Abstract
Taurine is an inhibitory neurotransmitter and is one of the most abundant amino acids present in the mammalian nervous system. Taurine has been shown to provide protection against neurological diseases, such as Huntington’s disease, Alzheimer’s disease, and stroke. Ischemic stroke is one of the leading causes of death and disability in the world. It is generally believed that ischemia-induced brain injury is largely due to excessive release of glutamate resulting in excitotoxicity and cell death. Despite extensive research, there are still no effective interventions for stroke. Recently, we have shown that taurine can provide effective protection against endoplasmic reticulum (ER) stress induced by excitotoxicity or oxidative stress in PC12 cell line or primary neuronal cell cultures. In this study, we employed hypoxia/reoxygenation conditions for primary cortical neuronal cell cultures as an in vitro model of stroke as well as the in vivo model of rat focal middle cerebral artery occlusion (MCAO). Our data showed that when primary neuronal cultures were first subjected to hypoxic conditions (0.3%, 24 h) followed by reoxygenation (21%, 24–48 h), the cell viability was greatly reduced. In the animal model of stroke (MCAO), we found that 2 h ischemia followed by 4 days reperfusion resulted in an infarct of 47.42 ± 9.86% in sections 6 mm from the frontal pole. Using taurine greatly increased cell viability in primary neuronal cell culture and decreased the infarct area of sections at 6 mm to 26.76 ± 6.91% in the MCAO model. Furthermore, levels of the ER stress protein markers GRP78, caspase-12, CHOP, and p-IRE-1 which were markedly increased in both the in vitro and in vivo models significantly declined after taurine administration, suggesting that taurine may exert neuroprotection functions in both models. Moreover, taurine could downregulate the ratio of cleaved ATF6 and full-length ATF6 in both models. In the animal model of stroke, taurine induced an upregulation of the Bcl-2/Bax ratio and downregulation of caspase-3 protein activity indicating that it attenuates apoptosis in the core of the ischemic infarct. Our results show not only taurine elicits neuroprotection through the activation of the ATF6 and the IRE1 pathways, but also it can reduce apoptosis in these models.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Endoplasmic Reticulum Stress
- Middle Cerebral Artery Occlusion
- Infarct Volume
- Primary Neuronal Culture
- Local Cerebral Blood Flow
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The endoplasmic reticulum (ER) is an important subcellular organelle that is responsible for intracellular calcium homeostasis, protein secretion, and lipid biosynthesis (Ma and Hendershot 2004; Anelli and Sitia 2008). ER stress plays a crucial role in hypoxia/ischemia-induced cell dysfunction (Azfer et al. 2006; DeGracia and Montie 2004). Cerebral hypoxia or ischemia leads to a decrease of oxygen and glucose availability which in turn induces the release of glutamate at the presynaptic level. The high levels of glutamate and the subsequent excessive activation of glutamatergic postsynaptic receptors are the main cause of the death of neurons (Choi and Rothman 1990; Nicholls and Attwell 1990). Overstimulation of glutamate receptors in neuronal injury has been observed in several neurodegenerative disorders and in acute insults, and this leads to massive brain cell death related to excitatory imbalance, which occurs in stroke and epilepsy (Lipton and Paul 1994; Mattson 2003). Hypoxia triggers the accumulation of unfolded proteins in the ER, leading to the unfolded protein response (UPR) (Kaufman 1999). Pathways that are initiated in response to the UPR include activation of PKR-like endoplasmic reticulum kinase (PERK), transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1), which in turn activate distinct signaling cascades mediating the ER stress response (Wang et al. 1998; Harding et al. 2000a). In normal neuronal homeostasis, PERK, ATF6, and IRE1 activities are inhibited by binding to glucose-regulated protein 78 (GRP78), an ER chaperone. In ER dysfunction, GRP78 dissociates from PERK, ATF6, and IRE1, inducing the dimerization and phosphorylation of PERK and IRE1, and cleavage of ATF6 (P90) to ATF6 (P50). Finally these components cause more apoptosis through the action of the CHOP protein. Taurine, 2-aminoethanesulfonic acid, is a free amino acid and the most abundant amino acid present in mammalian nervous system (Wu and Prentice 2010). It has been shown that taurine can provide protection against neurological diseases, including Huntington’s disease, Alzheimer’s disease, and stroke (Lousada 2004; Tadros et al. 2005; Takahashi et al. 2003). It has been proposed not only that taurine can protect neurons against glutamate-induced neurotoxicity by preventing glutamate-induced membrane depolarization and calpain activation due to elevation of intracellular [Ca2+] but also that it can upregulate Bcl-2 and prevent apoptosis (Wu et al. 2009). Membrane integrity, intracellular calcium homeostasis, osmoregulation, and antioxidant actions are also important functions of taurine in the brain (Balkan et al. 2002; Chen et al. 2001; Moran et al. 1987; Wade et al. 1988). It has been shown that not only does taurine have its own specific receptors on the cell membrane, but also it can elicit hyperpolarization by the inward movement of chloride through GABA and glycine receptors to reduce neuronal excitability (Hussy et al. 1997; Wang et al. 2007; Wu et al. 1992). Recently, it has been shown that taurine can reduce rat neurological deficits, brain infarct volume, and also caspase-3 activities in the ischemic penumbra 24 h after middle cerebral occlusion (MCAO) (Sun and Xu 2008). Stroke and especially the ischemic stroke is one of the leading causes of serious disability and death; there has been little progress toward the development of treatments to improve its prognosis (Weant and Baker 2012). Therefore, novel therapeutic strategies may be beneficial for improving clinical outcomes. In this study, we showed that taurine can exert a protective function against hypoxia by increasing the cell viability, decreasing infarct volume, and reducing ER stress both in vitro and in vivo.
