Abstract
Approximately 50% of the patients with colorectal cancer will develop peritoneal metastases at some point in their disease timeline. An aggressive locoregional therapy comprising of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) has led to a significant prolongation in overall survival and even cure in certain selected patients with this condition which portends a poor long-term outcome. For patients with a good performance status, a predicted peritoneal cancer index of <17–20 in whom complete removal of macroscopic disease is possible, CRS and HIPEC should be considered the standard of care. However, the percentage of patients that fall in this category is small, and strategies for improving outcomes in other patients are being developed. Neoadjuvant strategies comprising of systemic and intraperitoneal chemotherapy and new methods of intraperitoneal drug delivery like pressurized intraperitoneal aerosol chemotherapy (PIPAC) have shown promising results and are being evaluated prospectively. It is the patients with limited peritoneal disease that experience the maximum benefit from a curative approach; hence, the focus has shifted to the early detection and even prevention of the occurrence of colorectal peritoneal metastases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Peritoneal metastases (PM) of colorectal cancer are present in 5–10% of patients at the time of presentation for primary cancer treatment and in about 15–30% of patients with recurrent disease [1,2,3]. About 4–8% of these present with isolated peritoneal metastases with no evidence of other visceral metastases [4]. Though PM have poorer prognosis than other sites of metastases like the liver, over the past two decades the use of an aggressive locoregional strategy of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has shown a significant benefit in overall survival as compared to systemic chemotherapy alone in selected patients. The role of HIPEC is currently being evaluated in randomized controlled trials (PRODIGE 7-ACCORD 15 trial (NCT00769405)). Moreover, the patients who are candidates for such treatment are a small percentage of all patients with colorectal PM (CPM). Newer treatment strategies are being investigated to improve the outcomes in other patients. Since the patients who benefit most from such treatment are those with limited disease, the focus has been on more proactive approaches for prevention and early detection of colorectal PM (CPM). This chapter provides an approach to management of patients with CPM based on the current evidence and an update on the ongoing research in this field.
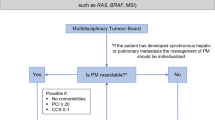
1.1 Approach to a Patient with CPM
Management of CPM requires a multidisciplinary team and is best carried out in centers experienced in delivering this form of treatment.
2 Pathophysiology of Peritoneal Dissemination
Understanding the disease biology forms the basis of treating CPM. The most common mechanism of dissemination of peritoneal metastases is by direct extension of the primary malignancy into the free peritoneal space. This can occur due to full-thickness involvement of the bowel wall (local peritoneal involvement) or due to spillage caused during surgery [5]. Once a viable, free cancer cell is present in the peritoneal cavity, adhesion to the peritoneal surface is required in order to ultimately invade the peritoneum, proliferate, and produce peritoneal deposits. In the postoperative period, production of reactive oxygen species and inflammatory cytokines leads to upregulation of specific cell surface adhesion molecules leading to increased adhesiveness of cancer cells. Surgical trauma caused to the peritoneum is also known to increase the adhesiveness and metastatic potential of free intraperitoneal cells [6]. This creates a milieu that favors the development of PM. The adhesion molecules that have been implicated in this process are CD44, integrin α2β1, and mucin 16 (MUC 16) [7].
In cases of full-thickness involvement of the bowel wall, PM are seen in the vicinity of the primary malignancy, layered out under the right hemidiaphragm or involving the pelvic peritoneum. Despite the fact that PM are present, cytological study of the peritoneal fluid is often negative. In women, a frequent site of the progression of peritoneal metastases is the ovaries, especially in the premenopausal women [8].
The most common site of metastatic spread from colorectal primary tumors is the liver. However, liver metastases have a more protracted course as compared to CPM which are more aggressive; Sugarbaker pointed out several differences in the biology of CPM and colorectal liver metastases (Table 12.1). Liver metastases arise as a result of portal dissemination and have a lower metastatic potential as compared to PM which spread more rapidly.
Franko et al. analyzed individual patient data for previously untreated patients enrolled in 14 phase III randomized trials done between 1997 and 2008. This analysis concluded that patients with colorectal PM have a significantly shorter overall survival than those with other isolated sites of metastases. In patients with several sites of metastasis, poor survival is a function of both increased number of metastatic sites and peritoneal involvement. The pattern of metastasis and, in particular, peritoneal involvement results in prognostic heterogeneity of metastatic colorectal cancer [9].
Apart from full-thickness bowel wall involvement which is associated with PM in 50% of the cases, the risk of metachronous PM in patients with mucinous or signet ring cell carcinoma is 11–36% and 9–36% in patients with a positive peritoneal cytology [10,11,12,13]. Mucinous histology is associated with a poorer overall and disease-free survival regardless of the presence of peritoneal dissemination.
Honore et al. performed a systematic review of the literature that included 16 clinical studies, all nonrandomized, 3 prospective and 13 retrospective, including 4395 patients. There were three situations that could result in a real higher risk of recurrent PM: synchronous PM, synchronous isolated ovarian metastases, and a perforated primary tumor [14]. The risk was similar in patients with spontaneous and iatrogenic tumor rupture.
Patients with BRAF mutation have a higher risk of developing PM though the prognosis of patients of CPM with or without this mutation is similar [15].
3 Clinical Presentation
Early peritoneal dissemination does not produce any symptoms. Symptoms occur when the disease is advanced and are usually nonspecific [16, 17]. Ascites is seen at presentation in 28–30% of the patients with synchronous metastases and small bowel obstruction in 8–20% of the patients [3]. Hence, the use of imaging studies and diagnostic laparoscopy should be made in patients with a high risk for peritoneal dissemination to detect it early.
3.1 Evaluating the Extent of Disease Spread
CT scan is the most commonly used imaging modality for evaluating the disease extent though its sensitivity is only 23–76%, and it has a limited value in detecting low-volume disease and small tumor nodules [18, 19]. In general, the use of contrast-enhanced CT scan assists in identifying primary lesions of bowel, solid organ metastases, and nodal metastases. Visible cardiophrenic angle lymph nodes on CT scan are strongly associated with the presence of peritoneal metastases. In a study of 114 patients, Elias et al. showed that the presence of these nodes had no prognostic impact after optimal cytoreductive surgery plus HIPEC [20].
Jacquet et al. compared the PCI predicted on preoperative CT scan with the surgical PCI and found that the accuracy of the CT scan was dependent on the lesion size. Small peritoneal nodules or masses less than 0.5 cm were detected in 28% of the patients preoperatively, moderate-sized nodules 0.5–5.0 cm were detected in 72%, and gross nodules greater than 5 cm were detected in 90% [21]. When the nine abdominopelvic regions were compared, the pelvic region was the least accurate. Similarly, other investigators have reported a sensitivity of 11% for nodules less than 0.5 cm, 37% for nodules 0.5–5.0 cm, and 94% for nodules greater than 5 cm [22]. Since here is such a strong relationship of extent of disease to outcomes, it is important to diagnose limited extent PM to improve outcomes [23,24,25]. Other imaging modalities have been investigated in an attempt to accurately predict the extent of PM, including diffusion-weighted MRI and PET-CT scan [25,26,27,28,29,30]. In a study by Low et al., MRI correctly categorized the tumor volume in 20 of 22 patients, with an overall sensitivity and specificity of 88% and 74% [28]. Espada et al. developed a scoring system with a diagnostic accuracy of 91% by evaluating DWI for detection of PM [31]. However, the sample size was small for both these studies, and other studies have not been able to replicate these results. In a recent meta-analysis based on 22 studies, MRI and PET-CT were shown to have similar per-patient diagnostic accuracy to CT scan in predicting the PCI, but the data was more robust for CT scan [32]. MRI requires 6 h of fasting and a stringent protocol and is more accurate when used by experienced radiologists. It has shown a greater accuracy in detecting small-volume disease [33, 34]. The use of these investigations needs to be individualized, keeping in mind that PM is usually more extensive than predicted by any one investigation [35].
The BIG-RENAPE and RENAPE working groups have developed the PeRitOneal MalIgnancy Stage Evaluation (PROMISE) Internet application (www.e-promise.org) to facilitate tabulation and automatically calculate the peritoneal cancer index (PCI) [36]. This application offers computer assistance to produce simple, quick, but precise and standardized pre-, intra-, and postoperative reports of the extent of peritoneal metastases. In addition to the radiological score, pathological and surgical scores can be generated as well. Not only the peritoneal metastases but other aspects like peritoneal thickening, involvement of adipose tissue, and fluid density are taken into consideration in this application. It can be used by less experienced centers as well and can help in research and multicentric studies related to peritoneal metastases [36].
CT scan may be required in the postoperative period for the management of complications. Dromain et al. reported CT findings in 51 patients in the first 15 days of CRS and HIPEC and found all the scans to have some abnormal findings. They concluded that findings like bowel and peritoneal thickening, increased intraperitoneal fat density, and compartmentalized ascites result from an inflammatory mesenteric reaction or inflammation of the small bowel or the peritoneum and do not require specific treatment. A knowledge of these findings is essential for appropriate management of these patients [37].
3.2 Diagnostic/Staging Laparoscopy
A diagnostic/staging laparoscopy allows direct visualization of the peritoneal surfaces and can pick up small peritoneal nodules that cannot be detected by imaging studies. Laparoscopy can be used for early diagnosis of PM as well as selection of patients for CRS and HIPEC. Laparoscopy has been shown to prevent an unnecessary laparotomy in 7–41% of the cases [38, 39]. The preferred site of port placement is the midline to facilitate resection of the port sites in future CRS. Laparoscopy allows sampling of the peritoneal fluid for cytology and biopsy of suspicious areas in evaluating response to chemotherapy [40]. It may be challenging to perform this procedure in patients with multiple prior surgeries. Extensive adhesions may preclude a thorough evaluation. Certain areas where a laparoscopic evaluation may be suboptimal are infiltration of the diaphragm muscle, involvement of the porta hepatis and pancreas, and in the region of the celiac axis. In addition, involvement of the ureters and pelvic sidewall may also be inaccurate. Iversen et al. reported that laparoscopy correctly predicted complete cytoreduction in only 29% of patients with recurrent colorectal cancers, compared to 33, 80, and 87.5% of patients with mesothelioma, PMP, and synchronous colorectal PC, respectively [41]. This has been attributed to the fact that recurrent CRC often tends to infiltrate retroperitoneal structures like the ureters or pancreas. However, these areas are more accurately assessed on imaging, and a combination of imaging techniques with laparoscopy should be used to select patients most likely to benefit from CRS and HIPEC [42].
4 Multimodality Treatment of Colorectal PM
The conventional treatment for colorectal PM is systemic chemotherapy. In the absence of definitive treatment, patients are administered systemic chemotherapy with the goal of obtaining some prolongation in survival and symptomatic relief or both. The treatment of colorectal PM with CRS and HIPEC has significantly improved the survival of these patients though this is possible only in selected patients who are in good general health and have no extra-abdominal disease, and the extent of PM is limited. This treatment may not be a replacement for systemic therapies, and majority of the patients require systemic chemotherapy in addition to CRS and HIPEC. The optimal treatment strategy needs to be individualized for each patient, and such decisions are best made by multidisciplinary teams at centers experienced in delivering this treatment.
4.1 Outcomes of Systemic Therapy as the Sole Treatment for CPM
Combination chemotherapy with or without targeted therapy is the cornerstone of treatment for colorectal PM. With the introduction of new agent like oxaliplatin and irinotecan, the overall survival which was rarely more than 12 months with 5-fluourouracil and leucovorin improved to almost 20 months (15.6 months with FOLFIRI regimen and 19.5 months with the FOLFOX regimen ) [42, 43]. It improved further with the addition of targeted agents like bevacizumab to 20.3 months with FOLFIRI and 21.3 months with FOLFOX [44, 45]. Similarly, the addition of cetuximab to FOLFIRI increased the median survival to 19.9 months and 22.8 months with FOLFOX. This benefit was seen only in KRAS non-mutated tumors [46, 47]. However, these studies were not carried out exclusively for patients with colorectal PM, and a large proportion of the patients in these studies had liver only metastases which is a more favorable prognostic group.
Franko et al. reported the outcome of patients with colorectal PM from a pooled analysis of two large phase III trials from the North Central Cancer Treatment Group (NCCTG) that included 2101 patients treated with systemic chemotherapy, out of which 1646 patients were undergoing evaluation of first-line therapy and 455 for second-line therapy [48]. Only 44 patients (2.1%) had PM which is a significantly low rate as compared to the expected incidence of 15–20%. Patients with PM had 30% reduction in overall survival as compared to those with other metastatic sites, with a median survival of 12.7 months compared to 17.6 months when patients had no PM (HR = 1.32, 95% CI, 1.15–1.50; P < 0.001). The authors opined that the presence of PM should not affect the choice of the chemotherapeutic regimen.
Klaver et al. reported the results of two similar studies from the Dutch Colorectal Cancer Group (DCCG) and came to the same conclusion as the North American group, both of which had a small percentage of patients with PM (4% and 6%, respectively) [49]. The proportion of patients with isolated PM were even lower—only 4/850 in the CAIRO study and 5/755 in the CAIRO 2 study [50, 51]. The studies analyzed the efficacy of different chemotherapy regimens in the first and subsequent lines of therapy in the metastatic setting. In the CAIRO study, median OS was 10.4 months for patients with PM vs. 17.3 months for patients with no PM, (P < 0.001), and in CAIRO 2, this was 15.2 months vs. 20.7 months, respectively (P < 0.001). These studies once again demonstrated the poor efficacy of modern chemotherapy regimens in patients with colorectal PM. There was no dose reduction in these patients or problem of tolerance, and the authors attributed the poor results to a biologically more aggressive nature of PM and a relative resistance to therapy.
