Abstract
Membranes are crucial for cellular life since they partition cells into different functional and physically separated compartments. Atomic force microscopy (AFM) makes it possible to observe, manipulate, and explore the cell membranes at a molecular resolution and therefore has produced a wealth of new opportunities in cell biology, including understanding the nanoscale organization and dynamics of cell membranes and cell walls, measuring cell mechanics and cell adhesion, and unraveling the molecular elasticity of cellular proteins and the mechanisms by which they assemble into nanodomains in the membrane. Single molecule force spectroscopy (SMFS) based on AFM enables the characterization of the mechanical response of biological matter at the nanometer scale. SMFS techniques exert and/or quantify forces to allow manipulation and characterization of the mechanical properties, functional state, conformations, and interactions of biological systems to molecular resolution. Here, we will mainly introduce the studies of cell membrane mechanics and dynamic process of endocytosis by AFM-based SMFS.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
AFM is an ideal method to study the surface topography of biological membranes. It allows membranes that are adsorbed to flat solid supports to be raster-scanned in physiological solutions with an atomically sharp tip [1]. Therefore, AFM is capable of observing biological molecular machines at work [2]. AFM offers great potential for characterizing single molecule and thus presents many advantages in scientific discoveries [3]. Paul Hansma’s group detected adhesive interaction forces that they attributed to either the rupture of individual hydrogen bonds or hydrogen interacting with ordered water layers near the surface [4]. After this early report, a significant amount of research effort was devoted to using the AFM cantilever as a force sensing device in biological research.
Gaub. H et al. reported the first biological application of SMFS on detection of ligand-receptor pairs interaction in 1994 [5]. And since then, SMFS measurements have particularly attracted scientists’ attention. We do not think it is exaggerated to state that many biologists dream to “play” with a single molecule. Along with optical tweezer experiments, AFM-based single molecule force measurements represent the dawn of what is now termed “single molecule experiments”. The conceptual advance is that single molecule experiments provided different and novel information that could not be assessed by bulk experiments [6]. Over the past two decades, the SMFS technique has been focused on the manipulation of proteins [7, 8], DNA [9, 10], sugars [11], and components of bacterial surfaces [12].
Taken advantages of AFM highly sensitive tip cantilever, force–distance curve could detect forces in a large range from 10 pN to 100 nN, which is wider than the range of other competing techniques [13, 14]. SMFS measurements have been performed on a large variety of biological systems. For example, force spectroscopy measurements were performed on membrane proteins providing information about folding and membrane insertion of the hydrophobic protein domains. Measurements of the interactions between biotin and avidin [5, 15], complementary strands of DNA [16], and an antibody with its antigen [17] were conducted, respectively. On living cell, detecting the mechanical properties and nanoscale forces arising in the cellular dynamic process including endocytosis and direct penetration plays a remarkable role on understanding the physiology and function of living cells and organisms [18, 19]. In this chapter, we will describe several force spectroscopy techniques including force–distance curve, nanoindentation, and force tracing as well as their applications on biological molecules and living cell membrane. We finally provide the practical protocols in SMFS techniques on preparing AFM tips and samples, conducting force–distance/tracing curves, and data analysis.
4.2 Principle
In force spectroscopy, the AFM cantilever is moved perpendicular to the sample (Z-direction), and its position is recorded. Molecules adsorbed on a surface are picked up by a microscopic tip (nanometers wide) that is located on the end of an elastic cantilever. In a more sophisticated version of this experiment (chemical force microscopy) the tips are covalently functionalized with the molecules of interest [20]. A piezoelectric controller then pulls up the cantilever. If some force is acting on the elastic cantilever, for example, because some molecule is being stretched between the surface and the tip, the cantilever will deflect upward (repulsive force) or downward (attractive force). Deflection is measured by the position of a laser beam reflected by the cantilever. The cantilever can be treated as a spring and described with Hooke’s law after its spring constant has been determined [21]. Therefore, force acting on the cantilever and force applied by the cantilever to the surface can be recorded with pN (10−12N) sensitivity [18], and cannot achieve much better resolution only because of thermal noise.
4.2.1 Force–Distance Curve
Force–distance curve is a plot of the deflection of the cantilever, measured by a position-sensitive photodetector, versus the extension of the piezoelectric scanner. In general, force–distance (F versus d) curves are used to measure the vertical force that the tip applies to the surface while a contact-AFM image is being taken. The force F between the tip and the sample is related to the cantilever’s deflection through Hooke’s law:
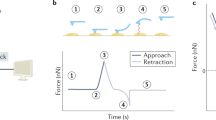
where k is the cantilever’s spring constant, V is the measured cantilever’s deflection in volt, and α is called the deflection sensitivity that converts cantilever’s deflection from volt to nanometers. In order to quantitatively measure interaction forces, it is necessary to calibrate the bending stiffness (or spring constant) of the AFM cantilever as accurately as possible. In a force–distance curve measurement, the sample is mounted on the sample stage that is drove by applying a voltage to the piezoelectric translator, while measuring the cantilever deflection [22]. The principle of force-distance detection of the typical ligand and receptor interactions on living cells by AFM is illustrated in Fig. 4.1. In this force-distance mode, the deflection angle of the cantilever is measured as a function of the vertical position of the cantilever [23]. The tip is moved toward the cell surface (solid line, 1–2) and then retracted (dotted line, 3–4) at a constant lateral position. During tip approaching, the ligand specifically binds with a receptor, which will lead to a force signal with a distinct shape. Upon tip retraction (3), the force increases until bond rupture occurs (4) at an unbinding force (fu). However, for some type of forces the force signal is detected during the approaching process. Usually, a specific type of force will shows a distinct force–distance curve, and the typical force–distance curves for the corresponding interaction pathway are shown in Fig. 4.2.