2 Material and Methods
All animal procedures were carried out in accordance with the Animal Use and Care Guidelines issued by the National Institutes of Health using a protocol approved by the Florida Atlantic University, Boca Raton, Animal Use and Care Committee.
2.1 In Vitro Study
2.1.1 Primary Neuronal Cell Culture
According to the method of Hartung et al., pregnant rats were sacrificed after isoflurane exposure, and embryos at 16–18 days were removed. Brains were isolated from the fetuses and placed in Basal Media Eagle (BME) supplemented with 2 mM glutamine, 26.8 mM glucose, and 20% heat-inactivated fetal bovine serum. This medium is referred to as growth media eagle (GME). The cortices were then dissociated by passing the tissue through a 14-G cannula. Cells were centrifuged at 300 g/min for 5 min at room temperature. The resulting pellet was resuspended in GME and plated on appropriate tissue culture plates pre-coated with 5 μg/ml of poly-d-lysine. Cells were maintained for 1 h in a humidified incubator (37°C, 99% humidity, and 5% CO2). Incubation medium was replaced with serum-free neurobasal medium supplemented glutamine, and the cells were then maintained in an incubator for 12–18 days until they were ready for handling (Hartung 1998).
2.1.2 Hypoxia and Reoxygenation
To generate hypoxic conditions, 14-day-cultured neurons in 6- or 96-well plates were placed in a hypoxia chamber with oxygen levels maintained at 0.3–0.4%. The level of oxygen was continuously monitored using an oxygen electrode. Primary cortical neuronal cultures in the absence or presence of taurine were subjected to 20 h of hypoxia. Reoxygenation was performed by removing cultured plates from the hypoxic chamber and transferring them into normal culture incubator remaining for another 20 h.
2.1.3 ATP Assay
Primary cortical neuronal cells in 96-well plates were treated with or without taurine (1, 5, and 10 mM) for 1 h, and then cells were subjected to hypoxia–reoxygenation conditions for 20 h to induce cell death. ATP solution (Promega) was added to each well, and cells were incubated for 10 min after which the amount of ATP was quantified through a luciferase reaction. The luminescence intensity was determined using a luminometer with lysates in a standard opaque-walled multi-well plate. The ATP content was determined by running an internal standard and expressed as a percentage of untreated cells (control).
2.2 In Vivo Study
2.2.1 Transient Focal Middle Cerebral Artery Occlusion (MCAO)
Male adult Sprague–Dawley rats (weighing 260–300 g, Harlem Chicago, IL) were given access to food and water ad libitum. Before surgery, rats were fasted overnight with free access to water prior to surgery, and the following day they were weighed and anesthetized by IP injection with ketamine hydrochloride (80 mg/kg body weight IP; Putney) and xylazine hydrochloride (20 mg/kg body weight IP; Vedco) (McCollum et al. 2010). During the experiment, core temperature was maintained at 37°C by a thermostatically controlled heating pad regulated via a rectal temperature probe (CMA 450). Local cerebral blood flow (LCBF) was monitored in the cerebral cortex of left hemisphere in the supply territory of the middle cerebral artery (MCA) by laser Doppler flowmeter (LDF) (Perimed Inc., OH, USA). Transient focal cerebral ischemia of the middle cerebral artery (MCA) for 2 h was induced by the suture occlusion technique (Longa et al. 1989; Sun et al. 2011). Briefly, the left common carotid artery and the left external carotid artery were exposed through a midline neck incision. A 4-0 monofilament nylon suture coated with silicon (Doccol Co., NM, USA) was inserted through an arteriectomy in the external carotid artery, gently advanced into the internal carotid artery, and positioned approximately 17 mm from the carotid bifurcation. LCBF was monitored continuously during the MCAO surgery. With the use of this technique, the tip of the suture occludes the origins of the MCA, the proximal anterior cerebral artery, and the posterior communicating artery. Reperfusion was accomplished by withdrawing the filament 2 h after MCAO (Longa et al. 1989; Sun et al. 2011).
2.2.2 Rat Treatment Schedules
After surgery, animals were allowed to recover from the anesthesia and given food and water ad libitum. Fifteen rats were randomly assigned as controls (MCAO rats which received only the vehicle, saline 0.9%), experimental (MCAO rats which received taurine, 40 mg/kg), and sham-operated (received the same surgical procedure without insertion of the silicon filament). Taurine was delivered subcutaneously to the experimental group 24 h after the reperfusion for 4 days.
