Abstract
Pathogenic infections cause tremendous health threats and socioeconomic burdens worldwide. Conventional methods for bacteria detection are laborious, time-consuming, expensive, and require particular devices and highly qualified specialists. Sensitive, selective, inexpensive, quick, and user-friendly biosensors are in urgent demand to prevent and detect bacterial infections in many fields, e.g., healthcare, food industry, or terrorism prevention. Among biorecognition elements utilized in biosensors, bacteriophages are highly promising due to their numerous advantages, such as host specificity, cheap and simple production, resistance to external factors, and ease of immobilization. Here we reviewed currently used methods for bacteria detection, pointing their advantages and disadvantages. We paid particular attention to bacteriophage-based methods, including phage-based sensors and phage display method.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Although quality of living is constantly improving through technological progress, bacterial infections remain a major problem in the modern world. Approximately 13% of the deaths are related to bacterial diseases (Punina et al. 2015). Furthermore, bacteria are also involved in specific types of cancers (Elsland and Neefjes 2018) and various metabolic disorders, including obesity, which affects 39% of adults (Castañer and Schröder 2018).
Bacteria are a significant threat for children or elders and in the developing countries, where respiratory diseases such as tuberculosis cause millions of deaths (Harding 2020). A major source of bacterial infections is food and water poisoning, causing 1.8 million casualties worldwide in 2005 (ECDC 2009). In a publication from 2009, it was shown that only in the USA number of infections and illnesses caused by foodborne pathogens reached 76 million. 325,000 cases were reported to hospitals, and 5200 died (Buzby and Roberts 2009). There are also socioeconomic costs related to outbreaks of an epidemic caused by food hazards. The report from 2011 showed 9.4 million episodes of foodborne illnesses in the USA (Scallan et al. 2011). 3816 people got sick, and 54 died due to Escherichia coli O104:H4 outbreak in Europe in 2011 (King et al. 2012). The total financial burden was estimated to reach 3 billion Euros. In 2015, the Center for Disease Control and Prevention (CDC) reported 15,202 foodborne infected patients, 950 hospitalizations, and 15 deaths (Wan et al. 2019). World Bank study conducted in 2018 in low- and middle-income countries estimated the cost of food-born illnesses at 110 billion USD and treatments cost at 15 billion USD annually (Jaffee et al. 2019).
Another major cause of bacterial infections is hospitals. According to a World Health Organization report from 2011, 4.1 million patients are affected by healthcare-related illnesses each year in Europe (World Health Organization 2011). Furthermore, only in the USA, nosocomial infections cause 100,000 deaths each year (Cimiotti et al. 2012). Due to the impact of the COVID-19 pandemic, the number of hospital-acquired infections (HAI) increased by 34–47% in 2020 compared to the number of cases that occurred in 2019 (Weiner-Lastinger et al. 2022). According to the report published by Quince Market Insights in July 2021, the HAI market reached over 12 billion USD in 2020 (Global hospital-acquired infections 2021).
The appearance of multidrug-resistant bacteria strains makes this problem even more urgent. The knowledge about antibiotic resistance mechanisms is still unsatisfying, and our main weapon against bacteria lost its potential (Paczesny et al. 2020). Due to the lack of enough funding in antibiotic development and the uncontrollable use of antibiotics, the danger of antibiotic resistance is increasing radically. The CDC’s latest estimation of death and infection in the USA conducted in 2018 suggested that more than 2.8 million antibiotic-resistant infections occur each year, and more than 35,000 people die because of it (US Department of Health and Human Services, CDC 2019). In 2015, antibiotic-resistant Gram-negative pathogens caused losses estimated at 287 million EUR (Touat et al. 2019). The cost of critical measures for antimicrobial resistance containment is estimated to be 9 billion USD globally (Jit et al. 2020). Research conducted by the World Bank Group estimated that the global economic cost of antibiotic resistance will range between 1.0 and 3.4 trillion USD in 2030, which is 1.1–3.8% of global GDP (Miller-Petrie et al. 2017).
Cost for biodefense and prevention from threats of biological warfare and bioterrorism also cause enormous expenses. For instance, statistics from 1997 indicated that the cost of prevention from brucellosis was estimated to be around $477.7 million per 100,000 persons exposed and anthrax was $26.2 billion per 100,000 people exposed (Kaufmann et al. 1997). These costs have multiplied over the last two decades (Christian 2013).
Therefore finding an efficient way to overcome problems caused by pathogens is paramount. There is also a need for a rapid and specific method to detect and recognize bacteria. Most methods that are currently in use rely on culturing, biochemical tests, or molecular protocols (e.g., PCR, polymerase chain reaction, amplification). Although these approaches are useful, there is still no method allowing to combine short time of the analysis and very low detection limit (e.g., 1 CFU (colony forming unit) per mL), even at the expense of the cost of analysis.
2 Culturing-Based Methods
The bacteria identification based on cultivation aims to get pure culture from repeated collection and seeding of an isolated colony. General-purpose agar-based media is commonly used to cultivate various pathogens, but some bacteria require more specific culture media for more accurate identification. For instance, “differential” culture media relies upon the metabolic difference of the pathogens by using a biochemical or pH indicator to detect them. “Selective” culture media has antimicrobials that inhibit the commensal flora from increasing the growth of certain bacteria of interest (Váradi et al. 2017).
Chromogenic media is frequently used as a microorganism identification method since it is cheap and straightforward. The chromogenic media method requires culturing bacteria samples using appropriate broth or agar media enriched with colorless or fluorescence chromogenic enzyme substrates. The substrates are colorized by specific bacterial enzymes (Váradi et al. 2017). The chromogenic media method is commonly employed in clinical laboratories since it requires a small workload and increases the chances of identification due to colored colonies, especially when multiple species are present in the sample.
Commercially available biochemical tests are frequently used after isolation to identify genus and species levels. Commercial kits such as Analytical Profile Index (API) kits can be applied to carry out the inoculation and reading of biochemical panels manually, so do automated tests such as the BD Phoenix or the Vitek 2. These systems can identify bacteria in 2–3 h and execute automated antimicrobial susceptibility testing (Jorgensen and Ferraro 2009).
Even though these methods cost less and provide quantitative and qualitative information about the bacteria, they require a lot of work and time for media preparation, dilution, plating, incubation, counting, isolation, and characterization. The main disadvantage of the chromogenic media method is that this method is usually time-consuming and requires up to a few days to obtain the results (Franco-Duarte et al. 2019). Also, in some cases, it requires additional examination using other analytical, often instrumental, methods. At times, biochemical properties inaccurately indicate the genomics of a given species (Janda and Abbott 2002), and results can be false positives considering similar species (Justé et al. 2008).
3 Molecular Methods
Molecular methods present multiple tools and techniques for bacteria characterization, detection, and identification (Ferone et al. 2020). They brought remarkable insights by detecting previously unidentified bacteria, classifying uncultivable bacteria, and allowing the metagenomics study of diverse bacterial communities on a large scale. Most molecular techniques for bacteria detection and identification are based on DNA analysis, extending from rather simple DNA amplification-based methods, such as polymerase chain reaction (PCR), real-time PCR, random amplification of polymorphic DNA PCR (RAPD-PCR) to more intricate approaches that rely upon restriction fragment analysis, targeted gene, and whole-genome sequencing (Galluzzi et al. 2007). Molecular methods can be classified as amplification methods (PCR, quantitative real-time PCR (qPCR), and reverse transcription PCR (RT-PCR)), DNA microarrays, hybridization-based detection methods (FISH), and whole-genome sequencing (WGS). These methods are culture-independent and enable bacteria identification at the genus level. It is crucial to understand the basic operating principles of each method, as well as their uses and limitations (Ferone et al. 2020).
Gene amplification and target gene sequencing is an effective method for bacteria identification. Over the past years, PCR amplification and gene sequencing have been utilized for detecting and identifying bacteria from colonies. Gene sequencing is a more objective method of bacteria identification, which does not regard fastidious growth or cell viability. This method provides reliable results and enables an increase in the diversity of bacterial taxa (Moshirabadi et al. 2019). Amplification methods provide relatively quick results, but there is a risk of cross-contamination associated with their sensitivity.
The 16S ribosomal RNA (16S rRNA) gene, the 26S rRNA gene, or particular genes encoding bacterial toxins are sequenced to detect bacteria. The 16S rRNA, a 1500 base pair gene common to all bacteria, is the most frequently utilized gene target for bacterial identification due to its high specificity to each species (Petti 2007). Real-time PCR is qualitative, more sensitive, and accurate compared to conventional PCR techniques. qPCR with fluorescence intensity enables the analysis of DNA amplification in real-time and does not require any post-PCR detection, which explains its broad usage in clinical and research fields. For instance, real-time-based 16S rRNA PCR was applied to identify and quantify microorganisms in chronic wound tissue and saliva sample (Melendez et al. 2010). Quantitative real-time PCR (qPCR) and reverse transcription real-time PCR (RT-qPCR), and other amplification methods were used to identify foodborne pathogens, such as Listeria monocytogenes, E. coli O157:H7, S. aureus, Campylobacter jejuni, Salmonella spp., and Shigella spp. (Law et al. 2014). Random amplification of polymorphic DNA (RAPD), on the other hand, uses short primers with random sequences that result in the amplification of arbitrary, repetitive regions of template DNA. Since the short primers for RAPD-PCR are intended to bind randomly to the template, this method does not oblige any prior information of the target genome sequence. RAPD-PCR can be utilized not only to detect bacterial genetic variability but also to discover and detect unidentified microorganisms (Franco-Duarte et al. 2019).
Microarray is an ordered assemblage of samples (DNA, RNA, protein, tissue) that can be probed with target molecules to generate gene expression or diagnostic information. Microarray analysis can simultaneously detect and characterize numerous bacteria. Several microarray methodologies are available for application, such as printed and in situ—synthesized microarrays, electronic and suspension bead microarrays, and high-density bead arrays. Generally, the ssDNA sequence is synthesized and immobilized as discrete features or spots on the microarray surface. The “unknown” target sequence of interest is fluorescently labeled and then hybridized to the probe microarray. Hybridization between the immobilized probe and the labeled target enhances the fluorescence intensity. The fluorescence scanner measures the intensity, and the collected data is analyzed further (Miller and Tang 2009).
Fluorescence in situ hybridization (FISH) is considered a less time-consuming and reliable cytogenetic technique for bacteria detection and identification at the genus or species level. The principle of the FISH method relies upon the binding of short (18–25 base pair), fluorescence-labeled target-specific DNA or nucleic-acid mimicking peptide-nucleic-acid (PNA) probes to the ribosomal RNA with subsequent analysis under the fluorescence microscope. The FISH analysis offers information on spatial resolution, morphology, identification, and fast differentiation of bacteria from a mixed-species solution. The method offers rapid and reliable detection at the genus and species level, minimal technical equipment necessity, and cost-effectiveness. The main drawbacks are a need for specifically targeted investigation, trained and experienced personnel, and lower sensitivity than PCR (Frickmann et al. 2017).
Whole-genome sequencing (WGS) is becoming a highly applicable technique that provides rapid detection and identification of bacteria, viruses, and fungi due to advancements in sequencing technologies (Tagini and Greub 2017). WGS technologies permit valuable data about difficult-to-grow pathogens and drug resistance, bacteria’s evolution and spread, possible virulence factors, candidate drug complexes, and a deep understanding of infection mechanisms. WGS technologies can compete with standard methods in speed, specificity, expense, and monitoring/investigating outbreaks of infectious diseases. Currently, WGS is commonly used in addition to real-time diagnostics in medical laboratories. Apart from detecting, identifying, and characterizing bacteria, WGS is applied to design diagnostic tools, assess multidrug resistance, examine and track the emergence of pathogens in hospital environments (Punina et al. 2015).
4 Probes for Bacteria Detection
Probes techniques such as Southern blot, Northern blot, and Western blot are relatively old yet not overused methods for detection. Southern blot was developed based on Southern sequencing, which was the first used DNA sequencing technique. This sequencing method relies on isolating the DNA from the “target” sample, amplification reaction using specific primers with controlled termination of amplification by dehydrogenated nucleoside triphosphates, agarose gel electrophoresis, and gel visualization by the usage of ethidium bromide (Yuen et al. 1993). Then, protocols were modified to detect specific DNA sequences in DNA samples. At first, the DNA sample is cut by restriction nucleases. DNA fragments are separated by size through agarose gel electrophoresis, then transferred to nitrocellulose membrane and crosslinked the membrane via exposure to ultraviolet radiation. The critical step in Southern blotting is exposing the crosslinked membrane to a hybridization probe—a single-stranded DNA fragment complementary to the sequence of interest, usually tagged with a fluorescent dye or radioactive marker. After hybridization, membranes are blocked, washed, and then visualized (Marcadet et al. 1989). The main advantage of Southern blot is that it detects unculturable, usually environmental, bacteria (Cecchini et al. 2012).
