Abstract
The growing industrial development and concomitant efforts to protect mother Earth from alleviating pollution levels has greatly demanded the use and disposal of milder chemicals and xenobiotics. Of the widely used xenobiotics, surfactants have an increasing market demand due to its prevalent role in pharmaceutical preparations, food industry as well as almost all foaming products. The use of biosurfactants in place of chemical surfactants has gained momentum owing to the low toxicity, higher biodegradability and better environmental compatibility of biosurfactants. The current review outlays the various biosurfactants produced and their significance in various industries. An outline of the various biosynthetic pathways, challenges and advancements in the synthesis of the two mostly used biologically synthesised biosurfactants—rhamnolipids and sophorolipids has been discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Surfactants find a myriad of applications in human life extending its role in household products to a vast array of industrial processes. The realm of surfactant activity is widespread to different industries including food (Kralova and Sjoblom 2009), environmental remediation (Cheng et al. 2017), textiles (Jing-xin 2004), fuel extraction (Torres et al. 2003; Chistyakov 2001), biotechnology (Singh et al. 2007), antimicrobials (Ginkel 1989) and many more. Chemical modification of renewable or non-renewable substrates is currently done to meet this great demand of surfactants (Behler et al. 2001). However, it leads to various environmental problems and health concerns owing to the indiscriminate use of chemical surfactants (Rebello et al. 2014).
Life cycle assessment of surfactant synthesis and disposal undoubtedly proves the levels of its toxicity at different ecosystems. The prevalence of some fluoridated surfactants such as PFOA commonly used in nonsticky utensils in human blood (Calafat et al. 2007), marine animals and birds (Groffen et al. 2017) are some instances to substantiate it. The LCA analysis of PFOA alone indicates that maximum exposure comes from emission on the application (117.0 t), followed by manufacture (3.9 t), consumer exposure (1.2 t), wastewater treatment plants (10.6 t) and environmental emission of 12.8 t (Meng et al. 2017). Surfactant synthesis, in turn, causes increased emission of greenhouse gases, which contribute to the increased global warming, ozone damage, and climatic variations. Surfactant toxicity is evident in almost all sectors of the ecosystem including both biotic (Meng et al. 2017) and abiotic elements of the atmosphere and water bodies (Pittinger et al. 1993).
1.1 Advantages of Biosurfactants
The choice of eco-friendly biosurfactants against chemical counterparts has attained relevance owing to its biodegradability, low toxicity, ecological acceptability, superior foaming ability, enhanced selectivity and increased specific activity (Cameotra et al. 2010). Since its first use in the 1980s, the field of biosurfactant research has gained great impetus and ecological significance due to increasing rates of pollutions associated with chemical surfactants. Figure 1 schematically represents the damage caused by surfactant chemical synthesis. Studies indicate that biosurfactants even in very small concentrations give stable emulsions at different environmental conditions compared to chemical surfactants requiring large concentrations (McClements and Gumus 2016).
Additionally, biosurfactants are found to be antibacterial (Mani et al. 2016; Ndlovu et al. 2017), antifungal (Chen et al. 2017), antiviral (Pang et al. 2017; Vollenbroich et al. 1997) and immunologic in nature, which adds an extra advantage to be used in medicine and therapeutics (Rodrigues et al. 2006). The antifungal role of surfactants in preventing various mycotic infections of plants is also evident by inhibitory action of surfactin and fengycin against Mycosphaerella fijiensis (Gonzalez-Jaramillo et al. 2017). Though economically biosurfactants are not a wise choice for synthesis than chemical surfactants, the growing interest for greener and biodegradable commodities has expected the biosurfactant market to rise in 2018–2020 approximately to $25 billion (http://www.transparencymarketresearch.com/specialty-and-biosurfactants-market.html; http://www.grandviewresearch.com/press-release/global-biosurfactants-market).
The current review targets to outlay the economised production of various biosurfactants focusing on the predominantly biosynthesised biosurfactants—rhamnolipids and sophorolipids. The review also outlays the biosurfactant utility in various sectors and biosynthetic pathways involved in their generation. The challenges faced by the biosurfactant industry are also described along with the progress developed by productive research.
1.2 Types of Biosurfactants
The term biosurfactant refers to amphipathic molecules derived from biological origin (plants or microbial) capable of reducing the surface tension of liquids. Though the term biosurfactant is used for microbially derived surfactants, any surfactant formed with any of the hydrophilic or hydrophobic part of biological origin is also included as a biosurfactant. Biosurfactants based on the mode of synthesis can be divided to first-generation biosurfactants usually termed green surfactants (involves chemical synthesis from renewable resources) and the second-generation biosurfactants or true biosurfactants (involves biological synthesis from renewable resources. The former category includes members such as alkyl polyglucosides, sucrose esters, etc., whose hydrophobic part alone is derived from a renewable resource. Such biosurfactants are produced using either plant- or animal-derived oils with biobased carbohydrates (Tmakova et al. 2017).
