Abstract
Inefficient wastewater treatment introduces huge amount of nutrients mainly phosphorus and nitrogen to the natural waterbodies. Excessive phosphate in the water leads to the growth of algae or eutrophication. One-third of the aquatic ecology has been destroyed by eutrophication worldwide including China, Japan, Europe, South Asia and South Africa. Artificial eutrophication affects the water ecology around the world by decreasing the quality standards of water and alters the ecosystem structure and function. Phosphorus is known to be a limiting factor, and it is crucial to remove the phosphate from the effluent prior to exoneration into waterbodies.
Intracellular phosphate content of certain important species of bacteria influences phosphate removal in wastewater treatment. A variety of polyphosphate-accumulating organisms (PAOs) are involved. Under alternating anaerobic and aerobic conditions, these PAOs store phosphate in the form of polyphosphate. Among PAOs, Accumulibacter sp., Pseudomonas sp., Aeromonas hydrophila, Tetrasphaera sp. and gram-positives are the major role players as phosphate removers. As compared to chemical method, biological way of nutrient removal proved to be cost-effective, and it reduces the sludge production. An integrative approach towards phosphoregulation is a key aspect of dealing with the problem.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Enhanced biological phosphorus removal (EBPR)
- Polyphosphate (poly P)
- Polyphosphate-accumulating organisms (PAOs)
6.1 Introduction
Wastewater management, eutrophication and phosphoregulation have an indispensable connection. Water scarcity is the immense problem faced worldwide due to increased growth of population, climate change and inefficient wastewater management. It was reported in the fourth World Water Development Report that only 20% of globally produced wastewater is currently receiving proper treatment (UN report 2012). There is an urgent need to conserve water and reuse the properly treated water for agricultural or other non-portable use.
To meet the needs other than drinking purposes, 1 billion gallons of treated wastewater have been used in the United States. An EPA estimate suggests that almost 91 billion is spent to assert and improve treatment systems all over the nation.
The discharge of untreated wastewater/improperly treated wastewater leads to the major calamity for water sources called “eutrophication” as this wastewater contains a huge amount of nutrients in it. According to UNEP newsletter and technical publication, the untreated wastewater or wastewater treated by conventional mechanical-biological techniques still contains 25–40 mg/l and 6–10 mg/l of nitrogen and phosphorus, respectively [1].
Eutrophication is the enrichment of waterbodies when a huge amount of nutrient-containing wastewater is dumped into it (Yewalkar-Kulkarni et al. 2016). It causes excessive growth of algae leading to the condition called algal bloom, causing the decrease in dissolved oxygen content and death of normal aquatic flora and fauna. Decomposition of these dead matters releases nutrients which amplify the process of eutrophication.
Dodds et al. (2008) showed that combined cost of $2.2 billion (approx.) was spent annually for recreational water usage, waterfront real estate, recovery of lost biodiversity and drinking water as a result of eutrophication in US freshwaters.
To check this deleterious effect, it is essential to control nutrient amount, and prime focus has been given to removal of phosphorus. Wastewater treatment plants or nutrient removal plants take advantage of polyphosphate-accumulating organisms (PAOs) which have the capability of accumulation of polyphosphate by removing phosphate from wastewater. Alternating anaerobic and aerobic condition is required for phosphate removal. Under anaerobic conditions PAOs uptake volatile fatty acid (VFA), e.g. acetate; polyhydroxybutyrate (PHB) is formed from this acetate and further used for cell growth and polyphosphate synthesis under aerobic conditions (Strom 2006). Based on the statistics for wastewater treatment, biological removal seems to be a simple and ecologically balanced way for phosphate removal and to curb eutrophication. Through this chapter we have tried to compile the issues regarding nutrients in wastewater; their ill effects, i.e. eutrophication; how this eutrophication and changing climate is related to each other; and the measures taken to get rid of eutrophication and reduce the nutrient loading into natural waterbodies by WWTPs. In this chapter, we have also illustrated the role of (meta)genomics approach to reveal the bacterial community structure so that the better modelling and designing of the WWTPs could be possible and best quality effluent would be generated to circumvent the harmful effects of effluent and sludge disposal to natural waterbodies. Not only the harmful effects of algal bloom but their use in WWTPs in nutrient sequestration is also discussed.
6.2 Nutrient Issues in Wastewater Management
The combined effluents that come from domestic use, industrial use, urban runoff and agricultural runoff are considered as wastewater. Wastewater management is a process of treatment of wastewater/sewage prior to introducing it to the waterbodies so that the ecology of the water should be maintained. Wastewater contains various pollutants such as:
-
Plant nutrients (phosphorus and nitrogen mainly)
-
Heavy metals (cadmium, mercury, nickel, lead and zinc)
-
Pathogens (bacteria, viruses and other microorganisms)
-
Other organic pollutants (UN report 2015)
All these pollutants have detrimental effects on both environment and human health; therefore, it is indispensable to treat the wastewater before disposing it to the natural water sources. If the wastewater management is neglected, then it will lead to two major impacts: one is chemical and nutrient contamination and the other one is microbial pollution.
6.2.1 Phosphate/Nutrient Induced in the Sewage
Wastewater comes from the industries, urban runoff and household; these are the major sources which introduce the nutrients into the sewage (Romero et al. 2013; UN report 2015). Household wastewater mainly consists grey water (kitchen and bathing wastewater) and black water (excreta, urine and faecal sludge) (UN report 2015). SeaWeb in their newsletter reported sewage and septic tanks as a major source of nutrient pollution as many soap and detergents contain phosphorus, whereas human excreta are known to be nitrogen rich [2]. In relation to microbiota, diversity and species richness are the factors to be considered for balancing the nutrient flow in any ecosystem. It has been found in many ecosystems that functional diversity dictates equilibrium in the ecosystem rather than species number (Mulder et al. 2012). This is an important factor as diversity is dictated by environment and nutrient cycling. Environmental sensing of phosphate has important roles in wastewater treatment (phosphate removal). High phosphate causes eutrophication in an aqueous environment. The intracellular phosphate content of certain important species influences phosphate removal in wastewater treatment (Ruiz-Martinez et al. 2015). Thus phosphate homeostasis could in part influence the rate of removal of phosphate in wastewater and reduce eutrophication.
6.2.2 Nutrient Management in Sewage
Various physical and chemical methods like filtration, membrane technologies and precipitation, respectively, are used to remove phosphate, but the biological method (EBPR) is known to remove phosphate in a cost-effective manner. It also reduces the phosphate level to acceptable standards (Strom 2006) and is also more environmentally friendly (Gunther et al. 2009). According to FAO Corporate Document Repository, after treatment of the sewage water by conventional methods, it still has the nutrients in concentration, phosphorus (P), 10 mg/l; potassium (K), 30 mg/l; and nitrogen (N), 50 mg/l [3]. EBPR is known to achieve around <0.1 mg/L effluent P levels (Barnard 2006). Phosphate removal by struvite precipitation was also studied recently (Lu et al. 2016).
6.2.3 Consequences of Untreated/Partially Treated Sewage Disposal to the Different Waterbodies
The biggest drawback of sewage disposal is that it demolishes the aquatic biodiversity by harmful effect of eutrophication. It also causes dissolved oxygen depletion to satisfy the BOD of organic matter present in sewage (Diaz and Rosenberg 2008). The introduction of sewage also leads to other harmful effects like the introduction of pathogenic organisms and production of unpleasant smelling gases, e.g. H2S (Klein and Perera 2002; Topare et al. 2011). The sewage disposal intensifies the presence of faecal coliforms (Rim-Rukesh and Agbozu 2013); these pathogenic organisms are known to cause waterborne diseases. According to an article in GE step ahead 2015, annually around 1 lakh causalities occur in India due to these waterborne diseases like cholera, jaundice, diarrhoea and typhoid [4]. The partially treated sewage when introduced into fresh waterbodies greatly reduces biological and physicochemical qualities of receiving waterbodies. Rim-Rukesh and Agbozu (2013) studied the impacts of partially treated wastewater on Epie Creek (Nigeria). In their study, they use Malaysian Water Quality Index (WQI) to assay the water quality. They report that Epie Creek is equitably polluted on the basis of their findings: dissolved oxygen (DO) 3.73–5.20 mg/l, chemical oxygen demand (COD) 17.3–53.2 mg/l, biochemical oxygen demand (BOD) 12.4–36.7 mg/l, total faecal coliforms 2,120–20,800 cfu/ml, total phosphorus (TP) 0.73–1.73 mg/l and ammoniacal nitrogen 4.10–5.0 mg/l. With the obvious effect of destroying the waterbody composition, untreated wastewater has long-time consequences including the destruction of waterbody ecosystem by altering the species distribution.
