Abstract
Knowledge on the fate and transport of heavy metals is essential for predicting the environmental impact of metal contamination on agricultural soils. This chapter presents an overview of various factors that are involved in controlling the retention and mobility of heavy metals in soils with a special reference to soil mineralogy. The bioavailability of most elements, in particular heavy metals, in soils is governed by adsorption-desorption, complexation, precipitation and ion-exchange processes. The most important surfaces involved in metal adsorption in soils are active inorganic colloids such as clay minerals, oxides and hydroxides of metals, metal carbonates and phosphates and organic colloids. In addition to soil mineralogy, other important parameters controlling heavy metal retention and their distribution are soil texture, structure, pH, redox condition, cation and anion concentration, ionic strength, organic matter, microbial and root activity and climatic conditions. However, the ultimate fate of elements depends on a combination of several factors that are working together in the soil system. Finally, several remediation strategies have also been highlighted based on the fundamental principles of metal immobilization on mineral containing soil amendments.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The concern over the ever-growing imbalance in the natural environments has been increasing over the last few decades. Lately, the threat of toxicity of heavy metals to plants and humans has become manifold due to environmental contamination resulting from the extensive use of heavy metals in industry and agriculture. Since soils are, inadvertently, the ultimate victim of all human activities, it is extremely important for us to protect soils from being degraded in order to give future generations a safe and sound habitat. In the order of the abundance of various metals, Pb, Cr, As, Zn, Cd, Cu and Hg are found in the contaminated sites (USEPA 1996; Wuana and Okieimen 2011). The harmful effects of these metals in posing human and animal health issues are well known. Most of these metals impose serious risk due to their potential bioaccumulation and biomagnification in the food chain. Depending on their chemical speciation the metals can even migrate into the ground water and create a more serious issue.
More than one heavy metal can co-exist in contaminated soils. The bioavailability of the metals present in such multi-element environments is regulated to a large extent by the competition for available adsorption sites. In other words, the bioavailability and bioaccessibility of heavy metals depend on the elements’ interaction with various soil components which control the metals’ retention and mobility in soils. Studies have demonstrated that numerous physical, chemical, biological factors can be involved in controlling the retention and mobility of heavy metals. This chapter aims to present an overview on these factors in relation to the mineralogical properties of soils. Several remediation strategies have also been highlighted based on the fundamental principles of metal immobilization on soil minerals.
2 Adsorption of Heavy Metals on Soil Minerals
The fate and bioavailability of most elements, in particular heavy metals, in soils is governed by adsorption–desorption processes. Their adsorption on soils and minerals are also different due to their hydrolysis behaviour (Naidu et al. 1998). Among various heavy metals, Cu and Pb are reportedly the least mobile, whereas Cd and Zn are considerably more mobile. In addition, highly toxic metalloids such as As, Cr and Se, which exist as anions in the environment, are highly mobile because these anionic species get repelled by the intrinsically negatively charged soil particles.
The adsorption of heavy metals on purified soil clay minerals has been studied extensively in the past (Sen Gupta and Bhattacharyya 2012; Bhattacharyya and Gupta 2008; García-Sánchez et al. 1999). Several chemical modification processes were also known to increase the adsorption capacity of heavy metals on clay minerals (Bhattacharyya and Gupta 2008; Sarkar et al. 2010, 2012, 2013a; Rusmin et al. 2015; Perelomov et al. 2016; Celis et al. 2000). The surfaces of clay minerals contain two major types of reaction sites, namely Bronsted and Lewis acid sites, and ion exchange sites. This could be further explained by a constant capacitance model that assumes two kinds of binding sites (Schindler et al. 1987; Angove et al. 1998). The first type of adsorption sites adsorb metals by ion exchange, whereas the second type of adsorption sites involve inner-sphere binding to ampholytic –OH groups. The hydroxyl groups located on the edges (due to silanol and aluminol groups) are responsible for many metal–clay interactions. The 1:1 type clay mineral (e.g., kaolinite) contains a net zero layer charge, but the small negative charge at the broken edges can participate in metal adsorption. Contrarily, 2:1 type clay minerals (e.g., montmorillonite) hold a net negative charge of 0.8 unit per unit cell, which makes it a better adsorbent of heavy metal cations.
