Abstract
Bacteriophage (phage) are obligate intracellular parasites that multiply inside bacteria by making use of some or all of the host biosynthetic machinery (i.e., viruses that infect bacteria). Bacteriophages are highly specific to their host pathogens.
An idea of using phages against bacterial infections, known as phage therapy, appeared practically simultaneously with the discovery of phages. However, after discovery of antibiotics, the interest of Western clinicians turned to use of broad-spectrum antibiotics for treating unknown infections that kill many types of bacterium. Phages, by contrast, kill just one species or strain. With the rise of antibiotic-resistance the researchers now realize that they need more precise ways to target multi-drug-resistant pathogenic bacteria to which bacteriophages are still active.
Recently, the nanosized dimensions of bacteriophages, coupled with the ease of use with which genetic modifications can be made to their structure and function, have put phages in the spotlight. This is because of their use in a variety of biosensors, including as recognition probes for pathogen detection. The popular bio-probes that have been employed on biosensor surface for pathogen detection are nucleic acids, antibodies, whole phages, phage-display peptides (PDPs), and most recently phage’s receptor binding proteins (RBPs). The efforts are now directed to development of enhanced detection technologies with high levels of reliability, sensitivity, and selectivity with short assay times.
This review summarizes experiences in development of phage-based therapeutics, prophylactic and diagnostic preparations, and their uses in different fields, such as medicine, veterinary, agriculture, food and water safety, etc.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
2.1 Bacteriophages
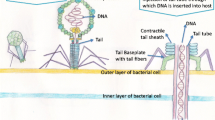
Bacteriophage, or phage for short, are viruses that infect only bacteria. In contrast to cells that grow from an increase in the number of their components and reproduce by division, viruses are assembled from pre-made components. Viruses are nucleic acid molecules surrounded by a protective coating. They are not capable of generating energy and reproduce inside of cells. The nucleic acid inside the coating, called the phage genome in a bacteriophage, encodes most of the gene products needed for making more phage. The phage genome can be made of either double- or single-stranded DNA or RNA, depending on the bacteriophage in question. The genome can be circular or linear. The protective coating or capsid surrounding the phage genome is composed of phage-encoded proteins.
Many important discoveries have been made using phage as model systems. From the discovery that a nonsense codon stopped protein synthesis, to the first developmental switch to be understood at the molecular level, phage have proven to be very useful.
2.2 Discovery of Bacteriophages
During the First World War a rather sensational news was spread out – the viruses “eaters of micro-organisms” had been discovered by Felix d’Herelle, who developed a phage preparation to treat World War I soldiers with dysentery [29]. Doctors all over the world were excited with this news, especially because of the announcement published in medical journals that bacteriophages are harmless for both humans and animals, and can be successfully applied as therapeutic means [2, 8, 14, 15, 56, 61, 67].
Before that, in 1915, a well-known British journal “The Lancet” published an article written by Frederick Twort about “the transmissible bacterial lyses” [73], in which Twort described his observation of “the eaten edges of the colonies of Staphylococcus”. He managed to filter the appropriate cultures of Staphylococcus and spotted the filtrate on the lawn of different Staphylococcus strains. Thus, he received a clear zone of lysis again and again. However, Twort could not explain the observed event and provided only its description. This was the very first publication on bacteriophages. French Canadian scientists Felix d’Herelle read this article and recalled in his mind his own observations in Mexico and Tunis [69]. He suspected that the filtered agent is a bacterial virus, an invisible invader that destroys bacteria.
The discovery, or rediscovery, of bacteriophages by Felix d’Herelle after Frederick Twort is frequently associated with an outbreak of severe hemorrhagic dysentery among French troops stationed at Maisons-Laffitte (on the outskirts of Paris) in July–August 1915 [67, 69]. Several soldiers were hospitalized, and d’Herelle was assigned to conduct an investigation of the outbreak. During these studies, he made bacterium-free filtrates of the patients’ fecal samples, and mixed and incubated them with Shigella strains isolated from the patients. A portion of the mixtures was inoculated into experimental animals (as part of d’Herelle’s studies on developing a vaccine against bacterial dysentery), and a portion was spread on agar medium in order to observe the growth of the bacteria. On these agar cultures, d’Herelle observed again the appearance of small, clear areas, which he initially called taches, then taches vierges (virgin spots), and, later, plaques [68]. D’Herelle’s findings were presented during the September 1917 meeting of the Academy of Sciences, and they were subsequently published in the meeting’s proceedings [31]. In contrast to Twort, d’Herelle had little doubt about the nature of the phenomenon, and he proposed that it was caused by a virus capable of parasitizing bacteria. The name “bacteriophage” (from “bacteria” and Greek φăγεῖν phagein “to eat”) was also proposed by d’Herelle [68].
The discovery of bacteriophages was inevitable. Similar phenomena had been observed in remote regions of the world by different scientists. At the end of the nineteenth century, N.F. Gamaleya (later Honorary Member of the Academy of the USSR) published an article in the Russian Archives of the Pathological and Clinical Medicine [20]. In this article he described the lysis of Bacillus antracis in distilled water, after which the water obtained an ability to lyse the other strains of Bacillus antracis. In 1917, a young Georgian scientist George Eliava had observed mysterious disappearance of Vibrio cholerae cells [22, 30].
The greatest merit of Felix d’Herelle is that he advanced an idea of using bacteriophages for treatment of human and animal bacterial diseases. For this idea nowadays he would deserve the Noble Prize to which he was nominated eight times since 1925, though he was never awarded one (cited by Hausler [29] according to Nobel Archives).
