Abstract
Spinal cord injury is a disease difficult to restore that function more than 2.5 million people worldwide. How to improve the prognosis of spinal cord injury is a matter concerned by clinicians and scientists. Complex pathophysiological processes after spinal cord injury are the important reason to hinder restore. To clarify its mechanism can provide ideas for clinical treatment. Neurotrophic factors , biological engineering and Chinese medicine have been applied to adjust the microenvironment after spinal cord injury. Transplanted cell can replace cells which were damaged and apoptosis in a certain extent, while secretion of a variety of nutritional factors improve the extracellular environment. Combined different therapy method can produce a synergic effect and improve the prognosis of spinal cord injury.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Spinal cord injury
- Regenerative repair
- Neurotrophic factors
- Cellular transplantation
- Traditional Chinese medicine
- Biological engineering
1 Introduction
People have realized for a long time that some symptoms, such as the partial or complete loss of sensory and motor function, chronic pain, spasticity and incontinence of defecation and so on, which can be caused by the spinal cord injury (SCI) [1]. Scientists all over the world are keen to find the solution to the problem of SCI patients which is still a worldwide challenge at present, because SCI may bring serious threat to the patient’s health and life, and cause heavy burden on the society and family. Spinal cord injuries can be subdivided into traumatic injuries (including traffic accidents, being struck by falling objects, crushing injuries, violence and sports-related injuries) and non-traumatic injuries (including inflammation, tumors, ossification, degenerative damage and vascular injury). About 73.7 % of the SCI was traumatic injury [2]. In order to increase the recovery degree of SCI, many treatment methods are developed. The defects of various methods have been constantly fixed. This article mainly discusses the research progress of the traumatic SCI and its regeneration repair.
2 Pathophysiological Changes of SCI
A series of pathophysiological changes after SCI is complex cascade reactions that can be divided into primary injury and secondary injury. At the instant of the injury, mechanical power of the external force or fracture causes the direct ruin of neurons and endothelial cells. The damage of cells and tissue necrosis produced in a very short time. The damaged cells and tissues will activate some complex molecular and cellular mechanisms and evolved into secondary injury and brought greater harm [3].
2.1 Primary Injury
In the primary injury, mechanical power direct shear membrane of nerve cells and endothelial cells. Due to the gray region is soft and rich in blood vessels, hemorrhage and necrosis will appear in these areas for the first time. In the central area of the spinal cord, tissue dislocation will lead to hemorrhage and the ruin of the neuron membrane and connective tissue after injury. The peripheral area of the spinal cord, tissue dislocation is minimal; axons near the spinal dura mater usually can survive. On the contrary, axon near the gray matter will be severely damaged after SCI [3, 4]. Therefore, as the damage continued, some special molecular and cellular mechanisms will be activated, and further evolved into the secondary injury.
2.2 Secondary Injury
In the secondary injury stage, damaged cells dysfunction of the reuptake of glutamate. A large number of glutamates will enter into the extracellular by exocytosis and cell lysis to over stimulate ionic glutamate receptors. Excitatory neurotoxicity will be required to lead to cell death [5]. Ischemia-reperfusion injury is another reason to cause cell death . The reactive oxygen species (ROS) and calcium overload produced by ischemia-reperfusion is directly related to the neuron apoptosis [6]. Wallerian degeneration is an important feature of the nervous system injury. It occurs in the axon stump distal to the site of injury and usually begins within 24–36 h after injury. The axonal skeleton of stump distal disintegrates, and the axonal membrane breaks apart. In the central nervous system (CNS), myelin sheath is constituted by oligodendrocytes. Unlike Schwann cells (SCs), oligodendrocytes are unable to clean up the myelin sheaths and their debris after Wallerian degeneration. Oligodendrocytes also can’t recruit macrophages for debris removal. Microglia plays a critical role in the central nervous system for debris removal but their recruitment and clearance is far slower than macrophage [7]. During this period, inflammatory cells begin to enter the ruin area and inflammation expands the extent of damage. So Wallerian degeneration can’t provide enough help for repair of SCI. The glial scar can limit inflammation but it is another important factor to further hinder chances for axonal regeneration and neuronal reinnervation. Early scar is comprised of oligodendrocytes fragments and myelin sheath which allow the axonal regeneration. Then meningeal cells from the surface of the CNS and precursor cells with multi-differentiating potential from the central tube migrate into the glial scar. Eventually astrocytes are seeping into the cavity cause by the primary injury to form a large amount of the glial scar. The glial scar and the surrounding environment are met with a variety of molecules called neurite growth inhibitor which can interrupt the growth cone of damaged neurons to disintegrate. To prevent the final scar formation is one of the essential parts to repair SCI [8].
Large number of experimental studies have shown that promoted regeneration after spinal cord injury SCI treatment and functional rehabilitation is the key. Factors limiting the central axonal growth or regeneration after spinal cord injury can be divided into two categories: First, the lack of neurotrophic factors and matrix components that guide and support nerve growth; Second, cysts and scar tissue resulting spinal cord stump and some inhibitory growth factor hinder and inhibit axonal regeneration. In recent years, research carried out in the treatment of spinal cord injury is mainly around these two aspects.
3 Using Neurotrophic Factor Treatment for SCI
Neurotrophic factors (NTFs) are a family of proteins that are responsible for the growth and survival of developing neurons and the maintenance of mature neurons. It includes Nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophins including neurotrophin-3 (NT-3) and neurotrophin-4/5 (NTF-4/5), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) and so on. The receptor of NTFs including Tyrosine receptor kinase (Trk) family and P75 receptor family. Trk receptor family has high affinity and high selectivity to the NTFs when P75 just opposite. Trk receptor mediated NTFs positive signal pathway of neurons. When P75 receptors coexpression with Trk, they will improve the affinity and selectivity of Trk to NTFs and promote the function of Trk receptor at the same time. When the Trk inactivation or low energy, p75 will mediate apoptosis [9].
3.1 Endogenous Neurotrophic Factors
NTFs and its receptors exist widely in the spinal cord. NTFs levels are low in the spinal cord except NT-3 is higher in the early development of the spinal cord [10]. Nerve growth factor is highly expressed in dorsal root ganglia after SCI [11]. Geng et al. [12] use enzymes linked immunosorbent assay (ELISA) to measure BDNF at 24 h after SCI which appeared incremental expression, returned to normal level after 28 days, has the time correlation. TrkC protein appears a downward trend within 7 days after spinal cord transection in the rat, began to rise after 7 days, rising speed at 14days is the most obvious which is consistent with the TrkC mRNA expression curve [13]. Unlike Trk, the expression change of P75 receptor mRNA is fluctuant after SCI, and positively correlated with neurons apoptosis. This fluctuation changes associated with the neurons apoptosis have significant relationship with regulation and repair after SCI [14]. So that NTFs and its receptor increases with time correlation after SCI, and it has different degree function to promote repair of the spinal cord. But due to its increase is limited, and cannot maintain the effective concentration for a long time, they limited to promoting the neural repair and functional recovery. Therefore, many scholars tried to utilize exogenous NTFs to further enhance the repair of SCI.
