Abstract
Ischemic stroke is one of the greatest contributors to death and long term disability in developed countries. Ischemia induced brain injury arises due to excessive release of glutamate and involves cell death due to apoptosis and endoplasmic reticulum (ER) stress responses. Despite major research efforts there are currently no effective treatments for stroke. Taurine, a free amino acid found in high concentrations in many invertebrate and vertebrate systems can provide protection against a range of neurological disorders. Here we demonstrate that taurine can combat ER stress responses induced by glutamate or by hypoxia/re-oxygenation in neuronal cell lines and primary neuronal cultures. Taurine decreased expression of ER stress markers GRP78, CHOP, Bim and caspase 12 in primary neuronal cultures exposed to hypoxia/re-oxygenation. In analyzing individual ER stress pathways we demonstrated that taurine treatment can result in reduced levels of cleaved ATF6 and decreased p-IRE1 levels. We hypothesized that because of the complex nature of stroke a combination therapy approach may be optimal. For this reason we proceeded to test combination therapies using taurine plus low dose administration of an additional drug: either granulocyte colony stimulating factor (G-CSF) or sulindac a non-steroidal anti-inflammatory drug with potent protective functions through signaling via ischemic preconditioning pathways. When primary neurons were pretreated with 25 mM taurine and 25 ng/mL G-CSF for I hour and then exposed to high levels of glutamate, the taurine/G-CSF combination increased the protective effect against glutamate toxicity to 88% cell survival compared to 75% cell survival from an individual treatment with taurine or G-CSF alone. Pre-exposure of PC12 cells to 5 mM taurine or 25 μM sulindac did not protect the cells from hypoxia/re-oxygenation stress whereas at these concentrations the combination of taurine plus sulindac provided significant protection. In summary we have demonstrated the protective effect of taurine in primary neuronal cultures against hypoxia with re-oxygenation through inhibition of ATF6 or p-IRE-1 pathway but not the PERK pathway of ER stress. Furthermore the combinations of taurine plus an additional drug (either G-CSF or sulindac) can show enhanced potency for protecting PC 12 cells from glutamate toxicity or hypoxia/re-oxygenation through inhibition of ER stress responses.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Stroke is the third cause of mortality world-wide and is a leading cause of disability (Go et al. 2014). Despite intense research efforts there are to date no effective therapies for stroke. Cerebral hypoxia/ischemia results in depleted oxygen and glucose availability and induces excessive release of glutamate and other neurotransmitters. Subsequent activation of post-synaptic glutamate receptors acts as a trigger for activation of major downstream signaling cascades resulting in neuronal cell death (Nicholls and Attwell 1990).
Taurine (2-amino ethanesulfonic acid) is one of the most abundant amino acids found in mammalian brain, skeletal muscle and cardiac muscle (Sturman 1993; Huxtable 1992). Taurine has been employed in the treatment of a range of neurological diseases including Alzheimer’s disease, Huntington’s disease and ischemic stroke (Takatani et al. 2004; Paula-Lima et al. 2005; Takahashi et al. 2003). The physiological functions of taurine include neuro-modulation, prevention of cellular calcium overload, osmoregulation, neurotransmission and neuroprotection (Oja and Saransaari 1996; Okamoto et al. 1983; Kumari et al. 2013). Taurine has also been reported to contribute to membrane stabilization and detoxification and to counteract the effects of oxidative stress in the brain (Moran et al. 1987; Chen et al. 2001). We have previously demonstrated that taurine can protect primary cortical neurons from hypoxia and glutamate induced endoplasmic reticulum stress (ER stress) induced by oxidative stress (Pan et al. 2010b).
Granulocyte colony stimulating factor (GCSF) is a growth factor that is clinically in use for treatment of neutropenia (Metcalf 1990). GCSF can cross the blood brain barrier and GCSF demonstrates important actions in the CNS through binding to the GCSF receptor on Neuronal cells. Increasing evidence indicates that GCSF is neuroprotective as well as neuroregenerative and GCSF has been found to elicit protection in a number of neurological disease models including those for Parkinson’s disease, Huntington’s disease and ischemic stroke (Schäbitz et al. 2003). A further additional candidate of great potential value for use in neuro-protection in the CNS is sulindac, a well-known anti-inflammatory drug and inhibitor of COX1 and COX2. It has been demonstrated that sulindac acts as an anti-cancer agent while also possessing the property of protecting normal cells by pro-survival pathways that include ischemic preconditioning pathways (Tinsley et al. 2011; Moench et al. 2009).
