Abstract
Stem cells present an enormous potential in a number of fields with a great impact on human health including Regenerative Medicine, drug discovery, toxicology studies and fundamental stem cell biology. Crucial to the accomplishment of this potential is the development of stem cell-based bioprocessing strategies based on the rational integration of cell culture procedures with separation methods towards the isolation of specific stem cell types from tissues and/or purification of stem cells and derivatives after in vitro culture. Separation methods/strategies have been applied to stem cells since many years ago, namely the isolation of hematopoietic stem/progenitor cells (HSPC) from bone marrow for the treatment of hemato-oncological diseases using density gradient centrifugation followed by immunoaffinity-based techniques. More recently, novel approaches have been proposed including affinity-based methods that take advantage of the use of more cost-effective ligands (e.g. aptamers, lectins), as well as novel biophysical-based methods requiring no cell labelling and integrated with microscale technologies. This chapter presents a critical assessment of these traditional and novel separation methodologies and their present or potential applications to the stem cell field. The techniques are grouped according to their fundamental principles, which are defined by the main physicochemical, biophysical and affinity properties of cells. Nevertheless, enormous challenges still need to be overcome in order to make available a wide range of strategies combining scalability potential with high-resolution abilities, allowing the cost-effective large-scale production of highly purified stem cell populations and/or derivatives. Further developments in this field are thus expected to greatly impact and potentiate the medical translation of stem cell-based therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Separation Technologies for Stem Cell-Based Therapies: Relevance and Challenges
The isolation and/or purification of stem cells and their derivatives to be used for cell therapy applications, including fully established stem cell-based therapies, as well as for fundamental biomedical research have been performed over the last decades (Diogo et al. 2012; Gonzalez-Gonzalez et al. 2012). Overall, separation techniques have been used for isolation of stem cell populations from tissues, for separating different stem cell populations from a heterogeneous cell mixture and for purification of stem cell derivatives obtained upon differentiation of stem cells in vitro. The most classical example of a stem cell-based separation is the isolation of human hematopoietic stem/progenitor cells (HSPC) from different sources such as bone marrow (BM), umbilical cord blood (UCB) and mobilized peripheral blood (mPB) for the treatment of hemato-oncological diseases. For this purpose, a separation strategy was conceived including a density gradient centrifugation followed by immunoaffinity-based techniques, including magnetic-activated cell sorting (MACS), and fluorescence activated cell sorting (FACS), for targeting of CD34+ cells. The main objective of these procedures is the enrichment of rare HSPC present in these sources for further transplantation to restore the blood and the immune system of cancer patients following high-dose chemotherapy or to treat autoimmune, metabolic and genetic diseases (Weissman and Shizuru 2008).
In addition to this widely established procedure, it is nowadays believed that the successful establishment of stem cell based-therapies at different stages of pre-clinical and clinical tests and other stem cell applications in the biomedical field is highly dependent on the development of more sophisticated and efficient separation technologies and strategies. According to the final stem cell-based therapy application envisaged, different challenges must be faced in this field. One of these challenges is the need for novel techniques with a higher resolution, either for depletion of contaminating cells, or for the separation of stem cell populations sharing similar physicochemical and affinity characteristics but presenting different clinical features. On the other hand, there is also the necessity of scaling-up the separation processes when the cellular product is intended to be used for clinical applications or as a tool for drug screening and pharmacological testing.
These challenges present a different relevance according to the stem cell-based therapy envisaged. Of note, several pre-clinical studies and clinical trials have pointed to the therapeutic potential of mesenchymal stem/stromal cells (MSC), based on their multilineage differentiation potential, but especially on their intrinsic immunomodulatory and pro-regenerative features (Caplan 2007, 2009; Santos et al. 2011; Uchida et al. 2000). These cells have been isolated from different sources such as BM, adipose tissue (AT) and umbilical cord matrix (UCM) typically based on their adhesion to plastic surfaces, which yields a very heterogeneous cell population. A more rational clinical use of MSC would thus strongly benefit from the development of novel high-resolution separation strategies to capture specific MSC sub-populations with defined properties from a variety of different sources. Other stem cell applications requiring the development of separation techniques with high-resolution abilities are the ones relying on the depletion of rare contaminating cancer stem cells (Geens et al. 2007) and the removal of tumorigenic stem cells from in vitro differentiating cultures of pluripotent stem cells (PSC), both embryonic (ESC) and induced pluripotent stem cells (iPSC) (Levenberg et al. 2010). Without these developments, the potential application of human PSC-derived tissue specific cells in clinical settings will remain hampered, among other reasons, by the presence of pluripotent cells or naïve proliferative progenitors that can form teratomas upon in vivo transplantation. Importantly, for all the applications that may be considered, separation technologies should be rationally integrated with cell production methods in wider bioprocessing strategies towards the large-scale manufacturing of stem cells and/or their progeny. Overall, the examples aforementioned illustrate the challenge and the relevance of separation technologies to potentiate the medical translation of stem cell-based therapies.
7.2 Cell Separation Technologies: An Overview
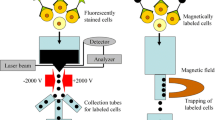
Cell separation technologies are selected according to general criteria including the final application of the cellular product, the cellular properties, the resolution capabilities required and the scalability of the process. When considering the cellular properties, different techniques have been explored that can take advantage over the differential physicochemical and biochemical characteristics of cells, including size, density or electrostatic and hydrophobic character, as well as the differential expression of cell-specific surface markers or adhesive properties (Fig. 7.1 and Table 7.1). The techniques that explore the physicochemical properties of cells are generally traditional methods such as centrifugation or membrane filtration. These techniques are characterized by a low resolution capacity and they are typically used at the first stages of cell processing for the separation of very distinct cell types and/or for cell concentration. Moreover, the differential adhesion of distinct cells to tissue culture plastic can also be explored as a low resolution separation/concentration method that is generally used during the first stages of the bioprocess. However, for similar cell phenotypes, high resolution techniques are required and in these cases cell separation has been generally performed by taking advantage of the differential number and type of molecules present on the cell surface that can be targeted by specific monoclonal antibodies, lectins and, more recently, by aptamers. This group of techniques is named immunoaffinity methods. Immunoaffinity cell separation strategies can be conceived by using a single specific ligand only (e.g. monoclonal antibody), but by targeting cells with several immunoaffinity ligands at the same time, or in sequential steps, more complex strategies can be conceived. Separation techniques taking advantage of the use of antibodies that bind to surface markers to specifically pick out cells of interest include the very widely used fluorescence-activated cell sorting (FACS), immunomagnetic cell sorting (MACS), affinity chromatography and aqueous two-phase systems (ATPS) using antibody-modified polymers. Nevertheless, although immunoaffinity separation methodologies typically provide a high resolution in cell separation, for many stem cell or stem-cell derived populations, surface markers for separation and analysis are limited. In addition, these immunoaffinity strategies rely on the formation of a complex cell-antibody or cell-antibody-magnetic particle, which could affect cell function (e.g. differentiation) or activation state (Chou et al. 2010). Alternative selection strategies avoiding the use of antibodies and magnetic particles have been more recently provided by novel “tag-less” methods, such as dielectrophoresis (DEP) (Pethig et al. 2010), integrated with microfluidics and other microfabricated structures, and also fluid flow fractionation (FFF) (Reschiglian et al. 2005). DEP and FFF do not require labelling of cells but they allow a selective cell separation based on the inherent biophysical properties of cells.
