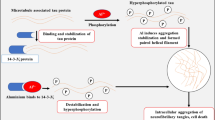
Abstract
Aluminum is believed to be a neurotoxicant for a lot of years and thought to be related with Alzheimer’s disease. In recent decades, aluminum nanoparticles have been utilized widely in many fields, and their potential adverse effect on health drew great concern. Al2O3 nanoparticles (ANPs) can be inhaled more deeply into the respiratory system, and translocated into the bloodstream to induce immunotoxicity and into the central nervous system to induce neurotoxicity, of which the possible mechanisms are summarized in this chapter. ANPs may induce pneumocyte apoptosis by triggering oxidative stress and inhibit or activate activity of cytokines, and the immunotoxicity induced by nanoalumina (Nano-Al) particles was higher than that of macro-sized alumina particles. Besides though blood compartment by which ANPs damage the blood-brain barrier, ANPs may enter into the central nervous system through the olfactory nerve. ANPs impair behavioral performance of model organisms and rodents. ANPs may induce neural cell death by triggering apoptosis, necrosis, and autophagy, complicate cell signal transmission pathways, and promote Aβ deposit and degeneration.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Wide Utilization of Aluminum Nanoparticles and Concern on Their Toxicity
9.1.1 Toxicity of Aluminum
Aluminum is a very abundant metal element in the environment, comprising 8 % of the earth’s crust and standing at third position in richness as elements. It is used extensively in many fields of modern life, such as industry, medicine, food, transportation, and living utensil, and may enter the human body from the environment via air, diet, drinking water, food, cosmetic, or medication. Concerns about aluminum toxicity have persisted since the demonstration as a potential neurotoxicant by Doellken more than 100 years ago [1]. This initial finding led to the extensive studies on increment of aluminum concentrations, senile plaque, and neurofibrillary tangles in the brain tissues of patients with Alzheimer’s disease (AD) [2, 3]. Various mechanisms have been proposed for aluminum-induced neurotoxicity, including free-radical damage via enhanced lipid peroxidation and impaired glucose metabolism, disturbed signal transduction and protein modification, alterations in the axonal transport, and abnormal phosphorylation level of neurofilaments [4–6]. Aluminum is relatively stable in the form of alumina (aluminum oxide).
9.1.2 Chemical Property and Utilization of Nano-Al
Aluminum oxide, commonly called alumina, is a chemical compound of aluminum and oxygen with the chemical formula Al2O3 and is the most commonly existing type of several aluminum oxides, specifically identified as aluminum(III) oxide. It occurs in its crystalline polymorphic phase α-Al2O3, in which it constitutes the mineral corundum, a lot of kinds of which form the precious gemstones ruby and sapphire. Al2O3 is basically used to produce aluminum metal generally via electrolysis process, as an abrasive owing to its hardness and as a refractory material owing to its high melting point [7]. The nanosized form of aluminum oxide, Nano-Al, has greatly enlarged its utilization in many more industries and fields, such as transparent ceramics, cosmetics, precision polishing materials, special glass, arms, aircraft, electronics, dispersion strengthening, nanocomposites, constituent of rocket propellant, constituent of explosive for military and for mini-bomb killing cancer tissue, solar cell, and drug delivery [8]. As of October 2013, the number of consumer products of nanosized materials has increased to 1628 since 2005 [9], in which alumina is among the most abundantly produced nanosized particles; the market volume is predicted to 5000 tons only in China in 2016 [10]. With this in mind, there has been a recent emergence of concern dealing with the potential for toxicity and the lack of data to substantiate or dismiss these concerns [11–13].
9.1.3 Elevation of Concern for Toxicity of Nano-Al
Aluminum oxide was deleted from the US Environmental Protection Agency’s chemicals lists in 1988, but its fibrous form is still on the EPA’s Toxics Release Inventory list, and toxicity of its nanosized particles is still far from being illuminated. With so wide utilization of Nano-Al, many people, production workers, and consumers are being exposed to alumina nanoparticles occupationally and environmentally, and in living condition, concerns regarding their safety and potential toxic effect have complicated their usage. Possible adverse effect of nanosized alumina on human being’s health has not been deeply investigated and elucidated, and the study on aluminum nanoparticles-induced adverse effect is rarely seen.
Toxicological studies [12, 14, 15] have shown increased toxicity of nanoparticles (<100 nm) compared to micrometer-sized particles of the same composition, which has raised concern about the impact on human health from nanoparticles.
In vitro and in vivo studies have shown that ANPs have multisystem and multi-organ toxicity on experimental animals, especially on the immune and nervous system. Thereby, we summarize the immune and neurological adverse effect of Nano-Al in this chapter based on the published data and our studies, even if the investigations were sparse.
