Abstract
Maintaining correct cellular function is a fundamental biological process for all forms of life. A critical aspect of this process is the maintenance of protein homeostasis (proteostasis) in the cell, which is largely performed by a group of proteins, referred to as the protein quality control (PQC) network. This network of proteins, comprised of chaperones and proteases, is critical for maintaining proteostasis not only during favourable growth conditions, but also in response to stress. Indeed proteases play a crucial role in the clearance of unwanted proteins that accumulate during stress, but more importantly, in the activation of various different stress response pathways. In bacteria, the cells response to stress is usually orchestrated by a specific transcription factor (sigma factor). In Escherichia coli there are seven different sigma factors, each of which responds to a particular stress, resulting in the rapid expression of a specific set of genes. The cellular concentration of each transcription factor is tightly controlled, at the level of transcription, translation and protein stability. Here we will focus on the proteolytic regulation of two sigma factors (σ32 and σS), which control the heat and general stress response pathways, respectively. This review will also briefly discuss the role proteolytic systems play in the clearance of unwanted proteins that accumulate during stress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sigma Factor
- Heat Shock Response
- General Stress Response
- Protein Quality Control
- Stress Response Pathway
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Like many living organisms, bacteria are constantly challenged with changing environmental conditions. In order to survive these changes, bacteria have developed a number of different cellular strategies. In cases where the stress is short-lived (e.g. heat-shock) they have developed sophisticated networks or programs to combat the effects of the stress, while in cases where the stress may be prolonged (e.g. when nutrients are depleted) they can enter a “hibernation”-like state, waiting for the return of better conditions. In fact, bacteria have developed several distinct pathways, each of which is tailored to a particular type of stress. In most cases, the response is controlled by a master regulator (or sigma factor), which in turn activates the expression of a particular set of genes (or regulon) that restore cellular homeostasis. In Escherichia coli, there are seven different sigma factors (σ70, σ54, σ38, σ32, σ28, σ24 and σ18), all of which compete for binding to the RNA polymerase (RNAP) core enzyme, for the transcription of a specific set of genes. As such, the cellular levels of these master regulators (and their affinity to RNAP) are crucial for the activation and/or maintenance of these different stress responses. Given this, it is not surprising that the active cellular concentration of these regulatory proteins is tightly controlled, not only at the transcriptional and translational levels, but also at the post-translational level through protein degradation. Hence proteases play a key role in the regulation of stress response pathways. This review will focus primarily on the general stress response and the heat shock response in E. coli. For a detailed description of the extracytoplasmic or extracellular stress response please refer to the accompanying review by Brachinger and Ades [1].
General Stress Response
As the name suggests, the general stress response is a common cellular response that is activated by a range of different conditions, from nutrient starvation and moderate temperature downshifts [2] to high osmolarity [3] and pH downshifts. It is characterised by a number of distinct morphological and physiological changes [4], which protects the cell from assault by these different stresses. As such, the general stress response acts as a pre-emptive measure to prevent subsequent cellular damage. This response occurs through the activation of a common set of genes that are up regulated by an alternative sigma factor subunit of RNAP, commonly referred to as the stationary-phase sigma factor (σ38), also known as σS [4–6]. The following section will describe some of these pathways, focusing in particular on the role of proteases in controlling the general stress response in E. coli.