2.3 Early Clinical Trials and Commercial Production of Bacteriophages
Not long after his discovery, d’Herelle used phages to treat dysentery in what was probably the first attempt to use bacteriophages therapeutically. The studies were conducted at the Hôpital des Enfants-Malades in Paris in 1919 under the clinical supervision of Professor Victor-Henri Hutinel, the hospital’s Chief of Pediatrics [68]. The phage preparation was ingested by d’Herelle, Hutinel, and several hospital interns in order to confirm its safety before administering it the next day to a 12-year-old boy with severe dysentery. The patient’s symptoms ceased after a single administration of d’Herelle’s anti-dysentery phage, and the boy fully recovered within a few days. The efficacy of the phage preparation was “confirmed” shortly afterwards, when three additional patients having bacterial dysentery and treated with one dose of the preparation started to recover within 24 h of treatment. However, the results of these studies were not immediately published and, therefore, the first reported application of phages to treat infectious diseases of humans came later from Richard Bruynoghe and Joseph Maisin [8], who used bacteriophages to treat staphylococcal skin disease. The bacteriophages were injected into and around surgically opened lesions, and the authors reported regression of the infections within 24–48 h. Several similarly promising studies followed [61, 63, 66]. Encouraged by these early results, d’Herelle and others continued studies of the therapeutic use of phages; for example, d’Herelle used various phage preparations to treat thousands of people having cholera and/or bubonic plague in India [37, 68, 69].
In 1916–1930 d’Herelle and his collaborators undertook numerous expeditions to China, Laos, India, Vietnam, and Africa to combat epidemics caused by cholera and plague with bacteriophages. According to Romanian medical historians Kazhal and Iftimovich [37] the first attempts to use cholera bacteriophage for treatment and prophylaxis were performed by Felix d’Herelle and George Eliava in 1931. According to these authors, the Institute of Vaccine and Sera in Tbilisi (nowadays known as the Eliava Institute of Bacteriophage, Microbiology and Virology) produced the first commercial anticholera phage preparation, which was reported to be successfully used for control of epidemics threatening the South-East territories of the USSR [37]. According to the estimations that were published that time, the application of bacteriophages caused the mortality of cholera in India to be reduced to 10 % [37]. This fact is described by d’Herelle himself in the book “Bacteriophage and the Phenomenon of Recovery”, published in the Russian language in 1935 in Tbilisi, Georgia.Footnote 1
According to d’Herelle [30], cholera epidemics occurred in Punjab region in 1927. Patients were treated orally with 2 ml of cholera phage diluted in 20 ml of water but the titer of phage in these preparations is unknown. If the patient vomited, a repeated dose of phage (5 ml diluted in 100 ml of water) was administered slowly with a teaspoon. The control group of patients treated themselves using a folk medicine (plant extracts). Of 14,450 people who lived in nine villages of Punjab region, only 73 have been treated with the phage. D’Herelle explained this by saying that people in India opposed any new medical measures, and rarely permitted him and his colleagues to use phages for treatment. Thus, only desperately ill patients were subjected to phage therapy. Altogether 118 persons were included in the control group in which 74 lethal outcomes (62.7 %) were registered, while in the experimental group the mortality rate was almost one-tenth of the control group, with 5 cases out of 73 (6.8 %) [30].
In his book, d’Herelle mentioned the establishment of two industrial centers for the production of bacteriophages against cholera in 1931 in India [30, 37]. D’Herelle’s commercial laboratory in Paris produced at least five phage preparations against various bacterial infections. The preparations were called Bacté-coli-phage, Bacté-rhino-phage, Bacté-intesti-phage, Bacté-pyo-phage, and Bacté-staphy-phage, and they were marketed by a company that later became the large French company L’Oréal [67–69].
The Oswaldo Cruz Institute in Rio-de-Janeiro, Brazil started production of the anti-dysentery bacteriophages in 1924 to combat dysentery in Latin American countries [17, 30]. Within a year, the institute produced 10,000 vials of phages, which were sent into the hospitals around Brazil [29]. Therapeutic phages were also produced in the United States. In the 1940s, the Eli Lilly Company (Indianapolis, Ind.) produced seven phage products for human use, including preparations targeted against staphylococci, streptococci, Escherichia coli, and other bacterial pathogens [67]. These preparations consisted of phage-lysed, bacteriologically sterile broth cultures of the targeted bacteria (e.g., Colo-lysate, Ento-lysate, Neiso-lysate, and Staphylo-lysate) or the same preparations in a water-soluble jelly base (e.g., Colo-jel, Ento-jel, and Staphylo-jel). They were used to treat various infections, including abscesses, suppurating wounds, vaginitis, acute and chronic infections of the upper respiratory tract, and mastoid infections. However, the efficacy of phage preparations was controversial [18, 41]. This could be caused by the absence of viable phages, low phage titer, or narrow strain range of phage in these preparations, which was discovered in the case of some commercial anti-staphylococcal phage preparations [59]. As a result with the advent of antibiotics, commercial production of therapeutic phages ceased in most of the Western countries.
2.4 Mass Application of Phage-based Products in Medicine
From a review of historic literature, it is apparent that phage therapy trials were active in the 1930s and 1940s throughout Georgia, Russia, Ukraine, Belarus, and Azerbaijan, in the Soviet Union. Observations of cases associated with road accidents and septic infection carried out in hospitals in Moscow in [38–40] led to the development of methods and instructions for their intramuscular and even intravenous use, which was crucial in cases of generalized infections. The results of these observations were reported at conferences held in March, June, and December of 1940. These methods and instructions were approved by the Soviet Supreme Red Army Military-Sanitarian Office and were applied to the treatment of soldiers in the Red Army during the Finnish Campaign and the Second World War. The war and the need for therapeutic preparations inspired Soviet doctors to perform new trials with phages and to develop novel methods for treatment of wound infections. This period was one of the most fruitful in the development of phage therapy in the former Soviet Union [38–40, 70–72].
In 1920s and 1940s, the intestinal infections caused by Salmonella and Shigella species were a huge problem all over the world [2, 10, 14, 15, 18, 34, 40, 50, 52]. The mortality rate was very high [34]. Therefore, the efforts of Soviet scientists were focused on development of therapeutic phages for treatment of intestinal infections. The broadest clinical study on the therapeutic effect of dysenteric phages was reported by Sapir [62]. The author described altogether 1,064 cases of dysentery that were treated with bacteriophages in two different clinics in Moscow. The effect of phage therapy against intestinal diseases was confirmed by numerous authors [25, 27, 32, 47, 55, 76]. Phage preparations were generally considered to be particularly efficient for the treatment of intestinal infections. However, it was recognized that use of phage preparations is much more reasonable for prophylactic purposes. Therefore, the phage preparations have been used extensively in the Soviet Union for prophylaxis in the regions with high incidence of infections, and, also in the communities where rapid spread of infections might occur such as kindergartens, schools, and military accommodation, etc. [1, 3, 5–7, 19, 33, 35]. The preventive measures against bacterial epidemics performed by use of phages are known under the term “prophylactic phaging”. Later on, mass prophylaxis of intestinal diseases was performed in the Red Army units by military doctors as well. For prevention of dysentery and typhoid epidemics, specific phages were also used with two tablets administered once every 5–7 days during the outbreak season [1, 3, 44].
