Summary
Scientific and market strategy is essential in developing biological hydrogen production processes. Plans for future research should be based on current knowledge, experience and techniques. This chapter focuses on the applied issues of photofermentative H2 production using purple non sulfur bacteria (PNSB) in combined systems, and in particular, the optimization of the process on real feedstock such as olive mill wastewater and dark fermenter effluents (DFEs) of thick juice, molasses, and potato steam peels. Based on the current state of the knowledge in the field, the future applicability and prospects of these systems are evaluated. Strategies to overcome the problems are outlined.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Laboratory scale biohydrogen studies have mostly been carried out with synthetic culture media. High production costs associated with these media are prohibitive for large scale processing, and as a result, the utilization of waste materials as renewable microbial substrate sources is increasingly being considered to address the economic restrictions of biological hydrogen production. Several researchers working on photofermentative hydrogen production have based their studies on the utilization of food and agricultural waste materials with high levels of organic compounds as feedstock (Rocha et al. 2001). This approach can potentially connect the benefits of energy production with waste management.
Availability, biodegradability, cost, organic acid content and the carbon to nitrogen ratio of feedstocks are some of the critical selection measures for finding the right waste material for photofermentative hydrogen production (Kapdan and Kargi 2006). The choice of waste material depends not only on its hydrogen production performance, but also on its abundance and environmental impact, which vary regionally. For example, olive mill wastewater, with a total annual production of 30 million m3, is an environmental concern in the Mediterranean region, (Ergüder et al. 2000; Sabbah et al. 2004) whereas tofu wastewater is a significant food waste for countries in Eastern Asia. As it can be expected, the optimum conditions and the economics for these widely varying applications can be completely different from one another; hence, biological H2 production technology must be developed specifically for the selected waste. To form an overall framework for a sustainable biohydrogen economy, it is crucial to exchange and accumulate the knowledge and expertise obtained from these diverse case studies.
The use of integrated processes, which combine multiple organisms and metabolic modes for hydrogen production, is a rather recent development in the biohydrogen field with the intent to reduce the amount of feedstock utilization and its related costs while improving the hydrogen production (Eroglu and Melis 2011). Thus, an increasing number of studies focused on the combination of dark and photo fermentation processes either towards sequential two-step or combined single-step processes (Argun et al. 2009; Zong et al. 2009; Özgür et al. 2010a, b; Yang et al. 2010; Avcioglu et al. 2011; Boran et al. 2012a; Rai et al. 2012). Sequential two-step processes are based on the utilization of dark fermenter effluents (DFEs) as a substrate source for photofermentative hydrogen production. Dark-fermentation can be used either as a pretreatment stage for improving the physicochemical properties of the waste material, or to produce hydrogen itself. On the other hand, the integration of dark-fermentation and photofermentation into a single stage has also been proposed as an alternative and less labor-intensive process option. Such an arrangement may also reduce or eliminate the need for external pH adjustment, as the acidic environment caused by the dark fermentation could be neutralized via photofermentation (Keskin et al. 2011).
The purpose of the present chapter is to highlight photofermentative hydrogen production using waste materials for future large-scale applications. Here, we would like to provide hints on how to choose photo-fermentable feedstocks and emphasize their key specifications. There is no general methodology to be followed for the development of photofermentative H2 production technology; however, we would like to share the experience we have gained in this area during a 3 year COST project (COST Action 841) “Biological and biochemical diversity of hydrogen metabolism” followed by a 5 year European Union 6th Frame Integrated Project, HYVOLUTION, “Nonthermal production of pure hydrogen from biomass”. The aim of the latter project was to prepare a blueprint for integrated biological hydrogen production processes composed of a dark fermentation step followed by photofermentation. The overall objective of our work-package was the utilization of the effluent of a thermo-bioreactor, for highly efficient and sustainable hydrogen production in photobioreactors (PBRs) by photosynthetic purple non sulfur bacteria (PNSB). Hence, the scope of this chapter is limited to a review of photofermentation and integrated dark and photofermentation processes, based on reported results from our work and from those of other researchers in this field.
II. Guidelines for Effective Photofermentative Hydrogen Production
What makes a waste material a good candidate as a resource for large-scale photofermentative hydrogen production? Advances reported in the relevant literature are primarily focused on finding ways to lower the operational costs of hydrogen production through the utilization of economical and regionally available feedstocks. A sustainable hydrogen economy cannot be solely based on wastes, hence, industrial and agricultural side products, crops, and biomass that do not strain the food supply must be also considered.
As mentioned previously, the optimal set of parameters for photofermentative hydrogen production from wastewater or other complex feedstock depend strongly on the individual application and therefore should be analyzed on a case-by-case basis. Nevertheless, based on accumulated insight gained from prior studies, a generally applicable set of guidelines to consider prior to developing such a waste-based photofermentative process can be proposed. The following properties are the most crucial parameters that need to be assessed before making any further plans about using that material as a substrate source.
A. Selection of the Microorganism
Photosynthetic purple non sulfur bacteria (PNSB) e.g. members of the Rhodobacter (Rb.) species are the most preferred ones for photofermentative hydrogen production. However, it should be emphasized that the hydrogen productivity on different feedstocks depends on the strain. Hence, screening of different PNSB strains for a specific feedstock is necessary. The absorption spectra of the feedstock and that of the PNSB should be compared to check if there is interference between the absorption spectrum of the medium and the bacteria. In PNSB a single photosystem, located in the intracellular membrane, is responsible for the absorption of the light energy. In practice, antenna pigments, namely carotenoids and bacteriochlorophylls, absorb the energy of a photon. These molecules show specific absorption spectra related to their characteristic color. Chlorophyll a, commonly available in all photosynthetic organisms, absorbs light at 375 nm, 590 nm, 800–810 nm and 830–890 nm, while the main peak is located at 850–890 nm (Weaver et al. 1975). Carotenoids, which are additionally located in the intercellular membrane of PNSB, are known to absorb light energy in the range of 400–550 nm (Uyar et al. 2007). It should also be noted that feedstocks with high absorbance values at wavelength ranges of 400–800 nm might interfere with the H2 production (Boran 2011). It has been reported that the percent penetration of light intensity for each 1 cm of depth is 70 % for thick juice DFE and 51 % for molasses DFE, which is significantly lower than that for artificial medium (89 %) (Boran et al. 2012a).
During photofermentation it is observed that H2 production starts above a certain critical cell concentration. However, when cell concentration exceeds a certain limit, hydrogen evolution stops. An optimum cell concentration of 0.5–0.7 gdcw per lc for the employed microorganisms has been reported (Gebicki et al. 2010; Androga et al. 2011a, b).
Outdoor production of hydrogen with photosynthetic bacteria is also strongly affected by fluctuations in temperature due to the day-night cycle and other seasonal, geographic and climatic conditions. The optimum temperature for hydrogen production by PNSB ranges between 30 °C and 35 °C depending on the strain used (Stevens et al. 1984). Although production at lower temperatures is possible, the significantly lower growth rate of the microorganisms below 20 °C renders the process very inefficient. It is also known that the PNSB may slow down or even turn off their hydrogen production at high temperatures. An actual maximum is not known yet, but temperatures higher than 45 °C should be avoided.
In outdoor applications, the light absorbed and the heat generated by bacteria cause the bioreactor temperature to rise up to 55 °C (18–20 °C higher compared to the air temperature) during the day. Reactor temperatures can be decreased by adapting a suitable cooling system or by using a proper shading material. Water spraying is an effective method for cooling solar glass bioreactors but not recommended for acrylic flat plate reactors. The temperature difference on the surface may cause cracking.
Thermo-resistant strains of PNSB (e.g. Rhodospirillum centenum) capable of growing at higher temperatures (optimum at 40–45 °C) are also known (Favinger et al. 1989). These microorganisms might be more interesting for regions of higher global radiation intensities if they could be used for biohydrogen production.
B. Composition of the Feedstock and Its Possible Adjustments
Purple non-sulfur bacteria are able to consume a wide variety of organic substrates including short-chain organic acids such as acetate, butyrate, propionate, and lactate, sugars such as glucose and sucrose, and mixtures of these. Short-chain organic acids are preferred for H2 production; however, H2 production performances of different strains vary depending on the type and the concentration of the substrate. The initial organic acid concentrations are known to have an impact on lag time, biomass growth rate, and H2 production, as will be discussed in Sect. IV. Feedstocks usually have very high organic acid concentrations that bring about the dilution requirement, which increases the water consumption rate. Recirculation of treated wastewater may decrease the fresh water consumption rate. The concentration of the nitrogen source, and especially the ammonium ion, NH4 +, also has a primary importance. It strongly influences the photo-fermentation process, as the nitrogenase enzyme is inhibited by the presence of ammonium. For this reason, the NH4 + content of the feedstock should be lowered by dilution or by pretreatment. It is known that the C/N ratio of substrate is one of the most important parameters affecting hydrogen productivity and yield in photo-fermentation. An optimum C/N ratio of 25 was reported when acetate and glutamate were used as C and N sources, respectively (Androga et al. 2011a).
During fermentation with artificial media, the addition of supplementary nutrients (iron, molybdenum, trace elements and vitamins) is necessary. Photo-fermentation experiments using various types of real feedstocks showed that most components necessary for growth and hydrogen production are readily available in the raw substrates. Yet, it was found that real feedstocks usually lack Fe and Mo, and supplementation of the media with these minerals was shown to enhance H2 production by PNSB.
Another parameter affecting the photo-fermentation process is the pH. pH adjustment requires either acid or base addition. However, due to large reactor areas, effective pH control is difficult. To keep the pH level between 6.5 and 8.0, phosphate buffer is used at a concentration range of 4–20 mM. Buffering improves H2 productivity, but if the buffer contains phosphate, its addition highly increases the environmental impact. More environmentally benign buffers like carbonate may be used to decrease this impact.
Is sterilization of the feedstock necessary? Small-scale experiments are usually carried out under sterile conditions. Yet, in large scale, sterilization would increase the cost substantially. However, contamination may halt the process during long-term operations, and should be avoided to obtain longer processes periods. Hence, a cheap sterilization method need to be applied in large scale.
Pretreatment may improve yield but brings additional cost to the process. Pretreatments include filtration, discoloration, purification, adsorption and chemical treatment. Discoloration significantly affects the absorption spectra of the feedstocks, thus the medium does not interfere with the absorption spectrum of the PNSB.
An anaerobic atmosphere is mandatory for hydrogen production by photosynthetic PNSB. Presence of N2, O2 and CO2 adversely affects the hydrogen production pathway (Koku et al. 2002); hence, these gases must be eliminated from the photobioreactor. An argon atmosphere is especially preferred in laboratory experiments. Argon sparging at the start-up decreases the lag-time to produce hydrogen. In large-scale applications, the system is completely filled with culture to eliminate air. Dissolved air could be consumed during the growth phase. A continuous diffusion of air into the reactor due to diffusion of air through reactor material or air leakage at the fittings will result in the complete cessation of hydrogen production. In many PNSB, a visual indication of such drastic air leakage is the gradual transformation of the culture medium color into a deep-red hue.
III. Utilization of Waste Materials for Photofermentative Hydrogen Production
A large variety of waste materials have been evaluated with respect to their hydrogen conversion via fermentative processes, as listed in Table 11.1. These studies also differ from each other in terms of the microorganism used. Hydrogen productivities of waste materials are mostly given in the literature as hydrogen production yield (units of H2 volume per volume of waste liquid, lH2 per lww), and hydrogen production rate (either units of H2 volume per culture volume over time, mlH2lc −1 h−1; or units of H2 volume per bacterial cell dry weight over time, mlH2gcell −1 h−1). It should also be pointed out that the variations in the process parameters such as reactor geometry, waste material, microorganism, light source and illumination period make the direct productivity comparison quite difficult.
