Abstract
With augmented population, hasty industrialization, and urbanization worldwide, land for agricultural production is declining faster, and there is a huge demand for ecologically viable and environmentally affable techniques in agriculture, competent of providing adequate sustenance for the increasing human inhabitants and of improving the quality as well as quantity of certain agricultural harvests. A great deal of endeavor focusing on soil biology and the agroecosystem as a whole is required, enabling better perception of the complex processes and communications governing the stability of agricultural lands and plant kingdom. The scientific advances in modern times, researching biodiversity, have revealed that microbial miscellany is of massive potential that can be explored through careful assortment of the same and their booming use may solve critical agricultural and environmental issues. Here, we promote the thought that considering the mechanism by which plants select and interact with their microbiomes may have a direct or indirect effect on plant health that further may lead to establishment of novel microbiome-driven strategies that can embark upon the development of a more sustainable agriculture.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Association
- Biofertilizers
- Crop production
- Inoculation
- Microorganisms
- PGPR
- Phyllosphere
- Rhizosphere
- Symbiosis
4.1 Introduction
The ultimate threats of the twenty-first century have become quite comprehensible in the last few decades. Climate change due to the enormous increase in the production of greenhouse gases is real (Crowley 2000). A typical characteristic of modern intensive agriculture worldwide, i.e., application of synthetic chemicals like fertilizers, fungicides, herbicides, and pesticides, has been reported as non-sustainable and having multiple harmful impacts on both human or animal and plant health as well as environmental well-being (Franks et al. 2006; Glick 2014). There is a legitimate need for renewable energy supplies (Cook et al. 1991; Jackson 1999). Under such circumstances, prospective alternatives to the use of chemical or synthetic inputs are microbial inoculants, environment-friendly microbial formulations that act as biofertilizers, phyto-stimulants, and/or microbial biocontrol agents (Olubukola et al. 2012).
Surplus microbes are there in each gram of soil, and microbial cells are found extensively in plant and animal tissues (Andreote et al. 2014). Microorganisms execute various metabolic activities indispensable for their own survival (Sengupta and Gunri 2015), and useful properties of such microbial inoculants could be manifested either by direct endorsement of plant growth through nutrient recycling or indirectly by defending plants from phytopathogens, or by invigorating tolerance to some of the abiotic strain in plants, which grow under nonoptimal ecological factors including soil, higher or lower temperature, acidity, salinity, drought, and heavy metals as well (Penrose and Glick 2003; Kang et al. 2014) .The varied community of microbes develop a metagenome of information that also extends to both outside and inside of the human body (Ahmed et al. 2011). Microbes are also capable of playing major roles in the development of soil aggregates that help in stabilization of the topsoil (van Veen et al. 1997) and improvement of soil health and can help in ecological detoxification, wastewater treatment, etc. (Ahmad et al. 2011). The mechanisms governed by microbes in the regulation of physiological processes of their hosts have been comprehensively studied in the light of latest findings on microbiomes. Even though there is no lucid depiction of the overall function of the plant microbiome, there is considerable confirmation that these communities are involved in infection control, enhanced nutrient attainment, and influence stress tolerance. Thus, currently, noteworthy venture is being exerted on research to build up such microbial inoculants which have positive plant growth properties in environmentally responsive sustainable cultivation (Barriuso et al. 2008a, b).
A large portion of favorable soil microorganisms are still undiscovered, and their environmental functions are pretty indefinite till date. Thus, enormous assays of microbial activities are the fundamental steps toward progress in innovative technologies for proficient exploitation of microorganisms for realization of sustainability in agriculture. Microbial involvement in combination with advancements in digital imaging, nanotechnology, and electronics may play a key role in solving universal challenges of the twenty-first century together with climate change (Ahmad et al. 2011).
This book chapter sums up features of microbial community that make up the plant microbiome and further presents a chain of studies recounting the underneath factors that contour the phylogenetic and useful plant-associated communities.
4.2 Microbial Interactions
Microbial populations interrelate and establish relations with each other and with higher organisms. Usually the relationship is nutritional, though other benefits may accrue, and the association can turn essential to the survival of one or both partners. There are several sorts of associations, viz., amensalism and competition, mutualism, parasitism protocooperation, synergism and commensalism, etc., between the organisms.
Odum (1971) has proposed the following relations:
-
(a)
Neutralism, where the two microorganisms perform entirely autonomously
-
(b)
Symbiosis, the two symbionts relying upon one another and mutually benefiting the affiliation
-
(c)
Protocooperation, a relationship of reciprocal advantage to the two species but devoid of the cooperation being mandatory for their survival or for performance of some response
-
(d)
Commensalism, in which only one species derives profit while the other is unaltered
-
(e)
Competition, a situation in which there is a repression of one organism as the two species fight for restraining quantities of O2, space, nutrients, or other common necessities
-
(f)
Amensalism, in which one species is covered up while the following is not affected, often the result of toxin production
-
(g)
Parasitism and Predation, the direct assault of one individual upon another
-
(h)
Synergism, in field conditions the probable synergistic effect in the plant between inducing virus and other non-linked viruses which could be brought to those plants from outside sources
4.3 Microbe–Plant Interactions
Plants are exposed to huge numbers of microorganisms that are present in the topsoil and are found on leaves and stems. Plants are the major resource of nutrients for microorganisms being the prime source of organic carbon. Plants provide nutrients through shedding of leaves, pollen, etc., or through exudates or dead tissues in an indirect manner (Sivakumar and Thamizhiniyan 2012). In few instances, nutrients are provided straightway to microbes that form close relations with plants. Associations with plants can vary from those that are tremendously damaging to the plant, such as those with dangerous pathogens, through exchanges, which do not come out to influence plant growth, to advantageous ones such as those formed with mycorrhizal fungi or nitrogen-fixing bacteria. For most microorganisms, exchanges with growing plants expand no further than the colonization of the surfaces of stems, leaves, and roots because these are regions where exudates are accessible.
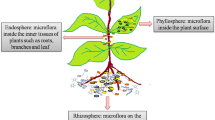
Such microbiomes in plants may form divergent communities, like the ones from the rhizosphere, endosphere, or the phyllosphere (Hirsch and Mauchline 2012; Hardoim et al. 2008) (Fig. 4.1). In each of these niches, the “microbial tissue” is established by, and responds to, specific selective pressures (Andreote et al. 2014).
4.3.1 Rhizosphere and Root Exudates
The rhizosphere is the frontier between plant roots and soil where communications among numerous invertebrates as well as microbes influence plant growth, biogeochemical cycles, and indulgence to biotic and abiotic strain (Philippot et al. 2013).
In rhizosphere microbial action is generally high. Hiltner (1904) observed the zone of extreme microbial commotion around the roots and named it as rhizosphere (as cited by Hartmann et al. 2008). Roots emit considerable amounts of sugars, amino acids, hormones, and vitamins, which promote such a widespread growth of fungi and bacteria that these organisms often form microcolonies on the surface of the roots. Primarily roots contain little or no microbial colonization but with advancement in plant growth, in the soil, root exudates, comprising a combination of 10 sugars, 10 organic acids, about 18 amino acids, mucilage, etc., along with other cell exerts or root caps that influence on microbial colonization (Griffin et al. 1976). The chemicals in the forms of root exudates, released in the proximity of plant rhizosphere, are known to belong from the vital group of carbohydrates, phenols, organic acids, protein, and lipid along with other cellular components (Nguyen 2003; Dini-Andreote and Elsas 2013). Root exudates have been grouped and are primarily classified into two major classes, viz., compounds of high molecular weight like polysaccharides and proteins and that of low molecular weight like amino acids, organic acids, sugars, phenolic compounds, and other secondary metabolites (Bais et al. 2006; Badri and Vivanco 2009; Narasimhan et al. 2003). From these molecules, few are linked with establishment of key portions of the microbial community (generally metabolized by a good number of soil organisms, e.g., glucose), but other compounds released are capable of activating precise groups of organisms (those related to signaling and chemo taxis, e.g., flavonoids) (Nguyen 2003; Jones et al. 2004).
