulate enzymes of S assimilation pathway such as ATP sulfurylase (ATP-S), APS kinase, PAPS reductase or APS reductase, sulfite reductase, serine acetyltransferase (SAT), and O-acetylserine/O-acetylhomoserine sulfhydrylase will widen the eyes view to exploit S as a more persuasive molecule in developing stress tolerance of plant (Anjum et al. 2015; Khan et al. 2016). Sulfur deficiency disrupts homeostasis of other essential nutrients, and thus the formation of basic structural components of the cell is hindered. A cross talk among GSH pools, miR395 levels, and ATP-S transcripts/activity regulates S pool and the pool of other essential nutrients within the plant which maintains the nutrient homeostasis for better plant development both under normal growth and abiotic stress condition (Anjum et al. 2015). This aspect should be considered for further studies.
Abbreviations
- ABA:
-
Abscisic acid
- ACS:
-
1-Aminocyclopropane carboxylic acid (ACC) synthase (ACS)
- APK:
-
APS kinase
- APR:
-
Adenosine-5′-phosphosulfate reductase
- APS:
-
Adenosine-5′-phosphosulfate
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- ATP:
-
Adenosine triphosphate
- ATPS:
-
ATP sulfurylase
- CAT:
-
Catalase
- CBL:
-
Cystathionine β-lyase
- CGS:
-
Cystathionine γ-synthase
- CSC:
-
Cysteine synthase complex
- Cys:
-
Cysteine
- Cyst:
-
Cystathionine
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- EF-TU:
-
Elongation factor-thermo unstable
- GAPDH:
-
Glyceraldehyde-3-P-dehydrogenase
- GB:
-
Glycine betaine
- GCL:
-
Glutamate-cysteine ligase
- Gly I:
-
Glyoxalase I
- Gly II:
-
Glyoxalase II
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GRX:
-
Glutaredoxins
- GSH:
-
Glutathione
- GSHS:
-
Glutathione synthetase
- GSSG:
-
Oxidized glutathione
- GST:
-
Glutathione S-transferase
- h-GSH:
-
Homo-GSH
- JA:
-
Jasmonates
- LOX:
-
Lipoxygenase
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- Met:
-
Methionine
- MG:
-
Methylglyoxal
- MRNA:
-
Messenger ribonucleic acid
- MS:
-
Methionine synthase
- NaHS:
-
Sodium hydrosulfide
- NPT:
-
Nonprotein thiol
- OAS:
-
O-Acetylserine
- OASS:
-
O-Acetylserine sulfhydrylase
- OAS-TL:
-
OAS(thiol)lyase
- OPH:
-
O-Phosphohomoserine
- PAPS:
-
3-Phosphoadenosine-5-phosphosulfate
- PCs:
-
Phytochelatins
- PEG:
-
Polyethylene glycol
- POD:
-
Peroxidase
- POX:
-
Peroxidases
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- SAT:
-
Serine acetyltransferase
- Ser:
-
Serine
- SiR:
-
Sulfite reductase
- SLG:
-
S-d-Lactoylglutathione
- SOD:
-
Superoxide dismutase
- SULTR:
-
Proton/SO4 2−cotransporter in plants
- SURE:
-
Sulfur-responsive element
- TBARS:
-
Thiobarbituric acid reactive substances
- TRX:
-
Thioredoxins
- γ-ECS:
-
γ-Glutamylcysteine synthetase
- γ-GluCys:
-
γ-Glutamylcysteine
References
Abdallah M, Dubousse L, Meuriot F, Etienne P, Avice JC, Ourry A (2010) Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J Exp Bot 61:2635–2646
Abdin MZ, Ahmad A, Khan N, Khan I, Jamal A, Iqbal M (2003) Sulphur interaction with other nutrients. In: Abrol YP, Ahmad A (eds) Sulphur in plants. Springer, Dordrecht, pp 359–374
Ahmad N, Malagoli M, Wirtz M, Hell R (2016) Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol 16:247. https://doi.org/10.1186/s12870-016-0940-z
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Anjum NA, Gill R, Kaushik M, Hasanuzzaman M, Pereira E, Ahmad I, Tuteja N, Gill SS (2015) ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front Plant Sci 6:210. https://doi.org/10.3389/fpls.2015.00210
Asgher M, Khan NA, Khan MIR, Fatma M, Masood A (2014) Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Saf 106:54–61
Asgher M, Per TS, Anjum S, Khan MIR, Masood A, Verma S, Khan NA (2017) Contribution of glutathione in heavy metal stress tolerance in plants. In: Khan M, Khan N (eds) Reactive oxygen species and antioxidant systems in plants: role and regulation under abiotic stress. Springer, Singapore, pp 297–313
