Abstract
Pollution is a phenomenon, which leads to ecological disequilibrium (alteration of biotic and abiotic) and may produce dangerous waste. Epidemiological studies, evaluate the relationship between the pollutants impacts over individual or collective risk and environmental factors. The rational use of pesticides in conjunction with other technologies may be justifiable in integrated pest management, the balance between benefits and effects being very complex. Pesticides are considered persistent pollutants, and may be classified according to chemical structure in the following main classes: organophosphates, carbamates, organochlorines, triazines, and pyrethroids. In this paper we present the mechanisms of action of the main pesticide classes in living organisms and especially in the human body. Organophosphate pesticides act on acetylcholinesterase, leading to development of cholinergic toxicity, because they decrease its enzymatic activity. The carbamate or phosphate pesticides inhibition of acetylcholinesterase, disrupts the equilibrium between acetylcholine synthesis and release on one hand and its hydrolysis on the other, and leads to its accumulation at synaptic level, with prolonged activation of cholinergic receptors. Organochlorine pesticides are highly lipophilic, and this property enhances their stability in living organisms and in the environment. They are largely stored in adipose tissue, a process called bioaccumulation, and this characteristic leads to the development of high toxicities in mammals. Triazines in high concentrations have been linked to increased cancer risk and incidence of birth defects. The pyrethroid insecticides acting on the sodium channels in the nerve membrane (neurotoxic), have high selectivity for insects, and do not have carcinogenic, mutagenic and teratogenic effects. Living organisms and humans are concurrently exposed to pesticides from more than one source, via the environment and food, and these may have a combined (synergistic or antagonistic) action, which can cause higher or lower toxic effects, in comparison with the situation of a single pesticide.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Organophosphate pesticides
- Organochlorine pesticides
- Triazine pesticides
- Pyrethrin pesticides
- Enzyme
- Action mechanism
16.1 Introduction
Our present society is facing severe environmental problems, at the local, regional and global level. Maintaining or improving of the quality of the environment may be materialized through environmental monitoring.
The monitoring activities must identify and describe the evolution of the environmental qualitative and quantitative parameters in relationship with the affecting factors.
Pollution is a phenomenon, which leads to ecological disequilibrium (alteration of soil, air, water and biota) and consists of the following elements [17]: pollution sources, pollutants transport and pollutants target.
The pollution affects air (climatic changes, stratospheric ozone depletion, acidification), waters (eutrophication, oxygen reduction), foods (reduced quality and quantity of nutrients), biota (habitat depletion and deterioration, species threatening) and may produces dangerous wastes [8].
The human body is exposed to an enormous number of non-nutrient compounds from the environment, many of which could be toxic. Epidemiological studies, evaluate the relationship between pollutants impacts over individual or collective risk and environmental factors.
16.2 Pesticides
Pesticides are among the environmental pollutant agents, which kill unwanted living organisms (animals or plants). Usually a pest is considered any living organism, which is interfering in a negative way with the human activity.
The rational use of pesticides in conjunction with other technologies may be justifiable in integrated pest management, the balance between benefits and effects being very complex.
The intended effects of pesticide use are as follows [9]:
-
the control of pests and vectors of diseases;
-
the control of organisms that harm other human activities.
On one hand, at community, national and global scales, there are three main benefit domains of pesticide use [10]:
-
social: health and quality of life;
-
economic: farm (agricultural) costs and profits;
-
environmental: terrestrial, aquatic and air.
On the other hand, the presence of pesticides in non-target species (including humans) can induces some perturbations, dependent on their nature, and their interactions with functional molecules.
Pesticides are a public health risk, and their use is the subject of some controversies concerning “what is acceptable risk” or “how can we minimize the risk” [16].
Pesticides are considered persistent pollutants, and may be classified according to their chemical structure in the following classes: organophosphates, carbamates, organochlorines, triazines, and pyrethroids.
16.2.1 Organophosphate Pesticides
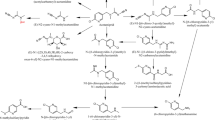
All organophosphate pesticides contain a central phosphorus (P) atom to which an oxygen or sulfur atom is doubly bound (Fig. 16.1). Two methoxy or ethoxy groups are also singly bonded to the central P atom, while a longer more complex structure (Z) is singly bonded to phosphorus, usually by an oxygen or sulphur atom [9, 11].
The toxicity of organophosphates is determined by the electrophilic magnitude of the central phosphorus atom, the steric nature of the substituents R1 and R2, and by the strength of the bond P – Z, and it is influenced by groups that increase the lability of the P – Z bond, which is broken during the inhibition process [32].