Thus, although systemic chemotherapy is widely used to treat colorectal PM, there is no strong evidence showing its efficacy in this pattern of spread of colorectal cancer, and there is a need for a more aggressive locoregional therapy that could address PM [52].
4.2 CRS and HIPEC for CPM: Current Evidence
In comparison to only systemic therapy, patients with PM treated with CRS and HIPEC can reach a median survival of 63 months and 2- and 5-year survival rates of 81% and 51%, respectively [53]. The aim of CRS is to achieve a complete resection of all macroscopic disease within the peritoneal cavity so that the residual microscopic disease can then be treated with HIPEC. Two retrospective and one prospective studies have looked at the role of cytoreductive surgery only, without adding any intraperitoneal chemotherapy treatment. In the patients that received complete resection of peritoneal disease, the 5-year survival ranged from 24 to 36% [54,55,56]. However, these studies included a heterogeneous group of patients, including 40–66% patients with the presence of distant metastases at the time of treatment of peritoneal metastases; the absolute numbers were quite small, ranging from 31 to 125 patients, and the data was collected over long periods ranging from 9 to 16 years [55, 57]. Considering these drawbacks and the nonrandomized nature of the studies, it is difficult to draw inferences, but these studies do show the beneficial effect of CRS in PM.
Several single-institution and multicentric studies have been published regarding the outcomes of this combined modality treatment, but few studies have compared CRS and HIPEC to systemic chemotherapy. Verwaal et al. conducted a phase III randomized trial comparing CRS and HIPEC to the then existing systemic chemotherapy 5-FU and leucovorin [58]. One hundred and five patients were randomized to either systemic chemotherapy with palliative surgery for prevention or treatment of complications, which was the standard treatment at the time of the study, or CRS and HIPEC with mitomycin C. The median overall survival was significantly better in the HIPEC group (22.2 months vs. 12.6 months; P = 0.028). This benefit was despite the fact that over half the patients in the HIPEC group did not receive a CC-0/CC-1 resection due to extensive disease, indicating that they were not good candidates for HIPEC. For the patients receiving CC-0 resection, the 5-year survival was 45%, and these findings were confirmed even after an 8-year follow-up, when more than 90% of the events had occurred [59]. The main criticism of this study is that although this was a randomized trial, it was performed in the era of 5-FU-leucovorin, and chemotherapy and targeted therapy for colorectal cancer have evolved since then with good long-term survival. To address this issue, Elias et al. compared 48 PM patients treated at various centers in France receiving palliative systemic oxaliplatin- and/or irinotecan-based chemotherapy to 48 patients who underwent additional CRS and HIPEC with oxaliplatin [53]. Both groups received a mean of 2.3 lines of chemotherapy. Two-year and 5-year overall survival rates were 81% and 51% for the HIPEC group versus 65% and 13% for the standard group, respectively. The median survival was 62.7 months in the HIPEC group, which compared favorably to 23.9 months in the standard group (P < 0.05). The results of this study showed that a median survival of 63 months and a 5-year survival of 51% could be achieved in patients with isolated colorectal PM which was significantly longer than the 24-month median survival achieved with systemic chemotherapy alone in patients with a similar disease extent.
Franko et al. performed a case-control study comparing 67 patients undergoing CRS and HIPEC in addition to systemic chemotherapy to 38 others receiving systemic chemotherapy alone and reported a significantly longer median survival in the CRS and HIPEC group (34.7 months vs. 16.8 months; P < 0.001) [60]. In another study by Franko et al., they performed a pooled analysis of the survival data of patients with PM from two phase III chemotherapy trials (N9741 and N9841) and compared the outcomes to non-PM metastatic colorectal cancer [48]. The median OS (12.7 vs. 17.6 months, hazard ratio [HR] = 1.3; P < 0.001) and PFS (5.8 vs. 7.2 months, HR = 1.2; P = 0.001) were shorter for PM versus non-PM patients, and this unfavorable prognostic influence of PM remained even after adjusting for other factors.
Cavaliere et al. reported the results of 120 patients treated with the Italian Society of Locoregional Treatment in Oncology (SITILO) protocol at six Italian centers. Patients were treated with CRS and HIPEC with cisplatin (CDDP) and mitomycin C (MMC), and only 11 underwent HIPEC with an oxaliplatin-based regimen [61]. A complete cytoreduction CC-0 was achieved in 85.2% of the patients. The 3-year survival was 25.8% and increased to 33.5% in patients who had an optimal cytoreduction (CC-0) (P < 0001). In a multicentric study of 523 patients from 23 French-speaking centers, Elias et al. reported a median overall survival of 30.1 months, 5-year overall survival of 27%, and a 5-year disease-free survival of 10% with CRS and HIPEC in PM [62]. The 5-year survival was 29% in patients with no residual disease and 14% in patients with residual disease <2.5 mm, and the group of patients with residual disease >2.5 mm had no 5-year survivors. On multivariate analysis, the independent variables for survival were completeness of CRS, extent of PM evaluated by PCI, lymph node positivity, and the use of adjuvant chemotherapy. This study showed that CRS and HIPEC could be performed with a low morbidity and mortality and resulted in a prolonged survival in patients with a PCI of <20. In another bi-institutional French study of 146 patients by Quenet et al., where they included only those patients who had completely resected PM and PCI < 25 treated with either oxaliplatin or oxaliplatin with irinotecan as the HIPEC agents, the median overall survival (OS) was 41 months and median relapse-free survival was 15.7 months, with a 5-year overall survival rate of 42% and 5-year relapse-free survival of 16% [63]. Lymph node metastases and PCI were the only independent prognostic variables, and there was no difference in the survival outcomes between the two HIPEC regimes [63]. Sugarbaker et al. presented their experience of CRS and HIPEC for PM in 318 patients [64]. The median survival was 21.5 months for the whole cohort, but in patients receiving CC-0/CC-1 resections, the median survival was 36.6 months, compared to 18.3 months and 7.6 months for CC-2 and CC-3 resections, again emphasizing the effect of completeness of CRS. This prognostic impact of completeness of CRS was maintained on multivariate analysis. The 3- and 5-year survival rates were 35% and 25%, which are quite encouraging considering the fact that the mean PCI was 15.2, which is quite high compared to other studies in colorectal PM. In another national patient cohort from Norway, Froysnes et al. reported a median survival of 47 months and a 5-year overall survival of 36% for their 118 patients; >95% of their patients had a CC-0 resection which further confirms the significant prognostic impact of a complete cytoreduction [65].
In a systematic review of CRS and HIPEC in colorectal PM, Baratti et al. reported that in the eight studies where patients underwent CC-0 or CC-1 cytoreduction, the median survival period ranged from 16 to 51 months (weighted average, 31.6 ± 10.3 months). The 5-year survival rates reported in nine series ranged from 22 to 50.5% (weighted average, 31.0 ± 9.4%) [66].
The results of all these studies (summarized in Table 12.2) suggest that CRS and HIPEC as a combined modality definitely offers a potential benefit in the scenario of PM, and possibly a major part of the benefit seems to be because of the cytoreduction. The role of HIPEC has been questioned for several reasons—a lack of uniformity of HIPEC protocols, drugs, and carrier solutions used, different methods of HIPEC administration (open, semi-open, closed techniques), heterogeneity of patient populations treated, and lack of randomized trials in the era of modern chemotherapy and targeted therapy. Future clinical trials will also have to address these concerns to establish the position of this promising treatment in the treatment of colorectal PM. Whether HIPEC adds a benefit over and above the CRS will be further clarified by the results of the hugely anticipated PRODIGE 7 trial (NCT00769405).
4.3 Role for HIPEC
Studies evaluating the drugs used during HIPEC have shown that the drug penetration is limited to a few cell layers and hence complete resection of all macroscopic disease is essential to have a beneficial effect of HIPEC. HIPEC has several theoretical benefits. HIPEC is performed immediately after the surgery which ensures free dispersion of the hyperthermia and chemotherapy prior to formation of peritoneal adhesions in which cancer cells may be trapped [67,68,69, 71]. Heat itself is cytotoxic and potentiates the cytotoxicity of chemotherapeutic agents. Animal studies have shown the additive effect of combining hyperthermia with intraperitoneal chemotherapy compared to either of them alone [71, 72].
However, its additive effect in humans with colorectal PM has not been conclusively proven. There is a lack of fundamental research on intraperitoneal chemotherapy which has moved very rapidly from the laboratory bench to the bedside [73].
Very few prospective clinical trials have been set up to determine the ideal parameters in terms of time, temperature, perfusion technique, and cytotoxic drug dose or type. There are no definitive guidelines for surgeons, and choice of technique is often determined by personal preference and experience [73]. However, for colorectal cancer, the protocol for oxaliplatin- and mitomycin C-based HIPEC has been standardized through consensus meetings and is widely adhered to (described later).
Conducting clinical trials in CRS and HIPEC is not only expensive, but the outcomes are difficult to evaluate, as pointed out by David Bartlett [74]. Unlike systemic therapy, the dose can be limited by a complication unrelated to the systemic effects of the drug in phase I studies which makes drawing conclusions difficult. In phase II and III studies, the concerns are patient accrual, funding, lack of endpoints other than DFS and OS, and comparison with the outcomes of systemic therapies which represents a “moving target” [74].
Since all aspects of intraperitoneal chemotherapy cannot be evaluated in clinical trials, researchers develop innovative animal experiments to study distinct aspects of HIPEC and other forms of intraperitoneal chemotherapy. Peritoneal metastases similar to that in humans can be induced in animals, and several small and large animal models have been developed and used for experimental purposes. Surgical techniques as well as various aspects of intraperitoneal chemotherapy have been studied [75, 76].
Most studies have successfully developed a clinically relevant model, and the focus of experimental research has now shifted toward enhancing and refining intraperitoneal chemotherapy [74]. Pelz et al. showed that HIPEC is an effective treatment for peritoneal metastases in animal models and reduced macroscopic and microscopic intraperitoneal tumor spread [73]. Another study showed that raised intra-abdominal pressure combined with hyperthermia increased the tissue concentration of oxaliplatin [77].
Klaver et al. compared CRS with CRS and heated saline perfusion, CRS and normothermic intraperitoneal chemotherapy, and CRS and HIPEC in a syngeneic rat CRC model. Every group consisted of 20 animals with a comparable PCI and surgical resection score. The primary endpoint was survival. The temperature for hyperthermia was set at 41–42°C as in the trial by Verwaal et al. [56]. A significant survival benefit was reported in both the HIPEC and the normothermic intraperitoneal chemotherapy groups, but with the latter achieving the best result [78].
These animal studies provide a proof of principle for both CRS and HIPEC. These studies do not evaluate pharmacokinetic aspects and tissue drug concentrations which are important.
The PRODIGE 7-ACCORD 15 trial (NCT00769405) has finished accrual, and the results will be available at the end of this year. Two hundred and sixty-four patients with CPM have been randomized to undergo CRS alone or CRS and HIPEC with oxaliplatin. The trial hypothesized that the addition of HIPEC to CRS should produce an overall survival benefit of 18 months over CRS alone. The secondary endpoints are recurrence-free survival, treatment toxicity, surgical morbidity, and factors influencing survival.
There are two concerns in the surgical community treating CPM.
-
1.
Should HIPEC be used in the treatment of CPM with CRS pending the results of PRODIGE 7?
Based on the above evidence, there seems to be a benefit of adding HIPEC to CRS as compared to performing CRS without HIPEC, since the reported survival in studies in which CRS and HIPEC both were used is longer. It is considered the standard of care in several countries for treating CPM with limited peritoneal spread. Hence, in the current scenario, when CRS is being performed for CPM, it should be coupled with HIPEC.
-
2.
How will the results of PRODIGE 7 influence current practice?
If the results of PRODIGE 7 favor the use of HIPEC, its role will be clearly established; however, if the result is negative, efforts will continue to determine the optimal drugs and regimens and methodology and to optimize other aspects to provide a clinical benefit of this therapeutic strategy which, in selected patients, has dramatically changed the prognosis of this disease.
At the same time, it is important to keep in mind that the role of CRS is already established and cannot be undermined even though the importance of HIPEC is reduced. Patients should continue to be treated in specialized centers in order to give them the benefit of a high quality of cytoreduction which deeply influences the prognosis of the disease in terms of disease-free and overall survival.
4.4 Systemic Chemotherapy in Addition to CRS and HIPEC: Before or After?
Both CRS + HIPEC and systemic therapy are increasingly used for the treatment of colorectal PM. Subsequently, combined treatment strategies have been introduced. However, there is a worldwide controversy on the indication, effectiveness, timing, and risks of perioperative systemic therapy as adjunct to CRS + HIPEC for PM. The rationale for using systemic therapy is the prevention of hematogenous spread as more than 50% of the patients treated with CRS and HIPEC develop extraperitoneal recurrence [79]. Several large studies have shown a benefit of adding systemic chemotherapy to CRS and HIPEC, whereas some others have not [18, 80,81,82,83]. In a study comprising of 231 patients with limited peritoneal disease treated with CRS and HIPEC at four expert French centers, patients who received early adjuvant systemic chemotherapy (within 3 months of surgery) experience a better DFS and OS compared to those who did not though this difference did not reach statistical significance [84]. The reasons for not administering adjuvant chemotherapy were a lack of evidence, delayed recovery from surgery, patient refusal, and early disease progression [84].
The use of perioperative systemic therapy in the neoadjuvant (neoadjuvant chemotherapy—NACT) or adjuvant setting has not been prospectively investigated for patients undergoing CRS + HIPEC [80]. A neoadjuvant treatment strategy in order to downstage intraperitoneal tumor load, limit extensiveness of CRS, and predict the biological behavior of the tumor may be of potential benefit in these patients. Additionally, in patients who proved to respond to neoadjuvant treatment, adjuvant systemic therapy in the same regimen may be of value by treating systemic micrometastases. In a systematic review of the role of neoadjuvant and adjuvant systemic chemotherapy as an adjunct to CRS + HIPEC, Waite et al. found seven eligible studies related to neoadjuvant chemotherapy, none of which showed strong evidence in favor of neoadjuvant systemic therapy [85].
