Abstract
Reef organisms are well known for engaging in photosymbiosis in which a heterotrophic protist or animal host partners with one or more kinds of photosynthetic microbes. This relationship provides metabolic advantages in nutrition and rapid calcification, often leading to secretion of massive skeletons in the host. In turn the symbiont receives protection, physical stability in the photic zone and direct access to the sun’s energy. On an evolutionary scale, this relationship provided strong selective pressures for producing the algal-host relationship and has occurred multiple times in geological history. Today, different kinds of algae (dinoflagellates, diatoms, chlorophytes, rhodophytes, and cyanobacteria) inhabit various hosts (foraminifera, corals, mollusks) in modern reefs, and multiple phylogenetically separate algae may have also inhabited phylogenetically distinct ancient animals and protists. The modern dinoflagellate photosymbiont Symbiodinium occurs in a wide variety of unrelated host organisms from protists to mollusks. Molecular data indicate this genus first evolved either after the end-Cretaceous mass extinction 65 my ago or in the Early Eocene some 55 my ago. Encysted dinoflagellates related to Symbiodinium have been traced to the Triassic, and photosymbiosis may have been involved in even earlier reef associations. In all fossils, however, the identity of ancient photosymbionts is difficult to establish because they rarely, if ever, fossilize. Nevertheless, indirect evidence indicates that photosymbiotic ecosystems existed at least as far back as the Cambrian. Inferential lines of evidence, including large colony size, massive skeletons, unusual or complex morphology, the biogeographic distribution of possible hosts and skeletal geochemistry are all consistent with active photosynthesis. In the following pages, we develop the hypothesis that photosymbiosis best explains both the successes and failures of reefs through geologic time. We then review the evidence that suggests photosymbiosis in reef organisms played significant roles through geologic time in both the evolution and extinction of organisms and the reefs they constructed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Photosynthesizing organisms have been essential throughout much of geological time for the building of reefs and deposition of carbonate platforms in the shallow, sunlit waters of Earth. In the Archean (3.5 to 2.5 billion years ago), stromatolites were constructed chiefly by photosynthetic cyanobacteria which trapped carbonate in their cells or mucilaginous secretions (Walter 1983; Allwood et al. 2007). Cyanobacteria built and continued to build reef-like structures in Precambrian to modern oceans (Fig. 3.1), initially in the absence of grazers and later on in environments where grazing animals were restricted (Dravis 1983; Dill et al. 1986; Riding 1992 Reid et al. 1995). They have also long been important as encrusters that cement reefs together.
Modern intertidal stromatolites growing at Carbala Point, Shark Bay, Western Australia . Each mushroom-shaped calcareous stromatolite contains chiefly cyanobacteria, although other microbes (foraminifera , diatoms, ciliates, dinoflagellates) and even animals live in and among them now. These are typically 0.5–1.0 + m in height. Microbial mats cover the areas between the stromatolites (Photo by J. H. Lipps 2002)
The first photosynthetic eukaryotes arose in the late Paleoproterozoic perhaps as long as 1.63 or more billion years ago (Butterfield 2015), as estimated from molecular evidence (Yoon et al. 2004), morphology (Knoll 2014) and the fossil record (Lipps 2006; Eme et al. 2014). Metazoans , however, did not appear until about 600 million years ago. Once heterotrophic eukaryotes, animals and microbes evolved, another kind of photosynthetic strategy appeared—photosymbiosis, the productive association of photosynthesizing unicellular algae or cyanobacteria with heterotrophic microbial eukaryotes and animals. This represented a powerful evolutionary strategy connecting the heterotrophs directly to the sun’s energy. Like symbioses in general, photosymbioses occur in both terrestrial and marine organisms (Margulis 1998; Douglas 2010). Among the many biotic relationships that evolved, photosymbiosis was particularly important in marine environments because it produced such profound biological, physical and chemical changes. While no direct evidence for photosymbiotic microbes exists in the fossil record , the process could have evolved among single-celled eukaryotes in the Precambrian even before animals appeared. Huge reefs, 300 m high and 8 km in diameter, were built by microorganisms and animals in the Neoproterozoic (Turner et al. 1993; Wood and Curtis 2014) and similar ones have been constructed ever since the Cambrian (Rowland and Gangloff 1988; Wood 1999; Rowland and Shapiro 2002), primarily by metazoan- or protistan-algal symbioses (Cowen 1983, 1988; Coates and Jackson 1987; Surge et al. 1997).
These associations result in not only reefs, but also in the production of prodigious amounts of carbonate sediment (Hallock and Schlager 1986) on reefs and banks (Lee and Anderson 1991; Hallock 1999; Lee 2006; Langer 2008). This biologic carbonate production annually accounts for very large amounts of reef-related sediments (Fig. 3.2; ∼30 × 106 metric tons of foraminifera alone, Langer 2008) and formation of a variety of carbonate rocks (James 1983). These, in turn, sequester many gigatons of carbon (Langer et al. 1997), thus helping to ameliorate effects of atmospheric CO2 buildup and global warming now and in the past. When photosymbiosis slows or fails, so does the production of massive amounts of biogenic carbonate .
Aerial view of the northeastern part of Eniwetak Atoll (ocean is to the left). Everything in this view except the vegetation on the islets is biogenic carbonate derived either from the complete skeletons or the broken debris of calcifying organisms. Loose sediment , carried from the reef and reef flat in large plumes (right, center), eventually ends up on the backreef and lagoon floor (Photo by J. H. Lipps, 1972)
At many times in the geologic
past, entire reef ecosystems
collapsed globally in response to environmental
changes, and mass extinctions
ensued (Fagerstrom 1987; Benton 2003; Erwin 2006; Stanley and Lipps 2011; Clarkson et al. 2015; see also Chap. 8). The breakdown of photosymbioses in today’s corals and foraminifera
is manifested by bleaching
(Stimson et al. 2002; Hallock et al. 2006), and mortality related to bleaching
likely accompanied extinctions
of many ancient reef ecosystems
. Subsequent diversifications of reef communities following those events may also have been in part due to the reacquisition of symbionts
as the environments ameliorated.
Modern reefs face ocean warming and acidification as CO2 increases in the atmosphere and oceans because of human activities. Degradation of coral-reef ecosystems is already obvious in the increasing incidence of bleaching (Glynn 1996; Douglas 2003; Hallock et al. 2006; van Oppen and Lough 2009), coral disease, ocean acidification (Kleypas et al. 1999; Pelejero et al. 2007), and general human destruction of reef structures (Lipps 2011). For these reasons and others, reef ecosystems appear to be moving toward massive failure (Pandolfi et al. 2005).
We regard reefs as photosynthetically -driven, closely integrated ecosystems much like rain forests on land (Reaka-Kudla 1997). Photosymbiosis is the primary driver of productivity through physiological and morphological adaptations today. In this chapter we develop the hypothesis that photosymbiosis was also integral to reef success and failure through geologic time. We argue that when photosymbiosis succeeded or failed in the past due to environmental perturbation , reefs and related carbonate platforms also succeeded or failed (Chap. 8). In the following pages, we review the occurrence of photosymbiosis on modern and ancient reefs and carbonate -platforms , and its relationship to macroevolutionary processes of diversification, radiation and extinction of reefs and the organisms themselves. While the specifics of this hypothesis must be tested by utilizing an increasingly robust database of taxonomic , paleogeographic, paleoecologic and phylogenetic molecular results, photosymbiosis is an important contributor to reef success today and available evidence indicates that this was also true in ancient reef ecosystems .
3.2 Photosymbioses in Modern, Shallow-Water Carbonate Environments
Photosymbioses by bacteria and single-celled algae living within microbes and larger invertebrates are mostly confined to warm, shallow-water, carbonate settings on reefs and platforms . An assemblage of symbionts living in one host is referred to as a “holobiont”, for example “the coral holobiont” (Knowlton and Jackson 2011). For heterotrophic microbes and metazoans , photosymbionts provide added metabolites, nutrients and enhanced calcification . These are particularly advantageous in oligotrophic tropical shallow waters. In kind, the photosymbionts benefit from the stable habitat , protection , and a supply of metabolic wastes, such as CO2 and nitrogenous compounds, provided by their host (Douglas 2003).
Today, photosymbionts include cyanobacteria, chlorophytes, rhodophytes , dinoflagellates and diatoms hosted by foraminifera (Hansen and Buchardt 1977; Hallock 1999; Lee 2006), radiolaria (Anderson 1983) and ciliates (Lobban et al. 2014) among the microbial forms, plus sponges, cnidarians (including corals), bivalves , tunicates, and possibly bryozoans among larger animals (Fig. 3.3). Fossil invertebrates such as brachiopods , bryozoans , gastropods , and other extinct forms may have hosted photosymbionts in the distant past. That so many different and unrelated lineages of algae and heterotrophs have adopted this cooperative strategy likely indicates a strong selective advantage for photosymbiosis (Baker 2003; Fautin and Buddemeier 2004).
Molecular phylogenetic diagram of the Eukarya showing the polyphyletic distribution of photosymbionts (gray arrows) and the common host either eukaryotic single-celled microbes or multicellular animals (black arrows). In addition to those taxa named, most other cnidarians, a tunicate, and possibly bryozoans may have hosted photosymbionts now or in the past. Other algae such as cyanobacteria (not shown; dates to 3000+ Ma) and the enigmatic acritarch cysts (not shown; dates to 1600 Ma) may include symbiotic forms as well. The oldest known geologic age based on fossils of each clade is indicated in the box near its root. Molecular or chemical biomarker dates are not included but may indicate earlier origins of most clades although they were not preserved as fossils until much later (Modified from Porter (2004) and Lipps (2006))
More than one kind of algal symbiont is often found in some hosts. Foraminifera, for example, host Symbiodinium , diatoms, rhodophytes , chlorophytes, and cyanophytes, each living alone (Hansen and Buchardt 1977; Hansen and Dalberg 1979; Lee 2006) or as one of multiple symbiont species (Lee 2006) or clades in a single foraminiferan or foraminiferal species (Fay et al. 2009). Sponges too host cyanophytes and dinoflagellates, among other symbionts . Even in modern reef settings, photosymbioses by unrelated symbionts and hosts are quite common (Fig. 3.3).
The most widespread modern photosymbionts are dinoflagellates commonly known as zooxanthellae or, more precisely, by the generic name Symbiodinium (Freudenthal 1962). Symbiont-bearing organisms may be called zooxanthellate (z-organisms) and those without symbionts are azooxanthellate (az-organisms). Symbiodinium densities measured within coral hosts range from hundreds of thousands to millions per square centimeter (Stimson et al. 2002) and thousands occur in single cells of some larger foraminifera (Fig. 3.4; Fay et al. 2009).