4.2.2 Force Extension
Because of the high efficiency and piconewton force sensitivity, force measuring mode of the AFM has been used to study the elastic properties of polymers and proteins with mechanical functions. In the force measuring mode [24], deflections result from the forced extension of a sample attached to the cantilever tip. The extension data is obtained by measuring the change in displacement between the two ends of the protein or other molecule of interest. The accuracy of this data is determined by the quality of the piezo-stage. In this configuration, the cantilever is mounted above an absorptive substrate whose height is controlled by a single axis piezoelectric positioner with angstrom resolution. When the both ends of a single polymer/protein are adsorbed between the cantilever and a substrate respectively, recession of the sample substrate causes elongation of the polymer or multimodular protein, resulting in a force signal that causes deflection of the cantilever. The schematic of the force-extension mode is shown in Fig. 4.3 [25]. The exact extension of the stretched protein can be obtained only if the attachment points can be precisely located, e.g., by using microfabricated cantilevers. The exact position of the tip on the soft cantilever, relative to the fixed surface, such as a cover slip, can also be determined directly with the use of a calibrated evanescent field [26]. To describe force-extension curves, the analytical approximation is often used to fit the worm-like chain (WLC) model to experimental force-extension curves [27]:
The parameters T is the absolute temperature, k B presents the Boltzmann constant, L is contour length, and ι p is persistence length.
4.2.3 Dynamic Process of Endocytosis on Living Cells
It is well known that fishing is an act of catching the fish via a bait attached on one end of the fish line. Inspired with the concept of “fishing,” nanoparticles and molecules could also be attached onto a tiny AFM tip to detect the force of single nanoparticle/molecule interaction with living cells under native conditions. Therefore, AFM-based SMFS was used to detect the dynamic processes on living cell membrane, such as the endocytosis of nanoparticle and transporting of biological molecules [28, 29]. The force signal is based on the detection of small shifts of the cantilever that occurs when a tip-tethered molecule/particle was taken by the living cells. The schematic of details about detecting the endocytosis by force spectroscopy on living cell is illustrated in Fig. 4.4. (1) The AFM tip tethered with nanoparticle/molecule initially moves toward the living cell which allows the nanoparticle/molecule to be contacted with the cell membranes. (2) As the tip lands on the cell surface, the nanoparticle/molecule on the pinpoint of AFM tip contact with the cell membranes and the cellular uptake occurs, which produces a force signal during approaching process. During the subsequent tip retraction process (3), a transient force signal is observed, which points to occurrence of the retraction of the nanoparticle/molecule from cell membranes (4). In most case, the force signals of both cellular uptake and retraction of nanoparticle could be simultaneously detected, which is clearly different from the sole unbinding event in the force–distance curve.
4.2.4 Force Tracing Based on AFM
According to the principle of force–distance curve, the AFM tip approaching process may disturb the dynamic process of the cellular uptake, a static AFM tip cantilever to hold the molecule/nanoparticle is necessary. A novel AFM-based technique, force tracing, was developed, which provides an unprecedented temporal-spatial resolution for the in situ observation of dynamic process on living cell membrane surface. The setup for force tracing with an AFM instrument is shown in Fig. 4.5 [30]. A laser beam is reflected by the AFM cantilever. The photodetector detects the laser position and records the change in the cantilever deflection. The AFM tip is approached to the cell surface with contact mode of AFM; subsequently, force–distance curves measuring are performed on the living cell to locate the contact point between the AFM tip and the cell surface. In a force-distance cycle, the AFM tip will stop at the position of Z scan start that is far from the contact point. The AFM tip is slowly moved to the contact point with the feedback system, and then turned off the feedback; thus, the AFM tip is suspended on the cell surface. Upon the application of an external force (the cells take in the molecule/particle tethered on AFM tip and stretch the bifunctional crosslinker), the cantilever is deflected, and the change of vertical position of the laser on the photodetector is acquired using a PCI card that can readily monitor rapid processes down to 1 μs; this card is suitable for recording the process of viral entry into living cells. The software is written in LabVIEW, and the sampling rate is set at suitable value for recording the cellular uptake dynamic process of different nanoparticles and molecules. The displacement of the molecule/nanoparticle during the trans-membrane process includes two parts (Bending distance of the cantilever d and the extension length of the PEG linker h):
4.2.5 Nanoindentation on Cell Membrane
Nanoindentation is one of the tools to analyze samples at a microstructural level, which allows to measure hardness and elastic properties of materials at sub-micrometer depths [31]. It has already been applied to the major classes of materials [32] and is increasingly being used to characterize biological and biomedical materials [33,34,35]. In a traditional indentation test, a hard tip whose mechanical properties are known (frequently made of a very hard material like diamond) is pressed into a sample whose properties are unknown. The load placed on the indenter tip is increased as the tip penetrates further into the specimen and soon reaches a user-defined value. At this point, the load may be held constant for a period or removed. The area of the residual indentation in the sample is measured and the hardness, H, is defined as the maximum load, P max, divided by the residual indentation area, A r :
A complete cycle of loaded and unloaded indentation will create a load–displacement curve (such as the one shown in Fig. 4.6) [36]. This curve can be used to extract mechanical properties of the material. It has already been applied to the major classes of materials [32] and is increasingly being used to characterize biological and biomedical materials [33,34,35]. The ability to conduct nanoindentation studies with nanometer depth and sub-nanonewton force resolution is possible using a standard AFM setup. The AFM allows nanomechanical studies to be conducted alongside topographic analyses, without the use of other dedicated instruments. Load–displacement curves can be collected similarly for a variety of materials, and mechanical properties can be directly calculated from these curves [37]. An AFM nanoindentation experiments is shown in Fig. 4.7 [38]. During its progression into the cell, the sharp pyramidal tip of the AFM cantilever penetrates first into the cell wall with a minor modification of its curvature (Regime A, Fig. 4.7a) and then bends the cell wall, acting on a thin viscoelastic shell (Regime B, Fig. 4.7b). For a given deflection of the cantilever (the contact force), the total displacement of the AFM piezo transducer is the sum of the cantilever deflection δ k = F/k, the depth of penetration of the tip inside the wall δ p , and the deformation (change of curvature) of the wall δ b . The force and penetration depth are measured, generating loading-unloading curves from which the hardness and elastic response of the material under load is evaluated. Furthermore, data can be collected with high spatial resolution, for example, the investigation of complex tissues and “mapping” of properties [39].
