Abstract
Pine wilt disease (PWD) is likely the most serious threat to pine forests worldwide. The causative agent of PWD, the pinewood nematode (PWN) Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle, engages in a symbiotic partnership with its insect vector, the Monochamus beetle, as well as associated bacteria and ophiostomatoid fungi, to successfully infect and kill its host pine trees. In this chapter, we focus on the interspecific communication between PWN and its associated partners, and the potential role of this communication in promoting pathogenicity and invasiveness of PWN. We describe the chemical and molecular signals positively influencing the survival, reproduction and spread of PWN. By considering life cycle of pinewood nematode and its interactions with other biological factors, many methods have been developed and used in infested areas. Removal of killed pines is the most important method in China. Direct trapping of vector adults were also used in recent years. Finally, concluding remarks and future perspectives were discussed in the chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Bursaphelenchus xylophilus
- Pine wilt disease
- Associated microbes
- Invasive species
- Ophiostomatoid fungi
- Monochamus
1 Introduction
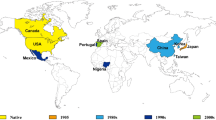
Pines represent one of the most important tree species in global ecosystems. They are not only a predominant component of parks, natural reserves, and urban ornamental landscapes, but also a valuable source of timber and various wood products, e.g. half of the global lumber supply is from pine trees (Williams 2005). In China, Masson pine forests, accounting for approximately 10% of the forest resource, provide the main source of wood for construction (Chen et al. 1997). The most serious threat to pine forests worldwide is pine wilt disease (PWD). PWD is caused by pinewood nematode (PWN) Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle, which is native to North America and has been a deadly exotic pest of pine forests in China since 1982 (Fig. 18.1). In China, most pine species, including Pinus massoniana, P. taiwanensis, P. yunnanensis, P. armandii, P. kesiya var. langbianesis and P. thunbergii, can be infected by PWN (Yang et al. 2003). The infested species are different to the pine species in North America (Williams 2005).
The first PWN infection in Chinese forests was reported in Nanjing, Jiangsu Province in 1982 (Yang et al. 2003; Xu and Ye 2003). By 2007, PWN infection had been found in 113 counties of 14 provinces, with the coastal regions being most seriously affected (provinces of Zhejiang, Jiangsu, etc.) (Yu et al. 2011). The Chinese government has invested heavily in stopping the spread of PWN and minimizing timber loss (Xu and Ye 2003). However, it has killed more than one million hectares of pine forests with losses of more than US $20 billion in 15 provinces in southern China in 2011 (State Forestry Administration of the People’s Republic of China 2011). It has infested to Shaanxi and Henan provinces in the northern China after 2005, suggesting that the nematode might infest more areas in the Northern Hemisphere in the future.
In addition to PWN, PWD involves several other key players, including the nematode’s insect vector, the Monochamus beetle (Coleoptera: Cerambycidae), as well as associated bacteria and ophiostomatoid blue-stain fungi, all of which likely contribute to the pathogenicity of the nematode. PWN engages in a mutualistic partnership with the Monochamus beetle in order to successfully dispersal and kill its host pines. The main vector beetle is M. alternatus in China, different to M. carolinensis in North America. The new native ophiostomatoid fungi induce PWN to produce greater numbers of offspring than in North America (Hyun et al. 2007; Zhao et al. 2013b) (Table 18.1). The nematode feeds on epithelial cells of the pine tree host, as well as ophiostomatoid (‘blue-stain’) fungi that proliferate on the damaged pine tree especially during later stages of infection. The Monochamus beetles reside within pine trees at larval stages. During the propagative phase of life cycle, PWN reproduces rapidly, developing from the egg through four larval stages (J1–J4) to the reproductive adult. In this life cycle, PWN has two different long-lived, dispersal stages, the third-stage (JIII) and fourth-stage juveniles (JIV, also referred to as ‘dauer juveniles’) (Pereira et al. 2013). Under unfavorable conditions such as food scarcity and low temperature, the nematode enters the dispersal phase, in which it moults from J2 into third-stage dispersal juveniles (JIII). When fifth-instar larvae of the beetle enter diapause in winter, the JIII would aggregate around the chambers of the beetle larvae. In the following spring, once overwintered beetles develop to late pupal or young adult stages, the JIII would moult into fourth-stage dispersal juveniles (JIV), which enter the tracheal system of the beetle ready for dispersal (Pereira et al. 2013). One adult Monochamus beetle can carry thousands of nematodes (1627 on average) in its tracheal system (Futai 2013). Bacterial accumulation surrounding PWN was revealed in the resin canals and along its body by transmission electronic microscopy (Suh et al. 2013). Nevertheless, the precise participation of these bacteria in the PWD disease mechanism is still a provocative issue.
With the introduction of PWN from North America to Japan in the early twentieth century and subsequently spread to other geographic locations, this pathogen has successfully exploited the lack of defenses in native pine species. Furthermore, as the nematode has existed in novel environments in Asian and European countries for ~100 years, it may have further evolved to exploit the native insect vectors, fungi, and bacteria more efficiently (Togashi and Jikumaru 2007). Therefore, we want to introduce the complex of PWN, insect vectors, fungi, and bacteria as follows.