In our previous investigations we demonstrated neuroprotection by taurine against ER stress induced by glutamate treatment of primary cortical neurons (Pan et al. 2010a, 2012). In our subsequent studies we have employed primary cortical neurons and PC12 cells to characterize the mechanisms of neuroprotection by taurine and taurine containing drug combinations (Pan et al. 2012). We have tested the hypotheses that other neuroprotective including sulindac and GCSF may demonstrate enhanced protection when combined with taurine using in vitro models of stroke (Pan et al. 2010b). To examine the potential of taurine plus sulindac to elicit protection of PC12 cells against hypoxia/re-oxygenation we have tested low doses of these drugs that show no protective effects individually to determine whether the combination of taurine and sulindac at these doses may elicit synergistic neuroprotective effects.
2 Materials and Methods
2.1 Primary Neuronal Cell Culture
Primary neuronal cultures were prepared by standard methods. Briefly pregnant rats were euthanized after isoflurane exposure and embryos at days E16–E18 were removed and brains were isolated from the fetuses. Brains were placed in Basal Medium Eagle supplemented with 2 mM glutamine, 6.8 mM glucose and 20% heat—inactivated fetal bovine serum. Cortices were dissociated by passing through a 14-G cannula. Cells were centrifuged at 300 g/min for 5 min at room temperature after which the pellet was re-suspended in GME for plating in tissue culture plates that had been pre-coated with 5 μg/mL of poly-D- lysine. Cells were then maintained for 1 h in a humidified incubator (37 °C, 99% humidity and 5% CO2) after which incubation medium was replaced with serum free neurobasal medium supplemented with 2% B27 and 500 μM glutamine. The cells were then maintained in an incubator for 12–18 days until they were ready for use in experimental analysis (Hartung 1998).
2.2 PC12 Cell Culture
PC12 cells were maintained at 37 °C/5% CO2 in F12-K medium supplemented with 2.5% (v/v) fetal bovine serum (FBS), 15% (v/v) penicillin—streptomycin solution. All experiments were performed on undifferentiated cells plated at a density of approximately 5 × 10e4 cells/ell for western blot for 4 h before starting the experiments. The 96 well plates or petri dishes were pre-coated with poly-d-lysine before plating.
2.3 Hypoxia and Re-oxygenation
To provide a hypoxic environment 14-day cultured neurons in 6 or 96 well plates were placed in a hypoxia chamber with oxygen levels maintained at 0.3–0.4%. The level of oxygen was continuously monitored using an oxygen electrode. Primary cortical neuronal cultures in the presence or absence of appropriate drug treatment conditions were subjected to 20 h of hypoxia. Re-oxygenation was carried out by removing the cultures from the hypoxia chamber and transferring them to a normal culture incubator for another 20 h. For taurine plus sulindac combination experiments, cells were pre-exposed to taurine or sulindac alone or both taurine and sulindac for 24 h prior to hypoxia/re-oxygenation exposure.
2.4 Glutamate Toxicity
To elicit glutamate induced toxicity neurons at 14 days in culture ere pre-incubated with different concentrations of drug treatment for 1 h. The neurons were then treated with 100 μM glutamate for another 1 h or 10 min.
2.5 ATP Assay for Measurement of Cell Viability
Primary cortical neuronal cells in 96 well dishes were subjected to drug treatment for 1 h and then cells were subjected to glutamate toxicity or to hypoxia/re-oxygenation to induce cell death. ATP solution (Promega) as added to each well and cells were incubated for 10 min after which the amount of ATP was quantified in a luciferase reaction. The luminescence intensity was measured using a luminometer with lysates in a standard opaque walled multi-well plate. The ATP content was determined by running an internal standard and expressed either in raw luminescence units or as a percentage of untreated cells (control).