Astolfi S, Zuchi S (2013) Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol Plant 35:175–181
Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI (2012) Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ Exp Bot 4:19–32
Bashir H, Ibrahim MM, Bagheri R, Ahmad J, Arif IA, Baig MA, Qureshi MI (2015) Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AOB Plants 7:1–13
Boaretto LF, Carvalho G, Borgo L, Creste S, Landell MG, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–175
Bottcher C, Westphal L, Schmotz C, Prade E, Scheel D, Glawischnig E (2009) The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21:1830–1845
Buchner P, Stuiver CE, Westerman S, Wirtz M, Hell R, Hawkesford MJ, Kok LJD (2004) Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol 136:3396–3408
Cabreiro F, Picot CR, Friguet B, Petropoulos I (2006) Methionine sulfoxide reductases. Ann N Y Acad Sci 1067:37–44
Calderwood A, Kopriva S (2014) Hydrogen sulfide in plants: from dissipation of excess sulfur to signaling molecule. Nitric Oxide 41:72–78
Cao MJ, Wang Z, Wirtz M, Hell R, Oliver DJ, Xiang CB (2013) SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J 73:607–616
Cao MJ, Wang Z, Zhao Q, Mao JL, Speiser A, Wirtz M, Xiang CB (2014) Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J 77:604–615
Capaldi FR, Gratão PL, Reis AR, Lima LW, Azevedo RA (2015) Sulfur metabolism and stress defense responses in plants. Tropl Plant Biol 8:60–73
Carciochi WD, Divito GA, Fernández LA, Echeverría HE (2017) Sulfur affects root growth and improves nitrogen recovery and internal efficiency in wheat. J Plant Nutr 40:1231–1242
Çevik S, Ünyayar S (2015) The effects of exogenous application of ascorbate and glutathione on antioxidant system in cultivated Cicer arietinum and wild type C. reticulatum under drought stress. J Nat Appl Sci 19:91–97
Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP (2012) Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 158:340–351
Chen J, Wang WH, Wu FH, You CY, Liu WT, Dong XJ, He JX, Zheng HL (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL (2015) Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:12516. https://doi.org/10.1038/srep12516
Chen J, Shang YT, Wang WH, Chen XY, He EM, Zheng HL, Shangguan Z (2016) Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front Plant Sci 7:1173. https://doi.org/10.3389/fpls.2016.01173
Chen Z, Chen M, Jiang M (2017) Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol Biochem 111:179–192
Cheng MC, Ko K, Chang WL, Kuo WC, Chen GH, Lin TP (2015) Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J 83:926–939
Christou A, Manganaris GA, Papadopoulos I, Fotopouls V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966
Colville L, Kranner I (2010) Desiccation tolerant plants as model systems to study redox regulation of protein thiols. J Plant Growth Regul 62:241–255
Cui W, Chen H, Zhu K, Jin Q, Xie Y, Cui J, Xia Y, Zheng J, Shen W (2014) Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo)glutathione and reactive oxygen species homeostases. PLoS One 9:e109669. https://doi.org/10.1371/journal.pone.0109669
D’Hooghe P, Escamez S, Trouverie J, Avice JC (2013) Sulphur limitation provokes physiological and leaf proteome changes in oilseed rape that lead to perturbation of sulphur, carbon and oxidative metabolisms. BMC Plant Biol 13:23. https://doi.org/10.1186/1471-2229-13-23
Daud MK, Mei L, Azizullah A, Dawood M, Ali I, Mahmood Q, Ullah W, Jamil M, Zhu SJ (2016) Leaf-based physiological, metabolic, and ultrastructural changes in cultivated cotton cultivars under cadmium stress mediated by glutathione. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-6739-5
Davidian JC, Kopriva S (2010) Regulation of sulfate uptake and assimilation the same or not the same? Mol Plant 3:314–325
Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS (2016) Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul 79:391–399