The second generation of biosurfactants are mostly produced biologically by fermentation using microbes, for example; rhamnolipids, sophorolipids, surfactin, etc. Chemical classification of biosurfactants greatly depends on the combinatorial linking of three different biomolecules, viz carbohydrates, lipids and proteins or their components to generate glycolipids, lipopeptides, oligopeptides, fatty acids/neutral lipids, phospholipids, polymeric molecules and particulate derivatives (Muthusamy et al. 2008). The glycolipids are of diverse kinds including sophorolipids (produced by Candida bombicola, Candida tropicalis), rhamnolipids (Pseudomonas aeruginosa, Burkholderia sp.), cellobioselipids (by Cryptococcus humicola, Ustilago maydis, Pseudozyma flocculosa), mannosylerythritol lipids (by Pseudozyma sp.) and trehalose lipids (Arthrobacter sp.). The major lipopeptides and oligopeptides include surfactins and subtilisin (Bacillus subtilis) and viscosin and syringomysin (Pseudomonas fluorescens). Table 1 lists the different types of biosurfactants produced by microbes and their industrial utility in various fields. Studies indicate that yet new types of biosurfactants are reported from new isolates as in the case of Wickerhamomyces anomalus CCMA 0358 (Souza et al. 2017).
Biosurfactants play diverse functions in the bacterial physiology, viz (a) as components of cell membrane (Cortes-Sanchez et al. 2013), (b) biomolecules enabling xenobiotic solubilisation and utilisation in hydrocarbon-loaded environment (Kaczorek et al. 2008), (c) as antibiotics (Magalhaes and Nitschke 2013), (d) hemolytic activity in human pathogenesis (Ashdown and Koehler 1990), (e) quorum signalling (Daniels et al. 2006), (f) regulating swarming (Wang et al. 2014), (g) biofilm formation (Bonnichsen et al. 2015), etc. They are produced either externally or intracellularly in response to various environmental factors, carbon sources and sometimes as factors to overcome stress.
1.3 Industrial Synthesis—Current Scenario
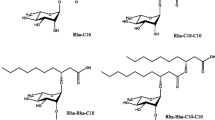
The effective utilisation and commercialization of a fermentation protocol is economic effectiveness, substrate access and high yield. The recycling of agro-industrial residuals for biosurfactants is a cost-effective strategy for economised production (Makkar et al. 2011). Figure 2 represents the structure of various commercially produced biosurfactants including both chemically modified and biosynthesized biosurfactants. According to biosurfactant market evaluation published in 2015, of the various biosurfactants produced industrially methyl ester ketone accounted for the highest biosurfactant market followed by alkyl polyglucosides and rhamnolipid (http://www.grandviewresearch.com/industry-analysis/biosurfactants-industry).
Biosurfactants are produced in various countries including companies such as BASF Cognis (Alkylpolyglucosides), Jeneil Biotech (rhamnolipids), Evonik (sophorolipids), etc. Of the commercially produced biosurfactants, methyl ester ketones and alkyl polyglucosides are derived from renewable resources like waste oil which further undergoes chemical modifications to yield the final biosurfactant product. However, rhamnolipids and sophorolipids are microbially derived, biologically synthesised rather than mere modification of biologically derived resources and thus are considered for study in this review as biosynthesized biosurfactants.
Various factors such as microbial type, carbon source, stirring, oxygen availability, physical factors (pH, temperature), aeration, nutrient level, bioprocess conditions, etc., greatly influence the production rate of biosurfactants. The biosynthesis and commercialization of biosurfactants is effective only to a small extent owing to poor yields and elevated post-production expenditure. Strategies adopted to overcome this crisis include the use of cheaper raw materials, high yielding fermentation protocols and use of novel or mutant hyperproducing strains (Muthusamy et al. 2008). Biosurfactants are produced using large number of carbon sources including vegetable oil, mineral oil (Stoimenova et al. 2009), glycerol (Putri and Hertadi 2015), palm oil (Radzuan et al. 2017), vineyard pruning (Vecino et al. 2017), SDS (Rebello et al. 2013), etc. Currently, biosurfactant research is advancing at a higher rate yielding new possibilities of replacing synthetic surfactants with eco-friendly candidates to a great extent in the near future.
1.4 Rhamnolipids
1.4.1 Chemistry and Biosynthesis
Rhamnolipids are microbial surface-active glycolipids containing one or more rhamnose moieties attached to a different type of fatty acids. The wide variety of rhamnolipids produced are critically analysed for their biopotential and applications (Chrzanowski et al. 2011). Chemically rhamnolipids contains dimers of 3-hydroxy fatty acids of different carbon lengths linked to a mono- or di-rhamnose moiety through a beta glycosidic bond. Naturally, they are released as mixtures of mono-rhamnolpids or di-rhamnolipids, for example rhamnolipid congeners Rha-C10-C10, Rha-C10-C12 and Rha-Rha-C10 were produced by Pseudomonas aeruginosa on growth on SDS as a carbon source (Rebello et al. 2013). The ratio of mono-rhamnolipid and di-rhamnolipid ratio generated is greatly influenced by carbon sources (Nicolo et al. 2017) and environmental factors.
Three main proteins, viz RhlA, RhlB and RhlC are involved in the biogenesis of rhamnolipids. RhlA utilises the b-hydroxydecanoyl–ACP precursors from the bacterial fatty acid synthetic pathway and convert them to hydroxyl-alkanoic acid (HAA), which serves the building blocks for mono- and di-rhamnolipids (Timmis 2002). Rhamnolipid formation utilises hydroxyl-alkanoic acid (HAA) to which two different rhamnose molecules are sequentially added to form mono-rhamnolipid and di-rhamnolipid respectively. The initial transfer of rhamnose to HAA is catalysed by RhlA. The transfer of dTDP-L-rhamnose to either HAA, or a previously generated mono-rhamnolipid is then catalysed by RhlB and RhlC (Deziel et al. 2003).