Separation methods in the stem cell field based on cell’s physicochemical, affinity and biophysical properties. Physicochemical-based methods such as density gradient centrifugation, take advantage of cell size, cell density, or the capacity to adhere to tissue culture plastic. Affinity-based methods, like MACS, employ specific monoclonal antibodies, lectins or aptamers targeting different types of molecules present on the surface of the cells; Biophysical-based methods, such as DEP, do not require cell labeling and promote selective separation based on inherent differences in cell’s biophysical properties
According to the final purpose and to the characteristics of the starting material, stem cells can be separated by negative or positive selection. A positive selection operation is more adequate for the isolation of specific and low proportion populations from a complex cell mixture. In the stem cell field, this strategy has been successfully used for the specific capture of CD34+ HSPC (either by FACS or MACS) from different sources. On the other hand, negative selection techniques are advantageous and required if the target cells have to be untouched (without magnetic particles or antibodies) for subsequent analysis or application in clinical settings. Moreover, negative selection techniques may also be required if no surface marker/monoclonal antibody specific for the cell of interest is known or available or if the main objective is the high-resolution depletion of an undesired cell type (e.g. tumorigenic cells). According to the desired cell phenotype, different separation techniques in different modes may be selected and integrated in order to take advantage of different cellular properties and achieve more efficient separation strategies. Importantly, these downstream processing techniques should also be strategically combined with cell culture operations in order to design a cost-effective and efficient bioprocess for production of stem cells and derivatives.
The following sections of this chapter describe the basic principles of traditional and novel cell separation techniques and their applications in the stem cell field. A critical assessment is provided here concerning their advantages and limitations considering the final usage of stem cells and/or their derivatives in particular for applications in stem cell-based therapies.
7.3 Stem Cell-Based Separation Technologies
7.3.1 Physico-chemical Methods
7.3.1.1 Centrifugation
One of the most traditional and widely used techniques for primary cell separation is discontinuous density gradient centrifugation (Fig. 7.1). In this separation method, two distinct solutions with different densities are put together forming a system with two immiscible layers. The two-layered system is generally composed of sucrose and a polymer, such as Percoll or Ficoll-Paque. After obtaining this system, cells are added to the less dense solution on the top and a centrifugation is performed causing the cells to cross the system and to be separated according to their densities. Thus, cells with higher density than the more dense solution beneath will cross the interface between the two immiscible layers and settle at the bottom whereas the cells that have a lower density will settle at the interface. This technique is characterized by a low resolution capacity and for that reason it is generally used for enrichment, concentration or as a preparative step before using other separation techniques with higher resolution capabilities, namely immunoaffinity-based methods, such as FACS or MACS (see following sections).
One of the most popular applications of density gradient centrifugation in the stem cell field is the enrichment of mononuclear cells from human UCB or BM by a Ficoll-Paque density gradient (1.077 g/mL) (Andrade et al. 2011; da Silva et al. 2005, 2009). In this case, erythrocytes and granulocytes sediment to the bottom layer, whereas lower density lymphocytes and other slowly sedimenting cells, including stem/progenitor cells, as well as platelets and monocytes, are retained at the interface between the plasma and the Ficoll-Paque. Cells can then be collected from the interface and subjected to subsequent isolation of HSPC or MSC populations using higher resolution immunoaffinity techniques. More recently, enrichment of mononuclear cells through density gradient-based separation has also been performed using commercially available equipment, the Sepax (Biosafe SA, Switzerland). This equipment is a fully-automated, closed, single-use and mobile system that can be used in GMP compliant environments or directly at bedside in the operating room for Regenerative Medicine applications. In addition to this application, gradient centrifugation was also already used with success for enrichment of human PSC derivatives. In particular, Percoll centrifugation was applied after differentiation of human ESC into cardiomyocytes using a monolayer adherent protocol (Laflamme et al. 2007). This purification methodology increased the purity of cardiomyocytes in cell suspension from 30 to 80 %.
An alternative type of centrifugation that has been recently applied for stem cell isolation is counter-flow centrifugal elutriation (CCE). In this case, cells are separated inside a centrifugal chamber where a continuous pumping of a fluid occurs. This technique was already used with success for the fractionation of umbilical cord (UC)-derived cells. In fact, through CCE it was possible to isolate a sub-population of small-sized UC-derived primary cells with MSC-like characteristics (Majore et al. 2009). This subpopulation exhibited a higher proliferative capacity as compared to the total UC-derived primary cultures and demonstrated a reduced amount of aging cells. The separation of this self-renewing MSC-like subpopulation by CCE provides a valuable tool to be used in Regenerative Medicine and may be an alternative to BM derived MSC.
7.3.1.2 Membrane Filtration
An alternative physico-chemical method for cell separation that has been recently used in the stem cell field is membrane filtration. In this case, cell separation is achieved based on cell size, according to the membrane pore size, but may also be based on the differential intensity of cell adhesion to the membrane. This technique is characterized by a high processing speed, simplicity, relatively low cost and, importantly, a high potential for scaling-up. In fact, the equipment necessary to perform this operation is already available at an industrial scale.
The isolation of CD34+ cells from mPB was already performed using unmodified polyurethane (PU) foaming membranes, as well as PU membranes modified with -COOH groups and coated with Pluronic F127 or hyaluronic acid at different blood permeation rates (Higuchi et al. 2006, 2008). The permeation ratio of CD34+ HSPC through the membranes was the lowest among blood cells regardless the type of PU membrane used while erythrocytes, platelets, T cells and B cells permeated more freely through the PU membranes. This behaviour was potentially due to the high expression of cell-adhesion molecules on the surfaces of the more primitive HSPC. More recently, the successful isolation of human adipose-derived stem cells (ADSC) with a superior capacity for osteogenic differentiation from a suspension of human adipose tissue was achieved by this technique also using PU membranes (Wu et al. 2012). Importantly, these cells were isolated in less than 30 minutes whereas the conventional method of adhesion to plastic surfaces (see following section) requires 5–12 days. Although these results are encouraging, many improvements are still needed to increase the potential of this technique for stem cell-based separation settings.
7.3.2 Cell Culture-Based Methods
The differential behaviour of different cell types in culture settings can also be used as a means to obtain their separation. The most classical example is the isolation of human MSC from different sources, namely BM, based on their ability to adhere to tissue culture plastic, which allows the separation of MSC from the majority of hematopoietic cells. In fact, these cells are mainly non-adherent, being eliminated during culture medium exchange (Lennon and Caplan 2006). Differential enzymatic treatment can also be used to eliminate the major cell contaminants in primary cultures of human MSC, namely monocytes, as these need longer incubation times with the enzymatic agent in order to be harvested from culture plastic. Another alternative and relatively straightforward method for the isolation of human MSC in culture settings is based on osmotic selection due to their uncommon resistance to osmotic lysis (Parekkadan et al. 2007).
7.3.3 Immunoaffinity Methods
As previously mentioned, high resolution cell separation can be performed by a group of techniques entitled immunoaffinity methods, in which the cells are targeted by specific immunoaffinity ligands, such as antibodies. This group of methods has been widely used for the high-resolution targeting of stem cells and their derivatives using specific cell surface markers (Table 7.2). One of the first immunoaffinity stem/progenitor cell selection strategies was developed in the hematological field and consists on the isolation and enrichment of human HSPC based on the expression of the surface marker CD34. CD34 antigen is indeed the most utilized in hematopoietic studies, identifying cells from the stem through progenitor states. However, since the expression of CD34 by truly self-renewing HSPC populations is not exclusive, a combination of CD34 and other antigens is often used for isolation and characterization of HSPC (Table 7.2). For example, the more immature hematopoietic stem cells (HSC) should possess a CD34+CD38− phenotype (da Silva et al. 2005). However, this primitive phenotype has been shown to inherently modulate in culture (da Silva et al. 2009), leading to erroneous quantification of engraftment competent cells upon ex-vivo cultures. Other phenotypes for human HSP have been proposed (Weissman and Shizuru 2008) including the expression of Thy-1 (or CD90). Another approach consists of the use of negative selection for lineage markers associated with terminal maturation of specific blood cell types (Lineage negative (Lin-) cells) combined with CD34 expression (Table 7.2).
