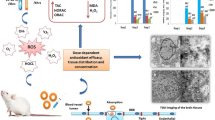
Abstract
Aluminum nanoparticles (AlNPs) are among the most abundantly produced nanosized particles in the market. There is limited information about the potential harmful effects of aluminum oxide due to its particle size on human health. Considering the toxic effects of Al on brain as its target tissue, in this study, the toxicity of nanoparticles, microparticles, and ionic forms of Al on rat brain and isolated mitochondria was evaluated. Sixty male Wistar rats were divided into ten groups (six rats each), in which group I was the control, and the other groups were administered different doses of Al nanoparticles, Al microparticles (AlMP), and Al ionic forms (2, 4, and 8 mg/kg, i.p.) for 28 days. After 24 h, the animals were killed, brain tissue was separated, the mitochondrial fraction was isolated, and oxidative stress markers were measured. Also, mitochondrial function was assayed by MTT test. The results showed that all forms of Al particles induced ROS formation, lipid peroxidation, protein oxidation, glutathione depletion, mitochondrial dysfunction, and gait abnormalities in a dose-dependent manner. In addition, Al particles decreased mitochondrial membrane potential. These data indicated that oxidative stress might contribute to the toxicity effects of Al. Comparison of oxidative stress markers between all forms of Al revealed that the toxic effect of AlNP on brain tissue was substantially more than that caused by AlMP and bulk form. This study showed more neurotoxicity of AlNPs compared to other forms on brain oxidative damage that probably is due to more penetration into the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials, engineered materials with dimensions less than 100 nm [1], have gained increasing attention because of their unique physicochemical properties [1] and high reaction activity [2, 3]. These properties lead to potential application in vast areas of research, industry [1], and commodities [4,5,6]. In fact, the use of nanoparticles or nanocomposites in industrial activities or for health products has been increased. Therefore, the survey of the effects of these nanomaterials, especially their toxic effects on experimental animals or humans, gained special attention [7]. On this basis, the Organization for Economic Cooperation and Development (OECD) has introduced 14 nanomaterials, including aluminum oxide nanoparticles (AlNPs), with high priority for study. AlNPs are one of the most plentiful produced nanoparticles which accounted for approximately 20% of the global market for nanoparticles in 2005 [8]. However, limited information is available on the potential hazards of AlNPs [1].
Aluminum oxide (Al2O3) nanoparticles have been used in different fields such as catalysis, ceramics, polymer modification, heat transfer fluids, and wastewater treatment. In addition, Al2O3 nanoparticles have shown vast biological applications in biosensors, biofiltration, drug delivery, and antigen delivery for immunization purposes [9]. Thus, the increase in annual production and applications of AlNPs leads to elevation of the occupational and environmental exposures to AlNPs. There are increasing evidences about neurotoxicity of Al in humans and rodents [10, 11]. It has been shown that Al accumulates in all regions of rat brain following chronic exposure, maximum being in hippocampus, which is the site of memory and learning [12,13,14,15]. Besides, neurological dysfunctions that are reported in dialysis patients probably are related to high Al concentrations in the dialysate and to the use of phosphate binding gels containing Al [16]. In addition, several studies exhibited that Al-contaminated drinking water is a risk factor for Alzheimer’s disease [17,18,19].
One of the most important mechanisms involved in the toxic effects of metal nanoparticles is their ability to cause oxidative stress [20,21,22,23] and mitochondrial dysfunction. Previous studies showed that Al exposure is associated with impairment of mitochondrial functions in vitro [24] and in vivo [25] and also impairs antioxidant defense system [26].
Mitochondria are the major sources of reactive oxygen species (ROS) and energy production in cells. Moreover, mitochondria are the main targets for ROS-induced cellular injury [27], because mitochondria lack protective structural proteins. Toxic materials could induce electron transport chain dysfunction and lead to increased ROS production and oxidation of mitochondrial DNA, proteins, and lipids. These events result in the opening of mitochondrial permeability transition pore and imitation of cell death signaling [28].