6.3 Eutrophication
The natural ageing process of waterbodies is called eutrophication. In this process of natural ageing, a large, profound, nutrient-poor lake successively turns in to be nutrient rich, and with the course of time, it becomes a pond and then converts to a marsh. The anthropogenic activities have increased the rate of this process, and it became so common that the term eutrophication itself sounds like a terrible condition of waterbodies. In the United States alone, eutrophication accounts for almost one-half of the impaired lake area and 60% of damaged river (Smith 2003). The United Nations Environment Programme (UNEP) in their newsletter and technical publication, volume 3, states that the eutrophic waterbodies are classified into four classes based on their nutrient concentrations (mainly nitrogen and phosphorus): these are oligotrophic, mesotrophic, eutrophic and hypereutrophic containing phosphorus and nitrogen (Table 6.1) [1].
6.3.1 Source of Nutrient to the Waterbodies
Nutrients can be deposited to the aquatic systems by two means either naturally or by human activities (anthropogenic). It will take centuries for a lake to become eutrophic by natural means (Gao 2015), but the anthropogenic activities speed up the process by heavy deposition of nutrients (Chislock et al. 2013; Erisman et al. 2013). Natural rock weathering (Carpenter 2008) and atmospheric depositions (Anderson 2002) are the examples of natural ways, while erosion and leaching from fertilized agricultural areas, development of aquaculture and sewage from cities, urban runoff and industrial wastewater are the main source of anthropogenic activities. Expansion of aquaculture plays a role in eutrophication by discharging the unused animal food and excreta of fish into the water (Klein and Perera 2002). It is quite easy to control the point source of nutrient pollution, whereas the non-point sources are difficult to control.
6.3.2 Consequences of Eutrophication
Ultimately whatever may be the source of nutrient to the waterbodies, the effect will almost always be the same. Availability of the high concentration of nutrients enhances the primary productivity of the waterbodies by increasing the metabolic rates which result in the increased production of phytoplankton and Cyanobacteria out of which some are poisonous and some are non-poisonous. Apart from nutrient availability, physical factors like temperature, renewal of water and light also play an essential role (Klein and Perera 2002). It was observed that in the Chesapeake Bay during the spring season owning to nutrient-rich environment, phytoplankton biomass increases (Anderson 2002). Blooms of Cyanobacteria result into foul-smelling scum and cause problems in drinking water by reducing its taste (Carpenter 2008); it also prevents the penetration of light to the bottom (Lehtiniemi et al. 2005). The major effect of eutrophication is depletion of oxygen and formation of dead zone near the bottom. After the eventual death of algal blooms during the process of decomposition, bacteria consume oxygen, and even some bacteria use sulphates (SO4 2−), and as a result, free S2− take up available dissolved O2 and make it unavailable for organisms (Klein and Perera 2002); this creates hypoxic condition and results into loss of actual biodiversity by killing normal aquatic flora and fauna. In 2005, 146 coastal marine dead zones had been documented around the world, out of which 43 were in the United States (Dybas 2005). The extensive dead zone was created during summer due to nutrient discharge from the Mississippi and Atchafalaya rivers in the northern Gulf of Mexico (Rabalais et al. 2002). Diaz in 2008 observed that dead zones have been reported to affect more than 245,000 km2, which accounts for more than 400 marine ecosystems. Smith (2003) mentioned there were 3,164 reported events of poisoning and 148 deaths of humans in the Asia-Pacific region alone. Economic losses may exceed US $1 million per event, and monitoring efforts may cost up to US $50,000 for each affected area. According to NOAA forecast, the prediction of dead zone ranges from 5204 to 6823 mi2 in the Gulf of Mexico in the year 2016. This forecast is based on the nutrient runoff, river and stream data from the United States Geological Survey (USGS). According to USGS around 20,800 metric tons of phosphorus and 146,000 metric tons of nitrate are received by the Gulf of Mexico from Mississippi and Atchafalaya rivers in May 2016. This makes 25% and 12% above the long term (1980–2015) of nitrate and phosphorus, respectively [5].
6.3.3 Correlation of Eutrophication and Climate Change
Not only by the excessive loading of nutrient by the anthropogenic activities but the climate change also affected the eutrophication. Climate change is mainly the consequence of pollution (especially introduction of greenhouse gases through the burning of fuels) caused by anthropogenic activities (Vijayavenkataraman et al. 2012). Basically, we look at eutrophication as the excessive growth of cyanobacteria (harmful algal blooms). The climate change (altered rainfall patterns, increased storms, melting glaciers and warming soil) increases the signs of eutrophication (Jeppesen et al. 2010; Jeppesen et al. 2011). Rising temperature correlates with eutrophication by various ways like increasing the nutrient loading due to increased mineralizing rate and reducing the water level which causes the concentration of existing nutrients; this is accompanied by low intense storm which causes nutrient loading due to soil erosion (Rustad et al. 2001; Brookshire et al. 2011). Paerl and Paul (2012) studied the anthropogenic and climatic control on a harmful algal bloom. They state that growth of cyanobacteria and the bloom-forming ability are highly influenced by the nutrient enrichment as well as climatic changes like hydrologic changes, global warming and increased frequencies and intensities of tropical storms and droughts. Temperature rise has a positive impact on the cyanobacterial growth (Watkinson et al. 2005). The cyanobacterial photoprotective and photosynthetic pigments absorb light and add on to the increased water temperature (Paerl and Paul 2012).
So far we have discussed the direct effect of climate change on eutrophication and growth of (cyano) harmful algal blooms, but climate change is also influenced indirectly by the consequence of eutrophication, i.e. dead zones. Recently, Altieri and Gedan (2015) reviewed the impact of climate change on dead zone. They also examined various climatic parameters like temperature, precipitation, wind, storm, ocean acidification and sea-level rise and concluded that it can affect the O2 availability and response to hypoxia. The main reason of dead zone is the appearance of a hypoxic condition in the waterbodies and death of aquatic fauna due to low oxygen availability. The temperature rise plays a key role in promoting the hypoxic condition. Warm water has low capacity to hold oxygen; thus the rise in temperature causes low availability to aquatic animals (Altieri and Gedan 2015). Another effect of the rise in temperature is that it causes stratification of the surface water and prevents mixing of oxygenated water (Cloern 2001). The climate change is also known to affect the time and rate of production of phytoplanktons (Winder and Sommer 2012). Similar kind of observation was made by Alheit et al. (2005); they observed early bloom formation in the Baltic Sea for the warmer period. As compared to phytoplanktons, their grazers are more sensitive towards temperature change; in many examples, it is seen that with the increase in temperature, grazing ability also increases. Climate warming also results in widening of existing dead zones and change in duration by the following ways: sea-level rise, season stretching and hypoxic thermal kill zones (Altieri and Gedan 2015).
Thus, climate change has an impact on eutrophication. It seems subtle if we take into consideration a short time period. But, over the course of time, this pools together to create more impact on enhancing rates of eutrophication.
6.3.4 Members of Algal Bloom
Among the bloom-forming organism, blue-green algae (cyanobacteria) usually override other algal species (Smith 2001; Wang and Lei 2016). Paerl and Paul (2012) reported about the algal bloom-forming toxigenic cyanobacteria, and these are Anabaena, Cylindrospermopsis, Microcystis and Oscillatoria (Planktothrix), while others are Phaeocystis and several dinoflagellates (Prorocentrum, Gymnodinium, Dinophysis) (Klein and Perera 2002). The composition slightly differs for different waterbodies, but the effects remain the same.
6.3.5 Remedy of Eutrophication
In order to mitigate the harmful effects of eutrophication, various approaches suggested by many people are compiled by Chislock et al. (2013). These approaches are:
-
Diversion of excess nutrients
-
Altering nutrient ratios
-
Application of potent algaecides and herbicides
But it seems to be more important to check the primary causative agent of eutrophication, i.e. nutrient loading. To circumvent eutrophication, only the control of reactive nitrogen is not enough. Measures to control phosphorus are essential and must be included in management programmes (Carpenter 2008). In the early 1990s, the deposition of about 50% of total P to Tar-Pamlico watershed of the Albemarle-Pamlico estuarine systems in North California by the largest phosphorus mine reduced to more than 90%, placing the example of point source control (Anderson 2002). This is the first line as the deposition of excessive phosphorus in any form is controlled beforehand. To eradicate phosphate from the point sources, various strategies are applied by the wastewater treatment plants; these are discussed in details in the other section of this chapter.