Schematic of load–displacement curve for an instrumented nanoindentation test. Reproduced from Ref. [36] by permission of Cambridge University
Sketch of the indentation of the cell wall by a pyramidal shape tip. a The tip penetrates the wall without noticeably change in the curvature of the cell wall. b For a deeper indentation, the wall curvature is modified by the pyramidal tip. Reprinted from Ref. [38], Copyright 2015, with permission from Elsevier
4.3 Applications of Force Spectroscopy on Cell Membranes
Membranes define the cell boundary and surround the various cellular compartments. Such compartmentalization has been essential for the development of life and reflects an important multifunctional tool of organisms. Cellular membranes allow the uptake of nutrients, small molecules and ions, release waste products, bind ligands, transmit signals, convert energy, sense the environment, maintain cell adhesion, control cell migration, and much more while forming a tight barrier [3]. How do the cell membranes fulfill those functions attracts the attention of biologists. Different microscopic approaches for characterizing cellular membranes have been developed. Here, we provide a flavor of the fascinating opportunities offered by the use of AFM-based SMFS as a nanobiotechnological tool in modern membrane biology. The application of SMFS on protein unfolding dynamics, cell adhesion, cell membrane protein, and dynamic endocytosis of living cells would be described.
4.3.1 Protein Unfolding Dynamics
Since the ability of the SMFS based on AFM to measure elasticity was reported in 1997 by Rief et al., the method has proved to stretch a variety of biological polymers, such as polysaccharides [40], DNA [41], and proteins [42]. Hermann E. Gaub used SMFS to investigate the mechanical properties of titin, the giant sarcomeric protein of striated muscle. Individual titin molecules were repeatedly stretched, and the applied force was recorded as a function of the elongation. At large extensions, the restoring force exhibited a sawtooth like pattern, with a periodicity that varied between 25 and 28 nm. Carrion-vazquez et al., compared the process of unfolding the protein by the mechanical SMFS and chemical method. They unfolded I27 domains by SMFS and chemical denaturant, respectively, the unfolding rates obtained by the two methods are same [43]. Furthermore, the transition state for unfolding appears at the same position on the folding pathway when assessed by either method, as shown in Fig. 4.8. They found that mechanical unfolding of a single protein by AFM does indeed reflect the same event that is observed in traditional unfolding experiments. The way is now open for the extensive use of AFM to measure folding reactions at the single molecule level. Recently, Hao Yu et al. reported the dynamics of the unfolding individual bacteriorhodopsin proteins [44]. They unfolded bacteriorhodopsin, a model membrane protein, with ultrashort cantilevers optimized for 1-ms SMFS and thereby uncovered previously unobserved protein dynamics and three major intermediates. Furthermore, the elucidated that the unfolding pathway with improved precision resolved a long-standing discrepancy in the size scale of the fundamental structural elements involved in bacteriorhodopsin unfolding, as deduced from experiments or molecular dynamics. By using ultrashort cantilevers optimized for improved spatiotemporal resolution, force spectroscopy allows to reveal a multiplicity of closely spaced and transiently occupied intermediate states, representing small changes in the molecular conformation. These researches initiate the mechanical unfolding of membrane proteins and, more broadly, enable experimental access to previously obscured protein dynamics.
Comparison of the folding pathway of an Ig domain denatured by an applied force or chemical denaturants. a Model of the stretching of a single Ig domain. Under an applied force, an Ig domain unravels, causing an increase in the end-to-end length, Δx. b Diagram of the folding pathway for an Ig domain as determined using AFM. The changes in free energy (ΔG) are plotted versus the reaction coordinate (end-to-end extension; Δx). Three distinct states are identified: native (N, Δx = 0), condensed denatured (CD, Δx = 25.5 Å), and extended denatured (ED, 25.5 < Δx < 284 Å). The transition state, ‡, is located 2.5 Å away from the native state and 23 Å away from the condensed denatured state. c The folding pathway determined by chemical denaturants. The changes in free energy (ΔG) between the native, N, and the denatured, D, state are shown vs. the reaction coordinate characterized by a fractional distance δ, where 0 < δ < 1 and δ‡ = m‡−N/mD−N = 0.1. The putative intermediate is not shown, as its position in the folding coordinate has not been determined. Reproduced from Ref. [43] with permission, Copyright (1999) National Academy of Sciences, U.S.A
4.3.2 Membrane Protein Mechanics Under Native Conditions
Membrane proteins, often arranged in lipid-protein domains, are involved in basic cellular activities, such as solute and ion transport, energy transduction in respiratory and photosynthetic systems, or sensory stimuli transduction, and information processing; hence, they are important drug targets. SMFS is a powerful tool to address the mechanics of single-membrane proteins in their native environment, i.e., embedded in the lipid bilayer and immersed in a physiological salt solution [45]. When the applied force overcomes the stability of the protein, it unfolds and gives rise to peaks in the force curve that corresponds to elements of secondary structure in the protein [46]. For the investigation of membrane proteins, both strategies of physisorption and covalent bonding via reactive groups have proven to be useful [47, 48]. While imaging the membrane proteins at high resolution, individual proteins were selected, scanning was halted, and the selected protein was then contacted by increasing the force beyond the adhesion threshold, which was found to differ from system to system but it lies typically in the range of 100–200 pN. Upon retracting the tip, a force distance trace was recorded as a protocol for the unfolding and extraction process. A subsequent imaging of the sample allowed the next experiments where a single-membrane protein was extracted would be identified, and the corresponding force distance traces to be selected for further analysis (Fig. 4.9) [49]. By using this technique, Gaub et al. analyzed in depth how the molecular interactions that stabilize the membrane structure of this and other rhodopsins protein are affected by extra- or intracellular unfolding, disulfide bonding, and oligomerization state [50]. SMFS was also used in unfolding and refolding experiments with a bacterial sodium/proton antiporter [51].
