Abstract
Purpose
Acidic soils exhibit high trace element availability compared to neutral pH soils, and thus, when trace metals are added (e.g. due to sewage sludge application), measures should be taken to reduce their mobility. In this experiment, we tested two such methods, liming and zeolite addition. The aim was to measure the availability, in ryegrass (Lolium perenne L.), of heavy metals (Cu and Zn) added to soil with sewage sludge in both acidic and limed soil.
Materials and methods
Thus, in this pot experiment, we used a soil at two pH values (original soil at pH 3.56 and limed to 6.5), two rates of sewage sludge (0 and 50 Mg ha−1) and three rates of zeolite (0, 2 and 5 Mg ha–1, referred to as Z-0, Z-1 and Z-2, respectively).
Results and discussion
We found that metal concentrations in plant decreased significantly with liming but zeolite did not further reduce metal levels. In metal extractions with DTPA, zeolite additions reduced metal concentrations. In the second sampling time (on day 100), metal levels were significantly reduced at Z-0 and Z-1 compared to day 50, but at Z-2, metals were either only slightly reduced or even unchanged.
Conclusions
We concluded that zeolite hindered metals from being strongly and irreversibly bound onto soil colloids. Zeolite at Z-2 kept metal availability relatively high over time, while metal availability at Z-0 and Z-1 was being reduced due to liming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Although sewage sludge may have beneficial effects to low organic matter content soils, such as those typically found in Greece, sludge-borne trace elements may adversely affect crop growth. This contamination risk further increases in acidic soils, which, contrary to what would be expected in the warm and dry Mediterranean climate (described as xeric according to soil taxonomy), account for c. 15% of the total land in Greece (Koukoulakis and Papadopoulos 2007, p. 30). In acidic soils, problems such as high trace element availability (which, in metal enriched soils, may constitute major health risk), Al toxicity to plant roots and low concentrations of nutrient base cations are factors that may dramatically diminish crop production. Thus, measures reducing metal availability such as liming are necessary. Liming tends to force the exchange of Al polymers from clay interlayer surfaces for added Ca2+ and to cause their subsequent precipitation as insoluble Al(OH)3, which reduces total acidity and increases soil cation-exchange capacity (CEC). Another measure may be the addition of zeolite (Gheshlaghi et al. 2008), a porous tectosilicate mineral with reported CEC up to 400 cmolc kg−1 (Essington 2004, p. 55), which is abundant in various provinces in Greece. Zeolite may function both as physical trap for contaminants (encaging heavy metal ionic species into its microscopic caves) and as chemically active negative-charged surface, electrostatically binding soil solution metal cations. However, the effect of the combination of both liming and zeolite application on metal availability is not yet well understood; For example, it has been reported that zeolite increases N uptake efficiency and thus plant biomass (Kavoosi 2007). This may cause, contrary to the expected availability reduction, an increase in sludge-borne trace element uptake with increased application of zeolite in limed soils. Thus, the aim of this study was to examine the role of liming and zeolite application in an acidic soil in sludge-added Cu and Zn availability to ryegrass.
2 Materials and methods
An acidic (pH 3.56) sandy loam (13% clay, 74% sand), with 1.18% organic C was obtained from Lepti, North Evros, Greece. The soil contained total concentrations of 22.10 mg Cu kg−1 and 32.38 mg Zn kg−1 (methods are explained below). The soil sample was air dried, sieved through a 2-mm sieve and measured for particle size distribution (with Bouyoucos hydrometer), pH (1:2.5 H2O), organic matter (wet oxidation), according to Rowell (1994) and total Cu and Zn concentrations with aqua regia (3-hour digestion with 3:1 concentrated HCl:HNO3) according to Ure (1995). Sewage sludge, collected from the wastewater treatment plant of Orestiada, Evros, which anaerobically treats municipal wastes, was air dried (while a portion of it was heated at 105°C for the determination of its water content), sieved through a 1-mm sieve and analysed for total Cu and Zn concentrations and organic matter with the same methods as those mentioned above. Zeolite was a clinoptilolite (Ca1.5K1.4Mg0.6Na0.5Al6.2Si29.8O72·20H2O), with CEC 226 cmolc kg−1.