9.1.4 Nano-Al Is Inhaled into the Respiratory System and Induces Oxidative Damage
Based on breathing air dynamics, nanosized particles can be inhaled more deeply into the respiratory system than large particles and deposited on the surface of the system. The lung tissue is considered the primary target organ for inhaled nanoparticles. Xiaobo Li et al. [16] performed H&E and TUNEL staining to detect pathology and programmed cell death in alumina nanoparticle (Al2O3 NPs)-exposed mice lung tissue. They found an inflammation and red blood cells located in the pulmonary mesenchyme; pneumorrhagia characterized by interstitial red blood cell distribution; massive lymphocyte infiltration, especially the subpleural area; lung cell degeneration; and massive bronchial epithelial cell apoptosis. By in vitro study using human bronchial epithelial cell (HBE cell), they also found significant increment of apoptosis (2.24 ± 0.17 %), increased activities of caspase-3 and caspase-9 in Al2O3 NP-treated cells, indicating HBE cell apoptosis is initiated by the intrinsic apoptotic pathway, marked damage to the mitochondrial membrane potential, significantly increased cytoplasmic cytochrome c, increased reactive oxygen species (ROS) level, and increased malondialdehyde (MDA). Moreover, they also found that Al2O3 NPs significantly triggered downregulation of mitochondria-related genes located in complex I, IV, and V. After having damaged epithelial cells of the lung tissue, alumina NPs may be translocated from the respiratory system to other organs and systems.
Direct input into the blood compartment from the lung tissue is certainly an important translocation pathway of NPs in mammals. However, since predictive particle deposition models indicate that respiratory tract deposits alone may be far from fully accounting for the NP burden in the body, especially in the central nervous system [17], we should consider as well input from other pathways.
9.2 Immune Toxicity of Nano-Al In Vivo and In Vitro Studies
9.2.1 Aluminum NPs and Alumina NPs Damage Alveolar Macrophages and Pneumocytes with Immune Response
Alveolar macrophages are very important first frontier immune cells against foreign materials which are inhaled into the respiratory system. In an in vitro study using human alveolar macrophages (U937) and human type II pneumocytes (A549) coculture treated with aluminum nanoparticle and Al2O3 NPs, Laura et al. [18] found that the macrophages as frontier immune cells were more susceptible to the NPs than the epithelial cells, but if the macrophages were not present, the pneumocytes showed significant cell death, indicating that the macrophages actively engulf exotic nanoparticles and interacted with toxicity of the NPs and protected the pneumocytes. The main function of macrophages is to destroy foreign material via phagocytosis. The authors assessed if the macrophages could still phagocytose bacteria named community-acquired methicillin-resistant Staphylococcus aureus (ca-MRSA) after treatment with the NPs and found that the Al2O3 NPs did not impair phagocytosis of macrophages to the bacteria, but the Al-NPs did, meaning that the Al-NPs alter the cell function and their higher immunotoxicity than the Al2O3 NPs. While ca-MRSA was exposed to Al-NPs, no decrease in bacterial numbers was observed following overnight incubation, indicating that the Al-NPs do not kill this specific strain of bacteria, and the reason of Al-NP-reduced phagocytosis may be due to the Al ions released by aluminum nanoparticles, which chemically alter the cellular environment and finally disrupt the phagocytic process. In a PCR assay, Al2O3 NPs alone induced slightly the NF-kB pathway, but the Al-NPs did not show this induction. ca-MRSA alone generated NF-kB pathway activation in cocultured cells, but when the Al-NPs and Al2O3 NPs were present with ca-MRSA and together treated the coculture, the cocultured cells did not generate activation of the NF-kB pathway, indicating that the NPs are capable of altering or abolishing the cells’ response to a pathogen via the NF-kB pathway. ca-MRSA infection alone induced inflammatory markers interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α) response in coculture; the NPs alone did not show this effect, but while the cocultured cells were infected by ca-MRSA and the NPs were present, the expression of IL-6 and TNF-α induced by ca-MRSA was abolished, showing that the NPs inhibited ca-MRSA-induced IL-6 and TNF-α expression. In the ELISA, Al-NPs inhibited the secretion of IL-6, IL-8, IL-10, IL-1, and TNF-α too, evidencing the results of the PCR assay.