2.2.3 Determination of Infarct Volume
Animals were sacrificed by isoflurane (Phoenix), and brains were removed for 2,3,5-triphenyltetrazolium chloride (TTC) staining and collecting samples for Western blot (Kramer et al. 2010). Using an adult rat brain slicer (Matrix, Zivic Instruments), brains were sectioned coronally into six 2 mm coronal slices (2, 4, 6, 8, 10, and 12 mm from the frontal pole) and incubated for 5 min in a 2% (wt/vol) solution of TTC (J.T. Baker, India) at 37°C. TTC, a water soluble salt, is reduced by mitochondrial dehydrogenases to formazan, which turns normal tissue deep red (Bederson et al. 1986; Rich et al. 2001). Thus, reduced TTC staining identifies regions of diminished mitochondrial function in the ischemic tissue. To assess lesion volume, TTC-stained slices were scanned using an HP ScanJet 5530 and analyzed by Image J analysis software (public domain software developed at NIH (http://rsbweb.nih.gov/ij/)). Lesion volume was determined as the percent of the total ipsilateral hemispheric volume as described previously (Swanson et al. 1990; O’Donnell et al. 2006). Briefly, to eliminate the effect of brain edema, the corrected infarct volume was calculated as follows: [(VR − VLn)/VR]100 in which VR is the volume of right hemisphere and VLn is the volume of nonlesioned tissue in left hemisphere (Schäbitz et al. 1999, 2000; O’Donnell et al. 2006). After the TTC experiment, while the sections were on ice, the ischemic parts of the left hemisphere (core and penumbra) and the right hemisphere (identical regions) were quickly dissected (Fig. 23.1a) (Ashwal et al. 1998).
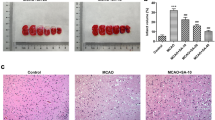
Effect of Taurine on cell viability in primary neuronal cell culture and TTC on MCAO model of stroke. (a) Core and penumbra of the lesion part of the left hemisphere. (b) Dose-dependent neuroprotection of taurine against hypoxia/reoxygenation. (1) Control; (2) hypoxia; (3) hypoxia + 1 mM taurine; (4) hypoxia + 5 mM taurine; (5) hypoxia + 10 mM taurine. Cell viability was measured by ATP assay. Control values were fixed at 100%. The values for Hyp and Tau + Hyp were normalized relative to the control values and represent mean ± SEM of five preparations (**p < 0.01 versus hypoxia). (c) Effects of taurine on infarct volume of 6 mm section on day 4 of reperfusion after 2 h of focal cerebral ischemia. Vehicle or taurine was injected subcutaneously 24 h after ischemia. The infarct zone was displayed by TTC staining in treated rats. Sham-operated group showed no infarct zone. Representative images are slices of 6 mm section from the frontal pole. Data were presented as mean ± SD, n = 16 (**p < 0.05 vs. vehicle)
2.2.4 Western Blot Analysis
Primary cortical neuronal cultures and rat brain samples were lysed in RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 1% (v/v) mammalian protease inhibitor cocktail and 1% (v/v) phosphatase inhibitor cocktail from Sigma and Thermo Scientific, respectively. Proteins in cell lysates were separated on a SDS-PAGE. After proteins were transferred to a nitrocellulose membrane, the membrane was then blocked in blocking buffer (20 mM Tris–HCl, 150 mM NaCl, 0.1% Tween-20, 5% milk) for 1.5 h at room temperature. After blocking, membranes were incubated overnight with following antibodies: GRP78 and p-IRE1(1:2,000; abcam), CHOP and p-Perk (1:1,000; Cell Signaling) and caspase-12 (1:500; Santa Cruz Biotechnology). Membranes were then incubated with ECL horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:3,000; GE Healthcare, UK) for 90 min in room temperature. GAPDH (1:3,000; Cell Signaling) and β-actin (1:2,000; Santa Cruz Biotechnology) were used as internal controls. Extensive washes with blocking buffer were performed between each step. The protein immunocomplex was visualized using ECL detection reagents purchased from Thermo Scientific. Quantitative Western blot results were obtained by densitometric analysis using Image Processing and Analysis in Java (Image J).
2.3 Data Expression and Statistical Analysis
All data were expressed as the mean ± SEM. The statistical significance of the data was determined with t-test or one-way ANOVA combined with Dunnett post hoc or Tukey test for comparison between groups.
3 Results
3.1 Primary Neuronal Culture Viability and Percent Area of Ischemic Injury in Rat Brain
Our data showed that different concentrations of taurine can attenuate cell death in hypoxia/reoxygenation. In order to determine the appropriate concentration of taurine in cultures, cortical neurons were exposed to hypoxia and reoxygenation in the presence of 1, 5, and 10 mM taurine as shown in Fig. 23.1b. After hypoxia and reoxygenation, viability of neurons without taurine treatment dropped to approximately 49% of control. Taurine treatment dramatically increased the cell viability. The presence of 1 mM taurine substantially increased the cell viability to greater than 70% of the control level. When taurine concentration was increased to 10 mM, cell viability was further enhanced to 85% of the control.