Adsorption reactions involving heavy metals are extremely rapid whereas desorption reactions can be orders of magnitude slower (McBride 1994). Additionally, adsorption–desorption reactions are often not completely reversible and this non-singularity or hysteresis can increase with increased residence time (or ageing) between the heavy metal and soil constituent surface (Glover et al. 2002; Rezaei Rashti et al. 2014). The adsorption–desorption of heavy metals on clay minerals depend on many environmental factors which consequently influence their mobility in soils. A complete understanding of the surface sequestration process in soils and minerals helps to better evaluate the bioavailability and potential toxicity of heavy metals.
3 Factors Affecting Retention of Heavy Metals in Soil
Mobility of heavy metal elements and subsequent retention in soil is controlled by a sequence of processes, beginning with desorption or dissolution followed by diffusion and convection. Further retention of elements at another location occurs due to re-adsorption or precipitation reactions. The key factors that control heavy metal retention in soils are summarized in Fig. 4.1. However, the ultimate fate of the element depends on a combination of several physical, chemical, biological and climatic factors.
3.1 Physical Factors
3.1.1 Texture
Soil texture plays an important role in the retention of heavy metals in soils. Texture reflects the distribution of various particle size fractions including the fine particles like clay and oxidic minerals. In general, soils high in clay-sized minerals tend to retain a higher concentration of elements than coarse-textured soils, which is attributed to the higher surface area and metal binding sites of the clay-sized fraction. The importance of clays in the retention of metals was experimentally proved by several researchers. For example, Andersson (1979) demonstrated the strong adsorption affinity of Pb and other metals to the clay fractions and ranked adsorption affinity in the order of clay > silt > sand. Similarly, for a given total Cd concentration, Cd availability was higher in sandy soils than in clay soils (Eriksson 1989). In another study, the ammonium acetate extractable Zn, Pb, Cu and Cd was always lower in loamy soils than in sandy soils (Scokart et al. 1983).
3.1.2 Structure
The soil physical structures which may influence metal mobility include the properties such as fracturing and permeability (Jones and Jarvis 1981). This plays a crucial role in maintaining flow velocity into and out of soil aggregates which may be helpful to predict diffusion of metal ions within the soils. For example, soils with a higher macro porosity and colloids with greater surface charge contributed a higher degree of Pb mobility and transport (Karthanasis 2001). While the presence of earthworms that tended to increase soil porosity and diffusivity resulted in increased plant-available Pb and Zn concentrations (Ireland 1975).
3.1.3 Water Content
High solubility of heavy metals is not manifested as significant mobility unless there is sufficient water movement in the soil pores. Under arid climatic condition, the net water flow through the soil profile is upward and the mobile metals that are carried to the surface become concentrated by evaporation. Conversely, under wet condition, mobile metals are carried downward as long as there is free drainage. However, the mobility of elements is ultimately governed by the individual character of the particular elements. Agricultural sites that were subjected to the use of arsenate, lead and copper as pesticides many decades ago still retained Pb and Cu in the soil surface, although arsenate moved deeper in the soil profile in some cases (McBride 1994). Even under continuous leaching, removal of a large portion of these less mobile elements by natural process could take over thousands of years (McBride 1994).