Ding X, Jiang Y, He L, Zhou Q, Yu J, Hui D, Huang D (2016) Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci Rep 6:35424. https://doi.org/10.1038/srep35424
Dixit G, Singh AP, Kumar A, Dwivedi S, Deeba F, Kumar S, Suman S, Adhikari B, Shukla Y, Trivedi PK, Pandey V, Tripathi RD (2015a) Sulfur alleviates arsenic toxicity by reducing its accumulation and modulating proteome, amino acids and thiol metabolism in rice leaves. Sci Rep 5:16205. https://doi.org/10.1038/srep16205
Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwivedi S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD (2015b) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Dos Santos CV, Cuiné S, Rouhier N, Rey P (2005) The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol 138:909–922
Droux M, Ruffet ML, Douce R, Job D (1998) Interactions between serine acetyl transferase and O-acetylserine (thiol) lyase in higher plants – structural and kinetic properties of the free and bound enzymes. Eur J Biochem 255:235–245
Du X, Jin Z, Liu D, Yang G, Pei Y (2017) Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol Biochem 120:112–119
Egesel CO, Gul MK, Kahriman F (2009) Changes in yield and seed quality traits in rapeseed genotypes by sulphur fertilization. Eur Food Res Technol 229:505–513
Erdala S, Turk H (2016) Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ Exp Bot 132:92–99
Fang T, Cao Z, Li J, Shen W, Huang L (2014) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76:44–51
Fatehi F, Hosseinzadeh A, Alizadeh H, Brimavandi T (2012) The proteome response of Hordeum spontaneum to salinity stress. Cereal Res Commun 39:6387–6397
Fatma M, Asgher M, Masood A, Khan NA (2014) Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63
Fu J, Liu CP, Zhang ZW, Xing MW, Xu SW (2013) Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res Vet Sci 95:495–501
Gallardo K, Courty PE, Le Signor C, Wipf D, Vernoud V (2014) Sulfate transporters in the plant’s response to drought and salinity: regulation and possible functions. Front Plant Sci 5:580. https://doi.org/10.3389/fpls.2014.00580
Genisel M, Erdal S, Kizilkaya M (2014) The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul 75:187–197
Gigolashvili T, Kopriva S (2014) Transporters in plant sulfur metabolism. Front Plant Sci 5:442. https://doi.org/10.3389/fpls.2014.00442
Gilabel AP, Nogueirol RC, Garbo AI, Monteiro FA (2014) The role of sulfur in increasing guinea grass tolerance of copper phytotoxicity. Water Air Soil Pollut 225:1806. https://doi.org/10.1007/s11270-013-1806-8
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Haag AF, Kerscher B, Dall’Angelo S, Sani M, Longhi R, Baloban M, Wilson HM, Mergaert P, Zanda M, Ferguson GP (2012) Role of cysteine residues and disulfide bonds in the activity of a legume root nodule-specific, cysteine-rich peptide. J Biol Chem 287:10791–10798
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012) Plant responses and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–316
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci 8:1061. https://doi.org/10.3389/fpls.2017.01061
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M (2017a) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18:200. https://doi.org/10.3390/ijms18010200
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017b) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23:249–268
Hashem A, Abd Allah EF, Alqarawi AA, Al Huqail AA, Egamberdieva D, Wirth S (2016) Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J Biol Sci 23:272–281
Hawkesford MJ (2012) Sulfate uptake and assimilation–whole plant regulation. In: De Kok LJ, Tausz M, Hawkesford MJ, Hoefgen R, McManus MT, Norton R, Rennenberg H, Saito K, Schnug E, Tabe L (eds) Sulfur metabolism in plants. Springer, Dordrecht, pp 11–24
Hirani AH, Li G, Zelmer CD, McVetty PBE, Asif M, Goyal A (2012) Molecular genetics of glucosinolate biosynthesis in brassicas: genetic manipulation and application aspects. In: Goyal A (ed) Crop plant. InTech, Rijeka, pp 189–216