6.4 Phosphoregulation
So far we have discussed the phosphate content in wastewater, phosphate pollution in the natural waterbodies and its harmful effects. But phosphate itself does not harm directly; it’s the excessive growth of algal bloom which is responsible for the detrimental effects on water ecosystem. In this section, we discuss the importance of phosphate in life, phosphate regulation in wastewater and other things.
6.4.1 Phosphate Flow in Ecosystems
Phosphorus is considered as the fifth most essential element for growth. Not only bacteria but plants as well as humans all require phosphorus in their nutrition. It plays a role in various biological processes like synthesis and maintenance of membrane and is involved in cell signalling as a second messenger and component of genetic material and energy metabolism (Santos-Beneit 2015). Bergwitz and Juppnner (2011) have reported that, if not properly regulated, phosphate can cause many diseases in humans like muscle myopathy, tumour-induced osteomalacia, cardiomyopathy, neuropathy and haemolysis. Misregulation of phosphate in the environment causes an environmental problem (Santos-Beneit 2015).
In general, bacteria utilizes the inorganic form of phosphorus (phosphate ion, i.e. Pi) present in its environment. In the case of unavailability of inorganic phosphate, the bacteria use organic compounds containing phosphate like phosphonates and glycerol-3-phosphate. They have two types of transporters for phosphate ion depending upon the availability of Pi; these are Pi-specific transporter (Pst) and low-affinity phosphate transporter (Pit) expressed under low and high availability of Pi, respectively. In the case of organic compounds containing phosphate, related transporters and enzymes are involved in their metabolism. Under the Pi-rich condition, the bacteria take up an excess amount of inorganic phosphate and with the help of enzyme polyphosphate kinase (ppk) forms a long polymer called polyphosphate (poly P) which severs as a source of energy under Pi-starving conditions (Fig. 6.1) (Santos-Beneit 2015).
6.4.2 Phosphorus Sequestration in Natural Waterbodies
We know that natural waterbodies receive phosphorus from both natural and anthropogenic activities (which may include the point source or non-point source) such as agricultural runoff and municipal and industrial sewage effluents (Howarth et al. 2000). As per United Nations Environment Programme (UNEP), the excessive nutrient loading can be prevented by wetlands; this ascertains a natural way to eradicate the problem of eutrophication. The basic concept is that wetland soil adsorbed the phosphorus present in the effluent/polluted water, whereas the nitrate is released as nitrogen in the atmosphere, after its conversion. Díaz et al. (2012) report the effectiveness of constructed wetland to reduce the agricultural runoff pollutants before discharging it in Sacramento-San Joaquin river system (California).
Many studies reported the phosphorus removal by adsorption methods, e.g. on activated aluminium oxide (Genz et al. 2004), oxide tailings (Zeng et al. 2004), zeolite (Karapinar 2009), ferrihydrite (Carabante et al. 2010) and titanium dioxide (Delaney et al. 2011). Zamparas et al. (2012) studied the effectiveness of modified bentonite (Zenith/Fe) in phosphorus sequestration in natural waterbodies and report 80% removal of phosphorus. Earlier used sand, gravels and soil do not remove nutrient with great efficiencies (Park 2009). Recent researches suggest that the industrial wastes and by-products would aid to improve the nitrogen and phosphorus removal if used as the substrate (Ahmad et al. 2016). Ahmad et al. (2016) reviewed the use of water treatment sludge as a substrate in constructed wetlands with improved efficiencies in nutrient removal.
Some materials can form hazardous species; e.g. it has been viewed in many cases that aluminium causes toxicity to living organisms (Haghseresht 2004). The agricultural by-products (ABPs) can be successfully used as biosorbent as these can be a prevalent source because of its low cost, eco-friendliness, and utilization of agricultural wastes (Nguyen (TAH) et al. 2012). The agricultural by-products are either used in its natural form or can be modified in order to increase their effectiveness (Table 6.2). The efficiency of ABPs is also governed by parameters like pH, temperature, adsorbent dosage, interfering ions and contact time (Nguyen (TAH) et al. 2012).
6.4.3 Phosphorus Sequestration Through Designed Bioreactors
Strom (2006) reported that phosphorous can be removed by various methods like filtration of particulate phosphate, membrane technologies, precipitation, crystallization, adsorption, constructed wetlands and enhanced biological phosphorous removal – EBPR. Various reactors and treatment techniques have been designed and practised with the aim of improving the effluent quality (to reduce the nutrient loading into natural water system). In this section, we discuss the bioreactors used in nutrient removal except for EBPR system; because of its huge serving in the nutrient removal, it is discussed separately. Seow et al. (2016) reviewed wastewater treatment technologies and discussed aerobic granulation, biofilm technology and microbial fuel cell techniques and their merits and demerits. All three techniques are used to treat various kinds of wastewater, but aerobic granulation shows effective results in phosphate/nutrient removal. The aerobic granulation technique is known to remove nutrients (nitrogen and phosphorus) from the slaughterhouse wastewater, livestock wastewater and domestic sewage with the values 99.3% and 83.5%, 73% and 70%, and maximum volumetric conversion rates for nitrogen and phosphorus were 0.17 and 0.24 kg/m3 in respective wastewater (Othman et al. 2013; Liu et al. 2015; Pronk et al. 2015). In the Netherlands under the trade name of Nereda®, a full-scale domestic sewage treatment plant is operated using aerobic granulation technique (Pronk et al. 2015).
Electrocoagulation is a technique used with increasing rate to treat not only the industrial wastewater but also raw domestic sewage as well as tertiary treated to reduce the microbes, nutrients and other personal care pollutants. Energy efficiency and less expensive nature make it popular to use in the wastewater treatment process. Percent mean reduction of phosphorus in both raw and tertiary-treated wastewater sample was found to be 95.79% and 96.33%, respectively (Symonds et al. 2015). Ozyonar and Karagozoglu (2011) perceived 98% phosphorus removal from domestic sewage by using electrocoagulation technique and concluded that aluminium electrode effectively removes phosphorus.
The microalgal culture is used to remove phosphorus because of its ability to assimilate this nutrient for its growth in photobioreactor which employed light source for the cultivation of microalgae. Abdel-Raouf et al. (2012) reviewed the use of microalgae in wastewater treatment. He states different treatment systems, like immobilized cell system, dialysis culture, tubular photobioreactor, stabilization pond and algal mat, and also the use of harvested biomass for energy production. The microalgal membrane technique is highly popular because of its ease in harvesting the biomass. Singh and Thomas (2012) worked on a unique microalgae membrane photoreactor (mMR) with the aim of furnishing the effluent obtained from domestic wastewater treating aerobic membrane bioreactor. They reported good rate of removal of NO3, NO2 and PO4 under both batch and continuous modes of operation. The algal-bacterial biofilm reactor reported to remove phosphorus, nitrogen and carbon from domestic wastewater at 10 days HRT (hydraulic retention time), 85 ± 9%, 70 ± 8% and 91 ± 3%, respectively (Posadas et al. 2013). Sukacova et al. (2015) studied the phosphorus removal by microalgal biofilm under different light conditions and reported 97 ± 1% of phosphorus under nonstop synthetic illumination.
6.4.4 Mechanism of Phosphate Sequestration
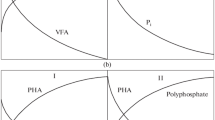
Wastewater treatment plants use enhanced biological phosphorus removal (EBPR) process to remove phosphate from sewage. The EBPR process is basically an activated sludge process functioning under anaerobic and aerobic conditions and introduces the influent wastewater in anaerobic phase (Kristiansen et al. 2013); this enriches the polyphosphate-accumulating organisms (PAOs). Under the anaerobic condition where the influent wastewater is mixed, the bacteria are able to uptake the carbon source/volatile fatty acid (e.g. acetate) and convert it into polyhydroxybutyrate (PHB), deriving the energy from stored poly P, and release orthophosphate in medium (Martin et al. 2006; Wong and Beiko 2015). The reducing equivalent required is obtained from either conversion of stored glycogen to PHB and CO2 or by partial oxidation of acetyl CoA through the TCA cycle (Skennerton et al. 2015). Under aerobic conditions poly P is synthesized by accumulating the phosphate present outside the cell; it also synthesizes glycogen by using the energy from anaerobically stored PHB. Thus the rate of P accumulation is much higher than the rate it is released during the anaerobic phase; this is how the PAOs are able to remove the higher amount of phosphate from the wastewater (Lu et al. 2016). This mechanism is diagrammatically represented (Fig. 6.2).