9.2.2 Alumina NP-Induced Immunotoxicity Is Complicated
In a repeated dose exposure experiment reported by Eun Jung Park et al. [19], 6-week-old male ICR mice were acclimatized for 1 week, and then Al2O3-NPs were administered orally at a dose of 1.5, 3, and 6 mg/kg for 13 weeks, and the control group was treated with autoclaved water. Blood (approximately 1.2 mL/mouse) was collected from the saphenous vein for biochemical and hemogram analysis, and then the mice were sacrificed, and the brain, thymus, lung, heart, liver, kidneys, spleen, and testis were collected for histological examinations. The levels of aspartate aminotransaminase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) in blood were significantly different between the Al2O3-NP-treated group and the controls. Compared with the controls, the levels of AST, ALT, and LDH decreased in the mice treated with 1.5 and 3 mg/kg Al2O3-NPs, but interestingly, these levels were markedly elevated in the 6 mg/kg Al2O3-NP-treated mice. In addition, with the same tendency, the accounted number of white blood cells (WBCs) and the proportion of lymphocytes in the WBCs in the mice treated with 1.5 and 3 mg/kg Al2O3-NPs were decreased compared with the control group, while those in the 6 mg/kg Al2O3-NP-treated group were significantly increased; the proportion of eosinophils in the WBCs in the mice treated with 1.5 and 3 mg/kg Al2O3-NPs was increased than in the control group, whereas that markedly decreased in the 6 mg/kg Al2O3-NP-treated group. The levels of IL-1β and TNF-α in the Al2O3-NP-treated groups did not show significant change compared with the control group, and granulocyte-macrophage colony-stimulating factor and transforming growth factor β were not detected at a significant level in all samples tested. However, the levels of IL-6 and monocyte chemotactic protein-1 increased in a dose-dependent manner. The results of Eun Jung Park et al. indicated that the immunotoxic effect of Nano-Al is complicated.
9.2.3 Al2O3 Nanoparticle Has Higher Immunotoxicity Than Micro-sized Alumina
In a 30-day Al2O3-NPs exposure study performed by Li Huan and colleagues [20], 70 healthy SPF ICR mice (3 months old) were treated with Al2O3 nanoparticle (13 nm diameter) at 25 mg/kg bw, 50 mg/kg bw, and 75 mg/kg bw as an exposure dose grade and Al2O3 nanoparticle (50 nm diameter) at 50 mg/kg bw and bulk Al2O3 at 50 mg/kg bw as comparison between nanosized alumina particle and micro-sized alumina particle, by nasal instillation, three times daily, continuously for 30 days. Superoxide dismutase (SOD) activity and glutathione (GSH) content in the spleen tissue and thymus tissue of Al2O3 particle-treated mice decreased significantly compared with blank and solvent controls, and in a dose-dependent and particle size-dependent manner, i.e., the higher the dose was, and the smaller the particle size was, the higher the SOD activity and GSH contents were. While MDA content in the spleen tissue and thymus tissue of Al2O3 particle-treated mice increased significantly compared with blank and solvent controls, and in a dose-dependent and particle size-dependent manner, the oxidative stress level was increased with increment of doses administered with alumina nanoparticles and with decrement of particle sizes. Inflammatory cytokines IL-1α, IL-1β, interferon-γ (IFN-γ), TNF-α, IL-2, and IL-10 contents in the spleen tissue and thymus tissue increased significantly, indicating immune response in Nano-Al-treated mice was upregulated. The results of this study showed that respiratory exposure to Al2O3 nanoparticle could initiate oxidative stress and immune response more strongly than the controls and bigger Al2O3 particles, implying Al2O3 nanoparticle has higher immunotoxicity than micro-sized alumina.
9.3 Neurotoxicity of Nano-Al
9.3.1 Nanoalumina Induces Neurobehavioral Impairment
In an in vivo study with male ICR mice [21], Zhang et al. compared the neurotoxicity of Nano-Al and nano-carbon (Nano-C) as reference of the same particle size and different chemical property and micro-alumina (Micro-Al) as reference of the same chemical property and different particle size. The animals were inoculated intranasally (i.n.) per day with Nano-Al, Nano-C, and Micro-Al at the dose of 100 mg/kg bw as experimental groups, whereas another group of animals that received 0.9 % saline were used as controls. The mice were sacrificed 10 days post-inoculation.
Tested with Morris water maze, treatment with Nano-Al dramatically lengthened the escape latency of animals (Nano-Al vs. control and Nano-C p < 0.01, respectively; Nano-Al vs. Micro-Al, p < 0.05). During the probe trial when the platform was removed, the Nano-Al-treated mice spent significantly less time in the target quadrant (Nano-Al vs. control, p < 0.01; Micro-Al vs. Control, p < 0.05) and exhibited fewer platform crossings (Nano-Al vs. control, p < 0.01; Micro-Al vs. Control, p < 0.05). In Nano-C-treated groups, both measurements were decreased but showed no significant difference from those of the control group (Nano-C vs. control, p > 0.05 for both parameters). In contrast, comparisons between Nano-Al- and Micro-Al-treated groups indicated that mice treated with Nano-Al required longer escape latency, spent less time in the target quadrant, and crossed the platform fewer times (Nano-Al vs. Micro-Al, p < 0.05). In an in vivo study with mice exposed to Nano-Al particles by the respiratory tract [22], Xin Zhang and colleagues reported that only in female mice the neurobehavioral changes and especially depression-like behavior appeared.