Fruit and vegetable, diary, and sugar wastes can be classified as the main groups of organic wastes used for hydrogen production. Some of the recent developments for each group will be explained in the sub-sections below, including an extended one for the “olive mill wastewater” as being one of the key research topics explored by the current chapter authors.
A. Olive Mill Wastewater (OMW)
Olives are one of the important agricultural crops across the Mediterranean area. During the extraction of olive oil, significant quantities of water are used especially for the continuous washing of the olive paste with warm water before the separation of oil from its olive paste (Kiritsakis 1991; Visioli et al. 1999). The olive oil manufacturing process generates a dark-colored, oily wastewater phase, the so called olive mill wastewater (OMW), which contains olive fruit juice, residual pulp, and process water in a moderately stable emulsion of oil (Tsagaraki et al. 2006; Eroglu et al. 2008a). OMW is generated in large amounts throughout the world, with over 30 million m3 per year from Mediterranean countries alone (Ergüder et al. 2000). Discarding this waste effluent to the environment requires critical precautions as it can create significant problems due to its high phenolic content (Eroglu et al. 2006, 2008a, 2009a).
Olive mill wastewater is a dark colored effluent with high organic content, with chemical oxygen demand (COD) values usually in between 50 and 200 g per l (Eroglu et al. 2009b; Yesilada et al. 1999). It is mostly composed of water (83–94 %), organic matter (4–16 %) and mineral salts (0.4–2.5 %) (Ramos-Cormenzana et al. 1996). The main organic constituents can be listed as oils, polysaccharides, proteins, organic acids, polyalcohols, and polyphenols (Cabrera et al. 1996). The characteristic dark color of OMW is mainly related to these phenolic constituents and lignin derivatives (Gonzalez et al. 1990). OMW also contains significant amounts of K, Ca, Na, Mg and Fe elements (Eroglu et al. 2004, 2009b).
Dilutions were optimized to obtain higher yields (Eroglu et al. 2004, 2009b). Various pretreatment stages enhanced hydrogen productivity (Eroglu et al. 2006, 2008a, 2009a). Different illumination regimes applied under indoor (Eroglu et al. 2010) or outdoor conditions (Eroglu et al. 2008b) also influenced the productivity. Iron and molybdenum are the other key components to supplement OMW for improving the yield (Eroglu et al. 2011).
Olive mill wastewater has been utilized as the sole substrate source for photofermentative hydrogen production (Eroglu et al. 2004) using the PNSB Rb. sphaeroides O.U.001, with glass column-photobioreactors (400 ml) under artificial illumination. Due to the dark color of the raw material, dilutions were needed for efficient hydrogen production. Dilutions of 1, 1.5, 2, 2.5, 3, 4, 5, 10, 20 % (v/v) in H2O, were investigated. Although bacterial growth could be achieved at all of these concentrations, hydrogen production could only be observed for dilutions at or below 4 %. The highest bacterial mass (0.553 gdcwlc −1) and hydrogen productivity were achieved with 4 % diluted OMW. On the other hand, the maximum hydrogen yield (13.9 lH2 per lOMW) was obtained with 2 % diluted OMW. In addition to the hydrogen production, significant reduction of the initial biochemical oxygen demand (58 %), chemical oxygen demand (35 %), and total phenolic content (60 %) were observed when 2 % diluted OMW was used (Eroglu et al. 2004). The physicochemical characteristics of the OMW, collected from different regions of Western Anatolia, were slightly variable. Four of these were compared on the basis of their photofermentative hydrogen production efficiencies. It was observed that the hydrogen productivity was directly related with the organic acid content and the carbon-to-nitrogen (C/N) molar ratio (Eroglu et al. 2009b). The highest hydrogen yield (19.9 lH2 per lOMW) was obtained with the OMW having the highest C/N molar ratio (73.8 MM−1), and the highest organic acid content, particularly acetic acid.
Figure 11.1 illustrates hydrogen production in a flat plate solar bioreactor (8 l) operating under outdoor conditions, utilizing 4 % diluted OMW in batch mode. Diurnal periods were 14 h light and 10 h dark. Bacterial growth stopped during the periods of darkness. Hydrogen yield was 11.4 lH2 per lOMW (Eroglu et al. 2008b).
Various two-stage hydrogen production processes with OMW have been reported (Eroglu et al. 2006, 2008a). In Eroglu et al. (2006), the first stage was based on the dark-fermentation of raw OMW by the mixed-cultures of activated sludge, which is followed by photofermentation via Rb. sphaeroides O.U.001. This approach converted the raw OMW to an effluent with favorable physicochemical properties for the photofermentation stage. The 50 % (v/v) diluted DFE achieved the highest photofermentative hydrogen production rate (8 mll−1 h−1), while the 4 % (v/v) diluted DFE achieved the highest hydrogen production potential (29 lH2 per lOMW). This two-stage process was mainly investigated for its potential to utilize raw OMW for photofermentative hydrogen production even at very high concentrations.
We proposed another two-stage process for enhancing the physicochemical properties of the raw OMW, which was based on a clay pretreatment step followed by photofermentation (Eroglu et al. 2006, 2008a). Clay pretreatment was investigated in detail to gain further insight into its overall effect on photofermentative hydrogen production (Eroglu et al. 2008a). The fundamental organic compounds of the clay pretreatment effluent were found as acetic, lactic, propionic, and butyric acids, which were favored during the photofermentative hydrogen production by Rb. sphaeroides O.U. 001. Hydrogen production with the effluent of the clay pretreatment process nearly doubled (31.5 lH2 per lOMW), in comparison with the raw olive mill wastewater (16 lH2 per lOMW) under the same experimental conditions. Clay pretreatment was mostly effective on the removal of unwanted compounds such as phenols, while the removal of desired substrates including organics acids, amino acids and sugars was minimal. The decrease in the phenolic content was also effective for color removal, which enhances the photosynthetic efficiency of the system by increasing the transmittance of light within the photobioreactor. Pretreatment methods applied to OMW include (i) oxidation with ozone, (ii) oxidation with Fenton’s reagent, (iii) photo-degradation via UV radiation, (iv) adsorption with clay, and (v) adsorption with zeolite (Eroglu et al. 2009a). Among these pretreatment processes, adsorption with clay was found to be the most applicable one for the photofermentation process. The effluents of the pretreatment stage by strong chemical oxidants were found to be unsuitable for either hydrogen production or bacterial growth, despite having the highest color removal (90 %).
B. Sugar Manufacturing Wastes
Sugar manufacture constitutes an important, widespread industry throughout the world. During the manufacturing of sugar, various wastes such as molasses, bagasse, and wastewater are produced. These wastes are known to have a high BOD content, which could be suitable for the growth of the photosynthetic microorganisms (Hampannavar and Shivayogimath 2010).
Singh et al. (1994) produced hydrogen via the utilization of potato starch, sugarcane juice and cheese whey by free and Ca-alginate immobilized cells of Rhodopseudomonas sp. Each feedstock was diluted with the Biebl and Pfennig medium (Biebl and Pfennig 1981). Among these three substrates, the maximum amount of hydrogen production was obtained with sugarcane juice, followed by potato starch and whey.
Almost no hydrogen gas was produced on sucrose by Rb. sphaeroides O.U.001, while very low hydrogen production rate (1 mlH2lc −1 h−1at 20 % (v/v) dilution) was obtained on diluted sugar refinery wastewater (SRW). Enrichment of the SRW with L-malic acid and sodium glutamate gave a higher hydrogen production rate and yield values as 5 mlH2lc −1 h−1and 4.63 lH2 per lSRW, respectively (Yetis et al. 2000). Extra nutrients were added to achieve a significantly higher carbon to nitrogen ratio (C/N: 70/2 MM−1). Continuous hydrogen production was achieved for 100 days with 20 % SRW supplemented with malate and glutamate mixture operating at fed-batch mode. The maximum hydrogen production yield of 8.61 lH2 per lSRW was observed at a dilution rate of 0.0013 h−1.
C. Dairy Product Wastes
Dairy product wastes, including milk or cheese residues and whey, are known to usually have high amounts of organic material content with a COD value varying between 5 and 50 g/l (Seifert et al. 2010a).
Zürrer and Bachofen (1979) used lactic acid containing whey and yogurt wastes as carbon sources for Rhodospirillum rubrum S-1. They reported very high hydrogen production yields of 47 and 45 lH2 per lww with whey and yogurt wastes, respectively. Sasikala and Ramana (1991) used the wastewater of a lactic acid fermentation plant for the production of hydrogen by Rb. sphaeroides O.U.001. Hydrogen production was observed for different dilutions of wastewater, ranging from 5 % to 100 %. The maximum hydrogen production rate was obtained as 5 mlH2h−1 l−1 while the hydrogen production yield was found to be 4.5 lH2 per lww.
Türkarslan et al. (1998) also investigated the hydrogen production potential of the dairy plant wastewater by Rb. sphaeroides O.U. 001. Milk factory waste was not sufficient for growth and hydrogen production by the photosynthetic bacteria. The dairy plant wastewater 30 % (v/v) with added malate produced hydrogen at a rate and yield of 5.5 mlH2l−1 h−1 and 2.0 lH2 per lww, respectively. Seifert et al. (2010a) reported the highest hydrogen production rate for 40 % (v/v) dairy wastewater after mixing with the Biebl and Pfennig medium. No hydrogen was produced from more concentrated dairy water due to the inhibitory effect of the high ammonium content. The highest hydrogen production yield (16.9 lH2 per lww) was achieved by 5 % (v/v) sterile dairy wastewater mixed with the Biebl and Pfennig medium (Biebl and Pfenning 1981).
D. Other Types of Waste Materials
Zhu et al. (1999) achieved high hydrogen production yields using immobilized cells of Rb. sphaeroides RV for hydrogen production from the wastewater of a tofu factory. They reported that 100 % (v/v) wastewater had hydrogen production rate and yield values of 59.0 mlH2lc −1 h−1 and 1.9 lH2 per lww, respectively. Several factors, such as the use of a cellular immobilization technique, the utilization of a Rb. sphaeroides RV strain capable of using significant amounts of glucose, and the feeding of the tofu wastewater only after the cells had begun to evolve hydrogen from a pre-culture of lactate medium, were given as the possible reasons for a higher hydrogen production rate. Mitsui and his colleagues isolated marine photosynthetic bacteria for photofermentative hydrogen production from orange processing wastes under outdoor conditions (Mitsui et al. 1983). These wastes were diluted with seawater until a total organic carbon (TOC) value of 430 ppm was obtained. They used 4 l outdoor reactors while marine photosynthetic bacteria were immobilized in agar plates. In addition to hydrogen production, they also observed a 90 % decrease in the initial BOD and 37 % decrease in TOC content of the wastewater.
Vatsala and Ramasamy (1987) investigated photofermentative H2 production from distillery waste by Rhodospirillum rubrum 11170 under both indoor and outdoor conditions. For the indoor conditions, 100 % (v/v) distiller waste resulted in a hydrogen production at a rate of 3 mlH2lc −1 h−1, while this rate was 0.8 mlH2lc −1 h−1 when 5 % distillery waste was used under outdoor conditions.
Brewery wastewater is also reported to be rich in organic content including organic acids, sugars, amino acids and etc., which would be favored during photofermentative hydrogen production. Seifert et al. (2010b) investigated the photofermentative hydrogen production potential of brewery wastewaters by using Rb. sphaeroides. They found the optimal dilution rate as 10 % (v/v), after mixing with the Biebl and Pfennig medium (Biebl and Pfenning 1981), which is mainly based on the high nitrogen content of the brewery wastewater. Diluted (10 %) brewery wastewater resulted in a hydrogen production yield of 0.22 lH2 per lww (2.24 lH2 per lmedia) (Seifert et al. 2010b).