6.4.5 Microbial Community Structure: Its Relation to Phosphorus
Enhanced biological phosphorus removal (EBPR) process is not performed by a single dominant bacterial species, but a mixed population of bacteria is able to utilize carbon source/VFA, store PHB, accumulate poly P, store and maintain glycogen, etc. Some heterotrophic bacteria are known to accumulate polyphosphate and use it as the source of phosphate and energy under phosphate-starving condition (Santos-Beneit 2015). Many researchers have found pure cultures, e.g. members of genera Acinetobacter, Tetrasphaera, Microlunatus and Lampropedia, with the traits of PAOs, but they were not found to be significant for wastewater treatment plants (Seviour et al. 2003). In 2009, Gunther et al. developed a dual polyphosphate/DNA fluorescent staining approach which explores the knowledge of noncultivable PAOs. Fluorescent staining determines the number of poly P granules, whereas DNA contents and cell size explain the active PAOs. For isolation and identification of the representative PAOs, cell sorting and terminal restriction fragment length polymorphism (T-RFLP) profiling of 16S rRNA gene were used. The dual staining divides the complex community into subcommunities on the basis of their growth rate and poly P content. 16s rRNA gene sequencing verified the staining technique specificity and generated clone library. This library showed a low diversity composed of phylotypes of Candidatus Accumulibacter and members of Pseudomonas and Tetrasphaera genera. The tetracycline (TC) and 4′-6′-diamidino-2-phenylindole (DAPI) are considered as a reliable method for the detection of PAO activity. In other studies, it was reported that the Accumulibacter sp. and Tetrasphaera sp. are the abundant poly P accumulators (Oehmen et al. 2007; Nielsen et al. 2010; Nguyen et al. 2011). The Tetrasphaera sp. comprises 30–35% of the microbial community, whereas Accumulibacter sp. forms only 3–10% (Gu et al. 2008; He et al. 2008; Nielsen et al. 2010; Nguyen et al. 2011). Sidat et al. (1999) carried out a study using a culture-dependent method and isolated 39 monocultures from the sludge procured from Johannesburg full-scale BNR system, out of which 24 isolates showed the phosphorus-accumulating ability. Gram negative forms 58% and gram positive forms 42% of total phosphorus-accumulating population isolated from sludge. Their study showed that gram positive and Pseudomonas sp. form the 50% of the population.
For successful operation of EBPR and its further optimization, it is essential to have the knowledge of PAO biodiversity (Blackall et al. 2002). To explore the microbial community dynamics, Ju and Zhang (2015) revealed a statistical method based on the correlation between bacterial community networks and the associated taxonomic affiliations through this predict co-occurrence and co-exclusion patterns of the community. Through this work they studied the 16s rRNA gene sequencing data, and species-species association (SSA) network was constructed which comprised of 3899 pairwise significant SSA correlations which connect 170 species-level OTUs. During their work, they found that apart from the closely related bacteria (taxonomically) which share the similar niche taxonomically, less related species were also observed and posed competition to community assembly.
6.5 GAOs as Competitor of PAOs
Glycogen-accumulating organisms (GAOs) are the potential competitor of PAOs for volatile fatty acids, and they do not accumulate phosphorus (Oehmen et al. 2006; Gu et al. 2008). Candidatus Competibacter phosphatis (B12%) and Defluviicoccus vanus (9%) were found to be the abundant GAOs in EBPR system (Saunders et al. 2003; Burow et al. 2007). If Competibacter sp. is present along with Accumulibacter sp. in WWTPs, then it will hamper the EBPR process by not achieving the target level of phosphorus removal (Zhang et al. 2011). Metabolically the GAOs are the same as that of PAOs except they do not accumulate poly P and use it as a source of energy under anaerobic phase (Cydzik-Kwiatkowska and Zielińska 2016).
6.6 Carbon and Phosphorus Metabolism in EBPR
EBPR system places a good example where one can study the correlated carbon and phosphorus metabolism. All PAOs have the same or related genes and their function for phosphate and central carbon metabolism. Here we take Accumulibacter as an example organism to discuss the metabolic process involved in EBPR.
The phosphate metabolism of PAO involves the gene for low-affinity phosphate transporters (pitA) and phosphate-specific transporters (pstABC) and genes responsible for the formation (ppk1) and hydrolysis (ppx) of polyphosphate. The metabolic pathways are almost similar for PHA production from acetate, glycolysis and the TCA cycle (Skennerton et al. 2015). There is a mystery regarding Accumulibacter carbon metabolism, either the EMP or ED pathway is followed during anaerobic glycolysis (Oehmen et al. 2007). Recently sequencing of all the Accumulibacter genomes showed the presence of the EMP pathway (Hesselmann et al. 2000). The source of reducing the power required during the formation of PHB presented another topic of conflict. Reducing power was either provided by degradation of store glycogen or by anaerobic operation of the citric acid cycle, i.e. TCA cycle (Skennerton et al. 2015). For anaerobic TCA to occur, it is mandatory to reoxidize the reduced quinones; quinol reductase helps in this process which is found in the genome of Accumulibacter UW-1 (Martin et al. 2006). A pictorial representation of this metabolic event that occur in Accumulibacter is given (Fig. 6.3). The biochemical network of EBPR is thus much more complex than just utilization and removal of phosphate as it has inputs from central energy metabolism. But, studying this in detail will help engineer bacteria through potentially suggesting a carbon source which ultimately assists phosphate removal. Such conditioned consortia would most likely have increased EBPR.
Metabolic event that occur in the Accumulibacter EBPR system. Events that occur in anaerobic conditions are depicted with black arrows and aerobic events with red arrows; black dot ( ) represents the inorganic phosphate, (
) represents the inorganic phosphate, ( ) represents the reducing equivalent, (
) represents the reducing equivalent, ( ) represents AMP, (
) represents AMP, ( ) represents ADP and (
) represents ADP and ( ) represents ATP
) represents ATP
6.7 Metagenomic Approach
Due to the immense contribution of (meta)genomic approaches in exploring the phylogenetic composition and functional prospective of a complex community in the EBPR process, this section is purely devoted to metagenomics and its application in WWT plants. Initially, the metagenomic approaches were used to identify the uncultivable microbes; now these approaches are used to study the dynamics of bacterial communities (Meena et al. 2015; Ambardar et al. 2016; Sharma and Lal 2017). The first metagenomic study was applied to obtain a draught genome of Accumulibacter phosphatis UW-1 from lab-scale EBPR reactors (Martin et al. 2006). Phylogenetic studies are not only limited to 16s rRNA gene but also carried out by using ppk1 gene. Study of these genes categorized the Accumulibacter into two clades, i.e. type I and type II, which is again subdivided into IA-IE and IIA-IIF, respectively (Flowers et al. 2013; Skennerton et al. 2015). Deep knowledge was gathered from the independent work of many researchers who use next-generation sequencing methods and expand our knowledge regarding the genetic variety of Accumulibacter; their work produces additional draught genomes, and these are as follows: one of A. phosphatis UW-2 from clade IA (Flowers et al. 2013), one of Accumulibacter sp. strain HKU-1 from Class IB (Mao et al. 2014) and one from clade IA, one from clade IC, three from clade IIC and three from clade IIF (Skennerton et al. 2015). Thus as a number of draught genomes for these bacteria increase, our knowledge and ability to build an effective as well as safe consortia for EPBR increase. It is quite difficult to compile the metagenomic studies; we have tried to gather the information and compile in a tabular form for easy understanding (Table 6.3).
6.8 Algae: Problem as Well as Boon
So far we have seen the algal community as the culprit of the worst situation of the aquatic systems called eutrophication, and we have also discussed its harmful effects. The ability of the algae to show robust growth in the presence of nutrient-rich conditions is only the dark shade of algae. This nature of algae can be exploited by using algae in the nutrient removal systems in WWT plants. This idea has been used in the early 1950s, and now this has been used in many lab scales and pilot-scale plants with more innovative techniques and improved efficiencies. Many researchers have reported the use of microalgal culture in the wastewater treatment with the ability of microalgae in nutrient stripping as well as removal of heavy metals and toxic compounds, and the biomass can be successfully used for other purposes (Kumar and Goyal 2010; Pittman et al. 2011; Abdel-Raouf et al. 2012; Ruiz-Martinez et al. 2012; Yewalkar-Kulkarni et al. 2016). The obtained biomass can be further used for the production of biofuels like biodiesel (Schenk et al. 2008), fertilizers and animal feed and pharmaceutical industry (Singh and Thomas 2012). The use of microalgae in wastewater treatment is popular because of its skill to use solar energy for biomass production which to some extent shorts down the cost of the process and can be grown in the outdoor solar bioreactors (photobioreactor) (Abdel-Raouf et al. 2012). For the enhanced removal of nutrient, either the monoculture or the polyculture is being used. Ruiz-Martinez et al. (2012) in their work used a polyculture with the idea that the strain will get selected and evolve according to the need and condition.