2.5 Bacteriophages Versus Antibiotics
To evaluate effect of phage therapy over 5,000 volumes of scientific journals, selected articles, books and thesis of dissertations published between 1920s and 1980s have been screened. The gathered information was included into a monograph: “A Literature Review of the Practical Application of Bacteriophage Research” [11]. The main conclusions derived from the literature are: (a) Success outcome of phage therapy is up to 95 %; (b) Rapid improvement and cure is achieved within 3–5–7 days; (c) After phage therapy of wounds no scars are left (positive cosmetic effect); (d) No or rare relapsed cases are registered; (e) No side effects (allergy, yeast or fungal infections, kidney or liver failure, etc.) were registered; (f) Mortality rate among children suffering with septicemia was minimized; (g) Due to “prophylactic phaging” number of disease cases was reduces three to six times; (h) In the “phaged” group only mild disease cases were observed; (i) Phage therapy gives an effect similar to vaccination; (j) Phage therapy reduces the number of hospital days.
In general the difference between phages and antibiotics is based on a few characteristics. Phages are live bacterial viruses with specific action towards the bacterial targets and do not harm the useful bacteria that live in and on the body. However, antibiotics have generalized action that is often harmful for human and animal gut microbiota and may cause side effects. Since the phages are parasites they may reproduce themselves only in presence of appropriate hosts. The phages are active against drug-resistant bacteria. Since phage and bacteria are co-evolving prey and predator system, the phages may easily overcome phage-resistance of the host bacterial cell, if such occurs.
2.6 Phage Preparations
During the long history of using phages as therapeutic agents in the former Soviet Union and Eastern Europe, phages have been administered to humans (i) orally, in tablet or liquid formulations (105–107 PFU/dose), (ii) rectally, (iii) locally (skin, eye, ear, nasal mucosa, etc.), in tampons, rinses, and creams, (iv) as aerosols or intrapleural injections, and (v) intravenously, albeit to a lesser extent than the first four methods, and there have been virtually no reports of serious complications associated with their use.
Presently, the following phage preparations are in use in Georgia:
-
Pyo-bacteriophage: active against Staphylococcus sp., Streptococcus sp, P. aeruginosa, Proteus mirabilis, Proteus vulgaris, E.coli causing purulent infections. The preparation is recommended for treatment and prophylaxis purulent-inflammatory infections of ear, throat, nose, bronchus, lungs; surgical infections (abscesses, phlegmona, osteomyelitis, peritonitis); urogenital infections (urethritis, cystitis, pyelonephritis); gynecology infections (colpitis, endometritis, etc.); enteral infections (gastro-enterocolitis, cholecystitis, disbiosis), and purulent-septical infection of newborns.
-
Intesti-bacteriophage liquid: active against Shigella sp, Salmonella sp., E.coli, Staphylococcus sp., Enterococcus sp., Proteus sp., Pseudomonas aeruginosa. This preparation is recommended for treatment and prophylaxis of intestinal disorders, bacterial dysentery, shigellosis, salmonellosis, typhoid fever, paratyphoid salmonella, disbiosis, enterocolitis, colitis, and dyspepsia.
-
Enko-phage: active against the following species: S. typhimurium, S. enteritidis, S. heidelberg, S. newport, S. cholerae suis, S. oranienburg S. dublin and S. anatum; Shigella flexneri (serovars 1, 2, 3, 4) and Shigella sonnei (six different serovars), enteropathogenic E.coli (serovars: O11, O55, O26, O125, O113, O 128, O18, O44, O25, O20); S. aureus, S. epidermidis and S. saprophyticus.
-
SES-bacteriophage: active against Staphylococci (S. aureus, S. epidermidis, and S. saprophyticus); Streptococci (S. pyogenes, S. sanguis, S. salivarius and S. agalactiae) and different serotypes of enteropathogenic E.coli (O11, O55, O26, O125, O113, O 128, O18, O44, O25, O20).
-
Fersis–bacteriophage: active against Staphylococci (S. aureus, S. epidermidis, and S. saprophyticus) and Streptococci (S. pyogenes, S. sanguis, S. salivarius and S. agalactiae).
2.7 Intravenous Staphylococcal Bacteriophages (IVSP) for Treatment of Septic Infections
The development of apyrogenic Staphylococcus phage for intravenous use is one of the significant achievements of the Eliava Institute. This preparation is unique and does not have analogues in the world. Before its mass application, it was tested on animals, healthy human volunteers, and in clinical trials including up to 1000 patients (adults and children). For intravenous administration, the IVSP was used in a dose of 0.5–1 ml per 1 kg of weight as transfusions combined with blood replacing compounds (saline solution, etc.). Higher doses (2 ml per 1 kg of weight) were not usually applied except for in rare cases, for example, in the cases of osteomyelitis. The IVSP was also successfully applied in treatments and prophylaxis of post-traumatic or post-operational infections. For gynecological purposes, 10–20 ml of phage was administered locally into the infected sites. To avoid the difficulties related to the development of antibodies, the IVSP was administered once per day, every day, for 3–18 days depending on the severity of the disease and other factors. During the intravenous phage administration of the IVSP, the doctors did not observe any life threatening side effects [60].
Interestingly, in vitro screening performed between 2004 and 2009 in epidemiologically unrelated 352 Staphylococcus clinical strains using staphylococcal phage strain Sb-1 (known also as ISB), which is a key component of the intravenous commercial phage preparations (ISVP), demonstrated that 98–99 % phage-susceptibility of the clinical strains [46]. Methicillin Resistant Staphylococcus Aureus (MRSA) is one of the most important health concerns worldwide. Bacteriophage therapy is considered to be an alternative to antibiotics. Therefore, the efficacy IVSP was tested also against 424 human MRSA strains isolated in the UK. The results were promising, as 98 % of MRSA appeared to be susceptible to IVSP [23].