A lack of response to NACT should not be considered an absolute contraindication to surgery, and patients with limited disease amenable to a complete cytoreduction and no extraperitoneal spread can still be treated with CRS and HIPEC with good long-term outcomes [86].
Ongoing clinical trials may provide more insight into patient selection and outcomes of neoadjuvant and adjuvant systemic chemotherapy with targeted therapy combined with CRS and HIPEC. The COMBATAC study (NCT01540344) is a phase II study that evaluates the effect as assessed by progression-free survival of perioperative systemic chemotherapy including cetuximab, combined with CRS and HIPEC in RAS wild-type colorectal PM patients.
The CAIRO 6 study (NCT02758951) is a prospective multicenter randomized parallel group study in which colorectal PM patients of non-signet histology, with PCI < 20 and in whom CC-0/CC-1 CRS seems likely, will be randomized to neoadjuvant combination chemotherapy plus bevacizumab and CRS + HIPEC followed by adjuvant combination chemotherapy (experimental arm) or CRS + HIPEC alone (control arm). The study will start as a randomized phase II study, and if the criteria of feasibility and safety are met, the study will continue as a phase III study with 3-year overall survival as primary endpoint. Clinical trials evaluating various aspects of treatment with CRS and HIPEC for CPM are listed in Table 12.3.
4.5 Long-Term Survival with CRS and HIPEC: Is There a Possibility of Cure?
Few patients undergoing CRS and HIPEC experience a prolonged DFS and OS. Goéré et al. analyzed the outcomes in 107 patients treated from 1995 to 2005 who had a follow-up of more than 5 years [87]. The median follow-up was 77 months, and the 5-year and 10-year survival rates were 35% and 15%, respectively. Patients who were disease-free for 5 years after treatment of colorectal PM or its recurrence were considered cured, and 17 patients (16%) belonged to this group; 14 of these 17 patients never developed recurrence. The analysis excluded patients who died in the perioperative period or due to other causes. Cured patients had a significantly lower median PCI than patients who were not cured, 4 (3–16) and 12 (2–36) (P = 0.0002), respectively. On multivariate analysis, a PCI of 10 or less was the only independent factor predicting cure. A similar cure rate has been reported in patients undergoing surgical resection of colorectal liver metastases [88,89,90]. Another study by the same authors confirmed these findings—the 5-year OS in patients undergoing CRS and HIPEC was not significantly different from those undergoing resection of liver metastases (36.5% and 38.5%, respectively) [91].
4.6 Role of Early Postoperative Intraperitoneal Chemotherapy (EPIC)
EPIC comprises of multiple intraperitoneal chemotherapy applications administered through drains placed during surgery. Typically, three to five instillations are performed starting on postoperative day 1. Some centers give multiple cycles of intraperitoneal chemotherapy combined with systemic chemotherapy, and this treatment continues for a few months after surgery—it is termed as sequenced intraperitoneal chemotherapy (SIPC) . 5-Fluorouracil (5-FU) alone or in combination with mitomycin C (MMC) is used. MMC 10–12 mg/m2 is administered on day 1 followed by 5-FU based on body surface area (500 mg/m2 and 800 mg/m2) or on body mass (15 mg/kg) from days 2 to 5 [92, 93]. Alternatively, only 5-FU is used for 3–5 days [94].
Most of the evidence comes from small retrospective studies that include patients with PM from various primary sites, and the role of EPIC has not been evaluated separately in those. Elias et al. performed a retrospective study of 64 patients who had PC arising from CRC; 19 (29.6%) of whom also had systemic metastases [79]. Seven patients were treated with CRS and EPIC and 27 patients with CRS and HIPEC. OS was lower in the EPIC group than in the HIPEC group, but not significantly.
They subsequently compared 23 patients undergoing CRS and HIPEC with oxaliplatin to 23 others receiving EPIC following CRS which showed similar results though the morbidity with EPIC was more [95].
Mahteme et al. compared 18 patients who underwent CRS with SIPC to historical controls with similar features treated with systemic chemotherapy alone [96]. The 2- and 5-year survivals in the SIPC group were 60 and 28%, respectively, whereas corresponding values in the control group were 10 and 5%, respectively. In all, 11 patients who were considered macroscopically tumor-free after CRS had a longer survival (34.5 months, 95% CI 28.7–75.7) than those who did not undergo CRS (10 months, 95% CI 15.7–70.0) (P = 0.02). Five patients in the CRS and SIPC group experienced long-term survival after surgery (median 8.3 years, range 6.8–9.1) [96].
In 1996, Elias et al. initiated a study comparing CRS and EPIC to CRS alone that had to be closed prematurely due to poor accrual and patient dissatisfaction in the control arm. The 2-year survival in this study was 60% in patients who underwent a complete cytoreduction compared to 10% in patients who received palliative therapy, demonstrating the benefit of a surgical intervention [97].
Glehen et al. reported results of a multi-institutional study of 506 patients who underwent CRS and HIPEC with or without EPIC from 28 institutions, in which 76% of the patients had HIPEC, 46% had EPIC, and 22% had both HIPEC and EPIC. A complete cytoreduction was obtained in 75%; HIPEC was commonly performed using mitomycin C (71%), mitomycin C and cisplatin (13%), and oxaliplatin (8%). EPIC was performed with 5-FU with or without mitomycin C (96%). With a median follow-up of 53 months, the median overall survival was 19.2 months and was 32.4 months in patients with a CC-0 resection and 34.8 months in patients with a low PCI. Moreover, no statistically significant difference was seen among patients treated with HIPEC, EPIC, or combined HIPEC/EPIC (overall survival, 19.2, 19.2, and 21.6 months, respectively) [98]. Cashin et al. performed a case-control study comparing 16 patients treated with CRS and HIPEC to 16 others treated with CRS and SIPC. The HIPEC group had a significantly better DFS and OS with a similar morbidity, and the authors recommended that it should be the preferred treatment for patients with CPM [99].
In another study comparing CRS and HIPEC with EPIC with CRS and HIPEC alone that included 69 patients with CPM, there was no difference in the two groups though the morbidity was higher in the group receiving EPIC [100].
The above evidence does not answer any of the questions pertaining to the use of EPIC.
-
Is EPIC an alternative to HIPEC?
-
Is there a role of EPIC in addition to HIPEC?
Currently the ICARuS trial (NCT01815359) which is a phase II trial is accruing patients in the United States at the Memorial Sloan Kettering Cancer Center. In this trial HIPEC with mitomycin C will be compared to EPIC with FUDR in patients with colorectal and appendiceal primary tumors following complete cytoreduction. The main caveat will be EPIC with FUDR which is not used at most centers.
Currently, several “expert” centers used EPIC in addition to HIPEC routinely; other centers don’t advocate its use.
5 Practical Concerns with CRS and HIPEC
5.1 Patient Selection for CRS and HIPEC
5.1.1 Patient-Related Factors
The two most important factors in selecting patients for the combined modality treatment are disease-specific factors (extent, histology) and the ability of the patients to withstand the procedure. Recently, there has been a lot of attention being paid to patient factors that can influence outcomes, and these need to be considered while selecting patients for CRS and HIPEC. The Eastern Cooperative Oncology Group (ECOG) performance score of 2 or less has been recommended as a cutoff in a Peritoneal Surface Oncology Group International (PSOGI) consensus statement in 2007 [101]. In one of the largest single-institution series to date, Levine et al. demonstrated that compared to patients with ECOG 0 or 1, ECOG 2 patients had a HR of 2.8, and ECOG 3 or 4 had a HR of 4.3 for a poorer overall survival following CRS and HIPEC [102]. Other studies have demonstrated similar findings and confirmed its impact in multivariate analyses [103, 104]. Diabetics are more likely to develop complications compared to nondiabetics (27.5% vs. 15.3%; P < 0.001), as shown in a retrospective series of 977 patients of which 91 were diabetic [105]. In this cohort, although the DFS of diabetics remained similar to nondiabetics, they had a significantly higher 30-day (8.8% vs. 2.7%; P = 0.007) and 90-day mortality rates (13.2% vs. 5.2%; P = 0.008). Similarly, age > 70 seems to have a higher 30-day (13.6% vs. 3.9%; P < 0.001) and 90-day (27.4% vs. 10.2%; P < 0.001) mortality rates, although these outcomes seem to improve with increasing surgical experience of a well-established program [105]. It is estimated that up to one-third of the patients with advanced colorectal cancer are malnourished [106]. Several methods of assessment of nutritional status have been used like the Subjective Global Assessment (SGA) scale , presence or absence of sarcopenia as assessed on CT scan, and preoperative serum albumin levels. Malnourished patients as assessed by SGA had longer hospital stay and poorer survival [107]. Sarcopenic patients had a significantly higher rate of reoperation (25.6% vs. 12.1%; P = 0.012) and higher complication rates (OR 0.93; P = 0.018) compared to non-sarcopenic patients in a retrospective study of 206 patients by Vugt et al. [107]. Valle et al. showed that a serum albumin level of <35 gm/dl was associated with a significantly higher rate of complications and enterocutaneous fistulas [108]. The presence of ascites appears to be a poor prognostic factor for most disease types treated with CRS and HIPEC. In one series of 1000 patients, the 229 patients who had malignant ascites significantly reduced the possibility of a CC-0/CC-1 resection (15% vs. 59%; P < 0.001) and were predictive of a worse overall survival [109].
5.1.2 CRS and HIPEC in the Elderly
Conventionally, age > 70 years has been considered a relative contraindication for performing CRS and HIPEC. Elderly patients have a reduced physical capacity to recover from surgery and other medical comorbidities [110, 111]. However, based on the favorable outcomes in elderly patients undergoing major oncologic procedures, experienced centers have used this treatment for selected patients over the age of 70 [112, 113]. Passot et al. reported outcomes in 188 patients over the age of 70 years undergoing CRS and HIPEC for various indications and reported a higher rate of “failure to rescue” in older patients leading to a higher mortality from surgical complications. The overall morbidity in both groups was similar. A PCI > 12 was an independent predictor of increased morbidity [114]. Another study of 85 patients over the age of 75 reported a similar morbidity and mortality compared to younger patients in carefully selected patients [115]. Selected patients over the age of 70 years with a good performance status and limited disease spread can be taken up for CRS and HIPEC in experienced centers where treatment is carried out by multidisciplinary teams.
5.1.3 Disease-Specific Factors
A consensus statement from representatives from the major peritoneal surface malignancy centers from around the world listed eight clinical and radiographic variables associated with increased chances of achieving a complete cytoreduction: [116]
-
Eastern Cooperative Oncology Group (ECOG) performance status 1 or less
-
No evidence of extra-abdominal disease
-
Up to three small, resectable parenchymal hepatic metastasis
-
No evidence of biliary obstruction
-
No evidence of ureteral obstruction
-
No evidence of intestinal obstruction at more than one site; small bowel involvement
-
No evidence of gross disease in the mesentery with several segmental sites of partial obstruction
-
Small-volume disease in the gastro-hepatic ligament
However, there are certain other factors that need to be considered.
5.1.4 Sugarbaker’s Peritoneal Cancer Index (PCI)
Though CRS and HIPEC can produce long-term survival reaching up to 50% at 5 years, this is only possible in selected patients. One of the two most important prognostic factors is the PCI. Elias et al. in a retrospective study of 180 patients demonstrated that there was no benefit of CRS and HIPEC in patients with a PCI of >17 even if complete cytoreduction could be obtained. The survival was similar to patients with palliative debulking in patients with a higher PCI [117]. This may not be an absolute contraindication as some selected patients with a higher PCI may still benefit from CRS and HIPEC especially patients with mucinous tumors. In another multicentric retrospective French study comprising of 523 patients, the 5-year survival of patients with a PCI of >20 was 10%. The authors considered a PCI of >20 with other poor prognostic factors like poor performance status, lymph node involvement, and poor response to chemotherapy as absolute contraindications for CRS and HIPEC [62].
Sugarbaker et al. in their study of 380 patients performed a receiver operating characteristic (ROC) curve analysis and identified PCI >12 as a predictive marker for disease recurrence with 100% specificity [64]. Similar findings were reported by a Norwegian study of 47 patients [63]. Another study of 72 patients found no benefit of CRS and HIPEC for a PCI of >16 [118]. CRS and HIPEC is not recommended for patients with a PCI of >17–20. A combination of imaging studies and diagnostic laparoscopy should be employed to select patients with limited disease extent and avoid unnecessary laparotomy in patients with more extensive disease. Long-term survival is possible only in patients with a PCI of <10.
5.1.5 Completeness of Cytoreduction
The second most important predictor of survival is the completeness of tumor removal. The commonly used score for this is the completeness of cytoreduction score (CCR) as defined by Sugarbaker. Only patients in whom a complete cytoreduction (CC-0/CC-1) is deemed possible should be taken up for surgery. The survival in patients having a CC-2/3 resection is similar to those receiving systemic therapy alone, and such procedures should not be undertaken [119]. In a large study, analysis of outcomes in 506 patients treated with CRS and HIPEC found completeness of cytoreduction to be the strongest predictor of survival on multivariate analysis (P < 0.0001) [98]. In the PRODIGE 7 trial, only patients who have residual disease <1 mm were considered to have a complete cytoreduction and subject to randomization.