Northern blot is commonly used to analyze the gene expression by detecting RNA in the sample. In principle, it is similar to Southern blotting, but electrophoresis gels have to contain formaldehyde to limit RNA secondary structure. Probes are complementary to the RNA sequence of interest. Still, they can be DNA, RNA, or oligonucleotides, usually labeled with radioactive isotopes, but chemiluminescence probes are becoming more and more common in use (Streit et al. 2009). Northern blot is not directly used for bacteria detection. Still, it allows detection of some particular bacterial small RNAs (sRNAs) in total RNA extract (Beckmann et al. 2010), which makes a fine way for examining gene expression. Its drawback is the impermanence of the analytical material, for it is tough to avoid RNase contamination.
Western blot, also known as the protein immunoblot, allows for the detection of specific proteins. In this method, proteins are separated by size via electrophoresis, usually in polyacrylamide gel, then transferred on the membrane and blocked. A protein of interest is targeted by incubation with a primary antibody. Then a secondary antibody targets the primary one. The secondary antibody is visualized through colorimetric, chemiluminescence, immunofluorescence, or radioactivity assays, indirectly detecting a target protein (Mahmood and Yang 2012). Because bacteria produce species-specific proteins, such as toxins, it is possible to detect and recognize them with Western blotting protocol (Bone et al. 2017). The main advantage of this technique is its simplicity and unambiguity of the results. Unfortunately, the analysis may require about a week to complete, making it an extremely lengthy procedure. Also, analyzed proteins tend to form complexes. This phenomenon may cause the antibody-binding site to become unavailable, or even worse—the protein complex may be visualized and mistakenly recognized as an additional target protein (Mahmood and Yang 2012).
5 Microscopic Methods
The optical microscope is a fundamental detection device for bacteria identification. Obtained images allow determining the shape, following motion, and categorizing species by their morphological contrast (Dige et al. 2007). However, only using microscopy for bacteria detection is not enough. In natural samples, smaller cells can be missed due to the density of larger cells. Distinguishing cells from other objects or living cells from dead cells can also be challenging (Franco-Duarte et al. 2019). Another major disadvantage of microscopy is that none displays the microorganisms’ phylogenetic diversity (Franco-Duarte et al. 2019). Also, microscopic techniques do not allow for the display of phylogenetic diversity.
In most cases, microscopic methods are used with fluorescent dyes due to more specific visualization and uncomplicated performance. Dyes such as 4′,6-diamidino-2-phenylindole (DAPI; maximum absorption is at 400 nm), acridine orange (absorption maximum 500 nm), SYBR® Green I (maximum absorption 497 nm) bind to the DNA of the bacteria and fluorescence after the UV exposure, making bacteria detectable (Franco-Duarte et al. 2019). Flow cytometry can also be applied for detecting individual cells. This method enables the possibility to count and evaluate individual cells’ size, shape, and features. Cells are suspended in a fluid flow and passed through a detector, collecting fluorescence or scattered light. Clausen et al. used a label-free technique of electrical impedance flow cytometry to distinguish Gram-negative from Gram-positive bacteria successfully and accurately determined the concentration of the bacteria solution (Clausen et al. 2018).
6 Spectroscopic Methods
Spectroscopy is the study of matter and its interactions with electromagnetic radiation. Spectroscopic techniques are used in nearly all technical areas of science and technology for quantitative and qualitative analyses (Ferone et al. 2020). This multivariate, reproducible methodology is used to solve numerous analytical problems due to its non-destructive, simple, and precise approach, enabling broad amounts of information acquired in a single measurement (Mariey et al. 2001). Spectroscopic techniques vary based on the examined species (molecular or atomic spectroscopy), the type of radiation–matter interaction to be monitored (absorption, emission, or scattering), as well as the used range of the electromagnetic spectrum. Spectroscopic methods require a combination of spectral pre-processing and different chemometric techniques to analyze and differentiate bacteria quantitively.
One of the latest developments in applying new spectroscopic techniques is the Fourier transform infrared spectroscopy (FTIR), an adjustable, rapid, non-invasive, and effortlessly operated method (Mariey et al. 2001). This method provides a label-free approach to the comprehensive interpretation of the chemical compounds and the physical state of the whole sample. It is possible to acquire precise, thorough information about nucleic acids, carbohydrates, lipids, and proteins only in one measurement with a small sample volume (Kosa et al. 2017). Also, FTIR enables an efficient biochemical characterization of the entire biological systems. The major advantage of the FTIR method is the capacity to examine numerous compounds at once. FTIR application to examine microorganisms leads to quite a complicated spectrum with the principal compounds’ overlapping absorption bands. Hence, a detailed statistical analysis is indispensable to extract only the essential data from spectra (Franco-Duarte et al. 2019). Also, this method does not require cell lysis to evaluate the biomolecules and is considered eco-friendly since toxic compounds are not used. Besides the achievement on the field of screenings, FTIR can be applied to monitor various processes in real-time (Kosa et al. 2017).
However, for the analysis of microbial diversity, regular infrared radiation is much more applicable (Santos et al. 2010). The near-infrared (NIR) spectral region was used in the food microbiology industry to detect and identify of Lactococcus lactis, Listeria innocua, Pseudomonas fluorescens, Pseudomonas putida, and Pseudomonas mendocina on chicken breast muscle (Alexandrakis et al. 2012). The major drawback of using NIR spectroscopy in food or microbiological analyses is the samples’ sensitivity to temperature shifts or the occurrence of photodegradation triggered by the light sources. Furthermore, the infrared (IR) signal is frequently dominated by the signal from the water, which is almost always present in the culture media and food products (Ferone et al. 2020).
Raman spectroscopy is another popular spectroscopic method recognized for its non-invasive and rapid recognition and characterization of various analytes, including bacteria. Its principle is the inelastic scattering of monochromatic light. Inelastic scattering implies the shifts of photon frequency in monochromatic light upon contacting the sample. The sample absorbs light photons and then reemits. The reemitted photons’ frequency is altered compared with the original monochromatic frequency (the Raman effect). This shift provides information about molecules’ vibrational, rotational, and other low-frequency transitions to create a structural fingerprint. This structural fingerprint can be used to distinguish microorganisms, with the accuracy that allows differentiating species, or even the strains, in a short period (Ferone et al. 2020). Additionally, the Raman signal is not affected by water, but fluorescence signals can give high background because of amino acids and nucleic acids (Ferone et al. 2020). Even though Raman spectroscopy has high specificity, it has inadequate sensitivity.
Surface-enhanced Raman spectroscopy (SERS) enables greater sensitivity in detecting low concentration analytes by intensifying electromagnetic fields created from the excitation of localized surface plasmons. Comparing to the standard Raman, a signal can be boosted from 103 to 106 times using SERS (Kneipp and Kneipp 2006). Bacteria detection using SERS can be carried directly by analyzing the intrinsic vibrational fingerprint of bacteria (Witkowska et al. 2017; Witkowska et al. 2018); or via indirect detection by using a nanotag as a quantitative reporter (Huang et al. 2021). SERS signal relies upon the active substrate’s material since each substrate has unique enhancement effects on the samples. The shape and size of the nanoparticles, the active substrate’s material, distance, and the number of probes adsorbed on the active substrate affect the signal (Ferone et al. 2020). Wei et al. successfully detected and identified E. coli, S. aureus, and Salmonella spp. using SERS coupled with silver colloidal nanoparticles. The distinctive differences of each pathogen were observed in the SERS spectral data, and a short time was required for the assay (Wei et al. 2018).
7 Chromatographic Methods
Mass spectrometry (MS) based techniques are an important microbial-typing tool because of the rapidity, low expense, ease of use, and effectiveness of all kinds of bacteria, archaea, and fungi. Mass spectrometry can be associated with multiple ionization and separation methods, such as gas chromatography (GC) and liquid chromatography (LC) (Fox 2006), matrix-assisted laser desorption ionization time-of-flight mode (MALDI-TOF) (Jang and Kim 2018), electromigration techniques (Buszewski et al. 2017), or electrospray ionization (ESI) (Zhang et al. 2011).
The LC combined with MS (LC-MS) transformed the analytical determination of metabolome, thus, enabled bacteria identification (Warren 2018). The use of relatively low temperatures and the fact that the sample doesn’t need to be volatile, the preparation process of the sample is much simpler, and the costs decreased. Samples are introduced into the solvent then separated within the column with the stationary phase (Holčapek et al. 2012). LC depends on the gravity force to move the mobile phase across the column, but for HPLC, pressures reach 50–350 bars. Moreover, it can be utilized at higher temperatures (high-temperature liquid chromatography) or in monolithic columns (Teutenberg 2009).
MALDI-TOF MS, a new generation tool, is widely used for the identification of microorganisms in the most advanced clinical laboratories. In this procedure, the microbial cells’ ionization is ionized by the laser pulses. Then the electric field accelerates created ions in a vacuum system (Doern and Butler-Wu 2016). An acquired spectra profile is unique for a particular microorganism and can be utilized as its molecular fingerprint. The identification is proceeded by comparing the molecular fingerprint with the database (Jang and Kim 2018). Nowadays, MALDI-TOF is commonly used as a complementary procedure to the culture methods. When combined, these two methods provide a quick and specific (to the species-level) detection (Váradi et al. 2017).
8 Electrokinetic Separation Methods
Capillary electrophoresis (CE)–MS merges the separation process of electrophoresis with MS detection. Comparing to GC and LC, it provides more efficient separation, faster analysis, allows for small volumes of sample required, inexpensive reagents, and separation of cations, anions, and uncharged molecules in one run. This method is applied to examine the metabolome of various bacteria, in which results were intriguing in the detection and quantification of numerous metabolite classes (Soga et al. 2002). CE lacks sensitivity due to the small sample volumes. At the same time, combined with MS, it has a limited number of accessible commercial libraries, and last but not least, decreased retention time reproducibility.
CE combined with capillary isoelectric focusing (CIEF) was used to isolate and identify bacteria species having different sizes and shapes (Armstrong et al. 1999). This experiment showed that intact biological cells could be successfully isolated via methods typically limited to macromolecules. Another combination is CE fused with fluorescence that can be utilized to monitor the separation process, operational conditions, and microbial dynamics regarding cell aggregation (Armstrong et al. 2002). The primary benefit of these methods is the capacity to control parameters (size, shapes, and charges) for isolation and detection.
Electrical field-flow fractionation (EIFFF) is an alternative method. It depends on the separation of sample components in a channel because the various electrical fields result in a distinct layer of each component. Two main walls of the channel are utilized by the EIFFF device to generate a difference in the potential, which allows for the separation of charges (Desai and Armstrong 2003).
9 Biosensors
Biosensors appear as the most promising devices for the detection of microorganisms. Biosensor-based methods are perceived to have great potential for further development (Arora et al. 2011; Velusamy et al. 2010). According to IUPAC, “a biosensor is a device which uses specific biochemical reactions mediated by isolated enzymes, immunosystems, tissues, organelles or whole cells to detect chemical compound usually by electrical, thermal or optical signals.” Antibodies, enzymes, and nucleic acids are commonly utilized as bio-receptors (Chen et al. 2017a).
Biosensors are divided into physical and chemical biosensors depending on the transducer used to detect the target analyte. Physical biosensors sense shifts in mass, resonance frequency, refractive index, fluorescence and are further categorized as optical and mechanical biosensors. Optical biosensors measure the analyte by its interaction with photons, such as fluorescence or phosphorescence emissions. Optical biosensors are divided into labeled and label-free. Mechanical biosensors detect the analytes by examining the shift in mass during the recognition stage. These sensors have several beneficial characteristics, such as no sample preparation step and label-free detection comparing to other sensors. The most frequently employed mechanical biosensors are quartz crystal microbalance or cantilever technology (Brindha et al. 2018).
Chemical biosensors detect the shifts in the chemical reactions during the interactions between the analytes and biorecognition elements. Chemical biosensors are further classified into electrochemical and biochemical sensors. Electrochemical biosensors analyze the differences in electrical properties, such as current, potential, or impedance at the electrode surface during the binding step. Based on the detection technique, electrochemical biosensors are categorized into labeled and label-free. Labels, such as enzymes, metal particles, or nanoparticles, are employed to target the analytes in labeled biosensors. In label-free biosensors, the attachment of biomolecules to the surface of the electrode cause shifts in electrical parameters. Electrochemical biosensors are categorized into amperometric, potentiometric, voltammetric, conductometric, and impedimetric (Gothandam 2018).