2.6 Western Blot Analysis
PC12 cultures were lysed in RIPA buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1% SDS) containing 1% mammalian protease inhibitor cocktail from Sigma and separated on SDS-PAGE followed by transfer to a nitrocellulose membrane. The membrane as then blocked in blocking buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20, 5% milk) for 1.5 h at room temperature. After blocking, membranes were incubated with primary antibodies for 1 h followed by a 1 h incubation with the corresponding HRP-conjugated secondary antibody at room temperature. Extensive washes with blocking buffer were performed between each step. The protein immuno-complex was visualized using ECL detection reagent purchased from Thermo Scientific. Quantitative Western Blot results were obtained by densitometric analysis using image processing and analysis in Java (Image J).
2.7 Statistical Analysis
All data were expressed as mean ± SEM. The statistical significance of the data was determined with Student’s t-test of by one- or two-way ANOVA combined with Dunnett post hoc or Tukey post hoc test to compare means between groups.
3 Results
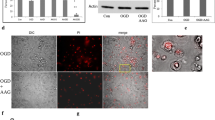
In cortical neurons treated with hypoxia and re-oxygenation and tested with a range of doses of taurine we previously established that culturing in the presence of 10 mM taurine would increase cell survival as measured by ATP assay from 49% without taurine up to the level of 85% cell viability. On exposure of cultures to hypoxia/re-oxygenation, we showed that expression of CHOP, caspase 12 and cleaved caspase 12 were highly induced and that pretreatment with taurine resulted in a significant reduction of CHOP, caspase 12 and cleaved caspase 12 pointing to a decrease in apoptosis resulting from ER stress (Pan et al. 2012). The major ER stress induced signaling pathways PERK (as measured by quantification of eIF2-alpha and ATF4), ATF6 and IRE1 (shown as cleaved ATF6 to ATF6 ratio) and levels of IRE-1 as measured by levels of phosphorylated IRE1 (pIRE-1) were substantially increased by hypoxia/re-oxygenation (Fig. 1a, b, c, d). Taurine pre- treatment resulted in a large decrease in cleaved ATF6/ATF6 ratio. pIRE-1 levels fell to less than 40% of hypoxia/re-oxygenation levels (Fig. 1c, d). In contrast to the evidence for inhibition of the ATF6 and IRE-1 pathways by taurine it was found that pre-treatment with taurine did not prevent the induction of the PERK pathway components p-eIF2-alpha or ATF4 (Fig. 1a, b).
Neuroprotective effect of taurine via hypoxia and glutamate induced ER stress pathways. Taurine has no effect on the PERK pathway (reflected in levels of P-eIF2-alpha and ATF4) after hypoxia/re-oxygenation and glutamate induced ER stress pathways. Taurine has no effect on the PERK pathway (reflected in levels of p-eIF2-alpha and ATF4) after hypoxia/re-oxygenation. Levels of expressed proteins were determined by Western blot and bar graphs reflect the densitometric data for the levels of the particular molecular target. (a) P-eIF-alpha Western blot results with arbitrary units. (b) ATF4 expression Western blot results with arbitrary units. (c) Ratio of cleaved ATF6 to ATF6 expression Western blot results with arbitrary units. (d) P-IRE-1 expression Western blot results with arbitrary units. (e) Neuroprotective effects of taurine plus GCSF against glutamate—induced excitotoxicity. Primary cortical neurons were pre-incubated with 25 mM taurine plus GCSF (25 ng/mL) for 1 h and then exposed to 100 μM glutamate for 4 h. Cell survival was measured by ATP assay. Values in bar graphs represent mean ± SEM, n = 3, *p < 0.05 and **p < 0.01 versus Normoxia, ##p < 0.01 versus Hypoxia (After Pan et al. 2010a and Pan et al. 2012)
To examine the potential of taurine or taurine in combination with GCSF to elicit protection of cultured neurons we preincubated cells for 1 h with taurine at a range of concentrations from 5 to 25 mM in combination with GCSF at 10 or 25 ng/mL and then subjected the cultures to excessive glutamate exposure to elicit excitotoxicity (data not shown). Exposure of cortical neurons to 100 μM glutamate for 4 h resulted in glutamate toxicity. Pre-exposure of cells to 25 mM taurine plus 25 ng/mL GSCF for 1 h resulted in protection against glutamate toxicity and increased cell survival to 88% compared to less than 75% cell survival with taurine or GCSF treatment alone (Fig. 1e).