Unzipping individual proteins from a regular array and subsequent high-resolution imaging of the respective vacancy. a A distinct relation between the number of force peaks and the related damage is observed on the hexagonally packed intermediate (HPI) layer [52]. Six single peaks and the vacancy corresponding to one hexamer indicate that each force peak corresponds to the removal of one HPI protein. Because one unit contains about 900 residues, only the peptide bridges, holding the HPI proteins together as hexamers, unfold. b Similar observations on the S-layer from c. glutamicum reveal more complex force peaks: dislodging two subunits required one small (arrow) and one fivefold larger force peak [53]. c Unzipping a single bacteriorhodopsin molecule produces a series of force peaks that can unambiguously be related to the seven-transmembrane α-helical structure of bacteriorhodopsin. Reprint from [49], Copyright (2008) by Annual Reviews
4.3.3 Cell Membranes Nanoindentation
The local mechanical properties of cells are frequently probed by force nanoindentation experiments with an AFM-based SMFS. Janshoff Andreas et al. used the typical force nanoindentation measurements to record the stress–strain relationship as well as viscoelastic correspondence principle applied to Hertzian contact mechanics [54]. The cholesterol repletion effect on the nanomechanical properties of human umbilical vein endothelial cell (EA.hy926) was studied using a control-based AFM nanomechanical measurements protocol [55]. Considering that the mechanical properties of the cell could be affected by cell adhesion that induce changes in the cytoskeleton, Samuel T. Souza et al. investigated the influence of the extracellular matrix (ECM) on the elastic properties of fixed macrophage cells using AFM [35]. It is found that there was an increase (about 50%) in the Young’s modulus of macrophages adhered to an ECM-coated substrate as compared with an uncoated glass substrate as shown in Fig. 4.10. In addition, cytochalasin D-treated cells have a 1.8-fold reduction of the Young’s modulus, indicating the contribution of the actin cytoskeleton to the elastic properties of the cell. Therefore, the cell adhesion influences the mechanical properties of the plasma membrane, providing new information toward understanding the influence of the ECM on elastic alterations of macrophage cell membranes. It also implied that cytoskeleton-mediated cell-matrix interactions directly affect biomechanical events in cells by modifying the physical properties of the cell cytoskeleton.
a An AFM image of a macrophage showing the indentation area. Twelve black dots represent the indentations performed on the central area of the cell. b Force-versus-displacement curves are measured in both the reference substrate and macrophages. (1) Fibronectin, (2) glass, (3) fibronectin + cytochalasin, and (4) glass + cytochalasin. The relation between load force and the indentation depth was calculated as the difference between these curves. Reprinted from Ref. [35], with kind permission from Springer Science + Business Media
4.3.4 Single Molecule Transporting via Transporter
Transporting individual molecules across cell membranes is a fundamental process of cellular metabolism. Although the crystal diffraction technique has greatly contributed to our understanding of the structures of the involved transporters, a description of the dynamic transport mechanism at the single molecule level has been extremely elusive. Using SMFS, the structure and dynamics of glucose cotransporter SGLT were studied by Peter Hinterdorfer et al. [56, 57]. Amino acid transporters across the plasma membranes, which mediate and regulate the flow of ionic nutrients into the cells [58, 59], have been classified into distinct families according to their substrate specificity, transport mechanism, and regulatory properties [60]. SMFS was applied to record the transporting events of single cysteine via amino acid transporters in cell membranes by attaching cysteine onto an AFM tip [29]. Cysteine was coupled to NHS-PEG-MAL and aldehyde-PEG-NHS via a thiol group and an amino group, respectively. For aldehyde-PEG-NHS linked cysteine, only the unbinding force “fu” was detected (Fig. 4.11b), which indicates the unbinding force between the amino acid transporter and a cysteine tethered on the AFM tip. For another NHS-PEG-MAL crosslinker tethered cysteine, both the “fi” and “fu” signals were found. The arrow head “fi” indicates the force signal of transporting the cysteine into the cell, and the arrow head “fu” indicates the force signal of pulling the cysteine out of the cell (Fig. 4.11d). The report also reached the conclusion that there are at least two sites of interaction between the transporter and the substrate, one is involved in the initial binding and another one is related to the translocation pathway [56].
Force spectroscopy of cysteine-modified AFM tip with different crosslinker. a Scheme of cysteine conjugation to the AFM tip via aldehyde-PEG-NHS. b The typical force-distance cycle of cysteine coated onto the AFM tip via cysteine-aldehyde coupling on the living HeLa cell in HBSS buffer showing the specific force signal in the retrace process. c Scheme of cysteine conjugation to the AFM tip via the crosslinker NHS-PEG-MAL. d The typical force-distance cycle on HeLa cells with a cysteine coated on the AFM tip via NHS-PEG-MAL. The living cells were pre-incubated in HBSS buffer to remove the extracellular amino acid and then to reduce the intracellular amino acid concentration before performing force spectroscopy. Reproduced from Ref. [29] by permission of The Royal Society of Chemistry
4.3.5 Single Nanoparticle Endocytosis by SMFS
The interactions between nanoparticles and cells determine the entrance of nanoparticles into cells, which is critical for most biomedical applications of nanoparticles [61,62,63,64], e.g., drug delivery [65], subcellular sensors [66], photodynamic therapies [67], and cytotoxicity [68]. Endocytosis works as one of the effective way for nanoparticles internalization [69, 70]. Both experimental and theoretical investigations show that the nanoparticle size [71, 72], shape [61], surface chemistry [65], and ligand arrangement affect its active endocytosis. The SMFS was applied to demonstrate the possibility of measuring the endocytosis force of living cells upon the uptake of single semiconductor quantum dots (QDs) at a single particle level under native conditions [73] (Fig. 4.12). In the force-distance cycle, both the force of cellular uptake of single QD and single QD detachment from the cell were recorded. The value of uptake forces “fi” (endocytosis) ranges from 22 to 80 pN with the loading rate of 1.2 × 105 pN/s, and the maximum of “fi” distribution is about 45 ± 5 pN. The value of unbinding forces ranges from 20 pN to 103 pN with the retracting rate of 1.2 × 105 pN/s, and the maximum of “fu” distribution is about 70 ± 5 pN.