3.2 Chemical Factors
3.2.1 Soil Reaction (pH)
Soil pH is generally considered to be the principal factor controlling elemental mobility. It governs elemental availability mainly by three ways: (1) by influencing the metal solubility; (2) controlling the precipitation–dissolution reaction, and (3) controlling the adsorption process. In general, the solubility of metals tends to increase at lower soil pH and decrease at higher pH values (Chuan et al. 1996; Ming et al. 2016). For metal cations, high pH would favor adsorption and precipitation as oxides, hydroxides and carbonates (Park et al. 2011a). Generally, in acidic pH, adsorption reaction becomes the important process in controlling elemental concentration in soil solution, whilst precipitation reaction takes the lead under alkaline conditions. Increasing solution pH tends to increase in the net negative charge of soil colloids and thus increases affinity of soils for metal cations (Naidu et al. 1998; Wu et al. 2003). The correlation between metal adsorption and pH is partly due to the competition of H+ (and Al3+) ions for binding sites at low pH leading to decreased metal adsorption (Basta and Tabatabai 1992).
3.2.2 Redox Condition
Soil redox potential is also crucial in controlling mobility of elements. Reduction–oxidation (redox) reaction is a process that involves flow of electrons from a reducing agent to an oxidizing agent. Redox reactions are governed by the free electron activity (pE) in soil solution, also expressed as Eh, the redox potential (Sposito 1983). High redox potential is recorded in well-aerated dry soils, whilst soils prone to waterlogging and high in organic matter content tend to have low Eh values. Some elements become more soluble and mobile in one oxidation state than another (e.g., Mn, Cr, As and Se) (Sarkar et al. 2012, 2013b). Transition metals (e.g., Fe, Mn) could facilitate the electron transfer reactions in the presence of organic acids and clay minerals to carry out reduction of metalloids (e.g., Cr) (Sarkar et al. 2013b). The elements that are classified as chalcophiles (e.g., Cu, Hg, Zn, Cd, As, Se and Pb) might form sulfide minerals in reducing environments which are insoluble in nature. The solubility of Pb, Cd and Zn could increase under reduced soil environment due to possible dissolution of Fe–Mn oxyhydroxides under reducing environments (McBride 1994). Under reducing environments Hg could form volatile organomercury compounds which might reduce soil bioavailability apparently.
3.2.3 Clay Content and Type
Heavy metals are less mobile in soils where a large quantity of binding sites for adsorption is available. Clay particles have surface functional groups that tend to adsorb heavy metal ions and make it immobile in nature. As described earlier, heavy metal adsorption can be described in two basic processes: nonspecific adsorption or ion exchange reaction and chemisorbed inner-sphere complex. Most phyllosilicate clay minerals such as vermiculite and montmorillonite carry permanent negative charge due to isomorphous substitution of cations within their mineral structure. A large portion of the metal binding capacity are due to the permanent and/or pH-independent charge. Further, cation adsorption by expandable layer silicates might occur largely in the inter-layer surfaces compared to the planar surfaces. However, penetration of water and metal cations between the layers of non-expandable phyllosilicates (e.g., kaolinite and serpentine) is difficult due to their low cation exchange capacity (Bhattacharyya and Gupta 2008).
3.2.4 Oxidic Material Content
Oxides and hydroxides of Fe and Mn occur in association with clay minerals as coatings on the phyllosilicates and also as crystals or free gels. Oxides concentrations are usually low under reducing conditions; therefore, influence of oxidic materials in controlling metal solubility is likely to be important under oxidizing environments. Hydroxides of Mn and Fe may reduce heavy metal ion concentration by both surface adsorption and precipitation reaction (Chuan et al. 1996). Surface adsorption on oxidic minerals followed by diffusion of metal ions into the small pores of mineral lattice structure might also contribute in elemental retention in soils (Backes et al. 1995). Preferential adsorptions of metals by different oxides are governed by the type of adsorbing surfaces and also by type of elements. For example, the preferential order of specific adsorption by hydrous oxides follows the order of Pb > Cu >> Zn > Cd. Oxides of Mn particularly have a strong affinity for Pb adsorption as compared to Cd. Further, Zn and Cu are probably adsorbed with equal affinity by Mn- and Fe-oxides. When multiple metals are present in the soil solutions, they might impart competition to each other for the active surfaces on clay minerals, and consequently one metal could become more mobile than the other (Ming et al. 2016).
