Abstract
Objective and methods
This article provides a summary of studies on pine wilt disease (PWD). PWD is a serious threat to forests, and the damage caused by this disease results in significant economic loss. In addition, PWD adversely affects not only animals and plants, but also the human environment. Having a better understanding over all possible interference and control measures strategies derived from knowledge of the complicated interrelation between the nematode, its vectors and the host pine trees is a precondition to effectively reduce the damage caused by the pine wood nematode (PWN). The references in this paper were collected from various sources, including PubMed, Google Scholar, and Web of knowledge before being organized by the authors.
Results and conclusion
Most papers discussing PWD have been conducted on the East Asia and European Union regions. Specific topics covered include: (1) damage and invasion of pine wilt disease, (2) the developmental cycle and transmission, (3) diagnosis method for PWN related to PWD and (4) control strategies to limit the spread of PWD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pine wood nematode (PWN), Bursaphelenchus xylophilus, is a lethal pest that infects pine wood trees. Although PWN supposedly originated in North America, pine wilt disease (PWD) was first recorded in Japan in the early twentieth century [1, 2]. Subsequently, the disease has spread to other East Asian countries including Korea and China. Asian pinus species, including P. massoniana, P. densiflora and P. thunbergii, are susceptible to B. xylophilus as a high-risk species. As a result, PWD, which is caused by B. xylophilus, has caused extensive damage in pine forests of East Asian countries, in particular Japan and Korea [3, 4]. In the 1990s, PWN was introduced into Portugal from East Asia where it caused major forest damage, and recently, it was introduced into Spain [5]. Accordingly, PWN is legally listed as a quarantine pest in many countries, and protecting pines against PWN is recognized as an urgent problem for forestry [6].
PWN is transmitted to dead or dying trees during activity of oviposition or maturation feeding of vector beetles. For example, Monochamus is a beetle species of a genus that is a major vector for Bursaphelenchus sp. [7, 8]. Significant efforts have been devoted to researching PWD, including investigations of the phoretic relationship between PWN and its insect vector as well as methods for diagnosis on PWN. These include review papers that provide an overview of PWD, how host pine trees respond to PWN infection, the infection history of PWD, and how to prevent the spread of PWD. Globalization and climate change are also increasing the opportunity for further incursion and expansion of PWN around the world. As such, we now recognize the importance of PWD, and research on PWD should continue.
Damage and invasion by pine wilt disease
An understanding of pine wilt disease (PWD) necessitates understanding the history of the invasion process. PWD is caused by the pine wood nematode (PWN), Bursaphelenchus xylophilus, and it results in an annual loss of millions of pine trees [9, 10]. PWN is thought to have originated in North America, namely Canada and the USA, and the first occurrence of PWD that resulted in damage to the pine forests of Japan was reported in 1905 [11]. Also, PWD caused by PWN, B. xylophilus, was first identified in Japan. Since then, the disease has spread from Japan to neighboring East Asian countries of China, Taiwan and Korea in 1980s [12,13,14,15]. In Europe, it was first observed in Portugal in 1999, it was also detected in Nigeria and Mexico in 1990s, [16, 17] and it has since been found to cause PWD in Spain from 2008 (Fig. 1) [18, 19].
