Abstract
Objectives
To assess the accuracy of FDG-PET/CT and MR with diffusion-weighted imaging (MR-DWI) for diagnosing peritoneal carcinomatosis (PC) from gastrointestinal malignancies.
Methods
Thirty consecutive patients referred for staging of gastrointestinal malignancy underwent FDG-PET/CT and MR-DWI in this retrospective study. Extent of PC was characterised by dividing the peritoneal cavity into three sites in each patient: right and left supramesocolic areas and inframesocolic level (total 90 sites). Presence of PC was confirmed either by surgery (18/30) or by follow-up (12/30).
Results
PC was confirmed in 19 patients (19/30). At a total of 90 sites, 27 showed proven PC. On a patient-based analysis, sensitivity, specificity, PPV, NPV and accuracy were respectively 84%, 73%, 84%, 73% and 80% for PET/CT and 84%, 82%, 89%, 75% and 83% for MR-DWI. On a site-based analysis, overall sensitivity and specificity of PET/CT (63%, 90%) and MR-DWI (74%, 97%) were not statistically different (P = 0.27). In the supramesocolic area, MR-DWI detected more sites involved than PET/CT (7/9 vs. 4/9). The sensitivities of PET and MR were lower for subcentimetre tumour implants (42%, 50%). Interobserver agreement was very good for PET/CT and good for MR-DWI.
Conclusions
FDG-PET/CT and MR-DWI showed similar high accuracy in diagnosing PC. Both techniques underestimated the real extent of PC because of decreased sensitivity for subcentimetre lesions.
Key Points
• FDG-PET/CT and MR-DWI showed similar high accuracy for diagnosing peritoneal carcinomatosis.
• In the supramesocolic area, MR-DWI could be more sensitive than PET/CT.
• Both techniques showed lower sensitivity for subcentimetre lesions.
• Interobserver agreement was very good for PET/CT and good for MR-DWI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early diagnosis of peritoneal carcinomatosis (PC) arising from gastrointestinal malignancy is essential. New curative approaches in the treatment of PC are now available, combining cytoreductive surgery with perioperative intraperitoneal chemotherapy [1, 2]. Patient selection for these new therapies is of the utmost importance. Hence, early identification of PC allows curative treatment, with an impact on patient outcome [2]. Multidetector computed tomography (MDCT) is the keystone imaging investigation in the management of patients with gastrointestinal malignancy, both at initial staging and during follow-up. Unfortunately, MDCT accuracy in the assessment of PC is limited with reported sensitivities ranging from 25 to 90% [3, 4]. The small size of the lesions and the poor soft-tissue contrast with MDCT are major limitations for the detection of PC [3]. In this setting, functional imaging tools such as FDG-PET/CT and magnetic resonance with diffusion-weighted imaging (MR-DWI) open new horizons in oncological imaging. Recent studies emphasised the incremental value of DWI addition to routine MRI for abdominal malignancy studies. Indeed, most solid tumours are characterised by high cellularity with consequent restriction of water movement, leading to high signal intensity [5]. The addition of diffusion-weighted sequences to conventional MR images has shown an incremental value in the staging of abdominal and pelvic tumours with detection of more involved sites [6–8]. Metabolic imaging with FDG-PET/CT has already proved its usefulness in the management of patients with digestive cancer [9]. However, there are only limited data available on the value of these techniques in assessing PC staging [6, 7, 10–12]. Moreover, to our knowledge, intraindividual head-to-head comparison of both FDG-PET/CT and MR-DWI has, to date, not been reported.
The purpose of the present study was to assess and compare the accuracies of MR-DWI and FDG-PET/CT for the diagnosis and extent of PC in patients with gastrointestinal malignancy.
Patients and methods
Patients
This study was approved by the local ethics committee of our institution. From November 2010 to June 2011, all patients referred for FDG-PET/CT with a new diagnosis or previous history of gastrointestinal malignancy were retrospectively selected, and included if additional MR-DWI was performed within 1 month. The inclusion criteria for the study were (1) initial staging for gastrointestinal malignancy in patients in whom surgical resection was planned and (2) suspected recurrence such as a rise in tumour markers with normal MDCT, equivocal clinical symptoms of PC or equivocal peritoneal lesion on MDCT without any other metastatic site during follow-up. Patients with unequivocal PC on previous MDCT were excluded.