Like bacteria microalgae are also known to store phosphorus in the form of polyphosphate (poly P) and will use it later on under the phosphate starvation conditions (Eixler et al. 2006; Larsdotter 2006; Powell et al. 2008, 2009, 2011; Rao et al. 2011). Apart from this biological way of phosphorus removal, chemical reactions are also known to remove the phosphate in the culture. As the photosynthesis occurs, it will reduce the CO2 which results in the increase in the pH. This increased pH causes the precipitation of phosphate after complex formation with metal ions (Pires et al. 2013). There are multiple safe ways to remove phosphorus, and it depends upon environmental conditions prevalent in waterbodies and level of eutrophication whether to use a single or a combination of ecologically safe methods.
6.8.1 Microalga Used in WWT
Cai et al. (2013) discussed the efficiency of microalgal species in nutrient removal from various wastewater sources. They account the efficacy of Chlorella sp., C. pyrenoidosa, C. sorokiniana, C. vulgaris, Scenedesmus sp., S. obliquus, Oscillatoria sp. and Arthrospira sp. in the removal of nitrogen and phosphorus. Likewise various others have reported different microalgae in wastewater treatment, Cyanobacteria, Phormidium sp. (Abdel-Raouf et al. 2012), Chlorella (Chlorella sp.), Scenedesmus dimorphus (S. dimorphus), Chlorella vulgaris (C. vulgaris), Scenedesmus quadricauda (S. quadricauda) (Singh and Thomas 2012), Spirulina platensis (Lodi et al. 2003), Chlamydomonas globosa, Chlorella minutissima and Scenedesmus bijuga (Bhatnagar et al. 2011). Many people have reported the effective removal of nutrient in wastewater with the aid of microalgae culture (Table 6.4).
6.8.2 Current Advances in the Phosphorus Sequestration by Algae
Pires et al. (2013) have reviewed the recent improvement in the use of microalgae culture in the wastewater treatment with the three different culturing techniques, i.e. suspended cells, immobilized cells and microalgae consortia (microalgae and bacteria). In suspended cell culture, the microalgae were found to remove average nutrient concentrations, 35.5 mg/l of NH4 +, 0.40 mg/l of NO3 − and 3.89 mg/l of PO4 3−, and it was observed that initial higher cell density gives better efficiencies of removal (Abdel-Raouf et al. 2012). For the immobilization of cells, alginate and carrageenan polymers were used (Pires et al. 2013), Chlorella vulgaris (Tam and Wong 2000), Dunaliella salina (Pires et al. 2013), Scenedesmus obliquus (Ruiz-Marin et al. 2010), Scenedesmus rubescens (Shi et al. 2007) and Scenedesmus sp. (Fierro et al. 2008; Zhang et al. 2008; He and Xue 2010). Various studies have been carried out using the microalgae and bacteria consortia. This proves to be an economical way to remove nutrients as microalgae provide O2 and bacteria provide CO2 by its action of degradation of organic compounds (Munoz and Guieysse 2006; Park et al. 2008; Subashchandrabose et al. 2011). De-bashan and Bashan (2004) reported 100% ammonium, 15% nitrate and 36% phosphorus removal from municipal wastewater by immobilizing the microalgae-bacterium consortium (Chlorella vulgaris/Azospirillum brasilense and Chlorella sorokiniana/Azospirillum brasilense) in alginate beads.
The expensive immobilizing material and its fragility during long-term operation use lead to the innovative idea of using biofilm photobioreactor (Guzzon et al. 2008; Boelee et al. 2011; Zamalloa et al. 2013). Posadas et al. (2013) studied the carbon, nitrogen and phosphorus removal with the efficiencies 91 ± 3%, 70 ± 8% and 85 ± 9%, respectively. A study of phosphorus removal under different light regime was carried out by using microalgal biofilm (biofilm photobioreactor), and it was reported that under continuous artificial illumination, this system can remove 97 ± 1% of total phosphorus from wastewater, while only 36–41% is removed when illuminated for 12 h. The species found to be present in biofilm were Phormidium autumnale, Pseudanabaena sp., Chroococcus sp., coccal green alga, Monoraphidium contortum, Diatoms, Scenedesmus acutus and Cymbella minuta; they were subject to seasonal variation in abundance, but some remains constant throughout the same like Pseudanabaena sp. remains always dominant and Cymbella minuta remains always rear (Sukacova et al. 2015).
Perspective
Huge amount of money is spent by the government for the construction and upgradation of wastewater treatment plants, but it did not achieve the appropriate goal of making wastewater fit for discharge. Phosphorus (nutrient pollution) in the waterbodies causes eutrophication which alters the water ecology. Wastewater treatment plants exploit the ability of PAOs for the phosphorus removal from wastewater. This proves to be effective, but still there is a scope of research for making these bacteria more efficient and achieve above the discharge limit. Metagenomics and genomic techniques revealed a lot of information about the community dynamics and give the insight about the molecular level which gives the idea of metabolic fluxes. For better understanding of this process, system biology approaches must be applied along with other fruitful strategies like metatranscriptomics, meta-metabolomics or community metabolomics, proteomics, etc.
References
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Ahmad T, Ahmad K, Alam M (2016) Sustainable management of water treatment sludge through 3 ‘R’concept. J Clean Prod 124:1–13. https://doi.org/10.1016/j.jclepro.2016.02.073
Albertsen M, Hansen LBS, Saunders AM, Nielsen PH, Nielsen KL (2012) A metagenome of a full-scale microbial community carrying out enhanced biological phosphorus removal. ISME J 6:1094–1106. https://doi.org/10.1038/ismej.2011.176
Alheit J, Möllmann C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N (2005) Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. ICES J Mar Sci J Conseil 62:1205–1215. https://doi.org/10.1016/j.icesjms.2005.04.024
Altieri AH, Gedan KB (2015) Climate change and dead zones. Glob Chang Biol 21:1395–1406. https://doi.org/10.1111/gcb.12754
Ambardar S, Gupta R, Trakroo D, Lal R (2016) High throughput sequencing: an overview of sequencing chemistry. Indian J Microbiol 56:394–404. https://doi.org/10.1007/s12088-016-0606-4
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25:704–726. https://doi.org/10.1007/BF02804901
Bai Y, Zhang Y, Quan X, Chen S (2016) Nutrient removal performance and microbial characteristics of a full-scale IFAS-EBPR process treating municipal wastewater. Water Sci Technol 73:1261–1268. https://doi.org/10.2166/wst.2015.604
Barnard J (2006) Requirements for achieving effluent phosphorus of less than 0.1 mg/L. WERF Workshop: 9–11
Benyoucef S, Amrani M (2011) Removal of phosphorus from aqueous solutions using chemically modified sawdust of Aleppo pine (Pinus halepensis Miller): kinetics and isotherm studies. Environmentalist 31:200–207. https://doi.org/10.1007/s10669-011-9313-1
Bergwitz C, Jüppner H (2011) Phosphate sensing. Adv Chronic Kidney Dis 18:132–144. https://doi.org/10.1053/j.ackd.2011.01.004
Bhatnagar A, Chinnasamy S, Singh M, Das KC (2011) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energ 88:3425–3431. https://doi.org/10.1016/j.apenergy.2010.12.064
Blackall LL, Crocetti GR, Saunders AM, Bond PL (2002) A review and update of the microbiology of enhanced biological phosphorus removal in wastewater treatment plants. A Van Leeuw J Microb 81:681–691. https://doi.org/10.1023/A:1020538429009
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2011) Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res 45:5925–5933. https://doi.org/10.1016/j.watres.2011.08.044
Brookshire ENJ, Gerber S, Webster JR, Vose JM, Swank WT (2011) Direct effects of temperature on forest nitrogen cycling revealed through analysis of long term watershed records. Glob Chang Biol 17:297–308. https://doi.org/10.1111/j.1365-2486.2010.02245.x
