Abstract
Bacterial infections were traditionally not considered as major causes of cancer. However, increasing evidence in the past decades has suggested that several cancers are highly associated with bacterial infection. The bacterial infections have evolved some unique strategies including lateral gene transfer, biofilm and microbiome to induce genome instability and chronic inflammation, as well as escape of immune surveillance for carcinogenesis. Here we summarize and highlight the recent progress on understanding of how bacterial infection plays a role in tumor formation and malignancy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Although viral infection is the main agent of infection-causing cancers in humans, and a number of bacterial pathogens have also been shown to make a significant contribution to cancer [1], research on effects of bacterial infection was left far behind than viral infection. The role of bacterial infection in inducing cancer is still a highly debated subject; in fact, several parameters must be met to be infectious cause of cancer. While the evidence of antibiotics such as aspirin could reduce risks of breast cancer for some time [2, 3], indicating that appropriate bacteria may contribute to the development and progress of particular cancer.
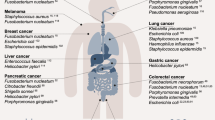
Early observations in 1772, Mycobacterium tuberculosis was the first bacterium thought to cause lung cancer, due to active tuberculosis in the lung cancer patient which was more frequently than the general population [4]. However, the Mycobacterium tuberculosis-cancer theory failed to stand in many cases test and appear to be the results of the malignancy instead of the cause. Despite the early mistake, the possible association of bacterial infection with carcinogenesis continues to be promulgated. A significant breakthrough was made when the chronic infection of Helicobacter pylori was identified to cause stomach ulcers followed by onset of gastric carcinomas or MALT (mucosa-associated lymphoid tissue) lymphomas [5, 6]. After the substantial progress in understanding the role of H. pylori on carcinogenesis, it has been estimated that bacteria account for at least half of organism infections in patients with malignancy [7]. Here we will address and highlight the relevance of H. pylori, oral bacteria, and some gram-positive bacteria with cancers (Table 11.1).
11.2 Helicobacter pylori and Cancers
Helicobacter is spiral-shaped, gram-negative bacterium [8, 9] and was firstly isolated and cultured from a human gastric biopsy by Marshall and Warren in 1982 [10]. The seminal discovery of this bacterium and its role in gastritis and peptic ulcer disease led to award of Nobel Prize of Medicine in 2005 for Marshall and Warren. Unlike other viruses and bacteria, H. pylori has the ability to colonize in highly acidic environment within the stomach [11]. The majority of H. pylori strains have expressed several virulence factors that have evolved to affect host cell signaling pathways, which included CagA (cytotoxin-associated gene A antigen), VacA (vacuolating cytotoxin), BabA (blood group antigen-binding adhesion), OipA (outer inflammatory protein), and IceA.
It has been estimated that nearly half of the world’s population is infected with H. pylori, and the majority of colonized individuals could develop chronic inflammation. Despite that H. pylori colonization does not absolutely cause symptoms [12], long-term carriage of H. pylori will significantly increase the risk of developing site-specific diseases. For example, around 10% of the infected individuals will develop peptic ulcer disease, 3% of them will develop gastric adenocarcinoma, and 0.1% of them will develop mucosa-associated lymphoid tissue (MALT) lymphoma [13]. However, due to the fact that individuals infected with H. pylori do not necessarily have antibodies against bacteria, or may not be detectable in blood, the number of cancer patients seropositive for H. pylori may be underestimated. It has been found that gastric MALT lymphoma can be completely cured by eradication of H. pylori at early stage and therefore is considered the first clonal lesion which can be eliminated by treatment with antibiotics [14].