In recent years, studies on size-dependent toxicity between micro- and nanoscale particles showed that toxicity of nanoparticles is higher compared to larger particles and affirmed the hypothesis that nanoparticles in general are more potent in causing damage. All these evidences suggested that nanoparticles of the same or different size exhibited varied toxic responses, but the studies with reference to their micron size are lacking [29].
The aim of this study was to compare the neurotoxicity of Al oxide as nano- and micrometer size, additionally ionic form of Al2O3 particles in rats on the basis of inducing mitochondrial oxidative damage.
Methods
Chemicals
Al2O3-NPs were purchased from Neutrino (Iran; 99.995% purity and density 3.6 g/cm3). Nanoalumina was used in the ultrasonicated form, with diameter < 20 nm (9.83 ± 1.61 nm) and specific surface area (SSA) 90–160 m2/g.
Animal Treatment
Male Wistar rats (200–250 g) were housed in an air-conditioned room with controlled temperature of 22 ± 2 °C and maintained on a 12:12-h light cycle with free access to food and water. All experimental procedures were conducted according to the ethical standards and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran.
All efforts were made to minimize the number of animals and their suffering. Animals were randomly divided into ten groups of six animals and the groups were as follows: control group, different doses (2, 4, 8 mg/kg) of Al bulk form groups, Al microparticles groups (AlMP) with size < 1000 nm, and different doses of AlNPs group (< 100 nm). The animals were maintained in their respective groups for 4 weeks. Food and fluid intake and body weights were measured weekly. At the end of the experimental period, the animals were anesthetized using ether and sacrificed by cervical decapitation. Brain tissue was separated, minced, and homogenized with a glass handheld homogenizer. Some parts were used for mitochondrial preparation using a differential centrifugation technique [30]. The isolation of mitochondria was confirmed by the measurement of succinate dehydrogenase [31]. The biochemical parameters determined included total protein, reactive oxygen species (ROS), lipid peroxidation, glutathione (GSH), and protein carbonyl. Brain-isolated mitochondrial function was assayed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. In addition, the assessment of mitochondrial membrane potential and the behavioral index evaluation were done.
The Behavioral Index (Gait Scores) Evaluation
At the end of animal treatment, the gait scores were evaluated according to the methods described by Mehri et al. [32]. Rats were placed in a clear plexiglass box and observed for 3 min. Following observation, a gait score was assigned from 1 to 4, where 1 = a normal, unaffected gait, 2 = a slightly affected gait (foot splay, slight hind limb weakness, and spread), 3 = a moderately affected gait (foot splay, moderate hind limb weakness, and moderate limb spread during ambulation), and 4 = a severely affected gait (foot splay, severe hind limb weakness, dragging hind limbs, and inability to rear).
Total Protein Assay
Protein concentrations were determined by the Coomassie Blue protein binding method as explained by Bradford [33].
Quantification of Reactive Oxygen Species Level
The ROS level measurement was performed using dichloro-dihydro-fluorescein diacetate (DCFH-DA) as an indicator. Briefly, DCFH-DA was added (final concentration, 10 μM) to samples (1 mg protein/ml) and then incubated for 10 min. The amount of ROS generation was determined through a Shimadzu RF5000U fluorescence spectrophotometer at 485 nm excitation and 520 nm emission wavelength; the results were expressed as the fluorescent intensity per 1 mg protein [34].
Measurement of Glutathione Content
GSH content was determined using DTNB as the indicator, and the developed yellow color was read at 412 nm on a spectrophotometer (UV-1601 PC, Shimadzu, Japan). GSH content was expressed as micromolar [35].
Measurement of Lipid Peroxidation
The content of malondialdehyde (MDA) was determined by the thiobarbituric acid (TBA) reactive substances expressed as the extent MDA of productions during an acid-heating reaction. The amount of MDA formed in each of the samples was assessed by measuring the absorbance of the supernatant at 532 nm with an ELISA reader (Tecan, Rainbow Thermo, Austria). Tetramethoxypropane was used as a standard and MDA content was expressed as micromolar [36].