IV. Photofermentative Hydrogen Production with Dark Fermenter Effluents
One of the advantages of using photofermentative hydrogen producing bacteria is that they can utilize organic acids produced by dark fermentative bacteria. Biohydrogen production by combination of dark and photofermentation has been considered as a promising route to increase the hydrogen yield, due to the potential of complete substrate oxidation. Dark fermentative biohydrogen production liberates reduced organic compounds like acetate, butyrate, lactate and propionate, which can be readily fermented by photofermentative bacteria to produce more hydrogen. This can be realized in a single-step (co-culture of dark and photo-fermentative bacteria) or in two steps (dark fermentation followed by photofermentation) (Fig. 11.2). Single-step processes require optimization of process conditions (i.e., pH, temperature, light intensity and biomass concentration) for both types of microorganisms grown in the same reactor at the same time. Selection of suitable microorganisms that can coexist is important.
In two-step processes, suitable microorganisms for different feedstocks can be selected independently for each step. Moreover, process conditions can be adjusted separately for higher efficiency. For example, effluents of dark fermentation can be supplemented with necessary nutrients or, they can be pre-treated to remove toxic or inhibitory compounds. It was reported that the supplementation of DFEs with appropriate amounts of iron and molybdenum, the co-factors of nitrogenase, increases the photofermentative hydrogen production significantly (Özgür et al. 2010a, b; Afşar et al. 2011; Afşar 2012). Ammonium, an additive for dark fermentation process, strongly inhibits the nitrogenase enzyme of PNSB, leading to cessation of photofermentative hydrogen production at concentrations higher than 2 mM (Yakunin and Hallenbeck 1998; Akköse et al. 2009). For successful process integration, the ammonium content of DFEs should be lowered. This can be achieved by: (i) lowering the amount of ammonium added during dark fermentation, (ii) diluting the DFEs with water, (iii) using/developing bacterial strains with ammonium tolerance or, (iv) pretreating the DFEs so that ammonium is removed. Androga et al. (2012) suggested a pretreatment method for high ammonium containing DFEs using clinoptilolite, a natural zeolite, to reduce the ammonium concentration. Pretreatment resulted in an 80 % decrease of the ammonium concentration of molasses DFE. After using the clinoptilolite-pretreated effluent, Rb. capsulatus produced hydrogen at a high yield (90 %) and productivity (1.16 mmol lc −1 h−1), while no hydrogen production was observed with the untreated one. If DFEs are too dark, as is the case for molasses DFE, such pretreatment techniques can also be useful for color reduction to increase light penetration through the PBR. Another important parameter for high efficiency photofermentative hydrogen production using DFEs is the concentration of the carbon (C) source. DFEs mainly contain acetate, lactate and butyrate, which are utilized by PNSB during photofermentation. The concentration of the carbon source is important for optimum metabolic processes that result in high hydrogen yields. Acetate at 30–40 mM (Özgür et al. 2010c), butyrate at 20 mM (Shi and Yu 2006) and lactate at 10–20 mM (Lo et al. 2011) were reported to be optimum for photobiological hydrogen production with different PNSB species. Dark fermenter effluents usually contain significant concentrations of acetate, which may decrease hydrogen production efficiency due to the diversion of the cell resources towards the synthesis of PHB. The effects of acetate concentration on hydrogen and PHB production in Rb. capsulatus were studied at 10–65 mM acetate and gene expression analysis of related genes (NifD for nitrogenase and PhaC for PHB synthase) were carried out (Özsoy 2012). Optimum acetate concentration for photofermentation with high hydrogen yield and low PHB amount was around 25–50 mM, where NifD expression was high. PhaC expression was highest at 65 mM meaning that high concentrations of acetate (above 50 mM) lead to direction of PNSB metabolism to PHB biosynthesis, decreasing the hydrogen yield. Adjustment of the carbon source concentration can be achieved through dilution with water. Buffering of the DFEs is also necessary to adjust and keep the pH at a tolerable range (6.0–8.0) for photofermentation. It was reported that 20 mM of potassium phosphate (pH 6.4) was required as a buffer supplement for molasses, thick juice, barley straw and potato steam peels DFEs (Özgür et al. 2010a, b; Afşar et al. 2011).
In the remainder of this section, photobiological hydrogen production studies on DFEs of a variety of agro-industrial wastes/wastewaters are reviewed and compared. Some of the applications are summarized in Table 11.2. Feedstocks used in sequential dark-photo fermentation processes are classified as lignocellulosic, starchy or sugar containing, based on their primary carbohydrate constituent.
A. DFEs Obtained from Lignocellulosic Feedstocks
Selection of cheap, renewable and nonfood resources is necessary for sustainable biohydrogen production. Lignocellulosic biomass, which constitutes a major portion of agricultural and forest wastes, and of industrial effluents from the pulp and paper industry, is of prime interest due to its abundance and nonfood nature. The main challenge in using lignocellulosic feedstock is that it is composed mainly of cellulose, hemicellulose, and lignin, which cannot be degraded by most of the fermentative bacteria utilized for biohydrogen production. Hence, pre-hydrolysis of such biomasses through physical, chemical or biological processes is necessary to produce monomeric carbohydrates from cellulose and hemicellulose (Ren et al. 2009).
Barley Straw DFE
Barley straw is a lignocellulosic agricultural residue of barley production. It has a dry matter content of 91.1 %: 38.9 % being glucan, 23.7 % xylan, 3.5 % other hemicelluloses and 22.8 % lignin (Foglia et al. 2011). The annual barley straw production was estimated to be 114 million tons in Europe, which corresponds to 511 TWh, assuming a lower heating value of 16.3 MJ per kg dry matter (Ljunggren et al. 2011). Worldwide annual yields of lignocellulosic biomass residues were estimated to exceed 220 billion tons, equivalent to 60–80 billion tons of crude oil (Ren et al. 2009). It has been considered as a feedstock for biofuel production through biochemical processes in several studies (Qureshi et al. 2010; Sigurbjornsdottir and Orlygsson 2012).
Biohydrogen production from barley straw DFE was carried out (Özgür and Peksel 2013). Barley straw hydrolysate, obtained after chemical and biological pretreatment, was fermented with a hydrogen-producing thermophilic dark fermentative bacterium, Caldicellulosiruptor saccharolyticus, and the effluent was used for photofermentative hydrogen production, in indoor batch cultures of Rb. capsulatus DSM1710 and Rb. capsulatus YO3 (uptake hydrogenase deleted strain of Rb. capsulatus MT1131). The effluent was diluted to have an initial acetate concentration of 30–35 mM and supplemented with potassium phosphate buffer. Good bacterial growth and hydrogen production with yields and productivities as high as 50 % and 0.58 mmol lc −1 h−1, respectively, were obtained with the Hup−strain. Addition of iron and molybdenum resulted in 30–50 % increase in productivity.
Miscanthus DFE
Miscanthus is a lignocellulosic energy crop mostly grown in USA and Europe as biomass feedstock for biofuel production, especially bioethanol. It is a perennial C4 grass that grows rapidly with dry weight yields of around 8–15 t ha−1, and requires only low inputs of nutrients for cultivation. Miscanthus hydrolysate, obtained after alkaline and enzymatic pretreatment, was used for biohydrogen production through sequential dark and photofermentation (Uyar et al. 2009; de Vrije et al. 2009). Dark fermentation, carried out using the thermophilic bacterium Thermotoga neopolitana on Miscanthus hydrolysate containing 10 g per l monomeric sugars in batch cultures, yielded 3.3 mol H2 per mole hexose with a productivity of 13 mmol H2lc −1 h−1. The effluent of dark fermentation, which mainly contained acetate (94 mM) together with some lactate (8.9 mM), was fed to PBRs operated at batch mode using Rb. capsulatus DSM155. Before being fed to the PBR, the effluent was centrifuged, diluted with water (1:1) to decrease the acetate concentration, supplemented with buffer to control pH, and sterilized. Effluents supplemented with iron and vitamins resulted in a hydrogen productivity of 19 mlH2lc −1 h−1.
B. DFEs Obtained from Sugar Processing Industry Intermediates
Intermediates from sugar beet processing industries, like molasses and thick juice, are very good sources for biohydrogen production due to their high content of fermentable sugars that can be readily utilized by microorganisms without any pretreatment. Molasses is the final effluent obtained in the manufacturing of sucrose by repeated evaporation, crystallization and centrifugation of juices from sugar cane and sugar beets. It contains more than 46 % total sugars (mostly sucrose), as well as nitrogenous compounds like amino acids and inorganic compounds, all of which make it an excellent substrate for fermentation. It is mainly used as animal feed, and as raw material for alcohol, yeast, and citric acid production. Thick juice is another by-product of sugar industry obtained after evaporation of sugar beet juice. It also has a high sugar content that can be utilized by microorganisms without any pretreatment. Unlike molasses, which can be stored for long times at room temperature without contamination, thick juice is contaminated easily, hence, it can only be used for biohydrogen production during sugar factory processing periods of the year.
Thick Juice DFE
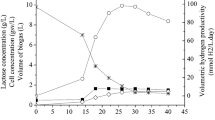
Biohydrogen production on thick juice through dark and photofermentation has been addressed within the context of the FP6 HYVOLUTION Project (Claassen and de Vrije 2006). The thermophilic dark fermentative bacterium, C. saccharolyticus, was utilized in a continuously operated 30 l CSTR dark fermenter, with an initial sucrose concentration of 10 g per l. The effluent of dark fermentation, containing acetate as the main product, was fed to photofermenters after necessary adjustments were made, including dilution to reduce acetate concentration to around 30 mM and ammonium concentration below 2 mM, and additions of buffer and iron. In batch operations using Rb. capsulatus DSM1710 and Rb. capsulatus hup- (YO3) strains, a 40 % molar yield with productivities of 0.4 mmol lc −1 h−1 and 0.6 mmol lc −1 h−1 was achieved with DSM1710 and YO3 strains, respectively. Promising results with continuously operated indoor 4 l panel PBRs were also reported on thick juice DFEs with a feeding rate of 10 % (vv−1) daily. During 25 days of continuous operation with the YO3 strain, stable biomass concentration of around 1 gdcw per lc was obtained with average hydrogen productivity and molar yield of 0.9 mmol lc −1 h−1 and 50 %, respectively (Özgür et al. 2010d). Continuous photofermentative hydrogen production under natural sunlight on thick juice DFEs was also reported (Özkan et al. 2012; Boran et al. 2012a). The DFEs were diluted and supplemented with buffer, Fe and Mo before feeding to the PBRs. It was reported that Rb. capsulatus YO3 strain grows and produces hydrogen successfully on thick juice DFE in outdoor conditions, unless the temperature of the reactor is kept above 35 °C. Temperature control was necessary as the reactor temperature exceeds 50 °C during summer, in Ankara, Turkey. To control the reactor temperature, chilled water was recirculated through the internal cooling coils. During 15 days of continuous operation inside panel PBRs with a daily feeding rate of 10 %, stable biomass concentration of around 1 gdcw per lc was achieved with a daily average hydrogen productivity of 1.12 mmol lc −1 h−1 and molar yield of 77 % (Fig. 11.3).
Continuous photofermentative hydrogen production and biomass growth in solar panel PBRs (4L) on molasses DFEs outdoors, during summer 2009 in Ankara, Turkey. (a) Rb. capsulatus hup-, (b) Rb. capsulatus DSM1710 (Reproduced with permission from Avcioglu et al. 2011).