Yinxia Li et al. [23] observed effects of acute exposure to Al2O3 NPs and bulk Al2O3 (micro-sized) on locomotion behaviors of nematodes. After exposure for 6 h, the significant decreases in head thrashes (p < 0.01) and body bends (p < 0.01) were observed in both nematodes exposed to 51–203.9 mg/L of Al2O3-NPs and nematodes exposed to the same doses of bulk Al2O3. Nevertheless, both head thrashes and body bends in Al2O3-NP-exposed nematodes were lower than those in bulk Al2O3-exposed nematodes. Moreover, after exposure for 48 h, the similar but more deteriorated locomotion behaviors were observed. The authors further examined the 10-day chronic neurotoxicity from 8.1 to 23.1 mg/L of Al2O3-NPs and same doses of bulk Al2O3 exposure on locomotion behavior of nematodes and got the similar results as the acute exposure experiment got. The authors concluded that Al2O3-NPs are more neurotoxic than bulk Al2O3, implying that alumina nanoparticles possess higher neurotoxicity than micro-sized alumina particles.
9.3.2 Inhaled Particles May Enter into the Central Nervous System via Mainly Two Routs: Blood Circulation and Olfactory Nerve
Chen Lei et al. [24] injected Alizarin Red S-labeled nanoalumina at the dose of 1.25 mg/kg into the mouse cerebral circulation via the carotid artery and detected the brain endothelium and astrocytes by staining for factor VIII as endothelium marker and GFAP as astrocyte marker, respectively. They found that Alizarin Red S-labeled-nanoalumina particles were colocalized with the brain endothelium 1 h after injection and were also appeared in astrocytes surrounding the cerebral vessels. Twenty-four hours after injection, Alizarin Red S-labeled-nanoalumina particles were diffused in brain parenchyma close to astrocytes. The distribution pattern of Alizarin Red S-labeled-nanoalumina particles in one week after injection was similar to that in 24 h after injection, indicating that nanoalumina particles passed the blood-brain barrier, entered into the brain tissue, and accumulated in the brain and not eliminated from the brain components. In order to examine how the nanoalumina particles impaired the BBB, the author further assessed the effects of nanoalumina on the levels of occludin and claudin-5, two types of important tight-junction proteins that regulate integrity and barrier function of the brain endothelium, in human cerebral microvascular endothelial cells (HCMECs). Following a 6-h exposure to nanoalumina, a dose-dependent decrease in expression of occludin and claudin-5 expression was detected in HCMECs. Nanoalumina also decreased tight-junction protein expression in vivo. A single dose of nanoalumina (1.25 mg/kg) was administered to mouse via the carotid artery, a gradual decrease in occludin for up to 30 days after injection was observed. Injection with 1.25 mg/kg nanoalumina significantly elevated blood-brain barrier permeability at day 3 after injection, and this effect was preserved for up to 30 days, indicating that only a single intravascular nanoalumina exposure can damage the BBB and the effect can last up to 30 days, and even the exposure dose is not high.
Though there was not a report on other pathways of Nano-Al entering the brain tissue except for blood circulation, there was a report on Nano-C entering the brain tissue via the olfactory nerve. Oberdörster et al. [25] exposed rats with Nano-C particles (36 nm) for 6 h, sacrificed the rats, and removed and examined the lungs, cerebrum, cerebellum, and olfactory bulbs of exposed rats at 1, 3, 5, and 7 days after exposure. The concentration of Nano-C particles in the lung tissue decreased from 1.39 μg/g on day 1 to 0.59 μg/g by day 7 after exposure, but the Nano-C particles in the olfactory bulb significantly and persistently increased, from 0.35 μg/g on day 1 to 0.43 μg/g by day 7, implying that the olfactory nerve is a channel for Nano-C entering into the brain tissue. Based on this fact, the authors concluded that the CNS can be targeted by airborne nanoparticles and that the most likely mechanism is from deposits on the olfactory mucosa of the nasopharyngeal region of the respiratory tract to subsequent translocation via the olfactory nerve. Depending on particle size, more than 50 % of inhaled nanoparticles can be deposited in the nasopharyngeal region during nasal breathing. The authors estimated that approximately 20 % of the nanoparticles deposited on the olfactory mucosa of the rat can be translocated to the olfactory bulb and then into other parts of the brain tissue. The increases of carbon nanoparticles in olfactory bulbs are consistent with studies in rodents that demonstrated that intranasally instilled solid ultrafine elemental particle translocates along axons of the olfactory nerve into the CNS [26].