Quantitative and qualitative compositions of exudes from plant roots are generally determined by the plant species, plant developmental stage, cultivar, and various environmental factors, including soil pH, temperature, type of soil, as well as presence of microorganisms in soil (Badri and Vivanco 2009; Uren 2000). These differences fabricate microbial communities in the rhizosphere that have a definite degree of specificity for each plant species.
4.3.1.1 Mechanism of Root Exudation
Plants communities employ a variety of transportation mechanisms to export and exude compounds in the soil rhizosphere (Badri and Vivanco 2009; Weston et al. 2012). Usually, roots can release root exudates through active or passive mechanisms by means of secretion or diffusions, respectively. Majority of low-molecular-weight organic compounds are released from plants through a passive process. Small polar and uncharged molecules are elated by direct passive diffusion, a procedure that depends on membrane permeability, the polarization of the exuded compounds, and cytosolic pH (Badri and Vivanco 2009). Plant root cells release additional substances, like resultant polysaccharides, proteins, and other metabolic derivatives, with the help of various membrane-bound proteins (Weston et al. 2012). These carrier proteins comprise the ATP-binding cassette (ABC) transporters (Badri et al. 2008, 2009a; Loyola-Vargas et al. 2007; Sugiyama et al. 2008), multidrug and toxic compound extrusion (MATE) family (Yazaki 2005), the key facilitator super family (Reddy et al. 2012), and the aluminum-activated malate transporter family (Weston et al. 2012). Though the detailed functions of these membrane-bound transport proteins are not well stated, they have been connected with the transfer of a wide range of compounds into the rhizosphere. Badri et al. (2008, 2009a) found that 25 ABC transporter genes were notably overexpressed in the Arabidopsis thaliana (L.) Heynh. roots and played significant roles in these discharge processes. Adding up to ABC transporters, MATEs are also dynamic transporters that export a large variety of substrates across membranes by using the electrochemical gradient of other ions (Weston et al. 2012). Many MATE genes play important role in exporting different compounds, such as plant-derived alkaloids, toxic compounds, antibiotics, citrate anions, and phenolic compounds, out of the cells of plant roots, which have been identified as well as characterized in Arabidopsis (Diener et al. 2001; Li et al. 2002; Liu et al. 2009), sorghum (Magalhaes et al. 2007), barley (Furukawa et al. 2007), and rice (Ishimaru et al. 2011).
4.3.1.2 Rhizosphere Microbes Influence Plant Root Exudation
Plant root exudation is also influenced by the microbes (fungi and bacteria), colonized in the rhizosphere (Jones et al. 2004; Leyval and Berthelin 1993; Matilla et al. 2010a, b). Several studies have shown that the arbuscular mycorrhizal fungi colonization can alter plant root exudation qualitatively, e.g., augmenting secretions of N, phenolics, and gibberellins and minimizing secretions of total sugars, potassium ions, and phosphorus (Jones et al. 2004).
Preceding studies have revealed that various ectomycorrhizal fungal taxa have discrete influence on profusion and specifications root exudes of plants (Fransson and Johansson 2010; Rosling et al. 2004). The inoculation with ectomycorrhizal fungus and/or rhizobacteria can modify root exudation in both quantitative and qualitative aspects (Leyval and Berthelin 1993). Another latest research has revealed that both the profusion and individuality of root-associated fungi influence plant root exudation rates (Meier et al. 2013). Furthermore, in reaction to pathogen attack, plants discharge compounds as root exudates, such as oxalic acids, phytoalexins, proteins, and other unknown substances (Nelson 1990; Steinkellner et al. 2007). In addition to fungi, bacteria influence plant root exudation too. For instance, A. thaliana was found to produce distinct root exudation profiles when cultured with Pseudomonas putida KT2440 compared with the plant without P. putida, suggesting that bacteria are also modulating plant root exudation (Matilla et al. 2010a, b). In addition to plant root exudation, the soil microbiome may also influence the plant metabolome (Badri et al. 2013b). Distinct soil microbiomes were applied to A. thaliana, and this not only affected plant growth but also influenced the leaf metabolome, which in turn influenced the feeding behavior of the larvae of the herbivore Trichoplusia ni (Badri et al. 2013b). Similarly, inoculation of Arabidopsis plants under drought stress with distinct microbial communities originating from pine, corn, and Arabidopsis soils demonstrated that a sympatric microbiome, with a history of Arabidopsis growth, was able to alter the plant’s ability to detect drought stress and increased its biomass production compared with the pine and corn microbial communities (Zolla et al. 2013). This may be due to the ability of soil microbes to modulate ethylene levels by degrading the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) using the enzyme ACC deaminase (Glick 2005). The plant hormone ethylene is involved in a large number of plant responses particularly related to plant stress, and its production is synchronized by nutrition, light, temperature, and even the status and levels of other plant hormones (Glick 2005). High levels of ethylene aggravate stress responses and even weaken plant root growth (Argueso et al. 2007). A large number of soil microbes are able to ease plant stress responses to ethylene production by catalyzing the cleavage of ACC, the direct precursor to ethylene, into α-ketobutyrate and ammonia (Glick 2005; Stearns et al. 2012). Thus, lowering plant ethylene levels improves the plants’ capacity to defend against a variety of abiotic and biotic stresses. ACC deaminase activity helps in ameliorating drought stress (Arshad et al. 2008), water stress, salinity stress (Mayak et al. 2004), and overall abiotic stress and also helps in growth promotion function of plants (Glick et al. 2007; Yang et al. 2009). For example, the soil bacterium Achromobacter piechaudii ARV8 that has ACC deaminase activity was able to increase tomato and pepper seedling biomass (Mayak et al. 2004). Recently, Stearns et al. (2012) studied the response of Brassica napus to ACC deaminase bacteria and showed that genes involved in auxin production were upregulated in the plant, while genes involved in ethylene stress response were downregulated. This provides a clear signal to the benefits ACC deaminase-containing bacteria have on the plant. Determining how the overall bacterial community is involved in mediating and reducing ethylene-mediated stress could create technologies to help the plant deal with abiotic stress.
4.3.1.3 Rhizospheric Interactions
4.3.1.3.1 Root Exudates and Plant–Microbe Interactions
In the last decade, the means by which root exudates mediate rhizospheric interactions have been extensively studied (Fig. 4.2) (Badri et al. 2013a; Broeckling et al. 2008; Chaparro et al. 2013; Doornbos et al. 2012; Micallef et al. 2009a, b).
Plant root-exuded phytochemicals can intervene a number of connections, such as plant–plant, plant–microbe, and plant–faunal. These interactions differ from neutral to advantageous or harmful (Mercado-Blanco and Bakker 2007; Raaijmakers et al. 2009). In few cases, microbes can change from pathogenic to symbiotic depending upon the environmental conditions (Newton et al. 2010). For example, rhizobia, symbiotic nitrogen (N)-fixing bacteria, range from a symbiotic to a neutral interaction with plants based on nitrogen levels in soils (Davidson and Robson 1986; Zahran 1999). Furthermore, under N-limiting conditions, legumes exude more flavones and flavonols to attract and initiate legume–rhizobia symbiosis (Coronado et al. 1995; Zhang et al. 2009).
In a similar way, mycorrhizal symbiotic relationships are governed by an equal exchange of nutrients and benefits for each member (Kiers et al. 2011). As, for example, in experiments on Medicago truncatula Gaertn., it was found that as more carbon was given to the mycorrhizal partner, the mycorrhiza in turn provided the plant with more phosphorous (Kiers et al. 2011).
4.3.1.4 Functions of Rhizosphere Microbiome
Microorganisms from the rhizosphere play significant roles in ecological vigor of the plant hosts. Significant microbial processes that are likely to take place in the rhizosphere consist of pathogenesis and its counterpart, plant protection/growth promotion, along with synthesis of antibiotics, colonization of plants, and recycling of natural resources (Kent and Triplett 2002). Plant–microbe interactions may thus be considered as advantageous, neutral, or detrimental to the plant, depending on the specific microorganisms and plants concerned and on the existing environmental situation (Bais et al. 2006). Exploring the microbes, through sorting out their probable interactions with plant communities, has opened up a new interesting area for experimentations in rhizosphere research.