Thick juice DFEs obtained with co-cultures of thermophilic dark fermentative bacteria, C. saccharolyticus and C. owensensis, were also utilized for continuous photofermentative hydrogen production by Rb. capsulatus DSM1710 using a pilot scale (90 l) solar tubular PBR with internal cooling coils. After a long lag phase (7 days), which was attributed to adaptation of bacteria to outdoor conditions, rapid bacterial growth with a growth rate of 0.025 h−1 was observed. Hydrogen production started after 9 days with daily average productivity and molar yield of 0.27 mmol lc −1 h−1 and 10 %, respectively, which are significantly lower compared to those obtained with panel PBRs. This was attributed to the tube diameter (6 cm), which is larger than the panel PBR thickness (2 cm), substantially lowering the light penetration through the deeper sites of the reactor (Boran et al. 2012b). It was also noted that the hydrogen permeability of the wall material used for the tubular reactor (low density polyethylene, LDPE) was around ten times higher than that of Plexiglass® used in panel PBR, with the added effect of wall thickness (150 μm for tubular PBR, 6 mm for panel PBR). Hence, both low light penetration and hydrogen leakage problems in tubular PBR resulted in lower hydrogen productivities. To increase the hydrogen production, lower tube diameters and continuous circulation to decrease the residence time of hydrogen within the reactor, were suggested, taking into consideration the energy requirements for circulation and land area needed. A direct correlation with the hydrogen yield factor (moles of hydrogen per gram cell dry weight) and daily-received light energy was reported, suggesting that hydrogen production efficiency is directly related to the light exposure of cells.
Molasses DFE
Due to its high fermentable sugar and nutritional content, molasses is one of the most promising substrates for sustainable fermentative biohydrogen production. There are a number of studies carried out on biohydrogen production from molasses through dark fermentation (Ren et al. 2006, 2009; Aceves-Lara et al. 2008; Guo et al. 2008; Wang and Jin 2009).
Özgür et al. (2010a) reported sequential operation of dark and photofermentation for biological hydrogen production from sugar beet molasses. An extreme thermophile C. saccharolyticus was used for the dark fermentation, and several photosynthetic bacteria (Rb. capsulatus DSM1710, Rb. capsulatus hup- mutant (YO3), and Rp. palustris) were used for the photofermentation. C. saccharolyticus was grown in a pH-controlled bioreactor, in batch mode, on molasses with an initial sucrose concentration of 15 g per l. The effects of addition of ammonium on dark and photofermentative processes were also determined. C. saccharolyticus fermentation yielded acetate and lactate as the main organic acids, with a hydrogen yield of 4.2 mol per mole sucrose in the absence of ammonium addition. Molasses DFE obtained without ammonium addition, which yielded highest hydrogen production in dark fermentation, was utilized for photobiological hydrogen production, under continuous illumination, in batch mode. Adjustments, including dilution, addition of buffer, iron and molybdenum, were carried out on DFE to improve the photofermentative hydrogen production. The highest hydrogen yield (58 % of theoretical hydrogen yield over consumed organic acids) and productivity (1.37 mmollc −1 h−1) was attained using a Hup- mutant of Rb. capsulatus. The overall yield increased from 4.2 mol H2 per mole sucrose in dark fermentation to 13.7 mol H2 per mole sucrose by sequential dark and photofermentation (corresponding to 57 % of the theoretical yield of 24 mol of H2 per mole sucrose).
Long term continuous hydrogen production under outdoor conditions from sugar beet molasses DFE in solar panel PBR using Rb. capsulatus DSM1710 and Rb. capsulatus hup- (YO3) was also reported (Avcioglu et al. 2011). The DFE obtained by a continuous dark fermentation process using thermophilic dark fermentative bacteria, C. saccharolyticus, contained mainly acetate as a carbon source, together with some lactate and formate. This DFE was fed to a PBR equipped with cooling coils to control the temperature below 35 °C using chilled water. The PBRs were successfully operated with a daily feeding rate of 10 %, for 55 days using Rb. capsulatus DSM1710, and 75 days for Rb. capsulatus hup-. An average biomass concentration of 0.9 gdcw per lc was achieved over the continuous operation, in outdoor conditions during summer 2009, in Ankara, Turkey (Fig. 11.3). The maximum hydrogen yield obtained using Rb. capsulatus hup- was 78 % (of the theoretical maximum) and the maximum hydrogen productivity was 0.67 mmol lc −1 h−1. The maximum hydrogen productivity and yield of the wild type strain on the molasses DFE were 0.50 mmol lc −1 h−1 and 50 %, respectively.
C. DFEs Obtained from Starch-Based Biomass
Starch-based biomass, like vegetable raw materials including potato and cereals and food wastes from the industry and household, contain high levels of carbohydrate and protein. Biohydrogen production studies from starch containing biomasses were reported to have promising hydrogen yields. Like lignocellulosic biomass, starchy biomass also needs pretreatment in order to hydrolyze starch to fermentable sugars, which is generally carried out through thermal and enzymatic pretreatment processes. However, some thermophilic dark fermentative bacteria can digest starch, eliminating the need for a pretreatment (Mars et al. 2010).
Potato Steam Peels (PSP) Hydrolysate DFE
Potato steam peel (PSP) waste is a co-product from the potato processing industry that is rich in starch, and available in large quantities. It is currently used as animal feed, but studies have shown that it is also a good source for fermentative hydrogen production (Claassen et al. 2005). A life cycle assessment (LCA) analysis made to evaluate the main environmental benefits and burdens of using PSP to produce hydrogen in a two-step fermentation process showed that it is more beneficial to use PSP for hydrogen production, and the protein-rich residue of this process for animal feed (Djomo and Humbert 2008). Results obtained from a dark fermentation process revealed that either PSP hydrolysate or untreated PSP are very suitable substrates for efficient fermentative hydrogen production at moderate substrate loadings (ca. 10 g per l of glucose), using thermophilic dark fermentative bacteria, C. saccharolyticus (Mars et al. 2010).
PSP hydrolysate, obtained after enzymatic liquefaction and saccharification steps, where starch is converted into glucose, was utilized for sequential dark and photofermentation (Afşar et al. 2011). Diluted hydrolysate (10 g C6 sugar per l) supplemented with yeast extract was fed to bioreactor with thermophilic dark fermentative bacteria, C. saccharolyticus. The DFE of PSP containing acetate (102 mM), lactate (28 mM) and NH4Cl (4.0 mM) was sterilized and diluted by three times with sterile dH2O. The effluent was also supplemented with 20 mM potassium phosphate buffer (pH 6.4), iron (Fe-citrate, 0.1 mM) and molybdenum (NaMO4.2H2O, 0.16 mM) before being fed to PBRs. Photobiological hydrogen production has been carried out in indoor, batch cultures of different PNSB strains (namely, Rb. capsulatus DSM1710, Rb. capsulatus hup- (YO3), Rb. sphaeroides O.U.001 (DSM5864), Rb. sphaeroides hup- (uptake hydrogenase deleted mutant of O.U.001) and Rp. palustris) under continuous illumination. Although the process was highly efficient in terms of acetate consumption (100 %), lower hydrogen yields (<25 %) and productivities (<0.55 mmol lc −1 h−1) were reported compared to the results obtained on molasses, thick juice or barley straw effluents.
Yokoi et al. (2002) used sequential hydrogen production from a media consisting of sweet potato starch residue as carbon source and corn steep liquor as a nitrogen source. The initial stage was composed of dark-fermentation of repeated batch cultures of Clostridium butyricum and Enterobacter aerogenes; this was followed by photofermentation using Rhodobacter sp. M-19. The overall hydrogen yield was observed to be the highest (4.5 mol H2 per mol glucose) when the DFE was supplemented with molybdenum and ethylene-diamine-tetra-acetic acid (EDTA).
Laurinavichene and her colleagues (2008) also applied a sequential two-step process to produce hydrogen from potato starch. Dark fermentation was performed using natural microbial consortia. Thereafter, the volatile fatty acid rich dark-fermentation effluent was used for photofermentative hydrogen production by Rb. capsulatus B-10. They observed that butyrate could not be consumed until the complete consumption of acetate, propionate, and lactate, after providing equimolar mixture of volatile fatty acids.
Cassava Starch DFE
Cassava is a starch containing root crop grown as a staple food and animal feed in subtropical and tropical regions of Africa, Latin America and Asia, with a total cultivated area over 18 million hectare. A high starch content (up to 90 %) together with its low agro-chemical requirements, high drought and heat tolerance make it an attractive plant for biofuel production (Jansson et al. 2009). Biohydrogen production from cassava starch through combination of dark and photofermentation has been reported (Su et al. 2009; Zong et al. 2009). Dark fermentation of raw cassava starch, after its gelatinization and enzymatic hydrolysis, was carried out at different initial starch concentrations (10–25 g per l) using hydrogen producing bacteria from preheated activated sludge (Su et al. 2009). Effluents of dark fermentation, which mainly consisted of acetate and butyrate, were used for photofermentative hydrogen production in batch cultures of Rp. palustris, after being diluted to optimal concentrations. The maximum hydrogen yield was reported to increase from 9.3 to 10.7 mmol H2 per g starch only in the dark fermentation to 17.5–18.0 mmol H2 per g starch in the combined dark and photo fermentation, which corresponds to 63.1–93.7 % improvement.
Ground Wheat Starch DFE
Waste ground wheat starch constitutes a reliable and renewable resource for biohydrogen production due to its high starch and gluten content (Kargi and Pamukoglu 2009; Argun et al. 2009). Ground wheat starch is reported to be rich in carbohydrates but deficient in nitrogen and phosphorous. Hence, supplementation with N and P maximizes the hydrogen yield by dark fermentation (Kargi and Pamukoglu 2009). The DFE of ground wheat starch containing 1950 ± 50 mg per l total volatile fatty acid (VFA), obtained by heat treated anaerobic sludge as the bacterial culture, were utilized for photofermentative hydrogen production by Rb. sphaeroides (NRRL-B1727) (Ozmihci and Kargi 2010). Continuous photofermentative hydrogen production at varying hydraulic retention times (HRT) (24–120 h) was carried out on the DFEs supplemented with several nutrients, such as MgSO4, EDTA, (NH4)2SO4 and KH2PO4 buffer to adjust the pH to 7.0 and C/N/P ratio to 100/1/0.3. The highest steady-state daily hydrogen production (55 ml per day) and hydrogen yield (185 ml H2 per g VFA) were obtained at 72 h HRT (3 days).
D. DFEs from Other Feedstocks
Cheese whey is a lactose-rich (about 5 %) byproduct of the cheese manufacturing industry and its removal represents a significant problem in the dairy industry. Utilization of cheese whey wastewater to produce biohydrogen through mesophilic and thermophilic dark fermentation processes using mixed microbial communities has been reported (Azbar et al. 2009a, b, c). Biohydrogen production through sequential operation of thermophilic dark fermentation and photo fermentation using cheese whey wastewater has been investigated (Azbar and Cetinkaya-Dokgöz 2010). After centrifugation and dilution at different degrees, the effluent containing mainly acetate, isobutyrate, and lactate was used for photofermentation by Rp. palustris. The highest hydrogen production (349 ml H2 per g COD) was obtained with the five-times diluted effluent. Overall hydrogen production performance of two-stage system was found to vary between 2 and 10 mol H2 per mole of lactose consumed. It was concluded that the dilution of anaerobic effluent helps to reduce the nitrogen and the volatile fatty acid content in the feeding, which can otherwise be inhibitory. It was also reported that addition of malate significantly improves the hydrogen production, hence, mixing of cheese whey effluent with malate containing material such as apple juice processing effluent, might be useful.
Rai et al. (2012) investigated sequential two-stage biohydrogen production on cheese whey using Enterobacter aerogenes in dark fermentation step, and Rhodopseudomonas BHU 01 in photofermentation steps. They showed that the cumulative H2 yield (dark and photo-fermentation) obtained by cultures immobilized by alginate entrapment was better (5.88 mol H2 per mole lactose) compared to the suspension cultures (3.40 mol H2 per mole lactose).