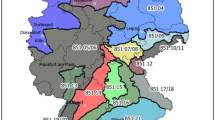
PWN, B. xylophilus, causes extensive damage to the pine forests of East Asian countries, specifically, Japan and Korea, because it occupies many pine species vulnerable to PWD. Since the introduction of PWD, the disease has led to a high mortality in pine trees in Japan for the past hundred years, and the annual loss of pine coverage has been more than 50 million m3 in 10 years from 2004 to 2014 (Fig. 2). To date, the total financial loss due to PWD in Japan has been estimated at 3.7 billion US dollars, assuming a market price of pine trees of US $100/m3 [20]. Pine trees infected with PWD were first discovered in Busan, Korea, in 1988. Due to strict control efforts during the period of severe invasion, the areas with major damage were limited to the southern regions in the twentieth century [21]. However, PWD has begun to gradually spread to the northern area of the country since 2010 as the average temperature has increased (Fig. 3). As a result, the number of pines infected by PWD has increased sharply since 2011, resulting in the largest losses of forest damage of 1.74 million pine trees in 2015. Since then, the number of pines infected with PWD decreased to less than half in 2019 due to intensive management. According to the Korean Forest Service, the total damage to forest products caused by PWD in the last decade is estimated to reach 8.4 billion won. In addition, environmental damages including loss of biodiversity and the cost of PWD control systems represent an even larger economic loss due to pine disease [22]. Moreover, even with quarantine efforts such as the restricted import of wood products to prevent PWN from reaching European countries, the disease has been found its way to Portugal and northern Spain. It has been predicted that by 2030, PWD could spread over 8–34% of Europe, and if PWN is not controlled, the cumulative value of lost forestry stock is estimated to reach € 22 billion [23, 24]. Therefore, PWD has become one of the most serious forest diseases in the world [25, 26].
The developmental cycle and transmission
Enormous efforts have been dedicated to studying PWD, including the pathological events after PWN infections and the phoretic relationship between the PWN and its vector beetles. These comprehensive research efforts have been able to determine the developmental cycle of PWD. The nematode developmental cycles shown in Fig. 4 illustrate how nematodes develop through four juvenile stages and reproduce within wood tissue, while food is available (which is called the propagative cycle). The first juvenile stage (J1) molts to the second juvenile stage (J2) in the egg. J2 hatches from the egg, and there are two more juvenile stages (J3 and J4) preceding the adult. Different types of juvenile stages appear under different conditions. In this life cycle, when the conditions are unfavorable and reach the dispersal cycle, PWN appears with the specialized third-stage juvenile (JIII). When the environment becomes dry or adverse because of nematode overpopulation, JIII survive at a higher ratio than other propagative forms because of their unique body structure. [27] Generally, this stage molts into the fourth-stage juvenile (JIV), which is transmitted by vector beetles to new trees [28,29,30,31]. However, in the absence of a suitable vector, the PWN population in the tree will ultimately die.
The developmental cycles of Bursaphelenchus xylophilus under two conditions. The solid arrows and dashed arrow appear the propagation cycle in pine trees and that for transmission to new host trees by insect vectors, respectively. The life cycle of PWN has juvenile stages of J1, J2, J3 and J4 under favorable conditions, and juvenile stages of JIII and JVI under unfavorable conditions [27]
The development of PWN is greatly influenced by temperature and seasonality [32]. From May through June, the JIV juveniles of the nematode invade the beetle's body. From early-June to late-July, the PWN infection of healthy pine trees occurs with adult beetles that have left dead pine trees [33, 34]. Under natural conditions, from mid-July, the population of propagative J3-stage juveniles rapidly increases and reaches a maximum level as the pine wilt disease became more advanced. In November and later, the PWN population in the dead tree declines, but the proportion of JIII juveniles in the population increases. During the winter and into the spring, the dispersal JIII-stage juveniles gather around the pupal chambers of the beetles. Thus, the transmission of PWD is associated with the pine host and insect vectors [35,36,37,38].
Since the nematode cannot spread over a long distance by itself, it needs an insect vector [39,40,41]. The JIII aggregate around the pupal chambers of the vector beetle. They molt to the JIV stage and then invade the insect vector’s body. A vector of the PWN, Monochamus beetles including Monochamus alternatus in Asia, M. carolinensis in the North America, and M. galloprovincialis in Southern Europe move from dead to healthy pines for prolonged feeding on young branches until their reproductive organs mature [42,43,44,45,46]. Then, JIV leave the beetle's body and invade new healthy trees. The Monochamus beetle is the most efficient vector for long distance transport of PWN. This results in PWN being transmitted to a new host tree or newly cut log by the beetle during oviposition, and then repeating the propagative cycle again.