When concerning to other multipotent stem cell types, such as human MSC, the scenario is different since few surface markers have been identified for the analysis and isolation of these cells. Moreover, the existence of universal markers for these stem cell types and their derivatives still remains elusive. For that reason, on a routine basis, most laboratories perform the isolation of MSC from BM samples or adipose tissue/UC based on its adherence to culture plastic (Lennon and Caplan 2006). Nevertheless, alternative immunoaffinity methods have been proposed in order to isolate MSC based on surface marker expression, either by positive or negative selection (Table 7.2) to obtain more homogeneous populations. One illustrative example is the purification of human MSC through positive selection using the STRO-1 (Goncalves et al. 2006; Gronthos and Zannettino 2008), CD73 (Barry et al. 2001) and CD105 (Tondreau et al. 2004) antibodies. Negative selection of human MSC has also been performed through the use of the CD45 surface marker (Jones et al. 2002). When considering other multipotent stem cell types, surface markers have also been identified for the analysis and isolation of neural stem cells (NSC) and neural progenitors from brain tissue such as Lex1 in mouse (Capela and Temple 2002) and CD133 in human (Uchida et al. 2000).
In the field of human PSC, expression of the surface markers SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 has been typically used for identification and separation of these cells, both ESC and iPSC. More recently, efforts have been devoted towards the identification of novel and more specific surface markers to ensure the complete depletion of these teratoma-forming cells from differentiating cultures, such as the SSEA-5 antigen (Tang et al. 2011), as well as novel lectin biomarkers (Wang et al. 2011c). In parallel, the design of complete immunophenotyping screens for identification and isolation of NSC, neurons and glia obtained during in vitro neural differentiation of human PSC has also been focused (Pruszak et al. 2007; Sundberg et al. 2011; Yuan et al. 2011). In what concerns to mouse ESC, SSEA-1 is generally used as universal surface marker for identification and separation of these cells.
The following sections will describe different immunoaffinity-based separation techniques that take advantage of these surface markers for targeting stem cells and their derivatives.
7.3.3.1 Fluorescence-Activated Cell Sorting (FACS)
Fluorescence-activated Cell Sorting (FACS) is one of the most widely used high-resolution techniques for isolation and purification of cells including stem cells and their derivatives. Cell separation in FACS relies on by Flow Cytometry exploiting cell’s size and light-scattering properties. However, the full potential of flow cytometry as a preparative separation technique is only achieved when fluorescently-labelled monoclonal antibodies are used to bind to specific antigen markers responsible for a particular surface phenotype. As previously mentioned, one of the major limitations associated to immunoaffinity techniques is the absence of surface markers to identify and isolate specific stem cell types or their derivatives. In FACS this limitation can be surpassed through the insertion of reporter constructs inside the cells to make fluorescent labels (e.g. a fluorescent protein expressed under the control of a specific promoter) (Aubert et al. 2003; Wang et al. 2011a). In addition, the use of reporter constructs also avoids the time-consuming step of immunostaining. Nevertheless, the genetic modification of stem cells to be used in clinical applications can raise important regulatory concerns and others that should be carefully analysed.
Flow cytometry is a powerful analytical and preparative technique with three major components: fluidics, optics and electronics (Brown and Wittwer 2000). After analysis and identification of the target population to be sorted, the cells in the liquid stream will be separated into small droplets through the use of mechanical vibrations. These droplets can be positively or negatively charged and when flowing parallel to electrodes are deflected into sampling tubes depending on the charge of the droplet (i.e. a cell generates a negative charge if fluoresces, and a positive charge if not). Since FACS allows the fractionation of one cell at a time this technique presents unique resolution capacities.
When concerning the stem cell field, FACS is widely used for sorting of human HSPC based on CD34 expression after removing the more mature cells and reducing the sample volume by density gradient centrifugation (Weissman and Shizuru 2008). This application is widely used in clinical settings. In fact, FACS would be very time consuming and expensive to process whole blood directly. Importantly, FACS was effective to obtain highly purified mPB CD34+CD90+ cells allowing the preparation of cancer-free transplants in breast cancer patients (Negrin et al. 2000). More recently, FACS has also been used for the depletion of PSC from heterogeneous cell populations obtained after cell differentiation, based on the expression of SSEA-4 in human and primate ESC (Fong et al. 2009; Shibata et al. 2006), TRA-1-60 in human ESC (Fong et al. 2009) and the expression of SSEA-1 in mouse ESC (Fukuda et al. 2006). It was also used for purification of ESC-derived neurons expressing the cell surface marker neural cell adhesion molecule (NCAM or CD56) (Pruszak et al. 2007) and for the isolation of ventricular-like cardiomyocytes differentiated from mouse ESC using a reporter cell line (Muller et al. 2000).
Although FACS presents an impaired high-resolution capacity, the equipment is large, very expensive and requires skilled technicians to operate it. In addition, it imposes significant contamination risks and a high shear stress to the cells. Importantly, the throughput of the technique is limited, with processing times of 3–6 hours including the pre-processing steps for immunostaining. Due to these characteristics, FACS is particularly adequate for purification of cells for biomedical research mainly, but very limited at a process scale for the manufacturing of cells for clinical applications. Therefore, other more scalable immunoaffinity techniques have been proposed.
7.3.3.2 Immunomagnetic Cell Separation
Magnetic-activated cell sorting (MACS) is a trademark name (Miltenyi Biotec) for a magnetic-based cell separation technique using small, magnetically susceptible beads bound to a monoclonal antibody. To achieve cell separation, cells are mixed with the beads and this mixture is then loaded into a column that is placed under the influence of a magnetic field. Due to this, the bead-carrying cells will be retained in the column whereas the unbound cells will be washed away (Fig. 7.1). The bead-carrying cells can then be recovered by elution after turning off the magnetic field. This technique can be used both for the enrichment of a target cell type (positive selection) or for the depletion of unwanted cells (negative selection).
Since the implementation of the original concept of MACS, this technology has been the focus of important developments (Grutzkau and Radbruch 2010). The most recent advances were the development of column-free systems (e.g. EasySep, Stem Cell Technologies) that rely on the use of very small submicron magnetic particles. Due to their biological and optical inertness, colloidal super-paramagnetic particles ranging from 20 to 100 nm have become the gold standard for magnetic cell separation (Grutzkau and Radbruch 2010). These microbeads are always in suspension allowing fast binding kinetics and short labelling procedures. Moreover, due to their small size, these particles do not saturate cell epitopes and thus they do not have to be removed for downstream applications. For example, these particle-antibody complexes do not interfere with subsequent flow cytometric analysis in opposition to cells labelled with microparticles, where the optical properties are changed. Until the introduction of this separation technology, cells labelled with submicron magnetic particles had to be magnetically separated on a column containing a magnetic matrix (e.g. StemSepTM, Stem Cell Technologies) requiring an extra step to remove the purified cells from the column. Other developments of MACS technology consisted on the development of multimagnetic devices that allow parallel processing of samples (autoMACSTM Pro, Miltenyi Biotec). In addition, for clinical applications requiring automated cell separation on a large scale, closed and sterile system, the target cells can be enriched from up to 1.2 × 1011 cells using the CliniMACS® system (Miltenyi Biotec). These automated systems have been widely used to enrich stem/progenitor cells from BM, UCB and mPB for use in hematopoietic cell transplantation (Weissman and Shizuru 2008) and for the isolation of highly purified UCB CD133+ cells from freshly isolated or cryopreserved samples (Bonanno et al. 2004).