3.2 Exogenous Neurotrophic Factors
Exogenous neurotrophic factors can reach the spinal cord by injecting into the vein, abdominal cavity, and muscle and subcutaneous tissue. But as NTFs concentrations decrease quickly, and difficult to enter into the blood-spinal cord and brain barrier by this administration route, NTFs eventually reached the injured area are extremely rare and can’t display the neural protective effect. NTFs can also be directly injected into the injured area, subarachnoid etc. Damaged area local drug delivery is advantageous to the formation of high concentration in the location, be helpful for neuronal survival. But the half-life of NTFs is short [15], so can’t maintain adequate concentration at a long time. Demand for NTFs after SCI has time correlation. The biological characteristics of the short half-life of NTFs lead to that single NTFs have been impossible to meet the needs of the repair of SCI. Although exogenous neurotrophic factor has a very good effect in inhibiting apoptosis of neurons, but each kind of NTF effect has a limit, and single neurotrophic factor is not sufficient to help neuron axons completing reconstruction.
3.3 Multiple Factors Combination Therapy
To meet the needs of the repair of SCI, the combined use of different NTFs or with other small molecules to repair SCI is a research hotspot in recent years. Methylprednisolone was believed to have nerve protective effect, but recently the research of Aomar et al. [16] think it has no improvement for neurological symptoms. However, Kim and Jahng [17] used BDNF and methylprednisolone for combined treatment of SCI which detected myelin regeneration, accorded with the results of immunohistochemical, and confirmed the combination therapy is possible and effective. Arvanian et al. [18] used NTF-3 and lysergic acid diethylamide (LSD) to combine treatment rats with hemisected SCI and found that compared to control group with the simple use of NTF-3 or LSD, combination group had earlier behavioral recovery time and better effect. Lee et al. [19]. reported that NTF-3 and thermo stabilized chondroitinase ABC (ChABC) combined use to treat the rats with SCI, the effect to restrain glycosaminoglycan’s side chain, the important component of glial scar is 3 times as monotherapy. Sharma [20] found after application of BDNF with high concentration in combination with GDNF after SCI, the damage of blood spinal cord barrier has been inhibited and edema also be alleviated. Thus the recovery of motor function has obviously improved. Nevertheless, some of the combined treatment effect can’t play the corresponding function even appear antagonism. Lang et al. [21] found that BDNF and CNTF combined treatment in the nerve root avulsion model showed no synergy. Donnelly et al. [22] also found small interference RNA and NTF-3 combination therapies with SCI did not show a better therapeutic effect. The synergy of NTFs to enhance the repair of SCI, but how to improve NTFs and additional small molecule combination plan needs further research.
3.4 Cooperate with Stem Cells
In addition to promoting the growth of axons, NTF can promote stem cell proliferation and differentiation. Therefore, NTFs can be followed in the stem cell transplants for the treatment of SCI. Tang et al. [23] developed NT-3-immobilized scaffolds which can sustain release of bioactive NT-3 to help with neural stem cell transplantation. And they observed the rate of survival and differentiation of stem cells had been strengthened considerably. Besides, for the half-life of NTFs is too short to maintain function for a long time, we can transfer NTFs gene to neural stem cells. So these neural stems cells will keep sustained secretion of NTFs to maintain the survival and the growth. I’ll consider this method later in the article.
3.5 Load in Biological Materials
To add NTFs into the biological materials and use the characteristics of biological materials to control the release of NTFs is a great method to control and guide the growth and differentiation of neuron, and help axon directional growth, reconstruct the original structure, and realize the functional recovery. See below for biological scaffold.
3.6 Neurotrophic Factor Receptor
Wang et al. [24] constructed a kind of immunoglobulin called p75NTR-ED-Fc for human p75 neurotrophic factor receptor (NTR), which can suppress the effect of p75NTR for improving the axonal regeneration and functional recovery after spinal cord injury. Research showed inhibition of p75 receptor, the spinal cord function has a certain degree of recovery while the axon length is actually increased. Figure 1 showed the function recovery after the hp75NTR-ED-Fc fusion protein therapy (Fig. 1).
Treated with hp75NTR-ED-Fc fusion protein promoted functional recovery after SCI. a The BBB scores in hp75NTR-ED-Fc-treated rats were consistently higher than those in SCI group. b Footprint analysis showed that the prints of all hind toes in hp75NTR-ED-Fc-treated rats were very visible, while in SCI rats, they were not clearly separated which indicated the signs of toe dragging. Fore paw footprints (red) and hind paw footprints (blue) from rats tested. “d” shows the vertical distance between the hind paws. c The distance of the base of support between the hind paws in hp75NTR-ED-Fc-treated rats was significantly reduced compared with SCI rats. *p < 0.05, **p < 0.01, ***p < 0.0001, compared with SCI group. (Reprint with permission from the article of Wang et al. [24])
4 Tissue Engineering
Although above-mentioned methods have made some important progress. However, there are still cannot achieve the structure and function of the damaged spinal cord repair completely. The main problems are: (1) the number of neuron rescues by various means is limited and no extensive growth potential of axonal fiber will give full play to. (2) It is very awkward that renewable fiber long distance search for the “target” and establish the functional synaptic. (3) Functional remodeling is needed for the regenerative repair of nervous structure to show the effective function. With the deepening of the research, people gradually realize that increase the number of regenerated fiber and promote synaptic connections and structure and function remodeling between renewable fiber and the “target” is essential to repair spinal cord injury. Nonetheless, in recent years, the rise of tissue engineering research offers hope for it.