In the rat MCAO stroke experiments, representative coronal brain sections from the control group (MCAO vehicle-treated) and experimental group (MCAO taurine-treated) stained with 1% TTC are shown in Fig. 23.1c. Four days of reperfusion following 2 h of ischemia resulted in an infarct of 47.42 ± 9.86% in the control group. Although in all sections the infarct volume was decreased in the taurine-treated group versus vehicle-treated group, only in sections 6 mm from the anterior pole (infarct volume of 26.76 ± 6.91%) was the difference significant (p < 0.05). The sham-operated group showed no ischemic injury as determined by TTC staining.
3.2 The ATF6 and IRE1 Pathways Were Inhibited by Taurine, But There Was No Effect on the PERK Pathway
PERK, ATF6, and IRE1 are the three major ER stress-induced signaling pathways. Since taurine can downregulate GRP78 in hypoxic conditions in cell culture and in a stroke model (data not shown), we aimed to further identify which signaling pathway is involved in the protection. The phosphorylation of elF2α, a downstream PERK pathway component, specifically regulates the translation of the transcription factor ATF4, leading to translational attenuation (Szegezdi et al. 2006). ATF4 is highly expressed after hypoxia/reoxygenation and increased by approximately threefold over control cultures. After treatment with taurine, followed by hypoxia/reoxygenation, however, the levels of ATF4 in cortical neurons is similar to that of hypoxia/reoxygenation alone (Fig. 23.2a), indicating that taurine does not inhibit the initiation of the PERK pathway under this condition. Similarly, the expression of ATF4 in the MCAO model does not change with taurine treatment in the core of the infarct by comparison with the vehicle-treated group (Fig. 23.2b). These results indicate that taurine has no observable effects on PERK pathway activation in either cortical neurons or in the MCAO stroke model.
Effect of taurine on ATF4 (from the PERK pathway) in neuronal cell cultures under hypoxia/reoxygenation condition and in rat MCAO stroke model. Taurine does not alter activity of the PERK pathway in primary neuronal cell culture and MCAO model. Norm normoxia, Hyp hypoxia (0.3% O2) for 20 h, reoxygenation for 20 h, Tau + Hyp neurons were treated with 10 mM taurine for 1 h, then hypoxia for 20 h, reoxygenation for 20 h, MCAO middle cerebral artery occlusion for 2 h followed by 4 days reperfusion, MCAO + taurine middle cerebral artery occlusion for 2 h followed by 4 days reperfusion, taurine was injected 24 h after reperfusion subcutaneously and injection continued for 4 days. (a) ATF4 expression analyzed by Western blot in primary neuronal cell culture. The bar graphs reflect the densitometric data from the experiment of ATF4 Western blot results with arbitrary units. (b) ATF4 expression analyzed by Western blot in MCAO rats in the core area. The values in bar graph represent mean ± SEM, (n = 3, **p < 0.01 versus norm)
We next examined the effect of taurine on the ATF6 pathway in cortical neurons subjected to hypoxia/reoxygenation and in the brain of rats subjected to MCAO occlusion. After dissociation from GRP78, ATF6 translocates from the ER to the Golgi apparatus where it is cleaved to its active form (Chen et al. 2002). Treatment with taurine considerably reduced the level of cleaved ATF6 in both primary neuronal cultures and in the core of the infarct of MCAO rats. Interestingly, the ratio of cleaved ATF6 to ATF6 in neurons and MCAO rats treated with taurine dramatically declined by approximately 50% relative to neurons under hypoxia/reoxygenation or MCAO rats, respectively, in the absence of taurine as shown in Fig. 23.3a, b. These results demonstrate that taurine can prevent the activation of the ATF6 pathway in vitro and in vivo. To determine if taurine can affect the IRE1 pathway, we tested the expression of p-IRE1 in rat primary cortical neurons under hypoxia/reoxygenation conditions and in the core of the infarct of MCAO rats with and without taurine treatment by Western blot analysis (Fig. 23.3). The results showed that phosphorylated IRE1 is highly expressed in cortical neurons under hypoxia/reoxygenation and in the core of the infarct in MCAO rat brain. Taurine decreases the expression of p-IRE1 to normal condition both in primary neuronal culture under hypoxia/reoxygenation levels (Fig. 23.3c) and in the core of the infarct of MCAO rats (Fig. 23.3d), demonstrating that taurine significantly inhibits the IRE1 pathway in ER stress.