3.2.5 Anions
Concentration of anions in the soil solution also controls the heavy metal solubility in soil. It is well established that various inorganic and organic anions form complexes with heavy metals and thus influences the metal solubility and subsequent mobility in soil. Precipitation of stable metal complex is governed by the type of anions present in the soil solution. For example, the mobility of Pb ions reduced in the presence of sulfate and phosphate anions due to the formation of sparingly soluble salts between Pb and these anions (Park et al. 2011a).
3.3 Biological Factors
3.3.1 Organic Matter
Organic matter affects the physical, chemical and biological conditions of soils. Decomposition of plant and animal residues leads to accumulation of organic matter in soils. Soil organic matter is composed of various functionally active compounds such as humic acid, fulvic acid and humin which are typically associated with soil inorganic colloids such as clay minerals. Organic matter reacts with heavy metals mainly by two major processes, including complexation or inner-sphere mechanism and adsorption or ion exchange reaction (Evans 1989). The active functional groups of organic matter are the negatively charged carboxyl, phenolic and amino groups that are involved in cation-binding reaction. These functional groups increase in number with the increase in humification processes. The increase in pH tends to increase in ionization of functional groups and organo-metal complexes thus become stable at higher pH values (Krishnamurti et al. 1997). There were also evidences where organic matter formed soluble organo-mineral complexes especially when organic component was dominated by the fulvic acid fraction (Temminghoff et al. 1997).
3.3.2 Microbial Activity
Microorganisms are considered to be the most important component controlling the biological activity in soils and influence the nutrient recycling in the system. Various functions are served by microbial transformation of metals. Generally, microbial transformation of metals is classified into two main categories: redox transformation of inorganic forms and transformations from inorganic to organic form, and vice versa (Bolan et al. 2013). Through oxidation of Mn, Fe, As and S, microorganisms can obtain energy. On the other hand, through dissimilatory reduction processes they can utilize metals as a terminal electron acceptor for anaerobic respiration. Soil microorganisms might also immobilize heavy metals by aiding the precipitation of hydrated ferric oxides and sulfides and also by exudation of metal complexing mucopolysaccharides (Park et al. 2011b). Bacterial cell walls may adsorb heavy metals from soil solutions due to the presence of surface functional groups (Mullen et al. 1989). Sometimes microorganisms might enhance metal solubility by the acidification of the soil (Ernst 1996).
3.3.3 Plant Root Activity
Plant root also plays an important role in controlling the metal bioavailability (Krishnamurti et al. 1997; Ernst 1996). The exudation of acidic chemicals (e.g., H2CO3) lowers the rhizospheric pH and hence increases metal bioavailability. Plant roots are also known to release chelating organic molecules that tend to solubilize metal cations from its insoluble forms, which results in greater bioavailability of metals in the solution. Further, symbiotic association of fungi with plant roots might facilitate metal solubilization over a large soil area.
3.4 Climatic Factors
Heavy metal uptake by plant roots was found to be positively correlated with temperature (Miller and Friedland 1994). This relationship was attributed to the increase rate of organic matter decomposition at higher temperature, which increased the mobilization of organo-metal complexes. Moreover, with an increase in temperature, the metal activity in the soil solutions and in plant roots might increase the absorption rate. Further, high evapo-transpiration rate at higher temperature also might contribute to an increased uptake of metals by plants.