2.8 Current Status of Development of Phage Preparations and Their Applications
The Eliava Institute was established by d’Herelle and George Eliava as the world center of bacteriophage research and during its best times employed approximately 700–800 people, including researchers, technologists and support personnel. At that time, the institute produced phage preparations (often several tons a day) against a dozen bacterial pathogens, including intestinal bacteria, staphylococci, Pseudomonas, Proteus, and many enteric pathogens [21].
Production and use of phages for therapy and prophylaxis never stopped at the Eliava Institute of Bacteriophage, however, the scale is much smaller than before the break off of the Soviet Union. The therapeutic and prophylactic phages are presently produced by a small spin-off company Eliava BioPreparations, Ltd established by the Eliava IBMV. In the past the scale of the phage production covered the greatest part of the Soviet countries, including the Caucasian and Middle Asian republics. Today, production of the Eliava BioPreparations, Ltd fully satisfies the Georgian market. In 2010, the company started to export the phages outside Georgia into Azerbaijan.
These achievements would be impossible without intensive research held at the Eliava Institute of Bacteriophage, most of which are conducted in close collaboration with the international scientific community. This helps the Georgian scientists to develop further, introduce modern techniques into their research, and, what is the most important, to design proper experiments corresponding to western standards. On another hand, the collaborative projects help the western researchers to avoid ‘discovery of wheel’, which may easily occur without having a background knowledge in development of phage preparations and their application in practice. Over 50 collaborative international projects have been accomplished during the period of 1995–2014, which resulted in a number of significant publications [9–11, 24, 26, 43, 45, 51, 53, 57, 67, 74, 78].
Thanks to grant support, several new phage preparations have been developed during the recent decades, these are: PhageBioDerm, Uro-phage [51, 67], Oseteophage and Micolyse [12, 13].
The PhageBioDerm is a novel wound-healing preparation consisting of a biodegradable polymer impregnated with an antibiotic and lytic bacteriophages, and was recently licensed for sale in the Republic of Georgia. The “Uro-phage” has been successfully used in a clinical trial for treatment of chronic bacterial prostatitis (CBP) caused by Gram-positive (Staphylococcus, Streptococcus and Enterococcus) and Gram-negative (E.coli, Pseudomonas and Proteus) bacteria [36]. A composite for treatment of dental infections (“Oseteophage”) was specifically formed by using a combination of hydraxyapatite, methyluracil, gelatin, and bacteriophages against Staphylococcus, Streptococcus, and E.coli. Clinical studies have approved that the preparation “Oseteophage” may be used to prevent dental archatropy and to stimulate bone regeneration [12]. Another novel bio-composite against oral infections caused by Staphylococcus, Streptococcus, and Candida albicans, and an antifungal extract of Pupulus nigra has been developed recently [13]. Gynecology is a promising area for application of bacteriophages therapy. A new combined preparation for treatment of bacterial and yeast diseases “Micolyse” was tested in a clinical experiment aimed at the treatment of pregnant women suffering from mixed bacterial and yeast infections.
Among other achievements in phage therapy, it is necessary to mention the treatment of cases of secondary infections associated with cystic fibrosis. Since 2008 phage preparations against secondary infections have been used in patients with cystic fibrosis. The study was performed in collaboration with the National Center of Cystic Fibrosis (Tbilisi, Georgia). Phages were applied to infants and adults via a nebulizer several times a day for 6–10 days. Simultaneously, patients were treated by conventional antibiotics, anti-mucus medications, and vitamins. Phage application caused a substantial decrease of bacterial counts in sputum samples and an improvement of the patient’s general health condition. Due to bacteriophage therapy, long-term remissions of infections were achieved [43].
While the phage preparations are in everyday use in Georgia and in a number of ex-Soviet countries, there are many obstacles for the clinical application of bacteriophages in the western countries, such as the perception of viruses as ‘enemies of life’ [75]. This is due to the lack of a specific frame for phage therapy in the current Medicinal Product Regulation [57] and the absence of well-defined and safe bacteriophage preparations. To evaluate the safety and efficacy of bacteriophages in the treatment of burn wound infections in a controlled clinical trial, a highly purified and fully defined bacteriophage cocktail (BFC-1) was prepared, which is active against the P.aeruginosa and the S.aureus strains currently circulating in the Burn Centre of the Queen Astrid Military Hospital [53]. Based on successive selection rounds, three bacteriophages were retained from an initial pool of 82 P. aeruginosa and 8 S. aureus bacteriophages, specific for prevalent P. aeruginosa and S. aureus strains in the Burn Centre of the Queen Astrid Military Hospital in Brussels, Belgium. This cocktail, consisting of P. aeruginosa phages 14/1 (family Myoviridae) and PNM (family Podoviridae) and S. aureus phage ISP (family Myoviridae) was produced and purified of endotoxin. Quality control included stability (shelf life), determination of pyrogenicity, sterility, cytotoxicity, confirmation of the absence of temperate bacteriophages, and transmission electron microscopy-based confirmation of the presence of the expected virion morphologic particles, as well as of their specific interaction with the target bacteria. Bacteriophage genome and proteome analysis confirmed the lytic nature of the bacteriophages, the absence of toxin-coding genes showed that the selected phages 14/1, PNM and ISP are close relatives of respectively F8, φKMV and phage G1. The bacteriophage cocktail is currently being evaluated in a pilot clinical study was cleared by a leading Medical Ethical Committee. No adverse reactions were observed [53]. The detailed description of the quality controlled small-scale phage preparation was considered as a first step to promote the concept of phage treatment in Western medicine. In addition, it supported the creation of a discussion platform for regulatory framework for approval of phage therapy [57, 74].