Symbionts may be genetically diverse complexes of closely related forms (Coffroth and Santos 2005). Genomic studies of Symbiodinium microadriaticum revealed the presence of a number of different clades (Blank and Trench 1985; Rowan and Powers 1991; LaJeunesse 2002; Fay et al. 2009; LaJeunesse et al. 2010). And, new Symbiodinium clades are recognized each year. Currently more than a dozen different genetic clades are known to live in many different hosts, both within and outside of cells. Some of these have been given formal or informal names or letters, and among those clades up to nearly 50 sub-strains also exist (van Oppen et al. 2009; LaJeunesse et al. 2010). The clades of Symbiodinium may live in the same host at the same time or in different hosts across many domains of eukaryotes (Fig. 3.3). Different clades may also be found in different parts of a single host . A single foraminiferan, for example, may contain several clades of Symbiodinium that live in different parts of its cell (Fay et al. 2009). Other organisms, like corals and the giant clam Tridacna, also contain Symbiodinium in several clades as noted above.
Symbionts live within the cells of the microbial eukaryotes or in special structures in animals (Farmer et al. 2001). In foraminifera , the symbionts live pressed against the interior of the test on the upper (or sunlit) part, and in Amphistegina the symbionts occupy cup-shaped depressions that may keep them separated from one another (Lee 2006). Although sponges, like foraminifera , are symbiotic with many algae (Knowlton and Rohwer 2003), only clionid sponges harbor Symbiodinium (Hill et al. 2011). Some reef bivalves also maintain Symbiodinium in special tubes either in the mantle or in the gills (Farmer et al. 2001; Vermeij 2013).
Photosymbionts produce photosynthates —organic compounds such as glycerol and triglycerides that are translocated within and between cells to supplement the host ’s nutrient requirements (up to 95 % of that required by the host , Lee 2006). Metabolic CO2 from the host is utilized by the algal symbionts in photosynthesis . Energy flow and carbon cycling is complex (Fig. 3.5), including the recycling and transport of carbon , and the dynamic energy flux on reefs due to these symbionts (Douglas 2003; Muscatine et al. 2005).
The flow of solar energy (joules) in photosymbiosis: an example from the dinoflagellate Symbiodinium to the host coral Pocillopora. Only a small amount is retained by the symbiont for growth and maintenance while the majority of it is translocated to the host which receives only a small amount from its feeding . The relative amounts of energy utilized by the host are shown in the lower part of the diagram. The host uses just under half to make mucus which is discharged to the environment where it is utilized as food by other organisms (Modified from Cowen 1988). Such diagrams are specific to individuals or species, but photosymbionts greatly enhance the energy flow in similar patterns for other organisms that have been measured
3.2.1 Photosymbiosis in Reef Organisms
Among life strategies both photosymbiotic and non-photosymbiotic organisms exist within taxonomic groups. In corals these are known as zooxanthellate and azooxanthellate (z-corals and az-corals) that today are about equally distributed among species (Cairns 1999, 2007). Zooxanthellate species and their photosynthetic symbionts are restricted to tropical latitudes and shallow depths, whereas azooxanthellate species can inhabit cold and deeper-water environments and expand their geographic distributions far outside the latitudinal ranges of zooxanthellate species (Stanley and Cairns 1988; Kiessling and Kocsis 2015). Molecular data (Barbeitos et al. 2010) suggested that coloniality was the original state of scleractinian corals and that the symbiosis between corals and photosynthetic partners was lost and gained repeatedly during their geologic history. Photosymbiotic organisms normally cannot live without their symbionts , but a few apozooxanthellate species are known to be capable of switching between a symbiotic and a non-symbiotic condition (Stanley and Cairns 1988; Lee 2006, 2011). Others can survive without zooxanthellae but they cannot secrete their carbonate shells as fast. For example, corals and foraminifera from which the symbionts have been removed by herbicides or by growing them in the dark fail to secrete skeletons and eventually die. Why more species are not facultatively zooxanthellate is unclear but it may have an evolutionary and genetic basis.
Oddly, while sunlight is required by photosymbionts , too much of it can kill or damage the host and symbionts due to intense light in very shallow waters. To deal with this, corals make colorful chromoproteins that take up substantial amounts of light (Smith et al. 2013) and foraminifera live in particular light ranges or behaviorally adjust light intensity by moving in or out from under overhangs that shade them (Hohenegger et al. 2000). Oxygen resulting from photosynthesis can also be damaging, and hosts have evolved certain antioxidants as protection (Furla et al. 2005). Carbon dioxide can also be limiting. Wooldridge (2014) coined the “CO2 (sink) limitation” model to explain that bleaching at least initially is caused by the host ’s failure to maintain a sufficient supply of CO2 which the algal partner needs.
Photosymbionts help calcifying organisms extract calcium (Ca2+) and acquire carbonate ions (CO3) from an ion-pumping mechanism that brings in Ca++ and exports 2 H+ ions , thus reducing acidity in the calcifying space and resulting in a transformation from CO2 to CO3 (Cohen et al. 2001) to facilitate construction of their carbonate skeletons . This has been demonstrated for corals and inferred for other organisms with symbionts (Hallock 1999). The mechanisms of the physio-chemical skeleton formation and the influence of light on the symbionts are not entirely resolved (Goreau and Goreau 1959; Carlon et al. 1996; Goreau et al. 1996; Marshall 1996; Gattuso et al. 1999), but clearly photosymbionts greatly enhance calcification in their skeletonized hosts (Cohen et al. 2001; Hohenegger 2006; Lee 2006; Cohen and Holcomb 2009; Ries et al. 2009; Lee 2011; McConnaughey 2012; see also Chap. 9). Thus carbonate production in these symbiotic organisms is greatest in the upper part of the top 10 m of sea water and falls off to about half that at depths of about 80 m, and then to very low values with depths increasing to 100 m or greater (Hohenegger 2006).
Some z-corals dwelling at greater depths (68–100 m) have adapted to the lower light levels by shifting toward the red end of the spectrum and by skeletal modifications causing the light to pass through their tissues multiple times thus increasing light harvesting efficiency (Kahng et al. 2012). This is directly due to decreasing photosynthetic activity of the symbionts caused by light attenuation with depth in clear tropical waters. The photosymbiotic foraminifera Cycloclepeus living at depths of over 100 m also demonstrates similar strategies—they harbor diatom symbionts that function optimally at the light spectra available at those depths and they possess very high surface-to-volume ratios to ensure adequate surface area for photosynthesis (Song et al. 1994).
As mentioned above, skeletal modifications evolved in scleractinian corals to support the dinoflagellate photosymbionts under a selection regime dominated by intense light and also when light is limited. These corals grow as plates and branches to maximize the surface area exposed to light at greater water depths. In addition, calcium carbonate skeletons have evolved to increase irradiance by multiple scattering. The carbonate crystals reflect incoming photons to increase the number of times they pass through the tissue. If it is not absorbed the first time, light bounces off the crystal structure of the calcium carbonate skeleton just underneath the living tissue. Then, it is transmitted back through the coral tissue where the symbionts live. This scattering process provides multiple opportunities for photons to be absorbed by algal pigments, reducing the effects of self-shading and increasing the amount of light absorbed per unit of pigment (Enríquez et al. 2005; Terán et al. 2010; Marcelino et al. 2013). Although not yet studied in detail (Lee 2006), foraminiferal tests of symbiont -bearing taxa also have similar complex internal structures (see the classic work of Carpenter et al. 1862 for detailed drawings of chamberlets, pores, canals, coiling and tubes) that may function to reflect, refract or redirect light within the tests.
Photosynthetic organisms can also live freely in sea water (as plankton , on floating mucus mats, or larger algae ) and on a variety of substrates (sediment , rocks, bio-mats) as non-symbiotic forms (Coffroth et al. 2006; Littman et al. 2008; Adams et al. 2009; Pochon et al. 2010; Takabayashi et al. 2012; Sweet 2014). When free-living, they can be dispersed by currents, surge, waves , and even other larger organisms like fish (Castro-Sanguino and Sanchez 2011). Subsequently, their hosts may acquire the symbionts directly, harvesting them from the surrounding environment. They may also be transmitted directly among corals and other metazoans from the parent to offspring and, in some cases, among asexually-dividing foraminifera (Lee 2006).
3.2.2 Photosymbiosis in Hypercalcifiers and Bleaching
On most reefs of the world, z-corals are framework producers and many are hypercalcifers, organisms that can rapidly secrete massive amounts of skeletal calcium carbonate . In the geologic past, Earth’s oceans experienced secular shifts in the Mg/Ca ratios driven by changing CO2 levels (Sandberg 1983) that led to alternating periods that favored or discouraged the precipitation of aragonite versus calcite . As a result, marine organisms were affected by these cycles depending on their preferred skeletal composition. Hypercalcifying organisms such as aragonitic scleractinean corals would be at a disadvantage in a calcite sea cycle, and this relationship may help explain selective patterns of extinctions (Stanley and Hardie 1999). An analysis of this selectivity showed a correspondence between extinctions and hypercalcifying organisms for some extinction events (Kiessling and Simpson 2011). However, the Phanerozoic correspondence for this is far from perfect (Kiessling et al. 2008).
Hypercalcifers today require vigorous water motion and generally prosper in the upper photic zone in optimal temperature ranges of 23–29 °C. While the thresholds change between species, morphologies and location, death will generally occur at prolonged temperatures below 14 °C or above 25 °C. Some photosymbiotic corals are genetically modified to live in warmer water in isolated pools on reefs (Barshis et al. 2013), so the temperature restrictions are not necessarily constant biologically or ecologically. Yet corals, giant clams and foraminifera lose the symbionts on which they depend when temperatures exceed the normal range. In such cases of bleaching , vast numbers of corals turn ghostly white and can die unless the thermal stress is short-lived and the corals can reestablish their photosynthetic relationship (van Oppen and Lough 2009).
Clades of Symbiodinium inhabiting hosts vary in their adaptability and response to thermal tolerance (Rowan 2004). Such holosymbionts may explain the survival of some species in bleaching events. Indeed the “Adaptive Bleaching Hypothesis” (Fautin and Buddemeier 2004) posits that, following bleaching , some corals (and presumably other organisms as well) have the ability to reestablish a symbiosis with new clades of symbionts that are better suited to the new post-bleaching environment. This pattern could explain why coral reefs seem so fragile in the short-term when rapid temperature changes can cause widespread mortality but robust in the longer geologic -term as more adaptable species survive and are, therefore, more likely to persist and be preserved.