Traditional MACS technology was already used for the separation of cardiomyocytes derived from human PSC after having identified VCAM1 as a cell surface marker (Uosaki et al. 2011). With this method it was possible to obtain more than 95 % of cells expressing TNNT2 (cardiac troponin – T). It was also used for the separation of undifferentiated mouse ESC from a pool of differentiated and undifferentiated cells in a batch system. By using a mathematical model it was predicted that MACS technology alone would be insufficient to achieve the necessary clearance of teratoma-forming undifferentiated cells for a therapeutic application (Schriebl et al. 2010). However, in a more recent work it was shown that by using MACS followed by selective killing of residual human ESC with a specific cytotoxic antibody, the required purity of human ESC-differentiated cells can be achieved (Schriebl et al. 2012).
When compared to other separation technologies, MACS may present undesirable biological effects from the use of magnetic particles, which can interfere with cell features and further cell analysis, for example using flow cytometry, since the cell’s optical properties can change. However, MACS is very easy to use, it is faster than FACS and provides comparable purity (especially if 2 consecutive cycles of magnetic separation are combined) and efficacy, and potentially conveys lower shear stress to the cells (Grutzkau and Radbruch 2010). For instances, concerning the enrichment of UCB CD34+ cells we recently reported average values of 69 % purity (%CD34+) after one round of purification (first column, cell recovery of 93 %). The purity of the cell population obtained could be further increased to 93 % (similar to the purities reached by FACS) after a second round of purification (second column, 52 % cell recovery) (Andrade et al. 2011). Both techniques can be strategically combined, by using MACS for pre-enrichment of rare cells and a subsequent FACS purification. However, both methods may be expensive and unsuitable for large-scale processing. Importantly, MACS has been considered the gold standard for stem cell isolation since it has been approved by FDA for clinical purposes, in particular for the enrichment of CD34+ cells in neuroblastoma ex-vivo therapy (Handgretinger et al. 2002).
7.3.3.3 Affinity Chromatography
One of the most important requirements when selecting a separation technique for stem cell-based isolation and purification for cell therapy applications is their potential for large scale bioprocessing. Chromatography is one of the most powerful and widely used separation and purification techniques in downstream processing of biomolecules and the adoption of this method for the separation of different cell types potentially offers many advantages with respect to scalability when compared to FACS and MACS. Since many years ago, several examples have been described in the literature regarding the use of packed bed chromatography for stem cell purification using for example a chromatographic column of avidin-coated Sephadex beads for CD34+ cell enrichment (Johnsen et al. 1999). However, this technique presents several important limitations such as the high shear stress conveyed to the cells, long processing times and the slow rate of diffusion within the pores of the matrix. Indeed, the large size of the cells, their low diffusivity as well as their complex surface structure and chemistry pose severe challenges. Therefore, novel alternatives have been proposed for efficient and gentle cell separation under the principles of chromatography. An alternative to packed bed chromatography can be potentially provided by immunoaffinity expanded bed chromatography (EBC) since this technique is characterized by a high interparticular porosity, high adsorbent surface area and a lower shear hydrodynamic environment. This makes EBC a potentially adequate technique for stem cell-based purification but until now it was only used for the recovery of other human cells, such as human monocytes, from a heterogeneous mixture of blood cells (Ujam et al. 2003). Another alternative can be provided by the use of monolithic chromatographic columns. In this case, columns are made of a continuous matrix rather than beads with porous channels. One possible type of these monolithic chromatographic columns are supermacroporous cryogels (Lozinsky et al. 2003). These columns are prepared by gelation or polymerization at sub-zero temperature under frozen conditions and they have large (10–100 μm) and interconnected pores, allowing micrometer size particles between 1 and 15 μm (like cells) to pass through the columns non-retained (Kumar and Bhardwaj 2008; Kumar and Srivastava 2010). In addition, the hydrophilic nature of pore walls results in a gentle separation system very well suited for large and fragile cells. Envisaging a precise fractionation, these chromatographic columns can be derivatized with a specific antibody ligand introduced at the surface of the pores, allowing the affinity capture of a specific cell type. The application of cryogel-based affinity chromatography was reported for the capture of CD34+ cells from UCB (Kumar and Srivastava 2010). Protein A is a protein obtained from Staphylococus aureus, which binds to the Fc portion of IgG from a wide range of species. When it is covalently coupled to Cryogel surfaces it can be used as an efficient adsorbent for cells that have been coated with a specific antibody (IgG type) that can thus be separated from cells that lack the surface antigen against which the antibody is directed. In this specific case, Protein A-captured CD34+ cells were recovered from the Cryogel by mechanical squeezing. Indeed, since these cryogels are elastic and soft they can withstand the pressure and can be compressed four-to-six fold without getting damaged and they re-swell to their original shape upon addition of more liquid. More than 95 % of bound cells were recovered through this method and these cells maintained their proliferative capacity and the expression of CD34 cell surface marker. Nevertheless, the application of this methodology for other stem cell types remains to be explored.
7.3.3.4 Aqueous Two-Phase Systems (ATPS)
An alternative separation method with a high scalable potential is aqueous two-phase systems (ATPS). ATPS a liquid-liquid fractionation technique used for recovery and primary purification of biological products, including for separation and/or purification of cells (Cabral 2007). This biphasic system is composed of two aqueous solutions at critical concentrations inducing the formation of two immiscible phases. The technique explores the differential partitioning of the biomolecules or cells between the two phases since they will preferentially partition to one of the phases and avoid the other based on their affinity for the compounds that constitute the two phases or the interface. The separation can be performed in one or more steps in negative and/or positive mode according to the required purity degree and cell yield. ATPS can be classified as polymer-polymer and also as polymer-salt systems. Most commonly used polymers include polyethylene glycol (PEG) and dextran whereas the most widely used salts are phosphates, sulphates and citrates (Cabral 2007).
In the case of the more traditional ATPS systems, the affinity of the molecules or cells for one of the two phases or interface is solely defined by their physicochemical properties such as hydrophobicity, size and net surface charge. Nevertheless, novel ATPS strategies for cell separation have been developed that include the use of antibody-conjugated polymers, namely utilizing temperature-sensitive polymers (Kumar et al. 2001). This combined strategy was first employed for type-specific separation of acute myeloid leukemia (KG-1) cells expressing the CD34 antigen in a PEG/dextran system using an antibody conjugated with a temperature-sensitive polymer, the poly-N-isopropylacrylamide (PNIPAM) (Kumar et al. 2001). Under these conditions, the target cells were purified with a high viability, a yield of 75 % and a purity of 80 %. Moreover, the use of PNIPAM allows the potential recovery and re-utilization of the antibody, which turns this method very cost-effective. This separation system was more recently adapted to stem/progenitor cell isolation in our laboratory, more precisely for the isolation of human CD34+ cells directly from whole UCB samples. In this case, the initial population of CD34+ cells (0.2 % of the initial sample) was enriched to values up to 42 % with a yield above 90 % in a single partitioning step. When compared with MACS technology, ATPS provides similar recovery yields (Sousa et al. 2011) and is a more simple method since avoids the use of magnetic particles. Moreover, when compared to FACS, ATPS is more scalable and can be used at an industrial scale. Nevertheless, ATPS still have not addressed the purity standards required for a clinical application (Ruiz-Ruiz et al. 2012) and these systems require the separation of the cells from the phase polymer, which consumes a significant amount of time. Indeed, a repetitive extraction may be required for a sufficient selectivity to be achieved. Considering these characteristics, ATPS can be an adequate solution for purification of stem cells and derivatives for applications in Regenerative Medicine when a precise fractionation is not required but a fast processing is needed. In particular, the ATPS separation method developed in our laboratory is expected to pave a new way to purify HSPC for use in a variety of clinical settings (Sousa et al. 2011).