2 Genetic Variation of Pinewood Nematode
In its native ranges, the pinewood nematode does not kill pine trees and rarely causes economic losses (Dwinell and Nickle 1989). The question then arises why pinewood nematode could spread uncontrollably and became highly pathogenic to pine trees when it was introduced into Asian regions. To answer this question, many studies have been conducted in China in the past 20 years. Currently, invasions and range expansion are thought to be related to genetic structure and variance (Cheng et al. 2008; Pereira et al. 2013).
2.1 Genetic Variation of Native and Invasive Nematode Populations
With such a successful invasive species, exploring the invasion process and its genetics is important. An amplified fragment length polymorphism (AFLP) survey was used to compare the genetic variation of native and invasive nematode populations in China and to examine the changes in genetic diversity during invasions. A total of 28 nematode populations were randomly collected from 28 geographical locations in Jiangsu, Guangdong, Chongqing in China. Chinese populations were found to have a slightly higher genetic diversity than American populations (Cheng et al. 2008). Moreover, genetic diversity differed significantly among geographic populations in China. Southern populations had a distinctly higher genetic diversity than others, and the genetic diversity of eastern coastal populations was obviously lower than that of eastern inland and western populations (Cheng et al. 2008). The 13 American populations formed a distinct group, however, the 28 Chinese samples were divided into two groups. One group was formed mainly by samples from Jiangsu, and the other mainly by samples from Zhejiang and Anhui (except samples from Mingguang, which clustered with Jiangsu). Samples from Guangdong Province were scattered across the two groups. Samples from Hubei, Guizhou and Congqing joined in the Jiangsu group. The two Japanese samples clustered with the Zhejing–Anhui group (Cheng et al. 2008). Based on the genetic relationships discovered among samples, two major invasion pathways in China are suggested. One might be from Guangdong to Anhui and Zhejiang, and the other from Guangdong to Jiangsu and then from Jiangsu to Hubei, Guizhong and Congqing. (Cheng et al. 2008). In addition, it was determined that there were no genetic bottlenecks caused by founder effect and genetic drift in Chinese populations. Multiple invasions with large amounts of nematodes from different sources led to high genetic diversity in the invasive populations. Maintenance of high genetic diversity during invasions may be one of genetic mechanisms in its invasion success. These findings provided basic background information for deep investigations to obtain a comprehensive interpretation of PWN invasion mechanisms (Xie et al. 2009).
PWN showed a low level of genetic diversity in locations where it was introduced recently, such as southwestern Europe. Bursaphelenchus mucronatus, an native species in the pine trees with pinewood nematodes together and an native species occurring with PWN in pine trees, displayed a high level of genetic diversity across Eurasia, possibly due to its longer time to adapt there (Pereira et al. 2013; Vieira et al. 2007). The genetic pressures of low population in PWN’s pathogenic life cycle, as well as the higher dispersal capacity of its vector beetle, may have resulted in a low genetic diversity in the nematode in China and Europe (Vieira et al. 2007).
2.2 Defensive Mechanism in PWN Responding to Host Defenses
Genomic expansions in detoxifying and antioxidant genes in PWN have likely been driven by the nematode’s need to respond to multiple layers of host defenses. PWN has numerous digestive proteases, expanded families of genes in the lysosome pathway, ATP binding cassette transporters and cytochrome P450 pathway genes (Kikuchi et al. 2011). PWN also rapidly secretes antioxidant proteins when invading a host pine. These proteins likely serve as a protective barrier that protects the nematode against a burst of reactive oxygen species generated by pine trees as a defensive mechanism during early infection. A total of 12 antioxidant proteins have been reported in the secretome of PWN, including peroxiredoxin, catalase, glutathione peroxidase, nucleoredoxin-like protein, superoxide dismutase, and thioredoxin (Shinya et al. 2013a, b). Intriguingly, the PWN secretome includes a number of proteins that potentially mimic host proteins, including two thaumatin-like proteins that show highest homology to pine tree proteins and a cystatin-like peptidase inhibitor with significant homology to a protein in the herbaceous plant Medicago truncatula (Shinya et al. 2013b). In plants, thaumatin-like proteins are classified as pathogenesis-related (PR) proteins and display antifungal activities by permeabilizing fungal membranes (Batalia et al. 1996). Plant cystatins play a crucial role in plant defenses against plant-parasitic nematodes (Arai et al. 2002). One of the thaumatin proteins (PR-5) and the cystatin-like peptidase (PR-6) were significantly overexpressed in susceptible pine trees during PWN infections (Hirao et al. 2012). Additionally, this phenomenon of molecular mimicry occurs in other specialized plant-parasitic nematodes, which are able to mimic endogenous host plant proteins. Wang and Lu have ever found the CLAVATA3/ESR (CLE)-like proteins using cyst nematodes (Wang et al. 2011a; Lu et al. 2009). The exact roles of the thaumatin and peptidase proteins are unknown so far; however, they probably co-evolve along with PWN and its native host pines. Because PWN invaded the pine species in Asia and Europe more recently, these pines may be more sensitive to the host mimic proteins mentioned above.