Symbiont -bearing protists and animals do not live well in areas affected by muddy or terrigenous sediments , an increase in nutrients (Hallock et al. 2006), elevated salinity , pollution , or warming temperatures (Douglas 2003). Corals and other carbonate -producing organisms capable of photosymbiosis are able to prosper in nutrient-deficient environments because of the efficient biochemical cycling of inorganic carbon and nitrogen by zooxanthellae (Hallock 2001). In contrast, low nutrients discourage macroalgae , a primary competitor for space on the reef. Normally, this will favor corals over macroalgae . However, the efficiency with which corals can produce carbonate in low-nutrient waters also makes them susceptible to even small changes.
3.3 Photosymbiosis in Ancient Fossils and Reef Environments
While photosymbiosis very likely occurred in many reefs and reef organisms of the geologic past, photosymbionts are not directly preserved among fossil organisms. As a result, inferring their presence in fossils depends on comparisons with modern animals in general (Cowen 1983, 1988), functional morphology in particular and, rarely, the presence of oxygen or carbon isotopes that are consistent with photosynthesis in the host skeletons (Dreier et al. 2014). Many genera of living scleractinians evolved in the early or middle Cenozoic (Budd 2000; Budd et al. 2011) and some species can be traced back millions of years. In these cases inferences about photosymbionts are more secure than for much older corals (e.g., tabulates and rugose corals). Thus confidence in biological uniformitarianism (i.e., modern biological processes are similar to those of the past) commonly decreases farther back in time. Many Mesozoic and Paleozoic taxa are extinct and many lack extant relatives, posing difficulties for inferring photosymbioses.
Although the dominant photosymbiont today is Symbiodinium , many reef organisms, including foraminifera , sponges, and even coral, also harbor other kinds of symbionts (Lee 2006, 2011; Ainsworth et al. 2010). These include other types of dinoflagellates that may have existed in earlier geologic time before the evolution of Symbiodinium.
The photosymbiotic hypotheses in fossils depend on a variety of indirect criteria. High levels of triaromatic dinosteroids are commonly associated with dinoflagellates in early Cambrian sediments , suggesting that their ancestry may extend to this time (Moldowan et al. 1996). Diagenetically unaltered fossil skeletons of Triassic and Jurassic corals (Stanley and Swart 1995), Paleozoic corals (Zapalski 2014), foraminifera (D’Hondt et al. 1994), and rudistid bivalves (Steuber 1996) have yielded stable isotopes of O and C that have been taken to indicate photosynthesis and hence the likelihood of ancient symbionts . Finally, large, thick and expansive skeletons (Figs. 3.6, 3.7, 3.8, 3.9, 3.10 and 3.11) suggest rapid skeletonization and, therefore, photosynthetic symbionts within the once-living organism. Regular annual bands within skeletons can provide actual linear extension rates and thus useful information about annual growth (Barnes and Lough 1993).
The giant clam Tridacna at the Palau Mariculture Demonstration Center, Palau. The valves of this clam are huge and massive , a characteristic of the skeletons of animals and protists that host symbionts . The photosymbiont Symbiodinium lives in the mantle tissue overlapping the edges of the valves (Photo by J. H. Lipps, 1992)
Large photosymbiotic foraminifera . (a) Marginopora, harboring the dinoflagellate Symbiodinium , ranges in size from 0.5 to 1.5 cm. It lives abundantly on sandy knolls, on algal turfs and Halimeda on reefs. (b) A living Alveolinella quoyi with pseudopodia extended hosts diatom symbionts . It lives on sandy slopes to at least 30 m depth and probably to the base of slopes on the floor of the lagoon , and usually at 40–50 m off reefs near Madang, Port Moresby, Papua New Guinea , and Lizard Island in the Great Barrier Reef , Australia . Smaller specimens may inhabit dead corals and coral rubble wherever it occurs, including just below low-tide level. (c) Three dead and cleaned tests of A. quoyi exhibit large size (up to 2.5 cm long), numerous long and narrow chambers and extended apertural faces with many large pores. In both Marginopora and Alveolinella, symbionts are concentrated in upper parts of the interior protoplasm of the tests creating the darker shades on the tests in these black and white images. These larger foraminifera exhibit massive amounts of CaCO3 making up their skeletons relative to non-symbiont bearing benthic foraminifera . (Photos by J. H. Lipps (1986) in Papua New Guinea at Motupore Island (top left) near Port Moresby, in the Madang Lagoon (bottom), and dead tests of Alveolinella quoyi from the Madang Lagoon. See Langer and Lipps (2003) for distributions in the Madang Lagoon)
Giant Pennsylvanian (Upper Carboniferous ) fusulinid foraminifera (Parafusulina). Scale = 1 cm. Fusulinids occur in thick, widespread limestone beds in the upper Paleozoic and closely resemble the living symbiont -bearing species Alveolinella (Fig. 3.8) in habitat , morphology , size, and internal complexities, all supporting the inference that fusulinids possessed photosymbionts (Photo by J. H. Lipps of University of California Museum of Paleontology specimens)
One of the largest calcareous foraminifera known, Praealveolina ranges to more than 10 cm in length. These were common in the later Cretaceous, and closely resemble the only large fusiform modern species Aleveolinella quoyi as well as the Paleozoic fusulinids, some of which attained even larger sizes. (a) This fusiform specimen is broken and about 3 cm are missing on the right end, making its total length and width (near the 4-cm mark) greater than 10 cm and 2.7 cm, respectively. (b) Broken end (2.6 cm in diameter) showing the complex inner structures of small chambers divided by partitions. Such complexities are indicative of photosymbionts contained within the test. Photos by Bruce Rubin of University of California Museum of Paleontology specimen A-9227
Photosymbiosis in fossil organisms may be inferred from morphologies to capture light along latitudinal or depth gradients (Cowen 1983, 1988; Wood 1999; Stanley and Lipps 2011; Groves et al. 2012). Flattened skeletal shapes and thin tissues spread symbionts over larger living areas within the host resulting in more efficient light capture (Wood 1999). These effects can be seen in flattened corals (Fig. 3.6), the expanded mantle of giant clams (Fig. 3.7) or flattened disc-like foraminifera (Figs. 3.8a and 3.10). Still others like large fusiform foraminifera (Figs. 3.8b, c, 3.9 and 3.11), expand their area through lengthening the skeleton which is occupied by symbionts in the upper part of the protoplasm below the upper test surface (Lipps and Severin 1986). High levels of corallite or modular integration (e.g., interconnection between coral polyps ) in colonial photosymbiotic organisms modify their shapes to maximize light and facilitate the transport of photosynthate (Coates and Oliver 1973). On modern reefs, modular organisms modify the colony according to light availability, although the resulting growth form is often a compromise for maximizing light and shedding sediments (Figs. 3.6 and 3.7).
Finally, most photosymbiotic reef organisms tend toward large size (Cowen 1988) at least in comparison to others in their group (Figs. 3.6, 3.7, 3.8, 3.9, 3.10 and 3.11). Increased size of symbiont -bearing taxa is true of most groups from foraminifera through corals to giant clams, although exceptions occur (Fig. 3.12). Therefore, massive skeletons and the large amounts of carbonate rock and sediment have been presumed to be a consequence of high calcification rates (James 1983).
Corculum (University of Montana Paleontology Center UMIP 14319), a modern photosymbiotic clam, has windows in its shell that allow light to pass to photosymbionts living in the mantle tissue inside the shell. When the clam burrows into sand and the valves are closed, the symbionts are still able to photosynthesize using light that passes through the windows (Photo courtesy of Kallie Moore). Scale bar = 2 cm
3.4 Important Photosymbiotic Taxa in Ancient Reef Ecosystems
In the previous section, we outlined several lines of evidence that suggest active photosymbioses in the past. The following is a brief overview of specific groups of fossil organisms considered to have been photosymbiotic and the evidence supporting this important relationship.
3.4.1 Foraminifera
In ancient and modern seas, these single-celled eukaryotes are abundant in shallow tropical and semitropical waters, occupying rather specific habitats on the reefs and platforms (Hohenegger et al. 1999; Langer and Lipps 2003; Hohenegger 2006). Some are truly giant and complex protists (Lipps and Severin 1986; Song et al. 1994; Hallock 1999; Lee 2006, 2011; Figs. 3.7, 3.8, 3.9, 3.10 and 3.11). Foraminifera have evolved particular morphologies ranging from the flattened tests in Marginopora or Cycloclepeus (Song et al. 1994) to large, complicated and elongate forms like Alveolinella, Praealveolina and fusulinids (Figs. 3.8, 3.9 and 3.11). Even some smaller foraminifera have morphologies associated with modern symbionts . For example, Amphistegina has tiny cups on the interior surface of its test that contain the symbionts (Lee 2006).
The first foraminifera that likely had photosymbionts were the mid-Paleozoic fusulinids (Fig. 3.9); they dominated carbonate banks and platforms until the end of that era (Vachard et al. 2010). Evidence for photosymbiosis in these foraminifera includes their relatively large size (for foraminifera ), complex internal morphology , ecologic and geographic distributions in tropical environments on reefs and carbonate banks , and overall similarity to modern symbiont -bearing alveolinellids (Lipps and Severin 1986; Severin and Lipps 1989; Lee 2006; Groves et al. 2012).
Large size in fusulinid foraminifera has also been attributed to high atmospheric oxygen levels (∼30 %) rather than symbiosis (Payne et al. 2012). When oxygen levels are high a large volume to surface area may allow oxygen to diffuse quickly into the interior of organisms and metabolic rates can be higher, yet foraminifera and other organisms grow to large sizes today when they possess symbionts (Lipps and Severin 1986; Song et al. 1994; Hallock 1999; Lee 2006, 2011; Vermeij 2013). Indeed the largest living fusiform foraminifera Alveolinella quoyi (Fig. 3.8b, c), which resembles the large fusulinids (Fig. 3.9), possesses diatom symbionts and can live to depths over 30 m or more. At lengths of 2–3 cm or more, A. quoyi achieves large sizes under today’s oxygen levels. The volume of the cytoplasm in A. quoyi rarely fills more than 45 % of available chamber volume (mean = 37 %; range = 17–100 %: Severin and Lipps 1989).