7.3.3.5 Other Immunoaffinity Techniques
Immunoadsorption techniques may also be applied for cell separation. One of the most traditionally used is the so-called Panning that consists on the covalent immobilization of antibodies to the surface of polystyrene flasks. The cells with surface receptors that bind to the immobilized ligand will tend to adhere to the plastic, while the loose cell fraction can be removed by gentle washing. One classical example of the use of this technique in the stem cell field is the isolation of CD34+ cells from human BM that was achieved with a purity of about 93 % and with a 74 % yield of the multipotent colony-forming units (CFU-GEMM) (Cardoso et al. 1995). This technique, however, is characterized by a low resolution and scalability.
The distinct transient interactions of different cell types with antibodies or lectins immobilized in a surface under fluid flow can also be used as a strategy for cell separation. It has been described that this characteristic, entitled rolling velocity, can be applied for cell separation when the velocity of a specific cell type is significantly lower than the velocity of a non-interacting cell near the surface (Hammer and Apte 1992). These differential interactions were already used for the separation of primitive populations of HSPC from adult BM and fetal liver (CD34+ and CD34+CD38−) from more differentiated cells (CD34− and CD34+CD38+) since the CD34+ and the CD34+CD38− cells were found to roll slowly especially on P-selectin and L-selectin immobilized in a parallel plate flow chamber when compared to more differentiated CD34− and CD34+CD38+ cells (Greenberg et al. 2000). The same basic principle was applied for developing an anti-CD34 antibody-immobilized cell-rolling column that can separate cells according to CD34 density on their surface (Mahara and Yamaoka 2010a). This strategy was already applied with success for the separation of different stem cell populations from BM namely MSC with distinct osteoblastic differentiation potential (Mahara and Yamaoka 2010b).
Novel filtration methods that take advantage of affinity interactions have also been applied to the stem cell field. As one example, a separation device that was developed for the isolation of MSC harvests cells via a nonwoven fabric filter composed of rayon and polyethylene, in a semi-closed system reducing contamination risks, without centrifugation (Ito et al. 2010). The filter selectively traps MSC among mononuclear cells based on affinity and not cell size.
7.3.4 Novel Stem Cell-Based Separation Methods
7.3.4.1 Aptamer-Based Separation
As previously mentioned, immunoaffinity methods are generally used for stem cell-based separation when a high resolution is required. However, this group of methodologies is very expensive mainly due to the necessity of using monoclonal antibodies which may turn unfeasible the application of these technologies on a large-scale for production of stem cells and their derivatives for Regenerative Medicine. In order to overcome this limitation, novel immunoaffinity alternatives have been recently proposed to the use of monoclonal antibodies, such as the use of synthetic peptides or highly-specific nucleic acids generated by combinatorial chemistry for cell capture, the so-called aptamers (Nery et al. 2009). The use of aptamers may be advantageous since their inherent flexibility enables the molecule to bind to target sites that are not normally accessible for typical antibodies. Due to this characteristic, aptamers can be potentially used to distinguish stem cells of the same lineage and with very similar molecular features but with different degrees of commitment. These novel ligands can potentially be adapted to all types of immunaffinity-based techniques. For example, aptamers can be bound to polymeric matrices such as cryogels, magnetic beads or polymers in order to directly replace them in affinity chromatography, immunomagnetic sorting or ATPS, respectively. Aptamers were already used for stem cell separation, in particular for the isolation of BM MSC (Guo et al. 2006) as an alternative to the traditional adherence to plastic surfaces (Lennon and Caplan 2006) or antibody-based separation. Biotinylated aptamers were developed for the recognition of the molecular signature of MSC that were then used for capturing MSC from BM using anti-biotin microbeads and using a cell sorter after being labelled with fluorescein isothiocyanate (FITC). A phenotypic characterization revealed that the purified cells were positive for CD29, CD44 and CD90 expression and most of the cells did not express CD45 being consistent with previous phenotypic characterisation of these cells (Lennon and Caplan 2006). Moreover, following re-plating, the purified cells revealed an increased proliferative capacity and also osteogenic and adipogenic differentiation ability when compared to MSC isolated trough the traditional plastic-adherence method.
7.3.4.2 Tag-Less Methodologies
In addition to the use of synthetic peptides and aptamers, other alternatives have been proposed to overcome the high-expensive nature of the immunoaffinity-based methods. One recent trend in this field is the development of novel tag-less separation methodologies in which no affinity ligands are required. In this case, cell separation is governed by the biophysical properties of cells. One possible tag-less methodology is Field Flow Fractionation (FFF). FFF encompasses a group of label-free and gentle separation techniques whose principles are based on cellular morphological and biophysical differences such as mass, charge, size, density, shape and rigidity. FFF is achieved within an empty capillary channel by the combined action of a transporting laminar flow of mobile phase and a field that is applied perpendicularly to the flow (Reschiglian et al. 2005). Different types of FFF have been used for stem cell separation (Comte et al. 2006; Guglielmi et al. 2004; Roda et al. 2009a, b) but the simplest variant is gravitational FFF (GrFFF) that makes use of the gravity field. GrFFF was already used for isolating human HSPC from mPB (Roda et al. 2009b) and human MSC from a variety of different sources (Roda et al. 2009a). Undifferentiated human HSPC have “simpler” biophysical properties when compared to more differentiated/committed cells with a spherical/ovoidal shape and with a low cytoplasm-to-nucleus ratio while committed cells acquire features related to their function that generally correspond to a more irregular shape and to more complex cytoplasm contents and a lower nucleus-to-cytoplasm ratio (Roda et al. 2009b). For this reason, in GrFFF, spherical particles elute later than non-spherical ones of similar size. In the case of human MSC, this technique was used for characterizing MSC populations from different sources, sorting different MSC subpopulations with a high differentiation potential and purifying MSC from epithelial contaminants (Roda et al. 2009a). As a major disadvantage, GrFFF is generally considered an analytical-scale methodology since a low number of cells can be isolated in each run.
Another label-free alternative for identification and separation of stem cells and stem cell-derived cells is dielectrophoresis (DEP). DEP devices consist of micro-channels filled with an adequate buffer solution into which the sample is injected. A non-uniform electric field is generated and the cells can be separated, moved or trapped (Fig. 7.1). The response of a cell to DEP-mediated forces depends on the polarization between the suspending medium and the intrinsic dielectrical properties of the cell such as cytoplasm, membrane and cell wall conductivities which are dependent on cell density, size, physiology and differentiation state. In the stem cell field, DEP has been used for enrichment of CD34+ HSPC from BM or mPB (Stephens et al. 1996; Talary et al. 1995). More recently, DEP was also applied to NSC populations derived from PSC (hPSNSC), which allowed to correlate the biophysical properties of cells with their differentiation potential. These studies indicated that the ultimate fate of these cells after differentiation can be predicted by distinct changes in their dielectrophoretic properties before the presence of cell-surface proteins can be detected (Flanagan et al. 2008; Labeed et al. 2011). In particular, recent data demonstrates that membrane capacitance, an electrophysiological property of cells, is inversely correlated with the neurogenic potential of human PSC-derived neural stem/progenitor cells (Labeed et al. 2011). This information indicates a potential mechanism to separate stem cells with different neuronal differentiation potential. DEP does not require a large number of cells nor expensive equipment, which are important advantages when compared to FACS.