4.3.1.4.1 Beneficial Functions
Plant beneficial microbial interactions can be more or less divided into three categories. Firstly, microorganisms those, in association with plants, are accountable for its nutrition (i.e., microorganisms that augment the supply of mineral nutrients to the plant). In this case, though majority of microorganisms may not intermingle directly with the plant, their impact on soil abiotic and biotic factor undoubtedly has impacts on plant growth. Again, there are group of microbes, documented as biocontrol agents, which can stimulate plant growth and development in an indirect manner, by prevention of activities of pathogens. The third group comprising microbes, known to produce phytohormones, is responsible for direct plant growth promotion. On the other hand, it seems that neutral connections are found broadly in the rhizosphere of all crop plants. Saprophytes are accountable for different crucial soil processes, like mineralization of associated soil nutrient or turnover processes and decomposition of organic residues in soil. While such organisms neither benefit nor harm the plants straightway, absence of such microbes would undoubtedly influence plant health and productivity, and their presence is evidently essential for soil dynamics (Brimecombe et al. 2007).
Bacteria, living in the rhizosphere, exert their favor on growth of the plants globally and are referred as PGPR, i.e., plant growth-promoting rhizobacteria (Kloepper and Schroth 1978). The number of bacteria, recognized as PGPR, has been found to increase on a recent time, due to various advanced studies in bacterial taxonomy and better understanding regarding mechanisms of actions of various PGPR, covering a broader collection of plant species as well. Presently, PGPR comprise members from varied bacterial taxonomic classes (Lucy et al. 2004), and we are going to discuss a few instances in order to illustrate the mode of functioning and biodiversity of such bacterial community. A wide range of beneficial PGPR have been utilized profitably for inoculation of crop plants that include members from the genera Azospirillum (Cassán and García Salamone 2008), Pseudomonas (Loper and Gross 2007), Bacillus (Jacobsen et al. 2004), Stenotrophomonas (Ryan et al. 2009), Serratia (De Vleeschauwer and Höfte 2007), Streptomyces (Schrey and Tarkka 2008), and Rhizobium (Long 2001). Rhizobium (Long 2001) and some fungi from the genera Trichoderma, Coniothyrium, and Ampelomyces have also been described to be beneficial for the host plant (Harman et al. 2004). The mode of functioning of these PGPR has complex mechanisms to promote plant growth, development, and protection. Important among them are biofertilization (improving nutrient availability to plants), phytostimulation (plant growth promotion through production of phytohormones), and biocontrol (control of diseases, primarily through production of various antibiotic as well as antifungal metabolites and lytic enzymes and induction of plant defense responses). The genera Bacillus and Pseudomonas are known to be the predominant ones among PGPR groups from the rhizosphere (Morgan 2005). It is mentioned that in several instances regarding individual benevolent connections between plants and microbes, a wide range of mechanisms are actually implicated therein (Muller et al. 2009). The direct plant growth promotion functions are complicated enough to discriminate from disease control, and the comparative significance on a specific method can differ within dissimilar pathogen systems (Chet and Chernin 2002).
4.3.1.4.2 Pathogen Inhibition
Bacteria and fungi live in the region of roots and get nourished on root exudates and dead root cells. Competition amid microbial species in this region is rigid. In the fight for perseverance and establishment in the niche, bacteria use a number of strategies.
4.3.1.4.3 Antagonism
Root colonization not only results in high PGPR inhabitant densities in the root system, it also delivers antagonistic metabolites that are concerned in straight inhibition of plant pathogens (Shoda 2000; Raaijmakers et al. 2002). This includes inhibitions of growth of microbes, i.e., antibiosis, by use of diffusible antibiotics or organic volatile compounds, biosurfactants, and toxins and the mechanism of parasitism, which perhaps could involve synthesis of extracellular enzymes that can degrade cell walls, such as chitinase and β1,3-glucanase (Compant et al. 2005; Haas and Défago 2005). Degradation of the pathogenicity factors for the pathogens, like toxic substances released by favorable organisms, has also been recorded as mechanism for protection (Haas and Défago 2005). To exhibit the function of antibiotics in the process of biocontrol, the mutants impaired in the process of biosynthesis or mutants with overproducing habit have been utilized together with, in few cases, reporter genes, or probes have been used to explain efficient production of the compound in rhizosphere. For example, Bacillus subtilis strains were found to develop a number of strong antifungal metabolites, viz., kanosamine, zwittermicin A, and lipopeptides from fengycin, iturin, and surfactin families (Emmert and Handelsman 1999; Ongena and Thonart 2006). Excess synthesis of the extracellular protease in Stenotrophomonas maltophilia W81, a mutant strain, has been reported to exert improved biocontrol of Pythium ultimum (Dunne et al. 2000). Release of glucanase and chitinase by Streptomyces and Trichoderma species has been reported to play a pivotal role in myco-parasitism of phytopathogenic fungi (Whipps 2001).
4.3.1.4.4 Colonization
For all thriving plant–microbe connections, the capability to colonize plant habitats is vital (Lugtenberg et al. 2002; Kamilova et al. 2005). Distinct bacterial cells can affix to surfaces and, after cell division and propagation, form dense aggregates normally referred to as macro-colonies or biofilms. Steps of colonization comprise of attraction, detection, adherence, incursion (pathogenic microbes and endophytes only), followed by colonization and growth, along with some other strategies for establishment of connections. Roots start cross talk with soil microbes by generation of signals that are accepted by the microbes, which in turn produce signals that set off colonization (Berg 2009). PGPR get to surfaces of the root through active motility using flagella and are guided by chemotactic responses (Pinton et al. 2007). This proves that ability of PGPR highly depends upon their capacity to take benefit of a precise situation or on their abilities to become accustomed to varying conditions or plant species. In the majority cases, after 2–3 weeks, the population of PGPR declines progressively with time after inoculation from 107,109 cells per gram dry soil to 105,106 cells per gram dry soil (DeFlaun and Gerba 1993). However such population threshold remains adequate to provide positive effects (Raaijmakers et al. 2002). As a result, the rhizosphere proficiency of the biocontrol agents involves successful root colonization along with the aptitude to live and proliferate by the side of growing plant roots over a long period, in presence of native microflora (Weller 1988; Lugtenberg and Dekkers 1999).
4.3.1.4.5 Competition
Competition for resources such as oxygen and nutrients occurs generally between soil-inhabiting organisms. For biocontrol, competition occurs, while antagonists compete straightway with the pathogenic microbes for various resources. Root-inhabiting microorganisms compete for appropriate sites at the surfaces of roots. Competition for nutrient elements, such as carbon, is considered to be accountable for the incidence of fungistasis, leading toward suppression of germination of fungal spore (Alabouvette et al. 2006). Given, the comparative profusion of substrates from the rhizosphere, the efficacies of uptake of nutrients, and catabolism by the bacterial community are a major factor for competitiveness (Chin-A-Woeng et al. 2003). The capacity for rapid growth when substrates are encountered is not the only factor affecting rhizosphere competence, as rhizobacteria deploy many other metabolic strategies. As, for example, the capacity for extracellular conversion of glucose to gluconic acid and 2-ketogluconic acid enables some bacteria, together with quite a few species from the genera Pseudomonas, in order to impound glucose successfully and gives some aggressive advantage over microbes that lack the capability to utilize these compounds (Gottschalk 1986).
Competition for tracer elements, like as iron, zinc, manganese, copper, etc., too occurs in soils. As, for instance, iron is an indispensable element for growth of all existing organisms and the lack of its bioavailable form in soil habitats results in an enraged competition (Loper and Henkels 1997). Siderophores, the compounds with lower molecular weight and higher affinity for iron, are synthesized by some of the microbes or mostly biocontrol agents in order to solubilize and obtain the ferric ions competitively under iron-restraining conditions that further render the very element unavailable to other microbes from soil that are unable to thrive without iron (Loper and Henkels 1997; Haas and Défago 2005). The microbes, having properties of siderophore production, on the contrary, can take up iron–siderophore complex by means of using a particular receptor located in the outer cell membrane. Suppression of the soilborne pathogens of various plants, by Pseudomonas, through siderophore production has also been reported by many authors (Loper 1988; Weger et al. 1988; Buysens et al. 1996).