Burow LC, Kong Y, Nielsen JL, Blackall LL, Nielsen PH (2007) Abundance and ecophysiology of Defluviicoccus spp., glycogen-accumulating organisms in full-scale wastewater treatment processes. Microbiology 153:178–185. https://doi.org/10.1099/mic.0.2006/001032-0
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19:360–369. https://doi.org/10.1016/j.rser.2012.11.030
Carabante I, Grahn M, Holmgren A, Hedlund J (2010) In situ ATR–FTIR studies on the competitive adsorption of arsenate and phosphate on ferrihydrite. J Colloid Interface Sci 351:523–531. https://doi.org/10.1016/j.jcis.2010.07.064
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci U S A 105:11039–11040. https://doi.org/10.1073/pnas.0806112105
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educ Knowl 4:10
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253. https://doi.org/10.3354/meps210223
Cydzik-Kwiatkowska A, Zielińska M (2016) Bacterial communities in full-scale wastewater treatment systems. World J Microbiol Biotechnol 32:1–8. https://doi.org/10.1007/s11274-016-2012-9
de Lima ACA, Nascimento RF, de Sousa FF, Josue Filho M, Oliveira AC (2012) Modified coconut shell fibers: a green and economical sorbent for the removal of anions from aqueous solutions. Chem Eng J 185:274–284. https://doi.org/10.1016/j.cej.2012.01.037
De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246. https://doi.org/10.1016/j.watres.2004.07.014
Delaney P, McManamon C, Hanrahan JP, Copley MP, Holmes JD, Morris MA (2011) Development of chemically engineered porous metal oxides for phosphate removal. J Hazard Mater 185:382–391. https://doi.org/10.1016/j.jhazmat.2010.08.128
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929. https://doi.org/10.1126/science.1156401
Díaz FJ, Anthony TO, Dahlgren RA (2012) Agricultural pollutant removal by constructed wetlands: implications for water management and design. Agric Water Manag 104:171–183. https://doi.org/10.1016/j.agwat.2011.12.012
Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, Thornbrugh DJ (2008) Eutrophication of US freshwaters: analysis of potential economic damages. Environ Sci Technol 43:12–19. https://doi.org/10.1021/es801217q
Dybas CL (2005) Dead zones spreading in world oceans. Bioscience 55:552–557. https://doi.org/10.1641/0006-3568(2005)0550552
Eixler S, Karsten U, Selig U (2006) Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 45:53–60. https://doi.org/10.2216/04-79.1
Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AMR, Leach AM, de Vries W (2013) Consequences of human modification of the global nitrogen cycle. Philos Trans R Soc Lond Ser B Biol Sci 368:20130116. https://doi.org/10.1098/rstb.2013.0116
Fierro S, del Pilar S-SM, Copalcua C (2008) Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour Technol 99:1274–1279. https://doi.org/10.1016/j.biortech.2007.02.043
Flowers JJ, He S, Malfatti S, del Rio TG, Tringe SG, Hugenholtz P, McMahon KD (2013) Comparative genomics of two ‘Candidatus Accumulibacter’clades performing biological phosphorus removal. ISME J 7:2301–2314. https://doi.org/10.1038/ismej.2013.117
Gao W, Chen Y, Liu Y, Guo HC (2015) Scientometric analysis of phosphorus research in eutrophic lakes. Scientometrics 102:1951–1964. https://doi.org/10.1007/s11192-014-1500-7
Genz A, Kornmüller A, Jekel M (2004) Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide. Water Res 38:3523–3530. https://doi.org/10.1016/j.watres.2004.06.006
AZ G, Saunders A, Neethling JB, Stensel HD, Blackall LL (2008) Functionally relevant microorganisms to enhanced biological phosphorus removal performance at full-scale wastewater treatment plants in the United States. Water Environ Res 80:688–698. https://doi.org/10.2175/106143008X276741
Günther S, Trutnau M, Kleinsteuber S, Hause G, Bley T, Röske Müller S (2009) Dynamics of polyphosphate-accumulating bacteria in wastewater treatment plant microbial communities detected via DAPI (4′, 6′-diamidino-2-phenylindole) and tetracycline labeling. Appl Environ Microb 75:2111–2121. https://doi.org/10.1128/AEM.01540-08
Guzzon A, Bohn A, Diociaiuti M, Albertano P (2008) Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res 42:4357–4367. https://doi.org/10.1016/j.watres.2008.07.029
Haghseresht F (2004) Comparison of the factors that affect performances of Phoslock and Alum. Phoslock Water Solutions Ltd. Internal Report. IR 002/04
He S, Xue G (2010) Algal-based immobilization process to treat the effluent from a secondary wastewater treatment plant (WWTP). J Hazard Mater 178:895–899. https://doi.org/10.1016/j.jhazmat.2010.02.022
He S, AZ G, McMahon KD (2008) Progress toward understanding the distribution of Accumulibacter among full-scale enhanced biological phosphorus removal systems. Microb Ecol 55:229–236. https://doi.org/10.1007/s00248-007-9270-x
Hesselmann RPX, Von Rummell R, Resnick SM, Hany R, Zehnder AJB (2000) Anaerobic metabolism of bacteria performing enhanced biological phosphate removal. Water Res 34:3487–3494. https://doi.org/10.1016/S0043-1354(00)00092-0
Howarth RW, Anderson DM, Church TM, Greening H, Hopkinson CS, Huber WC, Wiseman WJ (2000) Clean coastal waters: understanding and reducing the effects of nutrient pollution. National Academy Press, Washington, DC. ISBN: 0-309-06948-3, 405 p
Ismail ZZ (2012) Kinetic study for phosphate removal from water by recycled date-palm wastes as agricultural by-products. Int J Environ Stud 69:135–149. https://doi.org/10.1080/00207233.2012.656975
Jeppesen E, Moss B, Bennion H, Carvalho L, De Meester L, Feuchtmayr H, Liboriussen L (2010) Interaction of climate change and eutrophication. In: Kernan M, Battarbee RW, Moss B (eds) Climate change impacts on freshwater ecosystems. Blackwell Publishing Ltd, Somerset, pp 119–151. ISBN: 978-1-4051-7913-3
Jeppesen E, Kronvang B, Olesen JE, Audet J, Søndergaard M, Hoffmann CC, Beklioglu M (2011) Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 663:1–21. https://doi.org/10.1007/s10750-010-0547-6
Ju F, Zhang T (2015) Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant. ISME J 9:683–695. https://doi.org/10.1038/ismej.2014.162
Ju F, Guo F, Ye L, Xia Y, Zhang T (2014) Metagenomic analysis on seasonal microbial variations of activated sludge from a full-scale wastewater treatment plant over 4 years. Environ Microbiol Rep 6:80–89. https://doi.org/10.1111/1758-2229.12110
Karapınar N (2009) Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J Hazard Mater 170:1186–1191. https://doi.org/10.1016/j.jhazmat.2009.05.094
Klein G, Perera P (2002) Eutrophication and health. Office for Official Publications of the European Commission, Luxembourg. World Health Organization, Geneva
Kong Y, Ong SL, Ng WJ, Liu WT (2002) Diversity and distribution of a deeply branched novel proteobacterial group found in anaerobic–aerobic activated sludge processes. Environ Microbiol 4:753–757. https://doi.org/10.1046/j.1462-2920.2002.00357.x
Kong Y, Nielsen JL, Nielsen PH (2005) Identity and ecophysiology of uncultured actinobacterial polyphosphate-accumulating organisms in fullscale enhanced biological phosphorus removal plants. Appl Environ Microb 71:4076–4085. https://doi.org/10.1128/AEM.71.7.4076-4085.2005
Kristiansen R, Nguyen HTT, Saunders AM, Nielsen JL, Wimmer R, Le VQ, Nielsen KL (2013) A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal. ISME J 7:543–554. https://doi.org/10.1038/ismej.2012.136
Kumar R, Goyal D (2010) Waste water treatment and metal (Pb2+, Zn2+) removal by microalgal based stabilization pond system. Indian J Microbiol 50:34. https://doi.org/10.1007/s12088-010-0063-4
Larsdotter K (2006) Microalgae for phosphorus removal from wastewater in a Nordic climate, p 11. PhD thesis, School of Biotechnology, Royal Institute of Technology, Stockholm, Sweden. ISBN 91-7178-288-5