Imaging techniques
FDG-PET/CT
All FDG-PET/CT studies were performed from skull base to mid-thigh on a 16-slice PET/CT system (Gemini TF, Philips Medical Systems, the Netherlands) 60 min after intravenous injection of 3–3.5 MBq/kg of FDG. Unenhanced CT (120 kV, 100 mAs, collimation 16 × 1.5 mm, pitch of 0.69, slice thickness 3 mm at increments of 1.5 mm) was performed for attenuation correction purposes and the allowed precise anatomical localisation of PET data. All patients had a serum glucose level below 1.4 g/L at the time of injection.
MR with diffusion-weighted imaging
MRI studies were performed using a 1.5-T MRI system (MR 450, General Electric Healthcare, Milwaukee, WI, USA), with a 12-channel body coil and high performance gradient (50 mT/m). DW images were obtained by two acquisitions. First, respiratory-gated axial diffusion-weighted images of the liver were obtained with the following settings: slice thickness 5 mm, field of view (fov): 40 cm2, matrix 92 × 128 pixels, b = 800 s/mm2, number of excitations (NEX) 6, acquisition time 2 min 40 s. A second acquisition covering the entire abdomen and pelvis was performed with free breathing under the following settings: slice thickness 5 mm, fov 40 cm2, matrix 80 × 128 pixels, NEX 12, b = 800 s/mm2, time acquisition 3 min. Finally, conventional MR images were obtained with T2 single shot covering the entire abdomen and pelvis (TR 964 ms, TE 148 ms) and T1-weighted sequence gradient-echo (LAVA-Flex, General Electric Healthcare) with arterial and portal phase on the liver and delayed phase on the entire abdomen and pelvis. All patients received a single intravenous dose of meglumine gadoterate (0.1 mmol/kg; Dotarem; GuerbetGroup, France) and a single dose of glucagon (20 mg) was injected just before the first acquisition to minimise bowel motion. All patients had to drink a mannitol solution to obtain bowel distension (0.5–1.5 L mannitol 5%, 45 min before the study).
Image analysis
PET/CT and MR-DWI images were retrospectively and independently reviewed by two physicians specialised in nuclear medicine (MS and GP) and two radiologists (VB and VA), respectively, who all have a subspecialist expertise in oncological imaging. They were blinded to the results of previous examinations and patients’ clinical features. The conclusions of each physician were initially recorded. In the event of disagreement, images were then reviewed to reach consensus.
Findings of PC
Imaging diagnosis of PC relied on a visual analysis for both techniques. FDG-PET/CT diagnosis of PC was based on either intense focal or linear uptake regarding the peritoneum, the left subphrenic space or along the liver surface. Co-registered MDCT helped to classify slight to moderate focal FDG uptake as PC in the case of equivocal images, taking into account that partial volume effects might underestimate FDG uptake for small lesions.
Diffusion-weighted and conventional MR images were reviewed together. On the DW images, linear and/or nodular areas of hyperintensity located in the peritoneum were recorded as PC. Focal areas were recorded as peritoneal tumours if not related to a T2 shine-through effect from structures containing fluid. Conventional MRI provided anatomical localisation of hyperintense DW foci. In the case of DW hyperintensity without the corresponding lesions on conventional MR images, PC was considered only if the physician could rule out a T2 shine-through effect. Hyperintensity on DW images that were related to possible bowel air artefacts were dismissed and not recorded as peritoneal metastases. Nodular and/or linear findings on conventional MR images without hyperintensity on DW images were not considered to be peritoneal tumours. Even if suggestive of PC, ascites was not considered as representative of PC for PET/CT and MR interpretation in this study.
Assessment of PC extent
After discussion with surgeons, we decided to divide the peritoneal cavity into three sites. The transverse mesocolon defined supra- and inframesocolic spaces. The supramesocolic space was also divided into left and right areas, defined as the left and right sides of the falciform ligament. This simplified division adapted from Jacquet and Sugarbaker [13] was chosen to facilitate imaging interpretation and correlation with the standard of reference (see below). Lesion size measured as the largest diameter was evaluated either on pathological analysis if available or axial images of PET/CT or MR studies. The presence of visceral, lymphatic or bone metastases with both techniques was also considered for clinical purposes, but not taken into account in the present study, which focuses on PC.