1.4.2 Challenges in Rhamnolipid Production
Rhamnolipids are most explored biosurfactants and are produced mainly by Pseudomonas aeruginosa sp. utilising various carbon sources such as soybean (Giani et al. 1997), corn (Linhardt et al. 1989), olive (Robert et al. 1989), hexadecane (Tuleva et al. 2002), detergents such as SDS (Rebello et al. 2016), etc. In majority of the countries, high production costs hinders or slows down rhamnolipid industrial production to some extent. Problems related to costly raw materials, foam generation during production, low yield and increased post- production recovery charges are some factors contributing to such a scenario (Muthusamy et al. 2008).
The major challenges faced in its industrial production are (1) use of opportunistic pathogen Pseudomonas aeruginosa for biosurfactant production requires extra care in handling (Van Bogaert et al. 2007) (2) generation of excessive foam during large-scale production (3) formation of mixture of different congeners requires further purification thereby increasing cost (4) laborious and expensive downstream processing accounts for 70–80% of production cost (5) low yield (6) sophisticated fermentation, time-consuming production.
1.4.3 Bioreactors to Optimise Production
Rhamnolipids are produced both by shake flask or reactors as batch, continuous or fed-batch cultures. The latter technique proved superior to mere batch cultures, with approximately 1.3-fold increase in rhamnolipid yield and high substrate–product conversion ratios (Lee et al. 2004). Similarly fed-batch mode of recycling stationary phase P.aeruginosa into new culture media accounted for 100% increase in rhamnolipid yield in another study (dos Santos et al. 2016). Further advancements of fed-batch culture with a fill and take strategy yielded better rhamnolipid yields than conventional fed-batch modes (He et al. 2017). The use of semi-solid state fermentation technique using glycerol and wheat bran substrates for rhamnolipid production effectively reduced the foam generation associated with rhamnolipid synthesis (Wu et al. 2017). The excessive foam generated during rhamnolipid synthesis could be effectively overcome by methods of simple foam fractionating techniques reaching enrichment factors up to 200 (Beuker et al. 2016) or by use of stop valves as a foam breakers (Long et al. 2016).
Renewable resources such as agro wastes could help to reduce the cost of rhamnolipid synthesis to a factor of 10–30% compared to chemical surfactants (Satpute et al. 2017). With the increasing levels of glycerol generated as a by-product of biodiesel production, methods utilising glycerol for biosurfactant synthesis has also gained much interest (Randhawa and Rahman 2014). The continuous mode foam fractionation of biosurfactants from bioreactor has been found to be effective in controlling the foam generated during production as well as increasing the rate of mono-rhamnolipid production to a fivefold (Diaz De Rienzo et al. 2016). The use of hollow fibre reactors containing immobilised cells of Pseudomonas aeruginosa along with nitrate reduction instead of oxygen showed no problems associated with foam generation as observed in aerated systems (Pinzon et al. 2013).Thus, the compilation of information of different research around the world could surely help to economise rhamnolipid yields.
1.4.4 Genetic Engineering to Optimised Production
Attempts to resolve the first challenge of Pseudomonas aeruginosa pathogenicity has already resulted in the expression of its biosynthetic genes in E.coli, but the yields were less (Ochsner et al. 1994). The various recombinant DNA-based attempts to improve rhamnolipid production ranged from attempts to introduce LacZY genes of E.coli in Pseudomonas (Koch et al. 1988), transposome-mediated integration of rhlAB gene in E.coli (Wang et al. 2007) as well techniques to induce mutations in wild strains. Synthesis and expression of rhlAB gene and rhaBDAC gene cluster in recombinant E.coli under the influence of various synthetic promoters gave good rhamnolipid yields and they were further optimised by media engineering strategies (Gong et al. 2015). The recombinant expression of P.aeruginosa and Burkholderia rhlAB and rhlC genes in E.coli yielded di-rhamnolipid congeners to a greater extent (Du et al. 2017). Studies utilising mutation induced optimisation of rhamnolipids are also evident via transposon Tn5-GM-induced mutations of Pseudomonas (Koch et al. 1991), gamma ray mutations (Iqbal et al. 1995) and N-methyl-N-nitro-N-nitrosoguanidine-induced random mutagenesis (Tahzibi et al. 2004) yielding some successful reports on rhamnolipid yield.
The heterologous rhamnolipid production in Pseudomonas putida KT2440 by the introduction of rhlAB-genes helped to overcome pathogenicity of P.aeruginosa strains, quorum-sensing regulation and made possible biomass free production of the biosurfactant (Wittgens et al. 2011). The use of biofilms of the above recombinant P.putida KT2440 was also found to be a good source to produce mono-rhamnolipids (Wigneswaran et al. 2016). The carbon sources such as fatty acids transcriptionally delay the expression of rhlC gene and this could be used to control the ratio of monorhamnolipid to dirhamnolipids produced in the fermentation (Nicolo et al. 2017).