Diagnosis method for PWN related to PWD
The economic loss in forests resulting from the invasion of PWN into new areas has highlighted a need for accurate diagnosis of this species to prevent further spread. Traditionally, the detection of PWN has been based on the morphological characteristics after their extraction from wood samples. The PWN has been identified according to three characteristics: (1) in the male, flattened spicules with a disc-like cucullus at the tip, (2) in the female, an anterior vulval lip with a distinct flap-like overlap, and (3) a tail or posterior end of the body of the females that is usually round [47]. However, this method of identification is time consuming and requires a high level of taxonomical expertise. Besides, the morphological identification of PWN can sometimes be difficult or impossible as the species of the genus Bursaphelenchus are similar in morphology [48]. Accordingly, the need for more sensitive and accurate methodologies has led to the development of molecular detection using several DNA-based protocols. Identification methods based on molecular biology require expensive equipment and reagents, but can be made to be simpler, faster and more reliable. Several PCR-based methods have been designed to detect PWN with species-specific probes by targeting either internal transcribed sequence (ITS), intergenic spacer (IGS) regions in the ribosomal DNA (rDNA), satellite DNA (satDNA), or restriction enzymes species-specific pattern (RFLP), and real-time PCR technology based on a heat shock protein gene (hsp70) [49,50,51,52,53]. More recently, morphologically similar Bursaphelenchus sp. could be detected using multiples RT-PCR capable of detecting multiple species simultaneously [54, 55]. Also, method for direct detection of PWN have been developed using loop-mediated isothermal amplification (LAMP) tests and PCR amplification of the species-specific Mspl satDNA [56, 57]. Therefore, to prevent the spread of PWN between countries and economic and biological forest loss, accurate techniques to detect and identify PWN are required by using appropriate morphological and molecular methods.
Control strategies for limitation of spreading of PWD
PWD causes significant ecological and economic losses in natural coniferous forests in Asia (especially in Japan, China, and Korea) and Southern Europe (especially Portugal). Accordingly, PWN is among the most important pests included in the quarantine lists of many countries around the world [58]. Due to this serious damage, many scientists have tried numerous methods to manage PWD. In order to protect pine trees from PWD, there are methods to (1) control the pine nematodes themselves, (2) control the insect vectors, and (3) increase the resistance to PWN. There are several ways to carry out these strategies [59, 60].
Physical control is a highly effective control method. Physical tactics such as felling, crushing and burning infected pine trees could be used for large-scale treatment. An advantage of crushing is that the sawdust and chips from the diseased trees can be used, but the product is expensive because it requires intensive labor and machines must move through the forests. Burning infected trees is the most efficient method to control PWN, but its use is restricted to periods when the forest fire risk is low. Also, heat from the fires can damage other pine trees [61]. Although treating infected trees or dead trees regarding the insect vector and PWN can be used to prevent the spread of PWD, it is not a method to control or treat PWD before PWN infection. Therefore, to prevent PWD infection, chemical control methods such as tree injections and spraying nematicides and insecticides are more realistic [62].
Chemical controls are also major strategies for eradication, and these have been used for prevention. To protect the pine tree from the pine wilt, there are methods to control the pine wood nematode and the insect vector. Recent researches have shown that bacteria associated with pinewood nematode (PWN) affect pine wilt. Phytotoxins secreted by bacteria associated with PWN might be involved in the pathogenesis of PWD by inducing damage plant cells [63]. To control the nematode, there are methods such as trunk injection of nematicides or spraying the ground, and the target of the insect vector can be controlled by spraying or fumigating insecticides. Some trunk-injection agents containing mesulfenfos, morantel tartrate, levamisole hydrochloride, abamectin or emamectin benzoate as the antinematodal ingredient can be applied to the pine tree trunk [64,65,66,67,68]. These agents are known to be directly effective against PWN and safe for the environment. However, avermectin has been reported to produce resistance in nematodes, although PWN has not yet been reported to show resistance [69, 70]. Controlling insect vectors could be a more efficient way to prevent the spread of PWD. This practical method can be applied to hard-to-reach places and can be applied to a wider area. To control the dispersal of vector beetles, one or several of prothiophos, fenitrothion, fenthion, pyridaphenthion, thiacloprid, or chlorpyrifos-methyl chemicals can be included as major components of the insecticides used for preventative spraying against vector beetles [71,72,73]. However, chemical insecticides have been recently recognized as harmful substances that cause environmental pollution and bioaccumulation, and their use has decreased [74]. Due to these environmental risks, demand for alternative control agents or biological controls with low or no environmental risks has been increasing [75, 76]. An example is bioactive substances derived from plants or natural products that have nematicidal activity against PWN. Plant essential oils or plant extracts are potential sources of bioactive chemicals as natural products for PWN control because these have few harmful effects on non-target organisms and the environment [77,78,79,80,81,82].