Protein Carbonyl
Determination of protein carbonyl was done by spectrophotometric method using guanidine hydrochloride. The carbonyl content was determined by reading the absorbance at 365 nm wavelength [37].
Assessment of Mitochondrial Toxicity
Mitochondrial toxicity was assessed by measuring the reduction of MTT with minor modification of Ghazi-Khansari et al. 2006. This assay is a quantitative colorimetric method to determine the mitochondrial functionality that the yellow tetrazolium salt (MTT) is metabolized from mitochondrial succinate dehydrogenase to purple formazan. The product of formazan crystals was dissolved in 100 μL dimethyl sulfoxide, and the absorbance at 570 nm was measured with an ELISA reader (Tecan, Rainbow Thermo, Austria) [38].
Determination of the MMP
Mitochondrial uptake of the cationic fluorescent dye, rhodamine 123, has been used for the estimation of mitochondrial membrane potential. Rhodamine 123 (10 μM) was added to mitochondrial suspension in MMP assay buffer (220 mM sucrose, 68 mM d-mannitol, 10 mM KCl, 5 mM KH2PO4, 2 mM MgCl2, 50 μM EGTA, 5 mM sodium succinate, 10 mM HEPES, 2 μM rotenone). The fluorescence was monitored using Shimadzu RF-5000U fluorescence spectrophotometer at the excitation and emission wavelength of 490 and 535 nm, respectively [39].
Statistical Analysis
Results were expressed as mean ± SD, and statistical analyses were performed with ANOVA, followed by Tukey-Kramer test to compare the differences between means. For behavioral index assay, nonparametric test (Kruskal-Wallis test) was used for statistical analysis. Differences were considered statistically significant when P < 0.05.
Results
The effects of AlNP and Al bulk particles (AlBP) on body weight gain, food, and water intake and relative brain weight in rats are shown in Table 1. In all AlNP, AlMP, and AlBP-treated rats, water and pellet diet consumption significantly (P < 0.05) decreased that was accompanied with a decrease in body weight.
The Behavioral Index (Gait Scores)
Administration of AlNP, AlMP, and AlBP for 28 days induced progressive gait abnormalities in rats as shown in Fig. 1. Treatment of animals with AlNP induced more gait abnormalities than AlBP and AlMP groups (P < 0.5).
Reactive Oxygen Species Level
After 28 days, exposure of rats to different doses of AlBPs, AlMPs, and AlNPs showed an increase in ROS generation in brain tissues in a dose-dependent manner in comparison to control group. Also, AlNPs at doses of 2, 4, and 8 mg/kg showed significant increase in ROS formation in comparison to control group (P < 0.05). But, AlMPs and AlBPs at dose of 8 mg/kg showed significant (P < 0.05) increase in ROS formation in comparison to control group (Fig. 2).
Lipid Peroxidation
The administration of different doses of AlBPs, AlMPs, and AlNPs caused an increase in the level of MDA in the brain of rats compared to control groups. These effects were significant at doses of 4 and 8 mg/kg for all forms of Al (Fig. 3).
Protein Carbonyl
Concerning protein carbonyl content, it showed a significant increase at different doses of Al (BPs, MPs, and NPs) compared with control group. Although, AlNPs in all doses showed more toxic effects than other forms of Al (Fig. 4).
Effect of AlBPs, AlMPs, and AlNPs on protein carbonyl concentration in brain tissue of rats after 28 days. Mean ± SD with the same small letter on different pattern columns is not significantly different. Mean ± SD with the same capital letter on the same pattern column is not significantly different
Glutathione Content
Regarding the GSH concentration, the results showed a significant decrease at 8 mg/kg of AlBPs, AlMPs, and AlNPs (Fig. 5). An analysis of variance between control and groups which received different doses of AlBPs, AlMPs, and AlNPs showed a significant difference in all studied oxidative biomarkers in brain tissues, except in GSH level where no significant difference was observed between AlBP, AlMP, and AlNP groups.