Effect of taurine on ATF6 and p-IRE1 pathway in neuronal cell cultures under hypoxia/reoxygenation condition and in rat MCAO stroke model. Taurine does not alter activity of the PERK pathway, but inhibits the ATF6 and IRE1 pathway after hypoxia/reoxygenation and stroke. Norm normoxia, Hyp hypoxia (0.3% O2) for 20 h, reoxygenation for 20 h, Tau + Hyp neurons were treated with 10 mM taurine for 1 h, then hypoxia for 20 h, reoxygenation for 20 h, MCAO middle cerebral artery occlusion for 2 h followed by 4 days reperfusion, MCAO + taurine middle cerebral artery occlusion for 2 h followed by 4 days reperfusion, taurine was injected 24 h after reperfusion subcutaneously and injection continued for 4 days. (a) ATF6 expression in primary neuronal culture analyzed by Western blot. (b) ATF6 expression in the core of MCAO brain analyzed by Western blot. The bar graphs represent the ratio of cleaved ATF6 to ATF6 using the densitometric data from the experiment of ATF6 Western blot results with arbitrary units. (c) P-IRE1 expression in primary neuronal culture analyzed by Western blot. (d) P-IRE1 expression in the core of MCAO brain analyzed by Western blot. The bar graphs reflect the densitometric data from the experiment of P-IRE1 Western blot results with arbitrary units. The values in bar graph represent mean ± SEM, n = 3, **p < 0.01 versus norm; ##p < 0.01 versus hyp or MCAO
3.3 Taurine Can Decrease Apoptosis by Downregulation of Apoptotic Markers and Caspase-12 in Primary Neuronal Culture Induced by Hypoxia/Reoxygenation and in the Core of the Brain of MCAO
The Bcl-2 family plays crucial roles in the regulation of the mitochondrial pathways of apoptosis during experimental stroke. Bax is a member of the Bcl-2 family which translocates from the cytosol to the mitochondria after brain ischemia and causes release of cytochrome C which in turn activates caspase-3 (Sun et al. 2011). Caspase-3 is believed to be at the final stage of apoptosis. These results demonstrate that taurine can prevent the activation of caspase-3 by increasing the ratio of Bcl-2 to Bax in the core of the infarct in MCAO rats by more than fourfold (Fig. 23.4a, b). To determine the effect of taurine on apoptosis induced by ER stress, we measured the expression of CHOP by Western blot analysis in primary neuronal cultures after hypoxia/reoxygenation and in the MCAO stroke model. As shown in Fig. 23.4c, the expression of CHOP was upregulated after exposure to hypoxia/reoxygenation.
Effect of taurine on expression of Bcl2, Bax, caspase-3, CHOP and, caspase-12 in rat MCAO stroke model and CHOP and caspase-12 in neuronal cell cultures under hypoxia/reoxygenation conditions. (a) Bax and Bl2 expression in the core of MCAO brain analyzed by Western blot, the graph shows the ratio of BCl2 to Bax in the core of MCAO brain in MCAO (middle cerebral artery occlusion for 2 h followed by 4 days reperfusion) and MCAO + taurine (middle cerebral artery occlusion for 2 h followed by 4 days reperfusion, taurine was injected 24 h after reperfusion subcutaneously and injection continued for 4 days). (b) Caspase-3 expression in the core of MCAO brain analyzed by Western blot in MCAO and MCAO + taurine. (c) CHOP expression analyzed by Western blot in primary neuronal culture. Norm normoxia, Hyp hypoxia (0.3% O2) for 20 h, reoxygenation for 20 h, Tau + Hyp neurons were treated with 10 mM taurine for 1 h, then hypoxia for 20 h, reoxygenation for 20 h. The bar graphs reflect the densitometric data from the experiment of CHOP Western blot results with arbitrary units. (d) CHOP expression analyzed by Western blot in the core of the MCAO rat brain. (e) Caspase-12 expression analyzed by Western blot. The bar graphs reflect the densitometric data from the experiment of caspase-12 and cleaved caspase-12 Western blot results with arbitrary units. The values in bar graph represent mean ± SEM, n = 3, **p < 0.01 versus norm and ##p < 0.01 versus hyp or MCAO.1.3.3 Effect of TonEBP and dominant-negative TonEBP
Western blot analysis showed that taurine can decrease the levels of CHOP both in vitro in primary neuronal culture and in vivo in the MCAO stroke model (Fig. 23.4c, d). Taurine also significantly reduced the expression of caspase-12 and cleaved caspase-12 in vitro, demonstrating that taurine has the ability to inhibit the apoptosis induced by ER stress in hypoxia/reoxygenation (Fig. 23.4e).
4 Discussion
In the present study, the potential neuroprotective effects of taurine in an in vitro experimental model of brain ischemia/reperfusion and an in vivo model of MCAO stroke in rat were investigated. The main goal of this study was to investigate the effects of taurine on ER stress pathways in both the core of the brain infarct after MCAO and in primary neuronal cell culture after hypoxia/reoxygenation. We showed that taurine can not only protect primary neuronal cultures under hypoxia/reoxygenation conditions in a dose-dependent manner but also downregulate some ER stress and apoptotic markers in the brain in vivo after MCAO. Taurine as a neurotransmitter, neuromodulator, membrane stabilizer, and major intracellular free amino acid is employed in experimental therapies against neuronal damage, hypoxia, and epilepsy (Birdsall 1998). It has been shown that during cerebral ischemia, taurine may exert its neuroprotective function through both extracellular mechanisms by inhibiting calcium influx and intracellular mechanisms by protecting the mitochondrion through preventing mitochondrial dysfunction resulting from cytoplasmic calcium overload (El Idrissi and Trenkner 2004; Foos and Wu 2002; El Idrissi 2008; Huxtable 1992). Other functions of taurine, such as its role as an antioxidant, an osmoregulator, or an anti-inflammatory, contribute to its neuroprotective action (Huxtable 1992). During stroke, the levels of taurine in the extracellular fluid increases (Lo et al. 1998). The increases in the extracellular taurine levels under brain ischemia may constitute an important endogenous protective mechanism against neuronal damage (Saransaari and Oja 2000). However, intracellular taurine may be depleted resulting a disruption of intracellular homeostasis, leading to neuronal damage (Michalk et al. 1997; Huxtable 1992). Therefore, exogenous administration of taurine after brain ischemia may contribute to the recovery from ischemic damage by reducing the release of taurine, thus contributing to the restoration of intracellular homeostasis and the reduction of ischemic damage through both extracellular and intracellular mechanisms.