The relationship between H. pylori infection and gastric cancer has been widely studied for over four decades. Several studies have now provided clear notion that H. pylori infection is significantly associated with gastric cancer and eradication of H. pylori could significantly decrease the risk of gastric cancer in infected individuals without premalignant lesions [15,16,17]. Gastric cancer as the third most common cause of cancer deaths in the world; gastric adenocarcinoma accounts for over 95% of malignant neoplasms of the stomach, followed by gastrointestinal stromal tumors and mucosa-associated lymphoid tissue (MALT) lymphoma [18]. H. pylori infection is more prevalent (some up to 80%) in the developing countries where poor hygiene enhances person-to-person transmission through domestic contacts at an early age [8]. A recent prospective and population-based study in China showed that higher education, lifestyle changes, and sanitation habits could influence the rate of infection [9]. Although gastric cancer is relatively rare in United States, the incidence varies in many developed countries, for example, Korea, Mongolia, and Japan are the highest (29.9–41.8 per 100,000 persons), while Canada, Western Europe, and Australia are much lower [8, 19].
11.3 Oral Bacteria and Cancers
Many bacteria including Bacteroides fragilis, Enterococcus faecalis, Escherichia coli, Fusobacterium nucleatum, and Porphyromonas asaccharolytica have been shown to modulate tumorigenesis in colorectal cancer (CRC) [20, 21]. A recent review has summarized well how oral bacteria potentially induce colorectal cancer [22]; here we will address the key progress on the association between oral bacteria and cancer.
Although B. fragilis, E. faecalis, and E. coli present weak pathogenic features, two oral bacteria F. nucleatum and P. asaccharolytica were consistently identified in CRC patients and often synergistically promote oral and colon cancer progression [23]. In addition to F. nucleatum and P. asaccharolytica, other oral strains including Peptostreptococcus, Prevotella, Parvimonas, and Gemella were effectively used as biomarkers to detect CRC [24, 25]. Given the fact of the consistent co-occurrence of these oral bacteria in CRC, their potential roles in tumorigenesis were proposed to be synergistic activities of biofilm formation and anaerobic asaccharolytic metabolism. A “driver-passenger” model, namely, a “driver” organism such as P. gingivalis and F. nucleatum can produce virulence factors to help a “passenger” bacterial load and growth, has been proposed to induce this cancer-associated biofilm formation. The consistence of high abundance of F. nucleatum in many CRC and adenoma instead of healthy colon biofilm samples indicates that this oral organism plays an essential role in carcinogenesis [26, 27]. Besides F. nucleatum, other oral anaerobic bacteria such as Leptotrichia and Campylobacter have also been revealed by deep sequencing and pairwise correlation analysis of CRC and normal tissues [28].
Because there are so many anaerobic organisms that exist in colon tissue, it has been proposed that the asaccharolytic metabolism of these oral bacteria may play a role in carcinogenesis. Due to these bacteria usually that digest peptides and amino acids instead of sugar or carbon, they become typically proteolytic. The coordinated metabolism will promote growth of a diverse and cooperative polymicrobial ecosystem and continue the breakdown of host proteins to inhibit immune response [29]. A similar effect was observed when these oral organisms inhabit the colon [30]. Recent studies have shown that F. nucleatum can disrupt epithelial junctions through E-cadherin to alter mucosal environment where it facilitates growth of other anaerobic microbes once it localizes in colon tissue [31]. In addition, F. nucleatum-mediated degradation of host protein in the mouth and gut will build up a chronic inflammatory microenvironment to promote the development of CRC [32]. Another outcome of these metabolites was found to cause DNA damage in colon tissues by inducing polyamines and genotoxic ROS production, which will facilitate biofilm formation and promote cancer cell proliferation [33].
Given the recent advances in the integrated view of the oral microbiome in colorectal tumorigenesis, it has been accepted that all polymicrobes coordinate in concert rather than just a specific pathogen nor virulence factors to create an inflammatory microenvironment that leads to bacteria-associated cancers. In regard to how the microbe disseminates from the oral cavity to the colon, two hypotheses have been proposed. One possible hypothesis is that the microbe could disseminate from ulcerated gingival tissues into the bloodstream and then is located at colon tissue. The other possible hypothesis is that the oral bacteria are swallowed and colonized at colon tissue. However, these two routes remain to be further studied, and inflamed colon or perturbed community may contribute to colonization of oral microbes.