Organophosporus pesticides with p-CH3–S–aromatic group or p-NO2–aromatic group are the most toxic because these substituents enhance the electrophilicity of the P atom, by inductive and mesomeric effects. Mammals’ metabolic transformation (biotransformation) of these groups, leads to magnification of the electrophilic character of P atom and increases the pesticide toxicity (p-CH3–S group can be oxidized to p-CH3–SO group or p-CH3–SO2 group).
Also, the toxicity of organophosphate pesticides decreases, with increasing chain length of R1 and R2 groups, because it decreases the electrophilic magnitude of the central phosphorus atom.
The primary target of organophosphate pesticides is acetylcholinesterase leading to development of cholinergic toxicity, because they decrease its enzymatic activity [3, 31, 32].
Acetylcholinesterase is a serine enzyme with a hydroxymethyl group, present in the active site, which interacts with organophosphate pesticides to form an inactive complex (Fig. 16.2).
The physiological role of acetylcholinesterase is to catalyze the hydrolysis of acetylcholine, which is a major neurotransmitter in the central or peripheral nervous systems (Fig. 16.3). Acetylcholinesterase is a key enzyme responsible for terminating the nervous impulse.
Acetylcholinesterase inhibition causes acetylcholine accumulation in the synaptic cleft and the postsynaptic membrane is continuously stimulated. Lack of coordination of the neuromuscular system is a direct result of this mechanism of action, and the final response can be death [1, 30].
Acetylcholinesterase inhibition can also alter lymphocytic activity, and organophosphates have been associated with the development of immunological disorders [13, 20] or cancers [6].
Organophosphate pesticides are weak inhibitors of acetylcholinesterase when a sulfur atom is bound to phosphorus (P = S) in the general structure [29].
In phase I biotransformation processes, cytochromes P450 (CYP450) act as monooxigenases on organophosphate pesticides (P = S) and they are transformed by desulfurization, in the corresponding oxon analogs (P = O). Metabolic desulfurization process of organophosphorus pesticides is considered bioactivation [3, 11], because oxon compounds are up to 1,000 times stronger inhibitors of acetylcholinesterase, than the parent compounds (Fig. 16.4).
Organophosphates may also influence homeostasis of liver, brain and muscle where are glycogenesis (glycogen synthesis from glucose), gluconeogenesis (synthesis of glucose from nonzaharidic organic molecules), glycogenolysis (breakdown of glycogen to glucose) and glycolysis (glucose transformation to two molecules of pyruvic acid or lactic acid) are taking place, through pancreas control of glucose homeostasis, and by pancreatic control of insulin and glucagon secretion [23].
Exposure to organophosphate pesticides (dimethoate, acephate, malathion, dichlorvos) induces hyperglycemia through decreased hexokinase activity in favor of glycogen storage activation (increase glycogen synthase activity) and in detriment of glycolysis [28].
The induction of stress (the response to every situation which threatens homeostasis) or oxidative stress (alteration of the enzymes associated with antioxidant defenses) are the molecular mechanisms activated in pesticide toxicity, especially in presence of organophosphate pesticides [24].
Organophosphate pesticides induce reactive oxygen species generation and reactive nitrogen species generation, which contribute to the pathogenesis of acute pancreatitis through destruction of β-cells in diabetes and development of autoimmune diabetes [23]. Organophosphates are the most commonly used pesticides and are heavily used in agriculture and in urban settings as insecticides. Because of this, most of the population has been exposed to organophosphate pesticides in homes, outdoors, in workplaces, or through vegetal and animal foods consumption and in most of the subchronic or chronic disease cases there is no clear etiology because physicians cannot establish a direct cause-effect relationship between those and the pesticide toxicity.
16.2.2 Carbamate Pesticides
The carbamate pesticides are derivatives of carbamic acid, where one of the hydrogen atoms attached to nitrogen is replaced by a longer organic substituent (Fig. 16.5).
The carbamate insecticides inhibit acetylcholinesterase, like the organophosphate insecticides (the same inhibition mechanism). Organophosphate and carbamate pesticides are primarily recognized by their acetylcholinesterase inhibition [2,14].
The carbamate or phosphate pesticides inhibition of acetylcholinesterase, disrupts the equilibrium between acetylcholine synthesis and release on one hand and its hydrolysis on the other, and leads to its accumulation at synaptic level, with prolonged activation of cholinergic receptors [22].
Compared to organophosphate pesticides, the carbamate pesticides action is reversible, shorter in duration and milder in intensity.