4.1 Biological Scaffolds
A large number of cells necrosis and apoptosis after SCI lead to the formation of a spinal cord defect [25]. Survival neurons can’t meet the needs of rebuilding of neural pathways. And application of biological engineering technology to build the bridge can be attached on both ends of the injured spinal cord, lead to rebuilding orientation structure to targets axon and guiding axon directional growth. So as to realize the spinal cord regeneration and reconstruction of almost normal physiological structure [26]. Biological scaffold can be divided into natural biological material scaffold and synthetic biological material scaffold according to the source, and can be divided into the Hydrogel biological scaffold, spongy scaffold, tubular biological scaffold and membrane biological scaffold according to the form. The advantages of natural biological scaffold whose materials usually are saccharide and protein are good biocompatibility, biodegradable, degradation products can be absorbed and usually can’t produce inflammation. The main disadvantages of them are that the mechanical properties are poor and degradation process is not easy to be controlled. Agarose is linear polysaccharide which extracted from seaweed and curdlan. It is widely used drugs and macromolecular carrier. Above mentioned Lee et al. [19] used agarose contained NT-3 as a scaffold, to improve the thermostable ChABC local sustained delivery, and found the axonal regeneration and the motor function recovery in rats with SCI. The mechanism may be sufficient NT-3 and ChABC can promote new axons across the glial scar and reach its remote control targets [27]. Alginate is a linear polysaccharide produced by brown algae, are commonly used in spinal cord defect. Shahriari et al. [28] implants calcium alginate hydrogel in a rat model of spinal cord hemisection. And they use Fourier-transform infrared (FT-IR) spectroscopy to measure the stability of the implants and the effects on morphology and biochemistry of the injured tissue. The result shows that non-functionalized low-gelation soft Ca2+-alginate hydrogel has an enhanced long-term stability in vivo and help spinal cord repair by limiting demyelination and reducing scar. Grulova et al. [29] used the affinity-binding alginate scaffold with sustained delivery of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) to treat rats with spinal cord crushing and observed the recovery of function of rats. But commercial alginate products contain a large number of toxic materials so it should be super purified before use. And alginate degradation products also have slight cytotoxicity. Chitosan which also is called soluble chitin is obtained by deacetylation of chitin, the structure of which is similar to the plant fiber. Chitosan has excellent film-forming property and permeability, no or low immunogenicity. Yang et al. [30] found chitosan loaded with NT-3 can slow release of this neurotrophic factor to make an optimal microenvironment for regeneration. And they confirmed sensory and motor functional restorations in rats with SCI treated by this way. The drawback of chitosan scaffold is that the mechanical strength is negatively related to the biodegradability. So chitosan with superior mechanical strength will be difficult to degrade and can’t adapt the speed of tissue rebuilding. Self-assembling peptide (sapeptide) is a kind of emerging material consists of repeated sequences of short chains of amino acids, belongs to nano-scale hydrogel biological scaffold materials, with similar characteristics of the extracellular matrix. The advantage of self-assembling peptide scaffold is that can be injected in the damage area, to prevent iatrogenic injury. Its disadvantage is too sensitive to change of temperature and PH, so it is necessary for practical application to closely monitor and adjust the local microenvironment [31]. In China, Hou et al. [32] seeded the neural stem cells or motor neurons in the sapeptides scaffolds and found the sapeptides with motor neurons have a better effect for functional recovery after SCI in rats.
Sun et al. [33] tried to use the functional motifs containing cell adhesion peptide RGD and neurite outgrowth peptide IKVAV to change this weakness and had a good effect. Compared to the natural biological scaffolds, synthetic biological scaffold both have superior performance of biology, mechanics and material science, also has a unique degradation controllability at the same time. Its main drawback is that the concentration release of monomer produced from degradation often cause the immune reaction. Polycaprolactone which is a kind of aliphatic polyester with good biocompatibility and degradation performance, is widely used in many medical products, including wound dressings. Silva et al. [34] use a blend of starch with polycaprolactone to product a 3D scaffold. And they found that the scaffold can quickly restore spinal stability of rats with a T8-T9 spinal hemisection. Poly (lactic-co-glycolic acid) (PLGA) is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Depending on the ratio of lactide to glycoside used for the polymerization, different forms of PLGA can be obtained. It can be bending, expansion, deformation and changed of permeability by this way. Wen et al. [35] adopt scaffold which is modified by PLGA binding with an anti-Nogo receptor antibody containing BDNF and vascular endothelial growth factor (VEGF) to implant into the injured area created by a dorsal hemisection at T9-10 of the spinal cord in rats. And they observed the inhibition of inflammation and gliosis, and large numbers of new blood vessels and regenerated nerve fibers around the implants. There are some other synthetic biological scaffolds have also made very good progress, but to date the United States Food and Drug Administration (FDA) has not yet approved its wide application in the clinical.
5 Genetic Engineering and Protein Engineering
Genetic engineering and protein engineering both is an important branch of biological engineering. Genetic engineering which is also called gene splicing technology and recombinant DNA technology is a complex technology to manipulate gene at the molecular level and import the restructured exogenous gene to recipient cells for make the gene can be expressed, replication, transcription, translation in recipient cells. Protein engineering is the process of promoting useful or valuable proteins. In protein engineering, scientist uses detailed knowledge of the structure and function of the protein to ensure that desired changes. Genetic engineering is used to transfect the neural stem cells (NSCs) by neurotrophic factor gene, and make the cells can continue secrete NTFs to promote the repair of spinal cord. Protein expression carrier construction also is required to use genetic engineering. After SCI, apply recombinant proteins and monoclonal antibody target to inflammatory mediators maybe significantly reduce inflammation, improve the possibility of recovery of SCI [36]. Yune et al. [37] produced a fusion protein PEP-1–SOD1 by fusing a human SOD1 gene with PEP-1 in a bacterial expression vector. They confirmed this fusion protein has antioxidant agents that can protect neurons from ischemia reperfusion injury. With the progress of protein engineering, human knowledge of protein structure is more and more. A growing number of recombinant proteins will be utilized in SCI, help to get better curative effect in the treatment of SCI. The use of genetic engineering and protein engineering to construct a vaccine is also a hot spot of research. Mentioned above the Wang et al. [24] using genetic engineering to construct the p75 receptor immunoglobulin for promoting axonal regeneration and functional recovery by inhibiting p75 receptor. Vaccination also can be constructed for further inhibit factor, example Nogo-66 receptor (NgR) which a common receptor for three myelin associated inhibitors mediates their inhibitory activities on neurite outgrowth in the adult mammalian CNS. Wang et al. [38] uses 15 nm gold nanoparticles (GNPs) which can boost the immunogenicity of human NgR-Fc (hNgR-Fc) protein vaccine effectively, to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. We can also transfer some gene which is helpful for protecting the injured spinal cord into cells that can be transplanted. Zhang et al. [39] used rubrospinal neurons transferred by adenoviral cardiotrophin-1 (CT-1) gene to transplant into spinal cord with a shallow incision which was made on the left dorsal. They have demonstrated that adenoviral CT-1 gene transfer promoted the survival and regeneration of rubrospinal neurons and enhanced the partial functional recovery of forelimb usage after cervical spinal cord injury in adult rats.