1.4.5 Application Potential
Rhamnolipids aid the solubilisation and easy uptake of hydrophobic xenobiotic compounds, thus becoming useful in soil remediation (Chebbi et al. 2017) and enhanced microbial oil uptake (Safdel et al. 2017). Rhamnolipids also extend its activity in the field of medicine by differentiating fibroblasts and keratinocytes to help in early wound healing (Stipcevic et al. 2006), targeted killing of myofibroblasts as therapy of scars (Shen et al. 2016). The antimicrobial role of this biosurfactant facilitates its use against plant pathogens (Sha et al. 2012) as well as human pathogenic microbes (De Rienzo et al. 2016; Magalhaes and Nitschke 2013). Apart from the antimicrobial properties of rhamnolipids, the high emulsifying property makes it a cleansing agent in detergents and other cleansers. The biopesticidal properties of rhamnolipids enable the control of various insects also (Kim et al. 2010).
1.5 Sophorolipids
1.5.1 Chemistry and Biosynthesis
Sophorolipids are glycolipids produced extracellularly by various non-pathogenic yeasts such as Candida bombicola, Candida apicola, Candida bogoriensis (Tulloch et al. 1968) and Wickerhamiella domericqiae (Chen et al. 2006). Chemically, they contain a glucose disaccharide sugar sophorose, formed by a glucosyl-β-(1-2)glucosyl linkage between the sugar moieties. The complete sophorolipid is generated by joining the fatty acid with the sophorose moiety. In the majority of the sophorolipids, fatty acids of 22 carbon chain length are found, with the remaining 10% containing 24 carbon atom-based fatty acids. The non-pathogenicity of the host strains makes sophorolipids more advantageous than rhamnolipids derived from Pseudomonas aeruginosa, which are opportunistic pathogens. Sophorolipids are found as mixtures of free acidic, lactonised form and acetylated derivatives; with the lactonised form having better foaming and solubility agents (Elshafie et al. 2015). The greater degree of acetylation of sophorolipids makes them more water insoluble enabling its easy recovery. The acetylated derivatives of this biosurfactant are also a good antiviral agent and have cytokine stimulatory effect.
Biosynthesis of sophorolipids works in combination with fatty acid synthesis which yields the building blocks for sophorolipids, catalysed by the enzyme cytochrome P450 monooxygenases a member of CYP52 family. The first step in the sophorolipid synthesis is the formation of hydroxylated fatty acids from fatty acids (Van Bogaert et al. 2013). The formation of sophorolipids is further catalysed by two sequential glycosylation reactions to yield a β-D glucosyloxy fatty acid (Esders and Light 1972) which further gets acetylated on the breakdown of acetyl CoA as per the metaCyC sophorolipid biosynthetic pathway shown in Fig. 3 (Caspi et al. 2014). The acidic sophorolipids get lactonised by specific proteins secreted by S. bombicola (Ciesielska et al. 2016).
Biosynthetic pathway of sophorolipids (Caspi et al. 2014)
1.5.2 Challenges in Sophorolipid Production
The key hold-up to its economised synthesis is the increased expenditure associated with production. However, the use of low-cost renewable agro wastes, waste oil (Makkar et al. 2011), molasses and coconut oil (Hoa et al. 2017), etc., could reduce the cost of production to a large extent. The major companies manufacturing sophorolipids include Evonik, Japan-based Saraya, South Korea-based MG Intobio, Japan-based Allied Carbon Solutions and US-based Synthezyme. Most predominantly sophorolipids are used for the production of cosmetics, toiletries and in medical field. The ecofriendliness of sophorolipids as well as its high yield of around 400 g/L has aroused much industrial interest of these biosurfactants (Van Bogaert et al. 2011).
1.5.3 Bioreactors to Optimised Production
Sophorolipids are produced by Candida bombicola from fat- or oil-contaminated wastewater both in batch and continuous reactions (Daverey and Pakshirajan 2015). Solid-state fermentation of Starmerella bombicola using agro wastes such as molasses, oil cake and straw yielded 0.179 g of sophorolipid per gram dry matter (Jimenez-Penalver et al. 2016). The combinatorial use of fed-batch method along with ultrasounds greatly increased the sophorolipid yield from waste vegetable oil (Maddikeri et al. 2015). Pilot-scale optimisation of Starmerella bombicola lactone esterase overexpression strain was done to get a highly pure diacetylated sophorolipid, which was suitable for chemical modification to generate sophorolipid amine oxides (Delbeke et al. 2016). High cell density fermentation with increased nutrients and optimised physical parameters using Candida bombicola was done in a fermentor to achieve productivity of 200 g/L/day (Gao et al. 2013). This was found to be of much relevance for industrial synthesis.
1.5.4 Genetic Engineering
Most of the recombinant work on sophorolipids is related to the identification of genes involved in sophorolipid synthesis. The proteins encoded by YP52M1 gene cluster catalyse the hydroxylated fatty acids synthesis—an essential step for sophorolipid synthesis (Van Bogaert et al. 2013), while glucosyltransferase gene UGTA1 catalyses first addition of glucose to the fattyacid derivative generated in the former step (Saerens et al. 2011). The cloning and recombinant expression of the glucosyltransferase gene from C. bombicola in Sacharomyces cerevesiae was done to study the broad spectrum glycosylating activity of this enzyme gtf-1 on sterols and fatty acids (Solaiman et al. 2014). The further lactonization of sophorolipids is found to be catalysed by an esterase, entitled S. bombicola lactone esterase which was characterised to prove that the above protein generates the lactonised form of sophorlipids by a serine hydrolase mechanism (Ciesielska et al. 2016). Most of the scientific developments related to sophorolipids are mostly patented such as the generation of sophorolipid transport protein (Soetaert and Van Bogaert 2012) which regulates the secretion of sophorolipids.