16.2.3 Halogenated Pesticides
Halogenated compounds (organochlorine pesticides) are highly lipophilic, and this property enhances their stability in living organisms and in the environment. They are largely stored in adipose tissue, a process called bioaccumulation, and this characteristic leads to the development of high toxicities in mammals.
The toxicity of organochlorine pesticides is determined by their structure, the spatial arrangement of their atoms (symmetry and asymmetry), and the nature of the substituents [12, 19].
The main characteristics of organochlorine insecticides are:
-
preponderantly absorbed via the digestive system;
-
accumulate in lipid rich tissues (adipose tissue, liver, milk, brain);
-
interact with membrane lipoproteins (staple of the nerve membranes) causing the distortion of the nerve impulse transmission;
-
adverse health effects associated with organochlorine pesticide exposure may be: neurological deficiency, respiratory problems, dermal damage, memory disorders and cancer.
DDT (dichlorodiphenyltrichloroethane) is a penta chloro aromatic compound, which acts on enzyme systems or nervous tissue, and is highly insecticidal (Fig. 16.6).
DDT was the first widely used synthetic pesticide and the first pesticide used as insecticide in 1939 as an organic compound with extreme persistence, stability and low cost [19].
The use of DDT has been credited for the eradication of malaria in Europe and the United States and for revolutionizing agricultural production, but it has been associated with numerous pathologies in many animal populations [4].
In the developed countries DDT use has been restricted and continues to be used only as vector control.
DDT exerts its toxicity by binding to lipoproteins in the nerve cell membrane, disturbing the ionic homeostasis, especially the sodium/potassium balance across this membrane.
Also, DDT and its analogues can act through other mechanisms in the human body:
-
interact with the neuron membrane;
-
alter the transmission of the nervous impulses;
-
alter the mitochondrial phosphorylation (cell energy).
The highly chlorinated cyclic hydrocarbons (aldrin, dieldrin, heptachlor, endrin, alodan, chlordane) acts as lindane or DDT, to produce convulsivant actions (cyclodiene insecticides).
The presence of the double bonds in chlorinated cyclic hydrocarbons, increases pesticide toxicity, whereas presence of epoxide group in these compounds, decreased pesticide toxicity [18, 19].
Aldrin is a hexachlorinated cyclodiene and resembles the dieldrin structure, which possess a stable epoxide ring (Fig. 16.7). Dieldrin is a highly lipophilic compound and this property enhances its stability in the environment and in living organisms.
Dieldrin is an organochlorinated pesticide, and it is classified as chlorinated cyclodiene with an unusually stable epoxide ring compared to other compounds.
In living organisms, dieldrin induces reactive oxygen species (ROS) production and as a result can cause [18, 25]:
-
a decrease of dopamine level, altering the function of dopamine transporters;
-
perturbation of mitochondrial membrane potential, altering mitochondrial membrane perme-ability;
-
the release of cytochrome c from inner mitochondrial membrane into the cytosol, where it activates caspases;
-
caspase mediate hydrolytic cleavage and activation of protein kinase C, which acts on caspases by positive feedback (amplification of the apoptotic process);
-
activated protein kinase C induced DNA fragmentation, which contributes to apoptotic cell death.
Exposure to chlorinated pesticides has been associated with some pathological states: non-Hodgkin’s lymphoma, aplastic anemia, multiple myeloma, leukemia [6].
16.2.4 Triazine Pesticides
Triazines are moderately water-soluble herbicides (difficult to remove from potable water) and their chemical structure is based on an aromatic ring with three nitrogen atoms in alternating positions. Triazine derivatives possess some substituents (chlorine, amino groups) on the aromatic carbons (Fig. 16.8).
The best-known member of the triazines is atrazine, which is not very acutely toxic, but, in high concentrations, has been linked to increased risk of developing cancer and an increased incidence of birth defects [27].
16.2.5 Pyrethroid Pesticides
Pyrethroids are derivatives of natural pyrethrins and most of them contain cyclopropane carboxylic acid linked to alcohols through an ester or ether bond (Fig. 16.9).
Addition of an α-cyano group to general structure of pyrethrins, confers an increase of one order of magnitude of insecticidal activity for target or neurotoxic activity for non-target species [33].
Pyrethroids have 2–4 chiral carbons and the spatial conformation is determinant for their biological activity.
The pyrethroid insecticides acting on the sodium channels in the nerve membrane (neurotoxic), have high selectivity for insects, and do not have carcinogenic, mutagenic and teratogenic effects [5].
In humans the biotransformation of pyrethroids is rapid (ester hydrolysis or cytochrome P450 oxidation) and complete elimination from the body is realized in 2–8 days (detoxification).