In addition to colorectal cancer, the oral bacteria including Porphyromonas and Fusobacterium have also been found to strikingly associate with oral squamous cell carcinoma (OSCC is one of the most common cancers worldwide) and pancreatic cancer [34, 35]. In the epithelial and OSCC cell model, it has been found that P. gingivalis infection not only can upregulate the expression of B7-H1 and B7-DC receptors, which contribute to chronic inflammation [36], but also promote cellular invasion of OSCC cells through inducing metalloproteinase MMP-9 expression [37]. Further studies revealed that gingipains, a cysteine proteinase produced by P. gingivalis, plays a critical role in this process [37]. Similarly, F. nucleatum can also enhance tumor cell proliferation and migration through MMP-9 and MMP-13 [38].
11.4 Gram-Positive Bacteria and Cancer
Several lines of evidence have shown that many gram-positive microbes can cause serious infections and invasive bacterial disease in cancer patients. They include Staphylococci, Streptococci, and Enterococci. To understand the impact of these bacteria in patients with malignancy will help develop cancer therapeutic strategy and improve the survival of the cancer patient. We will describe the most recent progress on these three types of bacteria and their associated cancers below.
Staphylococcus aureus—Whether S. aureus is a cause of infection in cancer patients remains to be further demonstrated; it has a high relevance with the mortality of patients with pneumonia [39]. Cancer patients treated with antistaphylococcal antibiotics (i.e., daptomycin or ceftaroline) have shown a generally favorable outcome [40].
Streptococci—Among the Streptococci, viridans group streptococci (VGS) is the prominent member of the oral microbiome and is often found to correlate with high mortality of patients with pediatric acute myeloid leukemia [41, 42]. β-hemolytic streptococci GBS is found to associate with breast cancer [43], and S. pneumoniae affects the malignancy of patients with leukemia, lymphoma, or myeloma [44]. It has been shown that VGS is multidrug resistant including β-lactams, while GBS remains susceptible to β-lactams and could be treated with penicillins or cephalosporins, and S. pneumonia is susceptible to levofloxacin and vancomycin [45,46,47]. In addition, Streptococcus bovis in gastrointestinal microflora was found in blood and caused infective endocarditis [48, 49], which has been previously shown to associate with colorectal cancer [50], albeit no S. bovis DNA was identified in colorectal neoplastic tissues by using the PCR technique [51].
Enterococci—Although Enterococci is generally considered as low-virulence bacteria, E. faecium is found to associate with increased risk of mortality of patients with hematologic malignancies [52]. It is known that E. faecium is penicillin susceptible, while β-lactam or vancomycin is resistant for therapy of cancer patients.
Another example is Salmonella typhimurium (S. typhi) and gallbladder cancer (GBC), due to patients who are infected with this bacterium developed GBC more frequently [53, 54]. However, the causative links between this bacterial infection and cancer remain to be further demonstrated.
11.5 Potential Mechanisms of Bacterium-Associated Carcinogenesis
Chromosome instability is a common feature of cancer cells. Despite evidence of epidemiological studies of bacteria-associated cancer is persuasive, the molecular mechanisms of bacterial infection-causing genome instability still remain largely unclear. However, recent studies have shown that bacteria—their eukaryotic endosymbionts lateral gene transfer (LGT)—are a mean of causing genome instability [55]. Although the link between the LGT and tumor-causing ability has not been established, it is speculated that LGT may cause tumorigenesis. For example, in vitro experiments demonstrated that Bartonella henselae (a bacterium causing bacillary angiomatosis-peliosis tumor in humans [56]) is able to integrate their plasmid DNA into the human host genome [57]. Bioinformatics analysis revealed high incidence of LGT from Acinetobacter and Pseudomonas like DNA in the mitochondrial DNA of patients with acute myeloid leukemia [58]. In contrast, very few evidence of integration of H. pylori DNA was reported, despite the fact that infection with this bacterium is highly associated with gastric cancer.