7.3.4.3 Microfluidic Devices
One of the most recent trends in the field of cell separation is the adaptation of the different methodologies to microscale devices. Distinct flow channel designs have been developed for cell sorting based both on the physicochemical (Kim et al. 2008), affinity (Didar and Tabrizian 2010) and biophysical properties of cells. One illustrative example of a classical cell separation method that was recently incorporated into microfluidics is ATPS (Hardt and Hahn 2012). In this case, in opposition to the classic standard batch ATPS, a number of co-flowing streams of immiscible phases are guided through a microchannel while the biological samples partition between the phases. This type of continuous-flow process presents many advantages such as a more rapid mass transfer, an easy separation of the two phases, since they are recovered from different exit branches, and there is virtually no lower limit for the sample amount to be processed (Hardt and Hahn 2012). Microfluidics present important features for cell separation such as laminar flow, easy integration with mechanical, electrical and optical systems and a low cost fabrication (Wang et al. 2011b). In addition, the use of microfluidics allows handling of very small sample volumes and cell processing on closed systems, which avoids contamination. Although these microscale technologies are very recent, they can potentially be applied to stem cell separation. One example of application of microfluidic devices for stem cell-based separation reported in the literature combines the use of the microfluidic chip technology with optical tweezers, photonic devices that exploit a tightly focused laser beam to manipulate the dielectric properties in three dimensions in a non-invasive manner, for the isolation of human ESC from a mixture of different cells with similar sizes (Wang et al. 2011b). Digital image processing was used for recognition of cell size and fluorescence for separation of human ESC modified with green fluorescent protein (GFP). This microfluidic device thus presents a great potential for depletion of human ESC from cell suspensions obtained after differentiation to eliminate residual tumorigenic, undifferentiated cells.
As another potential application to the stem cell field, microfluidic systems can be used for the development of modern cytometers with enhanced portability for on-site measurements. As one example, micro-fabricated magnetoresistive sensors can be integrated within microfluidic channels for detection of magnetically labelled cells. It was recently described the real-time detection of single magnetically labelled cells with a magnetoresistive based cell cytometer (Loureiro et al. 2011). For KG1-a cells (CD34+) magnetically labelled to anti-CD34-conjugated beads (Miltenyi) and flowing through a 150 μm wide, 14 μm high microchannel, with speeds around 1 cm/s, bipolar signals with an average amplitude of 10–20 μV were observed. This system demonstrated to be effective for cell counting and has potential to be further exploited for stem cell-based separation.
Overall, despite the huge potential of these microscale devices to provide a high resolution separation it should be emphasized that the scaling up of these technologies is limited to the integration of several units in parallel configurations and for this reason the amounts of cells processed is not sufficient for a clinical application requiring 1–2 × 108 cells per patient. Thus, the application of these novel technologies is still for now restricted to the diagnosis field.
7.4 Large-Scale Manufacturing of Stem Cells and Derivatives for Cell Therapies: Bioprocess Integration
When considering the application of separation technologies for stem cell-based therapies, one of the most important issues to consider is their rational integration with culture technologies, especially bioreactor culture systems. A particularly relevant example in which the rational bioprocess integration will be required is the large-scale production of human PSC-derived cells for Regenerative Medicine applications. In fact, among other issues, the application of human PSC derivatives in clinical settings is critically hampered by the absence of highly-efficient separation techniques for purification of the desired cell phenotype after human PSC differentiation. An important aspect to consider when designing such a bioprocess is the integration of at least one high-resolution separation operation in a negative mode for the depletion of human PSC. This is a critical issue mainly because these cells can cause the formation of tumours upon transplantation but also because the presence of human PSC in culture, especially at high densities, can negatively influence the outcome of the differentiation process. Another critical issue associated to the use of PSC derivatives in cell therapies is the low efficiency of human PSC differentiation protocols, with the desired phenotype being obtained with relatively low yields. Due to this, high-resolution separation techniques must also be integrated in a positive mode after the differentiation process for capturing the desired cell phenotype with a relatively high yield and purity. A successful bioprocess for the in vitro production of PSC-derived cells should thus perform a rational integration of these purification operations with the different steps of PSC expansion, commitment and differentiation in bioreactors.
Another example of an integrated bioprocess based on stem cell technology includes a scalable bioreactor system towards the efficient production of human HSPC featuring in situ cell selection to maximize cell productivity. The major objective of this operation is the depletion of mature blood cells arising in culture and their overall effects on culture microenvironment, which is expected to result in a more efficient expansion of HSPC. In fact, these cells are known to secrete negative regulators inhibiting stem cell proliferation and/or inducing differentiation, which may be a major limitation on the expansion of the more primitive HSC. This separation can be performed by using our previously established immunoaffinity ATPS system for UCB HSPC isolation (Sousa et al. 2011). Therefore, the integration of this separation method in a bioreactor system will allow the in situ elimination of the more mature cells. The ability to successfully isolate, purify and expand the numbers of human HSPC ex-vivo, especially those from the UCB, will be an enormous boost to all current and future medical uses of these cells.
Overall, the ability to purify the cultured stem cell populations or their derived progeny along with cell production in a bioreactor, as well as at downstream processing, will represent a major breakthrough in terms of stem cell processing. The integration of these operations should thus be rationally performed in order to develop robust, scalable and cost-effective Bioprocesses towards the large-scale production of stem cells and their derivatives, which ultimately will have a major impact on the potential clinical use of these cells.
7.5 Conclusions and Future Trends
Considering the present and future applications of stem cells in Regenerative Medicine, drug screening, pharmacological testing as well as in fundamental studies on developmental biology and human disease mechanisms it has been recently identified the urgent necessity of developing novel separation techniques and strategies that can be successfully used for the isolation and purification of stem cells and their derivatives. According to the challenges to be faced in this field, separation techniques to be adopted should fulfill major requirements including a high-resolution capacity as well as a high potential for scalability. However, as described in previous sections, presently available techniques have a number of limitations such as their low selectivity, low scalability and contamination risks. Stem cell isolation from a variety of tissues has been performed over the years through the use of physicochemical-based methods, such as density gradient centrifugation, membrane filtration and adhesion-based separation, but these techniques are characterized by a low resolution capacity and they are only efficient for the separation of very different cell types. Affinity-based traditional techniques for cell purification, such as FACS and MACS, have also been systematically used in the stem cell field but these methods are not easily scalable and they are very expensive, due to the necessity of labeling the cells with specific targets for surface markers (e.g. monoclonal antibodies). In addition, cell labeling procedures (e.g. with magnetic particles) can potentially compromise the therapeutic application of cells. Furthermore, with the exception of the hematopoietic family, several stem cell (e.g. human MSC) and stem cell-derived populations (e.g. human PSC-derived cells) still lack a panel of surface markers that can be used as targets during affinity-based separation methods. A great effort should thus be performed towards the identification of novel more specific antigens that can distinguish similar cell populations of the same lineage. For that purpose, future efforts in this field should be focused on performing a thorough characterization of cell populations towards obtaining panels of novel surface markers similar to the ones already available in the haematological field. In addition, different techniques have been proposed to overcome some of the obstacles associated with affinity-based separation methods. Affinity chromatography and ATPS have been considered attractive alternatives since they present a higher potential for scalability but they still require cell labeling. An alternative to overcome this problem is the use of novel cell ligands, such as lectins and aptamers, that can potentially be less expensive for large-scale applications (i.e. aptamers are obtained by chemical synthesis). Microscale technologies based on microfluidic devices have also recently emerged as another powerful tool to overcome these limitations. As a novel trend in this field, microfluidic devices can be designed to perform a high-resolution separation based on the distinct biophysical properties of different cell populations after submitting cells to an electrical stimulation or a gravitational field. These novel “tag-less” biophysical techniques can be combined with other methodologies based on complementary cell properties, such as affinity characteristics, providing an integrated separation strategy. Moreover, the use of laminar flow that characterizes microfluidic systems potentiates a high-resolution separation and parallelization of microfluidic channels in compact arrays increasing the scalability and throughput of the method. Importantly, these devices can be operated as closed systems avoiding cell contamination. Future developments in this field should focus the integration of several microfabricated devices in lab-on-a -chip platforms to perform cell separation, culture, monitoring and concentration in a fully controlled, automated and closed system. Finally, when considering the different applications in stem cell-based therapies, future technological improvements should envisage the development of separation systems with unprecedented selectivity in order to deplete residual pluripotent and immature phenotypes that can cause tumours upon transplantation. Moreover, as an important future trend, integration of separation techniques with stem cell bioreactor culture and cryopreservation is an essential requisite for the translation of stem cells and stem cell-derived products to the clinics and drug discovery. Overall, these progresses are expected to boost the application of stem cell-based therapies in the near future.