Lehtiniemi M, Engström-Öst J, Viitasalo M (2005) Turbidity decreases anti-predator behaviour in pike larvae, Esox lucius. Environ Biol Fish 73:1–8. https://doi.org/10.1007/s10641-004-5568-4
Liu Y, Kang X, Li X, Yuan Y (2015) Performance of aerobic granular sludge in a sequencing batch bioreactor for slaughterhouse wastewater treatment. Bioresour Technol 190:487–491. https://doi.org/10.1016/j.biortech.2015.03.008
Lodi A, Binaghi L, Solisio C, Converti A, Del Borghi M (2003) Nitrate and phosphate removal by Spirulina platensis. J Ind Microbiol Biotechnol 30:656–660. https://doi.org/10.1007/s10295-003-0094-5
Lu YZ, Wang HF, Kotsopoulos TA, Zeng RJ (2016) Advanced phosphorus recovery using a novel SBR system with granular sludge in simultaneous nitrification, denitrification and phosphorus removal process. Appl Microbiol Biotechnol:1–8. https://doi.org/10.1007/s00253-015-7249-y
Lv JH, Yuan LJ, Chen X, Liu L, Luo DC (2014) Phosphorus metabolism and population dynamics in a biological phosphate-removal system with simultaneous anaerobic phosphate stripping. Chemosphere 117:715–721.0020doi:https://doi.org/10.1016/j.chemosphere.2014.10.018
Mao Y, Yu K, Xia Y, Chao Y, Zhang T (2014) Genome reconstruction and gene expression of “Candidatus Accumulibacter phosphatis” Clade IB performing biological phosphorus removal. Environ Sci Technol 48:10363–10371. https://doi.org/10.1021/es502642b
Martín HG, Ivanova N, Kunin V, Warnecke F, Barry KW, McHardy AC, Dalin E (2006) Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nat Biotechnol 24:1263–1269. https://doi.org/10.1038/nbt1247
Meena KK, Kumar M, Mishra S, Ojha SK, Wakchaure GC, Sarkar B (2015) Phylogenetic study of methanol oxidizers from Chilika-Lake sediments using genomic and metagenomic approaches. Indian J Microbiol 55:151–162. https://doi.org/10.1007/s12088-015-0510-3
Mulbry W, Kondrad S, Pizarro C, Kebede-Westhead E (2008) Treatment of dairy manure effluent using freshwater algae: algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Bioresour Technol 99:8137–8142. https://doi.org/10.1016/j.biortech.2008.03.073
Mulder C, Boit A, Mori S, Arie Vonk J, Dyer SD, Faggiano L, Marquet PA (2012) 1 Distributional (In) congruence of biodiversity-ecosystem functioning. Adv Ecol Res 46:1
Munoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815. https://doi.org/10.1016/j.watres.2006.06.011
Nguyen HTT, Le VQ, Hansen AA, Nielsen JL, Nielsen PH (2011) High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol Ecol 76:256–267. https://doi.org/10.1111/j.1574-6941.2011.01049.x
Nguyen TAH, Ngo HH, Guo W, Nguyen TV (2012a) Phosphorous removal from aqueous solutions by agricultural by-products: a critical review. J Water Sustain 2:193–207
Nguyen HTT, Nielsen JL, Nielsen PH (2012b) ‘Candidatus Halomonas phosphatis’, a novel polyphosphate-accumulating organism in full-scale enhanced biological phosphorus removal plants. Environ Microb 14:2826–2837. https://doi.org/10.1111/j.1462-2920.2012.02826.x
Nielsen PH, Mielczarek AT, Kragelund C, Nielsen JL, Saunders AM, Kong Y, Vollertsen J (2010) A conceptual ecosystem model of microbial communities in enhanced biological phosphorus removal plants. Water Res 44:5070–5088. https://doi.org/10.1016/j.watres.2010.07.036
Nielsen PH, Saunders AM, Hansen AA, Larsen P, Nielsen JL (2012) Microbial communities involved in enhanced biological phosphorus removal from wastewater—a model system in environmental biotechnology. Curr Opin Biotechnol 23:452–459. https://doi.org/10.1016/j.copbio.2011.11.027
Oehmen A, Saunders AM, Vives MT, Yuan Z, Keller J (2006) Competition between polyphosphate and glycogen accumulating organisms in enhanced biological phosphorus removal systems with acetate and propionate as carbon sources. J Biotechnol 123:22–32. https://doi.org/10.1016/j.jbiotec.2005.10.009
Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL, Reis MA (2007) Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res 41:2271–2300. https://doi.org/10.1016/j.watres.2007.02.030
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15:249–257. https://doi.org/10.1023/A:1023856702544
Othman I, Anuar AN, Ujang Z, Rosman NH, Harun H, Chelliapan S (2013) Livestock wastewater treatment using aerobic granular sludge. Bioresour Technol 133:630–634. https://doi.org/10.1016/j.biortech.2013.01.149
Ozyonar F, Karagozoglu B (2011) Operating cost analysis and treatment of domestic wastewater by electrocoagulation using aluminium electrodes. Pol J Environ Stud 20:173–179
Paerl HW, Paul VJ (2012) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363. https://doi.org/10.1016/j.watres.2011.08.002
Park WH (2009) Integrated constructed wetland systems employing alum sludge and oyster shells as filter media for P removal. Ecol Eng 35:1275–1282. https://doi.org/10.1016/j.ecoleng.2009.05.015
Park Y, Je KW, Lee K, Jung SE, Choi TJ (2008) Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia 598:219–228. https://doi.org/10.1007/s10750-007-9152-8
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2013) Wastewater treatment to enhance the economic viability of microalgae culture. Environ Sci Pollut Res Int 20:5096–5105. https://doi.org/10.1007/s11356-013-1791-x
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102:17–25. https://doi.org/10.1016/j.biortech.2010.06.035
Posadas E, García-Encina PA, Soltau A, Domínguez A, Díaz I, Muñoz R (2013) Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour Technol 139:50–58. https://doi.org/10.1016/j.biortech.2013.04.008
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42:5958–5962. https://doi.org/10.1021/es703118s
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae–defining the polyphosphate dynamics. Water Res 43:4207–4213. https://doi.org/10.1016/j.watres.2009.06.011
Powell N, Shilton A, Pratt S, Chisti Y (2011) Phosphate release from waste stabilisation pond sludge: significance and fate of polyphosphate. Water Sci Technol 63:1689–1694. https://doi.org/10.2166/wst.2011.336
Pronk M, de Kreuk MK, de Bruin B, Kamminga P, Kleerebezem R, van Loosdrecht MCM (2015) Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res 84:207–217. https://doi.org/10.1016/j.watres.2015.07.011
Rabalais NN, Turner RE, Wiseman WJ Jr (2002) Gulf of Mexico hypoxia, AKA “the dead zone”. Annu Rev Ecol Syst 33:235–263. https://doi.org/10.1146/annurev.ecolsys.33.010802.150513
Rao PH, Kumar RR, Raghavan BG, Subramanian VV, Sivasubramanian V (2011) Application of phycoremediation technology in the treatment of wastewater from a leather-processing chemical manufacturing facility. Water SA 37:07–14
Rim-Rukeh A, Agbozu E (2013) Impact of partially treated sewage effluent on the water quality of recipient Epie Creek Niger Delta, Nigeria using Malaysian Water Quality Index (WQI). J Appl Sci Environ Manag 17:5–12
Romero E, Garnier J, Lassaletta L, Billen G, Le Gendre R, Riou P, Cugier P (2013) Large-scale patterns of river inputs in southwestern Europe: seasonal and interannual variations and potential eutrophication effects at the coastal zone. Biogeochemistry 113:481–505. https://doi.org/10.1007/s10533-012-9778-0
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T (2010) Growth and nutrient removal in free and immobilized green algae in batch and semicontinuous cultures treating real wastewater. Bioresour Technol 101:58–64. https://doi.org/10.1016/j.biortech.2009.02.076
Ruiz-Martinez A, Garcia NM, Romero I, Seco A, Ferrer J (2012) Microalgae cultivation in wastewater: nutrient removal from anaerobic membrane bioreactor effluent. Bioresour Technol 126:247–253. https://doi.org/10.1016/j.biortech.2012.09.022
Ruiz-Martínez A, Serralta J, Romero I, Seco A, Ferrer J (2015) Effect of intracellular P content on phosphate removal in Scenedesmus sp. experimental study and kinetic expression. Bioresour Technol 175:325–332. https://doi.org/10.1016/j.biortech.2014.10.081
Rustad LEJL, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562. https://doi.org/10.1007/s004420000544