3 Interspecific Chemical Communication Between Pinewood Nematode, Its Insect Vector, and Associated Microbes
PWN engages in a symbiotic partnership with its insect vector, the Monochamus beetle, as well as associated bacteria and ophiostomatoid fungi, in order to successfully infect and kill its host pine trees. Here we focused on the interspecific communication between PWN and its associated partners, and the potential role of this communication in promoting pathogenicity and invasiveness of PWN. We describe the chemical and molecular signals positively influencing the survival, reproduction, and spread of PWN (Fig. 18.2). Knowledge of these signals could potentially be used to interfere with the proliferation and dispersal of PWN.
Multispecies interactions in PWD. Upon entering a healthy pine tree, JIV recover and become propagative Jn larvae, which are attracted to terpenes produced by the pine tree. PWN interacts with beneficial microbiota that may be introduced to the pine tree by the nematode, its vector beetles, or bark beetles. Blue stain fungi produce terpenes that may stimulate PWN propagation. Specific fungal isolates (Sporothrix sp. 1) induce the xylem tissue of the pine tree to produce diacetone alcohol (DAA), which may also increase PWN propagation and beetle larvae growth. Blue stain fungi also provide food to PWN at later stages of infestation after pine tree death. PWN may have acquired genes by HGT from associated microbiota or from pine trees that increase its pathogenicity. As conditions deteriorate inside the pine tree, PWN enters the dispersal phase of its life cycle. JIII form in response to unknown signals and aggregate around the pupal chamber of the vector beetle in response to terpene signals produced by the beetle larvae. Then, JIII molt to JIV in response to C16 and C18 FAEEs released from the surface of the emerging Monochamus beetle adult. Additional chemical or other types of signals may stimulate association of JIV with the beetle adult for dispersal to a new pine tree host. Red text indicates potential areas for future study. Purple circles, blue stain fungi; green circles, associated microbiota; blue circles, the pine trees; brown circles, insect vector; black circles, PWN. Abbreviations: PWD pinewood disease, PWN pinewood nematode, HGT horizontal gene transfer, FAEEs fatty acid ethyl esters
3.1 Horizontal Gene Transfer from Bacteria Benefit PWN
There is growing evidence that horizontal gene transfer (HGT) plays an important role in the evolution of nematode plant parasitism, including PWN (Davis et al. 2008; Wijayawardena et al. 2013). Genome sequencing and proteomic data mining of PWN provided strong evidence of multiple independent HGT events that potentially resulted in gene acquisition from both fungi and bacteria (Kikuchi et al. 2011; Shinya et al. 2013a). At least five groups of genes were acquired potentially from bacteria: (i) genes for a family of cell-wall degrading enzymes belonging to GH16 ß-1,3-glucanases, similar to those found in Gamma-Proteobacteria; (ii) a putative cystatin gene, which is similar to that found in Proteobacteria but divergent from PR-6 mentioned above; (iii) a gene for 6-phosphogluconolactonase similar to that from the Phylum Firmicutes; (iv) a HAD-superfamily hydrolase similar to the Firmicutes (Kikuchi et al. 2011; Shinya et al. 2013a) ; and (v) a marked expansion of genes for secreted peptidases and peptidase inhibitors (Shinya et al. 2013a). GH16 ß-1,3-glucanase is localized in the PWN’s esophageal gland cells and secreted through the stylet, suggesting an important acquired feature for weakening the fungal cell walls; ß-1,3-glucan is the main structural component of the cell walls of the blue-stain fungi, thus this enzyme permits PWN to feed on fungi such as Ophiostoma spp. (Kikuchi et al. 2005).
The repertoire of parasitism-related genes in PWN may reflect the diversity of food sources exploited during different nematode life stages, that is, plant tissues versus fungi, and the range of critical environments that the nematode confronts, for instance, resin canals of the host trees versus the tracheae of the insect vector (Kikuchi et al. 2011). However, little is known about the functional activities and evolutionary processes of these putative genes acquired by HGT from bacteria.
3.2 Host Pine Volatiles Mediate PWN Reproduction and Behavior
The ratios and concentrations of terpenes differ between healthy trees and nematode-infested trees with blue-stain ophiostomatoid fungi. The terpene ratio – α-pinene, β-pinene, and longifolene at 1:0.1:0.01 – found in healthy Pinus massoniana xylem attracts the propagative stages of PWN (Zhao et al. 2007). In addition, the propagation rate of PWN is higher when treated with a monoterpene ratio representative of blue-stain fungi-infected pines (α-pinene : β-pinene =1 : 0.8) than when treated with a monoterpene ratio representative of healthy pines or those damaged by M. alternatus feeding but without blue-stain fungi (α-pinene : β-pinene =1 : 0.1) (Niu et al. 2012).