5.1.6 The Peritoneal Surface Disease Severity Score (PSDSS)
The peritoneal surface disease severity score has been suggested as a method of preoperative prognostication of outcomes following CRS and HIPEC. The PSDSS incorporates clinical symptom severity, extent of disease as peritoneal cancer index (PCI) calculated on CT scan or laparoscopy, and primary tumor histology [120]. This score was validated by a study evaluating 1013 patients with PM and showed that PSDSS was capable of defining populations with a high or considerably lower likelihood of long-term survival after CRS/HIPEC [121]. However, other studies have not shown additional benefit of the PSDSS over PCI [122]. PCI continues to be used as the preferred tool in clinical practice and in clinical trials as well.
5.1.7 Response to Chemotherapy
Response to neoadjuvant chemotherapy (NACT) is predictive of a more favorable prognosis. In a study by Passot et al. of colorectal cancer patients receiving NACT prior to CRS and HIPEC, patients who had a complete or major response to chemotherapy had a significant improvement in survival compared to those who had a minor or no response (P = 0.0019). These survival differences were determined by an assessment of histopathologic specimens removed at the time of CRS and HIPEC. They concluded that histopathologic response to NACT was a new prognostic tool for the management of peritoneal metastases from colorectal cancer [123]. Paul Sugarbaker proposes a differential approach to patients depending on the response to chemotherapy [124]. Ten percent of the patients are expected to have a complete response, and in these patients, HIPEC may not add to the survival benefit that has been obtained with chemotherapy alone. CRS may be performed for staging purposes with a thorough exploration and generous biopsies. In situations where the surgeon is confident, the same can be performed laparoscopically. Seventy percent of the patients receiving neoadjuvant systemic chemotherapy have a minor response or no response. Though the probability of benefit of CRS and HIPEC is less in these patients, they should still undergo the procedure provided there is no extraperitoneal spread and a complete cytoreduction can be obtained [86]. If these patients received FOFOX as neoadjuvant therapy, then the drug for HIPEC should be mitomycin C with or without adriamycin instead of oxaliplatin. In those 20% of patients with a major response that falls short of complete response, CRS and HIPEC should be performed preferably using the same drug that was used for NACT [124].
5.1.8 Other Prognostic Factors
Several other factors have an impact on the outcomes of patients undergoing CRS and HIPEC.
Peritoneal lavage cytology to detect free intraperitoneal cancer cells is accepted as part of staging for ovarian epithelial cancers and has prognostic significance in gastric cancer [125,126,127,128]. In a large multicenter prospective study, EVOCAPE 2, peritoneal cytology was found to lack prognostic significance and furthermore did not predict for future development of PM in these same patients, including colorectal cancers [129]. However, it may have a role in predicting the risk of development of PM. There are currently two systematic reviews of intraoperative peritoneal lavage in colorectal cancer to determine risk of development of PM [130, 131]. Mohan et al. evaluated 18 studies (3197 patients) which evaluated the presence of free tumor cells and/or tumor-associated antigens (CEA, Ras, Ca 19-9) in peritoneal lavage, while Bosanquet et al. evaluated 12 studies (2580 patients) which used positive peritoneal lavage cytology, immunohistochemistry, or PCR [130, 131]. In both these studies, a positive peritoneal lavage portended a negative impact on prognosis and risk of peritoneal metastases. However, both reviews were limited by the heterogeneity of method of analysis of peritoneal lavage and therefore cannot be recommended for routine use in staging strategies.
Serum tumor markers are routinely used for surveillance in colorectal cancer patients. Pita-Fernandez et al. showed in a meta-analysis of 11 randomized studies comparing intensive follow-up compared to less intensive or no follow-up showed increased detection of asymptomatic recurrences and improved overall survival [132]. Serum carcinoembryonic antigen (CEA) is a widely used biomarker used for surveillance with sensitivity for relapse ranging from 41 to 97% [133]. Although not commonly used in the surveillance of colorectal cancer, serum Ca 19-9 has a higher specificity compared to serum CEA for peritoneal metastases [134]. Moreover, in a study of 105 patients, although CT scan was the most sensitive investigation to detect PM, about 27% of the patients had elevation of the CEA and/or Ca 19-9 as their earliest indicator of disease recurrence. In a study of over 870 Chinese patients, elevated CEA and CA 19-9 levels were risk factors for peritoneal metastases [135]. Thus, serum tumor markers can be used in surveillance for detection of PM, in particular early recurrence when imaging may be nondiagnostic.
Tumor histology seems to play an important role in outcomes. Although adenocarcinoma is the commonest histological subtype, mucinous adenocarcinoma and signet ring cell adenocarcinoma subtypes have more frequent peritoneal involvement [136]. The outcomes seem to be better for mucinous adenocarcinoma than for the other types. In a retrospective analysis of the Netherlands Cancer Registry, PM of mucinous adenocarcinoma had a median survival of 10.9 months vs. 7.4 months for adenocarcinoma vs. 6.6 months for signet ring histology (P < 0.0001) [137]. Multiple retrospective analyses have shown that the overall survival in signet ring histology PM patients undergoing CRS/HIPEC is dramatically worse than other subtypes, with median survival ranging 12–14 months and 5-year survival rates of 0–7% [138, 139]. In fact, in both the PSDSS and the colorectal peritoneal metastases prognostic surgical score (COMPASS) , signet ring cell histology has been given special consideration signifying poorer outcomes [120, 140].
5.2 Surgical Strategies for Obtaining a Complete Cytoreduction
Cytoreductive surgery attempts to remove all macroscopic disease using a combination of peritonectomy procedures and visceral resections which have been described by Sugarbaker [141]. When tumor involves visceral peritoneal surfaces, organ resections (splenectomy, large bowel or small bowel resection) are needed. When tumor involves parietal peritoneal surfaces, one of the five peritonectomies or stripping of the peritoneum is required [142]. One of the major limiting factors in obtaining a complete cytoreduction is the extent of small bowel involvement as resection of large portion has nutritional consequences [142].
5.2.1 Synchronous Resection of CPM and Liver Metastases
The presence of simultaneous liver and peritoneal metastases has been considered a contraindication for aggressive treatment at either of the disease sites [98, 143,144,145]. However, with reports of better results with liver resection done even in the presence of extrahepatic disease, including PM, these contraindications have become less absolute [145]. In a study by Kianmanesh et al., 43 patients had management of PM and liver metastases, 3 with liver resection prior to CRS/HIPEC, 10 done concurrently with CRS/HIPEC, and 2 done 2 months following CRS/HIPEC [146]. The survival of patients in the CRS + HIPEC and liver resection group was similar to the CRS + HIPEC alone group (median survival 36.0 vs. 35.3 months; P = 0.73). Three other studies, with sample size ranging from 14 to 37 patients, have addressed the outcomes of patients undergoing CRS + HIPEC with synchronous liver resection, in highly selected patient cohorts [147,148,149]. Elias et al. selected 24 young patients with good performance status, mild to moderate PM, moderate operative risk (no invasion of hilum, vena cava, hepatic veins, extensive PM), and responding or stable liver metastases after 3 months of systemic chemotherapy. At a median follow-up of 6.1 years, the only prognostic factor significant for recurrence was number of liver metastases >3 [147]. Maggiori et al. compared 37 patients with synchronous resection of liver metastases and PM with 61 patients with PM alone. CRS + HIPEC with liver resection fared worse in terms of overall survival compared to CRS + HIPEC alone (40% vs. 66%; P = 0.04). Moreover, patients with PCI < 12 and no liver metastases had a median OS of 76 months compared to PCI < 12 and 1–2 liver metastases (40 months) and PCI >12 or >3 liver metastases (27 months) [149]. Based on these studies, it appears that performing concurrent liver resection and CRS for more than three liver metastases does not confer a significant OS benefit and should be avoided. Elias and collaborators in a study of 287 patients with LM or PM or both found no difference in survival in the three groups of patients treated with liver resection, CRS and HIPEC, or both (Fig. 12.1) [150]. Based on this study, they developed a graphic nomogram that is simple to calculate and easy to use and can determine the prognosis of patients according to the number of LM, the PCI, or both. This nomogram needs to be validated in prospective studies.
Overall survival in patients with colorectal LM, PM, or both (From Ref. [151] with permission)
5.2.2 Resection of Ovaries
Synchronous ovarian metastases (OM) are reported in 1–9% of women undergoing surgical resection of a primary CRC, and metachronous OM occur in 1–7% [151, 152]. In patients with CPM, more than half of the women have OM diagnosed either before or synchronously shown in a study of 194 patients by Verwaal et al. [153]. These investigators recommended that a bilateral oophorectomy should be performed for all patients undergoing CRS and HIPEC. Patients with OM and PM have a similar OS and DFS when treated with CRS and HIPEC [154]. Women undergoing this treatment may not have completed their families and may be desirous of a future pregnancy. Of interest is the fact that in colorectal cancer, stromal involvement as opposed to capsular involvement is seen in majority of the patients as indicative to hematogenous spread [155]. Elias et al. evaluated the feasibility of ovarian preservation in 106 women aged less than 41 years undergoing CRS and HIPEC for PM [154]. Oophorectomy was done (1) when the ovary was macroscopically involved with tumor; (2) in case of clinical suspicion for tumor involvement based on intraoperative macroscopic inspection (presence of superficial tiny granulations or cysts); (3) systematically (contralateral oophorectomy) in patients who had previous unilateral oophorectomy at the time of initial surgery due to macroscopic involvement of one ovary, while the other macroscopically normal-appearing ovary was left in place; (4) when hysterectomy was needed due to tumor extent; and (5) in women who clearly did not want future pregnancy. Based on their findings, they recommend that a bilateral oophorectomy should be performed in all women who have suspicious involvement of both ovaries, when a hysterectomy is needed, and in women who do not wish to have anymore children. In women who have metastases in one ovary, the risk of contralateral ovarian metastases is 46% and a bilateral oophorectomy is recommended in these women as well. In women with grossly normal ovaries, the risk of occult metastases is 17% and the risk of future metastases to the ovary is over 50%. They recommend conservation of ovaries in some of these patients though pregnancy following CRS and HIPEC in patients with CPM has not been reported in the literature [154, 156].
5.2.3 Urological Procedures
Some patients with limited disease may require a resection of the kidneys, ureters, or bladder like nephrectomy, partial cystectomy, and resection of a segment of the ureter, to attain a complete cytoreduction [157]. If complete cytoreduction can be obtained, these procedures show a survival similar to other patients with limited CPM. In this setting, resection of the ureters is very common and is never itself a contraindication to CRS; similarly, a nephrectomy is performed when required for a complete cytoreduction. However, a total cystectomy though technically feasible could be considered unethical as it is unlikely to offer any oncological benefit. At the senior author’s institution, the procedure is never performed in the context of CRS/HIPEC. One small study reported increased morbidity with such procedures—the incidence of bowel fistulas and intra-abdominal abscesses was reported to be significantly higher though it was attributed to the extent of bowel resection rather than the urological procedure itself. Several other studies have reported no increase in morbidity [157,158,159].
5.3 HIPEC Methodology and Drugs
Several different HIPEC techniques have been elaborated for application in colorectal PM. Several drugs have been successfully used singly or in combination, at different concentrations, in different perfusates, for different durations, and at different effective temperatures [160]. Each modification of one of these parameters implies conducting a new pharmacokinetic study, which is not feasible. While this topic has been dealt with elsewhere, broadly the commonly used techniques are open and closed techniques. In an experimental study which compared the open to the closed technique, using intraperitoneal oxaliplatin at a temperature of 42°C, the systemic absorption and tissue concentration of oxaliplatin were higher by the open method. The closed method produced higher temperatures in the diaphragmatic cupolas, whereas the open technique performed better in other areas. Effective intraperitoneal hyperthermia could be achieved with both techniques, but systemic absorption and accumulation in the abdominal cavity were higher with the open technique [161]. There is no reported difference in the perioperative and survival outcomes between the two techniques [162]. In effect, it is very important to obtain a high and homogeneous temperature throughout the abdominal cavity, to routinely perform the same technique, which would render homogenous data for validation and analysis, as no prospective comparison of open and closed techniques of HIPEC in terms of survival, morbidity, or pharmacokinetics has ever been reported [163].
There are two commonly used regimens for HIPEC for colorectal PM: the first using mitomycin C (MCC) over 60 to 90 min at 41–43°C with a closed or open technique and the other using oxaliplatin (460 mg/m2 of oxaliplatin in 2 L/m2 of isoosmotic 5% dextrose) over 30 min, at a homogeneous temperature of 43°C (range, 42–44°C) with an open technique [164]. A bidirectional (intraperitoneal + systemic) intraoperative chemotherapy which combines intraperitoneal oxaliplatin preceded by an intravenous infusion of 5-FU (400 mg/m2) with leucovorin (20 mg/m2) is now mostly used for PM from CRC in Europe [164]. Current evidence does not show that one is superior to the other though there is a trend favoring the use of oxaliplatin. The various regimens in use of CPM are listed in Table 12.4. MMC has been used due to its high molecular weight, tissue penetration up to 5 mm, and a favorable pharmacokinetic profile that permits increased intraperitoneal concentration with limited systemic toxicity [165]. Oxaliplatin has a higher response rate when used intravenously in the metastatic setting as compared to MMC. Elias et al. have shown the efficacy and safety of intraperitoneal oxaliplatin in pharmacological and clinical studies [166]. Its efficacy and tolerance have been demonstrated in another phase I study from the United States [167]. Three retrospective studies have tried to compare outcomes of mitomycin vs. oxaliplatin HIPEC [168,169,170]. The Dutch study by Homes et al. included 95 patients from two centers and did not show any difference in the survival outcomes between the two regimens [168]. In another retrospective multicentric study, 539 patients were included with stratification as per the PSDSS and survival results analyzed. For favorable histologies and low-burden patients (PSDSS I/II), the outcomes seemed to be better with mitomycin C with a median OS of 54.3 months with mitomycin C and 28.2 months for oxaliplatin [169]. However, the retrospective nature of this study and the non-standardized dose of oxaliplatin preclude definite conclusions from this study. Another Australian study of 201 patients showed a survival benefit of performing HIPEC with oxaliplatin as compared to MMC [170]. Currently, three randomized controlled trials— PRODIGE 7, PHOPHYLOCHIP, and COLOPEC—are using oxaliplatin-based HIPEC in the experimental arm. There is a high incidence of hemorrhagic complications when oxaliplatin is used, and its cautious use in patients with a high PCI is recommended [171]. In a preliminary analysis of the PRODIGE 7 trial that has completed accrual, the 30-day grades 3–5 morbidity was similar in the both the arms, whereas the 60-day grades 3–5 morbidity was higher in the HIPEC arm (unpublished data). HIPEC was performed using oxaliplatin.