9.3.3 Nanoalumina Induces Cerebrovascular Damage via Autophagy
In an in vitro study performed by Lei Chen [24], HCMECs were treated with Alizarin Red S-labeled nanoalumina (1 μg/mL) for 6 h and stained with MitoTracker Red, MitoTracker Green, and MDC (marker for autophagic vacuoles). Alizarin Red S-labeled nanoalumina (1 μg/mL, stained red) was detected in HCMECs following a 2-h treatment. After a 12-h exposure, Alizarin Red S-labeled nanoalumina was visible as aggregates close to clustered mitochondria stained by MitoTracker Green, which indicates the loss of mitochondria membrane potential, and a notable increase of MDC intensity indicates enhanced autophagy.
In a same in vivo study performed by Chen Lei [24] described in Sect. 4.2, nanoalumina (1.25 mg/kg) was administered to mouse via the carotid artery, and 24 h later, mouse brains were taken out and subjected to mouse autophagy PCR array, and 84 autophagy-related genes were examined; 13 autophagy-related genes increased more than twofold. Then two frequently used autophagy marker proteins LC3 and p62 were also examined. Similar to the results obtained in HCMECs, in which a notable increase of MDC intensity showed an increased autophagy, an increase in LC3 and p62 proteins, which indicate autophagy too, was observed 24 h after nanoalumina injection and remained elevated for as long as 30 days after nanoalumina administration. Combining Chen Lei’s results, we could hypothesize that Nano-Al particles preserve continuously in blood circulation compartment and damage the BBB and are difficult to be discharged from circulation compartment and the brain tissue.
The effects of nanoalumina on protein degradation were assessed in HCMECs using DQ-BSA, a probe based on bovine serum albumin (BSA) labeled with red fluorescent BODIPY TR-X dye (Invitrogen). Treatment with nanoalumina dose-dependently elevated activity of DQ-fluorescence, and protein degradation activity was correlated with the formation of acidic vesicles, consistent with autophagolysosomes stained with LysoTracker Green.
9.3.4 Nano-Al Induces Neural Cell Death
In the same in vivo study as in Sect. 4.1 with male ICR mice [21], Zhang et al. compared the neurotoxicity of Nano-Al and Nano-C as reference of the same particle size and different chemical property and Micro-Al as reference of the same chemical property and different particle size. The animals were inoculated intranasally (i.n.) per day with Nano-Al, Nano-C, and Micro-Al at the dose of 100 mg/kg bw as experimental groups, whereas another group of animals that received 0.9 % saline were used as controls. The mice were sacrificed 10 days post-inoculation.
To investigate the potential mechanisms by which Nano-Al more strongly impaired the neurobehavior of animals than Nano-C and Micro-Al did, the changes in matrix metalloprotein (MMP) and ROS were observed. Treatment with 100 mg/kg Nano-Al but not with the same concentration of Nano-C resulted in a highly significant decrease in MMP. Alterations in mitochondrial potential may result in induction of cellular oxidative stress; indeed, ROS production measurements showed that Nano-Al led to a marked induction of oxidative stress.
Nano-Al-mediated MMP loss and significantly higher ROS production confirmed that Nano-Al may cause more severe neurotoxicity than Micro-Al. On the other hand, Nano-C with the same nanoparticle size resulted in only mild neurotoxicity that was not significantly different compared with controls treated with 0.9 % saline.
Both Nano-C and Nano-Al at 100 mg/kg bw were toxic and enhanced the necrotic rate, and the necrotic rate induced by Nano-Al was markedly higher than apoptotic rate it induced. Apoptosis was also observed in Nano-Al-treated mice and became more pronounced in Micro-Al-treated mice.
LC3 is a mammalian homolog of yeast Atg8, the only reliable marker of autophagosomes that indicate autophagy process. In order to recognize if autophagy exists in nanoparticle-induced neurotoxicity, the authors observed LC3 expression in the study and found its expression was low.
Lower expression of LC3 in the study suggests that autophagy may not be the major cell death mode in neural cells induced by Nano-C, Nano-Al, and Micro-Al. Furthermore, robust caspase-3 activation likely indicates significant apoptosis in Micro-Al-treated mice. The results also indicated that Nano-Al treatment led to neural cell death; and necrosis may be a major cell death mode in nanoparticle-induced neurotoxicity.
Furthermore, in an in vitro study performed by Zhang et al. [27], observed under light microscope, neural cells treated with Micro-Al presented shrink and irregular shape, indicating apoptotic-like cell death, while Nano-C particle-treated cells exhibited blackish color, flat, and swelling shape in cell body, indicating necrotic-like cell death. The Nano-C particles with the same size as Nano-Al could enter into the cell body and accumulate in the cell cytoplasma. Whereas treated by Nano-Al particles with the same chemical composition of Micro-Al and the same size with Nano-C, the neural cells displayed morphological characteristics of both Nano-C- and Micro-Al-treated cells, presenting both brownish particle accumulation and condensed nuclei and cell organelle disrupture and necrotic cell death.