Hopkins L, Parmar S, Blaszczyk A, Hesse H, Hoefgen R, Hawkesford MJ (2005) O-Acetylserine and the regulation of expression of genes encoding components for sulfate uptake and assimilation in potato. Plant Physiol 138:433–440
Howarth JR, Fourcroy P, Davidian JC, Smith FW, Hawkesford MJ (2003) Cloning of two contrasting high-affinity sulphate transporters from tomato induced by low sulphate and infection by the vascular pathogen Verticillium dahlia. Planta 218:58–64
Hu KD, Bai GS, Li WJ, Yan H, Hu LY, Li YH, Zhang H (2014) Sulfur dioxide promotes germination and plays an antioxidant role in cadmium-stressed wheat seeds. J Plant Growth Regul 75:271–280
Iqbal N, Nazar R, Syeed S, Masood A, Khan NA (2011) Exogenously- sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot 62:4955–4963
Iqbal N, Khan NA, Nazar R, da Silva JAT (2012) Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 78:84–90
Jankowski K, Budzyński W, Szymanowski A (2008) Effect of sulphur on the quality of winter rape seeds. J Entomol 13:521–534
Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414:481–486
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Kasajima I, Ohkama-Ohtsu N, Ide Y, Hayashi H, Yoneyama T, Suzuki Y, Naito S, Fujiwara T (2007) The BIG gene is involved in regulation of sulfur deficiency-responsive genes in Arabidopsis thaliana. Physiol Plant 129:351–363
Kataoka T, Watanabe-Takahashi A, Hayashi N, Ohnishi M, Mimura T, Buchner P, Hawkesford MJ, Yamaya T, Takahashi H (2004a) Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 16:2693–2704
Kataoka T, Hayashi N, Yamaya T, Takahashi H (2004b) Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol 136:4198–4204
Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T (2009) Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 57:313–321
Khan MIR, Asgher M, Iqbal N, Khan NA (2013) Potentiality of Sulphur-containing compounds in salt stress tolerance. In: Ahmad P, Azooz M, Prasad M (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 443–472
Khan NA, Khan MIR, Asgher M, Fatma M, Masood A, Syeed S (2014) Salinity tolerance in plants: revisiting the role of sulfur metabolites. J Plant Biochem Physiol 2:120. https://doi.org/10.4172/2329-9029.1000120
Khan MIR, Iqbal N, Masood A, Mobin M, Anjum NA, Khan NA (2016) Modulation and significance of nitrogen and sulfur metabolism in cadmium challenged plants. Plant Growth Regul 78:1–11
Koprivova A, Kopriva S (2008) Lessons from investigation of regulation of APS reductase by salt stress. Plant Signal Behav 3:567–569
Koprivova A, Kopriva S (2014) Molecular mechanisms of regulation of sulfate assimilation: first steps on a long road. Front Plant Sci 5:589. https://doi.org/10.3389/fpls.2014.00589
Kumar B, Singla-Pareek SL, Sopory SK (2010) Glutathione homeostasis: crucial for abiotic stress tolerance in plants. In: Pareek A, Sopory SK, Bohnert JH, Govindjee (eds) Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Springer, New York, pp 263–282
Lancilli C, Giacomini B, Lucchini G, Davidian JC, Cocucci M, Sacchi GA, Nocito FF (2014) Cadmium exposure and sulfate limitation reveal differences in the transcriptional control of three sulfate transporter (Sultr1;2) genes in Brassica juncea. BMC Plant Biol 14:132. https://doi.org/10.1186/1471-2229-14-132
Lee BR, Koprivova A, Kopriva S (2011) The key enzyme of sulfate assimilation, adenosine 5′-phosphosulfate reductase, is regulated by HY5 in Arabidopsis. Plant J 67:1042–1054
Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U (2008) Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105:8197–8202
Li ZG (2013) Hydrogen sulfide: a multifunctional gaseous molecule in plants. Russ J Plant Physiol 60:733. https://doi.org/10.1134/S1021443713060058
Li ZG (2015) Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal Behav 10:e1051278. https://doi.org/10.1080/15592324.2015.1051278