The paleobiogeography of fusulinids with smaller individuals in the Polar Regions and larger ones at the equator does not support the oxygen hypothesis for these foraminifera but rather suggests that they possessed symbionts (Zhang and Payne 2012). Fossil alveolinellids (Cretaceous to Neogene ) attained very large sizes too (some over 10 cm long; see Fig. 3.11) and all of these lived under a variety of atmospheric oxygen levels. Thus these observations cast “further doubt on the primary role of oxygen as a factor enabling gigantism in photosymbiotic species” (Vermeij 2013). As this variability is inconsistent with a tie to atmospheric O2 levels, we attribute large size in these symbiont -bearing foraminifera to photosymbiosis rather than oxygen availability.
On modern reefs, larger photosymbiotic foraminifera produce prodigious amounts of calcium carbonate on reefs and carbonate platforms , in some places contributing up to 25 % of the total (avg. ∼5 %: Langer et al. 1997; Langer 2008). In the past, vast amounts of carbonate rocks, forming banks and shelves, were similarly produced by large foraminifera that we interpret to have hosted or likely hosted symbionts . Their large size and extended pseudopods would have made them difficult to transport , enhancing their likelihood of in-situ deposition (Severin and Lipps 1989). Paleozoic fusiform fusulinids contributed to thick limestone blankets over many km2. Mesozoic and Cenozoic alveolinellids, orbitolinids, and others produced thick deposits of carbonate , and the giant, coin-like Nummulites (Fig. 3.10), dominated the former Tethyan Seaway in places like the Eocene of Israel. The enormous (up to 10 cm long) elongated foraminifera Praealveolinella (Fig. 3.11) appeared in the Cretaceous and surely had symbionts , as its size, carbonate content, distribution, and internal complexity attest.
The phrase “Power of the Pyramids” might be replaced with the “Power of Photosymbiosis ” since the huge monoliths of Egypt (Fig. 3.13) are made of nummulitic Eocene limestone blocks (Fig. 3.13 inset). Indeed the “power of photosymbiosis” made the pyramids possible in the first place, since nummulites very likely hosted photosymbionts . Photosymbiotic foraminifera have contributed to the formation of extensive carbonate rocks for ∼350 million years of geologic time with exceptions of the post-extinction periods (Chap. 8).
“The Power of Photosymbiosis ”—The Great Pyramid and Sphinx of Giza, Egypt. The Sphinx consists of several layers of marl and limestone with few nummulites in them as those foraminifera lived on a bank farther away (Gauri et al. 1990). The Great Pyramid was constructed chiefly of local limestone blocks containing abundant Nummulites (inset). The early historians, Herodotus (Greek, fifth century BCE), Strabo (Greek, second century BCE) and Pliny the Elder (Roman, 23–79 CE), considered stories that the nummulites were lentils dropped by the workmen as they ate which then petrified (Carpenter et al. 1862; Adams 1938; Lipps 1981). Even the earliest of these observers noted that the nummulites occurred widely in the region and hence were not the remains of lentils. The nummulites actually accumulated abundantly in the sediments of the Eocene Tethys Seaway. They likely hosted photosymbionts that made the growth of the large tests possible (Photos by J. H. Lipps, 2007)
3.4.2 Calcified Sponges
Ancient reefs were also built by a wide variety of calcitic or aragonitic sponges (demosponges, stromatoporoids , chaetetids and other groups). Archaeocyathids were calcitic sponges (Rowland 2001) that dominated reefs during the Early Cambrian (Fig. 3.14). They lived in tropical shallow waters where they produced small mounds, moderate-sized buildups , and even very large complexes, such as the Great Siberian Reef Complex, 200–300 km wide and 1500 km long (Rowland and Hicks 2004).
Cross sections of individual archaeocyathan skeletons in the Cambrian Montenegro member of the Poleta formation, White-Inyo Mountains, California. In many places, the archaeocyathans are associated with patch reef and large reef structures; they likely contained photosymbionts . Scale bar = 1 cm (Photo by J. H. Lipps, 1986)
Archaeocyaths included many species with cup-shaped skeletons that varied in morphology from nearly flat to lobate with flattened edges at the top of the cup to more tubular forms. Individuals ranged in size from a few cm up to 30+ cm and were attached to the substrate with holdfasts. Given their morphologies , shallow water habitats , tropical distribution, and reef-building abilities, archaeocyathids may have possessed symbiotic algae or perhaps cyanobacteria (Cowen 1983; Rowland and Gangloff 1988; Surge et al. 1997; Rowland and Shapiro 2002). However their small size, solitary growth form , low modular integration level and cryptic lifestyles led to the alternative hypothesis that they lacked symbionts and lived in environments with fluctuating nutrients and high input of terrigenous sediments (Wood 1993, 1999; Pratt et al. 2001; Zhuravlev 2001). Both symbiotic and asymbiotic forms may have inhabited the same reefs simultaneously, as the same major groups do today (e.g., foraminifera , sponges and corals).
Stromatoporoids were an important group of calcified sponges from Ordovician to Late Devonian time and during the late Mesozoic (Nestor et al. 2010). They were important reef-builders during mid-Paleozoic time, some reaching 10–20 m in diameter. These sponges secreted calcitic skeletons and like corals, appear to have been important in constructing impressive reefs during mid-Paleozoic time (Copper 2002). They lived together with calcareous algae , rugose corals and tabulate corals. Red algae , corals and stromatoporoids formed fringing and barrier reefs of the Silurian and Devonian. These reefs exceeded modern examples in size and volume during a mid-Paleozoic greenhouse time when tropical marine realms reached much higher latitudes than today.
Although stromatoporoids appear to have been reef builders, they yield equivocal evidence of photosymbiosis (Kershaw and Brunton 1999). Among the 5000 different species of living sponges, many harbor photosymbiotic organisms, especially cyanobacteria (Taylor et al. 2007) and some tropical examples show photosymbiotic activity with other organisms in very shallow settings (Steindler et al. 2002). A radical reinterpretation of stromatoporoids as cyanobacteria rather than metazoans would certainly imply photosynthesis (Kazmierczak 1976) but this interpretation is not widely accepted. Evidence supporting photosymbiosis in Paleozoic stromatoporoids includes growth forms like corals, modular integration and large size (Copper 2002). The limited data suggest that they were slightly slower growing than living corals (Gao and Copper 1997).
Paleoecologically , stromatoporoids in Paleozoic reefs appear to have been limited by nutrients and sediment influx and capable of growing between and over other organisms such as brachiopods , corals and red algae (Fig. 3.15). Some taxa suggest high levels of integration and are interpreted to have lived in shallow, open and sun-lit parts of ancient reefs. This contrasts with living sclerosponges , which are relegated to cryptic and/or deeper water environments.
Whether these organisms harbored photosymbionts is not clear. The large size, platy growth shapes (Fig. 3.16) and integration levels of mid-Paleozoic reef-dwelling stromatoporoids commonly resemble modern photosymbiotic scleractinians (Rosen 2000). Also, stromatoporoids provide evidence for the “thin tissue syndrome” (Wood 1999) and the “solar panel effect” (i.e., flattening with depth to maximize light-gathering capacity). Finally, feeding strategies and paleobiology indicated that at least some stromatoporoids were photoautotrophic (Brunton and Dixon 1994), an idea supported by the co-occurrence of these sponges with large photosymbiotic megalodontid bivalves , which also preferred warm, well-lit marine settings. However other evidence for photosymbiosis among mid-Paleozoic stromatoporoids is equivocal (e.g., are growth bands annual and what was their growth rate relative to corals: Kershaw 1998).
A vertical section cut through a whole stromatoporoid from the mid-Silurian Visby Formation, Gotland, Sweden. In this example, three different species of stromatoporoids grew together and were partly buried by sediment before the next growth , gradually building up the structure. Near the bottom a brachiopod lay on the lower growth of stromatoporoid and was then overgrown by the next stromatoporoid layer. This process illustrates how complex reef structures are built by various species (Photo courtesy of Steve Kershaw)
Other sponges which lived during the Permian , Carboniferous and Triassic appear to have been hypercalcifying and capable of building reefs. These include calcified chambered “sphinctozoan ” and chaetetid sponges. While some of these reached large size and were primary or secondary reef constructors, they show slow growth rates . Some calcified demosponges such as the Upper Triassic reef-adapted Stromatomorpha, are a mimic on Paleozoic stromatoporoids and they may have been photosymbiotic (Senowbari-Daryan and Stanley 2009).
3.4.3 Corals
Like their modern counterparts, corals in the geologic past constructed reefs. Morphological similarities and growth strategies suggest that they also share a photosymbiotic relationship. Their large size and corallite integration argue for rapid skeletonization . Also, what appear to be depth-related changes in colony shape (i.e., flatter colonies at depth: Dodge and Vaišnys 1980; Dustan 1982) and a high degree of corallite integration argue for photosymbiosis. The “edge zone” along the outer corallite wall where tissue extends is similar to modern, zooxanthellate corals. Like foraminifera and bivalves , they also are characterized by thin tissue syndrome (Cowen 1983), displaying large areas of thin tissue for harvesting light. Finally, like some zooxanthellate corals, they also contain abundant radiating features of the skeleton called pennular structures that radiate from the polyp centers and are thought to supplement nutrition in slightly deeper water (Wood 1999; Stanley 2006).
Many ancient corals contain alternating low-density and high-density layers of skeleton (Fig. 3.17a) that in modern corals are annual (Fig. 3.17b). They have been associated with variables of light, temperature , reproduction, nutrients , and other factors affecting the energy budget of coral growth (Buddemeier 1974). Such features in fossils of Permian , Triassic and Jurassic ages allow comparisons with fast-growing living reef species. Some massive Triassic corals contained annual bands almost identical to high and low density bands in living reef corals (Stanley and Helmle 2010). This may indicate ancient photosymbiosis in the Triassic.
Comparison of growth bands in a Late Triassic coral Ceriostella (UMIP 18001) (a) with a modern Montastraea (b) showing high density and low density annual banding . Scale bars = 1 cm (Photos from Stanley and Helmle (2010))
Many coral colonies, especially those on modern reefs, have large sizes and this has been used as a proxy for rapid growth and, therefore, photosymbiosis. Some Late Triassic colonies reached 5–10 m in height (Piller 1981; Stanley and Swart 1995), larger than some modern z-coral species. Also, they are similar to “microatolls ”, which form today as the colonies grow to sea level , their polyps die on top while the colony continues to expand laterally. This distinctive morphology records sea level , and in modern corals occurs in at least 43 (Rosen 1978) colonial or massive species (Scoffin and Stoddart 1978; Smithers and Woodroffe 2000). The oldest-known microatolls from the Late Triassic (Fig. 3.18) have flattened surfaces and a central cavity (Stanley 2005). Since microatolls today are only known in modern zooxanthellate species, their presence in fossil examples has been linked to photosymbiosis.