Force measurement of living cells uptaking single quantum dot. a Functionalization of AFM tips by QDs. The green dots show that QDs are covalently coupled to AFM tip via a heterobifunctional PEG, and the arrow indicates the details about the PEG and QD. b A real typical interaction force curve between single QD and the HeLa cell in DMEM at 37 °C. The long arrow indicates the uptaking force signal “fi.” The short arrow indicates the unbinding force signal “fu.” c The histogram of uptaking forces “fi” (n > 200). d The histogram of uptaking forces “fi” after blocking with cytochalasin B (n > 200). Statistical force values of uptaking e and unbinding f events for single Au NP with the diameters of 4, 12, and 17 nm. The vertical lines through boxes represent the distribution of interaction force values. The red, blue, and green box mean the main distribution of interaction force values for 4, 12, and 17 nm Au NP, respectively. The black line in every box represents the average of the force distribution, which increases 8–20 pN as the size of Au NP changes from 4 to 12 nm or 12 to 17 nm. Reproduced from Ref. [28, 73] by permission of The Royal Society of Chemistry
The endocytosis force of nanoparticles is related with the nanoparticle size and surface charge; therefore, it is necessary to detect the endocytosis process with different size particle. With the unique physical and chemical properties, gold nanoparticles (Au NPs) were selected to study the endocytosis process by SMFS. Au NPs with the diameters between 4 and 100 nm have been extensively used in the biomedical applications [74, 75], such as biosensors, drug and gene delivery, and novel photodynamic therapies [61, 71, 76, 77]. It is revealed that both the uptake and unbinding force values were dependent upon the size of gold nanoparticles [28]. In additional, comparing with the endocytosis force of 4 nm QD capped with mercaptoethylamine, 4 nm Au NP capped with cysteine showed a little bit lower endocytic force value. Because that there are net negative charges from the cysteine and the capped citrate ions on the surface of Au NPs, which can repel the negatively charged cell membranes and thus decrease the interaction force. However, on the surface of mercaptoethylamine capped quantum dot, there are lots of positive charges that enhance the interaction force considerably. The SMFS provides a suitable technique to detect the endocytosis force of single nanoparticle on living cell.
4.3.6 Single Organic/Inorganic-Nanoparticle Trans-membrane Dynamic Process
As the first step of cellular uptake, the trans-membrane dynamic process study is really important. Using the ultrafast and sensitive force tracing technique, the trans-membrane dynamic process of single Au NPs was directly recorded, and the crucial role of membrane cholesterol on the dynamic process was discovered [78]. Wang et al. tracked the dynamic process of cellular endocytosis of single Au NP with the average diameters of 5 nm, 10 nm, and 20 nm under physiological conditions, which could reveal new aspects of the dynamic mechanism of NPs entry into living cells. They show that the forces of Au NP endocytosis into the living cells vary from 20 pN to 140 pN, with the average values of 45 ± 14 pN, 67 ± 16 pN, 126 ± 25 pN for 5, 10, and 20 nm Au NPs, respectively. And the duration range between 20 ms and 160 ms, and the average duration for 5, 10, and 20 nm Au NPs are 45 ± 18 ms, 55 ± 18 ms, 81 ± 20 ms, respectively. However the average velocity of Au NP movement during the endocytosis process can be calculated as 0.489 μm s−1, 0.436 μm s−1, and 0.333 μm s−1 for 5, 10, and 20 nm Au NPs, respectively. It is noted that the force and duration are increased with the size of Au NP, but the average velocities decrease as the size of Au NPs increases. The case may be related to the size of the Au NP diameters, with the increase of the interaction area between Au NPs and the cell membrane as well as the local membrane curvature at the contact region becoming larger, which result in the increasing endocytosis force and duration from 5, 10 to 20 nm Au NPs.
As one of the organic materials, polymer plays more and more roles in many fields. Since fabricated by Tomalia et al. [79], dendrimers have been considered as one of the most promising molecular structures to elaborate nanostructured materials [80,81,82]. Szoka et al. reported that polyamidoamine (PAMAM) dendrimers were much superior to other polymeric scaffolds for drug and gene delivery due to their well-defined structure and homogeneously functionalized outer shell [83]. Recent studies demonstrated that dendrimers were capable to enter cells by endocytosis [81] (Fig. 4.13). Utilizing force tracing, the endocytosis process of single G5-PAMAM was studied. The biophysical parameters, including the force, duration, and speed of G5-PAMAM transporting via endocytosis were obtained at the single-particle and microsecond levels. The ∼19 ms of duration and ∼1.0 μm s−1 of speed unquestionably highlighted the fact that the invagination of the G5-PAMAM nanoparticle was quite a rapid process. Block experiments illustrate that the G5-PAMAM transporting through cell membranes relies on the caveolin-mediated/clathrin-mediated endocytosis, and macropinocytosis pathways with the main pathway being clathrin-mediated endocytosis and macropinocytosis, which is also consistent with the previous literature [84].
a Representative force tracing curves, the red arrows make the endocytic force signals clear. b The distribution of endocytic duration, (n ≈ 250). c The distribution of endocytic force, (n ≈ 250). Reproduced from Ref. [84] by permission of The Royal Society of Chemistry
4.3.7 Single Virus Trans-membrane Dynamic Process
As the smallest Trojan horses, viruses hijack the host cell machinery and then propagate and cause fatal harm to the host [85]. The first step in most viral infections is the penetration of the cell membrane via endocytosis. Endocytosis of a single live virion (Singapore grouper iridovirus, SGIV) through the apical membranes of a host cell was monitored by force tracing [30]. The force tracing test reveals that the maximum velocity during the cell entry of a single SGIV by membrane invagination is approximately 200 nm s−1, the endocytic force is approximately 60.8 ± 18.5 pN. And in the force tracing measurements, two major peaks for the viral displacement were identified at 81.0 ± 6.2 nm and 180.2 ± 21.6 nm. This could be attributed to the SGIV size of 200 nm. The majority of the virions are most likely attached to the side of the AFM tip, which corresponds to the peak in the distribution of the viral displacement at approximately 180.2 ± 21.6 nm. A peak at 81.0 ± 6.2 nm indicates that these virions are attached to the tip apex. The peaks in the time distributions were observed at 0.82 ± 0.06 s and 1.42 ± 0.68 s, corresponding to the two peaks in the displacement distribution.
Recently, Wang et al. used force tracing technique to directly record the endocytic force and wrapping time of single human enterovirus 71 (HEV71) virus-like particles via cell membranes. As shown in Fig. 4.14, while the membrane invaginates to form the vesicle packing virus and finish endocytosis process, the AFM tip will bend downward and a force signal could be detected. The force of virus infection ranges from 40 to 80 pN with a maximum distribution of 58 ± 16 pN. It suggests that the invagination of virus is driven by a force of about 60 pN. The time distribution of viral invagination (duration) is 279 ± 68 ms. The force tracing provides a potent technique to explore the fast dynamic process.