1.5.5 Application Potential
The anticancerous role of sophorolipid and its derivatives was effective in the treatment of pancreatic cancer and oesophageal cancer (Fu et al. 2008; Shao et al. 2012). Though the spermicidal and anti-HIV properties of this biosurfactant makes it a good topical contraceptive, the higher rates of cytotoxicity discourages its long-term use as a microbicidal contraceptive (Shah et al. 2005). They are used as surfactants, emulsifiers, antimicrobials, in environmental remediation of heavy metals, insoluble aromatic compounds and also in microbial-enhanced oil recovery (Elshafie et al. 2015). Moreover, it also is used in the generation of ω and ω-1hydroxy fatty acids, an essential component of perfumes (Van Bogaert et al. 2007).
2 Conclusions
Biosurfactant industrialised production is the need of the hour for the better ecological sustenance of the environment as well as the well-being of the human health. The cost factor of the biosurfactants could be overcome by using renewable resources as feedstock, response surface methodology-based optimisation, hyperproducing strains, better extraction protocols and cost-effective production protocols. The use of different expression systems using recombinant technology, in combination with media engineering and better extraction methods such as foam fractionation would help to achieve better yields. With successful attempts in the directions of rhamnolipids, sophorolipids and other biosurfactants the vision to replace chemical surfactants by biosurfactants would be possible in the near future.
References
Ashdown LR, Koehler JM (1990) Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J Clin Microbiol 28:2331–2334
Behler A, Biermann M, Hill K, Raths HC, Victor MES, Uphues G (2001) Industrial surfactant syntheses. Surfactant Sci Ser:1–44
Beuker J, Steier A, Wittgens A, Rosenau F, Henkel M, Hausmann R (2016) Integrated foam fractionation for heterologous rhamnolipid production with recombinant Pseudomonas putida in a bioreactor. AMB Express 6:11. https://doi.org/10.1186/s13568-016-0183-2
Bonnichsen L, Svenningsen NB, Rybtke M, de Bruijn I, Raaijmakers JM, Tolker-Nielsen T, Nybroe O (2015) Lipopeptide biosurfactant viscosin enhances dispersal of Pseudomonas fluorescens SBW25 biofilms. Microbiol 161:2289–2297
Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environ Health Perspect 115:1596–1602. https://doi.org/10.1289/ehp.10598
Cameotra SS, Makkar RS, Kaur J, Mehta SK (eds) (2010) Synthesis of biosurfactants and their advantages to microorganisms and mankind. In: Sen R. (eds) Biosurfactants. Adv Exp Med and Biol, Springer, New York, vol 672
Caspi R et al (2014) The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 42:D459–D471
Chebbi A et al (2017) Polycyclic aromatic hydrocarbon degradation and biosurfactant production by a newly isolated Pseudomonas sp. strain from used motor oil-contaminated soil. Int Biodeterior Biodegradation 122:128–140
Chen J, Song X, Zhang H, Qu Y (2006) Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb Technol 39:501–506. https://doi.org/10.1016/j.enzmictec.2005.12.022
Chen Q, Liu B, Wang J, Che J, Liu G, Guan X (2017) Antifungal Lipopeptides Produced by Bacillus sp. FJAT-14262 Isolated from Rhizosphere Soil of the Medicinal Plant Anoectochilus roxburghii. Appl Biochem Biotechnol 182:155–167
Cheng M, Zeng G, Huang D, Yang C, Lai C, Zhang C, Liu Y (2017) Tween 80 surfactant-enhanced bioremediation: toward a solution to the soil contamination by hydrophobic organic compounds. Crit Rev Biotechnol 20:1–14
Chistyakov BE (2001) Theory and practical application aspects of surfactants. In: Surfactants Chemistry, Interfacial Properties, Applications, Elsevier pp 511–618
Chrzanowski L, Lawniczak L, Czaczyk K (2011) Why do microorganisms produce rhamnolipids? World J Microbiol Biotechnol 28:401–419. https://doi.org/10.1007/s11274-011-0854-8
Ciesielska K et al (2016) Characterization of a novel enzyme–Starmerella bombicola lactone esterase (SBLE)—responsible for sophorolipid lactonization. Appl Microbiol Biotechnol 100:9529–9541. https://doi.org/10.1007/s00253-016-7633-2
Cortes-Sanchez AdJ, Hernandez-Sanchez H, Jaramillo-Flores ME (2013) Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol Res 168:22–32. https://doi.org/10.1016/j.micres.2012.07.002
Daniels R et al (2006) Quorum signal molecules as biosurfactants affecting swarming in Rhizobium etli. Proc Natl Acad Sci 103:14965–14970
Daverey A, Pakshirajan K (2015) Treatment of dairy wastewater containing high amount of fats and oils using a yeast-bioreactor system under batch, fed-batch and continuous operation. Desalination and Water Treat 57:5473–5479. https://doi.org/10.1080/19443994.2014.1003609