4.3.1.4.6 Induced Resistance
Bacteria, associated with plants, reduce the actions of pathogens by means of microbial antagonism along with by activating the plant to better defense mechanism, a phenomenon termed “induced systemic resistance (ISR)” (Shoda 2000; Van Loon 2007).
Sometimes, the methods of induced systemic resistance, elicited by plant growth-promoting rhizobacteria, overlap to some extent to that of systemic acquired resistance, i.e., SAR of pathogens. Both of the mechanisms stand for a condition of improved basal confrontation of the plant, which depends upon signaling compounds such as jasmonic acid, ethylene, and salicylic acid (Van Loon 2007). Natural defense response against stresses from biotic or abiotic origin such as physical stresses (heat or frost), inoculation by pathogenic or nonpathogenic organisms, and chemical molecules from natural or synthetic origins is exhibited by all plants (Alabouvette et al. 2006).
4.3.1.4.7 Plant Growth Promotion
4.3.1.4.7.1 Biofertilization
The system of escalating the performance of crop plants by PGPR is not finely comprehended yet. A number of PGPR inoculants are commercialized at present that appear to support augmentation in plant growth, through one of the following mechanisms:
-
1.
Production of bio-stimulants or phytohormones
-
2.
Inhibition of plant infection as bioprotectant
-
3.
Enhancement of nutrient acquirement as biofertilizers
PGPR as biofertilizer perform both directly and indirectly by serving to make nutrient available to the host plant and influencing growth of plant root and morphology positively or by additional favorable symbiotic interactions (Vessey 2003). The major instance of such kind of relationship is fixation of nitrogen by bacteria. The symbiosis between legume host and rhizobia is one of the significant examples of plant growth-promoting rhizobacteria (PGPR). Bacteria from this cluster can metabolize root exudates that are mainly carbohydrates and supply nitrogen to the host plant in return for production of amino acids. The free-living bacteria like Azospirillum, Burkholderia, and Stenotrophomonas have nitrogen-fixing ability as well (Dobbelare et al. 2003). One more nutrient element that can be provided to the crop plants through oxidation by bacteria is sulfate (Banerjee and Yesmin 2002). Bacteria can also supply plant nutrition by releasing phosphorous from organic sources like phytates and hence help in plant growth promotion indirectly (Unno et al. 2005). Use of Azospirillum resulted in augmentation of root growth and activities that increase uptake of phosphorous along with other macro- and microelements (Dobbelaere and Okon 2007). Pseudomonas fluorescens CHA0 has capability of acidification of its surroundings and solubilization of mineral phosphate, which strongly depends on its aptitude of gluconic acid production (De Werra et al. 2009).
4.3.1.4.8 Phytostimulation
Phytostimulation enhances plant growth in a direct way. Phytohormones [e.g., production of indole-3-acetic acid (IAA), auxins, cytokinins, and gibberellins] play an important role in processes of plant growth. Such phytohormones can be produced by the plants themselves as well as by their allied microbes, as, for example, Azospirillum spp., in addition to its capacity of fixing the atmospheric nitrogen (Steenhoudt and Vanderleyden 2000). Species from the genera Bacillus and Pseudomonas can synthesize the plant growth regulators or phytohormones that help crops in having greater amount of fine roots which have the effect of increasing the absorptive surface of plant roots for uptake of water and nutrients. They can produce phytohormones like gibberellins, cytokinins, indoleacetic acid, and ethylene production inhibitors. Indole-3-acetic acid is a phytohormone that is involved in cell division, root initiation, as well as enlargement of plant cells (Salisbury 1994). Auxins are most plentiful phytohormones quantitatively, which are exuded by Azospirillum spp., and their synthesis, more willingly than fixation of nitrogen, is the prime factor that is accountable for the encouragement of profuse rooting of plants and, thereby, enhanced plant growth (Bloemberg and Lugtenberg 2001). Furthermore, plant-associated bacteria can influence the hormonal balance of the plant. Ethylene is the significant instance to illustrate the fact that the stability is most imperative for the result of hormones: at lower level, it can endorse growth of plant in quite a few species together with Arabidopsis thaliana, whereas it is generally considered as an inhibitor toward plant development and known as a senescence hormone (Pierik et al. 2006). The general effect on the plant can be direct, that is, through plant growth promotion, or indirect, that is, through improving plant nutrition via the better development of the roots, and it is hard to differentiate between them. The increase in root IAA level for plantlets of lodgepole pine, infected with Paenibacillus polymyxa, as well as root concentration of dihydroxyzeatin riboside in case of plants inoculated using Pseudomonas fluorescens (Fuentes-Ramirez and Caballero-Mellado 2005), may be accredited to the orientation of plant hormone synthesis by the bacterial species. However, the uptake of bacterial synthesized phytohormones cannot be excluded, since both P. polymyxa and Pseudomonas produce IAA and cytokinins in vitro (Fuentes-Ramirez and Caballero-Mellado 2005).
4.3.1.4.9 Pathogenic Functions
Root exudates can attract both favorable and pathogenic populations (Schroth and Hildebrand 1964) that may be virulent for a few hosts. Many pathogens, fungi and bacteria, have evolved and exhibited a higher level of host specificity (Raaijmakers et al. 2009). Plants are also not out of defense. In fact, it is found that approximately 2% of the identified fungal species are capable of colonization in plants and thereby can cause infection in plant body (Buchanan et al. 2000). Though plants remain in constant contact with virulent fungal, bacterial, or viral pathogens, successful contamination is hardly recognized. This is because a common confrontation in opposition to most of such pathogens, named as “nonhost resistance” or “horizontal resistance,” is found in plant bodies (Heath 1981). This reinforces the concept that the plants are not always fit targets for infection by a definite group of pathogens owing to reflexive opposition mechanisms ensuing “basic incompatibility.” Such resistance mechanisms consist of configurational barriers and poisonous chemicals that are there in the strong plants, bound triumphant infection to specific pathogens, which have the abilities to conquer these factors and thus reveal “basic compatibility.” However, even if contact is recognized with the plant, pathogenic microbes are frequently confronted with toxic compounds named phytoanticipins (van Etten et al. 1994). This phrase consists of a range of components fashioned by various biosynthetic pathways that obtain antimicrobial characteristics. Such resultant metabolites of low molecular mass are primarily stored in inert forms in the organelles or vacuoles and are exuded upon demolition of the cells. While destruction of the integrity of the host plant tissue is a component of the colonization mechanism by fungal bodies, phytoanticipins symbolize a significant confrontation strategy in opposition to such pathogens. Though, in some cases, pathogenic bodies conquer the preformed hindrances from host plants and may expand virulent contamination leading toward ailment in plant bodies. Plant diseases participate directly in the eradication of ordinary possessions from agriculture. Particularly, soilborne pathogens impart more losses, as fungi remain most hostile from soil. Their detrimental effects range from placid symptoms to catastrophes where entire fields with agricultural produce can be ruined. Consequently, they become persistent and foremost threats toward stability of ecosystem and food production function worldwide. Most common bacterial agents comprise of Gram-positive bacterium Streptomyces scabies and the Gram-negative bacteria Ralstonia spp., Erwinia carotovora, and Pseudomonas. The oomycetes and fungal phytopathogens include members from the genus Rhizoctonia, Rhizopus, Fusarium, Pythium, Phytophthora, and Verticillium (Tournas and Katsoudas 2005). Among the woodland pathogens, the significant ones are the filamentous fungi like Phytophthora spp. (Rizzo et al. 2005) and Armillariella and Heterobasidion (Asiegbu and Nahalkova 2005).