One portion of the obtained soil was mixed with liming material (calcitic marble quarry tailings) consisting of 80% of CaCO3, at 72 mmolc CaCO3 kg−1 soil (equivalent to 5.76 Mg CaCO3 ha−1, assuming a bulk density of 1.4 g cm−3, a typical value for the sandy loam we sampled, and depth of incorporation 15 cm). Soil pH with this addition was corrected to the value of 6.5, as was predetermined in a preliminary 1-week incubation test in mixtures of the studied soil with increasing quantities of added lime.
Then, in both the original acidic and the limed soil, we produced the following mixtures: In 750 g of soil, we added the obtained sludge (which contained 147 mg Cu kg−1 and 487 mg Zn kg−1) at 50 Mg ha−1, equal to 17.86 g dry matter sludge per 750-g soil or 23.80 g sludge kg−1 soil, assuming, as previously, a bulk density of 1.4 g cm−3 and 15 cm of incorporation depth at field scale. As a result of the sludge addition, total (i.e. aqua regia digested) metal concentrations increased to 25.60 mg kg−1 for Cu and 43.98 mg kg−1 for Zn in the mixtures. As for zeolite, in the low application treatment, we added 0.72 g to the 750-g soil equal to 0.95 g zeolite kg−1 soil (2 Mg ha−1, according to the previous assumptions), and in the high application treatment, we added 2.38 g zeolite kg−1 soil (5 Mg ha−1). The experimental design more particularly was as follows:
-
Α1: Acidic control soil (no additions),
-
Α2: Acidic soil + sewage sludge at 50 Mg ha−1 with no zeolite (Z-0),
-
Α3: Acidic soil + sewage sludge at 50 Mg ha−1 + zeolite at 2 Mg ha−1 (Z-1),
-
Α4: Acidic soil + sewage sludge at 50 Mg ha−1 + zeolite at 5 Mg ha−1 (Z-2),
-
L1: Limed control soil (no additions other than lime),
-
L2: Limed soil + sewage sludge at 50 Mg ha−1 with no zeolite (Z-0),
-
L3: Limed soil + sewage sludge at 50 Mg ha−1 + zeolite at 2 Mg ha−1 (Z-2), and
-
L4: Limed soil + sewage sludge at 50 Mg ha−1 + zeolite at 5 Mg ha−1 (Z-2).
The treatments were replicated three times, placed in 1.5-L pots, watered to 67% of their water-holding capacity and left to equilibrate for 4 weeks. After that period, the pots were sown with 1 g of ryegrass (Lolium perenne L.) seeds and placed in a greenhouse at 20–22°C. On days 50 and 100 after sowing (after day 50, ryegrass was left to re-grow, so both plant samples were obtained from the same pot), ryegrass aerial biomass was harvested, washed with deionized water, put in pre-weighed paper bags and placed in an oven at 70°C for 48 hours. The biomass was recorded, and plant samples were ground to fine pounder. For the extraction of Cu and Zn, 0.5 g of the ground samples were weighed into porcelain crucibles, heated in a furnace at 500°C for 5 hours and then extracted with 20 mL 20% HCl. In certain treatments, plant yields were lower than 0.5 g, thus, for these samples, we used 0.1 g of ground material for the metal analyses. Soils were also sampled on days 50 and 100 and analysed for pH and diethylene triamine pentaacetic acid (DTPA)-extractable metals (according to Rowell 1994). Soil and plant extracts and digests were analysed in an atomic absorption spectrometer (Varian, SpectrAA-400 Plus, Australia). We also calculated the transfer coefficients, Tc, of metals as follows:
where metal uptake (in milligrams of metals per pot) is the product of metal concentration in plant (in milligrams per kilogram plant) and plant yield (in grams of plant per pot).
The experimental data were analysed for multi-factorial ANOVA [factor A being all eight combinations of pH, sludge and zeolite (A1-A4 and L1-L4), and factor B being time], and the method used to discriminate among the means was the Fisher’s least significant difference (LSD) procedure for a level of 95% (p < 0.05). The statistical package used was Statgraphics Plus 2.1. Error bars in the figures represent the standard error of the three replicates of each reported average value. Different letters in the figures indicate significant differences (at p < 0.05) within each sampling time, while asterisks indicate significant differences across sampling times when same treatments are compared.