Analytes in biosensors range from ions and molecules, through nucleic acids and proteins, up to the whole viruses and bacteria. Biosensors can detect bacteria by targeting bacterial components, such as DNA, RNA, intracellular proteins, exotoxins. This method requires sample processing and additional reagents, which raises costs and time. An alternative method to detect bacteria is to target whole bacteria cells. This direct method does not require additional reagents, which is more suitable for quick and inexpensive point of care testing. For the whole bacteria detection, impedimetric and optical methods are frequently applied (Ahmed et al. 2014).
Even though biosensors are rapid and specific, they are not consistently applied in bacteria detection due to cost, limit of detection, complex matrix, and difficulty in detecting more than one bacteria simultaneously (Velusamy et al. 2010). Primarily, much depends on the chosen type of bioreceptor element that can be more or less sensitive to contaminants (Neethirajan et al. 2018).
10 Bacteriophage-Based Methods
10.1 Bacteriophages
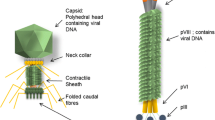
Bacteriophages are viruses that attach to particular bacterial receptor proteins to infect the host cells. Most known bacteriophages belong to Caudovirales, whose representatives are characterized by dsDNA genome and icosahedral, tailed capsid with the fibers attached to the tail (Ackermann 2007). The size of the virion is usually about 50–200 nm. However, some filamentous phages (e.g., M13) may reach even 400 nm length (Sharma et al. 2017). Recently, even bigger bacteriophages were discovered from marine water.
Based on their life cycles and means of propagation, phages are classified into two categories. Lysogenic (temperate or reductive) phages fuse their genetic materials into the bacterial genome and are inherited by daughter cells during binary fusion. Lytic (virulent or productive) phages undergo four steps process during infection: (1) binding to the receptors (protein or sugar moieties) of the bacterium due to host specificity properties; (2) injection of genomic materials into the cytoplasm of the bacteria; (3) viral replication via bacterial transcription, translation, and replication; (4) newly assembled phages leave the cell through bacterial lysis with the help of choline and endolysins proteins, causing the death of the host cell. This process is the basis of phage therapy for targeting pathogens (Kortright et al. 2019).
Bacteriophages are gaining recognition as a promising recognition element in the area of rapid detection of bacteria. Bacteriophages demonstrate advantageous qualities such as excellent specificity, robustness, toughness, and cheap preparation, making them popular biorecognition elements in biosensors and other assays for bacteria detection (Paczesny et al. 2020; Richter et al. 2018). The most crucial advantages of phage-based methods for bacteria detection are as follows:
-
Phages are ubiquitous and highly specific to bacteria (Koskella and Meaden 2013) but cause no major threat to humans (Tian et al. 2021).
-
Because of being “molecular parasites” (Breaker et al. 1994), phages need to infect a viable host to multiplicate by using its transcriptional machinery. This fact allows us to distinguish between living and dead bacteria, which is usually a significant issue for bacteria detection protocols. However, it may be phages absorbed on the surface of the dead cell (Krueger 1931).
-
Phages can self-amplify, which makes their “production” simpler and cheaper than, e.g., antibodies,
-
Phages targeting particular bacterial species may be isolated from various environments, such as hospital sewage water (Farooq et al. 2020), environmental water or sewage samples (Yan et al. 2017; Bhardwaj et al. 2016, 2017), or the soil (Cross et al. 2015). The isolation process is quick and cheap, can be provided in every biological laboratory without even identifying the isolates.
-
Phages display more shelf life due to their resistant nature to external factors, which decreases the environmental limitations and allows regeneration of the sensor surface (Zourob 2010).
-
Finally, phages are biological entities that evolve. This allows them to compete in the arms race with bacteria (de Jonge et al. 2019) and overcome developing resistance mechanisms. An intriguing example is the discovery of anti-CRISPR (Trasanidou et al. 2019).
Phage-based biosensors rely on two different approaches. The first one is the generation of the analytical signal upon the capturing of bacteria. These are usually surface-sensing elements or phage-based probes. Their main advantage is the speed of the analysis, yet a single event is difficult to detect. While being one of the major problems, this fact is responsible for relatively high detection limits (LOD). The sensitivity of these types of biosensors can be improved by using phage-based bioconjugates, layered sensors, and methods utilizing parts of phages without additional pre-incubation steps. This might be done by developments in biorecognition elements themselves (e.g., by ordering of phages within sensing layers) or by utilizing ultrasensitive transducers (e.g., optoelectronic-based).
Alternative designs are based on the infection of the target bacteria, which generates the measurable signal by affecting the cell’s metabolism (the release of progeny virions, products of reporter genes, or metabolites). These methods already showed some ultra-sensitivity, but they are lengthy. Schemes showing the phage-based approaches for bacteria detection are shown in Fig. 20.1.
Designs of most commonly used phage-based biosensors. (a) Bacteria detection by depositing phages on sensor surface or phage-based probes is rapid, but signal amplitude is usually low. On the contrary, (b) infecting bacteria and using its molecular mechanism increases sensitivity by producing progeny virions, expression of reporter genes, or releasing bacterial metabolites due to lysis. However, the process is time-consuming. The figure was inspired by (Paczesny et al. 2020) based on Creative Common CC BY 4.0 license
10.2 Methods Targeting Bacterial Metabolites
Certain metabolites can be used as target analytes in phage-mediated bacterial detection. These metabolites are discharged from the cytoplasm due to the cell membrane burst caused by the completion of the lytic cycle. Phage application allows more specificity and species-level recognition. For instance, T7 phage-based biosensor platform depended on the detection of β-galactosidase released during the lysis of bacterial cells. The released enzyme cleaved the substrate resorufin β-D-galactopyranoside resulting in a fluorescent product. This approach resulted in LOD of 10 CFU/mL E. coli BL21 within 8 h (Tilton et al. 2019). He et al. proposed a phage-affinity approach to detect P. aeruginosa based on bioluminescence detection of released intracellular adenosine triphosphate (ATP). The concentration of P. aeruginosa was determined via firefly luciferase-ATP bioluminescence reaction. The proposed method resulted in LOD of 2 × 102 CFU/mL. The separation and detection process required 2 h (He et al. 2017).
A further step for developing such an approach was introducing genetically modified phages to obtain both sensing and signal-generating elements. Reporter phages are genetically modified phages used as gene importers to inject a specific gene into the bacteria’s genome. The most suitable genes are fluorescent coding proteins or other easy-to-detect products expressed inside the host cells during the infection. Genes, such as bacterial lux (Loessner et al. 1996) or firefly luc (Lankes et al. 2007) bioluminescence genes, inaW gene-ice nucleation (Goodridge and Griffiths 2002), lacZ gene-β-galactosidase (Bremer et al. 1984), and Igfp gene (Poul and Marks 1999) were used as a reporter for various pathogens detection.
A review published by Pizarro-Bauerle and Ando (Pizarro-Bauerle and Ando 2020) presented the current state of the art of engineered bacteriophages’ practical applications. The report summarized the most recent applications of genetically modified phages in the clinical settings, food industry, agriculture, and material science. Here, we present the most significant reports consisting of biosensing using genetically modified phages.
A report from 2000 by Irwin et al. described the usage of Salmonella-targeting bacteriophage encoding ice nucleation protein (INP) to infect the bacteria. After supercooling with a phase-sensitive dye, the quantitative analysis of bacteria solutions was conducted. This allowed for the detection of Salmonella spp. with a minimum detectable level of about 2 CFU/mL within 3 h (Irwin et al. 2000).
Wisuthiphaet et al. developed an E. coli detection method by using a genetically modified T7-ALP phage that causes overexpression of alkaline phosphatase after the infection. The detection of E. coli BL21 bacteria was provided via fluorescence imaging of ELF-97 alkaline phosphatase substrate used to stain the bacteria retained on the filter. The LOD was around 102 bacteria per gram of model beverage and the time of analysis was about 6 h (Wisuthiphaet et al. 2019). Another recent publication by Wisuthiphaet et al. showed a rapid colorimetric pathogen detection method in a food matrix. T7 phage engineered with phoA gene was used to detect E. coli in food matrices. The technique consisted of phage-induced expression of an exogenous enzyme, alkaline phosphatase, incubation in nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) color dye, and filtration through a 0.2-micron polycarbonate membrane. Reported results showed LOD around 10 CFU/mL and 102 CFU/mL (coconut water and spinach leaves, respectively) within 5 h of enrichment and up to 2 h of incubation with phages (Wisuthiphaet et al. 2021).
A paper by Nugen et al. reported detection of E. coli using T7 containing NanoLuc luciferase expression cassette. This method requires the addition of luciferin NanoGlo substrate to detect the chemiluminescent signal (Pulkkinen et al. 2019). Modified phages were prepared by synthetic biology approach— PCR fragments and in vitro DNA assembly were used. This protocol provided relatively fast and straightforward preparation of modified phages. The LOD was about 5 × 102 CFU/mL after 2 h of incubation. Later on, the same research group proposed an approach to develop a new protocol for analysis of drinking water against generic E. coli (according to the US standards, there can be no “coliforms” in 100 mL of drinking water). They developed a phage-based membrane filtration approach by using luciferase and alkaline phosphatase for detection and quantification. 100 mL of drinking water sample was filtered on the cellulose filter. After 8 h of incubation, E. coli specific T7 phages carrying a reporter gene were added. In both cases, reporter genes were fused with genes encoding cellulose-specific carbohydrate-binding modules (CBM). After 1.5 h of incubation, enzymatic substrates were added to visualize the colonies. The overall time of this procedure was about 10 h, making it significantly faster than the plating method (24 h) and providing the most satisfying limit of detection of 1 CFU/100 mL (Hinkley et al. 2018a).
To make the analysis time shorter, the same group proposed another approach for detecting E. coli by using a genetically modified T7 coliphage carrying NanoLuc reporter gene. The authors managed to fuse NanoLuc with CBM and detected immobilized fusion protein resulted from a single CFU of E. coli. This approach enables rapid and low-cost detection of E.coli in 100 mL of the water sample. The final LOD was about 10 CFU/mL (Hinkley et al. 2018b). According to Wisuthiphaet, such detection limits can be acquired only in simple matrices. In complex matrices, the background signals may cover the signals from bacteria detection (Wisuthiphaet et al. 2019). Eventually, Hinkley et al. developed a rapid, selective, and sensitive phage-based detection by combining previously developed membrane filtration method along with phage infection to generate luminescence in the presence of E. coli. The proposed approach resulted in LOD around 20 CFU in 100 mL of water sample within 5 h (Hinkley et al. 2020).
Certain procedures can be done without any external substrates. Vinay and coworkers proposed the detection of E. coli and S. enterica ser. Typhimurium using HK620 and P22 phages with introduced gfp gene. Vinay et al. used genetically modified HK620 and P22 phages carrying gfp gene to detect E. coli and S. enterica Typhimurium by measuring fluorescence using flow cytometry. This method enabled rapid and sensitive detection as low as 10 cells/mL without enrichment procedure within 1 h (Vinay et al. 2015). Following research focused on the genetic modification of phages HK620 and HK97 to express the entire lux operon—luxAB genes coding luciferase and luxCDE coding fatty acids reductases. Although the reported LOD was not satisfying (104 bacteria/mL), incorporating the luxCDABE cassette into the COMBITOX instrument was successful. When upgraded, this instrument may become a useful tool for accommodating several bio-detector systems to detect bacteria, toxins, and heavy metals (Franche et al. 2017). A report published by Kim et al. presented quite a different approach for utilization of luxCDABE operon. Their phage-of-choice was phiV10 phage, targeting E. coli O157:H7. The amount of time required for the detection was 40 minutes, and the LOD of the biosensor was as low as 1 CFU/mL in the case of pure culture. For food samples, the LODs were 10 CFU/cm2 (romaine lettuce), 13 CFU/mL (apple juice), and 17 CFU/g (ground beef) (Kim et al. 2017). In 2016 Wu et al. fused the tetracysteine (TC)-tag with small outer capsid protein of the wild-type PP01 bacteriophage and used them to infect E. coli O157:H7 host cells. Then the progeny PP01-TC phages were fluorescently labeled, and a flow cytometry procedure was used to measure the fluorescence. The LOD in the complex fluid (apple juice) provided the LOD of 1 CFU/mL within 1 h (Wu et al. 2016). Recently, the same research group developed a rapid, sensitive, and multiplex detection method targeting E. coli O157:H7, S. typhimurium, and P. aeruginosa using dual-modified M13KE phage. The M13KE phage-displayed the targeting peptide on the minor coat protein pIII and the streptavidin-binding peptide on the major coat protein pVIII. The LOD of this method was 102 cells/mL in 40 mL of sample volume via flow cytometry (Wu et al. 2021).