4.3.2 Phyllosphere: Plant Community with Microbiome
A second component of plant–microbiome interaction consists of microbes colonizing the aboveground area or exterior of plant tissues, i.e., the phyllosphere. The phyllosphere is a massive ecology that is likely to attain an area of 6.4 × 108 km2 and is heavily colonized by microbes (Morris and Kinkel 2002). The terminology is generally used to describe the surface of the leaf (Vorholt 2012) though it is applicable to any aerial plant tissue.
The microbial communities from the phyllosphere have indispensable roles in plant growth and development. Protecting plant community from invading pathogens, fixation of atmospheric nitrogen, biosynthesis of phytohormones (Jones 1970; Freiberg 1998; Brandl et al. 2001; Kishore et al. 2005), carbon sequestration (Bulgarelli et al. 2013), etc., are some of such functions that are essential for sustainable agricultural practices.
Lindow and Brandl (2003) reported that community of phyllosphere is mainly comprised of bacteria, algae, fungi, and nematodes or protozoa in a few instances. Bacteria are the most plentiful community among these microbes that are found between 105 and 107 cells per cm2 (Beattie and Lindow 1995; Andrews and Harris 2000) in phyllosphere. These communities are sometimes to be found far away from the rhizosphere, prime resource of plant-associated microorganisms, and are found to exhibit higher rates of colonization, mostly promoted by the movement of airstream as well as vectors (Bulgarelli et al. 2013). Organisms from the phyllosphere can flourish and survive even under oligotrophic ecological surroundings with ultraviolet radiation, restricted nutrient accessibility, and varied pH, temperature, and moisture conditions (Andrews and Harris 2000). Air along with aerosols, earth, and moisture are the prime sources that frame the communities from the phyllosphere (Bulgarelli et al. 2013). The interaction among different ecological factors can amend the microbial communities from phyllosphere. Genomic structure of plants is one of the key drivers, determining the composition of bacterial communities in the phyllosphere in temperate (Redford et al. 2010) and tropical forests (Lambais et al. 2006). Diverse plant communities anchor different microbes, owing to the creation of precise niche and confined circumstances that are governed by the inherited and the efficient metabolic activities of the plants (Redford et al. 2010). The uniqueness of the phyllosphere was reported in plants of beans, lettuce, cucumber, maize, and grasses with alteration in the profusion and constitution of bacterial community (O’Brien and Lindow 1989; Kinkel et al. 2000; Rastogi et al. 2012). Geological remoteness is also another significant player in configuring microbial communities in the phyllosphere (Bokulich et al. 2014). The diverse bacterial communities anchored by grapevines manipulate superiority of the produced wine. In a more comprehensive outlook, the intraspecific alteration in the composition of the microbial community in the phyllosphere can be noticed, primarily governed by the heterogeneous nutritional condition, found in leaf surfaces, where the heterogeneous carbon sources like glucose, fructose, and sucrose lead to precise microbial colonization on the leaf veins, the regions close to the exterior appendages and stomata (Lindow and Brandl 2003; Vorholt 2012). According to Davey and O’Toole (2000) and Lindow and Brandl (2003), such heterogeneity in few instances is endorsed by the microbial association in biofilms that are general characteristic of organisms from the phyllosphere, functioning as the defender and aggregator of bacterial cells under the regular uncongenial circumstances. Regardless of such instances, it is likely to detect a “core” for the microbial population from the phyllosphere that colonize host plants, from the phyla Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (Redford et al. 2010; Vorholt 2012). These phyla consist of the most plentiful and well-examined microbes that signify the fact that additional researches regarding this issue should be planned to assess taxonomic ranks beyond phylum. Consequently, this core is assumed to be made of microbes depicting a coevolving history with the plant communities, along with the host structure that is complementary to the specifications found inside the bacterial cells. Such microbial reserve can be utilized for the benevolence of farming practices that endorse the movement and odd or synergistic associations, which may kindle plant growth and/or defense in opposition to attack of pathogenic communities.
4.3.3 Endosphere: A Forte Meant for Close Friends
The existence of microbial cells in plant inner tissues was explained long ago as the same as plant infection. At that time, the microbes within plant tissues were individuals that are able to contaminate the host plants, leading toward difficulties in growth and development of plants as well as losses in yield. Perhaps, such association was caused due to the accessibility of methods for detection of microbial connections that time that were merely proficient in making out microbes that are easy to cultivate or found in large quantities. The occurrence of nonpathogenic microbes within plant tissues was explained by De Bary (1866) for the first time, who revealed that microbes are present in microscopically examined plant tissues. Such examination remained unknown until the endophytes were defined. The endophytes are generally defined based on the capability to perceive the microbial cells from plant tissues that have been surface sterilized formerly (Hallmann et al. 1997). Petrini (1991) has given a functional description for endophytes as “organisms that at some part of their life cycle colonize internal plant tissues without causing apparent harm to the host.” On a more exhaustive examination, endophytes are divided in the subgroups “obligate” and “facultative” by the researchers. Endophytes, which depend on metabolism of host plants for their endurance, and are transmitted among plants through the activity of vectors or by vertical transmission, are classified as obligate ones (Hardoim et al. 2008). Endophytes, those living on the outer surface of the host plants at some point of their life cycle, are known as facultative ones. They are recruited by the host plants from neighboring communities from the soil mass, mainly from the rhizosphere. Endophytes are there in every plant inner tissues (Rosenblueth and Martinez-Romero 2006). The existence of endophytes in plants cultured in vitro has been explained, where such organisms seem to be closely connected with host plants not in by means of colonizing the culture media but preferably living inside the tissues of the plant (Almeida et al. 2009; Abreu-Tarazzi et al. 2010). Mendes et al. (2007) showed that the endophytic Burkholderia spp. have the capacity to regulate the growth of the pathogenic Fusarium moniliforme. Ferrara et al. (2012) showed that the endophytic diazotrophs from roots of sugarcane are capable of producing substances related to plant growth-promoting functions and can exude greater amount of amino acids that could aid in plant nutrition. Araújo et al. (2002) showed that the whole endophytic community is influenced by the occurrence of the pathogen and incidence of disease like variegated chlorosis in citrus is a consequence of the dealings between the endophytic community and pathogenic X. fastidiosa and not with the host only. The capability of genetically customized endophytes that generate the heterologous protein cry1Ac7 can control Diatraea saccharalis, a sugarcane pest (Quecine et al. 2014). Though numerous individual abilities have been explained for endophytes, such organisms, as a community, are competent enough for several other functions that cannot be detected from separate case studies on the microbes.
Numerous studies were made to find the origin of endophytic organisms (Hallmann et al. 1997; Saikkonen et al. 1998; Mitter et al. 2013). The origin of microbes, residing in rhizosphere or the seed-borne ones, is firmly associated to the strategy of preservation of the same within the host plants that confirms the diffusion of endophytic microbes between plants. The evidence of the mechanism of transmission as well as survival of specific endophytes and their interaction with plant bodies are indicated through their genomic organization. Dini-Andreote et al. (2012) studied over sizes and origins of numerous endophytic genomes. The scientists related the lifestyle of microbes to the genome size for detection of the deviation in ecological conditions as one of the key drivers of expansion or shrinking of genome. Endosymbionts usually possess more compacted genomes, while bacteria from niches of variable ecological conditions such as rhizosphere need to harbor the complete cache of genes to survive under diverse environmental situations, leading toward dominance of larger genomes. Apparently, endophytes appear to fit in the former portion of the theory since they exist within plant bodies, where the surroundings are more secure in comparison with the rhizosphere. Nevertheless, taking into account the origin and transmissions of endophytes, it is said that probably few endophytes must deal with distinct environments during their course of life cycle when they remain outside host plants. Mitter et al. (2013) showed a greater deviation in the genome size of bacterial endophytes, which suggests that the community of endophytes consist of microbes from various origin. Those with bigger genomes are likely to live in varied environments like soil or rhizosphere, and the ones with smaller genomes are to be transmitted vertically within stable surroundings.