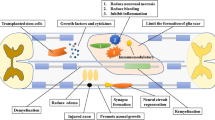
In conclusion, the main mechanisms of tissue engineering used to repair spinal cord injury and paraplegia recovery include: (1) Filling defect of the organizational structure: after spinal cord injury, due to the primary and secondary injury caused local tissue necrosis at the injury site. If not removed the necrotic tissue, it is easy to form scar tissue to impact new tissue ingrowth, is not conducive to the functional recovery. If cut it, the local tissue will form defects and adjacent tissues will collapse, that destroy the original anatomy. Therefore, researchers fill cells, nerve tissue and a variety of materials at the defect. (2) Create a suitable microenvironment: primary and secondary damage caused by a large number of inflammatory cells infiltration around the injury site and release large amounts of inflammatory factors after SCI, which caused the change of microenvironment is not conducive to the cells survival and axons growth or extend. Also, because a large number of cell necrosis, trophic factors secreted by the remaining cells cannot meet the needs of tissue regeneration. Therefore, transplant exogenous cells transfered by genetically engineered can produce large amounts of cytokines, in favor of the axon extension and regeneration. Scaffold material itself can also carry nutrition factor and blocking inflammatory cells infiltration, further create a suitable microenvironment. Material also can separate transplant cell from residual myelin and surrounding glial cells. In the process of material degradation, it provides a time window for the extension of new axons reduce the inhibition of surrounding cells or factors (3) a greater degree of repair organizational structure after SCI: mainly through the orderly proliferation, growth, migration and differentiation of seed cells on a biological scaffold with certain rules and the internal arrangement of the network structure, and try to restore the connection of the damaged spinal cord fiber and the corresponding neuronal network links. (4) Remodeling of damaged spinal cord functional linkages: the damage of spinal cord leads to tissue disintegration and apoptosis at injury site. The functions of the corresponding structure also will be destroyed or lost. Tissue engineering can fill the defect that formed by spinal cord injury and also can create a suitable microenvironment for the regeneration of their own or transplant tissue or cell. However, it is still not clear whether the functional regenerating organization ultimately can achieve partial or complete replacement for the original function. Remodeling functional structure mainly to establish a complete anatomical structures, remain intact nerve fibers communicating and ensure synaptic activity. Restoration of function mainly includes the improvement of incoming and outgoing signals of damaged spinal cord and the function of preliminary integration information. In addition to axoplasmic transport function of neurons and axons, etc. Spinal cord tissue engineering is still considered one of the most challenging therapeutic strategies to repair injured spinal structure.
6 Cellular Transplantation
SCI in addition to resulting in a large number of axons damaged, also will lead to a large number of cells death. The research of Liu et al. [40] showed that endogenous neural stem cells increased significantly at the injury site after mild spinal cord injury, and the endogenous neural stem cells in central canal of spinal cord can differentiated into different type of neural cells of adult rats. Proliferative activity of endogenous NSCs of spinal cord after injury was examined via DAPI staining as shown in Fig. 2. But they differentiate into astrocytes more than neurons, which may be associated with high expression of inhibitory Notch1 and Hes1 genes after injury (Fig. 2).
Proliferation of endogenous neural stem cells in central canal of T10 level after injury. a DAPI labeling endogenous neural stem cells of spinal cord 1 day after injury. b DAPI labeling endogenous neural stem cells of spinal cord 3 days after injury. c DAPI labeling endogenous neural stem cells of spinal cord 7 days after injury. d DAPI labeling endogenous neural stem cells of spinal cord 14 days after injury. * p < 0.05 indicated statistical significance compared with 1 day post-injury. # p < 0.05 indicated statistical significance compared with 3 days post-injury. & p < 0.05 indicated statistical significance compared with 7 days post-injury. Data represent mean ± SD of five independent samples in each group. [Reprint from the article of Liu et al. [40]
Because of the endogenous neural stem cells proliferation and differentiation slowly, we can transplant exogenous cells to help repair damaged parts. Mechanisms of cellular transplantation to repair SCI are not completely understood, but it can be summarized as the following aspects. (1) Through secreting NTFs to protect the remaining neurons. (2) To differentiate into neurons, oligodendrocytes and astrocytes, promote axonal regeneration and myelination under the specific environment of the spinal cord. (3) Improve the local microenvironment after SCI to reduce inflammatory reactions. (4) Promote cells to produce a variety of extracellular matrix, filled with gap in the spinal cord, is compatible with the axon regeneration [41, 42].
6.1 Transplantation Method
There are many ways to stem cell transplantation and basically has the following four. (1) Orthotropic transplantation on the injury site: Transplant stem cells directly into the damaged area around through surgery, can promote the improvement of the nerve cell function and recovery. However, to grasp the operation time is difficult, especially in emergency surgery. (2) Transplantation by lumbar puncture: this method which can choose the appropriate time for stem cell transplantation is injected stem cells into the cerebrospinal fluid through vertebral puncture, and stem cells will migrate to the injury site and repair the damaged nerve cells. This method is simple, easy to follow and has good repeatability. (3) Intravenous transplantation: inject stem cell which via proliferated and differentiated in vitro into the vein. And stem cell will be along with the circulation of the blood through the blood spinal cord barrier to reach the lesion site. (4) Intra-arterial transplantation: inject stem cell through arterial. The study of Amemori et al. [43] found that compared with implanted into the subarachnoid space, implanted stem cell into the lesion center can positive effect on the expression of endogenous neurotrophic and better to increase gray and white matter survival, axonal sprouting and reduced astrogliosis.
6.2 Transplantation Time
After SCI, gray and white matter immediately occur necrosis, spinal cord is in congestion and/or ischemia and edema are also found. It will reach its peak within 2–4 h, and extensive necrosis will appear in a few days in the injured spinal cord. However, after 12–14 days, injured spinal cord will be under a cyst. Glial cells will proliferate to form scars. That will severely harm the integrity of the spinal cord and blocks nerve cells regeneration and crossing. So stem cells need to be transplanted before that happens. But in the acute phase of SCI, a large number neurotoxic inflammatory factors produced in acute inflammation reaction, often cause the transplanted stem cells by death and/or differentiate into glial cells. This phase will last approximately 7–9 days [44]. After the acute phase, the nerve tissue become into the repair phase, which is conducive to the survival and differentiation of transplanted cells. So it advocates the delayed transplantation, namely transplantation after the acute phase. Research indicates that 1–2 weeks after SCI is the window phase for cell transplantation, and transplantation can achieve the best effect in this stage [41].