Wang et al. proposed the electrochemical detection of E. coli using T7 phage expressing the lacZ gene encoding β-galactosidase. The substrate was 4-aminophenyl-β-galactopyranoside (4-APG). 4-APG forms an electroactive product when cut by β-galactosidases. This product was detected by differential pulse voltammetry. The detection limit was in the range of 102 CFU/mL within 7 h (Wang et al. 2017).
FASTPlaqueTB assay for detecting Mycobacterium tuberculosis in sputum uses the lytic virulence of the phages as a “sensor.” Phage particles containing a luciferase gene are commonly used as reporters due to the highly sensitive detection of the bioluminescent signal luciferase generates. In addition, green fluorescent protein (GFP) and several other reporter genes are considered suitable. GFPs retain major advantages, such as high stability, low toxicity, and the fact that fluorescence is triggered by excitation light, eliminating the additional substrate as required for luciferases (Harada et al. 2018).
10.3 Detection of Progeny Virions
Bacteriophages offer a “built-in” amplification system—after the infection of the host cell and multiplication using its translational machinery, progeny virions destroy the host cell and are released. These progeny virions can be used for the detection of bacteria. Such amplification improves the detection limit, for much more objects can be detected.
To provide a faster time of analysis and better sensitivity, phage amplification methods for detecting progeny virions are usually combined with the PCR technique. Luo and others developed an assay that allows detection of 10 CFU Acinetobacter baumannii in 100 μL serum within 4 h without DNA extraction and purification process by using phage p53 at the concentration of 103 PFU/mL (Luo et al. 2018). Later, the same group used qPCR combined p53 phage recognition of A. baumannii LB8 isolated from sputum samples. They designed the primer pairs to recognize the phage or the bacteria. It allowed for a detection limit of around 1 CFU/mL within 6 h (Luo et al. 2020). A novel bacteria detection method for Salmonella Enteritidis based on phage vB_SenS_PVP-SE2 and qPCR was developed by Garrido-Maestu. The proposed method required 10 h to obtain LOD of 8 CFU in 25 g of chicken meat (Garrido-Maestu et al. 2019). Sergueev reported the detection of zoonotic bacteria Brucella abortus in mixed cultures and blood samples with the LOD of around 1 CFU/mL within 72 h (Sergueev et al. 2017). One of the most spectacular examples was published by Anany et al. (Anany et al. 2018), who developed a rapid and inexpensive method for constructing phage-based bioactive paper by utilizing inkjet printing to detect foodborne pathogens. The reported LOD of this dipstick assay was between 10 and 50 CFU/mL within 8 h.
Mido et al. proposed combining phage amplification with immunoassay protocol. Progeny MS2 phages coupled with specific antibodies were immobilized on the surface of magnetic beads. Upon the addition of the detector antibody, binding to MS2, the fluorescence was measured. A fluorescence-based method allowed for detection after 3 h of incubation with the LOD of around 102 cells/mL (Mido et al. 2018).
Phage titration is the most archaic and the simplest approach to detect and count progeny virions. Specific amounts of phages solution with different concentrations are dropped onto the agar plate with bacteria. Bacteria get lysed where virions are presented, which is visible as the holes in the bacterial layer. These holes are called plaques, and they mark the number of phages in the stock solution. Said et al. used this method to observe changes in Salmonella typhi pathogen during nutritional deficiency circumstances. The phage infectivity rate was much more suitable than the traditional plate culturing technique. This method enabled the detection of active pathogens, which are normally undetectable by conventional approaches (Ben et al. 2019). In 2006 Ulitzur and Ulitzur reported the usage of mutant phages (mutants that cannot form plaques at concentrations lower than their reversion rate and temperature-sensitive mutants) as a method for bacteria detection and determination of their antibiotic susceptibility. The method is based on plaque formation as the endpoint of the phage lytic cycle. The LOD ranged between 1 and 10 viable bacteria cells within 3–5 h (Ulitzur and Ulitzur 2006). Jassim and Griffiths reported an interesting P. aeruginosa detection method using Pseudomonas Phage NCIBM 10116 for standard plague counting method combined with live/dead fluorescent measurement. This resulted in the highly specific analysis in a reasonable period (1 cell/mL within 4 h) that allows viable cells monitoring (Jassim and Griffiths 2007).
10.4 Utilization of Whole Virions for Bacteria Detection for Biosensors
There are disadvantages to approaches depending on the completion of the phage lytic cycle. First, such methods require choosing only the virulent phages. Moreover, progeny phages are generated in rare cases while prophages are integrated into the host’s gene. Finally, bacterial phage-infections-preventing mechanisms, such as CRISPR-Cas, influence the process (Faure et al. 2019). Also, methods relying on genetically modified phages have some drawbacks that need to be considered. Reporter phage-based methods require deep understanding and expertise. It is also necessary to continuously adjust and enhance approaches depending on the target bacteria. The infection rate of genetically modified phages tends to be weaker than wild types (Pires et al. 2016). Finally, the release of genetically modified phages into the environment could cause unpredictable effects on the biosphere (Bárdy et al. 2016).
All this resulted in developing phage-based methods for bacteria detection, which generates an analytical signal upon capturing target cells. This can be done both in bulk via bioconjugates (cf. following section) and at the surface (Fig. 20.1).
A transducer is an essential part of a sensor that detects and converts signals from bio-receptors into measurable signals. The contact between the target analyte (here bacteria) and the surface is necessary for many analytical techniques, e.g., SERS, microbalance-based, magnetoelastic-based, or electrochemical methods. Phages immobilized at the surface must maintain their infectivity, binding affinity, and selectivity. The most frequently applied immobilization techniques include adsorption, entrapment, cross-linking, covalent coupling, and affinity. Physical adsorption is the fastest and simplest way, but there is the risk of desorption and low surface coverage. Hence, for phage immobilization purposes covalent bonding method is considered more favorable.
Richter et al. and Zhou et al. developed a phage deposition method on an electroconductive sensing layer. Experiments were carried out based on the surface charge and dipole moment of phage particles. The application of an alternating electric field on the sensing layer enabled head down–tails up orientation of phages considering the negative charges of the capsid. T4 phage was successfully oriented on a gold surface by utilizing an electric field coupled with chemical modification of the sensing layer. Chemical attachment of bacteriophage onto the biosensor surface considerably increases the overall detection’s stability and performance (Harada et al. 2018; Richter et al. 2016, 2017; Zhou et al. 2017).
Recently the number of reports on electrochemical approaches for bacteria detection increases rapidly (Farooq et al. 2020; Wang et al. 2017; Zhou et al. 2017; Neufeld et al. 2003; Neufeld et al. 2005; Li et al. 2018; Sedki et al. 2020; Xu et al. 2020). Electrochemical methods offer satisfying sensitivity, low-cost analysis, a vast field of possibilities for miniaturization. Here we present a couple of the latest reports on bacteriophage-based electrochemical methods for bacteria detections (Ferapontova 2020; Xu et al. 2019; Janczuk-Richter et al. 2019).
Sedki et al. described utilization of M13 phage immobilized on the electrodes combined with electrochemical impedance spectroscopy to target coliforms with the LOD of around 14 CFU/mL within 30 min (Sedki et al. 2020). This research presents a single phage balance highly specific with a wide range of hosts—multiple strains of E. coli can be detected, while there is no response to non-E. coli bacteria. Niyomdecha et al. used M13 phages displaying Salmonella-specific peptide immobilized on the electrode and applied it into a capacitive flow injection system. Their sensor provided measurements with sensitivity ranging from 2 × 102 to 1 × 107 CFU/mL within 40 min. The sensor was reusable up to 40 times, thanks to the alkaline eluting solution (Niyomdecha et al. 2018). Recently the review of available M13 phage-based biosensors was published (Moon et al. 2019).
Yue et al. developed a rapid and sensitive electrochemiluminescent (ECL) bioassay using highly specific virulent phage PaP1 as biorecognition element for detecting P. aeruginosa without employing labels. Glass carbon electrode was covered with phage-fused carboxyl graphene and luminol used as a source of chemiluminescence. This approach resulted in LOD of 56 CFU/mL and the amount of time required for detection was 30 minutes (Yue et al. 2017).
Xu et al. detected viable bacteria by chemically immobilizing T4 bacteriophages on the surface of the extended gate connected to a metal oxide semiconductor field-effect transistor (MOSFET) device. The obtained LOD was around 14 CFU/mL within 35 min (Xu et al. 2020).
An alternative approach is magnetoelastic biosensors. Mass sensitivity is a crucial measurement criterion for magnetoelastic biosensors. The amplitude of vibrations changes when the analyte is deposited on the sensing surface. The first sensor prepared according to this protocol, targeting methicillin-resistant S. aureus (MRSA) strain, reached the limit of detection of 3 × 103 CFU/mL within 30 min (Hiremath et al. 2015). In 2017 the same research group confirmed their sensor was detecting MRSA strain even in the presence of other competing bacteria (Hiremath et al. 2017). Chen et al. (2017b) and Mack et al. (2017) described the detection of S. enterica and S. typhimurium at the surface of chicken and lettuce, respectively.
Recently, Halkare et al. developed a new label-free method for detecting E. coli B40, using T4 phages as biorecognition elements on a plasmonic fiber-optic platform. The novelty of this method relies upon capturing the analyte before subjecting the sensing layer to bacteriophages. Application of this method resulted in detection concentration of 103–107 CFU/mL in environmental matrices within less than 4.5 h with high specificity to only E. coli B40 (Halkare et al. 2021).
Srivastava’s group proposed to use the sensing layers based on bacteriophages in SERS-based sensors. T4 phages were immobilized on a silicon platform along with the thin silver film. The authors reported the detection of E. coli in the concentration of 1.5 × 102 CFU/mL (Srivastava et al. 2015). In 2018 Rippa et al. immobilized bacteriophages on the surface of a substrate made of plasmonic nanocavities, obtaining the plasmonic quasicrystals (Rippa et al. 2018). The same group presented the meta structures for SERS-based detection, conjugated with Tbilisi bacteriophages targeting Brucella spp. With the sensitivity on the single-cell level, within 1 h, the authors were able to detect bacteria in the concentration of over 104 CFU/mL (Rippa et al. 2017). Lai et al. obtained an approximate LOD in detecting Bacillus spp. with gamma phages (Lai et al. 2017).
10.5 Phage-Based Bioconjugates
The micromolar concentration of a chemical compound makes over 1010 molecules or ions to be detected in every single mL. The situation looks quite different for bacteria when the need is to detect single cells in relatively large volumes (with the goal of 1 CFU/mL). Because of the small number of “objects,” the number of signal-generating events is low. The other problem is the relatively low probability of the attachment of bacteria to the sensing surface covered with immobilized phages and long search time.
Moving from the detection at the surface towards the bulk solves these issues. Bioconjugates offer shorter search time, more capturing events, and a broader range of analytical techniques for signal acquisition. The application of P9b phage conjugated with the gold nanoparticles in the SERS protocol for the detection of P. aeruginosa was reported in 2020 (Franco et al. 2020). Gold nanoparticles solution can also be used to prepare a colorimetric sensor because its color changes the aggregation. Another important feature of gold is that it covalently combines with the thiols (-SH) (Li et al. 2006). In the research by Peng and Chen, M13 phages were chemically modified to exposed SH groups. Phages were also genetically modified to display the receptors against different species of bacteria (i.e., P. aeruginosa, Vibrio cholerae, Xanthomonas campestris). The pellet of centrifuged bacteria with bounded M13 phages was resuspended in a buffer containing gold nanoparticles (AuNPs). AuNPs got attached to thiol groups presented on the viral capsids. The presence of phages in the pellet resulted in the change of color of gold nanoparticles solution, indirectly confirming the presence of target bacteria. The procedure took about 30 min and provided the limit of detection around 102 cells/mL (Peng and Chen 2019). SiO2@AuNP nanoparticles were also used to get bacteriophages immobilized on their surface. Darkfield microscopy was chosen for the analysis of the conjugates. While attached to S. aureus SA27 cells, the conjugates got aggregated, which caused a strong light scattering. The authors reported a detection limit of S. aureus of around 8 × 104 CFU/mL in up to 20 min (Imai et al. 2019).
Janczuk et al. proposed the utilization of bifunctional T4 phage-based bioconjugates to first magnetically separate target bacteria and then enumerate them using flow cytometry. The reported LOD of E.coli was around 104 CFU/mL. The separation protocol and flow cytometry analysis took about 15 minutes (Janczuk et al. 2017). Yan et al. combined bacteriophages deposited on the magnetic particles with immunoassay, targeting S. aureus in complex fluid (apple juice). This approach resulted in a LOD of around 9 × 103 CFU/mL within 90 min without any pre-enrichment (Yan et al. 2017).