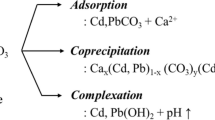
4 Stabilization/Immobilization of Heavy Metals in Soils
The key mechanisms of immobilization of heavy metals in soils are adsorption, surface complexation, precipitation and ion exchange (Fig. 4.2). These are achieved by applying various amendments to the contaminated soils (Table 4.1). One of the most effective physico-chemical processes controlling the behavior and bioavailability of heavy metals is adsorption (Wan Ngah and Hanafiah 2008). A charged solute (ions) can get attached to the charged soil surface due to electrostatic interaction (Bolan et al. 2003). This strategy of immobilization involves the addition of adsorbents (e.g., clay minerals, zeolites, fly ash, red mud and biochar) into the contaminated soil (Wuana and Okieimen 2011; Sarkar et al. 2012; Antoniadis et al. 2012; Taghipour and Jalali 2015; Usman et al. 2005; Zhang et al. 2016). The adsorbent can also provide surface complexation reaction. Through this reaction metals are redistributed from solution phase to the solid phase and reduce their bioavailability in the environment (Bolan and Duraisamy 2003). In this method, functional groups like hydroxyl, carboxyl, amino and phenoxyl on the surface of organic matter or clay minerals react with heavy metals and produce surface complexes (Harter and Naidu 1995). These complexes are of two types: (1) inner-sphere complexes, in which no molecule of the solvent is interposed between surface functional groups and ions, and (2) outer-sphere complexes, in which at least one molecule of the solvent comes between the surface functional groups and ion (Alloway 1995; Bolan et al. 2014). The outer-sphere complexes are less stable than inner-sphere complexes. Precipitation of heavy metals is another way to reduce metal bioavailability in soils. Precipitation can be achieved by adding various binding agents (e.g., cement, biochar, fly ash, lime, zeolite, manure, compost, chitosan and sewage sludge) (Bolan et al. 2014; Ling et al. 2008; Wuana and Okieimen 2011; Xi et al. 2014). Precipitation of hydroxides or sulfides within the solid matrix is one of the major mechanisms by which metals can be immobilized (Fu and Wang 2011). Hydroxide precipitation is relatively effective in the pH range of 8–11 (Huisman et al. 2006). Ion exchange is another way of remediating heavy metals in soils (Dabrowski et al. 2004). In this method, metal cations are replaced by surrounding phenolic groups, finally forming a chelate. The ion exchange agent could be naturally occurring inorganic zeolites or synthetically produced organic resins (Vazquez et al. 1994). However, this is a reversible process. Depending on the type of functional groups of exchanging ions, ion exchanger can be strongly acidic (sulfonate), weakly acidic (carboxylate), strongly basic (quaternary ammonium) and weakly basic (tertiary and secondary amines) (Hubicki and Kołodyńska 2012). Remediation of heavy metal contaminated soils with various organic and inorganic amendments has attracted attention due to the method’s low cost and environmental benefits.
5 Biological Quality of Metal Contaminated Soils
Metals can impose highly toxic effect on the native microorganisms in soils. The microorganisms also need metal ions at very low concentration for their nutrition. However, an excess amount of these essential metals can be detrimental for their cellular functions (Lemire et al. 2013). In a soil microhabitat, the degree of metal toxicity to microorganisms depends primarily on the types of metals, their speciations and the microorganisms themselves (Giller et al. 1998). A generalized toxicity profile of heavy metals represents that microorganisms (e.g., algae, bacteria, fungi, actinomycetes and protozoa) are more vulnerable to metal toxicity than the macro-biota (e.g., nematode and earthworm) (Fig. 4.3) (Vig et al. 2003). This chapter mainly emphasizes on the microorganisms due to their major role in the dynamics of soil biogeochemical processes in agricultural and environmental remediation perspective.
A generalized schematic presentation of different level of toxicity of metals on various soil biota (adapted and modified from Vig et al. 2003)
The heterogeneity of soil largely controls whether the toxicity of metals is mitigated or not. As described earlier, numerous factors including soil pH, organic matter and mineralogical composition are involved. Clay minerals and some of their modified products are efficient adsorbents of toxic metals in soils. This reduces metal bioavailability to the microorganisms and saves them from toxicity (Biswas et al. 2015; Mandal et al. 2016). In soils, the microorganisms often tend to nest with or in the vicinity of clay minerals and form micro-aggregates or biofilms (Almås et al. 2005; Biswas et al. 2017; Giller et al. 2009). Therefore, the application of clay minerals (as amendments) can be an efficient supplement in soil microsites to protect the microbial cells from toxic metals.