Several protective mechanisms of taurine such as improvement in osmotic status and calcium homeostasis in cell damage caused by hypoxia or glutamate excitotoxicity have been suggested (Chang et al. 2004; El Idrissi and Trenkner 1999; Michalk et al. 1997). We recently showed that ER stress inhibition may also be involved in taurine’s protective mechanisms under conditions of glutamate excitotoxicity and hypoxia (Pan et al. 2010, 2011). However, details of the relevant signaling pathways remain to be elucidated.
In this chapter, levels of cell viability as measured by the ATP assay were significantly enhanced by taurine after hypoxia/reoxygenation treatment, confirming the protective role of taurine. On the other hand, TTC results showed that posttreatment with taurine after MCAO could decrease the volume of cerebral damage, although this effect was not as strong as that of taurine pretreatment (Sun and Xu 2008; Sun et al. 2011). TTC staining clearly shows the stroke region which allows one to determine the exact size of cerebral infarction as well as to distinguish between the core of the infarct area, the penumbra, and healthy brain tissue (Benedek et al. 2006). Our data showed that using taurine (40 mg/kg), 24 h after reperfusion can still decrease lesion volume after 4 days. The colorless TTC is enzymatically reduced to a red formazan product by endogenous dehydrogenase enzyme complexes which are most abundant in mitochondria. Our ATP assay and TTC data confirm previous reports showing that taurine can regulate mitochondrial protein synthesis, enhance electron transport chain activity, and thereby increase the ATP levels and protect against excessive toxic superoxide generation (Schaffer et al. 2009; Jong et al. 2011). As a neuroprotective agent, taurine must pass through the blood–brain barrier (BBB) and enter into the brain under neuropathological conditions. On one hand, there are some reports of increases in radioactive taurine uptake in brain after systemic administration of radiolabeled taurine (Pasantes-Morales and Arzate 1981; Urquhart et al. 1974); on the other hand, in neuropathological conditions, the BBB may be ruptured and drugs can pass more freely. Moreover, taurine has been used with varying degrees of success in clinical therapy for epilepsy and other seizure disorders, and these data provide supporting evidence that taurine will cross the BBB and reach the damaged area when it is administrated subcutaneously after MCAO.
It is believed that brain ischemia followed by glutamate excitotoxicity leads to intracellular calcium overload and initiates a series of intracellular events, such as the release of apoptotic proteins leading to necrotic and apoptotic cell death (Nakka et al. 2008; Lipton 1999). Some reports have demonstrated that taurine can regulate intracellular calcium homeostasis through enhancing mitochondrial function, reducing the release of calcium from intracellular storage pools, and increasing the capacity of mitochondria to sequester calcium (Foos and Wu 2002; El Idrissi 2008; El Idrissi and Trenkner 2004). These data suggest that inhibiting intracellular calcium overload may be essential for the protection of taurine against MCAO. Taurine may block caspase-3 by regulating the release of mitochondrial cytochrome C. Cytochrome C release is regulated by the BCL-2 protein family of apoptotic regulators (Juin 1998). During brain ischemia, Bax expression is increased, and Bax protein translocates to mitochondria to induce cytochrome C release (Schäbitz et al. 2003; Gao and Dou 2000). We showed that 4 days after MCAO, taurine could decrease Bax protein expression, while Bcl-2 protein expression increased. Thus regulation of Bcl-2 and Bax has been demonstrated in our results, although the effect of taurine on intracellular calcium has not been directly investigated in this study. A high ratio of Bcl-2 to Bax can prevent release cytochrome C from mitochondria which results in decreased caspase-3 activity. As we showed in vitro in primary neuronal cultures, the proapoptotic factor CHOP is expressed at low levels under physiological conditions but is strongly induced in ER stress under hypoxic conditions (Nemetski and Gardner 2007; Oyadomari and Mori 2004; Paschen et al. 1998). We showed that an increase in CHOP was prevented by administration of taurine both in primary neuronal cultures and in the MCAO stroke model. Taurine can upregulate Akt phosphorylation to prevent ischemia-induced apoptosis (Taranukhin et al. 2008) and to attenuate ER stress (Yung et al. 2007). Taurine has also been shown to affect the pathways related to ER stress (Pan et al. 2010, 2011). Our current study demonstrated that taurine has beneficial effects on the protection against ER stress in the core of the MCAO infarct and on cortical neurons under hypoxia/reoxygenation. It has been shown that caspase-12, the first ER-associated member of the caspase family, is activated by ER stress (Yoneda et al. 2001; Nakagawa et al. 2000). We analyzed the expression of caspase-12 in the presence or in the absence of taurine in both in vivo and in vitro models. Our data demonstrated that the caspase-12 or cleaved caspase-12 expression was clearly reduced by taurine following hypoxia/reoxygenation in primary neuronal culture, but no change was seen in the MCAO stroke model. PERK, ATF6, and IRE1, three ER-resident transmembrane proteins, serve as the main proximal sensors of the ER stress response. In this chapter, we tried to identify which particular ER stress-induced pathway can be affected by taurine treatment in the brain of MCAO model and