Fruit and vegetable wastes (FVW) are produced in large amounts mainly from the domestic households, restaurants and market places. Due to their high biodegradability, they can cause several irritations in municipal landfills, which bring a need for their recycling (Bouallagui et al. 2005). FVW is mainly composed of easily biodegradable constituents such as sugars and hemicellulose (75 %) (Verrier et al. 1987). On the other hand, some vegetable processing effluents contain non-biodegradable organic constituents that should be minimized via pretreatment processes before being used for any bioprocesses (Bouallagui et al. 2005). Fascetti et al. (1998) used organic-acid rich (mostly lactic-acid and acetic-acid) DFE of fruit and vegetable wastes for photofermentative hydrogen production by Rb. sphaeroides RV. They achieved significant hydrogen production rates of around 100 mlH2 g−1.h−1 after the continuous processing of a 1 liter chemostat.
Abd-Alla et al. (2011) studied biohydrogen production from rotten date palm fruits in a three-stage process including dark fermentation by the facultative anaerobe E. coli EGY, followed by the strict anaerobe Clostridium acetobutylicum ATCC 824 and lastly by the PNSB Rb. capsulatus DSM1710, within the same reactor. They reported maximum hydrogen yield of 7.8 mol H2 per mole of sucrose corresponding to 162 l H2 per kg fresh rotten dates.
V. Optimization of Hydrogen Yield
A. Genetic Modifications
Genetic engineering is a promising tool to increase the yield and productivity of photofermentative hydrogen production (Vignais et al. 2006). Considering the H2 metabolism of PNSB, genetic modifications can be done to (i) inhibit H2 utilization by deletion of uptake hydrogenase, (ii) eliminate the light-dependency of hydrogen evolution by recombinant expression of hydrogen-evolving hydrogenases, (iii) optimize the flow of reducing equivalents to nitrogenase by the inhibition of PHB and CO2 fixation, (iii) eliminate/decrease the effect of environmental factors (e.g. NH4 +, temperature) by producing resistant mutants, (iv) reduce the size of antenna pigments to increase light utilization efficiency.
Deletion of Uptake Hydrogenase
The physiological function of uptake hydrogenase in most PNSB is to catalyze the conversion of molecular hydrogen to electrons and protons, which decreases the yield of H2 production. The uptake hydrogenase helps to maintain a redox balance. It was shown that the inactivation of uptake hydrogenase results in significant increase in total hydrogen production in these bacteria. Rb. sphaeroides O.U.001 is a purple non-sulfur bacterium producing hydrogen under photoheterotrophic conditions. Hydrogen is produced by Mo-nitrogenase enzyme and a substantial amount of H2 is re-oxidized by a membrane-bound uptake hydrogenase. To improve the hydrogen producing capacity of the cells, a suicide vector containing a gentamicin cassette in the hupSL genes was introduced into Rb. sphaeroiodes O.U.001 and the uptake hydrogenase genes were destroyed by site directed mutagenesis. The wild type and the mutant cells showed similar growth patterns but the total volume of hydrogen gas evolved by the mutant was higher than that of the wild type strain (Kars et al. 2008). A similar approach was adopted by Öztürk et al. (2006) to develop an uptake hydrogenase lacking strain of Rb. capsulatus MT1131. The mutant strain produced around 30 % more hydrogen than the wild type MT 1131. The hup- strain was also tested in large-scale photobioreactor outdoor conditions on acetate as carbon source and enhancement in H2 production rates and yields were observed (Androga et al. 2011a, b). The Rb. capsulatus hup- strain was also used for hydrogen production from real DFEs in a panel photobioreactor in outdoor conditions (Avcioglu et al. 2011). Similar studies involving the genetic manipulations of PNSB were reviewed by Kars and Gündüz (2010).
Expression of Hydrogen-Evolving Hydrogenases
Light is essential for efficient photobiological hydrogen production by PNSB but it is discouraging to know the fact that continuous H2 production in the outdoor using sunlight is impossible or rather inefficient during night. Therefore, one promising approach is to relieve the dependency of PNSB from light for hydrogen production by recombinant expression of hydrogen-evolving hydrogenases in these bacteria. Thus, continuous hydrogen production under light and dark conditions would be possible and certainly improve the efficiency of biological hydrogen production process. In a study by Kim et al. (2008), constitutive hydrogen evolution under both photoheterotrophic and dark fermentative conditions by recombinant Rb. sphaeroides was reported. They developed a recombinant Rb. sphaeroides KCTC 12085 strain that harbor, with all the accessory genes necessary, formate hydrogen lyase and Fe-only hydrogenase from Rs. rubrum, to enable dark fermentative hydrogen production from Rb. sphaeroides. The strain produced hydrogen during dark fermentative growth, and photofermentative hydrogen production increased by twofolds.
Redirecting the Electron Flow to Nitrogenase
The charge or redox status of the cells is very important for biohydrogen production, since nitrogenase needs electrons and ATPs in order to reduce protons to hydrogen. To optimize the flow of reducing equivalents to nitrogenase, genetic modifications were carried out targeting the CO2 fixation and PHB synthesis pathways, which compete for reducing equivalents. The Calvin-Benson-Bassham (CBB) pathway is not only the heart of photoautotrophic metabolism but also important in maintenance of redox homeostasis via CO2 assimilation. Öztürk et al. (2012) investigated the relationship between the redox balancing system and hydrogen production in various Rb. capsulatus strains whose CBB pathway was inactivated by deleting one of the key enzyme phosphoribulokinase (PRK). The results indicated that in the absence of the functional CBB pathway, the excess reducing equivalents were dissipated mainly through the nitrogenase in the form of hydrogen under nitrogen limiting conditions. The rate of hydrogen production was enhanced slightly for Rb. capsulatusHup-, PRK- and Rb. capsulatusHup-, PRK-, cbb3- mutants.
Spontaneous variants of Rb. capsulatus strains deficient in the CBB pathway have been shown to express nitrogenase structural genes to dissipate excess reducing equivalents, even in the presence of high concentrations of ammonium that is sufficient to repress nitrogenase expression in wild type (Tichi and Tabita 2000). In Rb. sphaeroides KD131, inactivation of PHB synthase resulted in a two-fold increase in hydrogen production on acetate and butyrate, in spite of depressed cellular growth and lower substrate utilization (Kim et al. 2011). It was shown that overexpression of rnf operon, which is thought to be dedicated to electron transport to nitrogenase, in Rb. capsulatus enhanced in vivo nitrogenase activity (Jeong and Jouanneau 2000). In another study done by Öztürk et al. (2006), a loss of function in the electron carriers in the membrane of Rb. capsulatus resulted in significant decrease in H2 production. These results suggest that the electron flow to nitrogenase is critical in the H2 production process and needs to be manipulated for the enhanced H2 production.
Improving the Ammonium Ion Tolerance
Ammonium is one of the substances regulating nitrogenase activity. Removal of ammonium inhibition on nitrogenase activity is important especially for integrated dark and photofermentation studies, in which the dark fermenter effluent is usually rich in ammonium. To develop mutants with ammonium insensitive-nitrogenase activity, Pekgöz et al. (2011) deleted genes expressing two regulatory proteins of ammonium-dependent nitrogenase regulation, GlnB and GlnK in Rb. capsulatus DSM1710. However, glnB mutants showed lower hydrogen production, while glnK mutants were unviable. This observation suggests that the GlnB/GlnK two component regulatory system most probably has roles in other metabolic pathways as well. An ammonia tolerant mutant strain of Rp. palustris has been developed through a mutation in nifA gene (NH4 +-dependent a transcriptional regulator of nitrogenase expression). The mutant strain constitutively expressed nitrogenase even in the presence of nitrogen (McKinley and Harwood 2010). Hydrogen production by NifA mutant Rp. palustris on undiluted vegetable waste derived medium has shown significant improvements in productivity, start-up time and substrate conversion efficiency at NH4 + concentrations as high as 6.1 mM (Adessi et al. 2012).
Proteomics and Microarray Analysis of Rb. capsulatus
For further improvement of biological hydrogen production, the understanding of whole genome expression profile of Rb. capsulatus under different stress conditions is required. Although the genome database is available, there is not enough information about the proteomics and transcriptomics of Rb. capsulatus. The first proteomic study, reported by Daldal’s group (Önder et al. 2010) revealed more than 450 proteins including the localization information. The data obtained by the proteome study of Rb. capsulatus SB1003 under different growth conditions, namely, those leading to aerobic respiratory, anaerobic photofermentative and anaerobic respiratory modes, contributed to the extent of the protein database and highlighted the proteins associated with these growth modes (Peksel 2012). A total 460 proteins were identified with 17 proteins being unique to particular growth conditions.
Understanding the whole genome expression profile under different stress conditions, such as temperature, UV, and high light intensity, is important for outdoor PBR applications. A custom designed Affymetrix Gene Chip for Rb. capsulatus DSM1710 (GEO Accession number: GPL18063) was constructed. The effects of temperature stress on transcriptome of Rb. capsulatus were investigated by comparing expression profiles under optimum hydrogen production condition (30 °C), heat (42 °C) and cold (4 °C) stress conditions (GSE53477) (Gürgan 2011). The influence of different nitrogen sources on transcriptome of Rb. capsulatus was investigated by comparing expression profile on 5 mM ammonium chloride and 2 mM glutamate. Carbon source was 40 mM acetate on both conditions. To study the effect of different acetate concentrations, 40 mM and 80 mM acetate were used with 2 mM glutamate as nitrogen source (GSE53303).
The data obtained from physiological, biochemical, proteomics and microarray studies has to be combined to further understand the metabolism of the microorganism and the genetic manipulation of the bacterial strains.
B. Photobioreactor Design
Photobioreactors (PBRs) are reactors that accommodate microbial culture systems to carry out light dependent biological reactions (Tredici 2004). In these reactors, light has to pass through the transparent reactor walls to reach the cells. A fundamental parameter for the evaluation of the photofermentation process is its photochemical efficiency (PE). This is defined as the conversion efficiency of the energy of the incident light to chemically bound energy in the hydrogen produced. In contrast to the performance of a fermenter, which is usually reported as a volumetric productivity, the performance of a PBR is usually given as an area related productivity – as hydrogen productivity per illuminated area or per land area occupied by the bioreactor. When designing an industrial process, however, not only the input energy of the incident light, but also the input energy for operation of the installation should be taken into consideration when evaluating the efficiency of the process in terms of energy conversion. When evaluating the economic efficiency of the process, costs of construction material, construction and maintenance as well as resources necessary for operation should also be taken into consideration. The ideal PBR requires low investment costs and low operational costs, but still should have a high productivity and be easily scalable. In practice it is not easy to provide a large surface-to-volume ratio for sufficient supply with solar light and enable at the same time a good mass and heat transfer.
In terms of reactor geometry, two main categories of PBRs can be distinguished as:
-
1.
Tubular reactors
-
2.
Flat panel reactors
Tubular Reactors
For hydrogen producing bacteria, residence time in the tubular part should be optimized since organic acids are already dissolved in the medium, but the gases produced (H2 and CO2) need to be removed. Therefore, a manifold configuration of the photo-bioreactor is preferred (Fig. 11.4).
Gas collection in a photo-bioreactor with a manifold configuration could be accomplished by tilting of the surface. Attachment of small gas bubbles to the wall of the tubes might be solved by large gas bubbles moving in the upward direction, as was done by Tredici and co-workers in the Near Horizontal Tubular Reactor (NHTR) (Chini Zittelli et al. 1999). In the NHTR designed by Tredici, gas is injected to create an airlift. In the case of hydrogen production, gas bubbles are only required to collect the small bubbles of hydrogen (and CO2) produced in the medium. The energy input for pumping liquid should be limited to enable a positive energy balance of the photofermentation process (Fig. 11.4).