Standard of reference
Peritoneal carcinomatosis was confirmed either by pathological data in the case of surgery or laparoscopic exploration, or patient follow-up with FDG-PET/CT and/or MR-DWI imaging after 3 months. For patients who underwent surgery, surgical and pathological reports were reviewed to determine the presence and extent of peritoneal dissemination with respect to the classification described above. The following imaging criteria were applied as the reference standard in patients without surgery, for both PET/CT and MR-DWI: lesions appearing significantly larger (at least a 20% increase) than during initial workup or showing a significant decrease after chemotherapy (at least a 30% decrease) were considered to be true positive; lesions initially detected that had resolved without therapy were considered to be false positive. In the case of mismatch between the two investigations, if lesions visible on the positive investigation were considered to be true positive (as defined above), the negative investigation was considered to be false negative. On imaging follow-up, no lesion appeared at a site that was classified as negative by both techniques in the initial evaluation.
We also classified each positive site as being involved with subcentimetre or supracentimetre implants according to the measurement of the largest implant either on histopathological evaluation or on imaging studies.
Data analysis
PET and MR results were compared with the reference standard. Descriptive statistical data (sensitivity, specificity, positive predictive value, negative predictive value and accuracy based on the consensus of both physicians) were determined on a patient-based and site-based analysis. McNemar’s test was used to compare sensitivity and specificity between PET and MRI. A kappa statistic was used to assess interobserver agreement for FDG-PET/CT and MR-DWI studies, for both patient-based and site-based analyses. The kappa value was interpreted as follows: poor agreement = less than 0.2, fair agreement = 0.2–0.4, moderate agreement = 0.4–0.6, good agreement = 0.6–0.8 and very good agreement = 0.8–1 [14].
Results
Patients
Thirty patients (11 women, 19 men; median age 63 years, range 25–88) were included in the present study and retrospectively analysed. Thirteen patients were referred for initial staging (43%, 13/30). Seventeen patients were referred to assess tumour relapse in a context of rising tumour markers (6/17), equivocal findings on CT (7/17) or equivocal clinical symptoms of PC such as abdominal pain or bowel occlusion (4/17). These 17 patients were previously treated either by surgery and chemotherapy (n = 12) or by chemoradiotherapy (n = 5). Primary tumours were colorectal cancers (n = 12), pancreatic cancers (n = 8), gastric cancers (n = 5), oesophageal cancers (n = 3) and small-bowel cancers (n = 2). The most frequent pathological type was adenocarcinoma (n = 28/30), including one gastric adenocarcinoma of the mucinous type. Two oesophageal cancers were squamous cell carcinomas. The median interval between the PET/CT and MR-DWI studies was 14 days (range 6–29) and none of the patients received chemotherapy during this interval period.
Presence of PC was assessed surgically in 18 patients (60%, 18/30) and by imaging follow-up in 12 patients (40%, 12/30). Median interval between the first imaging study (PET or MR-DWI) and surgery was 23 days (range 4–44). PC was confirmed in 19 patients (63%, 19/30). Out of a total of 90 potentially involved sites (3 sites per patient), 27 proved to be involved in PC (30%, 27/90).
Patient-based analysis
Table 1 shows the results of PET/CT and MR-DWI in the assessment of PC on a patient-based analysis. The accuracy was good for both PET/CT and MR-DWI at 80% and 83%, respectively (Fig. 1).