Despite its long (Eastern European) history, phage therapy is not currently authorized for routine use on humans in the West. Today, it is only approved in some former Soviet republics like Russia and Georgia, where commercial phage preparations are sold in pharmacies [11]. In Poland, a recent member of the European Union, phage therapy is considered an ‘Experimental Treatment’ covered by the Physician Practice Act (Polish Law Gazette N° 28 of 1997) and the declaration of Helsinki, administered only when other therapeutic options do not exist [28, 57]. In France, therapeutic made-to-order phage preparations from the Institute Pasteur (Paris and Lyon) were used until the beginning of the 1990s. Today, a French practitioner, Alain Dublanchet, still uses commercial phage preparations (purchased in Russia and Georgia) to treat severe infections [57]. Despite the absence of a specific framework for phage therapy [28, 57], a pilot clinical trial in burn wounds was approved by an ethical committee in Belgium [45]. In the United States, a Food and Drug Administration (FDA)-approved phase I clinical trial was conducted. No safety concerns were found [45]. Recently, a British phage therapy company conducted a phase I/II a clinical trial in chronic otitis. This study was approved through the UK Medicines and Healthcare products Regulatory Agency (MHRA) and the Central Office for Research Ethics Committees (COREC) ethical review process [45].
Some bacterial pathogens are evolving a resistance to antibiotics, due to overuse and bacterial evolution. In addition, the antibiotic pipeline is running dry, with only a few new antibacterial drugs expected to make it to the market in the foreseeable future. Bacteria that are resistant to all available antibacterial drugs, so-called superbugs, are emerging worldwide. Evolutionary ecology might inform practical attempts to bring these pathogens under stronger human control [77].
Now, faced with the looming spectra of antibiotic resistance, Western researchers and governments are giving phages a serious look. In March, the US National Institute of Allergy and Infectious Diseases listed phage therapy as one of seven prongs in its plan to combat antibiotic resistance. Phagoburn: the first large, multi-center clinical trial of phage therapy for human infections, is funded by the European Commission, which is contributing €3.8 million (US$5.2 million) to the study.
Recently, the EU Parliamentary Assembly published the document entitled as ‘Phage therapy, a public health issue’ (Doc. 13480, 08 April 2014) (assembly.coe.int). In September 2014, a report to the President on combating antibiotic resistance and ‘NATIONAL STRATEGY FOR COMBATING ANTIBIOTIC RESISTANT BACTERIA’ were published in the USA as well (http://www.cdc.gov/media/dpk/2014/dpk-carb.html). These documents underline the growing threat of antibiotic resistance and call for better attention to bacteriophages therapy as a potential alternative to antibiotics.
Although governments are starting to pay attention to phage therapy, pharmaceutical companies remain reluctant to get on board. One of the reasons for this reluctancy is the intellectual property rights; even after almost 100 years history of phage therapy companies are still not allowed to claim a treatment as intellectual property and therefore recoup its costs. It is likely that a 2013 ruling by the US Supreme Court against the patenting of natural genes would also apply to phages isolated from nature. An engineered phage could, in theory, be patented. Recently the researchers started to use a DNA-editing system called CRISPR to kill only antibiotic-resistant bacteria. Its principle is based on the following: the phage injects the bacterium with DNA, which the microbe transcribes into RNA. If part of the bacterium’s antibiotic-resistance gene matches that RNA sequence, an enzyme called Cas9 cuts up the cell’s DNA, killing it. In initial trials, the researchers found that their phage could kill more than 99 % of the E. coli cells that contained specific antibiotic-resistance gene sequences, whereas it left susceptible cells alone. Giving the phage to wax worm larvae infected with resistant E. coli increased the worms’ chance of survival. The researchers are now starting to test the system in mice (human trials are a long way off).
Phages have also emerged as promising tools for curing cancer. Thus, it was reported that phages play a potential protective role against oxidative stress [54]. Besides that, the engineered phages can be used as delivery vectors of anticancer agents [64]. It was shown that phages are inducing cytokines [58]. Phages may play a certain role for treatment of neurodegenerative diseases. Phage M13 has been used in treatment models for Alzheimer’s and Parkinson’s diseases because of its ability to bind to the typical b-amyloid and a-synuclein plaques in the brain, resulting in plaque disaggregation [42]. The human gut contains approximately 1015 bacteriophages (the ‘phageome’), probably the richest concentration of biological entities on earth [16]. In Barr et al. [4] demonstrated that phages adhering to mucus provide a non-host-derived immunity to the human gut.
2.9 Phage-based Techniques for Identification of Bacteria
An increasing number of disease-causing bacteria are resistant to one or more anti-bacterial drugs utilized for therapy. Early and speedy detection of these pathogens is therefore very important. Traditional pathogen detection techniques, that include microbiological and biochemical assays, are long and labor-intensive, while antibody or DNA-based methods require substantial sample preparation and purification. Biosensors based on bacteriophages have demonstrated remarkable potential to surmount these restrictions and to offer rapid, efficient and sensitive detection technique for antibiotic-resistant bacteria.
Typical biosensor has three associated components: the sensor platform functionalized with a bio-probe to impart specificity of recognition; a transduction platform that generates a measurable signal in the event of analyte capture; the amplifier which amplifies and process the signal to give a quantitative estimate of analyte capture. Biosensors can be directly applied for the detection of pathogen in processed food matrices. Biosensors do not require the time-consuming sample during the pre-enrichment and secondary enrichment steps; therefore, this can accurately predict the amount and kind of food contamination much faster compared to conventional microbiological, immunological, and molecular biological methods [65].
Viruses have been used in a variety of chemical and biological sensors, and new applications continue to emerge. Bacteriophages, in particular, are one of the most successful viruses yet to have been applied towards biosensors, and can be grouped into three categories, regardless of their filamentous or icosahedral classification; one of these categories groups specific peptides or proteins discovered through phage display technology are genetically introduced onto the extraluminal surface of the bacteriophage for the detection of target analyte. In general, biosensors based on the use of nonlytic bacteriophages (i.e., M13) form the majority of this class. The next subgroup has specific bacterial cell markers that are detected upon bacterial cell rupture via the use of a lytic phage, which infects the host, replicates its genome, and induces host cell lysis. The last grouping holds bacteriophages that act a nano-scaffolding material for the transportation of functional molecules or their immobilization in a variety of sensing devices [48].
The requirements to sensor recognition elements are high stability, ease of immobilization on sensor platform, and recognition specificity towards host with minimum cross-reactivity from interfering pathogens. All of the three features are characteristic to bacteriophages. In different identification techniques phages have been applied either as whole phages, or phage-display peptides (PDPs), or as phage’s receptor binding proteins (RBPs) [48].