However, the efficiency of a clay-based adsorption technique mostly lies on the properties of the clay mineral for a target toxic metal. Álvarez-Ayuso et al. (2003) reported that a 4% palygorskite amendment in a mining soil (pH = 5.4) immobilized 92% of the mobile (soluble and exchangeable) Pb, 77% of Cu, 76% of Zn and 48% of Cd. Several other clay minerals and zeolite were also used as the metal-immobilizer in soil; however, how the addition of clay minerals into soil would impact the microbial community and thus the overall microbial quality remained inconclusive. It was found that the toxicity of Cu (in terms of substrate-induced nitrification and substrate-induced respiration) could not be explained by the soil solution metal concentrations or exchangeable metal concentrations, but a significant relationship was found between the EC50 values for substrate-induced respiration and percent clay content (Broos et al. 2007). Therefore, it is highly important to conduct field scale studies in the toxicological perspective for the quality of soil biota while a raw and modified clay products is used as the metal adsorbent.
6 Conclusions
The fate and behavior of heavy metals and metalloids in the soil environment are governed by numerous physical, chemical and biological factors. The key physico-chemical properties that control heavy metal retention in soils are imparted by the clay minerals and oxidic particles. Additionally, organic colloidal particles also play an important role. The mineral and colloidal particles have numerous active sites on their surfaces which can retain heavy metals through adsorption, complexation, precipitation and ion exchange. Due to these properties mineral amendments can be applied to contaminated soils alone or in combination with another organic amendment for immobilizing heavy metals. This can be one of the cost effective strategies for heavy metal remediation in contaminated soils. However, further research is needed to investigate the bio-physico-chemical interactions of heavy metals where microorganisms also play a key role in the biogeochemical cycle of elements in soils.
References
Alloway B (1995) Heavy metals in soils. Blackie Academic and Professional, Glasgow, UK.
Almås ÅR, Mulder J, Bakken LR (2005) Trace metal exposure of soil bacteria depends on their position in the soil matrix. Environ Sci Technol 39(16):5927–5932
Álvarez-Ayuso E et al (2003) Palygorskite as a feasible amendment to stabilize heavy metal polluted soils. Environ Pollut 125(3):337–344
Andersson A (1979) Distribution of heavy metals as compared to some other elements between grain size fractions in soils. Swed J Agric Res 9:7–13
Angove MJ, Johnson BB, Wells JD (1998) The influence of temperature on the adsorption of cadmium(II) and cobalt(II) on kaolinite. J Colloid Interface Sci 204(1):93–103
Antoniadis V, Damalidis K, Dimirkou A (2012) Availability of Cu and Zn in an acidic sludge-amended soil as affected by zeolite application and liming. J Soils Sediments 12(3):396–401
Backes CA et al (1995) Kinetics of cadmium and cobalt desorption from iron and manganese oxides. Soil Sci Soc Am J 59:778–785
Basta NT, Tabatabai MA (1992) Effect of cropping systems on adsorption of metals by soils: II. Effect of pH. Soil Sci 153:195–204
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140(2):114–131
Biswas B et al (2015) Heavy metal-immobilizing organoclay facilitates polycyclic aromatic hydrocarbon biodegradation in mixed-contaminated soil. J Hazard Mater 298:129–137
Biswas B et al (2017) Structural changes in smectite due to interaction with a biosurfactant-producing bacterium Pseudoxanthomonas kaohsiungensis. Appl Clay Sci 136:51–57
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: a review involving specific case studies. Soil Res 41(3):533–555
Bolan NS, Adriano DC, Naidu R (2003) Role of phosphorus in (im)mobilization and bioavailability of heavy metals in the soil-plant system. Rev Environ Contam Toxicol 177:1–44