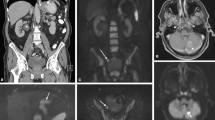
Imaging results in a 55-year-old man referred for initial staging of pancreatic cancer. PET/CT (a maximum intensity projection image, b axial PET/CT fusion) showed abnormal uptake in a large tumour of the pancreas tail (arrowhead) associated with a slight uptake in the right subdiaphragmatic area and an intense focal uptake in the pelvic area (arrows). MR-DWI (c fused T2/DWI, d DWI coronal view) also showed the pancreatic tumour (arrowhead) but identified clear perihepatic high signal (arrows) confirmed as peritoneal implants during laparoscopy
Both techniques missed PC in three patients. These false-negative results were related to subcentimetre implants in two patients and implants arising from a mucinous gastric adenocarcinoma in one patient. False-positive results were retrospectively related to small peritumoral lymph nodes that were misdiagnosed as small peritoneal implants in one patient (for both techniques). Physiological digestive uptake that had a focal appearance led to the two other false-positive results on PET. Regarding MR-DWI, a second false-positive result was retrospectively assigned to artefacts owing to the differences in susceptibility between tissue and air-filled gastrointestinal tract. Of note, one patient had ascites without evidence of PC on FDG-PET/CT and MR-DWI; pathological analysis after surgery confirmed the absence of PC.
The interobserver agreement was very good for PET studies (κ = 0.86) and good for MR-DWI studies (κ = 0.73).
Site-based analysis
Twenty-seven of the 90 potentially involved sites (30%) were positive for PC: inframesocolic sites (18/27), right supramesocolic sites (5/27) and left supramesocolic sites (4/27). Table 2 shows the site-based diagnostic results for the detection of PC in the 90 sites. The overall sensitivity and specificity were not statistically different between MR-DWI (74%, 97%) and PET/CT (63%, 90%, P = 0.27; Fig. 2). In the supramesocolic area, MR-DWI detected more sites than PET/CT (7/9 vs. 4/9 respectively). MR-DWI appeared especially more accurate than PET/CT in the right supramesocolic area (right supramesocolic area 5/5 vs. 3/5, left supramesocolic area 2/4 vs. 1/4, for MR-DWI and PET/CT respectively; Fig. 3). In the inframesocolic area, both techniques showed moderate sensitivities: 72% (13/18) for both techniques (Fig. 4).
Imaging results (a PET maximum intensity projection image, b PET/CT axial fusion, c fused T2/DWI) in a 65-year-old man with previously treated colon cancer with synchronous liver metastases who had increasing tumour markers. Both PET/CT (a MIP, b fused images) and MR-DWI (c fused T2/DWI) showed a focal lesion in the inframesocolic area anterior to the left psoas muscle (arrows). Surgical and histopathological findings confirmed the recurrence
Imaging results in a 57-year-old woman with a previously treated gastric cancer referred for a suspected recurrence (increased level of tumoral markers). PET/CT (a PET maximum intensity projection image) was negative, whereas MR-DWI showed high signal in the perihepatic space (b arrow). A consensus was found resulting in a “wait and see” attitude at the multidisciplinary meeting. Imaging follow-up at 3 months (c PET, d DWI) showed disseminated peritoneal lesions (arrows)
Imaging results in a 61-year-old man referred for initial staging of small-bowel cancer. PET/CT (a axial CT, b axial PET/CT fusion) showed a slight pathological uptake on pericolic subcentimetre nodules (arrows). MR-DWI (c axial T2, d axial DWI) showed moderate pericolic ascites but without high signal on DWI. Diffuse peritoneal implants were confirmed during laparoscopy
A relationship between tumour size and sensitivity was observed for both PET/CT and MR-DWI (Table 3). Sensitivity was lower when tumour implants were subcentimetre with both PET/CT and MR-DWI (42% and 50% respectively). Conversely, sensitivity increased to 80% and 93% (for PET/CT and MRI-DWI, respectively) when tumour implants were supracentimetre.
The interobserver agreement was very good for PET studies (κ = 0.92) and good for MR-DWI studies (κ = 0.78) on a site-based analysis.
Discussion
Diagnosis of PC is a challenge for imaging techniques because of its variable appearance and the small size of lesions. Reported sensitivity of MDCT in the assessment of PC varies from 25 to 90%, depending on site, size and morphology of tumour deposits, and adequacy of bowel opacification [3, 4]. Sensitivity of MDCT is reduced to 7–50% when the size of malignant implants is less than 1 cm [3, 4]. The main reason for this decreased sensitivity is that small tumour implants invaginated within peritoneal reflections or coating the bowel surface are difficult to distinguish from adjacent structures. Functional imaging with FDG-PET/CT and MR-DWI may overcome some limitations of MDCT. Indeed, both techniques show a high tumour-to-background ratio. FDG-PET/CT reflects increased glucose metabolism in tumours, whereas MR-DWI reflects tumoral cellularity [15, 16]. To our knowledge, this is the first study with intraindividual comparison of PET/CT and MR-DWI in the detection of PC.