Since some of the above control strategies may cause problems in the ecosystem, environmentally friendly control methods have replaced the use of chemical agents. Biological control intends to control or manage PWD by using natural organisms such as predators, parasites, entomopathogenic microorganisms and fungi, and entomophilic nematodes. These can be aimed at the PWN, insect vectors, or their ectosymbiotic bacteria [83, 84]. For predators, the following insects were confirmed to feed on larvae of beetles. Predators such as Alaus berus (Coleoptera: Elateridae) and Anisolabis maritima (Dermaptera: Anisolabidiae) prey on M. alternatus larvae in pupal chambers, while Temnochila japonica (Coleopetra: Trogossitidae), Inocellia japonica (Neoroptera: Inoceliidae), and Thanasinus lewisi (Coleoptera: cleridae) are predators of M. alternatus larvae under the bark [85]. Several natural parasites have been found in M. alternatus. They are insect parasitoids such as Scleroderma sp. [86, 87] and Dastarcus helophoroides [88,89,90,91] and parasitic fungi including Beauveria bassiana [92,93,94], B. brongniartii and Metarhizium anisopliae [95,96,97]. Whereas Esteya vermicola (Ophiostomataceae) is the reported endoprarasitic fungus of the PWN, B. xylophilus [98,99,100,101]. Steinernema sp., a parasitic nematode, is used for biological control of vector beetles [102]. However, bio-control strategies require a long time to control the beetles and the PWN. Thus, it will be necessary to use a combination of biological and chemical controls in order to achieve the desired objective of rapid control.
Conclusion
PWD by infection of the pine wood nematode B. xylophilus is a great threat to forest ecosystems and industries worldwide. B. xylophilus is a casual agent of PWD in East Asia and Europe, causing severe economic and ecological damage through deforestation and increases in pest control and forest management costs to pine forests in the world. To prevent the spread of PWD, it is crucial to identify the mechanism, such as the transmission ecology or developmental life cycle. This can help not only to understand PWD, but also to provide useful information for strategic and tactical management. In addition, it is possible to quickly prevent the spread of disease through a quick and accurate diagnosis of PWD. The diagnosis has changed from a morphological method to a method based on molecular biology, and further, the research to properly using these two methods has been conducted for rapid direct detection. Meanwhile, it is essential to build proper PWD control schemes in East Asia and Europe, as well as worldwide. Physical and chemical methods to control PWD show a high ability to control and cause mortality in the vector beetles or PWN, but these have a negative impact on the environment. Thus, biological control methods are becoming important, and research on them should be actively conducted. We now know that PWD is fatal disease in pine forests and that it can induce disastrous damage. Therefore, if we want to maintain healthy pine forests worldwide, it necessary to take further interest in and conduct research on PWD.