3 Results
3.1 Plant yields
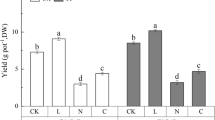
Plant yield increased significantly with liming even in the control on day 50 and yield was further enhanced in the sludge treatments, but there were no differences with added zeolite (Fig. 1 A). On day 100, acidic and limed controls had similar yields (the limed decreased over time from 0.90 to 0.52 g, LSDtime = 0.34, p < 0.05), but there were differences in the sludged treatments between the limed and the acidic soils (the limed were higher, see Fig. 1 B).
3.2 Soil pH
Liming was successful, as it increased pH to the target value of 6.5 (Fig. 2). It is noteworthy that on day 50 in the acidic treatment, sludge increased pH compared to the control, while in the limed treatment, it decreased pH significantly.
3.3 Metal concentrations in plants
Due to the low total Cu soil levels, plant Cu concentration did not decrease with liming in the control on day 50 (Fig. 3 A). In the sludged acidic soil, Cu concentration increased compared to control, but did not change with zeolite, while in the limed soil, only Z-2 was different (i.e. higher) than the control. On day 100 (see Fig. 3 B), Cu concentration in the limed control plants decreased significantly from day 50 (LSDtime = 0.327, p < 0.05). In the sludged soils on day 100, the differences in Cu concentrations between the limed and the acidic treatments were not significant, similar to day 50. Also, on day 100 in the acidic treatments, only Cu concentration at Z-2 was higher than the control because Z-0 and Z-1 decreased over time, while plant Cu concentration at Z-2 was retained high. Plant Zn concentration on day 50 in the sludged acidic soil was significantly reduced at Z-2 compared to Z-0 and Z-1 (Fig. 4 A), while in the limed soil, there were no zeolite effects. On day 100, plant Zn concentration in all studied treatments (except for the acidic control) decreased significantly compared to day 50 (LSDtime = 1.074, p < 0.05, see Fig. 4 B).
3.4 DTPA-extractable metals
As in the case of plant Cu concentration, on day 50, DTPA-extractable Cu did not decrease with liming in the control, but in the sludged acidic soil, Z-2 was lower compared to Z-0. Extractable Cu at Z-2 was even reduced back to the unamended control (Fig. 5 A). Over time, extractable Cu decreased at Z-0 and Z-1, but at Z-2, it increased (in the acidic soil from 0.46 to 0.60 mg kg−1 and in the limed from 0.48 to 0.64 mg kg−1, LSDtime = 0.032, p < 0.05, see Fig. 5 B). In DTPA-extractable Zn, similar to Cu, Z-2 in the acidic soil was significantly lower than Z-0, while in the limed soil, there was no zeolite effect (day 50, Fig. 6 A). Over time, extractable Zn was greatly reduced in the sludge treatments (LSDtime = 0.117, p < 0.05, see Fig. 6 B).
3.5 Transfer coefficients
Although on day 50 the acidic soil Cu Tc values seem to be higher than the limed soil values, the differences are not significant (Fig. 7). On day 100, increasing zeolite applications led to increasing Cu Tc values in the acidic soil, while in the limed treatment, the differences were not significant. Due to this unexpected increasing Tc trend, at Z-2, the differences between day 50 and day 100 were eliminated. This increasing trend in the acidic soil was also observed for Zn on day 100.
4 Discussion
Plant yield, as expected, increased with sludge due to added N. At Z-1 and Z-2 in the acidic soil, yields were retained relatively high on day 100, close to the yields recorded on day 50, probably due to better N economy (i.e. slower release of NH4–N from sludge, as also suggested by Ahmed et al. 2008), while at Z-0, yields decreased over time. This indicates that even at highly acidic problem soils, zeolite, although it does not directly increase yield, may improve growth conditions.