Force tracing curves based on AFM. a Typical force tracing curves showing viral invagination via cell membranes. b Distribution of force for cellular uptake of virus (n > 190 from about 2000 force tracing curves). c Distribution of time for viral invagination via cell membranes. Reproduced from Ref. [87] by permission of The Royal Society of Chemistry
4.4 Methods
For the force spectroscopy experiments, the functionalization of AFM tips with relevant biomolecules is required, which is critical for successfully performing a single-molecule experiment. While the sample preparing is of equal importance, such as preparing the living cell, pre-treatment of cells, and modifying the molecules on the support substrate. The quality and reproducibility of the tip and sample preparation govern the reliability of single molecule force spectroscopy measurements [86].
4.4.1 Conjugation of Molecules/Nanoparticles to AFM Tips
AFM tips modification is the essential procedure for the detection of single molecular force. Usually the biomolecules are conjugated to the AFM tips through bifunctional crosslinker via covalent bond, which is generally obtained using either thiol or silane surface chemistries [10, 87,88,89]. The biomolecules should be attached strongly at low surface density to achieve single molecule detection without unspecific adsorption. The methods mentioned above described are also available for attaching cells onto AFM cantilevers. The procedure of conjugating bifunctional crosslinker to the AFM tip is described in detail in Chap. 3. The quality of the surface modifications could be assessed using fluorescence or other surface analysis techniques [90, 91]. Most importantly, quantitative force measurements require accurate determination of the cantilever spring constant, which can be achieved using various methods, among which thermal tune is one of the most used approaches.
4.4.2 Preparing Supporting Surfaces and Living Cell Samples
Generally, mica, glass, and silicon could be used as supports for biomolecules and cell culture. For mica, the freshly pealed surface with positive charge can be modified with APTES, and the function of glass and silicon molecules is just the same as preparing AP-AFM tip cantilever (described in Chap. 3). Procedures are also available to immobilize cells on support substrates. The cells, using in force spectroscopy experiments, are sub-cultured every 2 or 3 days when the petri dish is covered by cells achieved 75% confluence [92]. The adherent Vero cells are washed with PBS (phosphate buffer solution) for three times and serum-free medium one time to remove cell debris and unattached cells before used in the force tracing experiments. As a general rule, it is recommended to keep functionalized tips and samples hydrated and in conditions where their functionality remains intact.
4.4.3 Recording Force Curves
Because of the short lifetime of functionalized tip (due to tip contamination or damage) and sample, they should be freshly prepared just before the experiments. Especially, when the sample surface is fragile and modified with loosely bound material, as often in the case for cell surfaces, the “activity” of the biological tip may be lost after recording a single image or a few force curves. In these conditions, it may therefore be useful to visualize the morphology of the sample surface with an unmodified tip to identify a region that is sufficiently smooth, homogeneous, and stable before engaging a functionalized tip on the same region. To measure discrete molecular interaction forces, it may sometimes be useful not only to dilute the surface density of biomolecules on the tip, but also to modulate the contact force and the contact time between tip and sample. To get reliable force data on a given system, users should record several hundred force curves using many independent tips and samples.
4.4.4 Data Analysis
The SMFS data usually are analyzed by the customized software written in MATLAB. By executing a command of “kspec19,” the files contain sensitivity of the AFM cantilever (Using the molecules or nanoparticles modified AFM tips to perform force spectroscopy on substrate without sample), and the force-distance cycle data will be loaded by the software. In addition, the spring constant of AFM cantilever used for collecting data is inputted. After then, the every force–distance curve will be showed successively, the force signal would be selected and detected by the software. While the measuring and statistics of data are accomplished.
4.5 Conclusions
AFM has become an invaluable tool in membrane biology research. The high temporal-spatial resolution SMFS allows the interaction between molecules and molecular assemblies on the nanoscale, conformational changes and dynamics of membrane proteins, free energy landscape of a protein including the native folded state and unfolded state, and the dynamic process of single molecule/nanoparticle transporting to be revealed. These features make the AFM unique and outstanding, promising highly exciting results in the future from the microscopic to the nanoscopic scales.
However, the technique of SMFS alone could not fully uncover the cell membrane dynamic process exactly. Developing new AFM-based approaches will contribute more to multifunctional tools that will reveal unique insights into pertinent questions in membrane biology.
References
Frederix P, Bosshart PD, Engel A (2009) Atomic force microscopy of biological membranes. Biophys J 96(2):329–338
Goksu EI, Vanegas JM, Blanchette CD et al (2009) AFM for structure and dynamics of biomembranes. Biochim Biophys Acta 1788(1):254–266
Muller DJ (2008) AFM: a nanotool in membrane biology. Biochemistry 47(31):7986–7998
Hoh JH, Cleveland JP, Prater CB et al (1992) Quantized adhesion detected with the atomic force microscope. J Am Chem Soc 114(12):4917–4918
Florin E, Moy V, Gaub H (1994) Adhesion forces between individual ligand-receptor pairs. Science 264(5157):415–417
Casuso I, Rico F, Scheuring S (2011) Biological AFM: where we come from—where we are—where we may go. J Mol Recognit 24(3):406–413
Kabaso D, Gongadze E, Elter P et al (2011) Attachment of rod-like (BAR) proteins and membrane shape. Mini Rev Med Chem 11(4):272–282