References
Linit M (1988) Nemtaode-vector relationships in the pine wilt disease system. J Nematol 20:227
Kikuchi T et al (2011) Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog 7(9):e100219
Evans H, McNamara D, Braasch H, Chadoeuf J, Magnusson C (1996) Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull 26:199–249
Ikegami M, Jenkins TA (2018) Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model–Pine Wilt disease as a model case. For Ecol Manag 409:343–352
Burgermeister W et al (1999) First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1:727–734
Sutherland JR (2008) Pine wilt disease. Springer, Berlin, pp 13–17
Ikeda T, Oda K (1980) The occurrence of attractiveness for Monochamus alternatus Hope (Coleoptera: Cerambycidae) in nematode-infected pine trees. J Jpn For Soc 62:432–434
Zhao LL, Wei W, Kang L, Sun JH (2007) Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J Chem Ecol 33:1207–1216
Shinya R, Morisaka H, Takeuchi Y, Futai K, Ueda M (2013) Making headway in understanding pine wilt disease: What do we perceive in the postgenomic era? J Biosci Bioeng 116:1–8
Proença DN, Grass G, Morais PV (2017) Understanding pine wilt disease: roles of the pine endophytic bacteria and of the bacteria carried by the disease-causing pinewood nematode. MicrobiologyOpen 6:e00415
Mamiya Y (1988) History of pine wilt disease in Japan. J Nematol 20:219
Yi CK, Byun BH, Park JD, Yang S, Chang KH (1989) First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res Rep For Res Inst (Seoul) 38:141–149
Han H, Chung Y-J, Shin S-C (2008) First report of pine wilt disease on Pinus koraiensis in Korea. Plant Dis 92:1251–1251
Zhao BG (2008) Pine wilt disease. Springer, Berlin, pp 18–25
Fonseca L et al (2012) The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 49:96–103
Khan FA, Gbadegesin RA (1991) On the occurrence of nematode induced pine wilt disease in Nigeria. Pakistan J Nematol 9:57–58
Dwinell L (1993) First report of pinewood nematode (Bursaphelenchus xylophilus) in Mexico. Plant Dis 77(8):846A
Abelleira A, Picoaga A, Mansilla J, Aguin O (2011) Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis 95:776–776
Robertson L et al (2011) Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 13:755–757
Hirata A et al (2017) Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 12(8):e082837
Kwon T-S, Shin JH, Lim J-H, Kim Y-K, Lee EJ (2011) Management of pine wilt disease in Korea through preventative silvicultural control. For Ecol Manag 261:562–569
An H, Lee S, Cho SJ (2019) The effects of climate change on pine wilt disease in South Korea: challenges and prospects. Forests 10:486
Soliman T et al (2012) Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 7(9):e45505
Matsuhashi S et al (2020) Developing a point process model for ecological risk assessment of pine wilt disease at multiple scales. For Ecol Manag 463:118010
Vicente C, Espada M, Vieira P, Mota M (2012) Pine wilt disease: a threat to European forestry. Eur J Plant Pathol 133:89–99
Roques A, Zhao L, Sun J, Robinet C (2015) Pine wood nematode, pine wilt disease, vector beetle and pine tree: how a multiplayer system could reply to climate change. In: Björkman C, Niemelä P (Eds) Climate change and insect pests, pp 220–234
Kondo E, Ishibashi N (1978) Ultrastructural differences between the propagative and dispersal forms in pine wood nematode, Bursaphelenchus lignicolus, with reference to the survival. Appl Entomol Zool 13:1–11
Jones JT, Moens M, Mota M, Li H, Kikuchi T (2008) Bursaphelenchus xylophilus: opportunities in comparative genomics and molecular host–parasite interactions. Mol Plant Pathol 9:357–368
Ryss AY, Kulinich OA, Sutherland JR (2011) Pine wilt disease: a short review of worldwide research. For Stud China 13:132–138