De Rienzo MAD, Stevenson PS, Marchant R, Banat IM (2016) Effect of biosurfactants on Pseudomonas aeruginosa and Staphylococcus aureus biofilms in a BioFlux channel. Appl Microbiol Biotechnol 100:5773–5779
Delbeke EIP et al (2016) Sophorolipid Amine Oxide Production by a Combination of Fermentation Scale-up and Chemical Modification. Ind Eng Chem Res 55:7273–7281. https://doi.org/10.1021/acs.iecr.6b00629
Deziel E, Lepine F, Milot S, Villemur R (2003) rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 149:2005–2013. https://doi.org/10.1099/mic.0.26154-0
Diaz De Rienzo MA, Kamalanathan ID, Martin PJ (2016) Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem 51:820–827. https://doi.org/10.1016/j.procbio.2016.04.007
Dos Santos AS, Pereira Jr N, Freire DMG (2016) Strategies for improved rhamnolipid production by Pseudomonas aeruginosa PA1. PeerJ 4:e2078. doi:https://doi.org/10.7717/peerj.2078
Du J, Zhang A, Hao J, Wang J (2017) Biosynthesis of di-rhamnolipids and variations of congeners composition in genetically-engineered Escherichia coli. Biotechnol Lett 39:1041–1048. https://doi.org/10.1007/s10529-017-2333-2
Elshafie AE, Joshi SJ, Al-Wahaibi YM, Al-Bemani AS, Al-Bahry SN, Al-Maqbali Da, Banat IM (2015) Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Front Microbiol 6. doi:https://doi.org/10.3389/fmicb.2015.01324
Esders TW, Light RJ (1972) Glucosyl-and acetyltransferases involved in the biosynthesis of glycolipids from Candida bogoriensis. J Biol Chem 247:1375–1386
Fu SL, Wallner SR, Bowne WB, Hagler MD, Zenilman ME, Gross R, Bluth MH (2008) Sophorolipids and their derivatives are lethal against human pancreatic cancer cells. J Surg Res 148:77–82. https://doi.org/10.1016/j.jss.2008.03.005
Gao R, Falkeborg M, Xu X, Guo Z (2013) Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Appl Microbiol Biotechnol 97:1103–1111. https://doi.org/10.1007/s00253-012-4399-z
Giani C, Wullbrandt D, Rothert R, Meiwes J (1997) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of L-rhamnose. United States Hoechst Aktiengesellschaft (Frankfurt am Main, DE) Patent
Ginkel CG (1989) Complete degradation of xenobiotic surfactants by consortia of aerobic microorganisms. Biodegradation 7(2):151–164
Gong Z, Peng Y, Zhang Y, Song G, Chen W, Jia S, Wang Q (2015) Construction and optimization of Escherichia coli for producing rhamnolipid biosurfactant. Chin J Biotechnol 31:1050–1062
Gonzalez-Jaramillo LM, Aranda FJ, Teruel JA, Villegas-Escobar V, Ortiz A (2017) Antimycotic activity of fengycin C biosurfactant and its interaction with phosphatidylcholine model membranes. Coll Surf B 156:114–122
Groffen T, Lopez-Antia A, D’Hollander W, Prinsen E, Eens M, Bervoets L (2017) Perfluoroalkylated acids in the eggs of great tits (Parus major) near a fluorochemical plant in Flanders. Belgium Environ Pollut 228:140–148. https://doi.org/10.1016/j.envpol.2017.05.007
He N, Wu T, Jiang J, Long X, Shao B, Meng Q (2017) Toward high-efficiency production of biosurfactant rhamnolipids using sequential fed-batch fermentation based on a fill-and-draw strategy. Coll Surf B 157:317–324. https://doi.org/10.1016/j.colsurfb.2017.06.007
Hoa NLH, Eun-Ki K, Ha TT, Duy ND, Khanh HQ, Dung NH (2017) Production and characterization of sophorolipids produced by Candida bombicola using sugarcane molasses and coconut oil. Asia Pac J Sci Technol 22:66–75
Iqbal S, Khalid ZM, Malik KA (1995) Enhanced biodegradation and emulsification of crude oil and hyperproduction of biosurfactants by a gamma ray-induced mutant of Pseudomonas aeruginosa. Lett Appl Microbiol 21:176–179
Jimenez-Penalver P, Gea T, Sanchez A, Font X (2016) Production of sophorolipids from winterization oil cake by solid-state fermentation: optimization, monitoring and effect of mixing. Biochem Eng J 115:93–100. https://doi.org/10.1016/j.bej.2016.08.006
Jing-xin Y (2004) Application of phosphate-type surfactants in the textile industry. Text Aux 3:008
Kaczorek E, Chrzanowski L, Pijanowska A, Olszanowski A (2008) Yeast and bacteria cell hydrophobicity and hydrocarbon biodegradation in the presence of natural surfactants: rhamnolipides and saponins. Bioresour Technol 99:4285–4291
Kim SK, Kim YC, Lee S, Kim JC, Yun MY, Kim IS (2010) Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae). J Agric Food Chem 59:934–938
Koch AK, Kappeli O, Fiechter A, Reiser J (1991) Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J Bacteriol 173:4212–4219
Koch AK, Reiser J, Kappeli O, Fiechter A (1988) Genetic Construction of Lactose-Utilizing Strains of Pseudomonas aeruginosa and Their Application in Biosurfactant Production. Nat Biotech 6:1335–1339
Kralova I, Sjoblom J (2009) Surfactants used in food industry: a review. J Dispers Sci Technol 30:1363–1383
Lee KM, Hwang S-H, Ha SD, Jang J-H, Lim D-J, Kong J-Y (2004) Rhamnolipid production in batch and fed-batch fermentation using Pseudomonas aeruginosa BYK-2 KCTC 18012P. Biotechnol Bioprocess Eng 9:267–273. https://doi.org/10.1007/bf02942342