3.3 Chemical Signals Synchronize the Development of PWN and Its Insect Vector
JIII aggregation and JIV formation in PWN are coordinated with the life cycle of the beetle vector (Zhao et al. 2007, 2013a; Ogura and Nakashima 2002). The JIII aggregate around the beetle’s pupal chamber in the pine tree, possibly in response to the specific ratio of volatile terpenes – α-pinene, β-pinene, and longifolene at 1:2.7:1.1 – produced by the Monochamus beetle larvae. This terpene ratio differs from that produced by pine trees, which only attracts the propagative larval stages of the nematode (Zhao et al. 2007). Near the time of emergence of adult beetles, PWN moults into JIV in response to long-chain C16 and C18 fatty acid ethyl esters (FAEEs) that are secreted from the body surface of the Monochamus beetle specifically during eclosion. Fatty acids, fatty acid methyl esters, and shorter-chain FAEEs do not induce JIV formation, suggesting that the C16 and C18 FAEEs represent a specific chemical signal (Zhao et al. 2013a).
The insulin/insulin-like growth factor 1 (IGF-1) and the nuclear hormone receptor DAF-12 (dauer formation 12) pathways play a key role in dauer formation in the free-living nematode C. elegans and in PWN (Ogawa et al. 2009; Zhi et al. 2012; Broughton and Partridge 2009). Treatment of PWN with the PI3 kinase inhibitor LY294002 promotes JIV formation, while ∆7-dafachronic acid blocks the effects of C16 and C18 FAEEs (Zhao et al. 2013a), suggesting a conserved role for the insulin/IGF-1 and DAF-12 pathways in JIV formation. Transcriptional changes in the insulin/IGF-1 and DAF-12 pathways correlate with dauer entry in both C. elegans and PWN (Zhao et al. 2013a), indicating conservation of the transcriptional response in the two nematodes.
Several factors may explain why Monochamus beetles are the sole vector for PWN. Importantly, Monochamus spp. are the only beetles present at the right time and place as conditions deteriorate inside pine trees. Because JIII are in the center of the xylem, they are able to aggregate around the pupal chamber in response to volatile terpenes produced by the beetle larvae and subsequently develop into JIV juveniles in response to the long-chain FAEEs produced by the emerging beetles. Furthermore, the beetles may secrete unidentified cues that induce the aggregation of the JIV juveniles in the tracheal system of the beetle for subsequent dispersal, although this possibility is yet to be determined.
3.4 Host Pine-PWN and Symbiotic Fungi
Although PWN feeds mainly on plant cells during the initial infection, the nematode feeds on blue-stain fungi, such as ophiostomatoid fungi, present within the wood later in infection and after the pine host is killed. Nematode reproduction increases when blue-stain fungi are the food source. Dead wood owing to PWD is often stained blue or blue-black because these dying pine trees are also infested with bark beetles (Coleoptera: Scolytinae) that carry a wide range of ophiostomatoid (blue-stain) fungi (Zhao et al. 2008). Blue-stain fungi facilitate infection by bark beetles by decreasing the tree’s vigor or by providing nutrition to bark beetles (Six et al. 2011). In addition to being carried by bark beetles, some evidence indicates that blue-stain fungi can also adhere to the body surface of adult M. alternatus and thus be transmitted to the twigs of healthy trees (Suh et al. 2013). Many genera of these fungi have been reported in different geographic regions (Suh et al. 2013; Wingfield 1987) (Table 18.1).
3.5 Associated Fungi Mediate the Development and Population of the Insect Vector
The type of fungi dominating dead wood may determine the number of PWN carried by the beetles emerging from the wood (Zhao et al. 2013b; Maehara Futai 1997). This surprising connection has been established for two fungi native to the invaded regions, namely Ophiostoma minus isolated in Japan and Sporothrix sp. 1 isolated in China (Zhao et al. 2008 ; Maehara Futai 1997). The presence of these fungi strongly and positively influenced PWN reproduction, and consequently the number of nematodes dispersed by the beetles (Zhao et al. 2008, 2013b; Maehara Futai 1997). The beetles in woods inoculated with O. minus carried a far greater number of PWN than those inoculated with Trichoderma sp. 2, Trichoderma sp. 3, O. minus and Trichoderma sp. (O + T), or uninoculated blocks (Maehara Futai 1997). Sporothrix sp. 1 positively affects the population and prevalence of the association of PWN with the native beetle M. alternatus in the xylem of trees (Zhao et al. 2013b). PWN produced greater numbers of offspring with a female-biased sex ratio and developed faster in the presence of Sporothrix sp. 1. The fragrant diacetone alcohol (DAA) released from wood infected by Sporothrix sp. 1 promoted fecundity of PWN and growth and survival of the beetle (Zhao et al. 2013b). That is, they determine the dynamics of PWD.