Metal-organic frameworks (MOF) crystallites were used to generate the analytical signal in phage-based bioconjugates. IRMOF-3 (Zn4O(NH2-BDC)3) (BDC = benzene-1,4-dicarboxylic acid) and NH2-MIL-53(Fe) (MIL = Matériaux de l′Institut Lavoisier) are examples of fluorescent MOFs. When conjugated to phages, they were used as probes for Staphylococcus arlettae and S. aureus detection (Bhardwaj et al. 2016, 2017). Upon capturing bacteria, MOF crystallites were partially concealed, and less excitation energy reached them. This resulted in a decrease in the fluorescence signal, which was reversely related to the number of bacteria in the sample. The obtained LODs were 102 CFU/mL and 31 CFU/mL, respectively.
Farooq et al. developed a highly efficient sensing surface integrating bacterial cellulose and carboxylated multiwalled carbon nanotubes (c-MWCNTs) with immobilized phages targeting S. aureus. They achieved the LOD of 3–5 CFU/mL within 30 min, which is the best balance between LOD and time for now-on, and were able to distinguish living and dead bacterial cells (Farooq et al. 2020).
Particles were also used as carriers for active compounds to be detected. In the interesting example, Cu3(PO4)2 nanoflowers were loaded with glucose oxidase, horseradish peroxidase, thionine, then gold nanoparticles were incorporated, and finally, T4 phages were attached to these gold nanoparticles. The detection process occurred at the surface of the electrode utilizing differential pulse voltammetry. First, bacteria were non-specifically immobilized at the surface. Loaded nanoflowers are bound only to bacteria selected by the used phages. In the vicinity of the electrode, the cascade of the electrochemical reactions involving electroactive compounds carried by nanoflowers occurred, resulting in signal generation. The LOD of this method was in the range of 1 CFU/mL and the time of analysis within 140 min (Li et al. 2018).
10.6 Parts of Virions as Sensing Elements
There are particular issues with whole-phage biosensors as sensing elements. First, the size of the virions marks the miniaturization limits. For instance, magnetophoretic separation requires the sub-micrometer size of virions conjugated with magnetic particles to maintain efficiency. The techniques such as surface plasmon resonance also need the binding to take place in the proper spacing from the transducer. Analytical signals can be hindered due to the size of phages that create a distance that is too long to be examined. Finally, lytic bacteriophages eventually destroy target bacterial cells, making prolonged analysis almost impossible. The first bacteria to be bounded may be lysed even before the end of the procedure.
These problems can be solved by preparing biosensors using only the bacteria-capturing parts of the virions. The recombinant tail fiber protein (P069) was used to detect P. aeruginosa in two approaches. The first one relied on the conjugation of P069 protein with the magnetic beads, then on adding them to the target bacteria in the sample. After the incubation and the magnetic separation, cells were washed and then lysed. Bacteria were detected indirectly, based on the amount of released ATP, recognized by using the bioluminescence protocol. In the second approach, the solid substrate was covered with the P069 fused with the fluorescent marker. The resulting parameters of the analyses were the following: the LOD of 6.7 × 102 CFU/mL within 60 min for the bioluminescent methods and LOD of 1.7 × 102 CFU/mL within 80 min (He et al. 2018).
Another solution made the cell-binding domain (CBD) of P108 bacteriophage fused with GFP for recognition of MRSA strains (Wang et al. 2020). Target cells were separated by magnetic beads conjugated with CBD and incubated. The analysis was provided with flow cytometry protocol. The procedure provided the LOD of around 40 CFU/mL and a time of analysis of about 1 h. The efficiency of CBD-GFP protein was compared with GFP-CTP1L-bacteriophage endolysin targeting Clostridium tyrobutyricum (Gómez-Torres et al. 2018). The GFP-CTP1L protein allowed for the recognition of 17 of 20 examined Clostridium strains. The suggested method also detects the clostridial spores in the sample.
Recently, Cunha et al. reported the development of a method as sensitive as magnetoresistive sensors, as portable as a lab-on-chip platform, and with the specificity comparable to the phage receptor-binding proteins. This method used protein gp18, a phage RBP, to detect E. faecalis I809 and protein gp109 for detecting S. aureus Sa12. In the case of both examined bacterial strains, the detection limit was about 10 CFU/mL within less than 2 h (Cunha et al. 2021).
Braun et al. created an approach for detecting pathogens by developing a single-tube centrifugation assay that simplifies the analysis of suspect colonies. Two types of enzyme-linked phage RBP assay (ELPRA) were used to identify vegetative cells of B. anthracis. Counting from the moment of colony collection, this assay can be completed within less than 30 min. The assay for now-on is rather qualitative than quantitative—allows to distinguish if B. anthracis spores were present in the sample (Braun et al. 2021).
10.7 Phage Display Method
Another aspect of phage-based recognition is a method developed by George P. Smith in the 80s, known as phage display (Smith 1985). This method allows using phages as universal recognition elements (not only for bacteria detection) instead of antibodies. The usage of antibodies is relatively expensive because of their preparation, and very often, the specificity of these sensors is not satisfying enough. G. P. Smith was the first one to obtain phages displaying specific peptides on the surface (Burton 1995).
Filamentous phages (M13, f1, or fd) are usually used in phage display (Ebrahimizadeh and Rajabibazl 2014), with several examples of using icosahedral phages (e.g., T4 or T7). Several types of filamentous phage-based phage display can be distinguished. Their classification is based on the surface protein used, i.e., pIII or pVIII (Nemudraya et al. 2016). These proteins were chosen because of their location in the virion and presence in a couple of many copies. pIII protein is located in the distal part of the virion in the number of copies of 3–5, while pVIII is present in about 2700 copies and is a major protein building viral capsid. Also, both of these proteins have an N-terminal signal sequence, so a foreign peptide sequence can be placed between the signal peptide and actual pIII/pVIII protein, forming the transcriptional fusion (Nemudraya et al. 2016).
The purpose of the phage display is to obtain a library of bacteriophages expressing various peptides. A library is defined as a heterogeneous mixture of phage clones carrying different genetic inserts (Burton 1995). First, a surface needs to be covered with objects to be detected. Then, bacteriophages with inserted sequences of random oligopeptides or proteins are incubated with their target. Once the incubation is over, unbound/unspecific virions are washed off. At the same time, bound phages are eluted and amplified (Nemudraya et al. 2016). Figure 20.2 presents this procedure schematically.
The scheme presents the biopanning of phage display library. Adapted from the ref. (Paczesny and Bielec 2020) on the Creative Common CC BY 4.0 license
This approach made a pathway to the alternative for using antibodies, so-called phage antibodies—phages with a domain of chosen oligopeptide or protein displayed. Because obtaining phage displaying molecule is a routine procedure, within a short time, it is possible to distribute a library of virions expressing numerous types of antibodies, making the research quicker and cheaper (Petrenko and Vodyanoy 2003).
Phage display is also a solution for one of the greatest limitations of phage therapy—bacteria getting resistant to phage infections (Labrie et al. 2010). When treated with the same bacteriophage, bacteria strain would eventually become resistant to infections by this particular phage to improve fitness. A phage requires a virulence factor, which is a surface receptor, lipopolysaccharide, pili, or secretion system for a successful infection (Nobrega et al. 2018). Phage display provides the selection of virions able to infect phage-resistant bacteria. Moreover, the correlation between phage and antibiotic sensitivity was observed. This phenomenon involves two strategies for fighting multidrug resistant bacteria strains—directly killing them by bacteriophages or using bacteriophages to make bacteria antibiotic-sensitive again (Kortright et al. 2019).
Once improved, phage display, formerly designed for molecular biology, became an attractive alternative approach for traditional blood morphology tests and in vivo blood analyses. One of the first came the antibodies targeting the antigens determining the blood types, which are anti-AB0 and anti-Rh antibodies (Marks et al. 1993). Antibodies against the cluster of differentiation (CD, AITP, GPIa, GPIII antigens, or 11-dehydro-thromboxane B2 cloning factor) were obtained. Another application is the diagnostic of immune diseases. In this field, the most important are antiTNFα or anti-CD52 antibodies, but also antibodies targeting some neurological disorders or tumors. Tumor targeting bacteriophage-based particles were already developed for various types of cancer diseased, e.g., B-cell lymphoma, colon, breast, or thyroid cancers (Bazan et al. 2012).
Also, modified bacteriophages were used as the nanocarriers for tumor-associated antigens (TAAs) or TAA-mimic molecules. The importance of this application is that, in general, tumors produce immunosuppressing factors that inhibit the immunological response. TAAs and TAA-mimic molecules are presented to immune system agents, first by exposure to the MHC (major histocompatibility complex), then through CD4+ and CD8+ T lymphocytes to B lymphocytes to induct cytotoxic response via production of TAA-specific antibodies (Goracci et al. 2020). TAA-displaying phages are sometimes considered anti-cancer vaccines (Fig. 20.3). It is also possible to modulate the activity of immune cells, CD11c/CD18 (integrin αXβ2) from the surface of APC (antigen-presenting cells) were fused with 12xhistidine tag and crosslinked to liposomes, creating the artificial tumor cells. By treating patients that way resulted in regression of primary cancer (Goracci et al. 2020).
Illustration of humoral response to tumor cells initiated by using tumor-associated antigens (TAA) -displaying bacteriophages. Phages displaying TAA are considered anti-cancer vaccines. The figure is inspired by (Goracci et al. 2020) on the Creative Common CC BY 4.0 license
10.8 Phage-Based Detection of Other Analytes
The detection capabilities of bacteriophages are not limited to just bacteria. They can be used to detect small molecules, such as ions (Yang et al. 2018) or organic compounds (Yoo et al. 2020). The report from Kim et al. (Kim et al. 2020) suggested the applications of bacteriophages to detect medical chemicals. The M13 phage variants were used: a wild-type virus and two displaying specific peptides changing the hydrophobic/hydrophilic balance of virions. A color pattern made an additional value for the determination of the response. This colorimetric method allowed the detection of endocrine-disrupting chemicals and some chemicals in a gaseous state (Yoo et al. 2020). Bacteriophages may also be used as indicators of pollution (Armon and Kott 1996). Many bacteriophages have the survival time related to the survival time of human viruses. Therefore they might be used to estimate the index of viral pollution in both air and water samples, e.g., to determine the drinking water quality.
11 Summary
Nowadays, there are a great many bacteria-sensing methods. The trick is to choose the one combining a short analysis time, high specificity, and detection limit as low as possible. Standard and the most often used detection methods require the isolation of the target bacteria, then culturing stage, usually followed by biochemical confirmation. They provide straightforward, cheap, and reliable protocols for bacteria identification. However, require trained operators, laboratory space, and up to a few days to obtain reliable results. Introducing new detection techniques to the standard diagnostics procedures may solve some of these problems. New molecular and instrumental techniques are advertised as extremely sensitive (e.g., PCR), label-free (e.g., SERS-based methods), or quick and easy to do (e.g., microscopic methods). But neither is universal, and each has some significant drawbacks, limiting its applicability. Most of them generate costs because they require expensive and sophisticated equipment and skilled personnel. The risk of false-positive results caused by the presence of the dead bacterial cells accompanies the nucleic acid-based and MS methods. Moreover, the PCR protocol for detecting the target bacteria requires particular probes (primers). Some mutations would prevent the pairing of the probes to the target DNA sequence, causing the failure of the identification. The availability of specific antigens limits immune-based tests. Therefore, various types of biosensors are becoming a valid alternative for molecular methods (Chen et al. 2017a). Among them, bacteriophage-based methods seem one of the most promising.
The critical milestone to be achieved in bacteria detection is developing the method that works in complex samples while providing the LOD of about 10 CFU/mL or less and the time of the analysis of 1 h or less (Paczesny et al. 2020). This is because 10 CFU/mL in the blood is a mark of sepsis in neonates (Opota et al. 2015). Because this is a stage of severe sepsis that may rapidly transform into septic shock, the only one-hour analysis would be efficient before using the antibiotics (Alam et al. 2018). The following goal will be to reach the detection limit of below 1 CFU/100 mL within a one-shift period (around 8 h). This is crucial for online analysis of drinking water (Hinkley et al. 2020).
Phage-based method might provide such characteristics. There are already reports showing some important advancements. LOD of 1 CFU per 100 mL was already reported in 2003 (Neufeld et al. 2003). Protocol taking only 30 min and allowing for LOD of 3–5 CFU/mL was developed in 2020 (Farooq et al. 2020). However, the main challenge in front of researchers working in the field is to bring phage-based methods to the market. According to our best knowledge, the Sample6 DETECT HT System (Microbiologique) is the only commercially available product. Scientists need to focus also on other factors, including copiability, compactness, user-friendliness, acuteness, price, and movability. All these features are not necessary to produce scientific publications but are crucial from the application point of view to solve the socioeconomic problem of bacterial infections.