The tubular PBR finally constructed and used for continuous outdoor experiments by our group (Boran et al. 2012a, b) had a volume of 85 l (Fig. 11.5). It is currently equipped with new manifolds with a tube length of 3 m. For up-scaling the reactor size, the tube length can be easily increased. However, it should be pointed out that the maximum allowable pressure for the tubes depends on the used tubing material and its wall thickness.
Tubular photo-bioreactor (85 L) operated at METU, Ankara (Reproduced with permission from Boran et al. 2012a).
Flat Panel Reactor
In flat panel reactors it is observed that the bacteria only settle in the dark. As soon as hydrogen is produced, the bacterial cells are ‘self-suspended’ due to the upward movement of the H2-bubblets onto which the cells tend to attach. To design a PBR as compact as possible, the design must aim to achieve a maximal ratio of illuminated surface area to land space covered by the reactors. The illuminated surface per ground space is higher in vertical reactors than in horizontal systems.
The performance of a photobioreactor is strongly dependent on the light availability for each single cell of the dense microorganism suspension within the panels. Self-shading due to light absorption by the pigments causes an exponential decay in light intensity with culture depth. If a fixed density of the culture is assumed, the spatial light distribution in the suspension can be regulated by means of the depth of the panels.
Since the bacteria are able to use diffuse light as well, the intensity of the incident light can be controlled by the orientation of the photo-bioreactor. If the illuminated surfaces of the panels are facing the east–west direction the heat input during noontime is reduced and the utilization of the sunlight is improved because purple bacteria mainly use long wave radiation, which dominate the morning and evening sky. Direct radiation of comparably low intensity enters the reactor in the mornings and evenings. At noontime, when the sun emits light with a high content of UV rays, which might damage the culture, mainly diffuse light impinges on the reactor surfaces. The optimal spacing between the panels depends on light distribution and temperature. One panel of the photobioreactor consists of a frame covered by a transparent plate on both sides. The height of the panels is limited to 1 m in order to reduce the deflection of the transparent plates and to guarantee the gas tightness of the enclosed volume. For this reactor PMMA plates of 2 mm in thickness were used since they are able to withstand the hydrostatic pressure at the bottom with a fairly low deflection.
The flat panel reactor used in the study (Gebicki et al. 2010) consists of four parallel panels, which are arranged vertically as shown in Fig. 11.6. Each panel has an illuminated area of 2 m2 and the plate thickness varies between 20 and 30 mm. This results in a total reactor volume of 112 l (4 × 28 l).
Modular arrangement of flat panel reactor (112 L) operated at RWTH, Aachen (Reproduced with permission from Gebicki et al. 2010).
VI. Efficiency Analysis
Cost efficiency of any biofuel production from biomass depends primarily on the feedstock cost. According to a biomass cost index (BCI) analysis for biohydrogen production by sequential dark and photofermentation using a variety of feedstock, where production, logistics and pretreatment costs were compared, the biomass cost for sugar and starch containing feedstocks are lower than lignocellulosic feedstocks, due primarily to higher pretreatment costs associated with lignocellulosic biomass (Diamantopoulou et al. 2011). In selecting the biomass for biofuel production, one should also consider the local feedstock availability to decrease the logistics cost.
An exergy analysis to determine the efficiency of the integrated dark and photofermentation has been carried out for different feedstocks based on the HYVOLUTION process described by Claassen and de Vrije (2006). It was shown that exergy efficiency is influenced by the used feedstock, applied process parameters, and process and heat integration (Modaressi et al. 2010). It was demonstrated by Foglia and colleagues (Foglia et al. 2010, 2011) that with proper heat integration of pretreatment, thermophilic dark fermentation, and photofermentation steps, and recirculation of process effluents, biohydrogen production from variety of feedstocks, including PSP, barley straw, and thick juice, through the HYVOLUTION process is technically feasible. However, techno-economical analysis of two-stage biohydrogen production process using barley straw as feedstock revealed that with the current technologies it is around 20 times more expensive compared to the bioethanol production process, due mainly to the low productivity, low energy efficiency, and high cost of buffer and base required to control the pH (Ljunggren et al. 2011). Improvements in bioreactor design and process conditions to decrease the chemical and water requirements are essential for the transition of two-stage biohydrogen production process from lab-scale to industrial scales.
VII. Future Prospects
The aim of photofermentation is the optimal conversion of organic acids present in wastewater or fermentation effluents to hydrogen and carbon dioxide, in a prolonged stable operation. A yield above 75 % is targeted with a high productivity. A long-term stable operation is limited by environmental factors, as photobiological hydrogen production has to be carried out in outdoor conditions relying on natural sunlight for an energy-efficient process. The application of the process in technical scale demands:
-
Stable microorganisms working under non sterile conditions with a high yield of capturing sunlight and with broad range of operational temperatures
-
Productive feedstocks with higher yields
-
Strain improvement to enhance the efficiency of the process under high/low light intensity and temperature variations
-
Reactors with a high illuminated surface per ground space, cheap and mechanically stable construction material
-
Plant design for the photofermentation, which guarantees well-defined operational conditions in all parts of the plant through development of a process control system. Design should include process control for pH, substrate concentration, temperature and liquid input/output control in each module.
The following items have to be investigated in the future to transfer the process to technical application:
-
(a)
Improvement of hydrogen productivity of the micro-organism(s):
The specific production rate of the microorganism has to be further increased to decrease the demand on reactor area. Their adaptability to fluctuating environmental conditions, like light intensity and temperature, has to be improved to increase the stability of the process. It is now clear that changes in metabolism alone, e.g. nitrogen-fixing conditions to induce nitrogenase biosynthesis, is not enough to force the phototrophs towards approaching the theoretical limits of hydrogen production. It is necessary to create mutants for high, stable and long-lasting hydrogen productivity. The PNSB could be genetically improved to produce hydrogen during night time as well by dark fermentation, which would increase the daily productivity.
-
(b)
Designed co-cultures:
Use of designed co-cultures should also be studied in photofermentation. By this approach, the effect of environmental conditions on photo-biohydrogen production can be partially avoided by using selected strains with different properties such as thermo-tolerant versus mesophilic strains, or strains adapted to high/low light intensity.
-
(c)
Nutrient requirements:
There are certain nutrients such as Mo and Fe, which are necessary to activate the nitrogenase of PNSB and there are certain compounds like ammonium that are inhibitors of nitrogenase activity. However, more attention should be paid to find out the possible other inhibitors of hydrogen production metabolism of PNSB, especially those that may potentially be present in feedstocks of different biomass sources. These may include alcohols (like ethanol), polyphenols and aldehydes.
-
(d)
Use of immobilized microorganisms:
Entrapment of the photosynthetic bacteria into a solid support improves the productivity as a higher cell density can be used compared to the suspension cultures (Elkahlout 2011). This also increases the hydrogen yield, as there is only limited bacterial growth in immobilized systems. A large-scale outdoor operation requires a special reactor design, which should be investigated in future studies. The immobilized systems will also require a novel feeding protocol.
-
(e)
Modeling:
Development of predictive models for photofermentation for design and process control purposes that should include new bioreactors, microorganisms, and changing environmental conditions is needed.
-
(f)
Optimization of reactor design:
The geometry of the PBRs should meet the requirements for locations with different process conditions: low temperature combined with low sunlight intensity (Central/Eastern Europe) versus high temperature combined with high sunlight intensity (Southern Europe). Outdoor tests have to be performed at larger scale (some 10 m2 reactors) to demonstrate their functional performance in the long run. Additionally, an improvement of the concept for the panel reactor is necessary to reduce the demand on construction material, to improve internal mixing and to allow for cooling. New construction materials have to be applied to minimize the Net Energy Ratio (NER). NER gives a monetary independent analysis for the viability of an energy conversion process. It is the relationship between the energy output and the energy content of all the materials of which the plant is constructed plus the energy needed for all operations, calculated for the lifetime of the system.
-
(g)
Optimization of the design of the plant:
Due to the inherent low productivity of the PNSB, a large illuminated area is necessary to produce an acceptable amount of hydrogen. Larger volumes of tubular and panel reactors may be obtained by repeating the original unit reactor operated in parallel. The concept for the arrangement of the reactors, the piping, and distribution of input and recirculation flow has to be demonstrated at large scale and has to be optimized as well. The optimal mixing of the reactor volume has to be ensured in the large-scale plant. This is simplified by dividing the whole reactor volume into smaller compartments, which are fed and mixed separately. The size of the compartments has to be defined. Smaller compartments are also favorable if a contamination emerges.
-
(h)
Concept for handling contamination:
Contamination may result in a breakdown of the hydrogen production. Since competing microorganisms will not stay in one module but spread out in the whole system a solution to handle contamination must be developed. The separation of the reactor into smaller units with independent feed inlets and effluent outlets might be a solution.
-
(i)
Heat economizing:
Operation of PBRs in outdoors is an energy requiring process due to the need of temperature control and recirculation. Exploitation of other renewable energy sources (sunlight, wind, geothermal energy, etc.) to supply energy to the PBR for recirculation or temperature control can be explored and implemented in the design of a biohydrogen plant. Heat economizing is necessary for the plant with the integration of cooling and heating streams in a heat exchange network.
-
(j)
Development of process control system:
Development of a complex process control system is inevitable. The variables that affect the PBR performance are pH, temperature, C/N ratio, and ammonium and acetate concentration in the effluent. In the lab-scale operation of the photobioreactor, the analysis of the feed and effluent may be carried out by means of an on-line HPLC. However, for a prototype PBR, practical devices and methods have to be developed to detect the control variables.
-
(k)
Stability in long-term operation:
Stability in long term operation has to be investigated at the molecular level. Metabolic understanding of the photo-sensing mechanism of bacteria may be helpful in the design of large-scale PBRs and process conditions. There are evidences that especially in complex media like real DFEs, photosynthetic PNSB conduct both dark and light fermentation in a competitive manner. If the light intensity, biomass concentration and temperature are not within the favorable intervals, bacteria switch off the photofermentative hydrogen production and proceed with dark fermentation alone. The understanding of hydrogen production switch-on/off mechanism may be beneficial for the development of long-term stable operations.