Puchner EM, Gaub HE (2009) Force and function: probing proteins with AFM-based force spectroscopy. Curr Opin Struct Biol 19(5):605–614
Lulevich V, Kim S, Grigoropoulos CP et al (2011) Frictionless sliding of single-stranded DNA in a carbon nanotube pore observed by single molecule force spectroscopy. Nano Lett 11(3):1171–1176
Kienberger F, Costa LT, Zhu R et al (2007) Dynamic force microscopy imaging of plasmid DNA and viral RNA. Biomaterials 28(15):2403–2411
Suzuki T, Iwazaki A, Katagiri H et al (1999) Enhanced expression of glucose transporter GLUT3 in tumorigenic HeLa cell hybrids associated with tumor suppressor dysfunction. Eur J Biochem 262(2):534–540
Puntheeranurak T, Stroh C, Zhu R et al (2005) Structure and distribution of the Bacillus thuringiensis Cry4Ba toxin in lipid membranes. Ultramicroscopy 105(1–4):115–124
Muller DJ, Dufrene YF (2011) Atomic force microscopy: a nanoscopic window on the cell surface. Trends Cell Biol 21(8):461–469
Clausen-Schaumann H, Seitz M, Krautbauer R et al (2000) Force spectroscopy with single bio-molecules. Curr Opin Chem Biol 4(5):524–530
Moy V, Florin E, Gaub H (1994) Intermolecular forces and energies between ligands and receptors. Science 266(5183):257–259
Lee G, Chrisey L, Colton R (1994) Direct measurement of the forces between complementary strands of DNA. Science 266(5186):771–773
Dammer U, Hegner M, Anselmetti D et al (1996) Specific antigen/antibody interactions measured by force microscopy. Biophys J 70 (5):2437–2441
Dufrene YF, Pelling AE (2013) Force nanoscopy of cell mechanics and cell adhesion. Nanoscale 5(10):4094–4104
Sharma S, Rasool HI, Palanisamy V et al (2010) Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 4(4):1921–1926
Stevenson J, Brown AJ (2009) How essential is cholesterol? Biochem J 420(2):e1–e4
Janmey PA, McCulloch CA (2007) Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9:1–34
Butt HJ, Cappella B, Kappl M (2005) Force measurements with the atomic force microscope: technique, interpretation and applications. Surf Sci Rep 59(1–6):1–152
Puntheeranurak T, Wimmer B, Castaneda F et al (2007) Substrate specificity of sugar transport by rabbit SGLT1: single-molecule atomic force microscopy versus transport studies. Biochemistry 46(10):2797–2804
Czajkowsky DM, Shao Z (1998) Submolecular resolution of single macromolecules with atomic force microscopy. FEBS Lett 430(1–2):51–54
Fisher TE, Marszalek PE, Oberhauser AF et al (1999) The micro-mechanics of single molecules studied with atomic force microscopy. J Physiol 520(1):5–14
Sarkar A, Robertson RB, Fernandez JM (2004) Simultaneous atomic force microscope and fluorescence measurements of protein unfolding using a calibrated evanescent wave. Proc Nat Acad Sci USA 101(35):12882–12886
Marko JF, Siggia ED (1995) Stretching DNA. Macromolecules 28(26):8759–8770
Shan YP, Ma SY, Nie LY et al (2011) Size-dependent endocytosis of single gold nanoparticles. Chem Commun 47(28):8091–8093
Shang X, Shan YP, Pan YG et al (2013) The force of transporting a single amino acid into the living cell measured using atomic force microscopy. Chem Commun 49(74):8163–8165
Pan Y, Wang S, Shan Y et al (2015) Ultrafast tracking of a single live virion during the invagination of a cell membrane. Small 11(23):2782–2788
Haque F (2003) Application of nanoindentation development of biomedical to materials. Surf Eng 19(4):255–268
Turnbull A, White D (1996) Nanoindentation and microindentation of weathered unplasticised poly-vinyl chloride (UPVC). J Mater Sci 31(16):4189–4198
Fang T-H, Kang S-H, Hong Z-H et al (2012) Elasticity and nanomechanical response of Aspergillus niger spores using atomic force microscopy. Micron 43(2–3):407–411
Roos WH, Wuite GJL (2009) Nanoindentation studies reveal material properties of viruses. Adv Mater 21(10–11):1187–1192
Souza ST, Agra LC, Santos CEA et al (2014) Macrophage adhesion on fibronectin evokes an increase in the elastic property of the cell membrane and cytoskeleton: an atomic force microscopy study. Eur Biophys J Biophys Lett 43(12):573–579
Oliver W, Pharr G. (2004) Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. J Mate Res 19(1):3–20
Kurland NE, Drira Z, Yadavalli VK (2012) Measurement of nanomechanical properties of biomolecules using atomic force microscopy. Micron 43(2):116–128
Digiuni S, Berne-Dedieu A, Martinez-Torres C et al (2015) Single cell wall nonlinear mechanics revealed by a multiscale analysis of AFM force-indentation curves. Biophys J 108(9):2235–2248
Bushby AJ (2001) Nano-indentation using spherical indenters. Nondestruct Test Eva 17(4–5):213–234
Askarova S, Sun Z, GY, Sun GY, Meininger GA, Lee JC-M (2013) Amyloid-b ppeptide on sialyl-lewisx-selectin-mediated membrane tether mechanics at the cerebral endothelial cell surface. PLOS One 0060972
Rief M, Clausen-Schaumann H, Gaub HE (1999) Sequence-dependent mechanics of single DNA molecules. Nat Struct Biol 6(4):346–349
Rief M, Gautel M, Oesterhelt F et al (1997) Reversible unfolding of individual Titin immunoglobulin domains by AFM. Science 276(5315):1109–1112
Carrion-Vazquez M, Oberhauser AF, Fowler SB et al (1999) Mechanical and chemical unfolding of a single protein: a comparison. Proc Nat Acad Sci U S A 96(7):3694–3699
Yu H, Siewny MGW, Edwards DT et al (2017) Hidden dynamics in the unfolding of individual bacteriorhodopsin proteins. Science 355(6328):945–950
Engel A, Muller DJ (2000) Observing single biomolecules at work with the atomic force microscope. Nat Struct Mol Biol 7(9):715–718
Garcia-Saez AJ, Schwille P (2007) Single molecule techniques for the study of membrane proteins. Appl Microbiol Biotechnol 76(2):257–266
Kada G, Kienberger F, Hinterdorfer P (2008) Atomic force microscopy in bionanotechnology. Nano Today 3(1–2):12–19
Chen J, Liu T, Gao J et al (2016) Variation in carbohydrates between cancer and normal cell membranes revealed by super-resolution fluorescence imaging. Adv Sci 1600270
Engel A, Gaub HE (2008) Structure and mechanics of membrane proteins. Annu Rev Biochem 77:127–148
Kessler M, Gottschalk KE, Janovjak H et al (2006) Bacteriorhodopsin folds into the membrane against an external force. J Mol Biol 357(2):644–654
Kedrov A, Ziegler C, Janovjak H et al (2004) Controlled unfolding and refolding of a single sodium-proton antiporter using atomic force microscopy. J Mol Biol 340(5):1143–1152