4.4 Conclusion
If microbiome–plant interactions are understood and described in a more improved and detailed manner, such data could be accessible for the invention of newer technologies, concentrating on a superior investigation of the characteristic in agricultural strata, influenced by microbes. Alteration in the configuration of microbial population, for example, by injection of distinct exogenous microbes or by means of influencing ecological circumstances toward benevolence of specific sets of microbiomes, heading toward improved plant opposition or effectiveness in the nutrient uptake could be a reality. In this manner, the progress of “microbiome-driven cropping systems” may effect in the subsequent uprising in agricultural field, offering a further sustained structure for plant production.
References
Abreu-Tarazi MF, Navarrete AA, Andreote FD, Almeida CV, Tsai SM, Almeida M (2010) Endophytic bacteria in long-term in vitro cultivated axenic pineapple microplants revealed by PCR DGGE. World J Microbiol Biotechnol 26:555–560
Ahmad I, Ahmad F, Pichtel H (eds) (2011) Microbes and microbial technology: agricultural and environmental applications doi 10.1007/978-1-4419-7931-5_1
Alabouvette C, Olivain C, Steinberg C (2006) Biological control of plant diseases: the European situation. Eur J Plant Pathol 114:329–341
Almeida CV, Andreote FD, Yara R, Ossamu FA, Azevedo JL, Almeida M (2009) Bacteriossomes in axenic plants: endohytes as stable symbionts. World J Microbiol Biotechnol 25:1757–1764
Andreote FD, Gumiere T, Durrer A (2014) Exploring interactions of plant microbiomes. Sci Agric 71(6):528–539
Andrews JH, Harris RF (2000) The ecology and biogeography of microorganisms on plant surfaces. Annu Rev Phytopathol 38:145–180
Araújo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JWL, Azevedo JL (2002) Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68:4906–4914
Argueso C, Hansen M, Kieber J (2007) Regulation of ethylene biosynthesis. J Plant Growth Regul 26(2):92–105. doi:10.1007/s00344-007-0013-5
Arshad M, Shaharoona B, Mahmood T (2008) Inoculation with Pseudomonas spp containing ACC-deaminase partially eliminates the effects of drought stress on growth yield and ripening of pea (Pisum sativum L). Pedosphere 18(5):611–620. doi:10.1016/S1002-0160(08)60055-7
Asiegbu FO, Nahalkova JLG (2005) Pathogen-inducible cDNAs from the interaction of the root rot fungus Heterobasidion annosum with scots pine (Pinus sylvestris L). Plant Sci 168:365372
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32(6):666–681. doi:10.1111/j1365-3040200901926x. PMID: 19143988
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Pena C, Jasinski M, Santelia D, Martinoia E, Sumner LW, Banta LM, Stermitz F, Vivanco JM (2008) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol 146(2):762–771. doi:10.1104/pp107109587 PMID:18065561
Badri DV, Quintana N, El Kassis EG, Kim HK, Choi YH, Sugiyama A, Verpoorte R, Martinoia E, Manter DK, Vivanco JM (2009) An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 151(4):2006–2017. doi:10.1104/pp109147462. PMID:19854857
Badri DV, Chaparro JM, Zhang R, Shen Q, Vivanco JM (2013a) Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem 288(7):4502–4512. doi:10.1074/jbcM112433300. PMID:23293028
Badri DV, Zolla G, Bakker MG, Manter DK, Vivanco JM (2013b) Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol 198(1):264–273. doi:10.1111/nph12124. PMID: 23347044
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57(1):233–266. doi:10.1146/annurevarplant57032905105159. PMID:16669762
Banerjee M, Yesmin L (2002) Sulfur-oxidizing plant growth promoting Rhizobacteria for enhanced canola performance US Patent 20080070784
Barriuso J, Ramos-Solabo B, Guierrez M (2008a) Protection against pathogen and salt stress by four plant growth promoting rhizobacteria isolated from Pinus sp on Arabidopsis thaliana. Biol Control 98(6):666–672
Barriuso J, Solano BR, Lucas JA, Lobo AP, Villaraco AG, Mañero FJG (2008b) In: Ahmad I, Pichtel J, Hayat S (eds) Ecology genetic diversity and screening strategies of Plant Growth Promoting Rhizobacteria (PGPR). WILEY-VCH Verlag GmbH, Co KGaA, Weinheim, pp 1–17
Beattie GA, Lindow SE (1995) The secret life of foliar bacterial pathogens on leaves. Annu Rev Phytopathol 33:145–172
Berg G (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
Bloemberg GV, Lugtenberg BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bokulich NA, Thorngate JH, Richardson PM, Mills DA (2014) Microbial biogeography of wine grapes is conditioned by cultivar vintage and climate. Proc Natl Acad Sci USA 111:E139–E148
Brandl MT, Quinones B, Lindow SE (2001) Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc Natl Acad Sci USA 98:3454–3459
Brimecombe MJ, De Leij FAAM, Lynch JM (2007) Rhizodeposition and microbial populations. In: Pinton R, Veranini Z, Nannipieri P (eds) The rhizosphere biochemistry and organic substances at the soil-plant interface. Taylor, Francis Group, New York
Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (2008) Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol 74(3):738–744. doi:10.1128/AEM02188-07
Buchanan R, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64(1):807–838
Buysens S, Heungens K, Poppe J, Höfte M (1996) Involvement of pyochelin and pyoverdin in suppression of Pythium induced damping off of tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol 62:865–871
Cassán F, García Salamone I (2008) Azospirillum sp: cell physiology plant response agronomic and environmental research in Argentina. Asociacion Argentina de Microbiologia, Buenos Aires
Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, Vivanco JM (2013) Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One 8(2):e55731. doi:10.1371/journalpone 0055731. PMID:23383346
Chet I, Chernin L (2002) Biocontrol microbial agents in soil. In: Bitton G (ed) Encyclopedia of environmental microbiology. Willey, New York, pp 450–465
Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Cook JH, Beyea J, Keeler KH (1991) Potential impacts of biomass production in the United States on biological diversity. Annu Rev Energ Environ 16:401–431
Coronado C, Zuanazzi J, Sallaud C, Quirion JC, Esnault R, Husson HP, Kondorosi A, Ratet P (1995) Alfalfa root flavonoid production is nitrogen regulated. Plant Physiol 108(2):533–542. doi:10.1104/pp1082533 PMID:12228491
Crowley TJ (2000) Causes of climate change over the past 1000 years. Science 289:270–277
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
Davidson IA, Robson MJ (1986) Effect of contrasting patterns of nitrate application on the nitrate uptake N2-fixation nodulation and growth of white clover. Ann Bot 57(3):331–338
De Bary A (1866) Morphologie und Physiologie Pilze Flechten und Myxomyceten Hofmeister’s handbook of physiological botany Engelmann Leipzig Germany
De Vleeschauwer D, Höfte M (2007) Using Serratia plymuthica to control fungal pathogens of plants. CAB Rev 2:4–6
De Werra P, Péchy T, Keel C, Maurhofer M (2009) Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl Environ Microbiol 75:4162–4174
DeFlaun MF, Gerba CP (1993) Monitoring recombinant DNA microorganisms and viruses in soil. In: Metting FBJ (ed) Soil microbial ecology: application in agricultural and environmental management. Marcel Dekker, Washington, DC, pp 131–150
Diener AC, Gaxiola RA, Fink GR (2001) Arabidopsis ALF5 a multidrug efflux transporter gene family member confers resistance to toxins. Plant Cell. Online 13(7):1625–1638. doi:10.1105/tpc1371625
Dini-Andreote F, van Elsas JD (2013) Back to the basics: the need for ecophysiological insights to enhance our understanding of microbial behaviour in the rhizosphere. Plant Soil 373:1–15
Dini-Andreote F, Andreote FD, Araújo WL, Trevors JT, van Elsas JD (2012) Bacterial genomes: habitat specificity and uncharted organisms. Microbial Ecol 64:1–7
Dobbelaere S, Okon Y (2007) The plant growth promoting effects and plant responses In: Elmerich C, Newton WE (eds) Associative and endophytic nitrogen fixing bacteria and cyanobacterial associations (Nitrogen fixation: origins applications and research progress) Heidelberg. Springer, Verlag