References
Ackermann H-W (2007) 5500 phages examined in the electron microscope. Arch Virol 152:227–243. https://doi.org/10.1007/s00705-006-0849-1
Ahmed A, Rushworth JV, Hirst NA, Millner PA (2014) Biosensors for whole-cell bacterial detection. Clin Microbiol Rev 27:631–646. https://doi.org/10.1128/CMR.00120-13
Alam N, Oskam E, Stassen PM et al (2018) Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med 6:40–50. https://doi.org/10.1016/S2213-2600(17)30469-1
Alexandrakis D, Brunton NP, Downey G, Scannell AGM (2012) Identification of spoilage marker metabolites in Irish chicken breast muscle using HPLC, GC-MS coupled with SPME and traditional chemical techniques. Food Bioprocess Technol 5:1917–1923. https://doi.org/10.1007/s11947-010-0500-8
Anany H, Brovko L, El Dougdoug NK et al (2018) Print to detect: a rapid and ultrasensitive phage-based dipstick assay for foodborne pathogens. Anal Bioanal Chem 410:1217–1230. https://doi.org/10.1007/s00216-017-0597-y
Armon R, Kott Y (1996) Bacteriophages as indicators of pollution. Crit Rev Environ Sci Technol 26:299–335. https://doi.org/10.1080/10643389609388494
Armstrong DW, Schulte G, Schneiderheinze JM, Westenberg DJ (1999) Separating microbes in the manner of molecules. 1. Capillary electrokinetic approaches. Anal Chem 71:5465–5469. https://doi.org/10.1021/ac990779z
Armstrong DW, Girod M, He L et al (2002) Mechanistic aspects in the generation of apparent ultrahigh efficiencies for colloidal (microbial) electrokinetic separations. Anal Chem 74:5523–5530. https://doi.org/10.1021/ac025726n
Arora P, Sindhu A, Dilbaghi N, Chaudhury A (2011) Biosensors as innovative tools for the detection of food borne pathogens. Biosens Bioelectron 28:1–12. https://doi.org/10.1016/j.bios.2011.06.002
Bárdy P, Pantůček R, Benešík M, Doškař J (2016) Genetically modified bacteriophages in applied microbiology. J Appl Microbiol 121:618–633. https://doi.org/10.1111/jam.13207
Bazan J, Całkosiñski I, Gamian A (2012) Phage displaya powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccines Immunother 8:1817–1828. https://doi.org/10.4161/hv.21703
Beckmann BM, Grünweller A, Weber MHW, Hartmann RK (2010) Northern blot detection of endogenous small RNAs (~14 nt) in bacterial total RNA extracts. Nucleic Acids Res 38:2–11. https://doi.org/10.1093/nar/gkq437
Ben SM, Ben SM, Achouri F et al (2019) Detection of active pathogenic bacteria under stress conditions using lytic and specific phage. Water Sci Technol 80:282–289. https://doi.org/10.2166/wst.2019.271
Bhardwaj N, Bhardwaj SK, Mehta J et al (2016) Bacteriophage conjugated IRMOF-3 as a novel opto-sensor for: S. arlettae. New J Chem 40:8068–8073. https://doi.org/10.1039/c6nj00899b
Bhardwaj N, Bhardwaj SK, Mehta J et al (2017) MOF-bacteriophage biosensor for highly sensitive and specific detection of staphylococcus aureus. ACS Appl Mater Interfaces 9:33589–33598. https://doi.org/10.1021/acsami.7b07818
Bone U, Steller E, Warner JF, et al (2017) Chapter 14: Microsatellite analysis for identification of individuals, vol 1606, pp 205–217. https://doi.org/10.1007/978-1-4939-6990-6
Braun P, Rupprich N, Neif D, Grass G (2021) Enzyme-linked phage receptor binding protein assays (Elpra) enable identification of bacillus anthracis colonies. Viruses 13:1462. https://doi.org/10.3390/v13081462
Breaker RR, Banerji A, Joyce GF (1994) Continuous in vitro evolution of bacteriophage RNA polymerase promoters. Biochemistry 33:11980–11986. https://doi.org/10.1021/bi00205a037
Bremer E, Silhavy TJ, Weisemann JM, Weinstock GM (1984) λ placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol 158:1084–1093. https://doi.org/10.1128/jb.158.3.1084-1093.1984
Brindha J, Chanda K, Balamurali MM (2018) Biosensors for pathogen surveillance. Environ Chem Lett 16:1325–1337. https://doi.org/10.1007/s10311-018-0759-y
Burton DR (1995) Phage display. Immunotechnology 1:87–94. https://doi.org/10.1016/1380-2933(95)00013-5
Buszewski B, Rogowska A, Pomastowski P et al (2017) Identification of microorganisms by modern analytical techniques. J AOAC Int 100:1607–1623. https://doi.org/10.5740/jaoacint.17-0207
Buzby JC, Roberts T (2009) The economics of enteric infections: human foodborne disease costs. Gastroenterology 136:1851–1862. https://doi.org/10.1053/j.gastro.2009.01.074
Castañer O, Schröder H (2018) Response to: comment on “The gut microbiome profile in obesity: a systematic review”. Int J Endocrinol 2018:9109451. https://doi.org/10.1155/2018/9109451
Cecchini F, Iacumin L, Fontanot M et al (2012) Identification of the unculturable bacteria Candidatus arthromitus in the intestinal content of trouts using dot blot and southern blot techniques. Vet Microbiol 156:389–394. https://doi.org/10.1016/j.vetmic.2011.11.020
Chen J, Andler SM, Goddard JM et al (2017a) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46:1272–1283. https://doi.org/10.1039/c6cs00313c
Chen I-H, Horikawa S, Bryant K et al (2017b) Bacterial assessment of phage magnetoelastic sensors for Salmonella enterica Typhimurium detection in chicken meat. Food Control 71:273–278. https://doi.org/10.1016/j.foodcont.2016.07.003
Christian MD (2013) Biowarfare and bioterrorism. Crit Care Clin 29:717–756. https://doi.org/10.1016/j.ccc.2013.03.015
Cimiotti JP, Aiken LH, Sloane DM, Wu ES (2012) Nurse staffing, burnout, and health care-associated infection. Am J Infect Control 40:486–490. https://doi.org/10.1016/j.ajic.2012.02.029
Clausen CH, Dimaki M, Bertelsen CV et al (2018) Bacteria detection and differentiation using impedance flow cytometry. Sensors (Switzerland) 18:1–12. https://doi.org/10.3390/s18103496
Cross T, Schoff C, Chudoff D et al (2015) An optimized enrichment technique for the isolation of Arthrobacter bacteriophage species from soil sample isolates. J Vis Exp 2015:1–9. https://doi.org/10.3791/52781
Cunha AP, Henriques R, Cardoso S et al (2021) Rapid and multiplex detection of nosocomial pathogens on a phage-based magnetoresistive lab-on-chip platform. Biotechnol Bioeng 118:3164–3174. https://doi.org/10.1002/bit.27841
de Jonge PA, Nobrega FL, Brouns SJJ, Dutilh BE (2019) Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol 27:51–63. https://doi.org/10.1016/j.tim.2018.08.006
Desai MJ, Armstrong DW (2003) Separation, identification, and characterization of microorganisms by capillary electrophoresis. Microbiol Mol Biol Rev 67:38–51. https://doi.org/10.1128/mmbr.67.1.38-51.2003
Dige I, Nilsson H, Kilian M, Nyvad B (2007) In situ identification of streptococci and other bacteria in initial dental biofilm by confocal laser scanning microscopy and fluorescence in situ hybridization. Eur J Oral Sci 115:459–467. https://doi.org/10.1111/j.1600-0722.2007.00494.x
Doern CD, Butler-Wu SM (2016) Emerging and future applications of matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in the clinical microbiology laboratory: a report of the Association for Molecular Pathology. J Mol Diagnostics 18:789–802. https://doi.org/10.1016/j.jmoldx.2016.07.007
Ebrahimizadeh W, Rajabibazl M (2014) Bacteriophage vehicles for phage display: biology, mechanism, and application, pp 109–120. https://doi.org/10.1007/s00284-014-0557-0
(2009) ECDC/EMEA joint technical report “The bacterial challenge: time to react”
Elsland D, Neefjes J (2018) Bacterial infections and cancer. EMBO Rep 19:1–11. https://doi.org/10.15252/embr.201846632
Farooq U, Ullah MW, Yang Q et al (2020) High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens Bioelectron 157:112163. https://doi.org/10.1016/j.bios.2020.112163
Faure G, Shmakov SA, Yan WX et al (2019) CRISPR–Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol 17:513–525. https://doi.org/10.1038/s41579-019-0204-7
Ferapontova EE (2020) Electrochemical assays for microbial analysis: how far they are from solving microbiota and microbiome challenges. Curr Opin Electrochem 19:153–161. https://doi.org/10.1016/j.coelec.2019.12.005
Ferone M, Gowen A, Fanning S, Scannell AGM (2020) Microbial detection and identification methods: bench top assays to omics approaches. Compr Rev Food Sci Food Saf 19:3106–3129. https://doi.org/10.1111/1541-4337.12618
Fox A (2006) Mass spectrometry for species or strain identification after culture or without culture: past, present, and future. J Clin Microbiol 44:2677–2680. https://doi.org/10.1128/JCM.00971-06
Franche N, Vinay M, Ansaldi M (2017) Substrate-independent luminescent phage-based biosensor to specifically detect enteric bacteria such as E. coli. Environ Sci Pollut Res 24:42–51. https://doi.org/10.1007/s11356-016-6288-y
Franco D, De Plano LM, Rizzo MG et al (2020) Bio-hybrid gold nanoparticles as SERS probe for rapid bacteria cell identification. Spectrochim Acta Part A Mol Biomol Spectrosc 224:117394. https://doi.org/10.1016/j.saa.2019.117394
Franco-Duarte R, Černáková L, Kadam S et al (2019) Advances in chemical and biological methods to identify microorganisms—from past to present. Microorganisms 7:130. https://doi.org/10.3390/microorganisms7050130
Frickmann H, Zautner AE, Moter A et al (2017) Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review. Crit Rev Microbiol 43:263–293. https://doi.org/10.3109/1040841X.2016.1169990
Galluzzi L, Magnani M, Saunders N et al (2007) Current molecular techniques for the detection of microbial pathogens. Sci Prog 90:29–50. https://doi.org/10.3184/003685007780440521
Garrido-Maestu A, Fuciños P, Azinheiro S et al (2019) Specific detection of viable Salmonella Enteritidis by phage amplification combined with qPCR (PAA-qPCR) in spiked chicken meat samples. Food Control 99:79–83. https://doi.org/10.1016/j.foodcont.2018.12.038
Global Hospital-Acquired Infections (HAI) market size to grow at a CAGR of 3.6% 2021 to 2030 (2021) https://www.globenewswire.com/news-release/2021/07/12/2261025/0/en/Global-Hospital-acquired-Infections-HAI-Market-Sizeto-Grow-at-a-CAGR-of-3-6-2021-to-2030.html
Gómez-Torres N, Dunne M, Garde S et al (2018) Development of a specific fluorescent phage endolysin for in situ detection of Clostridium species associated with cheese spoilage. Microb Biotechnol 11:332–345. https://doi.org/10.1111/1751-7915.12883
Goodridge L, Griffiths M (2002) Reporter bacteriophage assays as a means to detect foodborne pathogenic bacteria. Food Res Int 35:863–870. https://doi.org/10.1016/S0963-9969(02)00094-7
Goracci M, Pignochino Y, Marchiò S (2020) Phage display-based nanotechnology applications in cancer immunotherapy. Molecules 25:1–28. https://doi.org/10.3390/molecules25040843
Gothandam KM (2018) Food security and water treatment
Halkare P, Punjabi N, Wangchuk J et al (2021) Label-free detection of Escherichia coli from mixed bacterial cultures using bacteriophage T4 on Plasmonic fiber-optic sensor. ACS Sensors 6:2720–2727. https://doi.org/10.1021/acssensors.1c00801
Harada LK, Silva EC, Campos WF et al (2018) Biotechnological applications of bacteriophages: state of the art. Microbiol Res 212–213:38–58. https://doi.org/10.1016/j.micres.2018.04.007
Harding E (2020) WHO global progress report on tuberculosis elimination. Lancet Respir Med 8:19. https://doi.org/10.1016/S2213-2600(19)30418-7
He Y, Wang M, Fan E et al (2017) Highly specific bacteriophage-affinity strategy for rapid separation and sensitive detection of viable Pseudomonas aeruginosa. Anal Chem 89:1916–1921. https://doi.org/10.1021/acs.analchem.6b04389
He Y, Shi Y, Liu M et al (2018) Nonlytic recombinant phage tail fiber protein for specific recognition of Pseudomonas aeruginosa. Anal Chem 90:14462–14468. https://doi.org/10.1021/acs.analchem.8b04160