Chini B, Parenti M (2004) G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol 32(2):32–338
Scheuring S, Stahlberg H, Chami M et al (2002) Charting and unzipping the surface layer of Corynebacterium glutamicum with the atomic force microscope. Mol Microbiol 44(3):675–684
Brueckner BR, Noeding H, Janshoff A (2017) Viscoelastic properties of confluent MDCK II cells obtained from force cycle experiments. Biophys J 112(4):724–735
Yan B, Ren J, Liu Y et al. (2017) Study of cholesterol repletion effect on nanomechanical properties of human umbilical vein endothelial cell via rapid broadband atomic force microscopy. J Biomech Eng T Asme 139(3):034501–034501-5
Puntheeranurak T, Kasch M, Xia X et al (2007) Three surface subdomains form the vestibule of the Na+/Glucose cotransporter SGLT1. J Biol Chem 282(35):25222–25230
Wimmer B, Raja M, Hinterdorfer P et al (2009) C-terminal Loop 13 of Na+/Glucose cotransporter 1 contains both stereospecific and non-stereospecific sugar interaction sites. J Biol Chem 284(2):983–991
Christensen HN (1990) Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 70(1):43–77
Hundal HS, Taylor PM (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296(4):E603–E613
Palacin M, Bertran J, Zorzano (1998) Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 78(4):969–1054
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6(4):662–668
Dausend J, Musyanovych A, Dass M et al (2008) Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol Biosci 8(12):1135–1143
Klostranec JM, Chan WCW (2006) Quantum dots in biological and biomedical research: recent progress and present challenges. Adv Mater 18(15):1953–1964
Dixit SK, Goicochea NL, Daniel MC et al (2006) Quantum dot encapsulation in viral capsids. Nano Lett 6(9):1993–1999
Chavanpatil MD, Khdair A, Panyam J (2007) Surfactant-polymer nanoparticles: a novel platform for sustained and enhanced cellular delivery of water-soluble molecules. Pharmacol Res 24(4):803–810
Heller DA, Jeng ES, Yeung TK et al (2006) Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science 311(5760):508–511
Hirsch LR, Stafford RJ, Bankson JA et al (2003) Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Nat Acad Sci U S A 100(23):13549–13554
Liu N, Mu Y, Chen Y et al (2013) Degradation of aqueous synthesized CdTe/ZnS quantum dots in mice: differential blood kinetics and biodistribution of cadmium and tellurium. Part Fibre Toxicol 10:37
Shi X, von dem Bussche A, Hurt RH et al (2011) Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat Nano 6(11):714–719
Gao H, Shi W, Freund LB (2005) Mechanics of receptor-mediated endocytosis. Proc Nat Acad Sci U S A 102(27):9469–9474
Zhang S, Li J, Lykotrafitis G et al (2009) Size-dependent endocytosis of nanoparticles. Adv Mater 21(4):419–424
Wang Z, Tiruppathi C, Minshall RD et al (2009) Size and Dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 3(12):4110–4116
Shan Y, Hao X, Shang X et al (2011) Recording force events of single quantum-dot endocytosis. Chem Commun 47(12):3377–3379
Shukla R, Bansal V, Chaudhary M et al (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21(23):10644–10654
Jin H, Heller DA, Sharma R et al (2009) Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano 3(1):149–158
Kamei K, Mukai Y, Kojima H et al (2009) Direct cell entry of gold/iron-oxide magnetic nanoparticles in adenovirus mediated gene delivery. Biomaterials 30(9):1809–1814
Desai MP, Labhasetwar V, Walter E et al (1997) The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res 14(11):1568–1573
Ding B, Tian Y, Pan Y et al (2015) Recording the dynamic endocytosis of single gold nanoparticles by AFM-based force tracing. Nanoscale 7(17):7545–7549
Tomalia DA, Baker H, Dewald J et al (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17(1):117–132
Jain V, Bharatam PV (2014) Pharmacoinformatic approaches to understand complexation of dendrimeric nanoparticles with drugs. Nanoscale 6(5):2476–2501
Albertazzi L, Serresi M, Albanese A et al (2010) Dendrimer internalization and intracellular trafficking in living cells. Mol Pharm 7(3):680–688
Zong H, Thomas TP, Lee K-H et al (2012) Bifunctional PAMAM dendrimer conjugates of folic acid and methotrexate with defined ratio. Biomacromol 13(4):982–991
Haensler J, Szoka FC (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem 4(5):372–379
Saovapakhiran A, D’Emanuele A, Attwood D et al (2009) Surface modification of PAMAM dendrimers modulates the mechanism of cellular internalization. Bioconjug Chem 20(4):693–701
De Clercq E (2006) Antiviral agents active against influenza A viruses. Nat Rev Drug Discov 5(12):1015–1025
Hinterdorfer P, Dufrene YF (2006) Detection and localization of single molecular recognition events using atomic force microscopy. Nat Methods 3(5):347–355
Ebner A, Wildling L, Kamruzzahan ASM et al (2007) A new, simple method for linking of antibodies to atomic force microscopy tips. Bioconjug Chem 18(4):1176–1184
Kamruzzahan ASM, Ebner A, Wildling L et al (2006) Antibody linking to atomic force microscope tips via disulfide bond formation. Bioconjug Chem 17(6):1473–1481
Hinterdorfer P, Baumgartner W, Gruber HJ et al (1996) Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc Natl Acad Sci USA 93(8):3477–3481
Ebner A, Hinterdorfer P, Gruber HJ (2007) Comparison of different aminofunctionalization strategies for attachment of single antibodies to AFM cantilevers. Ultramicroscopy 107(101):922–927
Diao J, Ren D, Engstrom JR et al (2005) A surface modification strategy on silicon nitride for developing biosensors. Anal Biochem 343(2):322–328
Pan Y, Zhang F, Zhang L et al (2017) The process of wrapping virus revealed by a force tracing technique and simulations. Adv Sci 4:1600489
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31330082, 21773017 and 21673023) and Jilin Provincial Science Research Foundation of China (No. 20160520133JH).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shan, Y. (2018). Detection of Membrane Mechanical Properties and Endocytosis by Single Molecule Force Spectroscopy. In: Wang, H., Li, G. (eds) Membrane Biophysics. Springer, Singapore. https://doi.org/10.1007/978-981-10-6823-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-6823-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6822-5
Online ISBN: 978-981-10-6823-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)