Dobbelare S, Vanderleyden J, Okon Y (2003) Plant growth promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Doornbos R, Loon L, Bakker PHM (2012) Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere A review. Agron Sust Dev 32(1):227–243. doi:10.1007/s13593–011-0028-y
Dunne C, Moenne Loccoz Y, de Bruijn FJ, O’Gara F (2000) Overproduction of an inducible extracellular serine protease improves biological control of Pythium ultimum by Stenotrophomonas maltophilia strain W81. Microbiology 146:2069–2078
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease: a (gram) positive perspective. FEMS Microbiol Lett 171:1–9
Ferrara FIS, Oliveira ZM, Gonzales HHS, Floh EIS, Barbosa HR (2012) Endophytic and rhizospheric enterobacteria isolated from sugar cane have different potentials for producing plant growth-promoting substances. Plant Soil 353:409–417
Franks A, Ryan RP, Abbas A, Mark GL, O’Gara F (2006) Molecular tools for studying plant growth promoting rhizobacteria. In: Cooper JE, Rao JR (eds) Molecular approaches to soil rhizosphere and plant microorganisms analysis. Biddes Ltd Kings, Lynn, pp 116–131
Fransson PMA, Johansson EM (2010) Elevated CO2 and nitrogen influence exudation of soluble organic compounds by ectomycorrhizal root systems. FEMS Microbiol Ecol 71(2):186–196. doi:10.1111/j1574-6941200900795x. PMID:19889031
Freiberg E (1998) Microclimatic parameters influencing nitrogen fixation in the phyllosphere in a Costa Rican premontane rain forest. Oecologia 117:9–18
Fuentes-Ramirez LE, Caballero-Mellado J (2005) Bacterial biofertilizers. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 143–172
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48(8):1081–1091. doi:10.1093/pcp/pcm091. PMID: 17634181
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251(1):1–7. doi:10.1016/jfemsle2005 07030. PMID:16099604
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. In: Bakker PAHM, Raaijmakers JM, Bloemberg G, Hofte M, Lemanceau P, Cooke BM (eds) New perspectives and approaches in plant growth-promoting rhizobacteria research. Springer, Dordrecht, pp 329–339
Gottschalk G (1986) Bacterial metabolism. Springer, Berlin/Heidelberg/New York
Griffin GJ, Hale MG, Shay FJ (1976) Nature and quantity of sloughed organic matter produced by roots of axenic peanut plants. Soil Biol Biochem 8:29–32
Haas D, Défago G (2005) Biological control of soilborne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hardoim P, van Overbeek L, van Elsas J (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species – opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312(1-2):7–14
Heath MC (1981) A generalized concept of host-parasite specificity. Phytopathology 7:1121–1123
Hiltner L (1904) Uber neuere Erfahrungen und Probleme auf dem Gebiet der Bodenbakteriologie und unter besonderer Berücksichtigung der Gründüngung und Brachte. Arbeiten der Deutschen Landwirtschaftlichen Gesellschaft 98:59–78
Hirsch PR, Mauchline TH (2012) Who’s who in the plant root microbiome? Nat Biotechnol 30:961–962
Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011) A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem 286(28):24649–24655. doi:10.1074/jbcM111221168. PMID: 21602276
Jackson T (1999) Renewable energy Summary paper for the renewables series. Energy Policy 20:861–883
Jacobsen BJ, Zidack NK, Larson BJ (2004) The role of Bacillus-based biological control agents in integrated pest management systems: plant diseases. Phytopathology 94:1272–1275
Jones K (1970) Nitrogen fixation in phyllosphere of Douglas Fir Pseudotsuga-Douglasii. Ann Bot 34:239–244
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163(3):459–480. doi:10.1111/j1469–81372004 01130x
Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg B (2005) Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7:1809–1817
Kang SM, Khan AL, Waqs M, You YH, Kim JH, Kim GK, Hamayun M, Lee IJ (2014) Plant growth promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682
Kent AD, Triplett EW (2002) Microbial communities and their interactions in soil and rhizosphere ecosystems. Ann Rev Microbiol 56:211–236
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bucking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333(6044):880–882. doi:10.1126/science1208473. PMID:21836016
Kinkel LL, Wilson M, Lindow SE (2000) Plant species and plant incubation conditions influence variability in epiphytic bacterial population size. Microb Ecol 39:1–11
Kishore GK, Pande S, Podile AR (2005) Biological control of late leaf spot of peanut (Arachis hypogaea) with chitinolytic bacteria. Phytopathology 95:1157–1165
Kloepper JW, Schroth MN (1978) Plant growth promoting rhizobacteria on radishes In: Proceedings of the IVth international conference on plant pathogenic bacteria Argers France:Station de Pathologie Vegetale et Phytobacteriologyie INRA, pp 879–882
Lambais MR, Crowley DE, Cury JC, Bull RC, Rodrigues RR (2006) Bacterial diversity in tree canopies of the Atlantic forest. Science 312:1917–1917
Leyval C, Berthelin J (1993) Rhizodeposition and net release of soluble organic compounds by pine and beech seedlings inoculated with rhizobacteria and ectomycorrhizal fungi. Biol Fertil Soils 15(4):259–267. doi:10.1007/ bf00337210
Li L, He Z, Pandey GK, Tsuchiya T, Luan S (2002) Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277(7):5360–5368. doi:10.1074/jbcM108777200. PMID:11739388
Lindow SE, Brandl MT (2003) Microbiology of the phyllophere. Appl Environ Microbiol 69:1875–1883
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57(3):389–399. doi:10.1111/j1365-313X200803696x. PMID:18826429
Long SR (2001) Genes and signals in the Rhizobium-legume symbiosis. Plant Physiol 125:69–72
Loper JE (1988) Role of fluorescent siderophore production in biological control of Pythium ultirnum by a Pseudomonas fluorescens strain. Phytopathology 78:166–172
Loper JE, Gross H (2007) Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf5. Eur J Plant Pathol 119:265–278
Loper JE, Henkels MD (1997) Availability of iron to Pseudomonas fluorescens in rhizosphere and bulk soil evaluated with an ice nucleation reporter gene. Appl Environ Microbiol 63:99–105
Loyola-Vargas V, Broeckling C, Badri D, Vivanco J (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225(2):301–310. doi:10.1007/s00425–006–0349-2. PMID:16868775
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86(1):1–25
Lugtenberg BJJ, Dekkers LC (1999) What makes Pseudomonas bacteria rhizosphere competent. Environ Microbiol 1:9–13
Lugtenberg BJJ, Chin A, TFC W, Bloemberg GV (2002) Microbe plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek 81:373–383
Magalhaes JV, Liu J, Guimaraes CT, Lana UGP, Alves VMC, Wang Y-H, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39(9):1156–1161. doi:10.1038/ng2074. PMID: 17721535
Matilla MA, Ramos JL, Bakker PAHM, Doornbos R, Badri DV, Vivanco JM, Ramos-Gonzalez MI (2010a) Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. Environ Microbiol 2(3):381–382
Matilla MA, Ramos JL, Bakker PAHM, Doornbos R, Badri DV, Vivanco JM, Ramos-Gonzalez MI (2010b) Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. Environ Microbiol Rep 2(3):381–388. doi:10.1111/j1758–22292009 00091x. PMID:23766110
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42(6):565–572. doi:10.1016/jplaphy200405009. PMID:15246071
Meier IC, Avis PG, Phillips RP (2013) Fungal communities influence root exudation rates in pine seedlings. FEMS Microbiol Ecol 83(3):585–595. doi:10.1111/1574–694112016. PMID:23013386
Mendes R, Pizzirani-Kleiner AA, Araujo WL, Raaijmakers JM (2007) Diversity of cultivated endophytic bacteria from sugarcane: genetic and biochemical characterization of Burkholderia cepacia complex isolates. Appl Environ Microbiol 73(22):7259–7267
Mercado-Blanco J, Bakker P (2007) Interactions between plants and beneficial Pseudomonas spp: exploiting bacterial traits for crop protection. Antonie van Leeuwenhoek 92(4):367–389. doi:10.1007/s10482–007–9167-1
Micallef SA, Channer S, Shiaris MP, Colon-Carmona A (2009a) Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal Behav 4(8):777–780. doi:10.4161/psb489229. PMID:19820328