To study the protection by taurine or taurine combination therapy in cultured PC 12 cells exposed to hypoxia/re-oxygenation we pre-incubated cells for 30 min with 5 mM taurine in combination with sulindac at 25 μM and then subjected the cultures to 24 h hypoxia followed by 24 h of re-oxygenation. Pre-exposure of cultures to 5 mM taurine plus 25 μM sulindac protected the PC12 cells against hypoxia/reoxygenation and increased cell survival significantly compared to levels obtained for 5 mM taurine or 25 μM sulindac alone (Fig. 2). Hence taurine and sulindac in combination demonstrated a synergistic effect of protection of PC12 cells subjected to hypoxia/re-oxygenation at the low doses employed in this study (Fig. 2).
Synergistic neuroprotective effect of taurine plus sulindac enhances cell survival of PC12 cells subjected to hypoxia and re-oxygenation. PC12 cells were preincubated for 30 min with 5 mM taurine or 25 μM sulindac or a combination of 5 mM taurine plus 25 μM sulindac or with growth medium without drugs and then maintained in normoxic conditions or subjected to 24 h hypoxia with 24 h re-oxygenation. Cell viability was measured by ATP assay and expressed in raw luminescence units. Significant differences determined by ANOVA with post-hoc Tukey test.: *differs from # (p < 0.01); **differs from # (p < 0.05)
4 Discussion
Taurine, the most abundant free amino acid in the CNS is known to elicit protection for stroke and neurodegenerative disease (Birdsall 1998; Sun and Xu 2008; Sun et al. 2011). Taurine protection by antioxidant mechanisms has been previously demonstrated in myocardial mitochondria subjected to hypoxia re-oxygenation or to Mn-superoxide dismutase inhibition (Chen et al. 2009). Through its action as a GABA agonist taurine has been shown to increase GABA levels as well as to activate GABA receptors (Paula-Lima et al. 2005; Tadros et al. 2005). Taurine can also protect through preventing the increase in intracellular free calcium resulting from glutamate excitotoxicity.
We previously demonstrated that ER stress inhibition may underlie the protection by taurine against glutamate excitotoxicity (Pan et al. 2010b). In subsequent studies we have employing primary cortical neuronal cultures we demonstrated clear protection by taurine administration against cell death caused by hypoxia and re-oxygenation (Pan et al. 2012). Here we present analyses of the signaling pathways underlying the protection of cortical neurons against hypoxia/re-oxygenation that leads to ER stress pathway activation. Furthermore we analyze the contribution of individual ER stress pathways to hypoxia/re-oxygenation and determine the effect of taurine on inhibition of these pathways.
We have previously demonstrated that in primary cortical neurons treatment with excessive glutamate concentrations resulted in activation of intracellular components of an ER stress response including GRP78, CHOP, Caspase 12 and Bim (Pan et al. 2010a, 2012). The pro-apoptotic transcription factor CHOP is expressed at low levels in untreated cells and is known to be greatly induced by ER stress (Nemetski and Gardner 2007). We have shown that CHOP is increased by exposure to hypoxia/re-oxygenation and that taurine administration will decrease levels of CHOP to normoxic levels. Pro-caspase 12 resides on the ER membrane and activates caspase dependent apoptosis in response to ER stress. We demonstrated that caspase 12 or cleaved caspase 12 was induced by hypoxia/re-oxygenation and that taurine reduced levels of caspase 12 to levels found in normoxic conditions. Hence we have demonstrated the contribution of taurine to preventing cell death resulting from hypoxia/re-oxygenation through decreasing both caspase 12 and CHOP.