Futai K (2013) Pine wood nematode, Bursaphelenchus xylophilus. Annu Rev Phytopathol 51:61–83
Zhao L, Mota M, Vieira P, Butcher RA, Sun J (2014) Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol 30:299–308
Melakeberhan H, Webster J, Rutherford T (1992) Influence of temperature on reproduction of Bursaphelenchus xylophilus and Pinus sylvestris mortality. Nematologica 38:80–87
Mamiya Y, Enda N (1972) Transmission of bursaphelenchus lignicolus (nematoda: Aphelenchoididae) by monochamus alternatus (coleoptera: Cerambycidae). Nematologica 18:159–162
McGawley EC, Winchell K, Jones J, Birchfield W, Berggren G (1985) Population development and influence of Bursaphelenchus xylophilus on Gliocladium virens. J Nematol 17:69
Fukushige H, Futai K (1987) Seasonal changes in Bursaphelenchus xylophilus populations and occurrence of fungi in Pinus thunbergii trees inoculated with the nematode. Jpn J Nematol 17:8–16
Sikora E, Malek R (1991) Influence of temperature on development of pine wilt in Scots pine. J Nematol 23:188
Dwinell LD (1997) The pinewood nematode: regulation and mitigation. Annu Rev Phytopathol 35:153–166
Mamiya Y, Kobayashi K, Hoshizaki K, Yoshida A, Ohta K (2018) Population densities of the pine wood nematode, Bursaphelenchus xylophilus, in dead pine trees caused by pine wilt disease in cool areas of Japan. Nematol Res (Jpn J Nematol) 48:63–70
Mamiya Y, Kiyohara T (1972) Description of Bursaphelenchus lignicolus n. sp. (Nematoda: Aphelenchoididae) from pine wood and histopathology of nematode-infested trees. Nematologica 18:120–124
Luzzi M, Wilkinson R, Tarjan A (1984) Transmission of the pinewood nematode, Bursaphelenchus xylophilus, to slash pine trees and log bolts by a cerambycid beetle, Monochamus titillator, in Florida. J Nematol 16:37
Naves P, Camacho S, De Sousa E, Quartau J (2007) Transmission of the pine wood nematode Bursaphelenchus xylophilus through feeding activity of Monochamus galloprovincialis (Col, Cerambycidae). J Appl Entomol 131:21–25
Linit M (1990) Transmission of pinewood nematode through feeding wounds of Monochamus carolinensis (Coleoptera: Cerambycidae). J Nematol 22:231
Warren JE, Linit M (1993) Effect of Monochamus carolinensis on the life history of the pinewood nematode, Bursaphelenchus xylophilus. J Nematol 25:703
Maehara N, Futai K (2002) Factors affecting the number of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) carried by several species of beetles. Nematology 4:653–658
Kwon T et al (2006) Distribution patterns of Monochamus alternatus and M. saltuarius (Coleoptera: Cerambycidae) in Korea. J Korean For Soc. 95:543
Akbulut S, Stamps W (2012) Insect vectors of the pinewood nematode: a review of the biology and ecology of Monochamus species. For Pathol 42:89–99
Islam MS, Bakker J (2014) Pinewood nematode bursaphelenchus xylophilus–biology and durable control
Shin SC et al (2004) Development of an efficient PCR-based diagnosis protocol for the identification of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). Nematology 6:279–285
Tarès S, Lemontey J-M, De Guiran G, Abad P (1994) Use of species-specific satellite DNA from Bursaphelenchus xylophilus as a diagnostic probe. Phytopathology 84:294–298
Iwahori H, Tsuda K, Kanzaki N, Izui K, Futai K (1998) PCR-RFLP and sequencing analysis of ribosomal DNA of Bursaphelenchus nematodes related to pine wilt disease. Fundam Appl Nematol 21:655–666
Iwahori H, Kanzaki N, Futai K (2000) A simple, polymerase chain reaction-restriction fragment length polymorphism-aided diagnosis method for pine wilt disease. For Pathol 30:157–164
Cao A, Liu X, Zhu S, Lu B (2005) Detection of the pinewood nematode, Bursaphelenchus xylophilus, using a real-time polymerase chain reaction assay. Phytopathology 95:566–571