Given chronic inflammation which is also a common physiological response of host immunity to microbe infections, and is involved in generation of several mediators such as free radicals, prostaglandins, and cytokines, deregulation of these mediators by bacteria will lead to cell proliferation, angiogenesis, and oncogenic activation. Therefore, the specific modification of the inflammatory response by bacteria will lead to persistent infections and eventually development of cancer cells. For instance, ROS and IL-1β, as products of chronic inflammation, are shown to be upregulated by H. pylori in gastric epithelial cells for cell proliferation and angiogenesis [59, 60]. Epithelial cell infection with Pseudomonas aeruginosa or H. pylori is able to induce VEGF expression and trigger angiogenesis [61, 62]. In addition, H. pylori also plays a key role in activation of NF-κB through increasing the expression of IL-8 and TNF-α [63, 64]. Further studies have revealed that Toll-like receptors TLR4-mediated activation of NF-κB signaling facilitate the H. pylori colonization [65].
Deregulation of host cell proliferation and apoptosis is another common mechanism targeted by viral infection [66]. Emerging evidence has shown that bacteria could also evolve several strategies to control cell progression. For example, H. pylori-encoded CagE could promote the activation of Cyclin D1 in cell cycle [67]. The toxin CNF released by E. coli could not only induce G1-S transition and DNA replication but also inhibit cell apoptosis to stimulate cell progression [68, 69]. To block cell apoptosis, H. pylori-encoded Cag antigen also induces COX-2 expression to activate Bcl-2 and suppress apoptosis [70].
The evasion of the immune system is another key mechanism utilized by bacteria to promote cell malignancy. It has been demonstrated that bacteria have evolved multiple mechanisms to evade host immune response, including the modulation of bacteria surface, subversion of phagocytes, and blockade of innate immunity. To avoid surface signal recognition by immune system, many bacteria form carbohydrate-rich capsules or incorporate host proteins into capsules to mask their surface antigens from host receptors [71]. Since phagocytosis is one of the main ways used by host to counter bacterial infection, it is not surprising for bacteria to employ different strategies to escape. For example, Streptococcus pneumonia and Staphylococcus aureus produced immunoglobulin proteases to preclude the capture of antigens [72] or antibody-binding proteins to scavenge opsonizing antibodies [73]. To avoid the acquired immune response, H. pylori produce a vacuolating toxin VacA to block T cell proliferation and in turn inhibit the receptor-IL-2 signaling pathway and decrease of activated T cells [74, 75].
11.6 Future Perspective
Although it has been demonstrated that some bacterial infections associate with development of cancer, very few studies demonstrate the genomic instability of cells which was directly caused by bacteria or exposed to bacterial components through blood. More details about the molecular pathways involved in the induction of genomic instability in response to bacterial infection remain to be further explored. It still needs to answer why the causative relationship between the bacterial infection and cancers is only limited for a few cancers and whether the bacterial infection process is required to cause cancer. The association of oral organisms with colorectal cancer indicates that relocation of bacteria in inappropriate tissues and polymicrobial interactions together could be the key for bacteria to cause carcinogenesis. Along with the development of next-generation deep sequencing technology and bioinformatics analysis, it will provide a clear scenario about how bacterial infection contributes to cancer, which will facilitate to develop effective diagnostic and therapeutic strategies against infection-causing cancers.