Santos-Beneit F (2015) The Pho regulon: a huge regulatory network in bacteria. Front Microbiol 6:402. https://doi.org/10.3389/fmicb.2015.00402
Saunders AM, Oehmen A, Blackall LL, Yuan Z, Keller J (2003) The effect of GAOs (glycogen accumulating organisms) on anaerobic carbon requirements in full-scale Australian EBPR (enhanced biological phosphorus removal) plants. Water Sci Technol 47:37–43
Saunders AM, Albertsen M, Vollertsen J, Nielsen PH (2016) The activated sludge ecosystem contains a core community of abundant organisms. ISME J 10:11–20. https://doi.org/10.1038/ismej.2015.117
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43. https://doi.org/10.1007/s12155-008-9008-8
Seow TW, Lim CK, Norb MHM, Mubarakb MFM, Lam CY, Yahya A, Ibrahim Z (2016) Review on wastewater treatment technologies. Int J Appl Environ Sci 11:111–126
Seviour RJ, Mino T, Onuki M (2003) The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev 27:99–127. https://doi.org/10.1016/S0168-6445(03)00021-4
Sharma A, Lal R (2017) Survey of (meta) genomic approaches for understanding microbial community dynamics. Indian J Microbiol 57:23–58. https://doi.org/10.1007/s12088-016-0629-x
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423. https://doi.org/10.1007/s10811-006-9148-1
Sidat M, Bux F, Kasan HC (1999) Polyphosphate accumulation by bacteria isolated from activated sludge. Water SA 25:175–179
Singh G, Thomas PB (2012) Nutrient removal from membrane bioreactor permeate using microalgae and in a microalgae membrane photoreactor. Bioresour Technol 117:80–85. https://doi.org/10.1016/j.biortech.2012.03.125
Skennerton CT, Barr JJ, Slater FR, Bond PL, Tyson GW (2015) Expanding our view of genomic diversity in Candidatus Accumulibacter clades. Environ Microbiol 17:1574–1585. https://doi.org/10.1111/1462-2920.12582
Smith VH (2001) Blue-green algae in eutrophic fresh waters. Lake Line 21:34–36
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollut Res Int 10:126–139. https://doi.org/10.1065/espr2002.12.142
Strom PF (2006) Technologies to remove phosphorus from wastewater. Rutgers University, New Brunswick, p 18
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29:896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009
Sukačová K, Trtílek M, Rataj T (2015) Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res 71:55–63. https://doi.org/10.1016/j.watres.2014.12.049
Sydney EB, Da Silva TE, Tokarski A, Novak AC, De Carvalho JC, Woiciecohwski AL, Soccol CR (2011) Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy 88:3291–3294. https://doi.org/10.1016/j.apenergy.2010.11.024
Symonds EM, Cook MM, McQuaig SM, Ulrich RM, Schenck RO, Lukasik JO, Breitbart M (2015) Reduction of nutrients, microbes, and personal care products in domestic wastewater by a benchtop electrocoagulation unit. Sci Rep 5:9380. https://doi.org/10.1038/srep09380
Tam NFY, Wong YS (2000) Effect of immobilized microalgal bead concentrations on wastewater nutrient removal. Environ Pollut 107:145–151. https://doi.org/10.1016/S0269-7491(99)00118-9
Topare NS, Attar SJ, Manfe MM (2011) Sewage/wastewater treatment technologies: a review. Chem Commun 1:18–24
UN report (2012) Managing water under uncertainty and risk, The United Nations world water development report 4, UN Water Reports, World Water Assessment Programme
UN report (2015) Wastewater management-A UN-Water analytical brief, New York
Vijayavenkataraman S, Iniyan S, Goic R (2012) A review of climate change, mitigation and adaptation. Renew Sustain Energy Rev 16:878–897. https://doi.org/10.1016/j.rser.2011.09.009
Wang B, Lan CQ (2011) Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour Technol 102:5639–5644. https://doi.org/10.1016/j.biortech.2011.02.054
Wang LP, Lei K (2016) Rapid identification and quantification of Aureococcus anophagefferens by qPCR method (Taqman) in the Qinhuangdao coastal area: a region for recurrent Brown tide breakout in China. Indian J Microbiol 56:491–497. https://doi.org/10.1007/s12088-016-0619-z
Watkinson AJ, O’Neil JM, Dennison WC (2005) Ecophysiology of the marine cyanobacterium, Lyngbya majuscula (Oscillatoriaceae) in MoretonBay, Australia. Harmful Algae 4:697–715. https://doi.org/10.1016/j.hal.2004.09.001
Winder M, Sommer U (2012) Phytoplankton response to a changing climate. Hydrobiologia 698:5–16. https://doi.org/10.1007/s10750-012-1149-2
Wong DHJ, Beiko RG (2015) Transfer of energy pathway genes in microbial enhanced biological phosphorus removal communities. BMC Genomics 16:526. https://doi.org/10.1186/s12864-015-1752-5
Xu X, Gao Y, Gao B, Tan X, Zhao YQ, Yue Q, Wang Y (2011) Characteristics of diethylenetriamine-crosslinked cotton stalk/wheat stalk and their biosorption capacities for phosphate. J Hazard Mater 192:1690–1696. https://doi.org/10.1016/j.jhazmat.2011.07.009
Yewalkar-Kulkarni S, Gera G, Nene S, Pandare K, Kulkarni B, Kamble S (2016) Exploiting phosphate-starved cells of Scenedesmus sp. for the treatment of raw sewage. Indian J Microbiol:1–9. https://doi.org/10.1007/s12088-016-0626-0
Zamalloa C, Boon N, Verstraete W (2013) Decentralized two-stage sewage treatment by chemical–biological flocculation combined with microalgae biofilm for nutrient immobilization in a roof installed parallel plate reactor. Bioresour Technol 130:152–160. https://doi.org/10.1016/j.biortech.2012.11.128
Zamparas M, Gianni A, Stathi P, Deligiannakis Y, Zacharias I (2012) Removal of phosphate from natural waters using innovative modified bentonites. Appl Clay Sci 62:101–106. https://doi.org/10.1016/j.clay.2012.04.020
Zeng L, Li X, Liu J (2004) Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res 38:1318–1326. https://doi.org/10.1016/j.watres.2003.12.009
Zhang E, Wang B, Wang Q, Zhang S, Zhao B (2008) Ammonia–nitrogen and orthophosphate removal by immobilized Scenedesmus sp. isolated from municipal wastewater for potential use in tertiary treatment. Bioresour Technol 99:3787–3793. https://doi.org/10.1016/j.biortech.2007.07.011
Zhang Z, Li H, Zhu J, Weiping L, Xin X (2011) Improvement strategy on enhanced biological phosphorus removal for municipal wastewater treatment plants: full-scale operating parameters, sludge activities, and microbial features. Bioresour Technol 102:4646–4653. https://doi.org/10.1016/j.biortech.2011.01.017
Web Links
Halls S, Yamazaki K, Water quality: the impact of Eutrophication.http://www.unep.or.jp/ietc/publications/short_series/lakereservoirs-3/fwd.aspSeaWeb, Ocean issue briefs: nutrients. http://www.seaweb.org/resources/briefings/nutrient.phpFAO corporate document repository, Wastewater treatment and use in agriculture. http://www.fao.org/docrep/T0551E/t0551e03.htm#1.%20wastewater%20characteristics%20and%20effluent%20quality%20parametersGE Step Ahead, Why should we reuse wastewater. http://www.moneycontrol.com/gestepahead/article.php?id=954013&cid=2NOAA Headquarters, Average ‘dead zone’ predicted for Gulf of Mexico in 2016: outlook incorporates multiple hypoxia models for the second year. https://www.sciencedaily.com/releases/2016/06/160610094739.htm
Acknowledgment
Authors highly acknowledge Director, CSIR-NEERI for providing facilities for this work [KRC manuscript no. CSIR-NEERI/KRC/2017/July/EBGD/16]. Varsha Jha and Sampada (Puranik) Chande is supported by UGC Junior Research Fellowship and a postdoctoral fellowship from NIH-Training grant T32 at Yale School of Medicine respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jha, V., Puranik (Chande), S., Purohit, H.J. (2017). Seqestration Options for Phosphorus in Wastewater. In: Purohit, H., Kalia, V., Vaidya, A., Khardenavis, A. (eds) Optimization and Applicability of Bioprocesses . Springer, Singapore. https://doi.org/10.1007/978-981-10-6863-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-6863-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6862-1
Online ISBN: 978-981-10-6863-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)