Li ZG, He QQ (2015) Hydrogen peroxide might be a downstream signal molecule of hydrogen sulfide in seed germination of mung bean (Vigna radiata). Biologia 70:753–759
Li ZG, Ding XJ, Du PF (2013a) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013b) Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Li ZG, Long WB, Yang SZ, Wang YC, Tang JH, Chen T (2015) Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. In Vitro Cell Dev Biol-Plant 51:428–437
Li ZG, Min X, Zhou ZH (2016) Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci 7:1621. https://doi.org/10.3389/fpls.2016.01621
Liang T, Ding H, Wang G, Kang J, Pang H, Lv J (2016) Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol Environ Saf 124:129–137
Liu X, Yang Y, Deng X, Li M, Zhang W, Zhao Z (2017) Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ Exp Bot 135:13–20
Lou L, Kang J, Pang H, Li Q, Du X, Wu W, Chen J, Lv J (2017) Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int J Mol Sci 18:1628. https://doi.org/10.3390/ijms18081628
Lunde C, Zygadlo A, Simonsen HT, Nielsen PL, Blennow A, Haldrup A (2008) Sulfur starvation in rice: the effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective path ways. Physiol Plant 134:508–521
Ma D, Ding H, Wang C, Qin H, Han Q, Hou J, Lu H, Xie Y, Guo T (2016) Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS One 11:e0163082
Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA (2010) Ascorbate and glutathione: protectors of plants in oxidative stress. In: Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA (eds) Ascorbate–glutathione pathway and stress tolerance in plants. Springer, Berlin, pp 209–229
Mária V, Ladislav D, Pavel R (2017) Sulphur nutrition and its effect on yield and oil content of oilseed rape (Brassica Napus L.) Acta Univ Agric Et Silvic Mendel Brun 65:555–562
Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H (2003) Transcriptome profiling of sulfur-responsive genes in Arabidopsis reveals global effects of sulfur nutrition on multiple metabolic pathways. Plant Physiol 132:597–605
Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Yamaya T, Takahashi H (2004a) Induction of SULTR1;1 sulfate transporter in Arabidopsis roots involves protein phosphorylation/dephosphorylation circuit for transcriptional regulation. Plant Cell Physiol 45:340–345
Maruyama-Nakashita A, Nakamura Y, Yamaya T, Takahashi H (2004b) A novel regulatory pathway of sulfate uptake in Arabidopsis roots: implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. Plant J 38:779–789
Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Inoue E, Yamaya T, Takahashi H (2005) Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J 42:305–314
Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18:3235–3251
Mazid M, Khan ZH, Quddusi S, Taqi AK, Firoz M (2011) Significance of Sulphur nutrition against metal induced oxidative stress in plants. J Stress Physiol Biochem 7:165–184
Min Y, Ping QB, Xue-li M, Ping W, Mei-ling L, Lu-lu C, Lei-tai C, Ai-qing S, Zhen-lin W, Yan-ping Y (2016) Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.) J Integr Agric 15:2745–2758
Mobin M, Khan MN, Abbas ZK, Ansari HR, Al-Mutairi KA (2016) Significance of sulfur in heat stressed cluster bean (Cymopsis tetragonoloba L. Taub) genotypes: responses of growth, sugar and antioxidative metabolism. Arch Agron Soil Sci 63:288–295
Mukhtar I, Shahid MA, Khan MW, Balal RM, Iqbal MM, Naz T, Zubair M, Ali HH (2016) Improving salinity tolerance in chili by exogenous application of calcium and sulphur. Soil Environ 35:56–64
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015a) Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 7. doi:https://doi.org/10.1093/aobpla/plv069
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015b) Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot 112:44–54
Nahar K, Hasanuzzaman M, Alam MM, Fujita M (2015c) Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant 59:745–756