Mitochondrial Toxicity
As shown in Fig. 6, the effects of Al on mitochondrial function were assessed using MTT assay. A decrease in mitochondrial function in AlBPs, AlMPs, and AlNPs groups was observed as a consequence of oxidative damage. Mitochondrial dysfunction increased more in Al nanoparticles group than other groups, and this increase was significant in all doses (P < 0.05).
Mitochondrial Membrane Potential
Mitochondrial membrane potential (MMP) is a highly sensitive indicator of the mitochondrial inner membrane condition; therefore, the effect of AlBP, AlMP, and AlNP on MMP was measured by Rh123 staining. As shown in Fig. 7, AlNP treatment significantly decreased the MMP (P < 0.05), and potency of AlBP and AlMP in decreasing of MMP was lower as AlNP.
Effect of AlBPs, AlMPs, and AlNPs on mitochondrial membrane potential in brain tissue of rats after 28 days. Mean ± SD with the same small letter on different pattern columns is not significantly different. Mean ± SD with the same capital letter on the same pattern column is not significantly different
Discussion
Alumina nanoparticles (NPs) are among the most produced nanomaterials that its use is increasing in a wide range of industries and biological applications [40]. The most important mechanism, involved in the toxic effects of metal nanoparticles, is their ability to cause oxidative stress [20,21,22,23]. High Al concentrations cause oxidative stress that is one of the several mechanism suggestions to Al toxicity [41,42,43]. Neurodegeneration, one of the toxic effects of Al, was induced by an increase in Fe accumulation and oxygen reactive species (ROS) production [43]. The nervous system is particularly sensitive to oxidant-mediated damage because of the following: (a) its high oxygen consumption rate (approximately 20% of total oxygen consumed), (b) brain membranes are enriched in highly oxidizable polyunsaturated fatty acids, (c) brain antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) activities are comparatively lower than those found in other tissues, and (d) the brain content of iron is high [44].
In this research, we compared the neurotoxicity of nano- and micrometer and bulk form of Al in different doses to evaluate the impact of dose and particle size of Al on brain toxicity on the basis of oxidative damage as the most important mechanism for Al toxicity.
Aluminum is suggested as a contributing factor in the pathogenesis of Alzheimer’s disease, and Al exposure affects memory and neurologic function [45]. Our results showed that the treatment of animals with AlBPs, AlMPs, and AlNPs for 28 days caused gait abnormalities and at the end of the experiment, Al-exposed rats displayed severe abnormal gait scores.
Our data in this study showed that AlBPs, AlMPs, and AlNPs caused increasing oxidative stress markers. Oxidative damage induced by Al nanoparticles were more pronounced than equal doses of AlBPs and AlMPs. The ROS formation from a particular nanomaterial is dependent on the physical and chemical properties of the nanomaterials [46]. The critical chemical and physical structural determinants of the nanomaterial that lead to the generation of ROS and toxicity include molecular size, shape, oxidation status, surface area, bonded surface species, surface coating, solubility, and degree of aggregation and agglomeration [46,47,48,49,50,51]. In fact, because of the tiny size of nanomaterials, they can easily penetrate cell membranes and other biological barriers into living organisms and cause cellular dysfunction [48, 49]. The amount of cellular uptake decreases with the increase in particle size [52, 53]. Therefore, more toxic effects of AlNPs are probably due to its penetration to brain tissue and also the brain’s unique surface properties that lead to an increase in ROS production and oxidative stress.
Previous studies showed that exposure of cells to aluminum increased ROS formation [54,55,56]. Increased lipid peroxidation has been reported after long-term low-level aluminum exposure [57]. The product of lipid oxidation, malondialdehyde (MDA), has been quantified in rat brain exposed to aluminum [58]. Golub et al. observed no change in lipid peroxidation in both the brain and the liver in adult mice fed on high amounts of aluminum [59]. There was increased oxidative damage to mitochondrial proteins as revealed by the increased protein carbonylation [25].