6 Morbidity and Mortality
With an improvement in the patient selection, surgical techniques, perioperative management, and growing experience of certain “high-volume” or “expert centers,” there has been a considerable reduction in the morbidity and mortality from this procedure, and it is similar to that of other major gastrointestinal surgeries. Reported morbidity and mortality rates range from 23 to 45% and 0 to 12%, respectively [147, 176, 177,178,179]. The surgical complications include anastomotic leakage, bleeding, and wound infection, and chemotherapy-related complications include neutropenia, cardiac arrhythmia, or renal insufficiency. Other complications common to surgical procedures in general include thrombosis, lung embolism, or pneumonia [180]. A learning curve exists for CRS plus HIPEC, and it’s both the surgeon’s and the institutional experience that has an impact on the morbidity and mortality [181, 182].
Several factors have been associated with an increased risk of complications. They include the duration of surgery, the age, the number of visceral resections, the need for a stoma, an increasing dose of chemotherapeutic agent, and recurrent cancer [178, 182,183,184,185,186]. The most widely accepted factor prognostic of morbidity and mortality is the extent of the peritoneal disease measured by PCI, with an increased risk of grade 4 morbidity (life-threatening complications) when the PCI is greater than 12 [184, 187, 188]. One study found an extensive disease involvement in the left hemidiaphragm to be the only significant predictor of severe morbidity on multivariate analysis, probably because this procedure results in respiratory complications, and in a higher risk of pancreatic leak, bleeding, and intra-abdominal abscess, due to the dissection of the hilum of the spleen [188]. There is a high incidence of hemorrhagic complications when oxaliplatin is used and its cautious use in patients with a high PCI is recommended [171]. In an interim analysis of the PRODIGE 7, male sex, transverse colon primary tumors, ureteral anastomosis, two or more bowel anastomosis, and two or more sites of bowel suturing were associated with a greater 30-day grades 3–5 morbidity. This analysis which is at present under review for publication highlighted two important points. Firstly, the rate of gastrointestinal fistulas was higher in the HIPEC group as compared to the non-HIPEC group. Though this difference did not reach statistical significance, fistulas occurred even in the presence of a diverting ostomy. The presence of a stoma did not prevent fistulas but reduced the incidence of peritonitis. Hence, the authors recommend that a protective ostomy should be performed in case of more than two areas of intestinal stiches, of more than two bowel anastomoses, or in case of rectal resection (unpublished data). Secondly, adding HIPEC with oxaliplatin to CRS did not significantly increase the overall rate of postoperative complications, and at 30 days it resulted in a mortality rate similar to that of CRS alone. The dose of oxaliplatin used in this study was 460 mg/m2 for the open procedure and adapted to 360 mg/m2 for the closed procedure. However, it increased significantly the rate of grades 3–4 complications at 60 days. However, the authors suggest that this should be interpreted with caution since the actual number of patients experiencing such complications was small and other studies have not reported similar findings. In this study, the grades 3–4 hematological toxicity was higher in the HIPEC arm. It was not of and consequence as most of these patients were managed without any clinical consequences. Passot et al. have suggested that a better indicator of the quality of surgery is “failure to rescue” rather than the morbidity [189]. In experienced centers, patients with complications are managed better, leading to a reduction in the mortality from the procedure.
7 Reiterative Procedures
Though CRS and HIPEC are performed with the intent of cure, around 70–80% of the patients will develop recurrent disease, and about half of these recurrences are confined to the peritoneal cavity [190,191,192,193].
Over the years, evidence has accumulated showing the feasibility and survival benefit of a repeat CRS and HIPEC in selected patients [194, 195]. Bijelic et al. reported recurrent disease in 49 out of 70 patients with complete cytoreduction and perioperative intraperitoneal chemotherapy [193]. The median survival of patients who underwent a second surgery was significantly longer than that of patients who did not have a second operation (39 vs. 20 months; P = 0.0003). Diffuse peritoneal recurrence, isolated distant metastases, and diagnosis of recurrence within 6 months after CRS were associated with a worse prognosis. Median survival in complete secondary CRS was 42 months as compared to 30 months for the whole cohort [193]. In another small study by de Simone et al., the survival after the second procedure was similar to that after the procedure. However, patients with PM from other primary sites were included in this study [196]. The morbidity and mortality of such procedures are similar to that of the first procedure in high-volume centers [197]. In the largest multi-institutional study from 11 institutions across the world comprising of 189 patients, the reported median survival was 26.4 months, disease-free survival 10.1 months, and 5-year overall survival 20% following a repeat CRS and HIPEC [198]. The median PCI was 6.9 and 81% of the patients had a complete cytoreduction. A PCI of <10 during the second procedure, a complete cytoreduction, and absence of grades 3–5 morbidity were associated with a favorable prognosis. Patients who had positive nodes during the first procedure had poorer outcomes. Though this study had limitations like the lack of a control group, and its retrospective nature, it showed that long-term survival is possible with a repeat procedure in selected patients.
8 New Treatment Strategies for Patients with Extensive CPM
There are still a large proportion of patients with CPM that are not candidates for CRS and HIPEC. Systemic chemotherapy leads to a favorable response only in a small percentage of patients.
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is an innovative intraperitoneal chemotherapy concept that seems to enhance the effectivity of IPC by taking advantage of the physical properties of gas and pressure [199]. PIPAC pharmacokinetics permit the use of a minimal drug dose which reaches a higher intraperitoneal concentration than in HIPEC. Increased intra-abdominal pressure is known to increase tissue uptake and intra-tumoral drug concentration [200, 201]. In addition, there is micronization of the cytostatic agent which creates a thin film of microdroplets over the entire peritoneal cavity, increasing the contact surface area between drugs and tissues. It is given in multiple sittings usually at three-weekly intervals through a laparoscopic approach. Systemic chemotherapy is used with it. The reported toxicity profile is acceptable in preliminary studies [202]. In patients pretreated with surgery and multiple lines of chemotherapy, it has produced symptom control, clinical response, and a prolongation of survival [203]. Prospective studies are needed to further define and expand its role [203]. A new bidirectional chemotherapy (neoadjuvant intraperitoneal-systemic chemotherapy protocol (NIPS) ) was developed by Yonemura and his collaborators from Japan to induce a reduction of the peritoneal cancer index of patients with gastric PM [204]. NIPS can attack PM from both sides of peritoneum, not only from the peritoneal cavity but also from the subperitoneal blood vessels, and is considered a bidirectional chemotherapy [204]. Following a response to NIPS, selected patients become candidates for CRS and HIPEC. This treatment which has produced response rates of over 70% in patients with gastric PM is being investigated by Francois Quenet from Montpellier for CPM in the NIPOX trial (Fig. 12.2) [205]. In a pilot study, six patients with unresectable peritoneal disease of colorectal origin were included in the study. An intraperitoneal implantable chamber catheter was inserted during the laparotomy that evaluated the extent of the peritoneal disease (peritoneal cancer index 25 to 39). Patients then underwent intraperitoneal chemotherapy with oxaliplatin 85 mg/m2 in combination with systemic chemotherapy ( FOLFIRI or simplified LV/5-FU) and a targeted therapy every 2 weeks. Two patients completed the four intraperitoneal (IP) chemotherapy cycles without major toxicity. Two catheter perfusion incidents were reported due to the abdominal wall thickness. For one patient with aggressive disease, best supportive care was initiated after the first course of chemotherapy. The tolerance was acceptable for 85 mg/m2 IP oxaliplatin combined with systemic therapy in these patients. This study formed the basis for the NIPOX trial [206].
Patients with a PCI of >17 are given a combination of systemic chemotherapy and intraperitoneal chemotherapy through two intraperitoneal catheters with implantable chambers. Responders are subsequently evaluated for CRS and HIPEC. Simultaneously, a dose escalation study for intraperitoneal oxaliplatin is being performed. Along similar lines is the IPOXA trial (NCT02866903), which is a phase I/II trial studying the administration of IP oxaliplatin (normothermic port-directed) with systemic FOLFIRI and bevacizumab in CPM of uncertain respectability. Currently, this trial is looking at morbidity, dose-limiting toxicity, and overall response rates of this treatment strategy.
9 Preventive Strategies for CPM
Majority of the patients with PM eventually succumb to the disease. The most appropriate treatment strategy would be to prevent the occurrence of PM.
9.1 The Cautious and Proactive Surgeon
While surgical teams across the world have been focusing on developing the skill to perform CRS and setting up HIPEC centers, how the primary tumor is dealt with has become equally important once again, as improper surgical handling can lead to peritoneal dissemination even in patients without high-risk features (described below) for peritoneal spread [207]. Cancer spread following resection of the primary can occur in the following ways—through portal dissemination, lymph nodal recurrence, recurrence at the operative site, and peritoneal spread [207]. Whereas portal dissemination cannot be prevented, other recurrences could be minimized by proper surgical technique. Patients with colorectal have decreased local recurrence and a longer disease-free survival when the resection of the primary tumor is performed by experienced surgeons at high-volume centers [208]. Hermanek et al. reported a variation in local recurrence from 5 to 55% between different surgeons which resulted in a 5-year survival rate varying from 34 to 85% [209].
Turnbull in his publication on No-Touch Isolation Techniques described a mechanism of cancer dissemination that is no longer acceptable [210]. However, the results of using this technique were far superior to any others published in the same time period [210].
Sugarbaker suggested that proper surgical technique can prevent peritoneal dissemination to a certain extent. In order to limit peritoneal spread, “containment” should be one of the main goals of the gastrointestinal cancer surgeon. He described a technique called “centripetal surgery” in which one must move around the tumor mass with perfect hemostasis, adequate margins of dissection, and sufficient visualization so that the vital structures are not damaged. If all of these requirements are not met, the surgeon must approach the malignant disease from another anatomic site [211, 212].
The other important aspect of prevention is surgical handling of patients with positive peritoneal fluid cytology or with peritoneal nodules at presentation. Non-definitive procedures except those needed in the emergency setting, i.e., for perforated or obstructed tumors, should be avoided. Sugarbaker pointed out that the peritoneum itself acts as a first line of defense against carcinomatosis, and in its absence, cells become implanted wherever a raw surface is created [213]. Non-definitive surgery in these situations has some adverse consequences. These patients become poor candidates for subsequent curative approach using CRS and HIPEC, the lymph nodal clearance becomes more difficult, and there is tumor cell entrapment in avascular scar tissue which cannot be treated with chemotherapy. Retroperitoneal implantation of tumor cells can involve tubular structures like the ureters leading to obstruction. When such a situation is encountered during laparotomy or laparoscopy, further surgical intervention should stop and the patients should be referred to a center experience in treating peritoneal metastases [213].
Laparoscopic surgery minimizes surgical trauma and, compared with open surgery, has been associated with less peritoneal as well as metastatic tumor growth in several animal models [214]. The technique has raised concerns regarding the potential effect of a CO2 pneumoperitoneum on peritoneal cancer spread [215]. However, large clinical trials comparing open surgery with laparoscopic colectomy for colorectal cancer did not identify an increased risk of peritoneal recurrence associated with the laparoscopic approach [216]. The minimally invasive approach requires considerable amount of skill and should not be performed at the risk of compromising other oncological requirements like adequate margins, lymph node yield, and avoiding intraoperative tumor rupture and spill.
One of the most important prognostic factors determining the treatment outcomes in patients with PM is the disease extent determined by the peritoneal cancer index (PCI). In general, patients with less extensive disease have better outcomes, and one of the first treatment goals is to detect PM early in the course of disease evolution. In a study evaluating the Swedish registry data, which analyzed 11,124 patients with CRC treated between 1995 and 2007, PM was diagnosed in 8.3%, the prevalence of synchronous PM being 4.3%, and that of metachronous PM was 4.2%, with median time to recurrence around 14–16 months [217]. The known risk factors for peritoneal spread in patients with colorectal cancer are female sex; patients with primary mucinous adenocarcinomas; tumor stage T4; lymph node stage N2; a colonic primary, emergency surgery; and patients with positive resection margins [217, 218]. At the time of treatment of the primary malignancy, imaging modalities may fail to pick up low-volume disease, and during an open or laparoscopic resection, PM should be searched for and the extent documented in detail, especially in patients with known risk factors for peritoneal dissemination. For patients on surveillance also, the index of suspicion should be high. An elevation in tumor markers without evidence of disease on imaging should prompt the use of diagnostic laparoscopy for detection of early peritoneal cancer spread.
9.2 Role of HIPEC Is Prevention and Early Treatment of Peritoneal Metastases
One of the main concerns regarding the management of colorectal PM is that the disease is detected when the PM are extensive and patients are not eligible for a curative approach [218]. As the extent of the disease (PCI) and the completeness of resection are the main prognostic factors determining survival outcomes, it is obvious that survival results are dramatically better in patient detected with a low PCI [219].