6.3 Types of Transplant Cells
There are a lot of cells can be used for cell transplantation. Different cells have unique effects. The following are the main types of the source of the transplanted cells.
Embryonic stem cells (ESCs) are derived from a highly undifferentiated cells in the blastocyst. ESCs are highly totipotent that can be differentiation for a variety of cells. ESCs can adjust the external environment for nerve cell growth and increase the survival rate of nerve cells. The main mechanism is inducing neurons and glial cells regeneration, and repairing the demyelinated axons. Shroff and Gupta [45] for treatment of SCI, inject ESCs into patient’s body through a variety of methods. At first to make the body produce immune tolerance to embryonic stem cells by intramuscular injection. Then gradually adopt intramuscular, intravenous, brachial plexus block, intrathecal, caudal, epidural, popliteal block and/or deep spinal muscle and epidural catheter, in order to inject the ESCs as near the injured site as possible. Following the treatment, all patients showed improvement in different aspects including their power and movement of limbs, sitting balance, control and sensation of bowel and bladder. Vadivelu et al. [46] transplanted embryonic stem cell-derived neural lineage cells (ESNLCs) directly into the cavity of a contused spinal cord 9 days after injury. They observed axons had grown through long distances after transplanted ESNLCs. And they found ESNLCs prominent expression nerve glial antigen 2 (NG2) which can promote the expression of matrix metalloproteinase 9 (MMP-9) to make an increasingly inhibitory gradient of chondroitin sulfate proteoglycan (CSPG). CSPG is an essential part of the glial scar. That may be the mechanism of ESNLCs to help axons growth. However, the strong ability of ESCs for proliferation has shown tumorigenic potential. Salewski et al. [47] used an alternative method of clonal neurosphere generation to ensure the safety of the transplanted cells. They generated clonally derived definitive NSCs (dNSCs) from ESCs and proved that transplantation of these dNSCs can promote motor recovery in SCI mice without tumor formation.
Neural stem cells (NSCs) are pluripotent cells within the CNS which has the ability of self-renewal and multi-directional potential differentiation. The discovery of NSCs that broke the traditional concept of no regeneration of the CNS. Both embryonic and adult CNS can find the neural stem cells and they exist in specific niches where can provide a relatively stable environment for the survival, self-renewal and differentiation of the stem cell [48]. For the application of NSCs come from fetal brain or adult CNS the ethics are debatable. In order to solve the problems, the researchers used some methods including cell reprogramming or chemical induction indirectly obtained NSCs from other cells. Common sources include embryonic stem cells [47], human induced pluripotent stem cells [49] and immortalized cell lines [50]. NSCs under certain conditions can only be differentiated into neurons and glial cells, so it is easier to get the desired cell types. Relative to other cells transplantation, NSCs transplantation has unique advantages that NSCs can differentiate into all kinds of nerve cells have corresponding phenotype at the particular area in the spinal cord after transplantation, and may rebuild connection. NSCs also can make through passage, separation of self-renewal constantly and produce enough amount of transplanted cells. NSCs will generate into neurons and glial cells in terminal differentiation after transplanted into the body, which makes NSCs no longer have the ability of rapid proliferation of stem cells. No report of these cells to become cancerous, as a result, the NSCs is considered to be safe and reliable clinical cell transplantation.
Mesenchymal stem cells (MSCs) that originally obtained from the bone marrow are a kind of self-renewal and multi-directional differentiation potential of pluripotent stem cells. MSCs are not difficult to be amplified, preserved, donated and be accepted in ethic, so it is studied for the treatment of SCI. And among them, the bone marrow mesenchymal stem cells (BMSCs) have been widely concerned because it is easy to obtain, can be autologous transplantation, and minimize invasive operation. MSCs can play its role through diverse mechanisms after SCI. In the initial SCI, MSCs can secrete various NTFs to protect neurons from the effects of excitatory neurotoxin and promote axon growth. Zhou et al. [51] found that adipose tissue-derived MSCs (AT-MSCs) transplantation for SCI can improve the recovery of the spinal cord function in mice, and its mechanism is increasing the expression of BDNF and enhanced regrowth of serotonergic fibers. They also think the improvement is better than BMSCs transplantation. At the intermediate phase of SCI, MSCs can adjust immune by molecule secretions and cell-cell contact, and powerful alleviate inflammation to damage cells in the spinal cord. The previous view believes that MSCs can differentiate into neurons and oligodendrocytes directly participated in the reconstruction of the spinal cord and myelin formation. Matrix metalloproteinase also can be secreted to degrade extracellular matrix which have the benefit of axon growth. But the new research thinks that MSCs mediate functional recovery through a paracrine effect, rather than by transforming into and replacing damaged glia in the spinal cord [52]. MSCs are often used by transfection NTFs gene for more NTFs secreted and better neurotrophic effect. Zhang et al. [53] significantly improve functional recovery and nerve regeneration of rats with SCI by precise transplanting NT3 gene-transfected BMSCs. Compared with other cells, BMSCs is easy to extract and separate from the bone marrow, and it transplants back also have no risk of immune rejection. Therefore, BMSCs autologous transplantation clinical trials have developed for the treatment of SCI in many countries in recent years, and significant results have been achieved.
Schwann cells (SCs) are a kind of glial cell distributed in the peripheral nervous system (PNS) to form the myelin sheath surrounding the axons. SCs can secrete NTFs and extracellular matrix to support neurons. SCs also play an important role in the axon regeneration and the process of myelination after peripheral nerve injury . The CNS without SCs and this is one of the reasons for the CNS damage is difficult to restore. SCs is easy to obtain and separation, and can be auto-transplantation without immunological rejection. So SCs is considered to be an ideal and feasible transplanted cells. SCs secrete a number of NTFs and improve the extracellular microenvironment for neuronal survival and axonal regeneration [54]. But SCs grafted into the CNS is unable to long-term survival or unable to help complete the functional recovery of the spinal cord. So we need to combine a variety of method to realize the maximum potential of Schwann cells [55].
Olfactory ensheathing cells are a special type of glial cell that presents in the olfactory system, and distributed in the olfactory nerve, the olfactory bulb layer 1, 2 and olfactory epithelium. Olfactory ensheathing cells exist in both the central nervous system and peripheral nervous system at the same time. Many studies have shown that olfactory ensheathing cell transplantation can promote axon regeneration at a distance, make the demyelinated axons remyelination, and play a role in neuroprotection [56]. Many clinical trials also proved that OECs transplantation is safe and effective. Rao et al. injected OECs into the area surrounding the SCI under magnetic resonance imaging guidance in patients with cervical SCI, twice a week for four weeks. The recovery of patients has noticeable improvement after three months, but there is no further increase in a year. And no serious complications postoperatively were discovered during the follow-up period [57].