Abbreviations
- BCI:
-
Biomass cost index
- BOD:
-
Biological oxygen demand
- COD:
-
Chemical oxygen demand
- CSTR:
-
Continuously stirred tank reactor
- DFE:
-
Dark fermenter effluent
- FVW:
-
Fruit and vegetable wastes
- gdcw lc −1 :
-
Gram cell dry weight per liter of culture
- HRT:
-
Hydraulic retention time
- LDPE:
-
Low density polyethylene
- lH2 :
-
Liters of hydrogen
- lOMW :
-
Liters of olive mill wastewater
- M:
-
Molar
- OMW:
-
Olive mill wastewater
- PBR:
-
Photobioreactor
- PNSB:
-
Purple non-sulfur bacteria
- SRW:
-
Sugar refinery wastewater
- TOC:
-
Total organic carbon
- VFA:
-
Volatile fatty acids
References
Abd-Alla MH, Morsy FM, El-Enany AWE (2011) Hydrogen production from rotten dates by sequential three stages fermentation. Int J Hydrog Energy 36:13518–13527
Aceves-Lara CA, Latrille E, Bernet N, Buffière P, Steyer JP (2008) A pseudo-stoichiometric dynamic model of anaerobic hydrogen production from molasses. Water Res 42:2539–2550
Adessi A, McKinlay JB, Harwood CS, De Philippis R (2012) A Rhodopseudomonas palustris nifA* mutant produces H2 from NH4 +-containing vegetable waste. Int J Hydrog Energy 37:15893–15900
Afşar N (2012) A Global approach to the hydrogen production, carbon assimilation and nitrogen metabolism of Rhodobacter capsulatus by physiological and microarray analyses. Ph.D. thesis. Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey. http://etd.lib.metu.edu.tr/upload/12615030/index.pdf
Afşar N, Özgür E, Gürgan M, Akkose S, Yücel M, Gündüz U, Eroglu I (2011) Hydrogen productivity of photosynthetic bacteria on dark fermenter effluent of potato steam peels hydrolysate. Int J Hydrog Energy 36:432–438
Akköse S, Gündüz U, Yücel M, Eroglu I (2009) Effects of ammonium ion, acetate and aerobic conditions on hydrogen production and expression levels of nitrogenase genes in Rhodobacter sphaeroides OU001. Int J Hydrog Energy 34:8818–8827
Androga DD, Özgür E, Eroglu I, Gündüz U, Yücel M (2011a) Significance of carbon to nitrogen ratio on the long-term stability of continuous photofermentative hydrogen production. Int J Hydrog Energy 36:15583–15594
Androga DD, Özgür E, Gündüz U, Yücel M, Eroglu I (2011b) Factors affecting the long-term stability of biomass and hydrogen productivity in outdoor photofermentation. Int J Hydrog Energy 36:11369–11378
Androga DD, Özgür E, Eroglu I, Gündüz U, Yücel M (2012) Amelioration of photofermentative hydrogen production from molasses dark fermenter effluent by zeolite-based removal of ammonium ion. Int J Hydrog Energy 37:16421–16429
Argun H, Kargi F, Kapdan IK (2009) Hydrogen production by combined dark and light fermentation of ground wheat solution. Int J Hydrog Energy 34:4304–4311
Avcioglu SG, Özgür E, Eroglu I, Yücel M, Gündüz U (2011) Biohydrogen production in an outdoor panel photobioreactor on dark fermentation effluent of molasses. Int J Hydrog Energy 36:11360–11368
Azbar N, Cetinkaya-Dokgöz FT (2010) The effect of dilution and L-malic acid addition on bio-hydrogen production with Rhodopseudomonas palustris from effluent of an acidogenic anaerobic reactor. Int J Hydrog Energy 35:5028–5033
Azbar N, Çetinkaya Dokgöz FT, Keskin T, Korkmaz KS, Syed HM (2009a) Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int J Hydrog Energy 34:7441–7447
Azbar N, Dokgöz FT, Keskin T, Eltem R, Korkmaz KS, Gezgin Y, Akbal Z, Öncel S, Dalay MC, Gönen Ç, Tutuk F (2009b) Comparative evaluation of bio-hydrogen production from cheese whey wastewater under thermophilic and mesophilic anaerobic conditions. Int J Green Energy 6:192–200
Azbar N, Dokgöz FTÇ, Peker Z (2009c) Optimization of basal medium for fermentative hydrogen production from cheese whey wastewater. Int J Green Energy 6:371–380
Biebl H, Pfennig N (1981) Isolation of members of the family Rhodosprillaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes, vol 1. Springer-Verlag, New York, pp 267–273
Boran E (2011) Process development for continuous photofermentative hydrogen production. Thesis, Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey. (http://etd.lib.metu.edu.tr/upload/12612955/index.pdf)
Boran E, Özgür E, Yücel M, Gündüz U, Eroglu I (2012a) Biohydrogen production by Rhodobacter capsulatus in solar tubular photobioreactor on thick juice dark fermenter effluent. J Clean Prod 31:150–157
Boran E, Özgür E, Yücel M, Gündüz U, Eroglu I (2012b) Biohydrogen production by Rhodobacter capsulatus Hup− mutant in pilot solar tubular photobioreactor. Int J Hydrog Energy 37:16437–16445
Bouallagui H, Touhami Y, Cheikh RB, Hamdi M (2005) Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem 40:989–995
Cabrera F, Lopez R, Martinez-Bordiu A, Dupuy de Lome E, Murillo JM (1996) Land treatment of olive oil mill wastewater. Int Biodeter Biodegrad 38:215–255
Chini Zittelli G, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70:299–312
Claassen PAM, de Vrije T (2006) Non-thermal production of pure hydrogen from biomass: HYVOLUTION. Int J Hydrog Energy 31:1416–1423
Claassen PAM, Budde MAW, Niel EWJV, de Vrije T (2005) Utilization of biomass for hydrogen fermentation. In: Lens P, Westermann P, Haberbauer M, Monero A (eds) Biofuels for fuel cells: biomass fermentation towards usage in fuel cells. IWA Publishing, London, pp 221–230
de Vrije T, Bakker RR, Budde MA, Lai MH, Mars AE, Claassen PAM (2009) Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol Biofuels 2:12
Diamantopoulou LK, Karaoglanoglou LS, Koukios EG (2011) Biomass cost index: mapping biomass-to-biohydrogen feedstock costs by a new approach. Bioresour Technol 102:2641–2650
Djomo S, Humbert S (2008) Life cycle assessment of hydrogen produced from potato steam peels. Int J Hydrog Energy 33:3067–3072
Elkahlout KE (2011) Phototrophic hydrogen production by agar-immobilized Rhodobacter capsulatus. Ph.D. thesis, Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey. https://etd.lib.metu.edu.tr/upload/12613033/index.pdf
Ergüder TH, Güven E, Demirer GN (2000) Anaerobic treatment of olive mill wastes in batch reactors. Process Biochem 36:243–248
Eroglu E, Melis A (2011) Photobiological hydrogen production: recent advances and state of the art. Bioresour Technol 102:8403–8413
Eroglu E, Gündüz U, Yücel M, Turker L, Eroglu I (2004) Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int J Hydrog Energy 29:163–171
Eroglu E, Gündüz U, Yücel M, Turker L, Eroglu I (2006) Biological hydrogen production from olive mill wastewater with two stage processes. Int J Hydrog Energy 31:1527–1535
Eroglu E, Eroglu I, Gündüz U, Yücel M (2008a) Effect of clay pretreatment on photofermentative hydrogen production from olive mill wastewater. Bioresour Technol 99:6799–6808
Eroglu I, Tabanoglu A, Gündüz U, Eroglu E, Yücel M (2008b) Hydrogen production by Rhodobacter sphaeroides O.U.001 in a flat plate solar bioreactor. Int J Hydrog Energy 33:531–541
Eroglu E, Eroglu I, Gündüz U, Yücel M (2009a) Treatment of olive mill wastewater by different physicochemical methods and the utilization of their liquid effluents for biological hydrogen production. Biomass Bioenergy 33:701–705
Eroglu E, Eroglu I, Gündüz U, Yücel M (2009b) Comparison of the physicochemical characteristics and photofermentative hydrogen production potential of wastewaters produced from different olive-oil mills in Western-Anatolia Turkey. Biomass Bioenergy 33(4):706–711
Eroglu E, Gündüz U, Yücel M, Eroglu I (2010) Photosynthetic bacterial growth and productivity under continuous illumination or diurnal cycles with olive mill wastewater as feedstock. Int J Hydrog Energy 35:5293–5300
Eroglu E, Gündüz U, Yücel M, Eroglu I (2011) Effect of Fe and Mo addition on the photofermentative hydrogen production from olive mill wastewater. Int J Hydrog Energy 36:5895–5903
Fascetti E, D’Addario E, Todini O, Robertiello A (1998) Photosynthetic hydrogen evolution with volatile organic acids derived from the fermentation of source selected municipal solid wastes. Int J Hydrog Energy 23:753–760
Favinger J, Stadtwald R, Gest H (1989) Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. A Van Leeuw J Microbiol 55:291–296
Foglia D, Ljunggren M, Wukovits W, Friedl A, Zacchi G, Urbaniec K, Markowski M (2010) Integration studies on a two-stage fermentation process for the production of biohydrogen. J Clean Prod 18:S72–S80
Foglia D, Wukovits W, Friedl A, Ljunggren M, Zacchi G, Urbaniec K, Markowski M (2011) Effects of feedstocks on the process integration of biohydrogen production. Clean Technol Environ 13:547–558
Gebicki J, Modigell M, Schumacher M, van der Burg J, Roebroeck E (2010) Comparison of two reactor concepts for anoxygenic H2 production by Rhodobacter capsulatus. J Clean Prod 18:S36–S42
Gonzalez MD, Moreno E, Quvedo-Sarmiento F, Ramos- Cormenzana A (1990) Studies on the antibacterial activity of wastewaters from olive oil mills (alpechin): inhibitory activity of phenolic and fatty acids. Chemosphere 20:423–432
Guo WQ, Ren NQ, Wang XJ, Xiang WS, Meng ZH, Ding J, Qu YY, Zhang LS (2008) Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (EGBS) reactor. Int J Hydrog Energy 33:4981–4988
Gürgan Doğan M (2011) Microarray analysis of the effects of heat and cold stress on hydrogen production metabolism of Rhodobacter capsulatus. M. Sc. thesis Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey. http://etd.lib.metu.edu.tr/upload/12613668/index.pdf
Hampannavar US, Shivayogimath CB (2010) Anaerobic treatment of sugar industry wastewater by upflow anaerobic sludge blanket reactor at ambient temperature. Int J Environ Sci 1:631–639
Jansson C, Westerbergh A, Zhang J, Hu X, Sun C (2009) A potential biofuel crop in (the) People’s Republic of China. Appl Energy 86:S95–S99
Jeong HS, Jouanneau Y (2000) Enhanced nitrogenase activity in strains of Rhodobacter capsulatus that overexpress the rnf genes. J Bacteriol 182:1208–1214
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microbiol Technol 38:569–582
Kargi F, Pamukoglu MY (2009) Dark fermentation of ground wheat starch for bio-hydrogen production by fed-batch operation. Int J Hydrog Energy 34:2940–2946
Kars G, Gündüz U (2010) Towards a super H2 producer: improvements in photofermentative biohydrogen production by genetic manipulations. Int J Hydrog Energy 35:6646–6656
Kars G, Gündüz U, Rakhely G, Yücel M, Eroglu I, Kovacs KL (2008) Improved hydrogen production by uptake hydrogenase deficient mutant strain of Rhodobacter sphaeroides O.U.001. Int J Hydrog Energy 33:3056–3060
Keskin T, Abo-Hashesh M, Hallenbeck PC (2011) Photofermentative hydrogen production from wastes. Bioresour Technol 102:8557–8568
Kim EJ, Kim MS, Lee JK (2008) Hydrogen evolution under photoheterotrophic and dark fermentative conditions by recombinant Rhodobacter sphaeroides containing the genes for fermentative pyruvate metabolism of Rhodospirillum rubrum. Int J Hydrog Energy 33:5131–5136
Kim MS, Kim DH, Son HN, Ten LN, Lee JK (2011) Enhancing photo-fermentative hydrogen production by Rhodobacter sphaeroides KD131 and its PHB synthase deleted-mutant from acetate and butyrate. Int J Hydrog Energy 36:13964–13971
Kiritsakis A (1991) Olive oil. American Oil Chemists’ Society, Champaign
Koku H, Eroglu I, Gündüz U, Yücel M, Türker L (2002) Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrog Energy 27:1315–1329