The oldest reported microatolls (University of Montana Paleontology Center UMIP 6813) from the Triassic in Nevada. Like modern microatolls , they formed by growing to sea level to maximize solar radiation , and then spread laterally. Scale bars are 1 cm (Photo modified from Stanley (2005))
Cretaceous corals also possess colony shapes and corallite integration that suggest photosymbiosis. Jurassic corals likewise show high integration levels, annual banding and adaptation of colony shapes similar to those of modern reef-building corals (Leinfelder 2001; Barbeitos et al. 2010). Rosen and Turnšek (1989) characterized coral species that survived across the Mesozoic -Cenozoic boundary as either z-coral-like or az-coral-like on the basis of the indirect criteria described above.
A more direct line of evidence comes from isotopic studies of early Mesozoic corals. Thirteen specimens of Triassic scleractinians from reef complexes in Turkey and northern Italy and two specimens from the Jurassic of Poland showed that the isotopic signatures of the Triassic corals from Turkey were more like modern zooxanthellate corals, while the Jurassic samples were similar to azooxanthellate species (Stanley and Swart 1995). Isotopic analyses of skeletons of Late Triassic corals yielded a similar conclusion regarding photosymbiosis (Muscatine et al. 2005).
Photosymbiosis can be traced back to Paleozoic corals (both Rugosa and Tabulata) which were judged to have been photosymbiotic by paleoecological methods and isotope studies (Zapalski 2014). While the majority of rugose corals were solitary, some colonial species reached large sizes in mid-Paleozoic reefs and like living photosymbiotic species, possessed high levels of corallite integration . Tabulate corals (Fig. 3.19) lived on mid-Paleozoic reefs reached large sizes, some resembling colonies of modern z-corals. Growth rates of many Paleozoic corals (Gao and Copper 1997) also compare favorably to living z-corals and provide evidence for photosymbiosis. However corallum complexity of some Paleozoic species as judged by integration levels, are lower than for scleractinian corals (Coates and Jackson 1987). While these approaches are reasonable, such assessments are really historical hypotheses to be further tested.
Reconstruction of a Middle Silurian reef illustrating a coral-dominated ecosystem characterized by large colonies of tabulate and rugose corals along with crinoids, bryozoans, brachiopods and other invertebrate taxa. This was the closest approximation in the Paleozoic to scleractinian -dominated reefs (Illustration courtesy of Terry Chase)
3.4.4 Bryozoans
Starting in the late Cambrian (Landing et al. 2015), bryozoans have a detailed fossil record continuing to the present (Taylor and Waeschenbach 2015). Many species over this time are associated with reefs and some grow quite large. The large (up to 7 cm) calcareous bryozoan colonies formerly known as the Trepostomata, for example, may have possessed photosymbionts , and this idea was postulated for Permian examples (Håkansson and Madsen 1991). The large size is consistent with the rapid growth attributed to photosymbiosis. Stable isotope analysis, however, revealed that such Paleozoic bryozoans secreted calcite in isotopic equilibrium with seawater and so did not possess the signature of photosymbiosis (Key et al. 2005). Also, no modern bryozoans are reported to possess photosymbionts . Thus, the role of photosymbiosis in byrozoans remains speculative.
3.4.5 Brachiopods
Various Paleozoic productid brachiopods , including Richthofeniacea and Lyttoniacea, may have harbored photosymbionts based on (1) shell adaptations to expose much of the mantle to light, (2) massive calcification , (3) large size, (4) habitat , and (5) paleogeographic distributions in tropical seas of the Late Permian (Cowen 1983, 1988). Among these were the reef-dwelling richthofenid brachiopods (Fig. 3.20) Hercosia and Cyclacantharia (Grant 1972; Cowen 1983; Fagerstrom 1996; Cowan and Erickson 2010).
The conical silicified Middle Permian reef brachiopod Hercosestria from the Glass Mountains, south-west Texas. (a) The sieve-like covering over the ventral valve may have been covered with mantle tissue (UMIP 14291). (b) Several individual ventral valves of these brachiopods with their attachment spines and some of the sieve-like covering (UMIP 14292). Scale = 1 cm. University of Montana Paleontology Center (UMIP) (Photo courtesy of Kallie Moore)
Photosymbiosis is a logical hypothesis for the bizarre morphology exhibited by Late Permian lyttoniacean brachiopods which inhabited reef-like buildups (Cowen 1983). However, morphology alone is insufficient to support either a photosymbiosis hypothesis or the possibility that they may have combined photosymbioses with a filter feeding lifestyle.
Some Devonian brachiopods are relative giants, such as Stringocephalus in the Givetian, with shells up to 20 cm long; these could also have had algal symbionts (P. Copper, personal communication, 2011). However, unlike the majority of other photosymbiotic organisms that utilize aragonite , brachiopods secreted shells of calcite .
3.4.6 Mollusks
At least 17 independent bivalve groups may have developed photosymbiosis with algae in Earth’s history (Vermeij 2013). While evidence is commonly equivocal, these groups display many characters that would promote or result from photosymbiosis. One shelled mollusk may have had photosymbionts in the Eocene to Oligocene , the gastropod Velates which was large; it likely had exposed mantle tissue and inhabited well lit habitats (Vermeij 2013). Most photosymbiont -bearing taxa are attached to or buried in the substrate , hence bivalves dominate the shelled mollusks that hosted these symbionts . Many upright, Late Cretaceous rudistid bivalves built mounds and reef-like structures (Kauffman and Johnson 1988) and secreted large, thick shells of both calcite and aragonite (Fig. 3.21). Their fossils , based on calculated growth rates and modifications of the upper shell, strongly infer adaptation to light and photosymbiosis (Vogel 1975). However, some other rudistids may not have possessed photosymbionts (Steuber 2000). While some rudistids display growth rates comparable to the living photosymbiotic Tridacna (Fig. 3.7), other living bivalves like Corculum (Fig. 3.12) and Fragum are small and do not produce large or thick shells, yet they have unique “windows” in their shell, which would have allowed light to reach algal symbionts inside (Watson and Signor 1986; Farmer et al. 2001).
Other bivalves with algal symbionts , like the modern heart cockle Clinocardium, do not show any of the characteristics used to infer photosymbiosis (Jones and Jacobs 1992). While size is not always an indicator, it is an obvious characteristic of photosymbiosis for bivalves and other organisms.
Exclusive of rudistids , other giant, reef-dwelling bivalves existed on carbonate platforms through time. Giant clams occurred in the Devonian, Permian , Triassic , and Jurassic . Giant alatoform bivalves in the family Wallowaconchidae (Yancey and Stanley 1999) can be up to a meter in length and occur in reef-related, Upper Triassic carbonate rocks. Not only do these unique bivalves exhibit large size, they also display the “solar panel” effect, secreting a series of enveloping chambers along the margin of the shell (Fig. 3.22) where presumably photosymbionts were sequestered in the mantle tissue. Like living Corculum these bivalves may have hosted symbionts within the chambers and have been able to harvest light transmitted directly through the shell.
Giant wallowaconchid alatoform clams from the Late Triassic . (a) Reconstruction of the shell showing the conch with a cut-away view of the shell that reveals a series of concentric and overlapping chambers (arrow) connecting to the central body cavity . The chambers likely housed the photosymbionts inferred to have lived within the chambers of these clams, and the upper surface of the chambers were made of aragonite crystals perhaps oriented as in the modern Corculum (Fig. 3.12) to conduct light to the symbionts inside. Courtesy of Jose Garcia. (b) Field image of an individual clam cut at an oblique angle, showing the central body cavity and chambered, wing-like extensions (sample UMIP 23530). Upper left inset is a thin-section showing details of hollow chambers, now filled with micrite (sample UMIP 24206-F). Scale bar = 5 mm
3.5 Summary and Conclusions
Photosynthetic algae and microbial eukaryotes and metazoans have formed symbioses throughout much of geologic time (Cowen 1983; Coates and Jackson 1987; Talent 1988; Stanley and Lipps 2011). This cooperative relationship across many unrelated algae and hosts evolved very early on, and has been common and repetitive ever since. Although photosymbionts are not preserved in the fossil record , ample indirect evidence (e.g., isotopes , morphology , size and depth-related changes in host morphologies ) indicate that photosynthetic partnerships were strongly selected for the capture of energy directly from sunlight and a reliable nutrient supply for the hosts and a habitat with protection for the symbionts (Cowen 1983, 1988; Hallock 1999; Lee 2006; Vermeij 2013).
Photosymbioses evolved multiple times in geologic time and became quite common, only to go extinct during the extinction events of the past 543 Ma. Most likely these symbioses developed in single-celled hosts and sponges as the algae were harvested as part of the food supply and later sequestered within cells as happens today with the inclusion of both live chloroplasts (Lopez 1979; Cedhagen 1991) and free-living symbionts in the cytoplasm of certain foraminifera (Lee 2006; Fay et al. 2009; Lee 2011). In cases where they occupy those parts of present-day organisms that are irradiated by the sun, symbionts might have been acquired from the environment and transported to specific tissues. Perhaps the same mechanism coevolved in other partners as well. Did hosts acquire symbionts specifically for their own advantage and how complex was the evolution ? While modern corals will accept or reject certain algal symbionts , observations of infestations support the hypothesis that forming endosymbiotic associations leading to a “fit” host actually involves a complex series of co-evolutionary steps (Stat et al. 2006).
Although photosymbioses are inferred in the early Paleozoic , they were well-established and widespread by the early Mesozoic . Today’s prime photosymbionts are dinoflagellates in the genus Symbiodinium belonging to the order Suessiales, which includes closely related symbionts of various planktic organisms (Siano et al. 2010). In their life cycles, dinoflagellates often encyst and these are common as microfossils. The oldest Suessiales cysts that are morphologically similar to those of modern Symbiodinium symbionts first appeared in the Late Triassic when scleractinian corals radiated. The fossil record of these cysts tracks those of corals; both groups also experienced an extinction at the end of the Triassic and a recovery in the succeeding Jurassic (Stanley and van de Schootbrugge 2009). Photosymbiosis between scleractinian corals and dinoflagellate symbionts may have occurred in the Triassic (Stanley and Helmle 2010) based on the widespread development of reefs and thick reef-carbonate rocks during the Late Triassic interval (Stanley 1981; Riedel 1991; Kiessling 2010).
Molecular-clock data, based on sequences of chloroplasts in modern clades of Symbiodinium , place their origin in either the Paleocene or early Eocene, not long after the Cretaceous/Paleogene mass extinction . However, the diversification of the modern lineages of Symbiodinium did not occur until the mid-Miocene some 15 million years ago (Pochon et al. 2006; Stat et al. 2006), coincident with the evolution and expansion of the modern coral-reef ecosystems (Perrin 2002).