Studies have shown that aluminum ions may exert their toxic effects by oxidative damage to brain cell components and thus mediate neurotoxicity [26]. In some studies, the generation of ROS in neural stem cells upon the exposure to high concentrations of alumina NPs was observed [40, 60]. The oxidative damage was also observed in the studies of other metal oxide NPs [61]. Our study showed the increase in oxidative damage parameters such as LPO, ROS, and protein carbonyl, and decrease in antioxidant system (GSH content) that confirmed the previous studies.
Besides, oxidation of the thiol groups in the mitochondrial membrane proteins leads to structural changes in the mitochondrial permeability transition (MPT) pore that result in unlimited movement of water and solute, mitochondrial swelling, and mitochondrial membrane potential (MMP) collapse [62].
Our finding showed that AlNPs, AlMPs, and AlBPs decreased MMP, and AlNPs showed more toxic effects in decreasing of MMP than other groups.
The induction of MPT can be associated with the release of apoptogenic factor such as cytochrome c into cytosol and trigger of both necrosis and apoptosis pathways [63]. The increase in oxidative stress markers and also the collapse of MMP was directly related to increase in gait score in treated animals and decrease in weight gain and brain weight.
On the other hand, several studies showed that NPs are more toxic than other forms of metals [64, 65]. In our study, significant difference was observed between AlNPs other forms of Al in inducing oxidative stress.
Conclusion
Our study showed the role of mitochondrial oxidative damage in Al-induced neurotoxicity in rats. Also, the comparison of neurotoxicity between nanoparticles of Al with its ionic form and microparticles showed more neurotoxicity of nanoparticles than other forms that probably is related to its more penetration into the brain and more reactivity of nanoparticles.
References
Park E-J, Lee G-H, J-h S, Cho M-H, Lee B-S, Kim Y-B, Kim J-H, Kim Y, Kim D-W (2015) Comparison of the toxicity of aluminum oxide nanorods with different aspect ratio. Arch Toxicol 89(10):1771–1782
Yan L, Zheng YB, Zhao F, Li S, Gao X, Xu B, Weiss PS, Zhao Y (2012) Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chem Soc Rev 41(1):97–114
Amelia M, Lincheneau C, Silvi S, Credi A (2012) Electrochemical properties of CdSe and CdTe quantum dots. Chem Soc Rev 41(17):5728–5743
Joh DY, Kinder J, Herman LH, Ju S-Y, Segal MA, Johnson JN, Chan GK-L, Park J (2011) Single-walled carbon nanotubes as excitonic optical wires. Nat Nanotechnol 6(1):51–56
Tang F, Li L, Chen D (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 24(12):1504–1534
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Doudi M, Setorki M (2014) Acute effect of nano-copper on liver tissue and function in rat. Nanomedicine J 1 (5): 331-338
Oesterling E, Chopra N, Gavalas V, Arzuaga X, Lim EJ, Sultana R, Butterfield DA, Bachas L, Hennig B (2008) Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol Lett 178(3):160–166
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166
Perry CC, Keeling-Tucker T (1998) Aspects of the bioinorganic chemistry of silicon in conjunction with the biometals calcium, iron and aluminium. J Inorg Biochem 69(3):181–191
Becaria A, Campbell A, Bondy S (2002) Aluminum as a toxicant. Toxicol Ind Health 18(7):309–320
Lal B, Gupta A, Murthy R, Ali MM, Chandra S (1993) Aluminum ingestion alters behaviour and some neurochemicals in rats. Indian J Exp Biol 31(1):30–35
Julka D, Gill K (1996) Effect of aluminum on regional brain antioxidant defense status in Wistar rats. Res Exp Med 196(1):187–194
Kaur A, Joshi K, Minz RW, Gill KD (2006) Neurofilament phosphorylation and disruption: a possible mechanism of chronic aluminium toxicity in Wistar rats. Toxicology 219(1):1–10