A high dose of pyrethroids induces neurotoxicity in mammals (the non target species) in a similar manner to the effects it has in insects (the target species) for which pyrethroid pesticides possess a high selectivity. Compared to insects, mammals are three orders of magnitude less sensitive to pyrethroids and this characteristic supports the increasing substitution of other more toxic insecticide classes by these in order to decrease human occupational poisoning [26].
Target systems for pyrethroids in mammals are: protein phosphorylation (cell energy), noradrenaline release, membrane depolarization (permeability), nicotinic receptors, lymphocyte proliferation [7].
16.2.6 Action Mechanism Through the Reactive Oxygen Species
Paraquat (PQ) is a quaternary nitrogen herbicide, which is not absorbed from the gastrointestinal tract but is absorbed across the skin. Upon absorption, paraquat is little metabolized in the human body, and tends accumulates in the kidney and lung where it exerts its acute effects [15, 21].
In humans, chronic paraquat exposure is a possible etiological factor for Parkinson’s disease, which is a pathological state manifested by abnormalities of motor control: tremors, rigidity, and loss of reflexes.
In the human body, paraquat induces oxidative stress (production of reactive oxygen species) and alteration of the mitochondrial metabolism, manifested through changes in cell energy levels (Fig. 16.10).
Living organisms and humans are concurrently exposed to pesticides, both via the environment and food, and these may have a combined (synergistic or antagonistic) action, which can causes higher or lower toxic effects, in comparison with the situation of single pesticide [27].
The Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA) in the USA, the Health Council of the Netherlands, the Committee on Toxicity of Chemicals in Food, the Danish Veterinary and Food Administration, and other national or international organizations, recommended the introduction of the physiologically based toxicokinetic modelling (PBTK) as a tool in the risk assessment of pesticide mixtures.
These models require a large amount of data for construction, and once constructed and evaluated, they can be used for predicting the level of pesticides at the target site [7, 34, 35].
16.3 Conclusions
This paper presents some theoretical aspects of the pollutants (pesticides) and their action mechanisms in living organisms (mammals).
The carbamate or phosphate pesticides acetylcholinesterase inhibition, disrupt the equilibrium between acetylcholine synthesis, release and hydrolysis, and leads to their accumulation at synaptic levels, with prolonged activation of cholinergic receptors.
Organochlorine pesticides are highly lipophilic, and this property enhances their stability in living organisms and in the environment. This characteristic leads to the development of high toxicities in mammals.
Triazines can induces in high concentrations some disturbing links to cancer risk and incidence of birth defects.
The pyrethroid insecticides are high selectivity for insects, and are not carcinogenetic, mutagenetic and teratogenetic effects.
Living organisms and humans are concurrently exposed to pesticides and may have a combined (synergistic or antagonistic) action, which can causes higher or lower toxic effects, in comparison with the situation of single pesticide.
Pesticides are heavily used in agriculture and in urban as insecticides, and most of population has been contaminated to it in homes, outdoors, workplaces, or through vegetal and animal foods and most of subchronic or chronic diseases are not diagnosed, because cannot be established a direct cause-effect by physicians.
References
Aygun D, Doganay Z, Altintop L, Guven H, Onar M, Deniz T, Sunter T (2002) Serum acetyl cholinesterase and prognosing. J Toxicol Clin Technol 40:903–910
Badea M, Bala C, Rotariu L, Coman G, Gocan S, Marty JL (2008) Methyl paraoxon detection using HPLC-UV and Electric eel acetylcholinesterase based biosensors. J Environ Prot Ecol 9(4):763–772
Badea M, Florescu M, Coman G, Chesca A, Marty JL (2009) Comparative studies for pollutants detection using electrochemical sensors and enzyme-based biosensors. In: Vytas K, Kalcher K, Svancara I (eds) Sensing in electroanalysis. University Press, Pardubice
Beard J (2006) DDT and human health. Sci Total Environ 355:78–89
Carle RP, Calos R, Delabarre M, Escuret P, Fourcaud A (1982) Delatamethrin, Roussel Uclaf