Under transmission electron microscope, margination of condensed chromatin appeared in the Micro-Al-treated cells; the Nano-C-treated cells displayed nanoparticles inside the cytoplasma and nucleus, indicating the disrupture of cellular and nucleus membrane, while Nano-C-treated cells manifested the nanoparticles inside the cytoplasma with disrupted cell membrane, chromatin aggregation, and broken fragments surrounded by dissolved cytoplasma and organelles, indicating presentation of both the Nano-C- and Micro-Al-induced cell impairment features. Endocytosis appeared in Nano-Al-treated cells, and the cell bodies and their nuclei seemed to be disrupted and dissolved in the presence of the Nano-Al particles. Nanoparticles of alumina are located in the primary lysosome, secondary lysosome, and accumulated lysosomes in neural cells. The cell viability in Nano-Al-exposed cells was much lower than those in Micro-Al- and Nano-C-exposed cells (p < 0.05, p < 0.01). However, there was no significant difference between the viabilities of cells treated with Nano-C and Micro-Al (p > 0.05).
9.3.5 Aluminum Nanoparticles Induce Activation of MAPK Signal Pathway or Inhibit It
In a study performed by Jung-Taek Kwon [28], rats were exposed to Al-NPs by nasal instillation at 1 mg/kg body weight (low exposure group), 20 mg/kg body weight (moderate exposure group), and 40 mg/kg body weight (high exposure group), for a total of three times, with a 24-h interval after each exposure. Inductively coupled plasma mass spectrometry (ICP-MS) analysis indicated that the presence of aluminum in the olfactory bulb and the brain of Al-NP-treated mice was increased dose dependently. In microarray analysis, the regulation of mitogen-activated protein kinase (MAPK) activity was significantly overexpressed in the Al-NP-treated mice than in the controls (p = 0.0027). Moreover, Al-NPs induced the activation of ERK1 and p38 MAPK protein expression in the brain, but did not alter the protein expression of JNK, when compared with the controls. The results demonstrate that the nasal exposure of Al-NPs can permeate the brain via the olfactory bulb and modulate the gene and protein expression of MAPK and its activity. But another study confirmed that Al-NP exposure activated the JNK pathway [29].
But, in another study [30], the effect of Nano-Al on brain energy metabolism was evaluated in alumina NP-treated and NP-untreated mouse brain homogenates via western blot. The results indicated that Nano-Al inactivated MAPK and dephosphorylated it at Thr172 and reduced the expression of AMP-activated protein kinase (AMPK) in the brain compared to the untreated mice, while the total AMPK level remained unchanged. Similarly, the AMPK activity was also reduced in the brain homogenates of Nano-Al-treated mice analyzed through the CycLex® AMPK activity assay method. Additionally, the expression of p-AMPK was measured via immunofluorescence in the hippocampal cornu ammonis 1 (CA1) and cortical regions of nanoalumina-treated and nanoalumina-untreated mice. The images of immunofluorescence revealed that nanoalumina significantly inhibited the expression of p-AMPK, which supported western blot results, suggesting that alumina nanoparticles are involved in the disturbance of brain energy metabolism.
9.3.6 Nano-Al Can Induce Oxidative Stress in Neural Cells Both In Vitro and In Vivo
To analyze whether mouse hippocampal neural cell line, HT22 cells, can take up Nano-Al and increase their aluminum level, morin staining was performed in the same study as Sect. 4.5 [30]. Exposure of HT22 cells to Nano-Al caused an increased uptake of Nano-Al, which ultimately increased the aluminum abundance in cultured HT22 cells. Furthermore, to assess whether Nano-Al induces oxidative stress in HT22 cells, 8-oxo-guanine (8-OxoG) staining was performed using an anti-8-OxoG monoclonal antibody. The immunofluorescence images show that Nano-Al induced oxidative stress and produced a significantly high number of ROS in the Al-NP-treated HT22 cells in contrast to untreated HT22 cells.
In an in vivo study performed by the same authors, Nano-Al was peripherally administered to ICR female mice for three weeks. The immunohistological evaluations for abundance of brain aluminum were conducted in the hippocampal CA1, CA3, and dentate gyrus (DG) and cortical regions of the female mouse brain. The results indicated that exogenously administered nanoalumina significantly increased brain aluminum abundance compared with the untreated control mice. In vivo 8-OxoG staining was performed to analyze the extent of oxidative stress induced by nanoalumina, and it was evident from the immunostaining images that Nano-Al induced oxidative stress by increasing 8-OxoG expression in the brains of Al-NP-exposed mice. This trend was mainly observed in different parts of the hippocampus including CA1 and CA3, respectively, and DG and the cortical regions in Al-NP-treated mice, while no or only a few 8-OxoG appearances were seen in the hippocampus and cortical regions in the untreated control mice.