Hinkley TC, Singh S, Garing S et al (2018a) A phage-based assay for the rapid, quantitative, and single CFU visualization of E. coli (ECOR #13) in drinking water. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-33097-4
Hinkley TC, Garing S, Singh S et al (2018b) Reporter bacteriophage T7NLC utilizes a novel NanoLuc::CBM fusion for the ultrasensitive detection of: Escherichia coli in water. Analyst 143:4074–4082. https://doi.org/10.1039/c8an00781k
Hinkley TC, Garing S, Jain P et al (2020) A syringe-based biosensor to rapidly detect low levels of Escherichia Coli (ECOR13) in drinking water using engineered bacteriophages. Sensors 20:1953. https://doi.org/10.3390/s20071953
Hiremath N, Guntupalli R, Vodyanoy V et al (2015) Detection of methicillin-resistant Staphylococcus aureus using novel lytic phage-based magnetoelastic biosensors. Sensors Actuators B Chem 210:129–136. https://doi.org/10.1016/j.snb.2014.12.083
Hiremath N, Chin BA, Park MK (2017) Effect of competing foodborne pathogens on the selectivity and binding kinetics of a lytic phage for methicillin-resistant Staphylococcus aureus detection. J Electrochem Soc 164:B142–B146. https://doi.org/10.1149/2.0901704jes
Holčapek M, Jirásko R, Lísa M (2012) Recent developments in liquid chromatography-mass spectrometry and related techniques. J Chromatogr A 1259:3–15. https://doi.org/10.1016/j.chroma.2012.08.072
Huang L, Sun DW, Wu Z et al (2021) Reproducible, shelf-stable, and bioaffinity SERS nanotags inspired by multivariate polyphenolic chemistry for bacterial identification. Anal Chim Acta 1167:338570. https://doi.org/10.1016/j.aca.2021.338570
Imai M, Mine K, Tomonari H et al (2019) Dark-field microscopic detection of bacteria using bacteriophage-immobilized SiO2@AuNP Core-Shell nanoparticles. Anal Chem 91:12352–12357. https://doi.org/10.1021/acs.analchem.9b02715
Irwin P, Gehring A, Tu S-I et al (2000) Minimum detectable level of salmonellae using a binomial - based bacterial ice nucleation detection assay (BIND ®). J AOAC Int 83:1087–1095
Jaffee S, Henson S, Unnevehr L, Grace D, Emilie Cassou (2019) The safe food imperative: accelerating progress in low- and middle-income countries. World Bank Group, Washington, DC
Janczuk M, Richter Ł, Hoser G et al (2017) Bacteriophage-based bioconjugates as a flow cytometry probe for fast bacteria detection. Bioconjug Chem 28:419–425. https://doi.org/10.1021/acs.bioconjchem.6b00596
Janczuk-Richter M, Marinović I, Niedziółka-Jönsson J, Szot-Karpińska K (2019) Recent applications of bacteriophage-based electrodes: a mini-review. Electrochem Commun 99:11–15. https://doi.org/10.1016/j.elecom.2018.12.011
Janda JM, Abbott SL (2002) Bacterial identification for publication: when is enough enough? J Clin Microbiol 40:1887–1891. https://doi.org/10.1128/JCM.40.6.1887-1891.2002
Jang KS, Kim YH (2018) Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. J Microbiol 56:209–216. https://doi.org/10.1007/s12275-018-7457-0
Jassim SAA, Griffiths MW (2007) Evaluation of a rapid microbial detection method via phage lytic amplification assay coupled with live/dead fluorochromic stains. Lett Appl Microbiol 44:673–678. https://doi.org/10.1111/j.1472-765X.2007.02115.x
Jit M, Ng DHL, Luangasanatip N et al (2020) Quantifying the economic cost of antibiotic resistance and the impact of related interventions: rapid methodological review, conceptual framework and recommendations for future studies. BMC Med 18:38. https://doi.org/10.1186/s12916-020-1507-2
Jorgensen JH, Ferraro MJ (2009) Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49:1749–1755. https://doi.org/10.1086/647952
Justé A, Thomma BPHJ, Lievens B (2008) Recent advances in molecular techniques to study microbial communities in food-associated matrices and processes. Food Microbiol 25:745–761. https://doi.org/10.1016/j.fm.2008.04.009
Kaufmann AF, Meltzer MI, Schmid GP (1997) The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg Infect Dis 3:83–94. https://doi.org/10.3201/eid0302.970201
Kim J, Kim M, Kim S, Ryu S (2017) Sensitive detection of viable Escherichia coli O157:H7 from foods using a luciferase-reporter phage phiV10lux. Int J Food Microbiol 254:11–17. https://doi.org/10.1016/j.ijfoodmicro.2017.05.002
Kim C, Lee H, Devaraj V et al (2020) Hierarchical cluster analysis of medical chemicals detected by a bacteriophage-based colorimetric sensor array. Nanomaterials 10:121. https://doi.org/10.3390/nano10010121
King LA, Nogareda F, Weill FX et al (2012) Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin Infect Dis 54:1588–1594. https://doi.org/10.1093/cid/cis255
Kneipp K, Kneipp H (2006) Single molecule Raman scattering. Appl Spectrosc 60:322A–334A. https://doi.org/10.1366/000370206779321418
Kortright KE, Chan BK, Koff JL, Turner PE (2019) Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. https://doi.org/10.1016/j.chom.2019.01.014
Kosa G, Shapaval V, Kohler A, Zimmermann B (2017) FTIR spectroscopy as a unified method for simultaneous analysis of intra- and extracellular metabolites in high-throughput screening of microbial bioprocesses. Microb Cell Fact 16:1–11. https://doi.org/10.1186/s12934-017-0817-3
Koskella B, Meaden S (2013) Understanding bacteriophage specificity in natural microbial communities. Viruses 5:806–823. https://doi.org/10.3390/v5030806
Krueger AP (1931) The sorption of bacteriophage by living and dead susceptible bacteria. J Gen Physiol 14:493–516. https://doi.org/10.1085/jgp.14.4.493
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. https://doi.org/10.1038/nrmicro2315
Lai A, Almaviva S, Spizzichino V et al (2017) Bacillus spp. cells captured selectively by phages and identified by surface enhanced Raman spectroscopy technique. Proceedings 1:519. https://doi.org/10.3390/proceedings1040519
Lankes HA, Zanghi CN, Santos K et al (2007) In vivo gene delivery and expression by bacteriophage lambda vectors. J Appl Microbiol 102:1337–1349. https://doi.org/10.1111/j.1365-2672.2006.03182.x
Law JWF, Mutalib NSA, Chan KG, Lee LH (2014) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5:1–19. https://doi.org/10.3389/fmicb.2014.00770
Li ZP, Duan XR, Liu CH, Du BA (2006) Selective determination of cysteine by resonance light scattering technique based on self-assembly of gold nanoparticles. Anal Biochem 351:18–25. https://doi.org/10.1016/j.ab.2006.01.038
Li YY, Xie G, Qiu J et al (2018) A new biosensor based on the recognition of phages and the signal amplification of organic-inorganic hybrid nanoflowers for discriminating and quantitating live pathogenic bacteria in urine. Sensors Actuators B Chem 258:803–812. https://doi.org/10.1016/j.snb.2017.11.155
Loessner MJ, Rees CED, Stewart GSAB, Scherer S (1996) Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable listeria cells. Appl Environ Microbiol 62:1133–1140. https://doi.org/10.1128/aem.62.4.1133-1140.1996
Luo J, Jiang M, Xiong J et al (2018) Exploring a phage-based real-time PCR assay for diagnosing Acinetobacter baumannii bloodstream infections with high sensitivity. Anal Chim Acta 1044:147–153. https://doi.org/10.1016/j.aca.2018.09.038
Luo J, Jiang M, Hiong J et al (2020) Rapid ultrasensitive diagnosis of pneumonia caused by Acinetobacter baumannii using a combination of enrichment and phage-based qPCR assay, pp 1–25. https://doi.org/10.21203/rs.3.rs-16845/v1
Mack JD, Yehualaeshet T, Park MK et al (2017) Phage-based biosensor and optimization of surface blocking agents to detect Salmonella Typhimurium on Romaine lettuce. J Food Saf 37:e12299. https://doi.org/10.1111/jfs.12299
Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4:429–434. https://doi.org/10.4103/1947-2714.100998
Marcadet A, O’Connell P, Cohen D (1989) Standardized Southern blot workshop technique. Immunobiol HLA I:553–560. https://doi.org/10.1007/978-1-4612-3552-1_116
Mariey L, Signolle JP, Amiel C, Travert J (2001) Discrimination, classification, identification of microorganisms using FTIR spectroscopy and chemometrics. Vib Spectrosc 26:151–159. https://doi.org/10.1016/S0924-2031(01)00113-8
Marks JD, Ouwehand WH, Bye JM et al (1993) Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology 11:1145–1149. https://doi.org/10.1038/nbt1093-1145
Melendez JH, Frankel YM, An AT et al (2010) Real-time PCR assays compared to culture-based approaches for identification of aerobic bacteria in chronic wounds. Clin Microbiol Infect 16:1762–1769. https://doi.org/10.1111/j.1469-0691.2010.03158.x
Mido T, Schaffer EM, Dorsey RW et al (2018) Sensitive detection of live Escherichia coli by bacteriophage amplification-coupled immunoassay on the Luminex® MAGPIX instrument. J Microbiol Methods 152:143–147. https://doi.org/10.1016/j.mimet.2018.07.022
Miller MB, Tang YW (2009) Basic concepts of microarrays and potential applications in clinical microbiology. Clin Microbiol Rev 22:611–633. https://doi.org/10.1128/CMR.00019-09
Miller-Petrie M, Pant S, Laxminarayan R (2017) Drug-resistant infections. Disease control priorities. In: Major infectious diseases, vol 6, 3rd edn, pp 433–448. https://doi.org/10.1596/978-1-4648-0524-0_ch18
Moon JS, Choi EJ, Jeong NN et al (2019) Research progress of M13 bacteriophage-based biosensors. Nanomaterials 9:1448. https://doi.org/10.3390/nano9101448
Moshirabadi A, Razi M, Arasteh P et al (2019) Polymerase chain reaction assay using the restriction fragment length polymorphism technique in the detection of prosthetic joint infections: a multi-centered study. J Arthroplasty 34:359–364. https://doi.org/10.1016/j.arth.2018.10.017
Neethirajan S, Ragavan V, Weng X, Chand R (2018) Biosensors for sustainable food engineering: challenges and perspectives. Biosensors 8:23. https://doi.org/10.3390/bios8010023
Nemudraya AA, Richter VA, Kuligina EV (2016) Phage peptide libraries as a source of targeted ligands. Acta Nat 8:48–57. https://doi.org/10.32607/20758251-2016-8-1-48-57
Neufeld T, Schwartz-Mittelmann A, Biran D et al (2003) Combined phage typing and amperometric detection of released enzymatic activity for the specific identification and quantification of bacteria. Anal Chem 75:580–585. https://doi.org/10.1021/ac026083e
Neufeld T, Mittelman AS, Buchner V, Rishpon J (2005) Electrochemical phagemid assay for the specific detection of bacteria using Escherichia coli TG-1 and the M13KO7 phagemid in a model system. Anal Chem 77:652–657. https://doi.org/10.1021/ac0488053
Niyomdecha S, Limbut W, Numnuam A et al (2018) Phage-based capacitive biosensor for Salmonella detection. Talanta 188:658–664. https://doi.org/10.1016/j.talanta.2018.06.033
Nobrega FL, Vlot M, de Jonge PA et al (2018) Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol 16:760–773. https://doi.org/10.1038/s41579-018-0070-8
Opota O, Jaton K, Greub G (2015) Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin Microbiol Infect 21:323–331. https://doi.org/10.1016/j.cmi.2015.02.005
Paczesny J, Bielec K (2020) Application of bacteriophages in nanotechnology. Nanomaterials 10:1–25. https://doi.org/10.3390/nano10101944
Paczesny J, Richter Ł, Hołyst R (2020) Recent progress in the detection of bacteria using bacteriophages: a review. Viruses 12:845. https://doi.org/10.3390/v12080845
Peng H, Chen IA (2019) Rapid colorimetric detection of bacterial species through the capture of gold nanoparticles by chimeric phages. ACS Nano 13:1244–1252. https://doi.org/10.1021/acsnano.8b06395
Petrenko VA, Vodyanoy VJ (2003) Phage display for detection of biological threat agents. J Microbiol Methods 53:253–262. https://doi.org/10.1016/S0167-7012(03)00029-0
Petti CA (2007) Detection and identification of microorganisms by gene amplification and sequencing. Clin Infect Dis 44:1108–1114. https://doi.org/10.1086/512818