Sludge also altered pH within the two pH treatments (i.e. it increased pH in the acidic soil and reduced pH in the limed). A possible reason for that may be the nitrification of sludge-added NH4–N, a process with a known acidifying effect. Slight acidification of circum-neutral pH soils due to sludge-added NH4–N was also found by Stamatiadis et al. (1999). Nitrification is inhibited at very low pH values, such as those at control (as also agreed by Ste-Marie and Pare 1999). Thus, in the lime-sludged soils, nitrification occurred (slightly lowering pH), while in the acidic sludged soils, it did not (thus, NH4–N remained in soil and, as a base cation, slightly increased pH).
Liming, although expected to cause a significant decrease in metal concentrations in plant and soil (as reported by many, e.g. Sukreeyapongse et al. 2002), caused a slight (non-significant) decrease in most cases and a significant decrease in only five cases. However, zeolite exhibited a behaviour at Z-2 contrary to the expected: Increasing zeolite on day 100 did not depress Cu concentrations in plant, it rather enhanced the difference from the unsludged control to a significant level in the acidic treatment. It also helped plant Cu concentration in the acidic soil to be retained relatively high on day 100 compared to day 50 (the zeolite effect at Z-2 will be discussed in the next paragraph), while plant Cu concentration decreased over time in all the other sludged treatments. There are two possible reasons for the reduction of plant metals over time at Z-0 and Z-1. First, the decrease is probably connected with the similar decrease over time observed in the limed control in plant yields. This means that less rigorous plant growth may have resulted in lower metal plant concentrations. This connection was quantitatively assessed with regression analyses, where we found a significant relationship between yield and plant Zn concentration (R 2 = 0.648, at p < 0.001) and between yield and plant Cu concentration (R 2 = 0.433, p < 0.01). The relationship of metal concentrations in plants and yield was also recognised by Goecke et al. (2011), who found that Cu concentration in L. perenne was higher in pots exhibiting better plant growth. Second, the reduction over time may also indicate a possible depletion of metals from soil available pools. DTPA extractions confirmed a reduction of metals over time (with the exception of Cu at Z-2, which increased significantly over time, an effect that will be discussed in the next paragraph). It is noteworthy that on day 100 the DTPA-extractable Cu had no differences between any of the treatments, contrary to the findings in plant Cu, indicating that either the DTPA did not very successfully predict Cu concentrations in plants or that the differences observed in plant Cu were mainly due to growth variations. This was confirmed by the non-significant correlations between soil-extractable Cu and plant Cu concentrations (R 2 = 0.094, p = 0.524). DTPA-extractable Zn, on the other hand, more successfully predicted plant Zn levels, as also revealed by the significant correlation between the two (R 2 = 0.835, p < 0.001).
Our findings on Tc confirmed the observed trends in plant- and DTPA-extracted metals at Z-2, i.e. that zeolite protected the metals from being reduced over time. Sludge-added metals are reported to be considerably less mobile in soil than metals derived from inorganic sources, due to the binding capacity of organic phases also added to soil with sludge application. For example, Stietiya and Wang (2011) found that 75% of Cu and 8% of Zn were bound onto organic phases and that with organic matter oxidation the percentages fall to <10% for Cu and <3% for Zn. Apart from the presence of sludge-borne organic chelates, the role of inorganic colloids has also been recognised, especially some time (months or years) after the last application of an organic waste (when, as reported by Benke et al. 2008, it is expected that waste-added organic matter will have been decomposed to a great extent). Metals have the tendency to be diffused with time into less mobile (i.e. less accessible for plant uptake) soil pools due to their irreversible binding and their gradually increasing electrostatic attraction to non-exchangeable inorganic soil colloid sites. These inorganic phases tend to suppress the availability of sludge-added metals even within a matter of a few weeks. This relatively fast availability decrease over time occurs even more in the case of immobile (strongly bound) metals like Cu, as reported by Sanchez-Martin et al. (2007), who found a significant decrease of soluble and exchangeable Cu and its subsequent transfer into non-available soil pools within 6 months. Also, this decrease over time occurs especially when metal loads in sludge are low (as is the case with the sludge used in this study) because low sludge metal concentrations have low metal availability potential, as suggested by Smith (2009). (Thus, the observed metal availability reduction at Z-0 and Z-1 over time was not unexpected.) However, added zeolite at Z-2 seemed to make this expected metal “availability reduction” process considerably slower than in the non- or the low-zeolite treatments. We suggest two possible reasons for that. First, zeolite may have released back to the soil solution metals derived from sludge, initially sorbed onto it. We propose that because it has been reported in the literature that significant amounts of added metals to zeolite systems may be desorbed back to solution. For example, Hamidpour et al. (2010) in a batch test studied desorption and found that up to 40% of added Cd, a metal with chemical behaviour very similar to Zn, was desorbed from zeolite. In our work, this effect was much more evident (and thus significant) in the high zeolite treatment compared to Z-0 and Z-1, presumably due to higher zeolite content in the soil. Second, zeolite may have bound the studied metals less efficiently than indigenous soil colloid clay surfaces. This was also suggested by Usman et al. (2005), who, in a 120-day incubation experiment in soil mixtures, found that added zeolite caused only a very minor reduction in Cu and Zn availability (assessed in that study with H2O extractions) compared to added bentonite, a typical naturally occurring soil clay mineral.