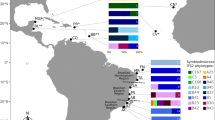
Because symbiont -host relationships are not monophyletic through geologic time, the coevolution with host species is unclear. Molecular studies of clade D Symbiodinium in reef corals, revealed “boom and bust” phases of diversification and extinction over the past 12 million years in response to climate change and the tectonic separation of the Caribbean and Pacific provinces by the emerging Central American Land Bridge (Thornhill et al. 2013). We suggest that in the perspective of deeper time, such “boom and bust” cycles characterized many photosymbionts and their hosts.
Symbionts from the Symbiodinium group have established relationships with a wide taxonomic variety of hosts such as sponges, corals and other cnidarians, benthic and planktonic foraminifera , giant clams, among others. Commonly the same clades may be associated with multiple unrelated hosts, e.g., foraminifera , jellyfish, milleporoid hydrozoans, octocorals, nudibranchs, tunicates and bivalves . Different molecular clades of Symbiodinium in corals, and perhaps other groups as well, have different ecological preferences for light, temperature , depth and, therefore, hosts (LaJeunesse et al. 2010; Kahng et al. 2012). In foraminifera , different clades may even occupy specific parts of the cell (Fay et al. 2009).
The geologic history of the successes and failures of reefs can be related directly to the acquisition or failure of photosymbioses (Talent 1988). Reefs did well during long periods of stable environmental conditions but became extinct when warming , acidification , and anoxia of the oceans occurred. Following these extinction events, newly acquired photosymbionts fueled the rapid diversification of reef organisms, for example, corals in the Triassic after the great Permian extinctions . Conversely, the loss of photosymbionts could have been a key strategy for surviving extinction crises (Barbeitos et al. 2010).
Based on our review of reef building and the calcifying organisms involved, we confirm our hypothesis that photosymbiosis was integral to the success of both present-day and ancient reefs. We find that the breakdown of the symbiosis most likely was tied to global environmental perturbations that led to mass extinctions . In concert with fluctuations in nutrient, sedimentation and other factors, such breakdowns might explain reef gaps, times of reduced carbonate sedimentation and drastic reductions in reef building observed in the geologic record.
References
Adams FD (1938) The birth and development of the geological sciences. Dover Pub. New York.
Adams LM, Cumbo VR, Takabayashi M (2009) Exposure to sediment enhances primary acquisition of Symbiodinium by asymbiotic coral larvae. Mar Ecol Prog Ser 377:149–156
Ainsworth TD, Thurber RV, Gates RD (2010) The future of coral reefs: a microbial perspective. Trends Ecol Evol 25:233–240
Allwood AC, Walter MR, Burch IW, Kamber BS (2007) 3.43 billion-year-old stromatolite reef from the Pilbara Craton of Western Australia: ecosystem-scale insights to early life on Earth. Precamb Res 158:198–227
Anderson OR (1983) Radiolaria. Springer-Verlag, New York
Baker AC (2003) Flexibility and specificity in coral–algal symbiosis: diversity, ecology and biogeography of Symbiodinium. Ann Rev Ecol Evol Syst 34:661–689
Barbeitos MS, Romano SL, Lasker H R (2010) Repeated loss of coloniality and symbiosis in scleractinian corals. Proc Nat Acad Sci 107:11877–11882
Barnes DJ, Lough JM (1993) On the nature and causes of density banding in massive coral skeletons. J Exp Mar Biol Ecol 167:91–108
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Knowles NT, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Nat Acad Sci 110:1387–1392
Benton MJ (2003) When life nearly died: the greatest mass extinction of all time. Thames & Hudson, London
Blank RJ, Trench RK (1985) Symbiodinium microadriaticum: a single species? Proc 5th Int Coral Reef Symp 6:113–117
Brunton, FR, Dixon OA (1994) Siliceous sponge-microbe biotic associations and their recurrence through the Phanerozoic as reef mound constructors. Palaios 9:370–387
Budd AF (2000) Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19:25–35
Budd AF, Klaus JS, Johnson KG (2011) Cenozoic diversification and extinction patterns in Caribbean reef corals: a review. Paleontol Soc Papers 17: 79–94
Buddemeier, RW (1974) Environmental controls over annual and lunar monthly cycles in hermatypic coral calcification. Proc 2nd Int Coral Reef Symp 2:259–267
Butterfield NJ (2015) Early evolution of the Eukaryota. Palaeontol 58: 5–17
Cairns SD (1999) Species richness of recent Scleractinia. Atoll Res Bull 459:1–46
Cairns SD (2007) Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull Mar Sci 31:311–322
Carlon DB, Goreau TJ, Goreau NI, Trench RK, Hayes RL, Marshall AT (1996) Calcification rates in corals. Science 274:117–118
Carpenter WB, Parker WK, Jones TR (1862) Introduction to the study of the Foraminifera. Q J Microsc Sci 2:1–319
Castro-Sanguino C, Sanchez JA (2011) Dispersal of Symbiodinium by the stoplight parrotfish Sparisoma viride. Biol Letters 299:282–286
Cedhagen T (1991) Retention of chloroplasts and bathymetric distribution in the sublittoral foraminiferan Nonionella labradorica. Ophelia 33:17–30
Clarkson MO, Kasemann SA, Wood RA, Lenton TM, Daines SJ, Richoz S, Ohnemueller F, Meixner A, Poulton SW, Tipper ET (2015) Ocean acidification and the Permo-Triassic mass extinction. Science 348:229–232
Coates AG, Jackson JBC (1987) Clonal growth, algal symbiosis, and reef formation by corals. Paleobiol 13:363–378
Coates AG, Oliver WA Jr (1973) Coloniality in zoantharian corals. In: Boardman RS, Cheetham AH, Oliver WA Jr (eds) Animal colonies: development and function through time. Dowden Hutchinson Ross, Stroudsburg, PA, pp 3–27.
Coffroth MA, Lewis CF, Santos SR, Weaver JL (2006) Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians. Current Biol 16:R985–R987
Coffroth MA, Santos SR (2005) Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156:19–34
Cohen AL, Holcomb MC (2009) Why corals care about ocean acidification: uncovering the mechanism. In: Doney SC, Balch WM, Fabry VJ, Feely RA (eds) The future of ocean biogeochemistry in a high CO2 world. Oceanogr 22:18–127
Cohen AL, Layne GD, Hart SR, Lobel PS (2001) Kinetic control of skeletal Sr/Ca in a symbiotic coral: implications for the paleotemperature proxy. Paleoceanogr 16:20–26
Copper P (2002) Silurian and Devonian reefs: 80 million years of global greenhouse between two ice ages. SEPM Spec Publ 72:181–238
Cowan HL, Erickson JM (2010) Rafinesquina alternata as a photosymbiont host: shedding new light on old questions. Geol Soc Amer Abstr Progr 42:287
Cowen R (1983) Algal symbiosis and its recognition in the fossil record. In: Tevesz MJS, McCall PL (eds) Biotic interactions in Recent and fossil benthic communities. Plenum Press, New York, pp 431–478
Cowen R (1988) The role of algal symbiosis in reefs through time. Palaios 3:221–227
D’Hondt S, Zachos JC, Schultz G (1994) Stable isotopic signals and photosymbiosis in late Paleocene planktic foraminifera. Paleobiol 20:391–406
Dill RF, Shinn EA, Jones AT, Kelly K, Steinin RP (1986) Giant subtidal stromatolites forming in normal saline waters. Nature 324:55–58
Dodge RE, Vaišnys JR (1980) Skeletal growth chronologies of Recent and fossil corals. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms: biological records of environmental change. Plenum Press, New York, pp 493–517
Douglas AE (2003) Coral bleaching––how and why? Mar Poll Bull 46:385–392
Douglas AE (2010) The symbiotic habit. Princeton University Press, Princeton
Dravis JJ (1983) Hardened subtidal stromatolites, Bahamas. Science 219:385–386
Dreier A, Loh W, Blumenberg M, Thiel V, Hause-Reitner D, Hoppert M (2014) The isotopic biosignatures of photo- vs. thiotrophic bivalves: are they preserved in fossil shells? Geobiol [DOI: 10.1111/gbi.12093]
Dustan P (1982) Depth-dependent photoadaptation by zooxanthellae of Montastrea annularis. Bull Mar Sci 68:253–264
Eme L, Sharpe SC, Brown MW, Roger AJ. (2014) On the age of eukaryotes: evaluating evidence from fossils and molecular clocks. Cold Spring Harb Perspect Biol 6:a016139
Enríquez S, Méndez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50:1025–1032
Erwin DH (2006) Extinction: how life on earth nearly ended 250 million years ago. Princeton Univ Press, Princeton
Fagerstrom JA (1987) The evolution of reef communities. Wiley, New York
Fagerstrom JA (1996) Paleozoic brachiopod symbioses: testing the limits of modern analogues in paleoecology. Bull Geol Soc Amer 108:1393–1403
Farmer MA, Fitt RK, Trench RK (2001) Morphology of the symbiosis between Corculum cardissa (Mollusca: Bivalvia) and Symbiodinium corculorum (Dinophyceae). Biol Bull 200:336–343
Fautin DG, Buddemeier RW (2004) Adaptive bleaching: a general phenomenon. Hydrobiol 530/531:459–467
Fay S, Weber M, Lipps JH (2009) The distribution of Symbiodinium diversity within individual host foraminifera. Coral Reefs 28:717–726
Freudenthal HD (1962) Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. nov., a zooxanthella: taxonomy, life cycle, and morphology. J Protozool 9:45–52
Furla P, Allemand D, Shick JM, Ferrier-Pages C, Richier S (2005) The symbiotic anthozoan: a physiological chimera between alga and animal. Integr Compar Biol 45:595–604
Gao JG, Copper P (1997) Growth rates of middle Paleozoic corals and sponges: Early Silurian of Eastern Canada. Proc 8th Int Coral Reef Symp 2:1651–1656