Castagnone C, Abad P, Castagnone-Sereno P (2005) Satellite DNA-based species-specific identification of single individuals of the pinewood nematode Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). Eur J Plant Pathol 112:191–193
Green M, Rott M, Leal I, Humble L, Allen E (2007) Application of a real-time PCR method for the detection of pine wood nematode, Bursaphelenchus xylophilus, in wood samples from lodgepole pine. Nematology 9:351–362
Filipiak A, Wieczorek P (2019) Tomalak M (2019) A fast and sensitive multiplex real-time PCR assay for simultaneous identification of Bursaphelenchus xylophilus, B mucronatus and B fraudulentus–three closely related species from the xylophilus group. Eur J Plant Pathol 155:239–251
Hu Y et al (2011) Direct PCR-based method for detecting Bursaphelenchus xylophilus, the pine wood nematode in wood tissue of Pinus massoniana. Forest Pathol 41:165–168
Cardoso JM, Fonseca L, Abrantes I (2012) Direct molecular detection of the pinewood nematode, Bursaphelenchus xylophilus, from pine wood, bark and insect vector. Eur J Plant Pathol 133:419–425
Mota MM, Futai K, Vieira P (2009) Integrated management of fruit crops nematodes. Springer, Berlin, pp 253–274
Kamata N (2008) Pine wilt disease. Springer, Berlin, pp 304–322
Xu F et al (2008) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Berlin, pp 379–388
Shin S-C (2008) Pine wilt diseas. Springer, Berlin, pp 26–32
Shin WS et al (2015) Development of effective screening method for efficacy test of trunk injection agents against pine wood nematode, Bersaphelenchus xylophilus in Japanese Black Pine, Pinus thunbergii. Korean J Pestic Sci 19:440–449
Zhang L et al (2012) Flagellin promotes propagation of pine wood nematode and its carrying Pseudomonas fluorescens GcM5-1A in callus of Pinus thunbergii through inducing cell death. Afr J Microbiol Res 6:1322–1328
Viglierchio D, Maggenti AR, Schmittt R, Paxman G (1977) Nematicidal injection: targeted control of plant-parasitic nematodes of trees and vines. J Nematol 9:307
Takai K, Soejima T, Suzuki T, Kawazu K (2000) Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag Sci 56:937–941
Takai K, Suzuki T, Kawazu K (2003) Development and preventative effect against pine wilt disease of a novel liquid formulation of emamectin benzoate. Pest Manag Sci 59:365–370
James R, Tisserat N, Todd T (2006) Prevention of pine wilt of scots pine (Pinus sylvestris) with systemic abamectin injections. Arboric Urban For 32:195
Shanmugam G, Lee SK, Jeon J (2018) Identification of potential nematicidal compounds against the pine wood nematode, Bursaphelenchus xylophilus through an In Silico approach. Molecules 23:1828
Scott J, Roush RT, Liu N (1991) Selection of high-level abamectin resistance from field-collected house flies, Musca domestica. Experientia 47:288–291
Gopal R, Pomroy W, West D (1999) Resistance of field isolates of Trichostrongylus colubriformis and Ostertagia circumcincta to ivermectin. Int J Parasitol 29:781–786
Lee S et al (2003) Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. J Korean For Soc 3(92):191–198
Han J-H et al International symposium on mites & whitefly, vol 2, pp 59–59
Xu F (2008) Pine wilt disease. Springer, Berlin, pp 323–333
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Park I-K, Kim J, Lee S-G, Shin S-C (2007) Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J Nematol 39:275
Lee CM et al (2015) Nematicidal and reproduction supression activity of actinomyces isolates against pine wood nematode, Bursaphelenchus xylophilus. Korean J Pesticide Sci 19:141–150
Prakash A, Rao J (1996) Botanical pesticides in agriculture. CRC Press, Boca Raton
Isman M in International symposium on development of natural pesticides from forest resources. 1–9 (Korea Forest Research Institute Seoul).