Linhardt RJ, Bakhit R, Daniels L, Mayerl F, Pickenhagen W (1989) Microbially produced rhamnolipid as a source of rhamnose. Biotechnol Bioeng 33:365–368
Long X, Shen C, He N, Zhang G, Meng Q (2016) Enhanced rhamnolipids production via efficient foam-control using stop valve as a foam breaker. Bioresour Technol 224:536–543. https://doi.org/10.1016/j.biortech.2016.10.072
Maddikeri GL, Gogate PR, Pandit AB (2015) Improved synthesis of sophorolipids from waste cooking oil using fed batch approach in the presence of ultrasound. Chem Eng J 263:479–487. https://doi.org/10.1016/j.cej.2014.11.010
Magalhaes L, Nitschke M (2013) Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control 29:138–142
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1:5. https://doi.org/10.1186/2191-0855-1-5
Mani P, Dinesh kumar G, Jayaseelan T, Deepalakshmi K, Ganesh Kumar C, Senthil Balan S (2016) Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech 6:163. https://doi.org/10.1007/s13205-016-0478-7
McClements DJ, Gumus CE (2016) Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv Coll Interface Sci 234:3–26. https://doi.org/10.1016/j.cis.2016.03.002
Meng J, Lu Y, Wang T, Wang P, Giesy JP, Sweetman AJ, Li Q (2017) Life cycle analysis of perfluorooctanoic acid (PFOA) and its salts in China. Environ Sci Pollut Res 24:11254–11264. doi:https://doi.org/10.1007/s11356-017-8678-1
Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: properties, commercial production and application. Curr Sci 94:736–747
Ndlovu T, Rautenbach M, Vosloo JA, Khan S, Khan W (2017) Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 7:108
Nicolo MS, Cambria MG, Impallomeni G, Rizzo MG, Pellicorio C, Ballistreri A, Guglielmino SPP (2017) Carbon source effects on the mono/dirhamnolipid ratio produced by Pseudomonas aeruginosa L05, a new human respiratory isolate. New Biotechnol. doi:http://dx.doi.org/10.1016/j.nbt.2017.05.013
Ochsner UA, Fiechter A, Reiser J (1994) Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem 269:19787–19795
Pang X, Zhao J, Fang X, Liu H, Zhang Y, Cen S, Yu L (2017) Surfactin derivatives from Micromonospora sp. CPCC 202787 and their anti-HIV activities. J Antibiot 70:105–108
Pinzon NM, Cook AG, Ju L-K (2013) Continuous rhamnolipid production using denitrifying Pseudomonas aeruginosa cells in hollow-fiber bioreactor. Biotechnol Prog 29:352–358. https://doi.org/10.1002/btpr.1701
Pittinger CA, Sellers JS, Janzen DC, Koch DG, Rothgeb TM, Hunnicutt ML (1993) Environmental life-cycle inventory of detergent-grade surfactant sourcing and production. J Am Oil Chem Soc 70:1–15
Putri M, Hertadi R (2015) Effect of Glycerol as Carbon Source for Biosurfactant Production by Halophilic Bacteria Pseudomonas stutzeri BK-AB12. Procedia Chem 16:321–327. https://doi.org/10.1016/j.proche.2015.12.059
Radzuan MN, Banat IM, Winterburn J (2017) Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour Technol 225:99–105
Randhawa KKS, Rahman PK (2014) Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front Microbiol 5:454–454
Rebello S, Asok AK, Joseph SV, Joseph BV, Jose L, Mundayoor S, Jisha MS (2013) Bioconversion of sodium dodecyl sulphate to rhamnolipid by Pseudomonas aeruginosa: a novel and cost-effective production strategy. Appl Biochem Biotechnol 169:418–430
Rebello S, Asok AK, Mundayoor S, Jisha MS (2014) Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett 12:275–287
Rebello S, Joseph BV, Joseph SV, Jose L, Mundayoor S, Jisha MSC (2016) Bioconversion of sodium dodecyl sulphate to rhamnolipids by transformed Escherichia coli DH5α cells—a novel strategy for rhamnolipid synthesis. J Appl Microbiol 120:638–646. https://doi.org/10.1111/jam.13032
Robert M, Mercade ME, Bosch MP, Parra JL, Espuny MJ, Manresa MA, Guinea J (1989) Effect of the carbon source on biosurfactant production by Pseudomonas aeruginosa 44T1. Biotechnol Lett 11:871–874
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618
Saerens KMJ, Roelants SLKW, Van Bogaert INA, Soetaert W (2011) Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res 11:123–132
Safdel M, Anbaz MA, Daryasafar A, Jamialahmadi M (2017) Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew Sustain Energy Rev 74:159–172
Satpute SK, Plaza GA, Banpurkar AG (2017) Biosurfactants production from renewable natural resources: example of innovative and smart technology in circular bioeconomy. Manag Syst Prod Eng 25:46–54. doi:https://doi.org/10.1515/mspe-2017-0007
Sha R, Jiang L, Meng Q, Zhang G, Song Z (2012) Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J Basic Microbiol 52:458–466
Shah V et al (2005) Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother 49:4093–4100