Treatment with combination therapies of drugs at low doses may show potential for achieving good efficacy while avoiding side effects that could result from high dose drug exposure. We have demonstrated that taurine in combination with GCSF is capable of eliciting protection of primary cortical neurons against glutamate excitotoxicity. We have extended our multi-drug studies to include pre-treatment of PC12 cells subjected to hypoxia/re-oxygenation with a combination of taurine plus sulindac. Individually 5 mM taurine and 25 μM sulindac did not show protection but the combination of taurine plus sulindac at these low doses showed significant protection. In conclusion taurine is effective in protecting neuronal cells against ER stress induced by glutamate toxicity or hypoxia/re-oxygenation and combination treatments using taurine plus GCSF and or taurine plus sulindac show very good potential for eliciting high level neuroprotection in cell culture models of glutamate excitotoxicity and hypoxia/re-oxygenation.
Abbreviations
- ER stress:
-
Endoplasmic reticulum stress
- GCSF:
-
Granulocyte colony stimulating factor
References
Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3(2):128–136
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY (2001) Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J Neurosci Res 66(4):612–619
Chen K, Zhang Q, Wang J, Liu F, Mi M, Xu H, Chen F, Zeng K (2009) Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res 1279:131–138
Go AS et al (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292
Hartung T (1998) Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol 5(3):221–225
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Kumari N, Prentice H, Wu JY (2013) Taurine and its neuroprotective role. Adv Exp Med Biol 775:19–27
Metcalf D (1990) The colony stimulating factors. Discovery, development, and clinical applications. Cancer 65(10):2185–2195
Moench I, Prentice H, Rickaway Z, Weissbach H (2009) Sulindac confers high level ischemic protection to the heart through late preconditioning mechanisms. Proc Natl Acad Sci U S A 106(46):19611–19616
Moran J, Salazar P, Pasantes-Morales H (1987) Effect of tocopherol and taurine on membrane fluidity of retinal rod outer segments. Exp Eye Res 45(6):769–776
Nemetski SM, Gardner LB (2007) Hypoxic regulation of Id-1 and activation of the unfolded protein response are aberrant in neuroblastoma. J Biol Chem 282(1):240–248
Nicholls D, Attwell D (1990) The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11(11):462–468
Oja SS, Saransaari P (1996) Taurine as osmoregulator and neuromodulator in the brain. Metab Brain Dis 11(2):153–164
Okamoto K, Kimura H, Sakai Y (1983) Taurine-induced increase of the Cl-conductance of cerebellar Purkinje cell dendrites in vitro. Brain Res 259(2):319–323
Pan C, Giraldo GS, Prentice H, Wu JY (2010a) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17
Pan C, Gupta A, Prentice H, Wu JY (2010b) Protection of taurine and granulocyte colony-stimulating factor against excitotoxicity induced by glutamate in primary cortical neurons. J Biomed Sci 17(Suppl 1):S18
Pan C, Prentice H, Price AL, Wu JY (2012) Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43(2):845–855
Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST (2005) Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 49(8):1140–1148
Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, Sommer C, Schwab S (2003) Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34(3):745–751
Sturman JA (1993) Taurine in development. Physiol Rev 73(1):119–147
Sun M, Xu C (2008) Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol 28(4):593–611
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40(5):1419–1429
Tadros MG, Khalifa AE, Abdel-Naim AB, Arafa HM (2005) Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharmacol Biochem Behav 82(3):574–582
Takahashi K, Ohyabu Y, Takahashi K, Solodushko V, Takatani T, Itoh T, Schaffer SW, Azuma J (2003) Taurine renders the cell resistant to ischemia-induced injury in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol 41(5):726–733
Takatani T, Takahashi K, Uozumi Y, Shikata E, Yamamoto Y, Ito T, Matsuda T, Schaffer SW, Fujio Y, Azuma J (2004) Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol 287(4):C949–C953
Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA (2011) Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 4(8):1275–1284
Acknowledgements
This work was supported by grant awards from the Department of Health, State of Florida and by a grant award from the AEURA Trust.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
Prentice, H. et al. (2017). Analysis of Neuroprotection by Taurine and Taurine Combinations in Primary Neuronal Cultures and in Neuronal Cell Lines Exposed to Glutamate Excitotoxicity and to Hypoxia/Re-oxygenation. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_18
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_18
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)