5 Conclusions
Zeolite retained Cu and Zn not as strongly as there would be expected and less efficiently than soil colloid surfaces. Thus, the added zeolite kept metal availability unchanged on day 100 compared to day 50, while at the same time Cu and Zn in the Z-0 and Z-1 treatments were being reduced, especially in the acidic treatments.
References
Ahmed OH, Aminuddin H, Husni MHA (2008) Ammonia volatilization and ammonium accumulation from urea mixed with zeolite and triple superphosphate. Acta Agr Scand B Soil Plant Sci 58:182–186
Benke MB, Indraratne SP, Hao X, Chang C, Goh TB (2008) Trace element changes in soil after long-term cattle manure applications. J Environ Qual 37:798–807
Essington ME (2004) Soil and water chemistry: an integrative approach. CRC Press, Boca Raton
Gheshlaghi ZT, McLaren RG, Adams JA (2008) Effect of treated zeolite, iron waste, and liming on phytoavailability of Zn, Cu, and Ni in long-term biosolids-amended soils. Austr J Soil Res 46:509–516
Goecke P, Ginocchio R, Mench M (2011) Amendments promote the development of Lolium perenne in soils affected by historical copper smelting operations. Int J Phytoremediat 13:552–566
Hamidpour M, Afyuni M, Kalbasi M, Khoshgoftarmanes AH, Inglezakis VJ (2010) Mobility and plant-availability of Cd(II) and Pb(II) adsorbed on zeolite and bentonite. Appl Clay Sci 48:342–348
Kavoosi M (2007) Effects of zeolite application on rice yield, nitrogen recovery, and nitrogen use efficiency. Commun Soil Sci Plant Anal 38:69–76
Koukoulakis PH, Papadopoulos AI (2007) Problem soils and their improvement. Stamoulis, Athens (in Greek)
Rowell DL (1994) Soil science: methods and applications. Prentice Hall, London
Sanchez-Martin MJ, Garcia-Delgado M, Rodriguez-Cruz MS, Ariezo M (2007) Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 142:262–273
Smith SR (2009) A critical review of the bioavailability and impacts of heavy metals in municipal solid waste compost compared to sewage sludge. Environ Int 35:142–156
Stamatiadis S, Doran JW, Kettler T (1999) Field and laboratory evaluation of soil quality changes resulting from injection of liquid sewage sludge. Appl Soil Ecol 12:263–272
Ste-Marie C, Pare D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589
Stietiya MH, Wang JJ (2011) Effect of organic matter oxidation on the fractionation of copper, zinc, lead, and arsenic in sewage sludge and amended soils. J Environ Qual 40:1162–1171
Sukreeyapongse O, Holm PE, Strobel BW, Panichsakpatana S, Magid J, Hansen HCB (2002) pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. J Environ Qual 31:1901–1909
Ure AM (1995) Methods of analysis for heavy metals. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie Academic and Professional, London, pp 58–102
Usman A, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J Soils Sediments 5:245–252
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Willie Peijnenburg
Rights and permissions
About this article
Cite this article
Antoniadis, V., Damalidis, K. & Dimirkou, A. Availability of Cu and Zn in an acidic sludge-amended soil as affected by zeolite application and liming. J Soils Sediments 12, 396–401 (2012). https://doi.org/10.1007/s11368-011-0446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0446-0