We found that FDG-PET/CT and MR-DWI are both accurate in the identification of patients with PC arising from gastrointestinal malignancy. Patient-based sensitivity and specificity were rather similar for both techniques, 84% and 73% for PET/CT and 84% and 82% for MR-DWI, respectively. However, PC extent was underestimated with both PET/CT and MR-DWI, as shown by limited site-based sensitivities (63% and 74%, respectively). This finding is mainly explained by a decreased detection capacity for subcentimetre implants for both investigations (42% and 50%, respectively).
In our study, there was a discrepancy between the limited sensitivities of site-based analyses and the good sensitivities of patient-based analyses with both techniques, meaning that the extent of PC was underestimated. Regarding the inframesocolic area, both techniques detected 13 of the 18 inframesocolic sites involved. Several factors can explain the false-negative results of PET/CT. Besides the limited spatial resolution of PET imaging, the variable physiological FDG uptake of the stomach and bowel is sometimes difficult to distinguish from PC [17], leading to either false-negative or false-positive results. Second, physiological movement of digestive loops during the 2-min bed position acquisitions can cause blurring artefacts, leading to an underestimation of FDG uptake.
Interestingly, MR-DWI appeared more sensitive than PET/CT in the detection of supramesocolic lesions, especially in the perihepatic area. Indeed, tumour implants localised in the right supramesocolic peritoneum are more conspicuous on MR-DWI [7, 18] because the liver has a low signal with high b value DWI sequences, thus providing high contrast between peritoneal implants and liver edges. This advantage may be less clear in the left supramesocolic area because of the persistent spleen hypersignal in the DW images. On the other hand, in the right supramesocolic area, PET/CT image interpretation is hampered partly because of the physiological liver uptake. Hence, only perihepatic lesions showing more intense uptake are distinguishable from the liver (Figs. 1, 3). In addition, the partial volume effect generally leads to an underestimation of tumour uptake for small lesions. Finally, respiratory motion causes blurring artefacts, leading to further uptake underestimation for small lesions. To overcome this, our MR protocol included respiratory triggered DW acquisition, which provides better image quality and signal-to-noise ratios than breath-hold DWI [19]. Respiratory-gated PET could be of great interest in the detection of perihepatic PC [20].
Despite high tumour-to-background ratio, we observed that PET/CT and MR-DWI were also hampered in the diagnosis of small peritoneal implants. Published data about the detection of small peritoneal tumours are scarce. Considering this specific issue, our results differ from those of Pfannenberg et al. [12], who reported PET/CT sensitivity of 72% for lesions smaller than 0.5 cm in 22 patients before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. This high sensitivity may be partially explained by the use of a multiphase, contrast-enhanced CT with bowel preparation in their PET/CT protocol. Furthermore, suspected lesions at CT were considered to be peritoneal tumours even in the absence of corresponding uptake. All these considerations raise the question of a “one-stop-shop” imaging protocol with the use of contrast-enhanced PET/CT with respiratory-gating and bowel preparation in the management of patients with known or possible PC.
We observed a good to very good interobserver agreement on a per-patient and per-site basis, with a trend towards PET/CT superiority over MR-DWI. Our results are similar to those of Satoh et al. [21] who investigated PET/CT and MR-DWI accuracies in the detection of PC in two different groups of patients. This underlines one potential advantage of functional imaging, where good interobserver agreement warrants uniform interpretation between physicians working in the same institution. Conversely, diagnosis of PC with MDCT in areas such as the right subdiaphragmatic space, the surface of the small bowel and root of the mesentery are difficult to assess with lower interobserver agreement (kappa values reported from 0.14 to 0.91 [4]).