References
Kuper H, Adami HO, Trichopoulos D (2000) Infections as a major preventable cause of human cancer. J Intern Med 248:171–183
Ness RB, Cauley JA (2004) Antibiotics and breast cancer – what’s the meaning of this? JAMA 291:880–881
Harris RE, Beebe-Donk J, Doss H, Burr DD (2005) Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review). Oncol Rep 13:559–583
Onuigbo WI (1975) Some nineteenth century ideas on links between tuberculous and cancerous diseases of the lung. Br J Dis Chest 69:207–210
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325:1127–1131
Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG (1991) Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338:1175–1176
Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, Akova M, Fourth European Conference on Infections in Leukemia Group ajvoEEIELN, Esgich/Escmid (2014) Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect 68:321–331
Mentis A, Lehours P, Megraud F (2015) Epidemiology and diagnosis of helicobacter pylori infection. Helicobacter 20(Suppl 1):1–7
Ding Z, Zhao S, Gong S, Li Z, Mao M, Xu X, Zhou L (2015) Prevalence and risk factors of helicobacter pylori infection in asymptomatic Chinese children: a prospective, cross-sectional, population-based study. Aliment Pharmacol Ther 42:1019–1026
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1:1311–1315
Weeks DL, Eskandari S, Scott DR, Sachs G (2000) A H+−gated urea channel: the link between helicobacter pylori urease and gastric colonization. Science 287:482–485
Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature reviews. Cancer 2:28–37
Peek RM Jr, Crabtree JE (2006) Helicobacter infection and gastric neoplasia. J Pathol 208:233–248
Sena Teixeira Mendes L, DA A, CW A (2014) Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut 63:1526–1527
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P (2005) Long term follow up of patients treated for helicobacter pylori infection. Gut 54:1536–1540
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS, China Gastric Cancer Study G (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187–194
Huret JL, Ahmad M, Arsaban M, Bernheim A, Cigna J, Desangles F, Guignard JC, Jacquemot-Perbal MC, Labarussias M, Leberre V, Malo A, Morel-Pair C, Mossafa H, Potier JC, Texier G, Viguie F, Yau Chun Wan-Senon S, Zasadzinski A, Dessen P (2013) Atlas of genetics and cytogenetics in oncology and haematology in 2013. Nucleic Acids Res 41:D920–D924
Song H, Ekheden IG, Zheng Z, Ericsson J, Nyren O, Ye W (2015) Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk western population. BMJ 351:h3867
Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD (2013) The gut microbiome modulates colon tumorigenesis. MBio 4:e00692–e00613
Sears CL, Garrett WS (2014) Microbes, microbiota, and colon cancer. Cell Host Microbe 15:317–328
Flynn KJ, Baxter NT, Schloss PD (2016) Metabolic and community synergy of oral bacteria in colorectal cancer. mSphere 1
Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, Nussbaum G, Elkin M (2015) Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 6:22613–22623
Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Xu X, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brunner N, Kristiansen K, Arumugam M, Sung JJ, Wang J (2017) Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66:70–78
Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, Amiot A, Bohm J, Brunetti F, Habermann N, Hercog R, Koch M, Luciani A, Mende DR, Schneider MA, Schrotz-King P, Tournigand C, Tran Van Nhieu J, Yamada T, Zimmermann J, Benes V, Kloor M, Ulrich CM, von Knebel DM, Sobhani I, Bork P (2014) Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 10:766
Whitmore SE, Lamont RJ (2014) Oral bacteria and cancer. PLoS Pathog 10:e1003933
Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, Stein E, Vadivelu J, Roslani AC, Malik AA, Wanyiri JW, Goh KL, Thevambiga I, Fu K, Wan F, Llosa N, Housseau F, Romans K, Wu X, McAllister FM, Wu S, Vogelstein B, Kinzler KW, Pardoll DM, Sears CL (2014) Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A 111:18321–18326
Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA (2013) Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 1:16
Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nature reviews. Immunology 15:30–44
Donaldson GP, Lee SM, Mazmanian SK (2016) Gut biogeography of the bacterial microbiota. Nature reviews. Microbiology 14:20–32
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14:195–206
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14:207–215
Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, Ivanisevic J, Cho K, Wick EC, Hechenbleikner EM, Uritboonthai W, Goetz L, Casero RA Jr, Pardoll DM, White JR, Patti GJ, Sears CL, Siuzdak G (2015) Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab 21:891–897
Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN (1998) The microflora associated with human oral carcinomas. Oral Oncol 34:304–308
Michaud DS (2013) Role of bacterial infections in pancreatic cancer. Carcinogenesis 34:2193–2197
Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J (2011) B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 216:1302–1310
Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, Morisaki I, Lamont RJ, Amano A (2014) Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol 16:131–145
Uitto VJ, Baillie D, Wu Q, Gendron R, Grenier D, Putnins EE, Kanervo A, Firth JD (2005) Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect Immun 73:1171–1179
Bodro M, Gudiol C, Garcia-Vidal C, Tubau F, Contra A, Boix L, Domingo-Domenech E, Calvo M, Carratala J (2014) Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer 22:603–610
Rolston KV, Besece D, Lamp KC, Yoon M, McConnell SA, White P (2014) Daptomycin use in neutropenic patients with documented gram-positive infections. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer 22:7–14
Dix D, Cellot S, Price V, Gillmeister B, Ethier MC, Johnston DL, Lewis V, Michon B, Mitchell D, Stobart K, Yanofsky R, Portwine C, Silva M, Bowes L, Zelcer S, Brossard J, Traubici J, Allen U, Beyene J, Sung L (2012) Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): results from the Canadian infections in AML research group. Clin Infect Dis: Off Publ Infect Dis Soc Am 55:1608–1614
Lewis V, Yanofsky R, Mitchell D, Dix D, Ethier MC, Gillmeister B, Johnston D, Michon B, Stobart K, Portwine C, Silva M, Cellot S, Price V, Bowes L, Zelcer S, Brossard J, Beyene J, Sung L (2014) Predictors and outcomes of viridans group streptococcal infections in pediatric acute myeloid leukemia: from the Canadian infections in AML research group. Pediatr Infect Dis J 33:126–129
Shelburne SA 3rd, Tarrand J, Rolston KV (2013) Review of streptococcal bloodstream infections at a comprehensive cancer care center, 2000–2011. J Infect 66:136–146
Domenech A, Ardanuy C, Grau I, Calatayud L, Pallares R, Fenoll A, Brueggemann AB, Linares J (2014) Evolution and genetic diversity of the Spain23F-ST81 clone causing adult invasive pneumococcal disease in Barcelona (1990–2012). J Antimicrob Chemother 69:924–931
Han SB, Bae EY, Lee JW, Lee DG, Chung NG, Jeong DC, Cho B, Kang JH, Kim HK (2013) Clinical characteristics and antimicrobial susceptibilities of viridans streptococcal bacteremia during febrile neutropenia in patients with hematologic malignancies: a comparison between adults and children. BMC Infect Dis 13:273
Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ, Active Bacterial Core surveillance/Emerging Infections Program N (2008) Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065
Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV (2013) Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.) Emerg Infect Dis 19:1074–1083
Noble CJ (1978) Carriage of group D streptococci in the human bowel. J Clin Pathol 31:1182–1186
Kupferwasser I, Darius H, Muller AM, Mohr-Kahaly S, Westermeier T, Oelert H, Erbel R, Meyer J (1998) Clinical and morphological characteristics in Streptococcus bovis endocarditis: a comparison with other causative microorganisms in 177 cases. Heart 80:276–280
Mc CW, Mason JM 3rd (1951) Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala 21:162–166
Tjalsma H, Scholler-Guinard M, Lasonder E, Ruers TJ, Willems HL, Swinkels DW (2006) Profiling the humoral immune response in colon cancer patients: diagnostic antigens from Streptococcus bovis. Int J Cancer 119:2127–2135