Elias et al. performed a seminal study that has formed a basis of two randomized trials. They devised a strategy of systematic second-look surgery in patients at high risk of developing PM. In a systematic review of the literature comprising 16 studies evaluating 4395 patients, the same authors concluded that the only three factors that were consistently associated with a high risk of developing PM were synchronous PM completely resected, isolated ovarian metastases, and perforated primary tumor [14]. Based on this, they devised a new strategy which evaluated the role of systematic second-look surgery in 41 patients without clinical, radiological, or biological evidence of recurrence [220]. Patients considered to have high risk of developing PM were based on the three criteria mentioned above, present at the time of surgery for the primary tumor: resected minimal synchronous macroscopic PM (n = 25), synchronous ovarian metastases (n = 8), and perforation of the colon (n = 8). PM was discovered and resected in 23 (55%) patients during the second-look surgery, in spite of normal investigations. The mean PCI was low (8 ± 6) and peritoneal deposits were resectable in all of the patients. Grades 3–4 morbidity rate was low (9.7%). After a median follow-up of 30 months, OS and DFS of all patients at 5 years were 90% and 44%, respectively. Peritoneal recurrences occurred in seven patients (17%), six of whom had macroscopic PM discovered during the second-look surgery (26%). Based on these encouraging results, the phase III randomized study ProphyloCHIP (NCT01226394) was initiated. In this trial, patients who are at high risk of peritoneal recurrence and are clinically disease-free after completing 6 months of adjuvant therapy are randomized to a standard follow-up comprising of a 3 monthly follow-up for 2 years and then a 6 monthly follow-up for 3 years or a systematic second-look surgery followed by HIPEC with oxaliplatin. Another similar study sponsored by the NCI was underway in the United States (NCT01095523) [221], in which patients with CRC at high risk of developing PM who underwent curative surgery and subsequently received standard of care adjuvant chemotherapy were randomized to routine surveillance or second-look surgery and HIPEC 1 year after the primary surgery. This study, however, was abandoned before recruitment was complete.
In a case-control study carried out by Sammartino et al. in patients with advanced (T3/T4, any N, M0) colonic cancer of mucinous or signet ring cell histology, or perforated primary tumor of any histology without PM or other metastases, patients were either treated with standard colectomy (n = 50) or with additional surgical procedures apart from a colectomy that included omentectomy, bilateral salpingo-oophorectomy, resection of the hepatic round ligament, appendectomy, and HIPEC with oxaliplatin (n = 25) at the time of diagnosis [222]. The study group comprised of 25 patients with mucinous or signet ring cell histology T3/T4, any N, and M0 colonic cancer who underwent hemicolectomy, omentectomy, bilateral salpingo-oophorectomy, hepatic round ligament resection, and appendectomy followed by HIPEC with oxaliplatin during the resection of the primary, while the control group of 50 patients was treated by standard surgical resection during the same time period. There was no increase in the morbidity due to the additional surgical procedures performed in the experimental group; however, the recurrence rate was significantly lower than that in the control group (4% versus 22%; P < 0.05). The OS was similar in both groups, but the DFS was significantly longer in the experimental group (36.8 versus 21.9 months; P < 0.01). Thus, this aggressive preventive surgical approach increased the disease-free survival significantly without increasing the morbidity. Based on these results, the PROMENADE trial (NCT02974556) has been initiated, which aims to determine the oncological effectiveness, compared to standard surgical treatment, of proactive management including target organ resection (omentectomy, bilateral adnexectomy, appendectomy, hepatic round ligament resection) and preventive HIPEC (intraperitoneal oxaliplatin with concomitant i.v. 5-fluorouracil/leucovorin) during a curative resection of high-risk (>/=5 mm tumor invasion beyond the muscularis propria) T3 and T4 colon cancer in preventing the development of peritoneal metastases. The primary outcome measure is incidence of PM at 36 months. Along the same lines, a Dutch study named the COLOPEC trial (NCT02231086) is a phase III randomized trial that aims to determine the oncological effectiveness of adjuvant HIPEC, using intraperitoneal oxaliplatin with concomitant i.v. 5-FU/LV, following a curative resection of a T4 or intra-abdominally perforated colon cancer in preventing the development of PM in addition to the standard adjuvant systemic treatment. However, in this trial the adjuvant HIPEC is given without any target organ resection, and the primary outcome measure is peritoneal recurrence-free survival at 18 months. Along the same lines, a multicentric phase III study is ongoing in China (NCT02179489) that will evaluate the disease-free survival of 300 patients at high risk of developing PM after HIPEC with mitomycin C after primary surgery (without target organ resection). Another study, the APEC trial (NCT02965248), is a phase II randomized study that plans to randomize 147 patients with colon cancer having T4NanyM0 or T3NanyM0 mucinous or signet ring adenocarcinoma undergoing an R0 resection into three arms, viz., (1) standard adjuvant chemotherapy only (control group), (2) HIPEC with raltitrexed (3 mg/m2) intraperitoneally for 60 min during surgery or within 10 days after the operation, or (3) HIPEC comprising oxaliplatin (130 mg/m2) during surgery for 30 min. The primary outcome measure is the incidence of PM at 3 years.
The above studies highlight the fact that the best way to deal with PM is to treat it early or prevent it and can potentially form the basis of future treatment for PM from CRC.
Conclusion
PM from colorectal cancer represents a subgroup of patients who are often diagnosed with advanced disease and have poor outcomes in spite of advances in modern chemotherapy and targeted therapy. The combined modality treatment of CRS and HIPEC offers a promising strategy especially if offered when the extent of peritoneal involvement is limited. Adequate patient selection is paramount in ensuring good results. Future strategies for prevention or early treatment of PM seem promising but require validation in the ongoing randomized trials. At the same time, new therapies for patients with extensive disease that is not amenable to aggressive therapy need to be further developed.
References
Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, De Hingh IH. Predictors and survival of synchronous peritoneal carcinomatoses of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–25.
Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–50.
Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–63.
Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705.
Shepherd NA, Baxter KJ, Love SB. The 36 prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112(4):1096–102.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Iuiter N, De Cuba E, Kwakman R, Kazemier G, Meijer G, Te Velde EA. Adhesion molecules in peritoneal dissemination: 35 function, prognostic relevance and therapeutic options. Clin Exp Metastasis. 2016. https://doi.org/10.1007/s10585-016-9791-9790.
Sugarbaker PH. Colorectal cancer: prevention and management of metastatic disease. Biomed Res Int. 2014;2014:782890. https://doi.org/10.1155/2014/782890.
Franko J, Shi Q, Meyers J, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17(2):1709–19.
Hompes D, Tiek J, Wolthuis A, et al. HIPEC in T4a colon cancer: a defendable treatment to improve oncologic outcome? Ann Oncologia. 2012;23(12):3123–9.
Pande R, Sunga A, Levea C, et al. Significance of signet-ring cells in patients with colorectal •• cancer. Dis Colon Rectum. 2008;51(1):50–5.
Noura S, Ohue M, Seki Y, Yano M, Ishikawa O, Kameyama M. Long-term prognostic value of conventional peritoneal lavage cytology in patients undergoing curative colorectal cancer resection. Dis Colon Rectum. 2009;52(7):1312–20.
Nishikawa T, Sunami E, Tanaka T, et al. Incidence and prognostic significance of positive peritoneal lavage in colorectal cancer. Surg Today. 2015;45(9):1073–81.
Honoré C, Goere D, Souadka A, Dumont F, Elias D. De nition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20(1):183–92.
Sasaki Y, Hamaguchi T, Yamada Y, Takahashi N, Shoji H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Nagai Y, Taniguchi H, Boku N, Ushijima T, Shimada Y. Value of KRAS, BRAF, and PIK3CA mutations and survival benefit from systemic chemotherapy in colorectal peritoneal carcinomatosis. Asian Pac J Cancer Prev. 2016;17(2):539–43.
Russell AH, Tong D, Dawson LE, Wisbeck WM, Griffin TW, Laramore GE, Luk KH. Adenocarcinoma of the retroperitoneal ascending and descending colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. Int J Radiat Oncol Biol Phys. 1983;9(3):361–5.
Russell AH, Tong D, Dawson LE, Wisbeck W. Adenocarcinoma of the proximal colon. Sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53(2):360–7.
Chua TC, Morris DL, Saxena A, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18:1560–7.
Sugarbaker PH, Re: Verwaal VJ, van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;88:276–8.
Elias D, Borget I, Farron M, Dromain C, Ducreux M, Goéré D, Honoré C, Boige V, Dumont F, Malka D, Pottier E, Caramella C. Prognostic significance of visible cardiophrenic angle lymph nodes in the presence of peritoneal metastases from colorectal cancers. Eur J Surg Oncol. 2013;39(11):1214–8. https://doi.org/10.1016/j.ejso.2013.08.006.
Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computer tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72(5):1631–6.
Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–33.
Esquivel J, Chua T, Stojadinovic A, Torres Melero J, Levine E, Gutman M, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010;102:565.
Coakley F, Choi P, Gougoutas C, Pothuri B, Venkatraman E, Chi D, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223(2):495.
Suzuki A, Kawano T, Takahashi N, Lee J, Nakagami Y, Miyagi E, Hirahara F, Togo S, Shimada H, Inoue T. Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging. 2004;31(10):1413–20. https://doi.org/10.1007/s00259-004-1577-y.
Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM. Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med. 2003;44(9):1407–12.
Satoh Y, Ichikawa T, Motosugi U, Kimura K, Sou H, Sano K, Araki T. Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol. 2011;196(2):447–53. https://doi.org/10.2214/AJR.10.4687.
Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the peritoneal cancer index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2014. https://doi.org/10.1245/s10434-014-4041-7.
Soussan M, Des Guetz G, Barrau V, Aflalo-Hazan V, Pop G, Mehanna Z, Rust E, Aparicio T, Douard R, Benamouzig R, Wind P, Eder V. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol. 2012;22(7):1479–87. https://doi.org/10.1007/s00330-012-2397-2.
Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D. Staging of peritoneal carcinomatosis: enhanced CT vs PET/CT. Abdom Imaging. 2008;33(1):87–93. https://doi.org/10.1007/s00261-007-9211-7.
Espada M, Garcia-Flores JR, Jimenez M, Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, Hernandez-Cortes G, Martinez-Vega V, Sainz De La Cuesta R. Diffusion-weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol. 2013;23(9):2636–42.
Laghi A, Bellini D, Rengo M, Accarpio F, Caruso D, Biacchi D, Di Giorgio A, Sammartino P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta-analysis. Radiol Med. 2017;122(1):1–15. https://doi.org/10.1007/s11547-016-0682-x.
Fujii S, Matsusue E, Kanasaki Y, et al. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol. 2008;18(1):18–23.
Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings—a feasibility study. AJR Am J Roentgenol. 2009;193(2):461–70.
Bhatt A, Goéré D. Cytoreductive surgery plus HIPEC for peritoneal metastases from colorectal cancer. Indian J Surg Oncol. 2016;7:177. https://doi.org/10.1007/s13193-016-0499-z.
Villeneuve L, Thivolet A, Bakrin N, Mohamed F, Isaac S, Valette PJ, Glehen O, Rousset P, BIG-RENAPE and RENAPE Working Groups. A new internet tool to report peritoneal malignancy extent. PeRitOneal Malignancy Stage Evaluation (PROMISE) application. Eur J Surg Oncol. 2016;42(6):877–82. https://doi.org/10.1016/j.ejso.2016.03.015.
Dromain C, Bisdorff A, Elias D, Antoun S, Boige V, Lasser P, Sigal R. Computed tomographic features of peritoneal carcinomatosis treated by intraperitoneal chemohyperthermia. J Comput Assist Tomogr. 2003;27(3):327–32.
Tabrizian P, Jayakrishnan TT, Zacharias A, et al. Incorporation of diagnostic laparoscopy in the management algorithm for patients with peritoneal metastases: a multi-institutional analysis. J Surg Oncol. 2015. https://doi.org/10.1002/jso.23924.
Jayakrishnan TT, Zacharias AJ, Sharma A, et al. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J Surg Oncol. 2014;12:270. https://doi.org/10.1186/1477-7819-12-270.
Valle M, Federici O, Garofalo A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging, and treatment. Surg Oncol Clin N Am. 2012;21:515–31. https://doi.org/10.1016/j.soc.2012.07.005.
Iversen LH, Rasmussen PC, Laurberg S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg. 2013;100:285–92. https://doi.org/10.1002/bjs.8908.
Seshadri RA, Hemanth Raj E. Diagnostic laparoscopy in the pre-operative assessment of patients undergoing cytoreductive surgery and HIPEC for peritoneal surface malignancies. Indian J Surg Oncol. 2016;7:230–5. https://doi.org/10.1007/s13193-015-0486-9.
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus Leucovorin, Irinotecan, and Oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. https://doi.org/10.1200/JCO.2004.09.046.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus Irinotecan, fluorouracil, and Leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. https://doi.org/10.1056/NEJMoa032691.
Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. https://doi.org/10.1200/JCO.2007.14.9930.
Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. https://doi.org/10.1056/NEJMoa0805019.
Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46. https://doi.org/10.1093/annonc/mdq632.
Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–7. https://doi.org/10.1200/JCO.2011.37.1039.
Klaver YLB, Simkens LHJ, Lemmens VEPP, et al. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012;38:617–23. https://doi.org/10.1016/j.ejso.2012.03.008.
Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370:135–42. https://doi.org/10.1016/S0140-6736(07)61086-1.
Tol J, Koopman M, Rodenburg CJ, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2007;19:734–8. https://doi.org/10.1093/annonc/mdm607.
Esquivel J. Current status and future directions of hyperthermic intraperitoneal chemotherapy (HIPEC). Intervent Oncol 360. 2014;2(6):E45–52.
Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–5. https://doi.org/10.1200/JCO.2008.19.7160.