Since it was first reported in 1985 in Science, one phage display technology has played a key role towards the development of antigen-specific peptides. Specifically, bacteriophages are used to choose select peptides with high specificity and affinity towards a desired antigen from a pool consisting of a large number of potential peptides. Their subsequent use for the recognition of specific target molecules and biomarkers has yielded a variety of economical, rapid, and efficient applications in fields such as vaccine development, enzyme inhibition, inflammation, and cancer research. In particular, the readily available information on the genomic makeup of various bacteriophages has enabled researchers to easily engineer foreign entities (i.e., proteins, peptides, and so on) on the extraluminal surface, which, in turn, has enabled their widespread use for phage display applications. While bacteriophages have continued to receive interest for these reasons, the last 10 years in particular have seen an emergence of novel bacteriophage-based biosensor applications in the form of nanotemplates or nanoprobes [48, 65].
Recently, the nanosized dimensions of bacteriophages, and ease with which genetic modifications can be made to their structure and function, have put them in the spotlight towards their use in a variety of biosensors. In particular, the expression of any protein or peptide on the extraluminal surface of bacteriophages is possible by genetically engineering the genome. In addition, the relatively short replication time of bacteriophages offers researchers the ability to generate mass quantities of any given bacteriophage-based biosensor. Coupled with the emergence of various biomarkers in the clinic as a means to determine pathophysiological states, the development of current and novel technologies for their detection and quantification is imperative [48].
Bacteriophages, conventionally used in phage display technology for the selection of peptides or antibody fragments, have emerged in the development of a variety of biomedical applications such as the fabrication of nanowire-based secondary battery cells, gene therapy constructs, sensors, and drug delivery vehicles.
Phage Receptor Binding Proteins (RBPs) are novel probes for pathogen detection. The unique host-specific recognition of the tailed phages comes from the RBPs located on the tail fibers and it is the binding of these proteins that trigger the translocation of the phage genetic material into the host. The phage’s RBPs generally recognize unique proteins or carbohydrate (polysaccharide) sequences on the surface of the host bacterium. Genetically engineered RBPs offer several advantages over the antibody or intact phage-based technology for pathogen detection due to the following characteristics: their agglutination ability towards bacterial cells is found to be similar to the monoclonal antibodies against the bacterial lipopolysaccharides; the RBPs offer better stability against environmental factors such as pH and temperature and resistance against gastrointestinal proteases; and their binding affinity can be easily tailored to the requirement and multi-valency can be imparted, if desired [49].
The most commonly employed techniques to enhancement the effect of bacterial detection are: surface Plasmon resonance (SPR), Raman spectrometry, fluorescence/phosphorescence spectrometry, and Bio- or chem-iluminescence, orientated immobilization to different polymeric and/or metal surfaces, etc.[49, 65].
The translation of bacteriophage-based biosensors from in vitro to in vivo settings must be preceded by toxicity examinations to eliminate any safety concerns. Furthermore, the sensitivity of the general public to genetically engineered bacteriophages must be taken into account, and this is arguably one of the largest hurdles in the field. However, by coupling the aforementioned advantages of bacteriophages with the continued development of micro-and nano-based measurements and analytical devices, the scientists predict a concurrent increase in safety awareness and subsequent improvements. Finally, it is important to note that the use of bacteriophages is not limited to the aforementioned categories. Bacteriophages with varying functions that express desired peptides or proteins on their surface can be engineered through the simple manipulation of the bacteriophage genome, and ultimately be used for the rapid and economical mass production of bacteriophage-based biosensors. Here, an appropriate understanding of the bacteriophage that covers a wide area of topics, such as the structure and function of individual components and its life cycle, must accompany such novel applications. In summary, we envision that the combination of such bacteriophages with analytical nanofabricated devices will emerge in novel applications as a powerful nanoscale tool for the detection of a wide spectrum of targets, ranging from biological agents (i.e., proteins, bacteria, spores, viruses, toxins, and so on) to chemical agents or even explosives [49].
Notes
- 1.
After the execution of George Eliava the book was impounded and access to it was limited to “for professional use only”. N.Ch.
References
Agafonov BI, Khokhlov DT, Zolochevsky MA (1984) Epidemiology of typhoidparatyphoid infections and their prophylactics. Mil Med J 6:36–40
Alessandrini A, Doria R (1924) Il batteriofago nella terapia del tifo addominale. Policlinico Sez Prat 31:109
Anpilov LI, Prokudin AA (1984) Prophylactic effectiveness of the dry polyvalent dysentery bacteriophage in organized communities. Mil Med J 5:39–40
Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J et al (2013) Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A 110:10771–10776
Belikova MA (1941) Experience of phage prophilaxis of summer dysentery among the young children performed in the city Stalingrad. J Microbiol Epidemiol Immunol 5–6:142
Blankov BI (1941) Analysis of the results of phage prophylaxis of dysentery among the contacting people. Report # 1. J Microbiol Epidemiol Immunol 5–6:125–131