Sánchez-Iglesias S, Soto-Otero R, Iglesias-Gonzalez J, Barciela-Alonso MC, Bermejo-Barrera P, Méndez-Álvarez E (2007) Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J Trace Elem Med Biol 21:31–34
Jack R, Rabin PL, McKinney TD (1984) Dialysis encephalopathy: a review. Int J Psychiatry Med 13(4):309–326
Neri L, Hewitt D (1991) Aluminium, Alzheimer’s disease, and drinking water. Lancet 338(8763):390
Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D (2000) Aluminum forms in drinking water and risk of Alzheimer’s disease. Environ Res 84(3):234–246
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55(2):187–196
Jones CF, Grainger DW (2009) In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev 61(6):438–456
Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F (2009) Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations—many questions, some answers. Mutation Res/Reviews in Mutation Res 681(2):241–258
Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S (2009) Role of oxidative damage in toxicity of particulates. Free radical research
Oberdörster G, Stone V, Donaldson K (2007) Toxicology of nanoparticles: a historical perspective. Nanotoxicology 1(1):2–25
Niu P, Niu Q, Zhang Q, Wang L, He S, Wu T, Conti P, Di Gioacchino M, Boscolo P (2005) Aluminum impairs rat neural cell mitochondria in vitro. Int J Immunopathol Pharmacol 18(4):683–689
Kumar V, Bal A, Gill KD (2008) Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res 1232:94–103
Kumar V, Gill KD (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83(11):965–978
Liang F-Q, Godley BF (2003) Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res 76(4):397–403
Halliwell B (1992) Reactive oxygen species and the central nervous system. In: Free radicals in the brain. Springer, pp 21–40
Chaves S, Lacava L, Lacava Z, Silva O, Pelegrini F, Buske N, Gansau C, Morais P, Azevedo R (2002) Light microscopy and magnetic resonance characterization of a DMSA-coated magnetic fluid in mice. Magnetics, IEEE Transactions on 38(5):3231–3233
Shaki F, Hosseini M-J, Ghazi-Khansari M, Pourahmad J (2012) Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochimica et Biophysica Acta (BBA)-General Subjects 1820(12):1940–1950
Lambowitz AM (1979) [34] Preparation and analysis of mitochondrial ribosomes. Methods Enzymol 59:421–433
Mehri S, Karami HV, Hassani FV, Hosseinzadeh H (2014) Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran Biomed J 18(2):101
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Gao X, Zheng CY, Yang L, Tang XC, Zhang HY (2009) Huperzine A protects isolated rat brain mitochondria against β-amyloid peptide. Free Radic Biol Med 46(11):1454–1462
Sadegh C, Schreck RP (2003) The spectroscopic determination of aqueous sulfite using Ellman’s reagent. MURJ 8:39–43
Zhang S, Fu J, Zhou Z (2004) In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol in Vitro 18(1):71–77
Lee YW, Hennig B, Yao J, Toborek M (2001) Methamphetamine induces AP-1 and NF-κB binding and transactivation in human brain endothelial cells. J Neurosci Res 66(4):583–591
Ghazi-Khansari M, Mohammadi-Bardbori A, Hosseini MJ (2006) Using Janus green B to study paraquat toxicity in rat liver mitochondria. Ann N Y Acad Sci 1090(1):98–107
Hosseini M-J, Shaki F, Ghazi-Khansari M, Pourahmad J (2013) Toxicity of vanadium on isolated rat liver mitochondria: a new mechanistic approach. Metallomics 5(2):152–166
Yang S-T, Wang T, Dong E, Chen X-X, Xiang K, Liu J-H, Liu Y, Wang H (2012) Bioavailability and preliminary toxicity evaluations of alumina nanoparticles in vivo after oral exposure. Toxicology Res 1(1):69–74
Kawahara M, Kato-Negishi M (2011) Link between aluminum and the pathogenesis of Alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis 2011