6.4 Combination Strategy
Biological engineering technology provides a great help for cell transplantation. Biological scaffold provides mechanical support for transplanted cell growth, proliferation. Biological scaffolds that load NTFs and chondroitin enzymes can create a perfect external environment, so as to promote the transplanted cells long-term survival, growth, proliferation and differentiation. And with the rapid progress of molecular biology and molecular mechanism of SCI, genetically modified technology in cell transplantation research has made significant progress. Through gene transfection technique, modified cells specifically expressed the required material, aimed at improving the local environment, which can greatly improve the curative effect of the transplanted cells. The combined use of different cells also can mutually optimize and create synergistic effects. Hu et al. [58] used co-transplantation oligodendrocyte progenitor cells (OPCs) and SCs to treat SCI. They believe the extracellular matrix produced by SCs can promote OPCs survival, proliferation and differentiation, and OPCs differentiation to oligodendrocytes can form the myelin sheath, which is beneficial to the spinal cord function recovery. And the experimental results accord with their ideal.
7 Electroacupuncture and Chinese Herbology
Traditional Chinese Medicine (TCM) is the national cultural treasures and the fruits of the Chinese people’s prevention and treating disease for thousands years. In recent years, many studies have shown that the TCM have surprising effects in many diseases. In the cognition of TCM, SCI is associated with mechanical injury of Du meridian, which leads to the disturbance of qi and blood, the stasis of meridian, and the instability in body warm-reinforcing and nourishing of qi and blood.
7.1 Electroacupuncture
The electroacupuncture therapy is an improved traditional Chinese acupuncture. It is a type of acupuncture where a small electric current is passed between pairs of acupuncture needles. Electroacupuncture has a good curative effect on the treatment in pain, neurosis, nerve palsy, gastrointestinal disease, high blood pressure and so on [59]. In the treatment of SCI, electroacupuncture is usually used to stimulate the Du meridian and Jiaji point. The position and function of the Du meridian describe in the traditional Chinese medicine is similar as the spinal cord. TCM holds that stimulate the Du meridian can restore the function of the Du meridian, namely the recovery of the spinal cord function. The Jiaji point locates at the T1 to the L5 spinous process adjacent to open 0.5-inch. Jiaji point near the Bladder Meridian of Foot-Taiyang and the Du meridian, so stimulation of the Jiaji point can help to restore the SCI.
In recent years, the study concluded that electroacupuncture can inhibit the formation of oxygen free radical and lipid peroxidation, inhibit apoptosis gene expression in neurons and increase the synthesis of NTFs and receptors expression [60]. Electroacupuncture can suppress the Notch signaling pathway to decrease the protein expression levels of Nogo-A and Nogo-66 receptor-1, so it can promote neural stem cell proliferation to help the recovery of the SCI [61, 62]. Changed microcirculation and neuronal morphology is another mechanism of electroacupuncture to improve the function of the spinal cord [63]. Jiang et al. [63] found to use different modalities of acupuncture to treat SCI in rats and found that electroacupuncture have better anti-inflammatory, antioxidant effect than other method of acupuncture which can protect neurons to improve recovery. In addition, electroacupuncture are effective to relieve pain by activating a variety of bioactive chemicals including opioids, serotonin and norepinephrine [64]. Combination of electroacupuncture treatment after cellular transplantation is a good strategy. Electroacupuncture can prolong the survival time of the transplanted cells, and promote the proliferation and differentiation of transplanted cells. Ding et al. [65] combinedly used electroacupuncture and grafted mesenchymal stem cells for SCI and detected that the expression of NTFs receptor TrkC is increased and remyelination and function in demyelinated spinal cord are improved.
7.2 Chinese Herbology
Chinese herbs have been used for centuries and formed a set of unique theory. According to the theory of Chinese herbology, the treatment of SCI mainly involves activating blood circulation, removing blood stasis, smoothing the meridian, nourishing kidney and benefitting qi (This is a traditional Chinese medicine theory, distinct from the theory of modern medicine). The commonly used prescription include the single prescriptions such as Salvia miltiorrhiza (Dan Sen), Panax notoginseng (San Qi), Panax ginseng (Ren Shen), Ligusticum wallichii (Chuan Qiong) or their active ingredients and the compound prescriptions such as Buyanghuanwu Decoction, Xuefuzhuyu Decoction, Fangjihuangqi Decoction and so on. Panax notoginsenoside is a compound isolated from Panax notoginseng. The study found that Panax notoginsenoside can inhibit the inflammatory cytokines release and signaling pathways in cell apoptosis for the effect of neuroprotection [66]. Panax notoginsenoside also caused an upregulation of NGF and BDNF for improving the hind limb motor function [67]. Ligustilide is one of the main active components of Angelica sinensis, which can reduce the generation of reactive oxygen species to reduce the neuron damage and promote restoration of the injury [68]. Compatibility of medicines is an important component of Chinese traditional medicine theory. Buyanghuanwu Decoction is composed of astragalus, Angelica sinensis, radix paeoniae rubra, lumbricus, Ligusticum wallichii, safflower, peach kernel. The study found Buyanghuanwu decoction can promote the proliferation and differentiation of neural stem cells [69], and also can decrease in expression of caspase-3 and Bax and increase in Bcl-2 expression to exert anti-apoptosis effect [70]. Buyanghuanwu Decoction also can be used to combine with embryonic neural stem cell transplantation, and that will be under a synergistic effect on the recovery of neurological function [71].