Micallef SA, Shiaris MP, Colon-Carmona A (2009b) Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J Exp Bot 60(6):1729–1742. doi:10.1093/jxb/erp053. PMID:19342429
Mitter B, Petric A, Shin MW, Chain PSG, Hauberg-Lotte L, Reinhold-Hurek B, Nowak J, Sessitsch A (2013) Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front Plant Sci 4:120
Morgan JAW (2005) Biological costs and benefits to plant-microbe interactions in the rhizosphere. J Exp Bot 56(417):1729–1739
Morris CE, Kinkel LL (2002) Fifty years of phyllosphere microbiology: significant contributions to research in related fields. In: Lindow SE, Hecht-Poinar EI, Elliott V (eds) Phyllosphere microbiology. APS Press, St Paul, pp 365–375
Müller H, Westendorf C, Leitner E, Chernin L, Riedel K, Schmidt S, Eberl L, Berg G (2009) Quorumsensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HROC48 FEMS. Microbiol Ecol 67:468–478. doi:10.1111/j.1574-6941.2008.00635.x. PMID: 19220861
Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant–microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132(1):146–153. doi:10.1104/pp102016295. PMID:12746520
Nelson E (1990) Exudate molecules initiating fungal responses to seeds and roots. Plant Soil 129:61–73
Newton AC, BDL F, Atkins SD, Walters DR, Daniell TJ (2010) Pathogenesis parasitism and mutualism in the trophic space of microbe–plant interactions. Trends Microbiol 18(8):365–373. doi:10.1016/jtim201006002. PMID:20598545
Nguyen C (2003) Rhizodeposition of organic C by plants:mechanisms and controls. Agronomie 23:375–396
O’Brien RD, Lindow SE (1989) Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology 79:619–627
Odum EP (1971) Fundamentals of ecology. Saunders, Philadelphia
Olubukola O, Babalola O, Glick BR (2012) The use of microbial inoculants in African agriculture. Food Agric Environ 10:540–549
Ongena M, Thonart P (2006) Resistance induced in plants by nonpathogenic microorganisms: elicitation and defense responses. In: Teixeira da Silva JA (ed) Floriculture ornamental and plant biotechnology: advances and topical issues. Global Science Books, London, pp 447–463
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15.
Petrini O (1991) Fungal endophytes of tree leaves. In: Fokkema NJ, van den Heuvel (eds) Microbial ecology of the leaves. Cambridge University Press, Cambridge, pp 185–187
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Pierik R, Tholen D, Poorter H, Visser EJW, Laurentius ACJ, Voesenek (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11:176–183
Pinton R, Veranini Z, Nannipieri P (2007) The rhizosphere biochemistry and organic substances at the soil plant interface. Taylor, Francis Group LLC, New York
Quecine MC, Araujo WL, Tsui S, Parra JRP, Azevedo JL, Pizzirani-Kleiner AA (2014) Control of Diatraea saccharalis by the endophytic Pantoea agglomerans 331 expressing cry1Ac7. Arch Microbiol 196:227–234
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81:537–547
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, van Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JHJ (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822
Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH (2012) The major facilitator superfamily (MFS) revisited. FEBS J 279(11):2022–2035. doi:10.1111/ j1742-4658201208588x. PMID:22458847
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010) The ecology of the phyllosphere: geographic and phyllogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893
Rizzo DM, Garbelotto M, Hansen EA (2005) Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annu Rev Phytopathol 43:309–335
Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts molecular plant-microbe interactions 19:827–837
Rosling A, Lindahl BD, AFS T, Finlay RD (2004) Mycelial growth and substrate acidification of ectomycorrhizal fungi in response to different minerals. FEMS Microbiol Ecol 47(1):31–37. doi:10.1016/S0168–6496(03)00222–8. PMID:19712344
Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, van der Lelie D, Dow JM (2009) Versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Microbiol Rev 7:514–525
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Ann Rev Ecol Syst 29:319–343
Salisbury FB (1994) The role of plant hormones. In: Wilkinson RE (ed) Plant-environment interaction. Dekker, New York, pp 39–81
Schrey SD, Tarkka MT (2008) Friends and foes:streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 94:11–19
Schroth MN, Hildebrand DC (1964) Influence of plant exudates on root infecting fungi. Annu Rev Phytopathol 2(10):11–32
Sengupta A, Gunri S (2015) Microbial intervention in agriculture: an overview. Afr J Microbiol Res 9(18):1215–1226
Shoda M (2000) Bacterial control of plant diseases. J Biosci Bioeng 89:515–521
Sivakumar PV, Thamizhiniyan P (2012) Enhancement in growth and yield of tomato by using AM fungi and Azospirillum. Int J Environ Biol 2(3):137–141
Stearns JC, Woody OZ, McConkey BJ, Glick BR (2012) Effects of bacterial ACC deaminase on Brassica napus gene expression. Mol Plant Microb Interact 25(5):668–676. doi:10.1094/MPMI-08-11-0213. PMID:22352713
Steenhoudt O, Vanderleyden J (2000) Azospirillum a free-living nitrogen-fixing bacterium closely associated with grasses: genetic biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Steinkellner S, Lendzemo V, Langer I, Schweiger P, Khaosaad T, Toussaint JP, Vierheilig H (2007) Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant–fungus interactions. Molecules 12(7):1290–1306. doi:10.3390/12071290. PMID:17909485
Sugiyama A, Shitan N, Yazaki K (2008) Signaling from soybean roots to Rhizobium:an ATP-binding cassette-type transporter mediates genistein secretion. Plant Signal Behav 3(1):38–40. doi:10.4161/psb314819. PMID:19704765
Tournas VH, Katsoudas E (2005) Mould and yeast flora in fresh berries grapes and citrus fruits. Int J Food Microbiol 105:1117
Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M (2005) Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of lupin analysed by phytate utilization ability. Environ Microbiol 7:396–404
Uren NC (2000) Types amounts and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface. Marcel Dekker, New York, pp 19–40
van Etten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus phytoanticipins. Plant Cell 6:1191–1192
Van Loon LC (2007) Plant responses to plant growth promoting bacteria. Eur J Plant Pathol 119:243–254
van Veen JA, van Overbeek LS, van Elisas JD (1997) Fate and activity of microorganisms introduced into soil. Microbiol Mol Biol Rev 61:121–135
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev 10:828–840
Weger LA, van Arendonk JJCM, Recourt K, van der Hofstad GAJM, Weisbeek PJ, Lugtenberg B (1988) Siderophore mediated uptake of Fe3+ by the plant growth stimulating Pseudomonas putida strain WCS358 and by other rhizosphere microorganisms. J Bacteriol 170:4693–4698
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
Weston LA, Ryan PR, Watt M (2012) Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J Exp Bot 63(9):3445–3454
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4. doi:10.1016/jtplants2008 10004. PMID:19056309
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8(3):301–307. doi:10.1016/jpbi200503011. PMID: 15860427
Zahran HH (1999) Rhizobium–legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63(4):968–989. PMID: 10585971
Zhang J, Subramanian S, Stacey G, Yu O (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57(1):171–183. doi:10.1111/j1365-313X2008 03676x. PMID:18786000
Zolla G, Badri DV, Bakker MG, Manter DK, Vivanco JM (2013) Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Appl Soil Ecol 68:1–9. doi:10.1016/japsoil201303007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sengupta, A., Gunri, S.K., Biswas, T. (2017). Microbial Interactions and Plant Health. In: Singh, D., Singh, H., Prabha, R. (eds) Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer, Singapore. https://doi.org/10.1007/978-981-10-5813-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-5813-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5812-7
Online ISBN: 978-981-10-5813-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)