References
Andrade PZ, da Silva CL, dos Santos F, Almeida-Porada G, Cabral JM (2011) Initial CD34+ cell-enrichment of cord blood determines hematopoietic stem/progenitor cell yield upon ex vivo expansion. J Cell Biochem 112(7):1822–1831
Aubert J, Stavridis MP, Tweedie S, O’Reilly M, Vierlinger K, Li M, Ghazal P, Pratt T, Mason JO, Roy D et al (2003) Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc Natl Acad Sci U S A 100(Suppl 1):11836–11841
Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J (2001) The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun 289(2):519–524
Bonanno G, Perillo A, Rutella S, De Ritis DG, Mariotti A, Marone M, Meoni F, Scambia G, Leone G, Mancuso S et al (2004) Clinical isolation and functional characterization of cord blood CD133+ hematopoietic progenitor cells. Transfusion 44(7):1087–1097
Brown M, Wittwer C (2000) Flow cytometry: principles and clinical applications in hematology. Clin Chem 46(8 Pt 2):1221–1229
Cabral JM (2007) Cell partitioning in aqueous two-phase polymer systems. Adv Biochem Eng Biotechnol 106:151–171
Capela A, Temple S (2002) LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35(5):865–875
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213(2):341–347
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217(2):318–324
Cardoso AA, Watt SM, Batard P, Li ML, Hatzfeld A, Genevier H, Hatzfeld J (1995) An improved panning technique for the selection of CD34+ human bone marrow hematopoietic cells with high recovery of early progenitors. Exp Hematol 23(5):407–412
Chou S, Chu P, Hwang W, Lodish H (2010) Expansion of human cord blood hematopoietic stem cells for transplantation. Cell Stem Cell 7(4):427–428
Comte I, Battu S, Mathonnet M, Bessette B, Lalloue F, Cardot P, Ayer-Le Lievre C (2006) Neural stem cell separation from the embryonic avian olfactory epithelium by sedimentation field-flow fractionation. J Chromatogr B Analyt Technol Biomed Life Sci 843(2):175–182
Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Fortunato F, Strazzer S, Salani S, Bresolin N, Comi GP (2005) Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. FASEB J 19(13):1860–1862
da Silva CL, Goncalves R, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G (2005) A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol 33(7):828–835
da Silva CL, Goncalves R, Porada CD, Ascensao JL, Zanjani ED, Cabral JM, Almeida-Porada G (2009) Differences amid bone marrow and cord blood hematopoietic stem/progenitor cell division kinetics. J Cell Physiol 220(1):102–111
Didar TF, Tabrizian M (2010) Adhesion based detection, sorting and enrichment of cells in microfluidic Lab-on-Chip devices. Lab Chip 10(22):3043–3053
Diogo MM, da Silva CL, Cabral JM (2012) Separation technologies for stem cell bioprocessing. Biotechnol Bioeng 109(11):2699–2709
Flanagan LA, Lu J, Wang L, Marchenko SA, Jeon NL, Lee AP, Monuki ES (2008) Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells 26(3):656–665
Fong CY, Peh GS, Gauthaman K, Bongso A (2009) Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Stem Cell Rev 5(1):72–80
Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, Sasai Y, Hashimoto N (2006) Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells 24(3):763–771
Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H (2007) The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod 22(3):733–742
Goncalves R, Lobato da Silva C, Cabral JM, Zanjani ED, Almeida-Porada G (2006) A Stro-1(+) human universal stromal feeder layer to expand/maintain human bone marrow hematopoietic stem/progenitor cells in a serum-free culture system. Exp Hematol 34(10):1353–1359
Gonzalez-Gonzalez M, Vazquez-Villegas P, Garcia-Salinas C, Rito-Palomares M (2012) Current strategies and challenges for the purification of stem cells. J Chem Technol Biotechnol 87(1):2–10
Greenberg AW, Kerr WG, Hammer DA (2000) Relationship between selectin-mediated rolling of hematopoietic stem and progenitor cells and progression in hematopoietic development. Blood 95(2):478–486
Gronthos S, Zannettino AC (2008) A method to isolate and purify human bone marrow stromal stem cells. Methods Mol Biol 449:45–57
Grutzkau A, Radbruch A (2010) Small but mighty: how the MACS-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytometry A 77(7):643–647
Guglielmi L, Battu S, Le Bert M, Faucher JL, Cardot PJ, Denizot Y (2004) Mouse embryonic stem cell sorting for the generation of transgenic mice by sedimentation field-flow fractionation. Anal Chem 76(6):1580–1585
Guo KT, SchAfer R, Paul A, Gerber A, Ziemer G, Wendel HP (2006) A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 24(10):2220–2231
Hammer DA, Apte SM (1992) Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J 63(1):35–57
Handgretinger R, Lang P, Ihm K, Schumm M, Geiselhart A, Koscielniak E, Hero B, Klingebiel T, Niethammer D (2002) Isolation and transplantation of highly purified autologous peripheral CD34(+) progenitor cells: purging efficacy, hematopoietic reconstitution and long-term outcome in children with high-risk neuroblastoma. Bone Marrow Transplant 29(9):731–736
Hardt S, Hahn T (2012) Microfluidics with aqueous two-phase systems. Lab Chip 12(3):434–442
Higuchi A, Iizuka A, Gomei Y, Miyazaki T, Sakurai M, Matsuoka Y, Natori SH (2006) Separation of CD34+ cells from human peripheral blood through polyurethane foaming membranes. J Biomed Mater Res A 78(3):491–499
Higuchi A, Sekiya M, Gomei Y, Sakurai M, Chen WY, Egashira S, Matsuoka Y (2008) Separation of hematopoietic stem cells from human peripheral blood through modified polyurethane foaming membranes. J Biomed Mater Res A 85(4):853–861
Ito K, Aoyama T, Fukiage K, Otsuka S, Furu M, Jin Y, Nasu A, Ueda M, Kasai Y, Ashihara E et al (2010) A novel method to isolate mesenchymal stem cells from bone marrow in a closed system using a device made by nonwoven fabric. Tissue Eng Part C Methods 16(1):81–91
Johnsen HE, Hutchings M, Taaning E, Rasmussen T, Knudsen LM, Hansen SW, Andersen H, Gaarsdal E, Jensen L, Nikolajsen K et al (1999) Selective loss of progenitor subsets following clinical CD34+ cell enrichment by magnetic field, magnetic beads or chromatography separation. Bone Marrow Transplant 24(12):1329–1336
Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D (2002) Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 46(12):3349–3360
Kim SM, Lee SH, Suh KY (2008) Cell research with physically modified microfluidic channels: a review. Lab Chip 8(7):1015–1023
Kumar A, Bhardwaj A (2008) Methods in cell separation for biomedical application: cryogels as a new tool. Biomed Mater 3(3):034008
Kumar A, Srivastava A (2010) Cell separation using cryogel-based affinity chromatography. Nat Protoc 5(11):1737–1747
Kumar A, Kamihira M, Galaev IY, Mattiasson B, Iijima S (2001) Type-specific separation of animal cells in aqueous two-phase systems using antibody conjugates with temperature-sensitive polymers. Biotechnol Bioeng 75(5):570–580
Labeed FH, Lu J, Mulhall HJ, Marchenko SA, Hoettges KF, Estrada LC, Lee AP, Hughes MP, Flanagan LA (2011) Biophysical characteristics reveal neural stem cell differentiation potential. PLoS One 6(9):e25458