3.6 Host Volatiles Positively Influence Interactions of Nematode and Fungi
The terpenes released from host pine trees influence the growth of ophiostomatoid fungi identified in dead trees and the interactions of PWN and fungi (Niu et al. 2012). The monoterpenes α-pinene and β-pinene inhibited the mycelial growth of associated fungi Sporothrix sp.2 and O. ips, but had no significant effects on the growth of Sporothrix sp.1, which is the best food resource for PWN in China. These results suggest that the native blue-stain fungus Sporothrix sp.1 is not sensitive to host signals and thereby improves PWN’s own propagation (Niu et al. 2012).
4 Monitoring and Prevention , and Semiochemical-Based Direct Control Tactics
In China, researchers pay more attention to the monitoring and preventing of the pine wilt disease, using effective and safe methods. By considering life cycle of pinewood nematode and its interactions with other biological factors, many methods have been developed and used in infested areas.
4.1 Spread Potential of Pine Wilt Disease in China
To determine the most appropriate control measures, it is important to anticipate where they could spread in the future. Using mathematical models to simulate the potential distribution ranges of the nematode and the disease development can help us to predict the areas at risk and consequently the areas where surveillance should be intensified.
A specific spread model was developed to describe the spread of the pine wilt disease symptoms at a larger spatial scale. It was fitted to the invasion history in China (Robinet et al. 2011), where the insect vector is mainly M. alternatus . It was later applied to Europe in order to determine the European ports from which the nematode and wilt disease could spread rapidly, and thus, where surveillance should be targeted more carefully (Robinet et al. 2011).
The model can simulate the potential long-distance spread of the nematode in China. The model showed that long distance dispersal appeared to be very important in China, representing more than 90% of the new infestations at an average distance of 111–339 km from the likely source populations (Robinet et al. 2009). The best factor that could explain these long-distance jumps was the human population density. This factor probably gave a good indication of the magnitude of human activity across the country and thus an indication of the amount of imports of wooden (potentially infested) materials (Robinet et al. 2009).
Climate constraints considered in the model were not the same between China and Europe. In China, the insect vector is not present throughout the country and its distribution is limited by both summer and winter temperatures (mean temperature in July above 21.3 °C and mean temperature in January above −10 °C). The only temperature constraint considered in the model applied to Europe is the temperature threshold for the disease expression (mean temperature of July above 20 °C), which is less restrictive than the temperature constrains used in China for the Asian vector. From the simulations of this model, climate change could affect more strongly the spread of the pine wilt disease in Europe than in China (Robinet et al. 2011). With 3 °C of temperature rise, the area suitable for the disease expression could expand by 40% in China against more than 100% in Europe (Robinet et al. 2011).
4.2 Early Detection of PWN
Early detection is of primary importance to enable rapid actions to prevent the spread and introduction of invasive species. We developed a kairomonal trapping technique that can be used to study within-tree horizontal and vertical distributions of PWN in infested stands in China (Zhao et al. 2007). It takes into account the changes of PWN within-tree distribution in relation to the development of symptoms in attacked pines. This technique provides a simple, effective, rapid and non-destructive sampling method. When 60–80% of the foliage has become pale green, PWN is recovered from larger diameter branches. As disease symptoms progress, PWN moves into and down the trunk. As the needles turn yellow, PWN was recovered from the trunk at 1–2 m above the ground (Fig. 18.3). This systematic sampling technique should greatly enhance early detection of PWN in field surveys, monitoring and phytosanitary inspections (Zhao et al. 2009).
After getting nematodes samples in trees, a real-time polymerase chain reaction (PCR) assay was developed to detect PWN. A set of primers and probes specific for PWN was designed to target the internal transcribed spacer (ITS) region. The assay was highly specific and sensitive, detecting as little as 0.01 ng of PWN DNA. The real-time PCR assay also successfully detected PWN in field samples, and it should be very useful for quarantine purposes (Cao et al. 2005). In addition, M. alternatus with nematodes together have also been ready for subsequent PCR detection of PWN (Wang et al. 2011b).
4.3 Removal of Killed Pines
The most important management tool for control is forest sanitation, especially the removal of killed pines to prevent their becoming breeding grounds for both vector beetles and the nematodes (Fig. 18.4). Entire Dead or dying trees should be removed immediately and should not be stored for later use as firewood. Even a single infected pine tree left can become an infection center that will devastate other pines nearby (Zhao et al. 2008).
In China, many treatments involving dead pines are used to halt the PWN disease cycle. The first is clear-cutting of affected areas to remove and treat dead pine trees. Secondly, heating and hydraulic pressing treatment of nematode-infected pine timbers can be conducted. Heating at 65–75 °C for 15 h, hydraulic pressing at 9 MPa, 157–168 °C for 10 min of PWN infested pine timbers (of different sizes) gave good control of both PWN and M. alternatus . In particular, treatment of diseased-infested boards less than 2.8-cm thick or 10 cm × 10 cm can kill 100% of the pests (Chen et al. 2000). Thirdly, the wilt-affected wood can be treated by submerging into hot water. Our trials in Nanjing showed that treating lumber with hot water to a core temperature of 60 °C for 2.30 ± 0.50 h could kill 100% of living nematodes or their beetle vectors (Zhao et al. 2008). Those methods could be used for the large-scale treatment of the trees killed by the PWN (Zhao et al. 2008).