Lukiw WJ, Pogue AI (2007) Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem 101(9):1265–1269
Wu Z, Du Y, Xue H, Wu Y, Zhou B (2012) Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiology Aging 33(1):199.e1–199.e12
Youdim M (1988) Iron in the brain: implications for Parkinson’s and Alzheimer’s diseases. Mount Sinai J medicine, New York 55(1):97–101
Sethi P, Jyoti A, Hussain E, Sharma D (2009) Curcumin attenuates aluminium-induced functional neurotoxicity in rats. Pharmacol Biochem Behav 93(1):31–39
Shaligram S, Campbell A (2013) Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol in Vitro 27(2):844–851
Ray PC, Yu H, Fu PP (2009) Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health Part C 27(1):1–35
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6(8):1794–1807
Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC (2008) Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett 463(1):145–149
Lu W, Senapati D, Wang S, Tovmachenko O, Singh AK, Yu H, Ray PC (2010) Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem Phys Lett 487(1):92–96
He C, Hu Y, Yin L, Tang C, Yin C (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13):3657–3666
Wang S-H, Lee C-W, Chiou A, Wei P-K (2010) Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnol 8(1):33
Campbell A, Prasad KN, Bondy SC (1999) Aluminum-induced oxidative events in cell lines: glioma are more responsive than neuroblastoma. Free Radic Biol Med 26(9):1166–1171
Strong MJ, Garruto RM, Joshi JG, Mundy WR, Shafer TJ (1996) Can the mechanisms of aluminum neurotoxicity be integrated into a unified scheme? J Toxicology Environ Health Part A 48(6):599–614
Prakash NT, Rao KJ (1995) Modulations in antioxidant enzymes in different tissues of marine bivalve Perna viridis during heavy metal exposure. Mol Cell Biochem 146(2):107–113
Kaiser R, Correa M, Spanevello R, Morsch V, Mazzanti C, Goncalves J, Schetinger M (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminium on different mouse brain regions. J Inorg Biochem 99:1865–1870
Candan N, Tuzmen N (2008) Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology 29(4):708–713
Golub MS, Han B, Keen CL, Gershwin ME (1992) Effects of dietary aluminum excess and manganese deficiency on neurobehavioral endpoints in adult mice. Toxicol Appl Pharmacol 112(1):154–160
Dong E, Wang Y, Yang S-T, Yuan Y, Nie H, Chang Y, Wang L, Liu Y, Wang H (2011) Toxicity of nano gamma alumina to neural stem cells. J Nanosci Nanotechnol 11(9):7848–7856
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4(1):26–49
Kowaltowski AJ, Castilho RF, Vercesi AE (2001) Mitochondrial permeability transition and oxidative stress. FEBS Lett 495(1–2):12–15
Garrido C, Galluzzi L, Brunet M, Puig P, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death & Differentiation 13(9):1423–1433
Karlsson HL, Gustafsson J, Cronholm P, Möller L (2009) Size-dependent toxicity of metal oxide particles—a comparison between nano-and micrometer size. Toxicol Lett 188(2):112–118
Zhao J, Wang Z, Liu X, Xie X, Zhang K, Xing B (2011) Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. J Hazard Mater 197:304–310
Acknowledgements
This study was extracted from Pharm.D. thesis of Mehdi Nazari.
Funding
This study was supported by a grant from the research council of Mazandaran University of Medical Sciences, Sari, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were conducted according to the ethical standards and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mirshafa, A., Nazari, M., Jahani, D. et al. Size-Dependent Neurotoxicity of Aluminum Oxide Particles: a Comparison Between Nano- and Micrometer Size on the Basis of Mitochondrial Oxidative Damage. Biol Trace Elem Res 183, 261–269 (2018). https://doi.org/10.1007/s12011-017-1142-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1142-8