Blankov BI, Zherebtsov ID (1941) Experience on the multiple phaging of the contacting population in the fight against dysentery. Report # 2. J Microbiol Epidemiol Immunol 5–6:131–136
Bruynoghe R, Maisin J (1921) Essais de the‘rapeutique au moyen du bacteriophage. CR Soc Biol 85:1120–1121
Ceyssens P-J, Glonti T, Kropinski AM, Lavigne R, Chanishvili N, Kulakov L, Lashkhi N, Tediashvili M, Merabishvili M (2011) Phenotypic and genotypic variations within a single bacteriophage species. Virol J 8:134
Chanishvili N, Chanishvili T, Tediashvili M, Barrow PA (2001) Phages and their application against drug-resistant bacteria. J Chem Technol Biotechnol 76:689–699
Chanishvili N (2012) A literature review of the practical application of bacteriophages research. Nova Science Publishers, New York
Chkonia I, Meipariani A, Jgenti D, Alavidze Z, Goderdzishvili M, Dzidzishvili L, Kvatadze N, Mamamtavrishvili D, Menabde G (2009) Development of technology for creation “Osteophage” a composite stimulating bone regeneration. Proc Georgian Acad Sci Biol Ser B 7(1–2):63–69
Chkonia I, Alavidze Z, Goderdzishvili M, Meiphariani A, Dzizdzishvili M, Kvatadze N, Jgenti D, Makhatadze N, Rigvava S, Karumidze N, Kusradze I, Dvalidze T, Barbakadze S, Kuchukhidze J, Turabelidze D (2013) Natural phage biocomposite for mixed infections. Microbiol Biotechnol 3:10–12
Costa Cruz J (1924) Le traitement des dysenteries bacillaires par le bacteriophage. CR Soc Biol 91:845
Compton A (1929) Antidysentery bacteriophage in treatment of dysentery: a record of 66 cases treated, with inferences. Lancet 2:273
Dalmassso M, Hill C, Ross RP (2014) Exploiting gut bacteriophages for human health. Trends Microbiol 22(7):399–406
Dublanchet A, Bourne S (2007) The epic of phage therapy. Can J Infect Dis Med Microbiol 18:15–18
Eaton MD, Bayne-Jones S (1934) Bacteriophage therapy: review of the principles and results of the use of bacteriophage in the treatment of infections. JAMA 23:1769–1939
Florova NN, Cherkass FK (1965) Results of mass application of polyvalent dysenteric bacteriophage. J Microbiol Epidemiol Immunol 3:118–125
Gamaleya NF (1898) Bacterial lyzins: the enzymes destroying bacteria. Russ Arch Pathol Clin Med Bacteriol 6:607–613
Georgadze IA (1974) Fifty years of the Tbilisi Scientific-Research Institute of Vaccine and Sera of the Ministry of Health of the USSR. In: Selected articles of the jubilee dedicated to 50th anniversary of the Tbilisi Institute of Vaccine and Sera. TIVS, Tbilisi
Georgadze IA, Makashvili EG (1979) George Eliava. In: Abashidze I, Metreveli R (eds) The Georgian Soviet encyclopedia, vol 4. State Education Commission’s Publishing House, Tbilisi, p 125
Giorkhelidze T, Koberidze T, Malkhazova I, Janelidze N, Tediashvili M, Chanishvili N, Hanlon G, Denyer S (2008) Screening results of the MRSA strains with Staphylococcus phages, phage, biology, ecology and therapy meeting, 12–15 June 2008, p 36
Glonti T, Chanishvili N, Taylor PW (2010) Bacteriophage-derived enzyme that depolymerizes the alginic acid capsule associated with cystic fibrosis isolates of Pseudomonas aeruginosa. J Appl Environ Microbiol 108:695–702
Gnutenko MP (1951) Treatment of S. typhi and paratyphi bacterial carriers with bacteriophages. J Microbiol Epidemiol Immunol 5:56–60
Goderdzishvili M, Meiphariani A, Chkonia I, Dzizdzishvili M, Kvatadze N, Jgenti D, Makhatadze N, Rigvava S, Karumidze N, Kusradze I, Dvalidze T, Gogbaidze G, Nikoladze B, Alavidze Z (2012) Development and initial testing of a phage cocktail to treat chronic bacterial prostatitis. Microbiol Biotechnol 3:15–18
Golubtsov GV (1940) Sero- and phage- therapies of dysentery among babies and infants. In: Selected articles of the 1st scientific conference of the Bashkir Medical University, Ufa, 23–25 Mar 1939, pp 13–16
Górski A, Miedzybrodzki R, Borysowski J, Weber-Dabrowska B, Łobocka M, Fortuna W, Letkiewicz S, Zimecki M, Filby G (2009) Bacteriophage therapy for the treatment of infections. Curr Opin Invest Drugs 10:766–774
Hausler T (2008) Viruses vs.superbugs. A solution to the antibiotics crises. Macmillan, London
d’Herelle F (1935) Bacteriophage and phenomenon of recovery. TSU Press, Tbilisi
d’Herelle F (1917) Sur un microbe invisible antagoniste des bacilles dysente’riques. CR Acad Sci (Paris) 165:373–375
Ionov ID, Erez SL, Goldenberg EY (1939) Specific therapy of dysentery. In: Variability of microorganisms and bacteriophage research (Proceedings of the scientific conference, 1936), Kiev, pp 337–346
Kagan MI, Kuznetsova EV, Teleshevskaya EA (1964) To the issue of epidemic effectiveness of the planned phaging in the day nurseries. J Microbiol Epidemiol Immunol 7:89–102
Karamov S (1938) Experience of phage therapy for treatment of typhoid fever. In: Selected articles of Azerbaijani Institute of Epidemiology and Microbiology, vol 6, Azerbaijan SSR, Baku, pp 101–105
Karpov SP (1946) The specific bacteriophage in relation to the issue of combating typhoid and paratyphoid diseases. J Microbiol Epidemiol Immunol 1–2:40–44
Karumidze N, Thomas JA, Kvatadze N, Goderdzishvili M, Hakala KW, Weintraub S, Alavidze Z, Hardies SC (2012) Characterization of lytic Pseudomonas aeruginosa bacteriophages via biological properties and genomic sequences. Appl Microbiol Biotechnol 94(6):1609–1617
Kazhal N, Iftimovich R (1968) From the history of fight against bacteria and viruses. Nauchnoe Izdatelstvo, Bucharest
Kokin GA (1941) Use of bacteriophages in surgery. Sov Med (“Sovietskaya Meditsina”) 9:15–18
Kokin GA (1946) Phage therapy and phage prophylaxis of gas gangrene. In: Smirnov E, Grigolava S, Orbeli L (eds) Experience of the Soviet military medicine during the great patriotic war 1941–1945, vol 3. MedGiz Publishing House, Moscow, pp 56–63
Krestovnikova VA (1947) Phage treatment and phage prophylactics and their approval in the works of the Soviet researchers. J Microb Epidemiol Immunol 3:56–65
Krueger AP, Scribner EJ (1941) Bacteriophage therapy. II. The bacteriophage: its nature and its therapeutic use. JAMA 19:2160–2277