9.3.7 Nano-Al Can Induce Aβ Production via Amyloidogenic Pathway in Mice
The same authors of Sect. 4.6 study further to investigate Aβ production via the amyloidogenic pathway as a consequence of Nano-Al treatment in mice. The western blot results showed that the administration of nanoparticles to mice enhanced the amyloidogenic pathway of Aβ production. Nano-Al upregulated the expression of the amyloid precursor protein (APP) and β-secretase beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) activity, which significantly increased the generation of Aβ in treated mice compared with untreated controls. Moreover, it also caused downregulation of the α-secretase enzyme sAPP-α, which is responsible for the generation of nontoxic Aβ peptides through a non-amyloidogenic pathway. The levels of soluble Aβ1–42 in the brain homogenates were measured through the ELISA method and revealed that Nano-Al significantly increased the production of Aβ1–42. Interestingly, Nano-Al also caused the formation of Aβ aggregation and plaques in the hippocampus and cortical regions of the brain in Al-NP-treated mice, which was measured immunohistopathologically both via the Aβ (6E10) antibody and thioflavin S staining. The effect of increase in the Aβ level produced by Nano-Al on the hyperphosphorylation of microtubule-associated tau at ser413 and synapse related proteins, including synaptophysin and postsynapse density protein 95 (PSD 95), was investigated via western blot. The result indicated that Aβ induced a significant increase in the expression level of p-tau (while the total tau protein level was unchanged) and downregulated the expression of synaptophysin and PSD 95 proteins in treated mice compared to the controls.
9.3.8 Nano-Al Induced Neurodegeneration In Vivo
The toxic effect of the administered Nano-Al on inducing neurodegeneration was further examined by Shahid Ali Shah, who analyzed the expression of apoptotic markers via western blot. The results indicated that Nano-Al significantly upregulated the expression of various apoptotic markers or apoptotic signals, such as cleaved caspase-3 and cleaved PARP-1, in the hippocampus and cortical sections of the mouse brain. However, caspase-3 and PARP-1 were less expressed in untreated mice. Additionally, Fluoro-Jade B (FJB) staining was performed to investigate the neurodegeneration in the hippocampus and cortical regions in mice by the administered Nano-Al. The staining of the cortical and hippocampal CA1, CA3, and DG regions of nanoalumina-treated mice revealed that the alumina nanoparticles induced neuronal cell death, as was evidenced by the number of positive FJB cells, whereas no such positive stainings were observed in the brain sections of the control mouse.
References
Doelken V. Ueber die wirkung des aluminiums mit besonderer berucksichtigung der durch das aluminium verursachten lasionen im centralnerven-system. Arch Exp Pathol Pharmakol. 1898;40:98–120.
Frisardi V, Solfrizzi V, Capurso C, Kehoe PG, Imbimbo BP, Santamato A, Dellegrazie F, Seripa D, Pilotto A, Capurso A, Panza F. Aluminum in the diet and Alzheimer’s disease: from current epidemiology to possible disease-modifying treatment. J Alzheimers Dis. 2010;20(1):17–30.
Walton JR. Brain lesions comprised of aluminum-rich cells that lack microtubules may be associated with the cognitive deficit of Alzheimer’s disease. Neurotoxicology. 2009;30(6):1059–69.
Esparza JL, Gomez M, Romeu MR, Mulero M, Sanchez DJ, Mallol J, Domingo JL. Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J Pineal Res. 2005;39(2):129–36.
Walton JR. An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J Inorg Biochem. 2007;101(9):1275–84.
Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R, Shankar SK, Stein R, Shanmugavelu P, Jagannatha Rao KS. Aluminium in Alzheimer’s disease: are we still at a crossroad? Cell Mol Life Sci. 2005;62(2):143–58.
Dörre E, Hübner H. Alumina processing, properties, and applications. Berlin: Springer; 1984.
Meziani MJ, Bunker CE, Lu F, Li H, Wang W, Guliants EA, Quinn RA, Sun YP. Formation and properties of stabilized aluminum nanoparticles. ACS Appl Mater Interfaces. 2009;1(3):703–9.
Nanotechnology – Project on Emerging Nanotechnologies. Inventory FindsIncrease in Consumer Products Containing Nanoscale Materials [http://www.nanotechproject.org/inventories/consumer/updates/]. Date accessed: 28 Apr 2016.