4.4 Direct Trapping of Vector Adults
In China, volatiles from a stressed host have been tested for their attractions to M. alternatus. Available results showed that some components of stressed host volatiles have strong trapping ability for M. alternatus. Thus, using attractant-based traps are an effective method for monitoring beetle population dynamics and for reducing populations of M. alternatus in pine forests (Zhao et al. 2008).
APF-I is a popular attractant in China for attracting female beetles before oviposition and the pubescent males. It is produced in Xiamen Sanyong Co., China. This is the most effective attractant appointed by Chinese government now. The effective distance is more than 150 m, and the effective period is more than 30d (20–25 °C). In Chongqing, Sichuan, Hunan, Gaungdong and Fujian provinces, APF-1 showed the trapping efficacy more than twice higher than terpines traps (Fig. 18.5).
4.5 Natural Enemies
Several natural enemies have been found in M. alternatus . They are insect parasitoids, such as Scleroderma guani and Dastarcus helophoroides, and parasitic fungi (Liou et al. 1999), bacteria and nematodes. Parasitic fungi include Beauveria bassiana, B. brongniartii, Metarhizium anisopliae, Isaria farinosa, Aspergillus flavus, Verticillium spp. and Acremorium sp., whereas parasitic bacteria include Serratia marcescens and the parasitic nematode, Steinernema feltiae (Zhao et al. 2008).
S. guani is one of the most successful natural parasites of M. alternatus larvae (Fig. 18.6). This parasitoid could be readily propagated on M. alternatus larvae. Following release of S. guani in pine forests, over 66.8% of M. alternatus larvae could be parasitized. Based on numerous field tests on release density, timing and method, the “release from a single tree method” was selected. When 5000 wasps per hectare were released using this method in a test forest in mid-July, parasitism rate of M. alternatus larvae was 66.82–84.21% (Fig. 18.6). This demonstrated that S. guani can provide good control of M. alternatus (Zhao et al. 2008).
4.6 Replanting in Clear-Cut Areas
Resistant pine species and other commercial crop were selected to protect water and soil. In clear-cut areas, there are often many openings with various size. These areas need to be reforested so that they can resume their ecological function in protecting water and soil in mountainous areas (Fig. 18.7). In a field trial beginning from 1984 in southern Nanjing, Jiangsu, two resistant pine species, including P. taeda, and three resistant Masson pine provenances, GX2, GX3 and GD5, were outplanted into plantations. By 1999, the areas replanted with P. taeda and GX2 provenances have reached 5340 and 1340 ha, respectively. In 2008, 24 years after the replanting, the two pines are still growing very well, and their selected resistance is stable and reliable. All these areas, which were previously damaged by the PWN, are again fulfilling their role in soil and water conservation (Li et al. 2013; Xu et al. 1999, 2004). Some special shrubs and commercial crops can also be used for replanting. For instance, tea trees might be good candidates, which can bring benefits for local people.
4.7 Selecting Bacteria to Reduce the Strong Pathogenicity of Chinese Pine Wood Nematodes
There are four dominant bacteria in U.S. pine wood nematode including Delfita tsurhatensis, Pseudomonas putida, Stenotrophomonas maltophilia and Pantoea sp. The pine seedlings treated with S. maltophilia withered more slowly than that treated with Pseudomonas fluorescents, a dominant bacterium in China. The four US bacteria also could live in Chinese nematodes. So this study provides a helpful reference for replacing virulent bacteria when released into the wild, in order to reduce the pathogenicity of Chinese pine wood nematodes (Zeng 2010). At present, Tianyilin Co. in Jiangsu Province can apply this bacterium to the infested forestry.
5 Concluding Remarks and Future Perspectives
In conclusion, Studies of multi-species interactions between PWN, its vector, and associated microbiota in China shed light on the potential factors that facilitate the propagation and spread of this invasive pathogen. Therefore, some methods developed based on the multi-species interactions in China were used in the infested regions effectively. By considering life cycle of pinewood nematode and its interactions with other biological factors, many methods have been developed and used in the infested areas. Removal of killed pines is the most important method in China. Direct trapping of vector adults is also used in recent years as a promising biocontrol tool. Selecting bacteria to reduce the strong pathogenicity of Chinese pine wood nematodes begin to show excited results in several regions. The number of infested regions changed to be less than before.