Russell AH, Tong D, Dawson LE, Wisbeck W. Adenocarcinoma of the proximal colon sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53:360–7. https://doi.org/10.1002/1097-0142(19840115)53:2<360::AID-CNCR2820530232>3.0.CO;2-U.
Mulsow J, Merkel S, Agaimy A, Hohenberger W. Outcomes following surgery for colorectal cancer with synchronous peritoneal metastases. Br J Surg. 2011;98:1785–91. https://doi.org/10.1002/bjs.7653.
Matsuda K, Hotta T, Takifuji K, et al. Clinical impact of a macroscopically complete resection of colorectal cancer with peritoneal carcinomatosis. Surgery. 2012;151:238–44. https://doi.org/10.1016/j.surg.2010.10.018.
Désolneux G, Mazière C, Vara J, et al. Cytoreductive surgery of colorectal peritoneal metastases: outcomes after complete cytoreductive surgery and systemic chemotherapy only. PLoS One. 2015;10:e0122816. https://doi.org/10.1371/journal.pone.0122816.
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43. https://doi.org/10.1200/JCO.2003.04.187.
Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal Carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32. https://doi.org/10.1245/s10434-008-9966-2.
Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–62. https://doi.org/10.1002/cncr.25116.
Cavaliere F, Valle M, De Simone M, et al. 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal chemohyperthermia: a S. I. T. I. L. O. multicentric. study. In Vivo. 2006;20(6A):747–50.
Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative Intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8. https://doi.org/10.1200/JCO.2009.23.9285.
Quenet F, Goéré D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without Irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294–301. https://doi.org/10.1097/SLA.0b013e3182263933.
Ihemelandu C, Sugarbaker PH. Management for peritoneal metastasis of colonic origin: role of cytoreductive surgery and perioperative Intraperitoneal chemotherapy: a single Institution’s experience during two decades. Ann Surg Oncol. 2017;24:898–905. https://doi.org/10.1245/s10434-016-5698-x.
Frøysnes IS, Larsen SG, Spasojevic M, et al. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: prognostic factors and oncologic outcome in a national patient cohort. J Surg Oncol. 2016;114:222–7. https://doi.org/10.1002/jso.24290.
Baratti D, Kusamura S, Pietrantonio F, et al. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit Rev Oncol Hematol. 2016;100:209–22. https://doi.org/10.1016/j.critrevonc.2016.01.017.
Zoetmulder FA. Cancer cell seeding during abdominal surgery: experimental studies. Cancer Treat Res. 1996;82:155–61.
Jacquet P, Averbach AM, Jacquet N. Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur J Surg Oncol. 1995;21(5):568–70.
Elias D, Di Pietrantonio D, Boulet T, Honore C, Bonnet S, Goere D, Kohneh-Shahri N, Raynard B. Natural history of complete cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2009;35(4):434–8.
Sugarbaker PH. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal cancer. Cancer Investig. 2005;23(2):155–72.
Shimizu T, Maeta M, Koga T. Influence of local hyperthermia on the healing of small intestinal anastomoses in the rat. Br J Surg. 1991;78(1):57–9.
Pelz JO, Doerfer J, Dimmler A, et al. Histological response of peritoneal carcinomatosis after hyperthermic intraperitoneal chemoperfusion (HIPEC) in experimental investigations. BMC Cancer. 2006;6:162. https://doi.org/10.1186/1471-2407-6-162.
Gremonprez F, Willaert W, Ceelen W. Intraperitoneal chemotherapy (IPC) for peritoneal carcinomatosis: review of animal models. J Surg Oncol. 2014;109(2):110–6.
Bartlett DL. HIPEC: the complexities of clinical trials. Ann Surg Oncol. 2008;15(5):1277–9. https://doi.org/10.1245/s10434-007-9768-y.
Zeamari S, Floot B, van der Vange N, et al. Pharmacokinetics and pharmacodynamics of cisplatin after intraoperative hyperthermic intraperitoneal chemoperfusion (HIPEC). Anticancer Res. 2003;23:1643–8.
Tang L, Mei LJ, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: evidence from an experimental study. J Transl Med. 2011;9:53.
Facy O, Al Samman S, Magnin G, et al. High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: an experimental study. Ann Surg. 2012;256:1084–8.
Klaver YL, Hendriks T, Lomme RM, et al. Hyperthermia and intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis: an experimental study. Ann Surg. 2011;254:125–30.
Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–6.
Ceelen W, Van Nieuwenhove Y, Putte DV, Pattyn P. Neoadjuvant chemotherapy with Bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21:3023–8. https://doi.org/10.1245/s10434-014-3713-7.
Yonemura Y, Canbay E, Ishibashi H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. ScientificWorldJournal. 2013;2013:978394. https://doi.org/10.1155/2013/978394.
Adachi T, Hinoi T, Egi H, et al. Oxaliplatin and molecular-targeted drug therapies improved the overall survival in colorectal cancer patients with synchronous peritoneal carcinomatosis undergoing incomplete cytoreductive surgery. Surg Today. 2015;45:986–92. https://doi.org/10.1007/s00595-014-1017-y.
Hompes D, Aalbers A, Boot H, et al. A prospective pilot study to assess neoadjuvant chemotherapy for unresectable peritoneal carcinomatosis from colorectal cancer. Color Dis. 2014;16:O264–72. https://doi.org/10.1111/codi.12560.
Maillet M, Glehen O, Lambert J, Goere D, Pocard M, Msika S, Passot G, Elias D, Eveno C, Sabaté JM, Lourenco N, André T, Gornet JM; BIG-RENAPE Working Group. Early postoperative chemotherapy after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for isolated peritoneal carcinomatosis of colon cancer: a multicenter study. Ann Surg Oncol. 2016;23(3):863–9. https://doi.org/10.1245/s10434-015-4914-4.
Waite K, Youssef H. The role of neoadjuvant and adjuvant systemic chemotherapy with cytoreductive surgery and heated intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review. Ann Surg Oncol. 2017;24:705. https://doi.org/10.1245/s10434-016-5712-3.
Passot G, Vaudoyer D, Cotte E, You B, Isaac S, Gilly FN, et al. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256:125–9.
Goéré D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal Carcinomatosis amenable to complete cytoreductive surgery and Intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–71. https://doi.org/10.1097/SLA.0b013e31827e9289.
Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. https://doi.org/10.1200/JCO.2007.11.0833.
Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–35. https://doi.org/10.1200/JCO.2008.19.9273.
Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–39. https://doi.org/10.1634/theoncologist.2012-0121.
Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–64. https://doi.org/10.1245/s10434-015-4387-5.
Sugarbaker PH, Graves T, DeBruijn E, Cunliffe W, Mullins R, Hull W, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. 1990;50:5790–4.
Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE, et al. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery. 1985;98:414–22.
Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–32.
Elias D, Benizri E, Dipietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2006;14:509–14.
Mahteme H, Hansson J, Berglund Å, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer. 2004;90(2):403–7. https://doi.org/10.1038/sj.bjc.6601586.
Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, Giovannini M, Lasser P. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting ran domized trials. Ann Surg Oncol. 2004;11:518–21. https://doi.org/10.1245/ASO.2004.09.008.
Glehen OKF, Sugarbaker PH, Elias D, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–92.
Duraj FF, Cashin PH. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal and hepatic metastases: a case-control study. J Gastrointest Oncol. 2013;4(4):388–96. https://doi.org/10.3978/j.issn.2078-6891.2013.026.
Lam JY, McConnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210:424–30.
Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol. 2007;14:128–33. https://doi.org/10.1245/s10434-006-9185-7.
Levine EA, Stewart JH, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–85. https://doi.org/10.1016/j.jamcollsurg.2013.12.013.
Ihemelandu CU, McQuellon R, Shen P, et al. Predicting postoperative morbidity following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CS+HIPEC) with preoperative FACT-C (functional assessment of cancer therapy) and patient-rated performance status. Ann Surg Oncol. 2013;20:3519–26. https://doi.org/10.1245/s10434-013-3049-8.
Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol. 2013;20:3497–503. https://doi.org/10.1245/s10434-013-3053-z.
Barbosa LRLS, Lacerda-Filho A, Barbosa LCLS. Immediate preoperative nutritional status of patients with colorectal cancer: a warning. Arq Gastroenterol. 2014;51:331–6. https://doi.org/10.1590/S0004-28032014000400012.
Vashi PG, Gupta D, Lammersfeld CA, et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr J. 2013;12:118. https://doi.org/10.1186/1475-2891-12-118.
van Vugt J, Braam HJ, van Oudheusden TR, Vestering A, Bollen TL, Wiezer MJ, de Hingh I, Bert van Ramshorst DB (2015) Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 22(11):3625.
Valle SJ, Alzahrani N, Alzahrani S, et al. Enterocutaneous fistula in patients with peritoneal malignancy following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: incidence, management and outcomes. Surg Oncol. 2016;25:315–20. https://doi.org/10.1016/j.suronc.2016.05.025.
Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol. 2014;21:1474–9. https://doi.org/10.1245/s10434-013-3224-y.
Faivre J, Lemmens VE, Quipourt V, Bouvier AM. Management and survival of colorectal cancer in the elderly in population based studies. Eur J Cancer (Oxford, England: 1990). 2007;43(15):2279–84.
Polanczyk CA, Marcantonio E, Goldman L, Rohde LE, Orav J, Mangione CM, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–43.
Pratt WB, Gangavati A, Agarwal K, Schreiber R, Lipsitz LA, Callery MP, et al. Establishing standards of quality for elderly patients undergoing pancreatic resection. Arch Surg (Chicago, Ill: 1960). 2009;144(10):950–6. 32.
Barrier A, Ferro L, Houry S, Lacaine F, Huguier M. Rectal cancer surgery in patients more than 80 years of age. Am J Surg. 2003;185(1):54–7.
Alyami M, Lundberg P, Kepenekian V, Goéré D, Bereder JM, Msika S, Lorimier G, Quenet F, Ferron G, Thibaudeau E, Abboud K, Lo Dico R, Delroeux D, Brigand C, Arvieux C, Marchal F, Tuech JJ, Guilloit JM, Guyon F, Peyrat P, Pezet D, Ortega-Deballon P, Zinzindohoue F, de Chaisemartin C, Kianmanesh R, Glehen O, Passot G, BIG-RENAPE and RENAPE Working Groups. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in the elderly: a case-controlled, multicenter study. Ann Surg Oncol. 2016;23(Suppl 5):737–45.
Cascales-Campos PA, López-López V, Muñoz-Casares FC, Feliciangeli E, Torres Melero J, Barrios P, Morales R, Ramos I, Ortega G, Camps B, González-Bayón L, Bretcha-Boix P, Farré-Alegre J, González-Moreno S, Gil J. Morbidity and mortality outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients aged 75 years and over: Spanish group of peritoneal cancer surgery (GECOP) multicenter study.Surg Oncol. 2016;25:111–6
Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98:263–7.
Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22(9):2958–64. https://doi.org/10.1245/s10434-015-4387-5.
Vaira M, Cioppa T, D’Amico S, de Marco G, D’Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24(1):79–84.
Mirnezami R, Moran BJ, Harvey K, et al. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J Gastroenterol. 2014;20(38):14018–32. https://doi.org/10.3748/wjg.v20.i38.14018.
Pelz JOW, Stojadinovic A, Nissan A, Hohenberger W, Esquivel J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol. 2009;99:9–15. https://doi.org/10.1002/jso.21169.
Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) multiinstitution evaluation of the peritoneal surface disease severity score (PSDSS) in 1,013 patients with colorectal cancer with peritoneal Carcinomatosis. Ann Surg Oncol. 2014;21(13):4195–201. https://doi.org/10.1245/s10434-014-3798-z.
Ng JL, Ong WS, Chia CS, Tan GHC, Soo K-C, Teo MCC. Prognostic relevance of the peritoneal surface disease severity score compared to the peritoneal cancer index for colorectal peritoneal carcinomatosis. Int J Surg Oncol. 2016;Article ID 2495131, 7 p. https://doi.org/10.1155/2016/2495131.
Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E, Glehen O. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21:2608–14.
Sugarbaker PH. Preoperative assessment of cancer patients with peritoneal metastases for complete cytoreduction. Indian J Surg Oncol. 2016;7(3):295–302.
Benedet JL, Bender H, Jones H, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO committee on Gynecologic oncology. Int J Gynaecol Obstet. 2000;70:209–62.
La Torre M, Ferri M, Giovagnoli MR, et al. Peritoneal wash cytology in gastric carcinoma. Prognostic significance and therapeutic consequences. Eur J Surg Oncol. 2010;36:982–6. https://doi.org/10.1016/j.ejso.2010.06.007.
Katsuragi K, Yashiro M, Sawada T, et al. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007;97:550–6. https://doi.org/10.1038/sj.bjc.6603909.
Euanorasetr C, Lertsithichai P. Prognostic significance of peritoneal washing cytology in Thai patients with gastric adenocarcinoma undergoing curative D2 gastrectomy. Gastric Cancer. 2007;10:18–23. https://doi.org/10.1007/s10120-006-0402-7.
Cotte E, Peyrat P, Piaton E, et al. Lack of prognostic significance of conventional peritoneal cytology in colorectal and gastric cancers: results of EVOCAPE 2 multicentre prospective study. Eur J Surg Oncol. 2013;39:707–14. https://doi.org/10.1016/j.ejso.2013.03.021.
Bosanquet DC, Harris DA, Evans MD, Beynon J. Systematic review and meta-analysis of intraoperative peritoneal lavage for colorectal cancer staging. Br J Surg. 2013;100:853–62. https://doi.org/10.1002/bjs.9118.
Mohan HM, O’Connor DB, O’Riordan JM, Winter DC. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: a systematic review. Surg Oncol. 2013;22:e1–6. https://doi.org/10.1016/j.suronc.2013.01.001.
Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–56. https://doi.org/10.1093/annonc/mdu543.