Zhou X, Arends JP, Span LF, Friedrich AW (2013) Algorithm for pre-emptive glycopeptide treatment in patients with haematologic malignancies and an Enterococcus faecium bloodstream infection. Antimicrob Resist Infect Control 2:24
Welton JC, Marr JS, Friedman SM (1979) Association between hepatobiliary cancer and typhoid carrier state. Lancet 1:791–794
Tewari M, Mishra RR, Shukla HS (2010) Salmonella typhi and gallbladder cancer: report from an endemic region. Hepatobiliary Pancreat Dis Int: HBPD INT 9:524–530
Robinson KM, Sieber KB, Dunning Hotopp JC (2013) A review of bacteria-animal lateral gene transfer may inform our understanding of diseases like cancer. PLoS Genet 9:e1003877
Koehler JE, Sanchez MA, Garrido CS, Whitfeld MJ, Chen FM, Berger TG, Rodriguez-Barradas MC, LeBoit PE, Tappero JW (1997) Molecular epidemiology of bartonella infections in patients with bacillary angiomatosis-peliosis. N Engl J Med 337:1876–1883
Schroder G, Schuelein R, Quebatte M, Dehio C (2011) Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. Proc Natl Acad Sci U S A 108:14643–14648
Riley DR, Sieber KB, Robinson KM, White JR, Ganesan A, Nourbakhsh S, Dunning Hotopp JC (2013) Bacteria-human somatic cell lateral gene transfer is enriched in cancer samples. PLoS Comput Biol 9:e1003107
Handa O, Naito Y, Yoshikawa T (2011) Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep: Commun Free Radic Res 16:1–7
El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398–402
Martin C, Thevenot G, Danel S, Chapron J, Tazi A, Macey J, Dusser DJ, Fajac I, Burgel PR (2011) Pseudomonas aeruginosa induces vascular endothelial growth factor synthesis in airway epithelium in vitro and in vivo. Eur Respir J 38:939–946
Takayama S, Takahashi H, Matsuo Y, Okada Y, Takeyama H (2010) Effect of Helicobacter bilis infection on human bile duct cancer cells. Dig Dis Sci 55:1905–1910
Mori N, Wada A, Hirayama T, Parks TP, Stratowa C, Yamamoto N (2000) Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-kappaB in gastric epithelial cancer cells. Infect Immun 68:1806–1814
Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S (2000) Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol 95:2768–2776
Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, Vaughan TL, McColl KE, Lissowska J, Zatonski W, Schoenberg JB, Blot WJ, Mowat NA, Fraumeni JF Jr, El-Omar EM (2007) A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology 132:905–912
Coschi CH, Dick FA (2012) Chromosome instability and deregulated proliferation: an unavoidable duo. Cell Mol Life Sci: CMLS 69:2009–2024
Lax AJ (2005) Opinion: bacterial toxins and cancer – a case to answer? Nature reviews. Microbiology 3:343–349
Patyar S, Joshi R, Byrav DS, Prakash A, Medhi B, Das BK (2010) Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci 17:21
Oswald E, Sugai M, Labigne A, Wu HC, Fiorentini C, Boquet P, O’Brien AD (1994) Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci U S A 91:3814–3818
Lax AJ, Thomas W (2002) How bacteria could cause cancer: one step at a time. Trends Microbiol 10:293–299
Finlay BB, McFadden G (2006) Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767–782
Sarantis H, Grinstein S (2012) Subversion of phagocytosis for pathogen survival. Cell Host Microbe 12:419–431
Nitsche-Schmitz DP, Johansson HM, Sastalla I, Reissmann S, Frick IM, Chhatwal GS (2007) Group G streptococcal IgG binding molecules FOG and protein G have different impacts on opsonization by C1q. J Biol Chem 282:17530–17536
Torres VJ, VanCompernolle SE, Sundrud MS, Unutmaz D, Cover TL (2007) Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. J Immunol 179:5433–5440
Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R (2003) Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099–1102
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhu, C., Wang, Y., Cai, C., Cai, Q. (2017). Bacterial Infection and Associated Cancers. In: Cai, Q., Yuan, Z., Lan, K. (eds) Infectious Agents Associated Cancers: Epidemiology and Molecular Biology. Advances in Experimental Medicine and Biology, vol 1018. Springer, Singapore. https://doi.org/10.1007/978-981-10-5765-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-10-5765-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5764-9
Online ISBN: 978-981-10-5765-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)