Nahar K, Hasanuzzaman M, Fujita M (2016) Physiological roles of glutathione in conferring abiotic stress tolerance to plants. In: Gill SS, Tuteja N (eds) Abiotic stress response in plants. Wiley, Weinheim, pp 151–179
Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87
Nishida S, Duan G, Ohkama-Ohtsu N, Uraguchi S, Fujiwara T (2016) Enhanced arsenic sensitivity with excess phytochelatin accumulation in shoots of a SULTR1; 2 knockout mutant of Arabidopsis thaliana (L.) Heynh. Soil Sci Plant Nutr 62:367–372
Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian JC, Sacchi GA (2006) Heavy metal stress and sulfate uptake in maize roots. Plant Physiol 141:1138–1148
Nocito FF, Lancilli C, Giacomini B, Sacchi GA (2007) Sulphur metabolism and cadmium stress in higher plants. Plant Stress 1:142–156
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Nuruzzaman M, Sharoni AM, Satoh K, Al-Shammari T, Shimizu T, Sasaya T, Omura T, Kikuchi S (2012) The thioredoxin gene family in rice: genome-wide identification and expression profiling under different biotic and abiotic treatments. Biochem Biophys Res Commun 423:417–423
Osman AS, Rady MM (2012) Ameliorative effects of sulphur and humic acid on the growth, anti-oxidant levels, and yields of and pea (Pisum sativum L.) plants grown in reclaimed saline soil. J Hortic Sci Biotechnol 87:626–632
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509
Reich M, Aghajanzadeh T, Helm J, Parmar S, Hawkesford MJ, De Kok LJ (2017) Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 411:319–332
Riemenschneider A, Wegele R, Schmidt A, Papenbrock J (2005) Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J 272:1291–1304
Rochaix JD (2011) Assembly of the photosynthetic apparatus. Plant Physiol 155:1493–1500
Romero LC, Aroca MÁ, Laureano-Marín AM, Moreno I, García I, Gotor C (2014) Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant 7:264–276
Salvagiotti F, Castellarín JM, Pedrol HM (2009) Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crops Res 113:170–177
Scherer HW (2008) Impact of sulphur on N2 fixation of legumes. In: Khan NA, Singh S, Umar S (eds) Sulphur assimilation and abiotic stresses in plants. Springer-Verlag, New York, pp 43–54
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Bot 57:711–726
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037. https://doi.org/10.1155/2012/217037
Shen J, Xing T, Yuan H, Liu Z, Jin Z, Zhang L, Pie Y (2013) Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by MicroRNA expressions. PLoS One 8:e77047. https://doi.org/10.1371/journal.pone.0077047
Sheng H, Zeng J, Liu Y, Wang X, Wang Y, Kang H, Fan X, Sha L, Zhang H, Zhou Y (2016) Sulfur mediated alleviation of Mn toxicity in polish wheat relates to regulating Mn allocation and improving antioxidant system. Front Plant Sci 7:1382. https://doi.org/10.3389/fpls.2016.01382
Shi H, Ye T, Chan Z (2014) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.) Plant Physiol Biochem 74:99–107
Shi H, Ye T, Han N, Bian H, Liu X, Chan Z (2015) Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol 57:628–640
Shibagaki N, Rose A, Mcdermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify SULTR1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29:475–486
Siddiqui MH, Mohammad F, Khan MMA, Al-Whaibi MH (2012) Cumulative effect of nitrogen and Sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 249:139–153
Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT (1995) Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci U S A 92:9373–9377
Song Y, Cui J, Zhang H, Wang G, Zhao FJ, Shen Z (2012) Proteomic analysis of copper stress responses in the roots of two rice (Oryza sativa L.) varieties differing in Cu tolerance. Plant Soil 366:647–658
Srivalli S, Khanna-Chopra R (2008) Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stress in plants. Springer, Berlin, pp 207–225
Srivastava S, D’souza SF (2010) Effect of variable sulfur supply on arsenic tolerance and antioxidant responses in Hydrilla verticillata (Lf) Royle. Ecotoxicol Environ Saf 73:1314–1322