Bolan NS et al (2013) Microbial transformation of trace elements in soils in relation to bioavailability and remediation. In: Whitacre MD (ed) Reviews of environmental contamination and toxicology. Springer, New York, pp 1–56
Bolan N et al (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166
Broos K et al (2007) Soil factors controlling the toxicity of copper and zinc to microbial processes in Australian soils. Environ Toxicol Chem 26(4):583–590
Celis R, HermosÍn MC, Cornejo J (2000) Heavy metal adsorption by functionalized clays. Environ Sci Technol 34(21):4593–4599
Chuan MC, Shu GY, Liu JC (1996) Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water, Air Soil Pollut 90:543–556
Dabrowski A et al (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56(2):91–106
Eriksson JE (1989) The influence of pH, soil type and time on adsorption and uptake by plants of Cd added to the soil. Water Air Soil Pollut 48:317–335
Ernst WHO (1996) Bioavailability of heavy metals and decontamination of soils by plants. Appl Geochem 11:163–167
Evans LJ (1989) Chemistry of metal retention by soils. Environ Sci Technol 23:1046–1056
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92(3):407–418
García-Sánchez A, Alastuey A, Querol X (1999) Heavy metal adsorption by different minerals: application to the remediation of polluted soils. Sci Total Environ 242(1–3):179–188
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30(10–11):1389–1414
Giller KE, Witter E, McGrath SP (2009) Heavy metals and soil microbes. Soil Biol Biochem 41(10):2031–2037
Glover LJ, Eick MJ, Brady PV (2002) Desorption kinetics of cadmium2+ and lead2+ from goethite. Soil Sci Soc Am J 66(3):797–804
Harter RD, Naidu R (1995) Role of metal-organic complexation in metal sorption by soils. Adv Agron 55:219
Hubicki Z, Kołodyńska D (2012) Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. In: Ayben Kilislioglu A (ed) Ion exchange technologies. InTech, doi:10.5772/51040
Huisman JL, Schouten G, Schultz C (2006) Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 83(1):106–113
Ireland MP (1975) The effect of the earthworm Dendrobaena rubida on the solubility of lead, zinc and calcium in heavy metal contaminated soil in Wales. J Soil Sci 26:313–318
Jones LHP, Jarvis SC (1981) The fate of heavy metals. In: Greenland DJ, Hayes MHB (eds) The chemistry of soil processes. Wiley, Chichester
Karthanasis AD (2001) Mineral controls in colloid mediated transport of metals in sub surface environments. In: 6th international conference on biogeochemistry of trace elements, Guelph
Krishnamurti GSR et al (1997) Kinetics of cadmium release from soils as influenced by organic acids: Implication in cadmium availability. J Environ Q 26:271–277
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11(6):371–384
Ling W et al (2008) Use of bentonite to control the release of copper from contaminated soils. Soil Res 45(8):618–623
Mandal A et al (2016) Surface tailored organobentonite enhances bacterial proliferation and phenanthrene biodegradation under cadmium co-contamination. Sci Total Environ 550:611–618
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York
Miller EK, Friedland AJ (1994) Lead migration in forest soils: response to changing atmospheric inputs. Environ Sci Technol 28:662–669
Ming H et al (2016) Competitive sorption of cadmium and zinc in contrasting soils. Geoderma 268:60–68
Mullen MD et al (1989) Bacterial sorption of heavy metals. Appl Environ Microbiol 55(12):3143–3149
Naidu R, Sumner ME, Harter RD (1998) Sorption of heavy metals in strongly weathered soils: an overview. Environ Geochem Health 20(1):5–9
Park JH et al (2011a) Environmental monitoring of the role of phosphate compounds in enhancing immobilization and reducing bioavailability of lead in contaminated soils. J Environ Monit 13:2234
Park JH et al (2011b) Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J Hazard Mater 185(2–3):829–836