Feasibility analysis and development tendency prediction of nano-alumina projects in China and World (2016 edition) http://www.baogaochina.com/List_YeJinBaoGao/33/NaMiYangHuaLvHangYeXianZhuangYuFaZhanQianJing.html
Maynard AD. Nanotechnology: the next big thing, or much ado about nothing? Ann Occup Hyg. 2007;51(1):1–12.
Stanley JK, Coleman JG, Weiss CA, Steevens JA. Sediment toxicity and bioaccumulation of nano and micron-sized aluminum oxide. Environ Toxicol Chem. 2010;29(2):422–9.
Balasubramanyam A, Sailaja N, Mahboob M, Rahman MF, Hussain SM, Grover P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol In Vitro. 2010;24(6):1871–6.
McLeish JA, Chico TJ, Taylor HB, Tucker C, Donaldson K, Brown SB. Skin exposure to micro- and nano-particles can cause haemostasis in zebrafish larvae. Thromb Haemost. 2011;103(4):797–807.
Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut. 2009;157(5):1619–25.
Li X, Zhang C, Zhang X, Wang S, Meng Q, Wu S, Yang H, Xia Y, Chen R. An acetyl-L-carnitine switch on mitochondrial dysfunction and rescue in the metabolomics study on aluminum oxide nanoparticles. Part Fibre Toxicol. 2016;13:4. doi:10.1186/s12989-016-0115-y.
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65(20):1531–43.
Braydich-Stolle LK, Speshock JL, Castle A, Smith M, Murdock RC, Hussain SM. Nanosized aluminum altered immune function. ACS Nano. 2010;4(7):3661–70.
Park EJ, Sim J, Kim Y, Han BS, Yoon C, Lee S, Cho MH, Lee BS, Kim JH. A 13 week repeated dose oral toxicity and bioaccumulation of aluminum oxide nanoparticles in mice. Arch Toxicol. 2015;89:371–9.
Huan L, Yong D, Xiaohong W, Yaxian P, Cuicui G, Wang H, Wenhui W, Qi Z, Qiao N, Qinli Z. Impact of Nano-alumina particles on variations of immune indices in mice. J Toxicol. 2015;29(2):114–8.
Zhang QL, Li MQ, Ji JW, Gao FP, Bai R, Chen CY, Wang ZW, Zhang C, Niu Q. in vivo toxicity of nano-alumina on mice neurobehavioral profiles and the potential mechanisms. Int J Immunopathol Pharmacol. 2011;24(1S):23–9.
Zhang X, Xu Y, Zhou L, Zhang C, Meng Q, Wu S, Wang S, Ding Z, Chen X, Li X, Chen R. Sex-dependent depression-like behavior induced by respiratory administration of aluminum oxide nanoparticles. Int J Environ Res Public Health. 2015;12(12):15692–705.
Yinxia L, Shunhui Y, Quili W, Meng T, Yuepu P, Dayong W. Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans. J Hazard Mater. 2012;219– 220:221–30.
Chen L, Yokel RA, Hennig B, Toborek M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol. 2008;3:286–95.
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–45.
Wang B, Feng WY, Wang M, Shi JW, Zhang F, Ouyang H, Zhao YL, Chai ZF, Huang YY, Xie YN, Wang HF, Wang J. Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res. 2007;118(3):233–43.
Zhang Q, Xu L, Wang J, Sabbioni E, Piao L, Di Gioacchino M, Niu Q. Lysosomes involved in the cellular toxicity of nano-alumina: combined effects of particle size and chemical composition. J Biol Regul Homeost Agents. 2013;27(2):365–75.
Kwon J-T, Seo G-B, Jo E, Lee M, Kim H-M, Shim I, Lee B-W, Yoon B-I, Kim P, Choi K. Aluminum nanoparticles induce ERK and p38MAPK activation in rat brain. Toxicol Res. 2013;29(3):181–5.
Kleinmana MT, Araujob J, Nelc A, Sioutasd C, Campbelle A, Conga PQ, Lia H, Bondya SC. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178(2):127–30.
Shah SA, Yoon GH, Ahmad A, Ullah F, Ul Amin F, Kim MO. Nanoscale-alumina induces oxidative stress and accelerates amyloid beta (Aβ) production in ICR female mice. Nanoscale. 2015;7:15225.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Niu, Q., Zhang, Q. (2017). Combined Effect on Immune and Nervous System of Aluminum Nanoparticles. In: Otsuki, T., Petrarca, C., Di Gioacchino, M. (eds) Allergy and Immunotoxicology in Occupational Health. Current Topics in Environmental Health and Preventive Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-10-0351-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-0351-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-0349-3
Online ISBN: 978-981-10-0351-6
eBook Packages: MedicineMedicine (R0)