Laurinavichene TV, Tekucheva DN, Laurinavichius KS, Ghirardi ML, Seibert M, Tsygankov AA (2008) Towards the integration of dark and photo fermentative waste treatment. 1. Hydrogen photoproduction by purple bacterium Rhodobacter capsulatus using potential products of starch fermentation. Int J Hydrog Energy 33:7020–7026
Ljunggren M, Wallberg O, Zacchi G (2011) Techno-economic comparison of a biological hydrogen process and a 2nd generation ethanol process using barley straw as feedstock. Bioresour Technol 102:9524–9531
Lo YC, Chen CY, Lee CM, Chang JS (2011) Photo fermentative hydrogen production using dominant components (acetate, lactate, and butyrate) in dark fermentation effluents. Int J Hydrog Energy 36:14059–14068
Mars AE, Veuskens T, Budde MAW, van Doeveren PFNM, Lips SJ, Bakker RR, de Vrije T, Claassen PAM (2010) Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int J Hydrog Energy 35:13206–13213
McKinley LB, Harwood CS (2010) Carbon dioxide as a central redox cofactor recycling mechanism in bacteria. PNAS 107:11669–11675
Mitsui A, Philips EJ, Kumazawa S, Reddy KJ, Ramachandran S, Matsunaga T, Haynes L, Ikemoto H (1983) Progress in research toward outdoor biological hydrogen production using solar energy, sea water, and marine photosynthetic microorganisms. Ann N Y Acad Sci 413:514–530
Modaressi A, Wukovits W, Foglia D, Friedl A (2010) Effect of process integration on the exergy balance of a two-stage process for fermentative hydrogen production. J Clean Prod 18:S63–S71
Önder O, Sunar SA, Selamoglu N, Daldal F (2010) A glimpse into the proteome of phototrophic bacterium Rhodobacter capsulatus. Adv Exp Med Biol 675:179–209
Özgür E, Peksel B (2013) Biohydrogen production from barley straw hydrolysate through sequential dark and photofermentation. J Clean Prod 52:14–20
Özgür E, Mars A, Peksel B, Lowerse A, Afşar N, Vrije T, Yücel M, Gündüz U, Claassen PAM, Eroglu I (2010a) Biohydrogen production from beet molasses by sequential dark and photofermentation. Int J Hydrog Energy 35:511–517
Özgür E, Afşar N, Vrije T, Yücel M, Gündüz U, Claassen PAM, Eroglu I (2010b) Potential use of thermophilic dark fermentation effluents in photofermentative hydrogen production by Rhodobacter capsulatus. J Clean Prod 18:S23–S28
Özgür E, Uyar B, Öztürk Y, Yücel M, Gündüz U, Eroglu I (2010c) Biohydrogen production by Rhodobacter capsulatus on acetate at fluctuating temperatures. Resour Conserv Recy 54:310–314
Özgür E, Uyar B, Gürgan M, Yücel M (2010d) Hydrogen production by Hup- mutant and wild type strains of Rhodobacter capsulatus on dark fermenter effluent of sugar beet thick juice in batch and continuous photobioreactors. In: Detlef S, Grube T (eds) Hydrogen production technologies, part-1, Proceedings of WHEC 2010, Essen. Forschunszentrum Jülich GmbH, Verlag, GmbH, pp 228–233
Özkan E, Uyar B, Özgür E, Yücel M, Eroglu I, Gündüz U (2012) Photofermentative hydrogen production using dark fermentation effluent of sugar beet thick juice in outdoor conditions. Int J Hydrog Energy 37:2044–2049
Ozmihci S, Kargi F (2010) Bio-hydrogen production by photo-fermentation of dark fermentation effluent with intermittent feeding and effluent removal. Int J Hydrog Energy 35:6674–6680
Özsoy B (2012) Hydrogen and poly-hydroxy butyric acid production and expression analyses of related genes in Rhodobacter capsulatus at different acetate concentrations. M.Sc. thesis, Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey. http://etd.lib.metu.edu.tr/upload/12614076/index.pdf
Öztürk Y, Yücel M, Daldal F, Mandaci S, Gündüz U, Turker L, Eroglu I (2006) Hydrogen production by using Rhodobacter capsulatus mutants with genetically modified electron transfer chains. Int J Hydrog Energy 31:1545–1552
Öztürk Y, Gökce A, Peksel B, Gürgan M, Özgür E, Gündüz U, Eroglu I, Yücel M (2012) Hydrogen production properties of Rhodobacter capsulatus with genetically modified redox balancing pathways. Int J Hydrog Energy 37:2014–2020
Pekgoz G, Gündüz U, Eroglu I, Yücel M, Kovacs K, Rakhley G (2011) Effect of inactivation of genes involved in ammonium regulation on the biohydrogen production of Rhodobacter capsulatus. Int J Hydrog Energy 36:13536–13546
Peksel B (2012) Proteome analysis of hydrogen production mechanism of Rhodobacter capsulatus grown on different growth conditions. M.Sc. thesis, Graduate School of Natural and Applied Sciences, METU, Ankara, Turkey (http://etd.lib.metu.edu.tr/upload/12614133/index.pdf)
Qureshi N, Saha BC, Dien B, Hector RE, Cotta MA (2010) Production of butanol (a biofuel) from agricultural residues: part I – Use of barley straw hydrolysate. Biomass Bioenergy 34:559–565
Rai PK, Singh SP, Asthana RK (2012) Biohydrogen production from cheese whey wastewater in a two-step anaerobic process. Appl Biochem Biotechnol 167:1540–1549
Ramos-Cormenzana A, Jimenez B, Pareja G (1996) Antimicrobial activity of olive mill wastewaters (alpechin) and biotransformed olive oil mill wastewater. Int Biodeter Biodegrad 38:283–290
Ren N, Li J, Li B, Wang Y, Liu S (2006) Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int J Hydrog Energy 31:2147–2157
Ren N, Wang A, Cao G, Xu J, Gao L (2009) Bioconversion of lignocellulosic biomass to hydrogen: potential and challenges. Biotechnol Adv 27:1051–1060
Rocha JS, Barbosa MJ, Wijffels RH (2001) Hydrogen production by photosynthetic bacteria: culture media, yields and efficiencies. In: Miyake J, Matsunaga T, San Pietro A (eds) Biohydrogen II – an approach to environmentally acceptable technology. Elsevier Science Ltd., London, pp 3–32
Sabbah I, Marsook T, Basheer S (2004) The effect of pretreatment on anaerobic activity of olive mill wastewater using batch and continuous systems. Process Biochem 39:1947–1951
Sasikala K, Ramana CV (1991) Photoproduction of hydrogen from waste water of a lactic acid fermentation plant by a purple non-sulfur photosynthetic bacterium, Rhodobacter sphaeroides O.U. 001. Indian J Exp Biol 29:74–75
Seifert K, Waligorska M, Laniecki M (2010a) Hydrogen generation in photobiological process from diary wastewater. Int J Hydrog Energy 35:9624–9629
Seifert K, Waligorska M, Laniecki M (2010b) Brewery wastewaters in photobiological hydrogen generation in presence of Rhodobacter sphaeroides O.U.001. Int J Hydrog Energy 35:4085–4091
Shi X, Yu H (2006) Continuous production of hydrogen from mixed volatile fatty acids with Rhodopseudomonas capsulata. Int J Hydrog Energy 31:1641–1647
Sigurbjornsdottir MA, Orlygsson J (2012) Combined hydrogen and ethanol production from sugars and lignocellulosic biomass by Thermoanaerobacterium AK54, isolated from hot spring. Appl Energy 97:785–791
Singh SP, Srivastava SC, Pandley KD (1994) Hydrogen production by Rhodopseudomonas at the expense of vegetable starch, sugarcane juice and whey. Int J Hydrog Energy 19:437–440
Stevens P, Vertonghen C, De Vos P, De Ley J (1984) The effect of temperature and light intensity on hydrogen production by different Rhodopseudomonas capsulata strains. Biotechnol Lett 6:277–282
Su H, Cheng J, Zhou J, Song W, Cen K (2009) Improving hydrogen production from cassava starch by combination of dark and photo fermentation. Int J Hydrog Energy 34:1780–1786
Tichi MA, Tabita FR (2000) Maintenance and control of redox poise in Rhodobacter capsulatus strains deficient in the Calvin-Benson-Bassham pathway. Arch Microbiol 174:322–333
Tredici MR (2004) Mass production of microalgae: photobioreactors. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing, Oxford, pp 178–214
Tsagaraki E, Lazarides HN, Petrotos KB (2006) Olive mill wastewater treatment. In: Oreopoulou V, Russ W (eds) Utilization of by-products and treatment of waste in the food industry. Springer, New York, pp 133–157
Türkarslan S, Yigit DO, Aslan K, Eroglu I, Gündüz U (1998) Photobiological hydrogen production by Rhodobacter sphaeroides O.U.001 by utilization of waste water from milk industry. In: Zaborsky OR (ed) Biohydrogen. Plenum Press, London, pp 151–156
Uyar B, Eroglu I, Yücel M, Gündüz U, Turker L (2007) Effect of light intensity, wavelength and illumination protocol on hydrogen production in photobioreactors. Int J Hydrog Energy 32:4670–4677
Uyar B, Schumacher M, Gebicki J, Modigell M (2009) Photoproduction of hydrogen by Rhodobacter capsulatus from thermophilic fermentation effluent. Bioprocess Biosyst Eng 32:603–606
Vatsala TM, Ramasamy V (1987) Photo-hydrogen production from distillery waste. In: Veziroğlu TN (ed) Proceedings of the 8th Miami international conference on alternative energy sources, vol 2. Miami, Florida, pp 618–623
Verrier D, Ray F, Albagnac G (1987) Two-phase methanization of solid vegetable wastes. Biol Waste 22:163–177
Vignais PM, Magnin J-P, Willison JC (2006) Increasing biohydrogen production by metabolic engineering. Int J Hydrog Energy 31:1478–1483
Visioli F, Romani A, Mulinacci N, Zarini S, Conte D, Vincieri FF, Galli C (1999) Antioxidant and other biological activities of olive mill waste waters. J Agric Food Chem 47:3397–3401
Wang X, Jin B (2009) Process optimization of biological hydrogen production from molasses by a newly isolated Clostridium butyricum w5. J Biosci Bioeng 107:138–144
Weaver PF, Wall JD, Gest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105:207–216
Yakunin AF, Hallenbeck PC (1998) Short-term regulation of nitrogenase activity by NH4 + in Rhodobacter capsulatus: multiple in vivo nitrogenase responses to NH4 + addition. J Bacteriol 180:6392–6395
Yang H, Guo L, Liu F (2010) Enhanced bio-hydrogen production from corncob by a two-step process: dark- and photo-fermentation. Bioresour Technol 101:2049–2052
Yesilada Ö, Sık S, Sam M (1999) Treatment of olive oil mill wastewater with fungi. Turk J Biol 23:231–240
Yetis M, Gündüz U, Eroglu I, Yücel M, Turker L (2000) Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides O.U.001. Int J Hydrog Energy 25:1035–1041
Yokoi H, Maki R, Hirose J, Hayashi S (2002) Microbial production of hydrogen from starch-manufacturing wastes. Biomass Bioenergy 22:389–395
Zhu H, Suzuki T, Tsygankov AA, Asada Y, Miyake J (1999) Hydrogen production from tofu wastewater by Rhodobacter sphaeroides immobilized in agar gels. Int J Hydrog Energy 24:305–310
Zong W, Yu R, Zhang P, Fan M, Zhou Z (2009) Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenergy 33:1458–1463
Zürrer H, Bachofen R (1979) Hydrogen production by the photosynthetic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 37:789–793
Acknowledgements
This work was supported by European Cooperation in Science and Technology (COST Action 841), European Commission – Research: The Sixth Framework Program for Research and Technological Development Sustainable Energy Systems EU FP6-SES IP HYVOLUTION (contract no. 019825), Turkish State Planning Organization (DPT) (BAP-08-11-DPT.2005K120600), Turkish Scientific Research Council (TÜBİTAK) (108T455) and Middle East Technical University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Eroglu, I., Özgür, E., Eroglu, E., Yücel, M., Gündüz, U. (2014). Applications of Photofermentative Hydrogen Production. In: Zannoni, D., De Philippis, R. (eds) Microbial BioEnergy: Hydrogen Production. Advances in Photosynthesis and Respiration, vol 38. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8554-9_11
Download citation
DOI: https://doi.org/10.1007/978-94-017-8554-9_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8553-2
Online ISBN: 978-94-017-8554-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)