Chitwood DJ (2002) Phytochemical based strategies for nematode control. Annu Rev Phytopathol 40:221–249
Lee D, Lee S, Lee S, Choo H (2010) Nematicidal activity of some herval extracts against the pine wood nematode, Bursaphelenchus xylophilus. Korean J Soil Zool 14:43–49
Shin JH et al (2016) Nematicidal activity of Eclipta prostrata extract and terthiophene against pine wood nematode, Bursaphelenchus xylophilus. Korean J Pestic Sci 20:56–65
Pavaraj M, Bakavathiappan G, Baskaran S (2012) Evaluation of some plant extracts for their nematicidal properties against root-knot nematode, Meloidogyne incognita. J Biopestic 5:106
Sousa E, Rodrigues J, Bonifácio L, Naves P, Rodrigues A (2011) Management and control of the Pine Wood Nematode, Bursaphelenchus xylophilus, in Portugal. Nematodes: morphology, functions and management strategies
Yang Z-Q, Wang X-Y, Zhang Y-N (2014) Recent advances in biological control of important native and invasive forest pests in China. Biol Control 68:117–128
Shimazu M (2008) Pine wilt disease 351–370 (Springer)
Xu F (1998) Advances in the research in the natural enemy of Monochamus alternatus in the world. World For Res 11:41–45
Xu K et al (2002) The techniques of Scleroderma guani Xiao et Wu to control pine sawyer beetles. J Nanjing For Univ 26:48–52
Inoue E (1993) Dastarcus longulus, a natural enemy of the Japanese pine sawyer. For Pests 42:171–175
Ogura N, Tabata K, Wang W (1999) Rearing of the colydiid beetle predator, Dastarcus helophoroides, on artificial diet. Biocontrol 44:291–299
Huang H et al (2003) Dastarcus helophoroides—a natural enemy of Monochamus alternatus. J Guandong For Sci Technol 3:11–15
Li X-J, Dong G-P, Fang J-M, Liu H-J, Guo W-L (2018) Parasitic behaviour of Dastarcus helophoroides (Fairmaire)(Coleoptera: Bothrideridae) induced changes in free animo acid pools in hemolymph of host Monochamus alternatus Hope (Coleoptera: Cerambycidae). Oriental Insects 52:329–341
Shimazu M, Kushida T, Tsuchiya D, Mitsuhashi W (1992) Microbial control of Monochamus alternatus Hope (Coleoptera: Cerambycidae) by implanting wheat-bran pellets with Beauveria bassiana in infested tree trunks. J Jpn For Soc 74:325–330
Maehara N, Kanzaki N (2014) Effect of aging in adult Monochamus alternatus (Coleoptera: Cerambycidae) on the susceptibility of the beetle to Beauveria bassiana (Ascomycota: Hypocreales). J For Res 19:357–360
Álvarez-Baz G, Fernández-Bravo M, Pajares J, Quesada-Moraga E (2015) Potential of native Beauveria pseudobassiana strain for biological control of Pine Wood Nematode vector Monochamus galloprovincialis. J Invertebr Pathol 132:48–56
Higuchi T et al (1997) Development of biorational pest control formulation against longicorn beetles using a fungus, Beauveria brongniartii (Sacc.) Petch. J Ferment Bioeng 84:236–243
Ansari M, Shah F, Tirry L, Moens M (2006) Field trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biol Control 39:453–459
Shang Y, Feng P, Wang C (2015) Fungi that infect insects: altering host behavior and beyond. PLoS Pathog 11:100537
Liou J, Shih J, Tzean S (1999) Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol Res 103:242–248
Wang CY et al (2008) High infectivity of an endoparasitic fungus strain, Esteya vermicola, against nematodes. J Microbiol 46:380
Wang CY et al (2011) Biological control of the pinewood nematode Bursaphelenchus xylophilus by application of the endoparasitic fungus Esteya vermicola. Biocontrol 56:91–100
Wang CY et al (2018) Using the nematophagous fungus Esteya vermicola to control the disastrous pine wilt disease. Biocontrol Sci Tech 28:268–277
Phan LK (2008) Pine wilt disease. Springer, Berlin, pp 371–379
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2020R1A6A1A06046235)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Bit-Na Kim, Ji Hun Kim, Ji-Young Ahn, Sunchang Kim, Byung-Kwan Cho, Yang-Hoon Kim, and Jiho Min declares that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kim, BN., Kim, J.H., Ahn, JY. et al. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 12, 297–304 (2020). https://doi.org/10.1007/s13530-020-00068-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-020-00068-0