Gattuso J-P, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Amer Zool 39:160–183
Gauri KL, Chowdhury AN, Kulsreshtha NP, Punuru AR (1990) Geologic features and durability of limestones at the sphinx. Environ Geol Water Sci 16:57–62
Glynn P (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol 2:495–509
Goreau TF, Goreau NI (1959) The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. Biol Bull 117:127–167
Goreau TJ, Goreau NI, Trench RK, Hayes RL (1996) Calcification rates in corals. Science 274:117
Grant RE (1972) The lophophore and feeding mechanism of the Productidina (Brachiopoda). J Paleontol 46:213–249
Groves JR, Pike M, Westley K (2012) A test for the possibility of photosymbiosis in extinct fusuline foraminifera: size and shape related to depth of habitat. Palaios 27:739–752
Håkansson E, Madsen L (1991) Symbiosis - a plausible explanation of gigantism in Permian trepostome bryozoans. In: Bigey FP, d’Hondt J-L (eds), Bryozoaires actuales et fossiles. Nantes: Société des Sciences Naturalles de l’Ouest de la France. Memoire hors series 1:151–159
Hallock P (1999) Symbiont-bearing foraminifera. In: Sen Gupta BK (ed), Modern foraminifera. Kluwer Academic Pub, Dordrecht, pp 123–139
Hallock P (2001) Coral reefs, carbonate sediments, nutrients, and global change. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic/Plenum Pub, New York, pp 387–427
Hallock P, Schlager W (1986) Nutrient excess and the demise of coral reefs and carbonate platforms. Palaios 1:389–398
Hallock P, Williams DE, Fisher EM, Toler SK (2006) Bleaching in foraminifera with algal symbionts: implications for reef monitoring and risk assessment. Anuario Instit Geociênc 29:108–128
Hansen HJ, Buchardt B (1977) Depth distribution of Amphistegina in the Gulf of Elat, Israel. Utrecht Micropaleontol Bull 15:205–239
Hansen, HJ, Dalberg P (1979) Symbiotic algae in milioline foraminifera: CO2 uptake and shell adaptations. Bull Geol Soc Denmark 28:47–55
Hill M, Allenby A, Ramsby B, Schönberg C, Hill A (2011) Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: evidence of heteroplasmy and putative host-specific symbiont lineages. Mol Phylogen Evol 59:81–8
Hohenegger J (2006) Morphocoenoclines, character combination, and environmental gradients: a case study using symbiont-bearing benthic foraminifera. Paleobiol 32:70–99
Hohenegger J, Yordanova E, Nakano Y, Tatzreiter F (1999) Habitats of larger foraminifera on the upper reef slope of Sesoko Island, Okinawa, Japan. Mar Micropaleontol 36:109–168
Hohenegger J, Yordanova E, Hatta A (2000) Remarks on West Pacific Nummulitidae (Foraminifera). J Foram Res 30:3–28
James NP (1983) Reef environment. In: Scholle PA, Bebout DG, Moore CH (eds) Carbonate depositional environments. Amer Assoc Petrol Geol Mem 33, Tulsa, pp 345–440
Jones DS, Jacobs DK (1992) Photosymbiosis in Clinocardium nuttalli: implications for tests of photosymbiosis in fossil molluscs. Palaios 7:86–95
Kahng SE, Hochberg EJ, Apprill A, Wagner D, Luck DG, Perez D, Bidigare, RR (2012) Efficient light harvesting in deep-water zooxanthellate corals. Mar Prog Ser 455:65–77
Kauffman EG, Johnson CC (1988) The morphological and ecological evolution of Middle and Upper Cretaceous reef-building rudistids. Palaios 3:194–216
Kazmierczak J (1976) Cyanophycean nature of stromatoporoids. Nature 264:49–51
Kershaw, S (1998) The applications of stromatoporoid palaeobiology in palaeoenvironment analysis. Palaeontol 41:509–544
Kershaw, S, Brunton FR (1999) Palaeozoic stromatoporoid taphonomy: ecologic and environmental significance. Palaeogeogr Palaeoclim Palaeoecol 149:313–328
Key MM Jr, Jackson PMW, Håkansson E, Patterson WP, Moore DM (2005) Gigantism in Permian trepostomes from Greenland: testing the algal symbiosis hypothesis using δ13 C and δ18 O values. In: Moyano G, Cancino JM, Jackson PMW (eds) Bryozoan studies 2004. Balkema Publ; Leiden, pp 141–151
Kiessling W (2010) Reef expansion during the Triassic: spread of photosymbiosis balancing climatic cooling. Palaeogeogr Palaeoclim Palaeoecol 290:11–19
Kiessling W, Aberhan M, Villier L (2008) Phanerozoic trends in skeletal mineralogy driven by mass extinctions. Nature Geosci 1:527–530
Kiessling W, Kocsis ÁT (2015) Biodiversity dynamics and environmental occupancy of fossil azooxanthellate and zooxanthellate scleractinian corals. Paleobiol 41:402–414
Kiessling W, Simpson C (2011) On the potential for ocean acidification to be a general cause of ancient reef crises. Global Change Biol 17:56–67
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Knoll AH (2014) Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb Perspect Biol 6: a016121
Knowlton N, Jackson JBC (2011) Evolutionary diversity and ecological complexity of coral reefs. Paleontol Soc Pap 17:111–120
Knowlton N, Rohwer F (2003) Multispecies microbial mutualisms on coral reefs: the host as a habitat. Amer Nat 162 (4 Suppl):S51–62
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt MK (2010) Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800
Landing E, Antcliffe JB, Brasier MD, English AB (2015) Distinguishing earth’s oldest known bryozoan (Pywackia, Late Cambrian) from pennatulacean octocorals (Mesozoic–Recent). J Paleontol 89:292–317
Langer MR (2008) Assessing the contribution of foraminiferan protists to global ocean carbonate production. J Eukary Microbiol 55:163–169
Langer MR, Lipps JH (2003) Foraminiferal distribution and diversity, Madang reef and lagoon, Papua New Guinea. Coral Reefs 22:143–154
Langer MR, Silk MT, Lipps JH (1997) Global ocean carbonate and carbon dioxide production: the role of reef foraminifera. J Foram Res 27:271–277
Lee JJ (2006) Algal symbiosis in larger foraminifera. Symbiosis 42:63–75
Lee JJ (2011) Fueled by symbiosis, Foraminifera have evolved to be giant complex protists. In: Seckbach J, Dubinski Z (eds) All flesh is grass: plant-animal interrelationships. Springer, New York, pp 427–452
Lee JJ, Anderson OR (1991) Symbiosis in foraminifera. In: Lee JJ, Anderson OR (eds) Biology of Foraminifera. Academic Press.
Leinfelder RR (2001) Jurassic reef ecosystems. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic/Plenum Pub, New York, pp 251–309
Lipps JH (1981) What, if anything, is micropaleontology? Paleobiol 7:167–199
Lipps JH (2006) Major features of protistan evolution: controversies, problems and a few answers. Anuario Inst Geociênc 29:55–80
Lipps JH (2011) Reef restoration—the good and the bad, a paleobiologic perspective. Paleontol Soc Papers 17:139–152
Lipps JH, Severin K (1986) Alveolinella quoyi, a living fusiform foraminifera, at Motupore Island, Papua New Guinea. Sci New Guinea 11:126–137
Littman RA, van Oppen MJH, Willis BL (2008) Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). J Exp Mar Biol Ecol 364:48–53
Lobban CS, Schefter M, Donaldson TJ (2014) Cluster dynamics in Maristentor dinoferus, a gregarious benthic ciliate with zooxanthellae and a hypericin-like pigment, in relation to biofilm grazing by the fish Ctenochaetus striatus. Symbiosis 63:137–147
Lopez R (1979) Algal chloroplasts in the protoplasm of three species of benthic Foraminifera: taxonomic affinity, viability and persistence. Mar Biol 53:201–211
Marcelino LA, Westneat MW, Stoyneva V, Henss J, Rogers JD, Radosevich A, Turzhitsky V, Siple M, Fang A, Swain TD, Fung J, Backman V (2013) Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. www.PLoS One doi: 10.1371/journal.pone.0061492
Margulis L (1998) Symbiotic planet: a new view of evolution. Basic Books, Amherst, MA
Marshall AT (1996) Calcification in hermatypic and ahermatypic corals. Science 271:637–639
McConnaughey TA (2012) Zooxanthellae that open calcium channels: implications for reef corals. Mar Ecol Prog Ser 460:277–287
Moldowan JM, Dahl J, Jacobson SR, Huizinga BJ, Fago FJ, Shetty R, Watt DS, Peters KE (1996) Chemostratigraphic reconstruction of biofacies: molecular evidence linking cyst-forming dinoflagellates with pre-Triassic ancestors. Geol 24:159–162
Muscatine L, Goiran C, Land L, Jaubert J, Cuif J-P, Allemand D (2005) Stable isotopes (−13C and −15N) of organic matrix from coral skeleton. Proc Nat Acad Sci 102:1525–1530
Nestor H, Copper P, Stock CW (2010) Late Ordovician and Early Silurian stromatoporoid sponges from Anticosti Island, eastern Canada: crossing the O/S mass extinction boundary. Nat Res Council, Res Press, Ottawa
Pandolfi, JM, Jackson JBC, Baron N, Bradbury RH, Guzman HM, Hughes TP, Kappel CV, Micheli F, Ogden JC, Possingham HP, Sala E (2005) Are US coral reefs on the slippery slope to slime? Science 307:1725–1726
Payne JL, Groves JR, Jost AB, Nguyen T, Moffitt SE, Hill TM, Skotheim JM (2012) Late Paleozoic fusulinoidean gigantism driven by atmospheric hyperoxia. Evol 66:2929–2939
Pelejero C, Calvo E, Mcculloch MT, Marshall JF, Gagan MK, Lough JM, Opdyke BN (2007) Preindustrial to modern interdecadal variability in coral reef pH. Science 309:2204–2207
Perrin C (2002) Tertiary: the emergence of modern reef ecosystems. SEPM Spec Publ 71:587–621
Piller WE (1981) The Steinplatte reef complex, part of an Upper Triassic carbonate platform near Salzburg, Austria. SEPM Spec Publ 30:261–290
Pochon X, Montoya-Burgos JI, Stadelmann, B, Pawlowski JP (2006) Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol Phylogen Evol 38:20–30
Pochon X, Stat M, Takabayashi M, Chasqui L, Chauka LJ, Logan DDK, Gates RD (2010) Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. J Phycol 46:53–65
Porter SM (2004) The fossil record of early eukaryotic diversification. In: Lipps JH, Waggoner BM (eds) Neoproterozoic—Cambrian biological revolutions. Paleontol Soc Papers 10:35–50.