also in the cortical neuronal culture model under hypoxia/reoxygenation. Under ER stress conditions, PERK has proved to be responsible for repressing global protein synthesis via phosphorylation of a subunit of eIF2a (Kumar et al. 2001; Harding et al. 2000b). Phosphorylation of eIF2a, on the other hand, can also indirectly control genetranscription by positively regulating the translation of transcription factors as has been shown for mammalian ATF4 (Szegezdi et al. 2006). Since p-eIF2a and ATF4 are two downstream proteins in the PERK pathway of ER stress, it is appropriate to measure expression levels of these two proteins in order to determine the extent of PERK pathway response in the presence or in the absence of taurine treatment. We found that in the MCAO model of stroke and after hypoxia/reoxygenation in primary cell culture, there was a strong increase in ATF4 expression, indicating that the PERK pathway is activated in both models. However, there were no significant alterations of ATF4 protein levels in taurine-treated groups both in vitro and in vivo. These results suggest that taurine may have neither suppressed nor facilitated the activation of the PERK pathway, which plays an important role in attenuating protein translation to restore neuronal homeostasis during ER stress. After dissociation of GRP78, ATF6 translocates from the ER to the Golgi apparatus where it is cleaved to its active form (cleaved ATF6) (Chen et al. 2002). The ratio of cleaved ATF6 to full-length ATF6 demonstrates that taurine clearly inhibits ATF6 cleavage in both MCAO stroke model and in primary neuronal cultures under hypoxia/reoxygenation. The levels of p-IRE1 in both the MCAO stroke model and in the hypoxia/reoxygenation model of primary neuronal cultures were measured to test whether taurine has an effect on the IRE1 pathway. The results indicate that the elevation of p-IRE1 is strongly suppressed by taurine treatment, either using in vivo or in vitro experiments. These findings provide strong evidence that activation of the IRE1 pathway can be inhibited by taurine. Furthermore, the results indicating suppression of both CHOP and caspase-12 by taurine treatment provide substantial evidence that taurine can contribute to an effective inhibition of ER stress induced by hypoxia/reoxygenation.
5 Conclusion
In summary, as it is shown in Fig. 23.5, we demonstrated that taurine can exert its protective effect on CNS neurons both in the in vitro model of hypoxia/reoxygenation and in vivo model of the MCAO through suppression of ER stress. Moreover, the effect of taurine treatment on the three ER stress-induced signaling pathways showed that taurine significantly inhibited apoptosis by activation of the ATF6 and the IRE1 pathway, but not the PERK pathway.
Abbreviations
- ER:
-
Endoplasmic reticulum
- MCAO:
-
Middle cerebral artery occlusion
- GRP78:
-
Glucose-regulated protein 78
References
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29:1037–1046, discussion 1047
Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE (2006) Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291:H1411–H1420
Balkan J, Kanbağli O, Hatipoğlu A, Kücük M, Cevikbaş U, Aykaç-Toker G, Uysal M (2002) Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci Biotechnol Biochem 66:1755–1758
Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM (1986) Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304–1308
Benedek A, Móricz K, Jurányi Z, Gigler G, Lévay G, Hársing LG, Mátyus P, Szénási G, Albert M (2006) Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res 1116:159–165
Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3:128–136
Chang L, Xu J, Yu F, Zhao J, Tang X, Tang C (2004) Taurine protected myocardial mitochondria injury induced by hyperhomocysteinemia in rats. Amino Acids 27:37–48
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY (2001) Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J Neurosci Res 66:612–619
Chen X, Shen J, Prywes R (2002) The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277:13045–13052
Choi DW, Rothman SM (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13:171–182
DeGracia DJ, Montie HL (2004) Cerebral ischemia and the unfolded protein response. J Neurochem 91:1–8
El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328
El Idrissi A, Trenkner E (1999) Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 19:9459–9468
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res 29:189–197
Foos TM, Wu J-Y (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Gao G, Dou QP (2000) N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem 80:53–72
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000a) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000b) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904
Hartung T (1998) Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol 5:221–225
Hussy N, Deleuze C, Pantaloni A, Desarménien MG, Moos F (1997) Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol 502(Pt 3):609–621
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Jong CJ, Azuma J, Schaffer S (2011) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42:2223–2232
Juin P (1998) Induction of a caspase-3-like activity by calcium in normal cytosolic extracts triggers nuclear apoptosis in a cell-free system. J Biol Chem 273:17559–17564
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C, Beyer C, Kipp M (2010) TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J Neurosci Methods 187:84–89
Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, White BC, Krause GS, DeGracia DJ (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2alpha kinase, PERK. J Neurochem 77:1418–1421