Carozza SE, Li B, Wang Q, Horel S, Cooper S (2009) Agricultural pesticides and risk of childhood cancers. Int J Hyg Health 212:186–195
Clewell RA, Clewell HJ (2008) Development and specification of physiologically based pharmacokinetics models for use in risk assessment. Regul Toxicol Pharmacol 50:129–143
Colbeck I, Draghici C, Perniu D (2004) Pollution and environmental monitoring. Editura Academiei Romane, Bucuresti
Coman G, Draghici C (2004) Pollutants and their impact on human body. Transilvania University Press, Brasov
Cooper J, Dobson H (2007) The benefits of pesticides to mankind and the environment. Crop Prot 26:1337–1348
Costa L (2006) Current issues in organophosphate toxicology. Clin Chim Acta 366:1–13
Duarte CT, Roman R, Tinoco R, Duhalt RV (2009) Halogenated pesticide transformation by laccase-mediator system. Chemosphere 77:687–692
Fabry G, Duhayon S, Mertens C, Lison D (2008) Risk of leukemia among pesticide manufacturing workers. Environ Res 106:121–137
Ferrari A, Venturino A, Pechen A (2007) Effects of carbaryl and azinphos methyl on juvenile rainbow trout detoxifying enzymes. Pesticide Biochem Physiol 88:134–142
Franco R, Li S, Rodriguez H, Burns M, Panayiotidis M (2010) Molecular mechanisms of pesticide neurotoxicity. Chem Biol Interact 188:289–300
George J, Shukla Y (2011) Pesticides and cancer. J Proteomics 74:2713–2722
Haiduc I (1996) Environmental chemistry. Babes-Bolyai University Press, Cluj-Napoca
Kanthasamy AG, Kitazava M, Kanthasamy A, Anantharam V (2005) Dieldrin-induced neuro toxicity. Neurotoxicology 26:701–719
Kaushik P, Kaushik G (2007) An assessment of structure and toxicity correlation in organochlorine pesticides. J Hazard Mater 143:102–111
Mahajan R, Blair A, Lynch CF, Schroeder P, Hoppin JA, Slander DP, Alavanja MCR (2006) Fonofos exposure and cancer incidence, Agricultural Health Study. EnvironHealth Prospect 114(12):1838–1842
Moretto A, Colosio C (2011) Biochemical and toxicological evidence of neurological effects of pesticides. Neurotoxicology 32:383–391
Pope C, Karanth S, Liu J (2005) Pharmacology and toxicology of cholinesterase inhibitors. Environ Toxicol Pharmacol 19:433–446
Rahimi R, Abdollahi M (2007) A review of mechanisms involved in hyperglycemia induced by organo phosphorus pesticides. Pesticide Biochem Physiol 88:115–121
Ranjbar A, Solhi H, Mashayekhi F, Rezaie A (2005) Oxidative stress in acute human poisoning with organophosphorus insecticides. Environ Toxicol Pharmacol 20:88–91
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920
Ray DE, Fry JR (2006) A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther 111:174–193
Reffstrup TK, Larsen JC, Meyer O (2010) Risk assessment of mixtures of pesticides. Regul Toxicol Pharmacol 56:174–192
Rezc R, Mornagui B, Arbi AE, Kamoun A, Fazaa SE, Gharbi N (2006) Effect of subchronic exposure to malathion on glycogenphosphorylase and hexokinase activities in rat liver using native PAGE. Toxicology 223:9–14
Sanchez LS, Roman R, Duhalt RV (2012) Pesticide transformation by a variant of CYPBM3 with improved peroxygenase activity. Pesticide Biochem Physiol 102:169–174
Singh K, Singh KD (2000) Toxicity to the snail Limmaea acuminate of plant-derived molluscicides in combination with synergists. Pest Manag Sci 56:889–898
Singh S, Kumar V, Thakur S, Banerjee B, Chandna S, Pasha S, Jain S, Rai A (2011) DNA damage and cholinesterase activity in occupational workers exposed to pesticides. Environ Toxicol Pharmacol 31:278–285
Tahara M, Kubota R, Nakazava H, Tokunaga H, Nishimura T (2005) Use of cholinesterase activity as an indicator for the effects of combinations of organophosphorus pesticides in water from environmental sources. Water Res 39:5112–5118
Wolansky MJ, Harrill JA (2008) Neurobehavioral toxicity of pyrethroid insecticides in adult animals. Neurotoxicol Teratol 30:55–78
US EPA (2005) Guidelines for Carcinogen Risk Assessment. US EPA, Washington, DC
ATSDR (2004) Guidance manual for the assessment of joint toxic action of chemical mixtures. Division of Toxicology, Atlanta
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Coman, G., Farcas, A., Matei, A.V., Florian, C. (2013). Pesticides Mechanisms of Action in Living Organisms. In: Simeonov, L., Macaev, F., Simeonova, B. (eds) Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6461-3_16
Download citation
DOI: https://doi.org/10.1007/978-94-007-6461-3_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6460-6
Online ISBN: 978-94-007-6461-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)