Pratt BR, Spincer BR, Wood RA, Zhuravlev AYu (2001) Ecology and evolution of Cambrian reefs. In: Zhuravlev AYu, Riding R (eds) The ecology of the Cambrian radiation. Columbia Univ Press, New York, pp 254–274
Reaka-Kudla ML (1997) The global diversity of coral reefs: a comparison with rain forests. In: Reaka-Kudla ML, Wilson DE, Wilson EO (eds) Biodiversity II: Understanding and protecting our biological resources. Joseph Henry Press, Washington, DC, pp 83–108
Reid RP, Macintyre IG, Browne KM, Steneck, RS, Miller T (1995) Modern marine stromatolites in the Exuma Cays, Bahamas: uncommonly common. Facies 33:1–17
Riding R (1992) Temporal variation in calcification in marine cyanobacteria. J Geol Soc London 149:979–989
Riedel P (1991) Korallen in der Trias de Tethys: Stratigraphische Reichweiten, Diversitätsmuster, Entwicklungstrends und Bedeutung als Rifforganismen. Mitteil Gesellsch Geol Bergbaustud Österreich 37:97–118
Ries JB, Cohen AL McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geol 12:1131–1134
Rosen BR (1978) The nature and significance of microatolls, appendix: determination of a collection of coral microatoll specimens from the northern Great Barrier Reef. Proc Trans Royal Soc London B 284:115–122
Rosen BR (2000) Algal symbiosis, and the collapse and recovery of reef communities: Lazarus corals across the K-T boundary. In: Culver SJ, Rawson PA (eds) Biotic response to global change: the last 145 million years. Cambridge Univ Press, Cambridge, pp 164–180
Rosen BR, Turnšek D (1989) Extinction patterns and biogeography of scleractinian corals across the Cretaceous/Tertiary boundary. In: Jell PA, Pickett JW (eds) Fossil Cnidaria 5. Mem Assoc Australas Paleontol 8:355–370
Rowan R. (2004) Coral bleaching—thermal adaptation in reef coral symbionts. Nature 430:742–742
Rowan R, Powers DA (1991) A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbioses. Science 251:1348–1351
Rowland SM (2001) Archaeocyaths—a history of phylogenetic interpretation. J Paleontol 75:1065–107
Rowland SM, Gangloff RA (1988) Structure and paleoecology of Lower Cambrian reefs. Palaios 3:111–135
Rowland SM, Hicks M (2004) The Early Cambrian experiment in reef-building by metazoans. In: Lipps JH, Waggoner BM (eds) Neoproterozoic—Cambrian biological revolutions. Paleontol Soc Papers 10:107–130
Rowland SM, Shapiro SH (2002) Reef patterns and environmental influences in the Cambrian and earliest Ordovician. SEPM Spec Publ 72:95–128
Sandberg PA (1983) An oscillating trend in Phanerozoic nonskeletal carbonate mineralogy. Nature 305:19–22
Scoffin TP, Stoddart DR (1978) The nature and significance of microatolls. Proc Trans Royal Soc London B 284:99–122
Senowbari-Daryan B, Stanley GD Jr (2009) Taxonomic affinities and paleogeography of Stromatomorpha californica Smith, a distinctive Upper Triassic reef-adapted demosponge. J Paleontol 83:783–793
Severin KP, Lipps JH (1989) The weight-volume relationship of the test of Alveolinella quoyi: implications for the taphonomy of large fusiform foraminifera. Lethaia 22:1–12
Siano AR, Montresorb M, Proberta I, Nota F, DeVargas C (2010) Pelagodinium gen. nov. and P. beii comb. nov., a dinoflagellate symbiont of planktonic foraminifera. Protist 161:385–399
Smith EG, D’Angelo C, Salih A, Wiedenmann J (2013) Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 32:463–474
Smithers SG, Woodroffe CD (2000) Microatolls as sea-level indicators on a mid-ocean atoll. Mar Geol 168:61–78
Song, Y, Black RG, Lipps JH (1994) Morphologic optimization in the largest living foraminifera: implications from finite element analysis. Paleobiol 20:14–26
Stanley GD Jr (1981) Early history of scleractinian corals and its geological consequences. Geol 9:507–511
Stanley GD Jr (2005) Coral microatolls from the Triassic of Nevada: oldest scleractinian examples. Coral Reefs 24:247
Stanley GD Jr (2006) Photosymbiosis and the evolution of modern coral reefs. Science 312:857–860
Stanley GD, Cairns SD (1988) Constructional azooxanthellate coral communities: an overview with implications for the fossil records. Palaios 5:233–242
Stanley GD Jr, Helmle KP (2010) Middle Triassic coral growth bands and their implication for photosymbiosis. Palaios 25:754–763
Stanley GD Jr, Lipps JH (2011) Photosymbiosis: the driving force for reef success and failure. Paleontol Soc Papers 17:33–60
Stanley GD Jr, Swart PK (1995) Evolution of the coral-zooxanthellae symbiosis during the Triassic: a geochemical approach. Paleobiol 21:179–199
Stanley GD Jr, van de Schootbrugge B (2009) The evolution of the coral-algal symbiosis. In: van Oppen MJH, Lough JM (eds) Coral bleaching: patterns, processes, causes and consequences. Ecol Stud Series 205:7–19
Stanley SM, Hardie, LA (1999) Hypercalcification: paleontology links plate tectonics and geochemistry to sedimentology: GSA Today 9:1–7
Stat M, Carter D, Hoegh-Guldberg, O (2006) The evolutionary history of Symbiodinium and scleractinian hosts—symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol Syst 8:23–43
Steindler L, Beer S, Ilan M (2002) Photosymbiosis in intertidal and subtidal tropical sponges. Symbiosis 33:263–273
Steuber T (1996) Stable isotope sclerochronology of rudist bivalves: growth rates and Late Cretaceous seasonality. Geol 24:315–318
Steuber T (2000) Skeletal growth rates of Upper Cretaceous rudist bivalves: implications for carbonate production and organism-environment feedbacks. Geol Soc London Spec Publ 178:21–32
Stimson J, Sakai K, Sembali H (2002) Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching induces mortality. Coral Reefs 21:409–421
Surge DM, Savarese M, Dodd JR, Lohmann KC (1997) Carbon isotopic evidence for photosymbiosis in Early Cambrian oceans. Geol 25:503–506
Sweet MJ (2014) Symbiodinium diversity within Acropora muricata and the surrounding environment. Mar Ecol 35:343–353
Takabayashi M, Adams LM, Pochon X, Gates RD (2012) Genetic diversity of free-living Symbiodinium in surface water and sediment of Hawai’i and Florida. Coral Reefs 31:157–167
Talent JA (1988) Organic reef-building: episodes of extinction and symbiosis? Senckenberg Lethaea 69:315–368
Taylor MW, Hill RT, Piel J, Thacker RW, Hentschel U. (2007). Soaking it up: the complex lives of marine sponges and their microbial associates. The ISME J 1:187–190
Taylor PD, Waeschenbach A (2015) Phylogeny and diversification of bryozoans. Palaeontol 58:585–599
Terán E, Méndez ER, Enríquez S, Iglesias-Prieto R (2010) Multiple light scattering and absorption in reef-building corals. Appl Optics 49:5032–5042
Thornhill DJ, Lewis AM, Wham DC, LaJeunesse TC (2013) Host-specialist lineages dominate the adaptive radiation of reef coral endosymbionts. Evol 68:352–367
Turner EC, Narbonne GM, James NP (1993) Neoproterozoic reef microstructure from the Little Dal Group, northwestern Canada. Geol 21:259–262
Vachard D, Pille L, Gaillot J (2010) Palaeozoic foraminifera: systematics, palaeoecology and responses to global changes. Rev Micropaléontol 53:209–254
van Oppen MJ, Baker AC, Coffroth MA, Willis BL (2009) Bleaching resistance and the role of algal endosymbionts. Ecol Stud 205:83–102
van Oppen MJH, Lough JM (2009) Coral bleaching: patterns, processes, causes, and consequences. Springer-Verlag Berlin, Heidelberg
Vermeij GJ (2013) The evolution of molluscan photosymbioses: a critical appraisal. Biol J Linnean Soc 109:497–511
Vogel K (1975) Endosymbiotic algae in rudists. Palaeogeogr Palaeoclim Palaeoecol 17:327–332
Walter MR (1983) Archean stromatolites: evidence of the Earth’s earliest benthos. In: Schopf JW (ed) Earth’s earliest biosphere. Princeton University Press, Princeton, pp 187–213
Watson ME, Signor PW (1986) How a clam builds windows: shell microstructure in Corculum (Bivalvia: Cardiidae). Veliger 28:348–355
Wood R (1993) Nutrients, predation and the history of reef-building. Palaios 8:526–543
Wood R (1999) Reef evolution. Oxford University Press, Oxford
Wood R, Curtis A (2014) Extensive metazoan reefs from the Ediacaran Nama Group, Namibia: the rise of benthic suspension feeding. Geobiol DOI: 10.1111/gbi.12122
Wooldridge SA (2014) Differential thermal bleaching susceptibilities amongst coral taxa: re-posing the role of the host. Coral Reefs 33:15–27
Yancey TE, Stanley, GD Jr (1999) Giant alatoform bivalves in the Upper Triassic of western North America. Palaeontol 42:1–23
Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya, D (2004) A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol 21:809–818
Zapalski MK (2014) Evidence of photosymbiosis in Palaeozoic tabulate corals. Proc R Soc B 281: 20132663. http://dx.doi.org/10.1098/rspb.2013.2663
Zhang Y, Payne JL (2012) Size-frequency distributions along a latitudinal gradient in Middle Permian fusulinoideans. PLoS One 7(6):e38603. doi:10.1371/journal.pone.0038603
Zhuravlev AY (2001) Paleoecology of Cambrian reef ecosystems. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic/Plenum Publishers, New York, pp 121–157
Acknowledgements
We are indebted to our numerous colleagues and students. Prof. Dennis Hubbard and Dr. Caroline S. Rogers provided significant editorial assistance that greatly improved our paper. Lipps particularly thanks Drs. Michele Weber, Scott Fay, Richard Cowen, Martin Langer, Ken Severin, Bernard Ormsby and Yan Song for discussion of this problem and assistance in the field and lab. Lipps’s research on larger foraminifera was supported by NSF EAR 84–08001. Stanley is indebted to Kallie Moore for photo assistance and thanks go to Jose Garcia for his artwork. Steve Kershaw provided helpful discussions on Paleozoic stromatoporoids .
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Lipps, J.H., Stanley, G.D. (2016). Photosymbiosis in Past and Present Reefs. In: Hubbard, D., Rogers, C., Lipps, J., Stanley, Jr., G. (eds) Coral Reefs at the Crossroads. Coral Reefs of the World, vol 6. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7567-0_3
Download citation
DOI: https://doi.org/10.1007/978-94-017-7567-0_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7565-6
Online ISBN: 978-94-017-7567-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)