Shao L, Song X, Ma X, Li H, Qu Y (2012) Bioactivities of Sophorolipid with Different Structures Against Human Esophageal Cancer Cells. J Surg Res 173:286–291. https://doi.org/10.1016/j.jss.2010.09.013
Shen C et al (2016) Targeted killing of myofibroblasts by biosurfactant di-rhamnolipid suggests a therapy against scar formation. Sci Rep 6:37553
Singh A, Van Hamme JD, Ward OP (2007) Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol Adv 25:99–121
Soetaert W, Van Bogaert I (2010) Sophorolipid transporter protein. U.S. Patent Application 13/514, 908
Solaiman DKY, Liu Y, Moreau RA, Zerkowski JA (2014) Cloning, characterization, and heterologous expression of a novel glucosyltransferase gene from sophorolipid-producing Candida bombicola. Gene 540:46–53. doi:https://doi.org/10.1016/j.gene.2014.02.029
Souza KST et al (2017) New glycolipid biosurfactants produced by the yeast strain Wickerhamomyces anomalus CCMA 0358. Coll Surf B 154:373–382
Stipcevic T, Piljac A, Piljac G (2006) Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burn 32:24–34. https://doi.org/10.1016/j.burns.2005.07.004
Stoimenova E, Vasileva-Tonkova E, Sotirova A, Galabova D, Lalchev Z (2009) Evaluation of different carbon sources for growth and biosurfactant production by Pseudomonas fluorescens isolated from wastewaters. Zeitschrift fur Naturforschung C 64:96–102
Tahzibi A, Kamal F, Mazaheri Assadi M (2004) Improved production of rhamnolipids by a Pseudomonas aeruginosa mutant. Iran Biomed J 8:25–31
Timmis KN (2002) Pseudomonas putida: A cosmopolitan opportunist par excellence. Environ Microbiol 4(12):779–81
Tmakova L, Sekretar S, Schmidt S (2017) Plant-derived surfactants as an alternative to synthetic surfactants: surface and antioxidant activities. Chem Pap 70:188–196. https://doi.org/10.1515/chempap-2015-0200
Torres LG, Orantes JL, Iturbe R (2003) Critical micellar concentrations for three surfactants and their diesel-removal efficiencies in petroleum-contaminated soils. Environ Geosciences 10(1):28–36
Tuleva BK, Ivanov GR, Christova NE (2002) Biosurfactant production by a new Pseudomonas putida strain. Zeitschrift Fur Naturforschung C 57:356–360
Tulloch AP, Spencer JFT, Deinema MH (1968) A new hydroxy fatty acid sophoroside from Candida bogoriensis. Can J Chem 46:345–348. https://doi.org/10.1139/v68-057
Van Bogaert INA, Holvoet K, Roelants SLKW, Li B, Lin YC, Van de Peer Y, Soetaert W (2013) The biosynthetic gene cluster for sophorolipids: a biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol Microbiol 88:501–509
Van Bogaert INA, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34. https://doi.org/10.1007/s00253-007-0988-7
Van Bogaert INA, Zhang J, Soetaert W (2011) Microbial synthesis of sophorolipids. Process Biochem 46:821–833. https://doi.org/10.1016/j.procbio.2011.01.010
Vecino X, Rodriguez-Lopez L, Gudiia EJ, Cruz JM, Moldes AB, Rodrigues LR (2017) Vineyard pruning waste as an alternative carbon source to produce novel biosurfactants by Lactobacillus paracasei. Ind Eng Chem Res 55:40–49
Vollenbroich DM, Vater J, Kamp RM, Pauli G (1997) Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 25:289–297
Wang Q, Fang X, Bai B, Liang X, Shuler PJ, Goddard WA, Tang Y (2007) Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng 98:842–853. https://doi.org/10.1002/bit.21462
Wang S et al (2014) Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in pseudomonas aeruginosa. Appl Environ Microbiol 80:6724–6732. https://doi.org/10.1128/aem.01237-14
Wigneswaran V, Nielsen KF, Sternberg C, Jensen PR, Folkesson A, Jelsbak L (2016) Biofilm as a production platform for heterologous production of rhamnolipids by the non-pathogenic strain Pseudomonas putida KT2440. Microb Cell Fact 15:181. https://doi.org/10.1186/s12934-016-0581-9
Wittgens A et al (2011) Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb Cell Fact 10:80. https://doi.org/10.1186/1475-2859-10-80
Wu J, Zhang J, Wang P, Zhu L, Gao M, Zheng Z, Zhan X (2017) Production of rhamnolipids by semi-solid-state fermentation with Pseudomonas aeruginosa RG18 for heavy metal desorption. Bioprocess Biosyst Eng. doi:https://doi.org/10.1007/s00449-017-1817-8
Acknowledgements
Sharrel Rebello (SERB-NPDF- File no PDF/2015/000472) and Raveendran Sindhu (DBT Bio-CARe File no BT/Bio-CARe/06/890/2011-2012) acknowledge for the respective fundings.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rebello, S., Aneesh, E.M., Sindhu, R., Binod, P., Pandey, A. (2018). Biosynthesis and Technological Advancements of Biosurfactants. In: Varjani, S., Parameswaran, B., Kumar, S., Khare, S. (eds) Biosynthetic Technology and Environmental Challenges. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7434-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-7434-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7433-2
Online ISBN: 978-981-10-7434-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)