Ksendzovsky A, Stuart Walbridge BS, Saunders RC, Asthagiri AR, Heiss JD, Lonser RR (2012) Convection-enhanced delivery of M13 bacteriophage to the brain. J Neurosurg 117(2):197–203
Kutateladze M, Adamia R (2010) Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol 28:591–595
Kurochka VK, Karniz AF, Khodyrev AP (1987) Experiences of implementation of preventive anti-epidemic measures in the center of intestinal infections with water transmission mechanism of morbidity. Mil Med J 7:36–37
Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11:69–86
Kvachadze L, Balarjishvili N, Meskhi T, Tevdoradze E, Skhirtladze N, Pataridze T, Adamia R, Topuria T, Kutter E, Rohde C, Kutateladze M (2011) Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb Biotechnol 4(5):643–650
Lipkin YaO, Nikolskaya PV, (1940) Experience of phage therapy of dysentery. In: Selected articles of the Kuibishev Red Army Military-Medical Academy, vol 4. KRAMMA, Kuibishev, Russian SSR, pp 193–198
Lu TK, Bowers J, Koeris MS (2013) Advancing bacteriophage-based microbial diagnostics with synthetic biology. Trends Biotechnol 31(6):325–327
Lu TK, Koeris MS (2011) The next generation of bacteriophage therapy. Curr Opin Microbiol 14:524–531
Manolov DG, Sekunova VN, Somova EE (1948) Experience of therapy of typhoid fever by intravenous administration of the phage. J Microbiol Epidemiol Immunol 4:33
Markoishvili K, Tsitlanadze G, Katsarava R, Morris G, Sulakvelidze A (2002) A novel sustained-release matrix based on biodegradable poly (esteramide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int J Dermatol 41:453–458
Melnik MI, Nikhinson IM, Khastovich RI (1935) Phage prophylaxis of dysentery. In: Proceedings of the Mechnikov Institute in Kharkov, vol 1(1), MIKH, Kharkov, Ukrainian SSR89
Merabishvili M, Pirnay J-P, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L, Lavigne R, Volckaert G et al (2009) Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944
Miedzybrodzki R, Switala-Jelen K, Fortuna W, Weber-Dabrowska B, Przerwa A, Lusiak-Szelachowska M, Dabrowska K, Kurzepa A, Boratynski J, Syper D, Pozniak G, Lugowski C, Gorski A (2008) Bacteriophage preparation inhibition of reactive oxygen species generation by endotoxin-stimulated polymorphonuclear leukocytes. Virus Res 131(2):233–242
Mikaelyan VG (1949) Phage therapy of the bacterial carriers of typhoid bacteria. In: Proceedings of the Yerevan Medical Institute, vol 6. YMI, Yerevan, pp 54–59
Parfitt T (2005) Georgia: an unlikely stronghold for bacteriophage therapy. Lancet 365:2166–2167
Pirnay J-P, De Vos D, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M, Zizi M, Laire G, Lavigne R, Huys I, Van den Mooter G, Buckling A et al (2011) The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharm Res 28:934–937
Przerwa A, Zimecki M, Switała-Jeleń K, Dabrowska K, Krawczyk E, Łuczak M, Weber-Dabrowska B, Syper D, Miedzybrodzki R, Górski A (2006) Effects of bacteriophages on free radical production and phagocytic functions. Med Microbiol Immunol 195:143–150
Rakieten ML (1932) Studies with Staphylococcus bacteriophage. I. The preparation of polyvalent Staphylococcus bacteriophage. Yale J Biol Med 4:807–818
Ministry of Health, Ministry of Medical Production of the USSR, Chief Agency of Microbiological Production (1985) Report of the clinical trials.
Rice TB (1930) Use of bacteriophage filtrates in treatment of suppurative conditions: report of 300 cases. Am J Med Sci 179:345–360
Sapir IB (1939) Observations and recommendations related to phage therapy of dysentery. In: Proceedings of the Moscow Institute of Infectious Diseases after I.I. Mechnikov, pp 135–151
Schless RA (1932) Staphylococcus aureus meningitis: treatment with specific bacteriophage. Am J Dis Child 44:813–822
Shoae-Hassani A, Sharif S, Verdi J. (2011) The neurosteroid dehydroepiandrosterone could improve somatic cell reprogramming. Cell Biol Int. 35(10):1037–41. doi: 10.1042/CBI20100927
Singh A, Poshtiban S, Evoy S (2013) Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors 13:1763–1786
Stout BF (1933) Bacteriophage therapy. Tex State J Med 29:205–209
Sulakvelidze A, Alavidze Z, Morris JG Jr (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659
Summers WC (1999) Felix d’Herelle and the origins of molecular biology. Yale University Press, New Haven
Summers WC (2001) Bacteriophage therapy. Annu Rev Microbiol 55:437–451
Tsulukidze AP (1938) Application of phages in urology. Urology (“Urologia”) 15:10–13
Tsulukidze AP (1940) Phage treatment in surgery. Surgery (“Khirurgia”) 12:132–133
Tsulukidze AP (1941) Experience of use of bacteriophages in the conditions of war traumatism. Gruzmedgiz, Tbilisi
Twort F (1915) An investigation on the nature of ultramicroscopic viruses. Lancet 11:1241
Verbeken G, Huys I, Pirnay J-P, Jennes S, Chanishvili N, Scheres J, Górski A, De Vos D, Ceulemans C (2014) Taking bacteriophage therapy seriously: a moral argument. BioMed Res Int 2014:1–8
Villareal LP (2005) Overall issues of viral and host evolution. In: Villareal IP (ed) Virus and evolution of life. ASM Press, Washington, DC, pp 1–28
Vlasov KF, Artemenko EA (1946) Treatment of chronic dysentery. Sov Med (“Sovetskaya Medicina”) 10:22–28
Williams PD (2010) Darwinian interventions: taming pathogens through evolutionary ecology. Trends Parasitol 26:83–92
Yilmaz C, Colak M, Yilmaz BC, Ersoz G, Kutateladze M, Gozlugol M (2013) Bacteriophage therapy in implant-related infections: an experimental study. J Bone Joint Surg Am 95(2):117–125
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Chanishvili, N. (2015). Bacteriophage-based Products and Techniques for Identification of Biological Pathogens. In: Camesano, T. (eds) Nanotechnology to Aid Chemical and Biological Defense. NATO Science for Peace and Security Series A: Chemistry and Biology. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7218-1_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-7218-1_2
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7217-4
Online ISBN: 978-94-017-7218-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)