Nicholson BD, Shinkins B, Pathiraja I, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;CD011134. https://doi.org/10.1002/14651858.CD011134.pub2.
Park IJ, Choi G-S, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29:4303–8.
Liu F, Yu J, Liang Y, et al. Associated risk factors of peritoneal metastasis in colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:254–6.
Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651–7. https://doi.org/10.1093/annonc/mdt591.
Razenberg LGEM, van Gestel YRBM, Lemmens VEPP, et al. The prognostic relevance of histological subtype in patients with peritoneal metastases from colorectal cancer: a nationwide population-based study. Clin Colorectal Cancer. 2015;14:e13–9. https://doi.org/10.1016/j.clcc.2015.05.011.
Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21:1456–62. https://doi.org/10.1245/s10434-013-3328-4.
Van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol. 2015. https://doi.org/10.1002/jso.23784.
Simkens GA, van Oudheusden TR, Nieboer D, et al. Development of a prognostic nomogram for patients with peritoneally metastasized colorectal cancer treated with cytoreductive surgery and HIPEC. Ann Surg Oncol. 2016;23:4214–21. https://doi.org/10.1245/s10434-016-5211-6.
Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
Cotte E, Passot G, Mohamed F, Vaudoyer D, Gilly FN, Glehen O. Management of peritoneal carcinomatosis from colorectal cancer: current state of practice. Cancer J. 2009;15:243–8.
Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18-21.
Elias D, Ouellet J-F, Bellon N, et al. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:567–74. https://doi.org/10.1002/bjs.4071.
Kianmanesh R, Scaringi S, Sabate J-M, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. https://doi.org/10.1097/01.sla.0000255561.87771.11.
Elias D, Benizri E, Pocard M, et al. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol. 2006;32:632–6. https://doi.org/10.1016/j.ejso.2006.03.013.
Varban O, Levine EA, Stewart JH, et al. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer. 2009;115:3427–36. https://doi.org/10.1002/cncr.24385.
Maggiori L, Goéré D, Viana B, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013. https://doi.org/10.1097/SLA.0b013e3182778089.
Elias D, Faron M, Goéré D, Dumont F, Honoré C, Boige V, Malka D, Ducreux M. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. 2014;21(6):2052–8. https://doi.org/10.1245/s10434-014-3506-z.
Goere D, Daveau C, Elias D, et al. The differential response to chemotherapy of ovarian metastases from colorectal carcinoma. Eur J Surg Oncol. 2008;34:1335–9.
Segelman J, Floter-Radestad A, Hellborg H, Sjovall A, Martling A. Epidemiology and prognosis of ovarian metastases in colorectal cancer. Br J Surg. 2010;97:1704–9.
Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. 2011;98:287–92.
Eveno C, Goéré D, Dartigues P, Honoré C, Dumont F, Tzanis D, Benhaim L, Malka D, Elias D. Ovarian metastasis is associated with retroperitoneal lymph node relapses in women treated for colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2013;20(2):491–6. https://doi.org/10.1245/s10434-012-2623-9.
Moore RG, Chung M, Granai CO, et al. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol. 2004;93:87–91.
Ortega-Deballon P, Glehen O, Levine E, et al. Childbearing after hyperthermic intraperitoneal chemotherapy: results from an international survey. Ann Surg Oncol. 2011;18:2297.
Braam HJ, van Oudheusden TR, de Hingh IH, Nienhuijs SW, Boerma D, Wiezer MJ, van Ramshorst B. Urological procedures in patients with peritoneal carcinomatosis of colorectal cancer treated with HIPEC: morbidity and survival analysis. Anticancer Res. 2015;35(1):295–300.
Honore C, Souadka A, Goere D, Dumont F, Deschamps F, Elias D. HIPEC for peritoneal carcinomatosis: Does an associated urologic procedure increase morbidity? Ann Surg Oncol. 2012;19(1):104–9, 15.
Leapman MS, Jibara G, Tabrizian P, Franssen B, Yang MJ, Romanoff A, Hall SJ, Palese M, Sarpel U, Hiotis S, Labow D. Genitourinary resection at the time of cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis is not associated with increased morbidity or worsened oncologic outcomes: a case-matched study. Ann Surg Oncol. 2014;21(4):1153–8.
Elias D, Antoun S, Goharin A, Otmany AE, Puizillout JM, Lasser P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig. 2000;1(5):431–9.
Ortega-Deballon P, Facy O, Jambet S, Magnin G, Cotte E, Beltramo JL, Chauffert B, Rat P. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol. 2010;17(7):1957–63.
Glehen O, Cotte E, Kusamura S, Deraco M, Baratti D, Passot G, Beaujard A-C, Noel GF. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242–6. https://doi.org/10.1002/jso.21061.
Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC, Lowy AM. Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. Recent Results Cancer Res. 2007;169:75–82.
Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247–52. https://doi.org/10.1002/jso.21051.
Kuzuya T, Yamauchi M, Ito A, et al. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J Pharm Pharmacol. 1994;46:685–9.
Elias D, Bonnay M, Puizillou JM, Antoun S, Dermirdjian S, El Otomany A, et al. Heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Surg Oncol. 2002;13:267–72.
Stewart JH, Shen P, Russell G, et al. A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann Surg Oncol. 2008;15(8):2137–45. https://doi.org/10.1245/s10434-008-9967-1.
Hompes D, D'Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of oxaliplatin or mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109:527–32. https://doi.org/10.1002/jso.23546.
Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, González-Moreno S, Goodman M, Halkia E, Kusamura S, Moller M, Passot G, Pocard M, Salti G, Sardi A, Senthil M, Spiliotis J, Torres-Melero J, Turaga K, Trout R. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with mitomycin C versus oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol. 2014;110:779–85. https://doi.org/10.1002/jso.23728.
Leung V, Huo YR, Liauw W, Morris DL. Oxaliplatin versus mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43(1):144–9. https://doi.org/10.1016/j.ejso.2016.09.015.
Charrier T, Passot G, Peron J, Maurice C, Gocevska S, Quénet F, Eveno C, Pocard M, Goere D, Elias D, Ortega-Deballon P, Vaudoyer D, Cotte E, Glehen O. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy with oxaliplatin increases the risk of postoperative hemorrhagic complications: analysis of predictive factors. Ann Surg Oncol. 2016;23(7):2315–22. https://doi.org/10.1245/s10434-016-5143-1.
Van der Speeten K, Stuart OA, Chang D, et al. Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2011;68:147. https://doi.org/10.1007/s00280-010-1460-4.
Witkamp AJ, van Coevorden F, Kaag MM, van Slooten GW, Beijnen JB, Boot H, et al. Dose finding study of hyperthermic intraperitoneal chemotherapy with mitomycin C in patients with carcinosis of colorectal origin. Eur J Surg Oncol. 1998;24:214.
Turaga K, Levine E, Barone R, et al. Consensus guidelines from the American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21:1501. https://doi.org/10.1245/s10434-013-3061-z.
Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13(2):267–72.
Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–6.
Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–13.
Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion. Cancer. 2006;106:1144–53. https://doi.org/10.1002/cncr.21708.
Franko J, Gusani NJ, Holtzman MP, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15:3065–72. https://doi.org/10.1245/s10434-008-0105-x.
Glockzin G, Ghali N, Lang SA, et al. Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. J Surg Oncol. 2009;100:306–10. https://doi.org/10.1002/jso.21332.
Smeenk RM, Verwaal VJ, Zoetmulder FAN. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–14. https://doi.org/10.1002/bjs.5863.
Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg 2007;204:943-53-5. doi: https://doi.org/10.1016/j.jamcollsurg.2006.12.048.
Elias D, Goere D. Peritoneal carcinomatosis of colorectal origin: recent advances and future evolution toward a curative treatment. Recent Results Cancer Res. 2007;169:115–22.
Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–9.
Jacquet P, Stephens AD, Averbach AM, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77:2622–9. https://doi.org/10.1002/(SICI)1097-0142(19960615)77:12<2622::AID-CNCR28>3.0.CO;2-T.
Hansson J, Graf W, Påhlman L, et al. Postoperative adverse events and long-term survival after cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2009;35:202–8. https://doi.org/10.1016/j.ejso.2008.04.002.
Verwaal VJ, Tinteren H, van Ruth SV, Zoetmulder FAN. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85:61–7. https://doi.org/10.1002/jso.20013.
Saxena A, Yan TD, Morris DL. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative Intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J Surg. 2010;34:70–8. https://doi.org/10.1007/s00268-009-0206-0.
Mercier F, Cotte E, Glehen O, Passot G. Why morbidity is not an adequate metric for evaluation of surgical quality. Ann Surg. 2017. https://doi.org/10.1097/SLA.0000000000002256.
Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol. 2008;98:295–9.
Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg. 1999;23:23–9.
Verwaal VJ, Boot H, Aleman BM, et al. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol. 2004;11:375–9.
Bijelic L, Yan TD, Sugarbaker PH. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol. 2007;14:2281–8.
Saxena A, Yan TD, Morris DL. Critical assessment of preoperative and operative risk factors for complications after iterative peritonectomy procedures. Eur J Surg Oncol. 2010;36:309–14.
Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38:509–15.
Vaira M, Robella M, Mellano A, Sottile A, De Simone M. Iterative procedures combining cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for isolated peritoneal recurrence. Int J Hyperthermia. 2014;30(8):565–9. doi: https://doi.org/10.3109/02656736.2014.974693.
Golse N, Bakrin N, Passot G, Mohamed F, Vaudoyer D, Gilly FN, Glehen O, Cotte E. Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol. 2012;106(2):197–203. https://doi.org/10.1002/jso.23062.
Alzharani N, Huang Ye, Baratti D, Deraco M et al. Repeat Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in colorectal cancer in 189 patients (PSOGI collaboration). J Peritoneum. 2016;1(1):1 (abstract).
Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21(2):553–9. https://doi.org/10.1245/s10434-013-3213-1.
Esquis P, Consolo D, Magnin G, et al. High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg. 2006;244(1):106–112. https://doi.org/10.1097/01.sla.0000218089.61635.5f. [PMC free article] [PubMed][Cross Ref].
Jacquet P, Stuart OA, Chang D, Sugarbaker PH. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs. 1996;7(5):596–603. doi: https://doi.org/10.1097/00001813-199607000-00016. [PubMed] [Cross Ref].
Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128. https://doi.org/10.1186/s12957-016-0892-7.
Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis. 2016;18(4):364–71. https://doi.org/10.1111/codi.13130.
Yonemura Y, Elnemr A, Endou Y, et al. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012;2012:148420. https://doi.org/10.1155/2012/148420.
Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, Takao N, Ichinose M, Liu Y, Li Y. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol. 2017;24(2):478–85. https://doi.org/10.1245/s10434-016-5487-6.
Sgarbura O, Samalin E, Carrere S, Mazard T, de Forges H, Alline M, Pissas MH, Portales F, Ychou M, Quénet F. Preoperative intraperitoneal oxaliplatin for unresectable peritoneal carcinomatosis of colorectal origin: a pilot study. Pleura Peritoneum. 2016;1(4):209–15.
Sugarbaker PH. It’s what the surgeon does’nt see that kills the patient. J Nippon Med Sch. 2000;67:5–8.
Renzulli P, Lowy A, Maibach R, et al. The influence of the surgeon’s and the hospital’s caseload on survival and local recurrence after colorectal cancer surgery. Surgery. 2006;139:296–304.
Hermanek P, Wieblet H, Staimmer D, Riedl S. SGCRC: prognostic factors of rectum carcinoma-experience of the German multicentre study SGCRC. Tumori. 1995;81(supplement):60–64.
Turnbull RB, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of notouch isolation technic on survival rates. Ann Surg. 1967;166(3):420–7.
Sugarbaker PH. Carcinoma of the colon-prognosis and operative choice. Curr Probl Surg 1981;18:755–802. 4.
Averbach AM, Jacquet P, Sugarbaker PH. Surgical technique and colorectal cancer: impaction on local recurrence and survival. Tumori. 1995;81(supplement):65–71.
Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol. 2007;95(2):93–6.
Carter JJ, Feingold DL, Kirman I, et al. Laparoscopic-assisted cecectomy is associated with decreased formation of postoperative pulmonary metastases compared with open cecectomy in a murine model. Surgery. 2003;134:432–36. 54.
Canis M, Botchorishvili R, Wattiez A, et al. Cancer and laparoscopy, experimental studies: a review. Eur J Obstet Gynecol Reprod Biol. 2000;91:1–9. 55.
Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC trial group. J Clin Oncol. 2007;25:3061–8.
Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705. https://doi.org/10.1002/bjs.8679.
Van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, Creemers GJ, de Hingh IH. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963–9. https://doi.org/10.1016/j.ejso.2013.10.001.
González-Moreno S. Peritoneal surface oncology: a progress report. Eur J Surg Oncol. 2006;32(6):593. https://doi.org/10.1016/j.ejso.2006.03.001.
Elias D, Honoré C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289–93. https://doi.org/10.1097/SLA.0b013e31822638f6.
Ripley RT, Davis JL, Kemp CD, et al. Prospective randomized trial evaluating mandatory second look surgery with HIPEC and CRS vs. standard of care in patients at high risk of developing colorectal peritoneal metastases. Trials. 2010;11:62. https://doi.org/10.1186/1745-6215-11-62.
Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012:1–7. https://doi.org/10.1155/2012/141585.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhatt, A., Mehta, S., Quénet, F. (2018). Multimodality Treatment for Colorectal Peritoneal Metastases. In: Bhatt, A. (eds) Management of Peritoneal Metastases- Cytoreductive Surgery, HIPEC and Beyond. Springer, Singapore. https://doi.org/10.1007/978-981-10-7053-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-7053-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7052-5
Online ISBN: 978-981-10-7053-2
eBook Packages: MedicineMedicine (R0)