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S et al (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25(9):1015–1024
Lennon DP, Caplan AI (2006) Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol 34(11):1604–1605
Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP, Langer R (2010) Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Protoc 5(6):1115–1126
Loureiro J, Andrade PZ, Cardoso S, da Silva CL, Cabral JM, Freitas PP (2011) Magnetoresistive chip cytometer. Lab Chip 11(13):2255–2261
Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B (2003) Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol 21(10):445–451
Mahara A, Yamaoka T (2010a) Antibody-immobilized column for quick cell separation based on cell rolling. Biotechnol Prog 26(2):441–447
Mahara A, Yamaoka T (2010b) Continuous separation of cells of high osteoblastic differentiation potential from mesenchymal stem cells on an antibody-immobilized column. Biomaterials 31(14):4231–4237
Majore I, Moretti P, Hass R, Kasper C (2009) Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun Signal 7:6
Muller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, Muller OJ, Schlenke P, Frese S, Wobus AM et al (2000) Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J 14(15):2540–2548
Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, Hu WW, Johnston LJ, Shizurn JA, Stockerl-Goldstein KE et al (2000) Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant 6(3):262–271
Nery AA, Wrenger C, Ulrich H (2009) Recognition of biomarkers and cell-specific molecular signatures: aptamers as capture agents. J Sep Sci 32(10):1523–1530
Parekkadan B, Sethu P, van Poll D, Yarmush ML, Toner M (2007) Osmotic selection of human mesenchymal stem/progenitor cells from umbilical cord blood. Tissue Eng 13(10):2465–2473
Pethig R, Menachery A, Pells S, De Sousa P (2010) Dielectrophoresis: a review of applications for stem cell research. J Biomed Biotechnol 2010:182581
Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O (2007) Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells 25(9):2257–2268
Reschiglian P, Zattoni A, Roda B, Michelini E, Roda A (2005) Field-flow fractionation and biotechnology. Trends Biotechnol 23(9):475–483
Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF (2001) Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 412(6848):736–739
Roda B, Lanzoni G, Alviano F, Zattoni A, Costa R, Di Carlo A, Marchionni C, Franchina M, Ricci F, Tazzari PL et al (2009a) A novel stem cell tag-less sorting method. Stem Cell Rev 5(4):420–427
Roda B, Reschiglian P, Alviano F, Lanzoni G, Bagnara GP, Ricci F, Buzzi M, Tazzari PL, Pagliaro P, Michelini E et al (2009b) Gravitational field-flow fractionation of human hemopoietic stem cells. J Chromatogr A 1216(52):9081–9087
Ruiz-Ruiz F, Benavides J, Aguilar O, Rito-Palomares M (2012) Aqueous two-phase affinity partitioning systems: current applications and trends. J Chromatogr A 1244:1–13
Santos F, Andrade PZ, Abecasis MM, Gimble JM, Chase LG, Campbell AM, Boucher S, Vemuri MC, Silva CL, Cabral JM (2011) Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods 17(12):1201–1210
Schriebl K, Lim S, Choo A, Tscheliessnig A, Jungbauer A (2010) Stem cell separation: a bottleneck in stem cell therapy. Biotechnol J 5(1):50–61
Schriebl K, Satianegara G, Hwang A, Tan HL, Fong WJ, Yang HH, Jungbauer A, Choo A (2012) Selective removal of undifferentiated human embryonic stem cells using magnetic activated cell sorting followed by a cytotoxic antibody. Tissue Eng Part A 18(9–10):899–909
Shibata H, Ageyama N, Tanaka Y, Kishi Y, Sasaki K, Nakamura S, Muramatsu S, Hayashi S, Kitano Y, Terao K et al (2006) Improved safety of hematopoietic transplantation with monkey embryonic stem cells in the allogeneic setting. Stem Cells 24(6):1450–1457
Sousa AF, Andrade PZ, Pirzgalska RM, Galhoz TM, Azevedo AM, da Silva CL, Aires-Barros MR, Cabral JM (2011) A novel method for human hematopoietic stem/progenitor cell isolation from umbilical cord blood based on immunoaffinity aqueous two-phase partitioning. Biotechnol Lett 33(12):2373–2377
Stephens M, Talary MS, Pethig R, Burnett AK, Mills KI (1996) The dielectrophoresis enrichment of CD34+ cells from peripheral blood stem cell harvests. Bone Marrow Transplant 18(4):777–782
Sundberg M, Andersson PH, Akesson E, Odeberg J, Holmberg L, Inzunza J, Falci S, Ohman J, Suuronen R, Skottman H et al (2011) Markers of pluripotency and differentiation in human neural precursor cells derived from embryonic stem cells and CNS tissue. Cell Transplant 20(2):177–191
Talary MS, Mills KI, Hoy T, Burnett AK, Pethig R (1995) Dielectrophoretic separation and enrichment of CD34+ cell subpopulation from bone marrow and peripheral blood stem cells. Med Biol Eng Comput 33(2):235–237
Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR et al (2011) An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol 29(9):829–834
Tondreau T, Lagneaux L, Dejeneffe M, Delforge A, Massy M, Mortier C, Bron D (2004) Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy 6(4):372–379
Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL (2000) Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A 97(26):14720–14725
Ujam LB, Clemmitt RH, Clarke SA, Brooks RA, Rushton N, Chase HA (2003) Isolation of monocytes from human peripheral blood using immuno-affinity expanded-bed adsorption. Biotechnol Bioeng 83(5):554–566
Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK (2011) Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One 6(8):e23657
Wang P, Rodriguez RT, Wang J, Ghodasara A, Kim SK (2011a) Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 8(3):335–346
Wang X, Chen S, Kong M, Wang Z, Costa KD, Li RA, Sun D (2011b) Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 11(21):3656–3662
Wang YC, Nakagawa M, Garitaonandia I, Slavin I, Altun G, Lacharite RM, Nazor KL, Tran HT, Lynch CL, Leonardo TR et al (2011c) Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res 21(11):1551–1563
Weissman IL, Shizuru JA (2008) The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112(9):3543–3553
Wu CH, Lee FK, Suresh Kumar S, Ling QD, Chang Y, Wang HC, Chen H, Chen DC, Hsu ST, Higuchi A (2012) The isolation and differentiation of human adipose-derived stem cells using membrane filtration. Biomaterials 33(33):8228–8239
Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA et al (2011) Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One 6(3):e17540
Acknowledgments
The authors acknowledge the funding from Fundação para a Ciência e Tecnologia (FCT) through the Compromisso para a Ciência Program (2007), MIT-Portugal Program and projects PTDC/EBB-BIO/101088/2008, PTDC/EQU-ERQ/105277/2008, PTDC/EQU-EQU/114231/2009.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Diogo, M.M., da Silva, C.L., Cabral, J.M.S. (2014). Separation Technologies for Stem Cell Bioprocessing. In: Al-Rubeai, M., Naciri, M. (eds) Stem Cells and Cell Therapy. Cell Engineering, vol 8. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7196-3_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-7196-3_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7195-6
Online ISBN: 978-94-007-7196-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)