A comprehensive understanding of the biological interactions involved will continuously facilitate the prevention and management of PWD in the future. (Arai et al. 2002). Comparative genomics-, transcriptomics-, and proteomics-based approaches can be used to track the intrinsic low genetic diversity in the isolates of PWN compared with closely related native species of B. mucronatus isolated from different countries. Thus, the nucleotide sequences for genes related to the intrinsic invasiveness would be disturbed as a control target; (Batalia et al. 1996). Analysis of the adaptive evolution of gene expression related to reproduction, dispersal, and virulence of PWN with different beetle vectors and ophiostomatoid fungi over numerous generations is necessary. Tools to disrupt the association of the nematode with its vector, as well as their dispersal and spread, could offer a method to exploit the chemical signals involved in synchronization. (Broughton and Partridge 2009). Moulting to JIII is induced environmentally by deteriorating conditions in the pine tree or a lack of food resources. Additional unidentified chemical cues may also, for example, promote the propagative nematodes to moult to JIII. Further investigation regarding chemical communication in PWN could be equally concerned. With the increasing number of identified chemical signals, this technique may enable the development of methods to disrupt the development and behavior of PWN and its vector in the field.
References
Arai S, Matsumoto I, Emori Y, Abe K (2002) Plant seed cystatins and their target enzymes of endogenous and exogenous origin. J Agric Food Chem 50:6612–6617
Batalia MA, Monzingo AF, Ernst S, Roberts W, Robertus JD (1996) The crystal structure of the antifungal protein zeamatin, a member of the thaumatin-like, PR-5 protein family. Nat Struct Biol 3:19–23
Broughton S, Partridge L (2009) Insulin/IGF-like signaling, the central nervous system and aging. Biochem J 418:1–12
Chen L, Chen Q, Liu W (1997) Forest diversity and its geographical distribution in China. Sci Press, Beijing
Cheng XY, Cheng FX, Xu RM, Xie BY (2008) Genetic variation in the invasive process of Bursaphelenchus xylophilus (Aphelenchida: Aphelenchoididae) and its possible spread routes in China. Heredity 100:356–365
Davis EL, Hussey RS, Mitchum MG, Baum TJ (2008) Parasitism proteins in nematode-plant interactions. Curr Opin Plant Biol 11:360–366
Dwinell LD, Nickle WR (1989) An overview of the pinewood nematode ban in North America. Gen. Tech, Rep. SE-55. US. Department of agriculture forest service, southeastern forest experiment station: Asheville, NC, 13 pp
Futai K (2013) Pine wood nematode, Bursaphelenchus xylophilus. Annu Rev Phytopathol 51:61–83
Hirao T, Fukatsu E, Watanabe A (2012) Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol 12:13
Hyun MW, Kim JH, Suh DY, Lee SK, Kim SH (2007) Fungi isolated from pinewood nematode, its vector Japanese pine sawyer, and the nematode-infected Japanese black pine wood in Korea. Mycologia 35:159–161
Kikuchi T, Shibuya H, Jones JX (2005) Molecular and biochemical characterization of an endo-beta-1,3-glucanase from the pinewood nematode Bursaphelenchus xylophilus acquired by horizontal gene transfer from bacteria. Biochem J 389:117–125
Kikuchi T, Cotton JA, Dalzell JJ, Hasegawa K, Kanzaki N, McVeigh P, Berriman M (2011) Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog 7:e1002219
Li QQ, Ye JR, Zhu LH, Wu XQ, Zhu XF (2013) Resistance determination of wilt-resistant Pinus densiflora tissue culture seedling to Bursaphelenchus xylophilus. J Northeast For Univ 41:45–47
Liou JY, Shih JY, Tzean SS (1999) Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol Res 103:242–248. doi:10.1017/s0953756298006984
Lu SW, Chen S, Wang J, Yu H, Chronis D, Mitchum MG, Wang X (2009) Structural and functional diversity of CLAVATA3/ ESR (CLE)-like genes from the potato cyst nematode Globodera rostochiensis. Mol Plant-Microbe Interact 22:1128–1142
Maehara N, Futai K (1997) Effect of fungal interactions on the numbers of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), carried by the Japanese pine sawyer, Monochamus alternatus Coleoptera: Cerambycidae. Fundam Appl Nematol 20:611–617
Niu H, Zhao L, Lu M, Zhang S, Sun J (2012) The ratio and concentration of two monoterpenes mediate fecundity of the pinewood nematode and growth of its associated fungi. PLoS One 7:e31716
Ogawa A, Streit A, Antebi A, Sommer RJ (2009) A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol 19:67–71
Ogura N, Nakashima T (2002) In vitro occurrence of dispersal fourth stage juveniles in Bursaphelenchus xylophilus co-incubated with Monochamus alternatus. Jpn J Nematol 32:53–59
Pereira F, Moreira C, Fonseca L, van Asch B, Mota M, Abrantes I, Amorim A (2013) New insights into the phylogeny and worldwide dispersion of two closely related nematode species, Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. PLoS One 8:e56288