Pires DP, Cleto S, Sillankorva S et al (2016) Genetically engineered phages: a review of advances over the last decade. Microbiol Mol Biol Rev 80:523–543. https://doi.org/10.1128/mmbr.00069-15
Pizarro-Bauerle J, Ando H (2020) Engineered bacteriophages for practical applications. Biol Pharm Bull 43:240–249. https://doi.org/10.1248/bpb.b19-00914
Poul MA, Marks JD (1999) Targeted gene delivery to mammalian cells by filamentous bacteriophage. J Mol Biol 288:203–211. https://doi.org/10.1006/jmbi.1999.2678
Pulkkinen EM, Hinkley TC, Nugen SR (2019) Utilizing in vitro DNA assembly to engineer a synthetic T7 Nanoluc reporter phage for Escherichia coli detection. Integr Biol 11:63–68. https://doi.org/10.1093/intbio/zyz005
Punina NV, Makridakis NM, Remnev MA, Topunov AF (2015) Whole-genome sequencing targets drug-resistant bacterial infections. Hum Genomics 9:19. https://doi.org/10.1186/s40246-015-0037-z
Richter Ł, Matuła K, Leśniewski A et al (2016) Ordering of bacteriophages in the electric field: application for bacteria detection. Sensors Actuators B Chem 224:233–240. https://doi.org/10.1016/j.snb.2015.09.042
Richter Ł, Bielec K, Leśniewski A et al (2017) Dense layer of bacteriophages ordered in alternating electric field and immobilized by surface chemical modification as sensing element for bacteria detection. ACS Appl Mater Interfaces 9:19622–19629. https://doi.org/10.1021/acsami.7b03497
Richter Ł, Janczuk-Richter M, Niedziółka-Jönsson J et al (2018) Recent advances in bacteriophage-based methods for bacteria detection. Drug Discov Today 23:448–455. https://doi.org/10.1016/j.drudis.2017.11.007
Rippa M, Castagna R, Pannico M et al (2017) Octupolar metastructures for a highly sensitive, rapid, and reproducible phage-based detection of bacterial pathogens by surface-enhanced Raman scattering. ACS Sensors 2:947–954. https://doi.org/10.1021/acssensors.7b00195
Rippa M, Castagna R, Zhou J et al (2018) Dodecagonal plasmonic quasicrystals for phage-based biosensing. Nanotechnology 29:405501. https://doi.org/10.1088/1361-6528/aad2f5
Santos C, Fraga ME, Kozakiewicz Z, Lima N (2010) Fourier transform infrared as a powerful technique for the identification and characterization of filamentous fungi and yeasts. Res Microbiol 161:168–175. https://doi.org/10.1016/j.resmic.2009.12.007
Scallan E, Hoekstra RM, Angulo FJ et al (2011) Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis 17:7–15. https://doi.org/10.3201/eid1701.P11101
Sedki M, Chen X, Chen C et al (2020) Non-lytic M13 phage-based highly sensitive impedimetric cytosensor for detection of coliforms. Biosens Bioelectron 148:111794. https://doi.org/10.1016/j.bios.2019.111794
Sergueev KV, Filippov AA, Nikolich MP (2017) Highly sensitive bacteriophage-based detection of Brucella abortus in mixed culture and spiked blood. Viruses 9:144. https://doi.org/10.3390/v9060144
Sharma S, Chatterjee S, Datta S et al (2017) Bacteriophages and its applications: an overview. Folia Microbiol (Praha) 62:17–55. https://doi.org/10.1007/s12223-016-0471-x
Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317
Soga T, Ueno Y, Naraoka H et al (2002) Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem 74:2233–2239. https://doi.org/10.1021/ac020064n
Srivastava SK, Ben HH, Kushmaro A et al (2015) Highly sensitive and specific detection of E. coli by a SERS nanobiosensor chip utilizing metallic nanosculptured thin films. Analyst 140:3201–3209. https://doi.org/10.1039/c5an00209e
Streit S, Michalski CW, Erkan M et al (2009) Northern blot analysis for detection and quantification of RNA in pancreatic cancer cells and tissues. Nat Protoc 4:37–43. https://doi.org/10.1038/nprot.2008.216
Tagini F, Greub G (2017) Bacterial genome sequencing in clinical microbiology: a pathogen-oriented review. Eur J Clin Microbiol Infect Dis 36:2007–2020. https://doi.org/10.1007/s10096-017-3024-6
Teutenberg T (2009) Potential of high temperature liquid chromatography for the improvement of separation efficiency—a review. Anal Chim Acta 643:1–12. https://doi.org/10.1016/j.aca.2009.04.008
Tian F, Li J, Nazir A, Tong Y (2021) Bacteriophage—a promising alternative measure for bacterial biofilm control. Infect Drug Resist 14:205–217. https://doi.org/10.2147/IDR.S290093
Tilton L, Das G, Yang X et al (2019) Nanophotonic device in combination with bacteriophages for enhancing detection sensitivity of Escherichia coli in simulated wash water. Anal Lett 52:2203–2213. https://doi.org/10.1080/00032719.2019.1604726
Touat M, Opatowski M, Brun-Buisson C et al (2019) A payer perspective of the hospital inpatient additional care costs of antimicrobial resistance in France: a matched case–control study. Appl Health Econ Health Policy 17:381–389. https://doi.org/10.1007/s40258-018-0451-1
Trasanidou D, Gerós AS, Mohanraju P et al (2019) Keeping crispr in check: diverse mechanisms of phage-encoded anti-crisprs. FEMS Microbiol Lett 366:1–14. https://doi.org/10.1093/femsle/fnz098
Ulitzur N, Ulitzur S (2006) New rapid and simple methods for detection of bacteria and determination of their antibiotic susceptibility by using phage mutants. Appl Environ Microbiol 72:7455–7459. https://doi.org/10.1128/AEM.00761-06
US Department of Health and Human Services, CDC (2019) Antibiotic resistance threats in the United States. Centers Dis Control Prevention, pp 1–113
Váradi L, Luo JL, Hibbs DE et al (2017) Methods for the detection and identification of pathogenic bacteria: past, present, and future. Chem Soc Rev 46:4818–4832. https://doi.org/10.1039/c6cs00693k
Velusamy V, Arshak K, Korostynska O et al (2010) An overview of foodborne pathogen detection: in the perspective of biosensors. Biotechnol Adv 28:232–254. https://doi.org/10.1016/j.biotechadv.2009.12.004
Vinay M, Franche N, Grégori G et al (2015) Phage-based fluorescent biosensor prototypes to specifically detect enteric bacteria such as E. coli and Salmonella enterica Typhimurium. PLoS One 10:1–17. https://doi.org/10.1371/journal.pone.0131466
Wan MLY, Forsythe SJ, El-Nezami H (2019) Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges. Crit Rev Food Sci Nutr 59:3320–3333. https://doi.org/10.1080/10408398.2018.1490885
Wang D, Chen J, Nugen SR (2017) Electrochemical detection of Escherichia coli from aqueous samples using engineered phages. Anal Chem 89:1650–1657. https://doi.org/10.1021/acs.analchem.6b03752
Wang Y, He Y, Bhattacharyya S et al (2020) Recombinant bacteriophage cell-binding domain proteins for broad-Spectrum recognition of methicillin-resistant Staphylococcus aureus strains. Anal Chem 92:3340–3345. https://doi.org/10.1021/acs.analchem.9b05295
Warren CR (2018) A liquid chromatography–mass spectrometry method for analysis of intact fatty-acid-based lipids extracted from soil. Eur J Soil Sci 69:791–803. https://doi.org/10.1111/ejss.12689
Wei C, Li M, Zhao X (2018) Surface-enhanced Raman scattering (SERS) with silver nano substrates synthesized by microwave for rapid detection of foodborne pathogens. Front Microbiol 9:2857. https://doi.org/10.3389/fmicb.2018.02857
Weiner-Lastinger LM, Pattabiraman V, Konnor RY et al (2022) The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network. Infect Control Hosp Epidemiol 43(1):12–25. https://doi.org/10.1017/ice.2021.362
Wisuthiphaet N, Yang X, Young GM, Nitin N (2019) Rapid detection of Escherichia coli in beverages using genetically engineered bacteriophage T7. AMB Express 9:55. https://doi.org/10.1186/s13568-019-0776-7
Wisuthiphaet N, Yang X, Young GM, Nitin N (2021) Application of engineered bacteriophage T7 in the detection of bacteria in food matrices. Front Microbiol 12:691003. https://doi.org/10.3389/fmicb.2021.691003
Witkowska E, Korsak D, Kowalska A et al (2017) Surface-enhanced Raman spectroscopy introduced into the International Standard Organization (ISO) regulations as an alternative method for detection and identification of pathogens in the food industry. Anal Bioanal Chem 409:1555–1567. https://doi.org/10.1007/s00216-016-0090-z
Witkowska E, Korsak D, Kowalska A et al (2018) Strain-level typing and identification of bacteria—a novel approach for SERS active plasmonic nanostructures. Anal Bioanal Chem 410:5019–5031. https://doi.org/10.1007/s00216-018-1153-0
World Health Organization (2011) Report on the burden of endemic health care-associated infection worldwide. In: WHO Libr Cat. Data
Wu L, Song Y, Luan T et al (2016) Specific detection of live Escherichia coli O157: H7 using tetracysteine-tagged PP01 bacteriophage. Biosens Bioelectron 86:102–108. https://doi.org/10.1016/j.bios.2016.06.041
Wu L, Hong X, Luan T et al (2021) Multiplexed detection of bacterial pathogens based on a cocktail of dual-modified phages. Anal Chim Acta 1166:338596. https://doi.org/10.1016/j.aca.2021.338596
Xu J, Chau Y, Lee YK (2019) Phage-based electrochemical sensors: a review. Micromachines 10:855. https://doi.org/10.3390/mi10120855
Xu J, Zhao C, Chau Y, Lee YK (2020) The synergy of chemical immobilization and electrical orientation of T4 bacteriophage on a micro electrochemical sensor for low-level viable bacteria detection via differential pulse voltammetry. Biosens Bioelectron 151:111914. https://doi.org/10.1016/j.bios.2019.111914
Yan C, Zhang Y, Yang H et al (2017) Combining phagomagnetic separation with immunoassay for specific, fast and sensitive detection of Staphylococcus aureus. Talanta 170:291–297. https://doi.org/10.1016/j.talanta.2017.04.007
Yang T, Zhang XX, Yang JY et al (2018) Screening arsenic(III)-binding peptide for colorimetric detection of arsenic(III) based on the peptide induced aggregation of gold nanoparticles. Talanta 177:212–216. https://doi.org/10.1016/j.talanta.2017.07.005
Yoo YJ, Kim WG, Ko JH et al (2020) Large-area virus coated ultrathin colorimetric sensors with a highly Lossy resonant promoter for enhanced chromaticity. Adv Sci 7:2000978. https://doi.org/10.1002/advs.202000978
Yue H, He Y, Fan E et al (2017) Label-free electrochemiluminescent biosensor for rapid and sensitive detection of pseudomonas aeruginosa using phage as highly specific recognition agent. Biosens Bioelectron 94:429–432. https://doi.org/10.1016/j.bios.2017.03.033
Yuen LKW, Ross BC, Jackson KM, Dwyer B (1993) Characterization of Mycobacterium tuberculosis strains from Vietnamese patients by Southern blot hybridization. J Clin Microbiol 31:1615–1618. https://doi.org/10.1128/jcm.31.6.1615-1618.1993
Zhang JI, Talaty N, Costa AB et al (2011) Rapid direct lipid profiling of bacteria using desorption electrospray ionization mass spectrometry. Int J Mass Spectrom 301:37–44. https://doi.org/10.1016/j.ijms.2010.06.014
Zhou Y, Marar A, Kner P, Ramasamy RP (2017) Charge-directed immobilization of bacteriophage on nanostructured electrode for whole-cell electrochemical biosensors. Anal Chem 89:5734–5741. https://doi.org/10.1021/acs.analchem.6b03751
Zourob M (2010) Recognition receptors in biosensors. Springer, New York
Acknowledgments
The National Science Centre supported this work within the PRELUDIUM BIS grant according to decision number 2020/39/O/ST5/01017.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Paczesny, J., Wdowiak, M., Ochirbat, E. (2022). Bacteriophage-Based Biosensors: Detection of Bacteria and Beyond. In: Hameed, S., Rehman, S. (eds) Nanotechnology for Infectious Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-16-9190-4_20
Download citation
DOI: https://doi.org/10.1007/978-981-16-9190-4_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9189-8
Online ISBN: 978-981-16-9190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)