Perelomov L et al (2016) Uptake of lead by Na-exchanged and Al-pillared bentonite in the presence of organic acids with different functional groups. Appl Clay Sci 119(Part 2):417–423
Rezaei Rashti M et al (2014) Cadmium desorption behaviour in selected sub-tropical soils: Effects of soil properties. J Geochem Explor 144(Part B):230–236
Rusmin R et al (2015) Structural evolution of chitosan–palygorskite composites and removal of aqueous lead by composite beads. Appl Surf Sci 353:363–375
Sarkar B et al (2010) Remediation of hexavalent chromium through adsorption by bentonite based Arquad® 2HT-75 organoclays. J Hazard Mater 183(1–3):87–97
Sarkar B et al (2012) Organoclays reduce arsenic bioavailability and bioaccessibility in contaminated soils. J Soils Sediments 12(5):704–712
Sarkar B, Naidu R, Megharaj M (2013a) Simultaneous adsorption of tri- and hexavalent chromium by organoclay mixtures. Water Air Soil Pollut 224(12):1–10
Sarkar B et al (2013b) Manganese(II)-catalyzed and clay-minerals-mediated reduction of chromium(vi) by citrate. Environ Sci Technol 47(23):13629–13636
Schindler PW, Leichti P, Westall JC (1987) Adsorption of copper, cadmium and lead from aqueous solution to the kaolinite/water interface. Neth J Agric Sci 35:219–230
Scokart PO, Meeus-Verdinne K, de Borger R (1983) Mobility of heavy metals in polluted soils near zinc smelters. Water Air Soil Pollut 20:451–463
Sen Gupta S, Bhattacharyya KG (2012) Adsorption of heavy metals on kaolinite and montmorillonite: a review. Phys Chem Chem Phys 14(19):6698–6723
Sposito G (1983) The chemical forms of trace metals in soils. In: Applied environmental geochemistry. London, Academic Press
Taghipour M, Jalali M (2015) Effect of clay minerals and nanoparticles on chromium fractionation in soil contaminated with leather factory waste. J Hazard Mater 297:127–133
Temminghoff EJM, van der Zee SEATM, de Haan FAM (1997) Copper mobility in a copper-contaminated sandy soil as affected by pH and solid and dissolved organic matter. Environ Sci Technol 31:1109–1115
USEPA 1996 Recent developments for in situ treatment of metals contaminated soils. Report, U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response
Usman A, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil (8 pp). J Soils Sediment 5(4):245–252
Vazquez G et al (1994) Adsorption of heavy metal ions by chemically modified Pinus pinaster bark. Bioresour Technol 48(3):251–255
Vig K et al (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8(1):121–135
Wan Ngah WS, Hanafiah MAKM (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99(10):3935–3948
Wu ZH et al (2003) Effects of organic acids on adsorption of lead onto montmorillonite, goethite and humic acid. Environ Pollut 121:469–475
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20
Xi Y, Wu X, Xiong H (2014) Solidification/stabilization of Pb-contaminated soils with cement and other additives. Soil Sediment Contam Int J 23(8):887–898
Zhang C et al (2016) Active capping technology: a new environmental remediation of contaminated sediment. Environ Sci Pollut Res 23(5):4370–4386
Acknowledgements
BS acknowledges the Department of Education and Training, Australian Government, for awarding him the Endeavour Research Fellowship. SS and BBB acknowledge their parent organization, Indian Council of Agricultural Research (ICAR). SM is grateful to the Department of Education, Australian Government, for providing her an Australian Post-graduate Award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sarkar, S., Sarkar, B., Basak, B.B., Mandal, S., Biswas, B., Srivastava, P. (2017). Soil Mineralogical Perspective on Immobilization/Mobilization of Heavy Metals. In: Rakshit, A., Abhilash, P., Singh, H., Ghosh, S. (eds) Adaptive Soil Management : From Theory to Practices. Springer, Singapore. https://doi.org/10.1007/978-981-10-3638-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-3638-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3637-8
Online ISBN: 978-981-10-3638-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)