Lousada PR (2004) Taurine prevents the neurotoxicity of -amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J 18:511–518
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Lipton S, Paul R (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Lo EH, Pierce AR, Matsumoto K, Kano T, Evans CJ, Newcomb R (1998) Alterations in K + evoked profiles of neurotransmitter and neuromodulator amino acids after focal ischemia-reperfusion. Neuroscience 83:449–458
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Ma Y, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28:51–65
Mattson MP (2003) Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med 3:65–94
McCollum M, Ma Z, Cohen E, Leon R, Tao R, Wu J-Y, Maharaj D, Wei J (2010) Post-MPTP treatment with granulocyte colony-stimulating factor improves nigrostriatal function in the mouse model of Parkinson’s disease. Mol Neurobiol 41:410–419
Michalk DV, Wingenfeld P, Licht C (1997) Protection against cell damage due to hypoxia and reoxygenation: the role of taurine and the involved mechanisms. Amino Acids 13:337–346
Moran J, Salazar P, Pasantes-Morales H (1987) Effect of tocopherol and taurine on membrane fluidity of retinal rod outer segments. Exp Eye Res 45:769–776
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Nakka VP, Gusain A, Mehta SL, Raghubir R (2008) Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 37:7–38
Nemetski SM, Gardner LB (2007) Hypoxic regulation of Id-1 and activation of the unfolded protein response are aberrant in neuroblastoma. J Biol Chem 282:240–248
Nicholls D, Attwell D (1990) The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11:462–468
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE (2006) Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26:1234–1249
Pan C, Giraldo GS, Prentice H, Wu J-Y (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17
Pan C, Prentice H, Price AL, Wu J-Y (2011) Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 2012(43):845–855
Pasantes-Morales H, Arzate ME (1981) Effect of taurine on seizures induced by 4-aminopyridine. J Neurosci Res 6:465–474
Paschen W, Gissel C, Linden T, Althausen S, Doutheil J (1998) Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res 60:115–122
Rich PR, Mischis LA, Purton S, Wiskich JT (2001) The sites of interaction of triphenyltetrazolium chloride with mitochondrial respiratory chains. FEMS Microbiol Lett 202:181–187
Saransaari P, Oja SS (2000) Taurine and neural cell damage. Amino Acids 19:509–526
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87:91–99
Schäbitz W-R, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, Sommer C, Schwab S (2003) Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34:745–751
Schäbitz WR, Li F, Irie K, Sandage BW, Locke KW, Fisher M (1999) Synergistic effects of a combination of low-dose basic fibroblast growth factor and citicoline after temporary experimental focal ischemia. Stroke 30:427–431, discussion 431-2
Schäbitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S (2000) Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 31:2212–2217
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429
Sun M, Xu C (2008) Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol 28:593–611
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Tadros MG, Khalifa AE, Abdel-Naim AB, Arafa HMM (2005) Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharmacol Biochem Behav 82:574–582
Takahashi K, Ohyabu Y, Takahashi K, Solodushko V, Takatani T, Itoh T, Schaffer SW, Azuma J (2003) Taurine renders the cell resistant to ischemia-induced injury in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol 41:726–733
Taranukhin AG, Taranukhina EY, Saransaari P, Djatchkova IM, Pelto-Huikko M, Oja SS (2008) Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids 34:169–174
Urquhart N, Perry TL, Hansen S, Kennedy J (1974) Passage of taurine into adult mammalian brain. J Neurochem 22:871–872
Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL (1988) A possible role for taurine in osmoregulation within the brain. J Neurochem 51:740–745
Wang G-H, Jiang Z-L, Fan X-J, Zhang L, Li X, Ke K-F (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52:1199–1209
Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17:5708–5717
Weant KA, Baker SN (2012) New windows, same old house: an update on acute stroke management. Adv Emerg Nurs J 34:112–121
Wu J-Y, Prentice H (2010) Role of taurine in the central nervous system. J Biomed Sci 17(Suppl 1):S1
Wu J-Y, Wu H, Jin Y, Wei J, Sha D, Prentice H, Lee H-H, Lin C-H, Lee Y-H, Yang L-L (2009) Mechanism of neuroprotective function of taurine. Adv Exp Med Biol 643:169–179
Wu JY, Tang XW, Tsai WH (1992) Taurine receptor: kinetic analysis and pharmacological studies. Adv Exp Med Biol 315:263–268
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940
Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ (2007) Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J 21:872–884
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Gharibani, P.M. et al. (2013). The Mechanism of Taurine Protection Against Endoplasmic Reticulum Stress in an Animal Stroke Model of Cerebral Artery Occlusion and Stroke-Related Conditions in Primary Neuronal Cell Culture. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 776. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6093-0_23
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6093-0_23
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6092-3
Online ISBN: 978-1-4614-6093-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)