Robinet C, Roques A, Pan H, Fang G, Ye J, Zhang Y, Sun J (2009) Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS One 4:e4646. doi:10.1371/journal.pone.0004646
Robinet C, Van Opstal N, Baker R, Roques A (2011) Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol Invasions 13:2981–2995
Shinya R, Morisaka H, Takeuchi Y, Futai K, Ueda M (2013a) Making headway in understanding pine wilt disease: what do we perceive in the postgenomic era? J Biosci Bioeng 116:1–8
Shinya R, Morisaka H, Kikuchi T, Takeuchi Y, Ueda M, Futai K (2013b) Secretome analysis of the pine wood nematode Bursaphelenchus xylophilus reveals the tangled roots of parasitism and its potential for molecular mimicry. PLoS One 8:e67377
Six DL, Poulsen M, Hansen AK, Wingfield MJ, Roux J, Eggleton P, Paine TD (2011) Anthropogenic effects on interaction outcomes: examples from insect-microbial symbioses in forest and savanna ecosystems. Symbiosis 53:101–121
State Forestry Administration of the People’s Republic of China (2011) Announcement of state forestry administration of the people’s republic of China, No. 3, 2011
Suh DY, Hyun MW, Kim JJ, Son SY, Kim SH (2013) Ophiostoma ips from pinewood vematode vector, Japanese pine sawyer beetle (Monochamus alternatus), in Korea. Mycobiology 41:59–62
Togashi K, Jikumaru S (2007) Evolutionary change in a pine wilt system following the invasion of Japan by the pinewood nematode, Bursaphelenchus xylophilus. Ecol Res 22:862–868
Vieira P, Burgermeister W, Mota M, Metge K, Silva G (2007) Lack of genetic variation of Bursaphelenchus xylophilus in Portugal revealed by RAPD-PCR analyses. J Nematol 39:118–126
Wang J, Replogle AMY, Hussey R, Baum T, Wang X, Davis EL, Mitchum MG (2011a) Identification of potential host plant mimics of CLV3/ESR (CLE)-like peptides from the plant-parasitic nematode Heterodera schachtii. Mol Plant Pathol 12:177–186
Wang XR, Zhu XW, Kong XC, Mota MM (2011b) A rapid detection of the pinewood nematode, Bursaphelenchus xylophilus in stored Monochamus alternatus by rDNA amplification. J Appl Entomol 135:156–159
Wijayawardena BK, Minchella DJ, DeWoody JA (2013) Hosts, parasites, and horizontal gene transfer. Trends Parasitol 29:329–338
Williams CG (ed) (2005) Landscapes, genomics and transgenic conifers forests. Springer, Dordrecht
Wingfield MJ (1987) Fungi associated with the pinewood nematode, Bursaphelenchus xylophilus, and cerambycid beetles in Wisconsin. Mycologia 79:325–328
Xie B, Cheng X, Shi J, Zhang Q, Dai S, Cheng FX (2009) Mechanisms of invasive population establishment and spread of pinewood nematodes in China. Sci China C Life Sci 52:587–594
Xu R, Ye W (2003) Biological invasion: theory and practice. Science Press, Beijing
Xu FY, Ge MH, Zhang P, Zhao Z, Sun Z (1999) Studies on provenance of masson pine resistance to pine wood nematode (PWN) and resistance mechanisms. In: Futai K, Togashi K, Ikeda T (eds) Sustainability of pine forests in relation to pine wilt and decline. Proceedings of the international symposium, Tokyo, 27–28 October 1998, Shokado, Kyoto, Japan, pp 213–216
Xu FY, Ge MH, Xu KQ, Zhang P, Xie CX (2004) Studies on the techniques of integrated management to PWN in Jiangsu. In: Proceedings of the international symposium on ecology and management of pine wilt disease. Seoul, 7–9 October 2004, pp 63–75
Yang BJ, Tang J, Wang YY, Pang HY, Wang LF (2003) Pine wilt disease. Chin For Pub House, Beijing
Yu MJ, Xu XH, Ding P (2011) Economic loss versus ecological gain: the outbreaks of invaded pinewood nematode in China. Biol Invasions 13:1283–1290
Zeng FL (2010) Study on biocontrol potential of bacteria carried by pine wood nematode. Master thesis of Nanjing For. Unniv. Nanjing
Zhao LL, Wei W, Kang L, Sun JH (2007) Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J Chem Ecol 33:1207–1216
Zhao BG, Futai K, Sutherland JR, Takeuch Y (2008) Pine wilt disease. Springer, Berlin
Zhao L, Zhang S, Wei W, Hao H, Zhang B, Butcher RA, Sun J (2013a) Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr Biol 23:2038–2043
Zhao L, Lu M, Niu H, Fang G, Zhang S, Sun J (2013b) A native fungal symbiont facilitates the prevalence and development of an invasive pathogen-native vector symbiosis. Ecology 94:2817–2826
Zhi X, Zhou XE, Melcher K, Motola DL, Gelmedin V, Hawdon J, Xu HE (2012) Structural conservation of ligand binding reveals a bile acid-like signaling pathway in nematodes. J Biol Chem 287:4894–4903
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd. 2017
About this chapter
Cite this chapter
Zhao, L., Sun, J. (2017). Pinewood Nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle. In: Wan, F., Jiang, M., Zhan, A. (eds) Biological Invasions and Its Management in China. Invading Nature - Springer Series in Invasion Ecology, vol 13. Springer, Singapore. https://doi.org/10.1007/978-981-10-3427-5_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-3427-5_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3426-8
Online ISBN: 978-981-10-3427-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)