Sun XM, Lu B, Huang SQ, Mehta SK, Xu LL, Yang ZM (2007) Coordinated expression of sulfate transporters and its relation with sulfur metabolitesin Brassica napus exposed to cadmium. Bot Stud 48:43–54
Takahashi H (2010) Regulation of sulfate transport and assimilation in plants. Int Rev Cell Mol Biol 281:129–159
Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2000) The role of three functional sulfate transporters involved in uptake and translocation of sulfate in Arabidopsis thaliana. Plant J 23:171–182
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Tong Y, Gabriel-Neumann E, Ngwene B, Krumbein A, George E, Platz S, Rohn S, Schreiner M (2014) Topsoil drying combined with increased sulfur supply leads to enhanced aliphatic glucosinolates in Brassica juncea leaves and roots. Food Chem 152:190–196
Walker KC, Booth (2003) Sulphur nutrition and oilseed quality. In: Abrol YP, Ahmad A (eds) Sulphur in plants. Springer, Dordrecht, pp 323–339
Wang F, Chen F, Cai Y, Zhang G, Wu F (2011) Modulation of exogenous glutathione in ultrastructure and photosynthetic performance against Cd stress in the two barley genotypes differing in Cd tolerance. Biol Trace Elem Res 144:1275–1288
Wawrzyńska A, Lewandowska M, Sirko A (2010) Nicotiana tabacum EIL2 directly regulates expression of at least one tobacco gene induced by sulphur starvation. J Exp Bot 61:889–900
Wei B, Zhang W, Chao J, Zhang T, Zhao T, Noctor G, Liu Y, Han Y (2017) Functional analysis of the role of hydrogen sulfide in the regulation of dark-induced leaf senescence in Arabidopsis. Sci Rep 7:2615. https://doi.org/10.1038/s41598-017-02872-0
Wirtz M, Birke H, Heeg C, Müller C, Hosp F, Throm C, Konig S, Feldman-Salit A, Rippe K, Petersen G, Wade RC, Rybin V, Scheffzek K, Hell R (2010) Structure and function of the hetero-oligomeric cysteine synthase complex in plants. J Biol Chem 285:32810–32817
Wojtyla L, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front Plant Sci 7:66. https://doi.org/10.3389/fpls.2016.00066
Wu JC, Sun SH, Ke YT, Xie CP, Chen FX (2011) Effects of glutathione on chloroplast membrane fluidity and the glutathione circulation system in young loquat fruits under low temperature stress. Acta Hortic 887:221–225
Yatusevich R, Mugford SG, Matthewman C, Gigolashvili T, Frerigmann H, Delaney S, Koprivova A, Flugge UI, Kopriva S (2010) Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J 62:1–11
Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29:465–473
Yoshimoto N, Inoue E, Saito K, Yamaya T, Takahashi H (2003) Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol 131:1511–1517
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zhang J, Zhao QZ, Duan GL, Huang YC (2011) Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ Exp Bot 72:34–40
Zhu DB, Hu KD, Guo XK, Liu Y, Hu LY, Li YH, Wang SH, Zhang H (2015) Sulfur dioxide enhances endogenous hydrogen sulfide accumulation and alleviates oxidative stress induced by aluminum stress in germinating wheat seeds. Oxidative Med Cell Longev. https://doi.org/10.1155/2015/612363
Acknowledgments
The authors acknowledge Khursheda Parvin and Sayed Mohammad Mohsin, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan, for critic reading and formatting of the manuscript.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hasanuzzaman, M., Hossain, M.S., Bhuyan, M.H.M.B., Al Mahmud, J., Nahar, K., Fujita, M. (2018). The Role of Sulfur in Plant Abiotic Stress Tolerance: Molecular Interactions and Defense Mechanisms. In: Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B. (eds) Plant Nutrients and Abiotic Stress Tolerance. Springer, Singapore. https://doi.org/10.1007/978-981-10-9044-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-9044-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9043-1
Online ISBN: 978-981-10-9044-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)