This study has several limitations. First, it is a retrospective study that encompasses a small population. Second, about one third of the patient population did not undergo surgical and histopathological evaluation. The standard of reference in these patients was based on follow-up with PET/CT and/or MR-DWI, with consequent difficulties in the definitive classification of some small lesions. Third, the image analysis only relied on a visual interpretation. No quantitative assessment and comparison of FDG uptake and apparent diffusion coefficient values were taken into account, as no lesion-based analysis was performed. We chose to classify PC according to a site-based analysis only because precise correlation of each tumour implant visible on imaging with the reference standard was not feasible. Finally, a bowel preparation was not performed on FDG-PET/CT, which could partially explain the low sensitivity we observed in the inframesocolic area.
In conclusion, we showed that FDG-PET/CT and MR-DWI had similar high accuracy in the detection of patients with PC arising from gastrointestinal malignancy, with a slightly better interobserver agreement for PET/CT than for MR-DWI. MR-DWI appeared to be more accurate in the evaluation of the right supramesocolic area, where FDG-PET/CT accuracy seemed to be hampered by respiratory motion and physiological liver uptake. Both techniques underestimate the real extent of the disease, raising the problem of decreased sensitivity for subcentimetre lesions. Even if PET/CT and MR-DWI are more accurate than MDCT, laparoscopic or surgical evaluation remains mandatory in therapeutic planning. FDG-PET/CT and MR-DWI can, however, facilitate patient selection for peritonectomy and hyperthermic intraperitoneal chemotherapy.
At this point, it remains difficult to determine the method of choice in the evaluation of PC arising from gastrointestinal malignancy. FDG-PET/CT, a whole body imaging investigation, remains a very sensitive tool in the detection of extra-abdominal metastases. MR-DWI of the abdomino-pelvic cavity coupled with liver exploration could have an advantage over FDG-PET/CT because of its superior sensitivity in the detection of liver metastases.
References
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H et al (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M et al (2004) Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 22:3284–3292
de Bree E, Koops W, Kroger R, van Ruth S, Witkamp AJ, Zoetmulder FA (2004) Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol 86:64–73
Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E, Chi D et al (2002) Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 223:495–499
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S et al (2008) Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol 18:18–23
Low RN, Sebrechts CP, Barone RM, Muller W (2009) Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings – a feasibility study. AJR Am J Roentgenol 193:461–470
Kyriazi S, Collins DJ, Morgan VA, Giles SL, deSouza NM (2010) Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics 30:1269–1285
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH et al (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49:480–508
Berthelot C, Morel O, Girault S, Verriele V, Poirier AL, Moroch J et al (2011) Use of FDG-PET/CT for peritoneal carcinomatosis before hyperthermic intraperitoneal chemotherapy. Nucl Med Comm 32:23–29
Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J et al (2008) Staging of peritoneal carcinomatosis: enhanced CT vs PET/CT. Abdom Imaging 33:87–93
Pfannenberg C, Konigsrainer I, Aschoff P, Oksuez MO, Zieker D, Beckert S et al (2009) (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 16:1295–1303
Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–374
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Tohma T, Okazumi S, Makino H, Cho A, Mochiduki R, Shuto K et al (2005) Relationship between glucose transporter, hexokinase and FDG-PET in esophageal cancer. Hepatogastroenterology 52:486–490
Sanchez Salmon A, Barandela Salgado J, Ruibal Morell A (2006) PET in abdominal pathology: advantages and limitations. Abdom Imaging 31:174–181
Bozkurt M, Doganay S, Kantarci M, Yalcin A, Eren S, Atamanalp SS et al (2011) Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values. Eur J Radiol 80:224–228
Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS (2009) Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol 192:915–922
Bettinardi V, Picchio M, Di Muzio N, Gianolli L, Gilardi MC, Messa C (2010) Detection and compensation of organ/lesion motion using 4D-PET/CT respiratory gated acquisition techniques. Radiother Oncol 96:311–316
Satoh Y, Ichikawa T, Motosugi U, Kimura K, Sou H, Sano K et al (2011) Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol 196:447–453
Acknowledgements
We are indebted to Dr Bernard Uzzan, who carefully reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soussan, M., Des Guetz, G., Barrau, V. et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol 22, 1479–1487 (2012). https://doi.org/10.1007/s00330-012-2397-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2397-2