8 Conclusion
SCI brings a series of functional impairment and pain to the patient caused devastating shock and heavy burden. Owing to the unique nature of the central nervous system and complex pathophysiological mechanisms after SCI, it is difficult to form a suitable environment conducive to recovery of SCI. On the contrary, scar formation and low levels of NTFs hinder the repair of the spinal structure. The treatment of SCI is creating a suitable microenvironment on the damaged area and supplement the new cells to instead the damaged and apoptotic cells to support the reconstruction of the original structure of the spinal cord. Supplementary exogenous NTFs is the principal part of creating suitable microenvironment. Nonetheless, management strategies of different NTFs at different times and different site need to be more sophisticated regulation. Biological scaffold that can cope with the delivery of NTFs, while cell transplantation can also offer support. The presence of a large number of cells destroyed or apoptosis after SCI, and the insufficient endogenous stem cells in the central nervous system makes it is necessary to transplant exogenous cells. Exogenous cells can supplement the lack of cell, while the secretion of extracellular matrix can play a role in improving the microenvironment. And genetic engineering can help the transplanted cells be best to play this role. Chinese medicine is one kind of magical drugs and treatment methods. Continue to clarify the biological mechanisms of Chinese medicine also can provide excellent ideas for the treatment of SCI. At present the main problems or challenges existing in the research are: first, the cells transplanted into the defect is not able to completely differentiate to form the cell suiting the functional and the structure. Such as how to regulate the biology behavior including proliferation and differentiation of NSCs as seed cells, so that it can provide important cell structure basis and neurotrophic support for the reconstruction of spinal cord tissue engineering, and avoid unfavorable side for SCI repair, such as the transplanted neural stem cells in a quiescent state or mostly differentiate into glial cells and so on. Secondly, the number of new tissue growth in both ends of the fiber is limited and it is difficult to form synaptic connections. Thirdly, how to exclude or counteract the cytokines or negative components that inhibit axonal regeneration and the formation of synapses in the environment. Fourthly, the transplantation related issues such as immune rejection, revascularization and so on are also very important issues. Finally, more important is to repair the injured spinal structure but not functional rehabilitation. After various conditions stimulus in vivo and vitro environmental reached the remodeling of structural and functional, and ultimately to achieve important goals that the function of patients with paraplegia rehabilitation and quality of life improved obviously. We still have a hard and slow way to get that.
After comprehensive analysis of large number of domestic and foreign basis and clinical research about SCI, the author put forward the treatment of spinal cord injury with paraplegia rehabilitation treatment, which should be taken to a new comprehensive treatment strategies and methods called “5R-Combined Strategies”. The concrete content includes: (1) Rescue: including spinal surgical fixation, decompression, neural protection and stress protection and so on; (2) Regeneration: a various kinds of treatment for promoting damaged spinal cord regeneration. Main idea is to promote spinal cord neurons axon regrowth, such as use NTFs, transgenic therapy; (3) Replacement: It is all kinds of neurons, stem cells or other cells for the transplant therapy; (4) Remyelination: It is mainly to promote form new myelin of the demyelinated spinal cord fibers and restore conduction function of damaged nerve fiber, including the appropriate drugs, factors and myelin forming cells therapy; (5) Rehabilitation: including almost all of the neurological rehabilitation physiotherapy methods and measures. We think we should establish the following concepts: (1) Rehabilitation is not an adjuvant therapy. Breaking the idea in traditional sense that the rehabilitation dispensable; (2) Adhere to the principle of individual rehabilitation. According to each the actual injury patients and the development of the situation, make a scientific and reasonable rehabilitation methods and embodiments; (3) early intervention and perseverance principle. Breaking the traditional concept that the rehabilitation is the late treatment and establishing the concept of lifelong rehabilitation; (4) Rehabilitation techniques adhere to integration of Chinese and western, and value both physical and chemical. Breaking the traditional concept that the rehabilitation just includes physiotherapy and physical exercise therapy. Rehabilitation equipment should adjust local conditions and personalized; (5) Rehabilitation theory need innovation and absorbs new knowledge. Such as the present, in particular, should be focused on the psychological rehabilitation of paraplegics, truly improve physical and mental health of paraplegics. 5R-Combined Strategies also should adjust the local conditions and personalized. In summary, the combination of a variety of methods for clinical therapy of SCI is the development direction in the future, and basic research continuously investigation of the downstream mechanism of each method can provide fresh ideas for clinical combined strategy.
References
Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–53.
Yang R, et al. Epidemiology of spinal cord injuries and risk factors for complete injuries in Guangdong, China: a retrospective study. PLoS ONE. 2014;9:e84733.
Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–13.
Blight A. Mechanical factors in experimental spinal cord injury. J Am Paraplegia Soc. 1988;11:26–34.
Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221.
Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–49.
Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–79.
Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–35.
Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23.
Ba YC, Dai P, Zhou HL, Liu J, Wang TH. Spatiotemporal changes of NGF, BDNF and NT-3 in the developing spinal cords of embryonic chicken. Neurochem Res. 2010;35:273–8.
Brown A, Ricci MJ, Weaver LC. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exp Neurol. 2007;205:283–6.
Geng SJ, et al. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol. 2010;222:256–66.
Qian DX, Zhang HT, Cai YQ, Luo P, Xu RX. Expression of tyrosine kinase receptor C in the segments of the spinal cord and the cerebral cortex after cord transection in adult rats. Neurosci Bull. 2011;27:83–90.
Li HP, et al. P75 neurotrophin receptor mRNA sequential expression and significance after Cauda equina compression in rats. Zhongguo Gu Shang. 2011;24:509–13.
Ejstrup R, et al. Pharmacokinetics of intravitreal glial cell line-derived neurotrophic factor: experimental studies in pigs. Exp Eye Res. 2010;91:890–5.
Aomar MM, et al. Assessment of neurologic function and complications in a retrospective cohort of patients with acute spinal cord injury due to trauma treated with large-dose methylprednisolone. Rev Esp Anestesiol Reanim. 2011;58:583–8.
Kim DH, Jahng TA. Continuous brain-derived neurotrophic factor (BDNF) infusion after methylprednisolone treatment in severe spinal cord injury. J Korean Med Sci. 2004;19:113–22.
Arvanian VL, et al. Combined treatment with neurotrophin-3 and LSD facilitates behavioral recovery from double-hemisection spinal injury in neonatal rats. J Neurotrauma. 2006;23:66–74.
Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–5.
Sharma HS. Selected combination of neurotrophins potentiate neuroprotection and functional recovery following spinal cord injury in the rat. Acta Neurochir Suppl. 2010;106:295–300.
Lang EM, Asan E, Plesnila N, Hofmann GO, Sendtner M. Motoneuron survival after C7 nerve root avulsion and replantation in the adult rabbit: effects of local ciliary neurotrophic factor and brain-derived neurotrophic factor application. Plast Reconstr Surg. 2005;115:2042–50.
Donnelly EM, et al. Lentiviral vector-mediated knockdown of the NG2 [corrected] proteoglycan or expression of neurotrophin-3 promotes neurite outgrowth in a cell culture model of the glial scar. J Gene Med. 2010;12:863–72.
Tang S, et al. The effects of controlled release of neurotrophin-3 from PCLA scaffolds on the survival and neuronal differentiation of transplanted neural stem cells in a rat spinal cord injury model. PLoS ONE. 2014;9:e107517.
Wang YT, et al. Ameliorative effects of p75NTR-ED-Fc on axonal regeneration and functional recovery in spinal cord-injured rats. Mol Neurobiol. 2015;52:1821–34.
Obermair FJ, Schroter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiol (Bethesda). 2008;23:296–304.
Koffler J, Samara RF, Rosenzweig ES. Using templated agarose scaffolds to promote axon regeneration through sites of spinal cord injury. Methods Mol Biol. 2014;1162:157–65.
Francis NL, Hunger PM, Donius AE, Wegst UG, Wheatley MA. Strategies for neurotrophin-3 and chondroitinase ABC release from freeze-cast chitosan-alginate nerve-guidance scaffolds. J Tissue Eng Regen Med. 2014.
Shahriari D, Koffler J, Lynam DA, Tuszynski MH, Sakamoto JS. Characterizing the degradation of alginate hydrogel for use in multilumen scaffolds for spinal cord repair. J Biomed Mater Res A. 2015;104:611–9.
Grulova I, et al. Delivery of alginate scaffold releasing two trophic factors for spinal cord injury repair. Sci Rep. 2015;5:13702.
Yang Z, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:13354–9.
Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A. 2015;104:1002–16.
Hou T, et al. Cellular prostheses fabricated with motor neurons seeded in self-assembling peptide promotes partial functional recovery after spinal cord injury in rats. Tissue Eng Part A. 2012;18:974–85.
Sun Y, et al. Functional self-assembling peptide nanofiber hydrogels designed for nerve regeneration. ACS Appl Mater Interfaces. 2015;8:2348–59.
Silva NA, et al. Benefits of spine stabilization with biodegradable scaffolds in spinal cord injured rats. Tissue Eng Part C Methods. 2013;19:101–8.
Wen Y, et al. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2015;364:17–28.
de Rivero VJ, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res. 2016;167:35–45.
Yune TY, et al. Systemic administration of PEP-1-SOD1 fusion protein improves functional recovery by inhibition of neuronal cell death after spinal cord injury. Free Radic Biol Med. 2008;45:1190–200.
Wang YT, et al. The use of a gold nanoparticle-based adjuvant to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials. 2011;32:7988–98.
Zhang ZF, et al. Protective effects of adenoviral cardiotrophin-1 gene transfer on rubrospinal neurons after spinal cord injury in adult rats. Neurotox Res. 2003;5:539–48.
Liu Y, et al. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol. 2015;8:3835–42.
Cusimano M, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–60.
Lu P, Kadoya K, Tuszynski MH. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr Opin Neurobiol. 2014;27:103–9.
Amemori T, et al. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015;6:257.
Garbossa D, et al. Recent therapeutic strategies for spinal cord injury treatment: possible role of stem cells. Neurosurg Rev. 2012;35:293–311 (Discussion 311).
Shroff G, Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann Neurosci. 2015;22:208–16.
Vadivelu S, et al. NG2+ progenitors derived from embryonic stem cells penetrate glial scar and promote axonal outgrowth into white matter after spinal cord injury. Stem Cells Transl Med. 2015;4:401–11.
Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015;24:36–50.
Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701–13.
Hong JY, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289:32512–25.
Amemori T, et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Ther. 2013;4:68.
Zhou Z, et al. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15:434–48.
de Almeida FM, Marques SA, Ramalho BS, Massoto TB, Martinez AM. Chronic spinal cord lesions respond positively to tranplants of mesenchymal stem cells. Restor Neurol Neurosci. 2015;33:43–55.
Zhang RP, Wang LJ, He S, Xie J, Li JD. Effects of magnetically guided, SPIO-labeled, and neurotrophin-3 gene-modified bone mesenchymal stem cells in a rat model of spinal cord injury. Stem Cells Int. 2016;2016:2018474.
Ghosh M, et al. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. 2012;60:979–92.
Wang X, Xu XM. Long-term survival, axonal growth-promotion, and myelination of Schwann cells grafted into contused spinal cord in adult rats. Exp Neurol. 2014;261:308–19.
Tetzlaff W, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–82.
Rao Y, et al. Clinical application of olfactory ensheathing cells in the treatment of spinal cord injury. J Int Med Res. 2013;41:473–81.
Hu JG, et al. Cotransplantation of glial restricted precursor cells and Schwann cells promotes functional recovery after spinal cord injury. Cell Transplant. 2013;22:2219–36.
Lin LL, et al. Systems biology of meridians, acupoints, and chinese herbs in disease. Evid Based Complement Altern Med. 2012;2012:372670.
Wu MF, et al. Neuroprotective effects of electroacupuncture on early- and late-stage spinal cord injury. Neural Regen Res. 2015;10:1628–34.
Tan F, et al. Electroacupuncture attenuates cervical spinal cord injury following cerebral ischemia/reperfusion in stroke-prone renovascular hypertensive rats. Exp Ther Med. 2014;7:1529–34.
Geng X, et al. Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res. 2015;10:394–403.
Jiang DX, et al. Electroacupuncture improves microcirculation and neuronal morphology in the spinal cord of a rat model of intervertebral disc extrusion. Neural Regen Res. 2015;10:237–43.
Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503.
Ding Y, et al. Combination of electroacupuncture and grafted mesenchymal stem cells overexpressing TrkC improves remyelination and function in demyelinated spinal cord of rats. Sci Rep. 2015;5:9133.
Ning N, Dang X, Bai C, Zhang C, Wang K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol. 2012;139:504–12.
Wang B, Li Y, Li XP, Li Y. Panax notoginseng saponins improve recovery after spinal cord transection by upregulating neurotrophic factors. Neural Regen Res. 2015;10:1317–20.
Xiao W, Yu A, Liu D, Shen J, Xu Z. Ligustilide treatment promotes functional recovery in a rat model of spinal cord injury via preventing ROS production. Int J Clin Exp Pathol. 2015;8:12005–13.
Liu B, Cai G, Yi J, Chen X. Buyang Huanwu decoction regulates neural stem cell behavior in ischemic brain. Neural Regen Res. 2013;8:2336–42.
Xian-Hui D, Xiao-Ping H, Wei-Juan G. Neuroprotective effects of the Buyang Huanwu decoction on functional recovery in rats following spinal cord injury. J Spinal Cord Med. 2014;39:85–92.
Zhang M, Chai Y, Liu T, Xu N, Yang C. Synergistic effects of Buyang Huanwu decoction and embryonic neural stem cell transplantation on the recovery of neurological function in a rat model of spinal cord injury. Exp Ther Med. 2015;9:1141–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liu, C., Wu, Y. (2017). Spinal Cord Injury and Regenerative Repair. In: Fu, X., Liu, L. (eds) Advanced Trauma and Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-10-2425-2_21
Download citation
DOI: https://doi.org/10.1007/978-981-10-2425-2_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2424-5
Online ISBN: 978-981-10-2425-2
eBook Packages: MedicineMedicine (R0)