The Master Regulator of the General Stress Response, σS
SigmaS (σS) was first discovered as the master regulator of stationary-phase [4]. It is an inducible subunit of RNAP, which is related to the constitutively expressed vegetative or housekeeping sigma-factor, σ70, and as such competes for binding to the RNAP core enzyme [7] (Fig. 5.1a). Under normal cellular conditions (i.e. in rapidly growing cells) the levels of σS are low [8]. However, during stationary phase and in response to a number of different stresses, such as anaerobiosis [9], oxidative stress [10], and osmotic stress [5] the cellular levels of σS rise rapidly (Fig. 5.1a, b). As a result of this increase in the cellular concentration of σS, RNA polymerase is directed to a specific set of promoters resulting in the expression of downstream σS-dependent genes, which control the metabolic state of a cell (Fig. 5.1) [11, 12]. Indeed, σS has been implicated in the regulation (either directly or indirectly) of approximately 500 genes, which equates to approximately 10 % of the E. coli genome [12–14]. These genes are expressed not only during the transition into stationary-phase, but also in response to a number of different stresses [12], i.e. under conditions of nutrient limitation, in which the cells switch from optimal growth to a “maintenance” state. Similarly, σS also controls the expression of genes that mediate programmed cell death, in which the sacrifice of a small population of cells under extreme stress provides a supply of nutrients to other cells permitting their survival [15]. In addition to these survival mechanisms, σS also controls virulence genes in pathogenic enteric bacteria [reviewed by 16].
In E. coli, the levels of SigmaS are controlled not only at the transcriptional and translational level, but also through protein degradation. (a) During exponential phase transcription is largely restricted to housekeeping genes. This is due, partly to the greater abundance of σ70, but also to its higher affinity for RNAP core enzyme, in comparison to most alternative sigma factors including σS. During stationary phase, despite its weak affinity for RNAP (relative to σ70), the rapid increase in the level of σS, permits competitive binding to RNAP and hence, transcription switches to general stress genes. (b) Different environmental stresses modulate the cellular levels of σS, by targeting different processes; (i) reduced growth rate stimulates transcription, (ii) high cell density or low temperature stimulate rpoS translation, (iii) carbon starvation or high temperature inhibits σS degradation and (iv) high osmolarity or pH, both stimulate rpoS translation and inhibit σS degradation
Given such an important role in the cell, the levels of σS are tightly controlled, not only at the transcriptional level, but also at the level of translation and protein activity. In E. coli, the gene encoding σS (rpoS) is located downstream of nlpD, a gene of unknown function. Although some transcription of rpoS occurs via the nlpD promoter, most transcription occurs from a promoter located in nlpD, 567 nucleotides upstream of the AUG of rpoS. This long 5′ untranslated region (UTR) plays a crucial role in the regulation of σS translation (see below). Consistently, deletion of this region results in a 20-fold reduction of σS expression, during both exponential and stationary-phase [17]. Although the regulation of σS largely occurs at the translational and post-translational levels (see below), the transcription of rpoS is also controlled by various regulators. For example, cyclic adenosine monophosphate (cAMP) and catabolite response protein (CRP) negatively regulate rpoS transcription [4], while in contrast the two-component system (BarA/UvrY) is a positive regulator of rpoS transcription. Similarly, (p)ppGpp is also reported to increase the cellular levels of rpoS mRNA, however currently it remains unclear if this effect is due to an increase in stability or elongation of the mRNA [17].
Translational Control of σS
At the translational level, the expression of σS is stimulated by a variety of different conditions, including hyperosmotic shift [3, 18], low temperature [2], and acid pH [19]. This activation, of rpoS translation, is regulated by the structural rearrangement of the rpoS mRNA [19], which is mediated by the RNA-chaperone, Hfq [20] and several regulatory small RNAs [21–24]. Specifically, the long 5′ UTR of rpoS mRNA, is proposed to form an intra-molecular stem loop structure, which under normal conditions occludes the ribosome-binding site (RBS) and hence limits σS translation [18]. The translation of σS, can however be stimulated by various non-coding small RNAs (sRNAs), such as DsrA and RprA, in the presence of the RNA chaperone, Hfq [25–28]. Hfq is a small RNA-binding protein that not only stabilises the sRNAs, but also enhances RNA-RNA interactions [29–32]. E. coli strains bearing mutations in hfq are sensitive to multiple stresses and hence exhibit a similar phenotype to rpoS mutant strains [31]. Consistently, Hfq plays a role in the translation of rpoS [20], however the mode of action by which Hfq functions is currently unclear. Despite this, a number of models have currently been proposed. The first, suggests that Hfq acts on rpoS mRNA directly by stabilising the secondary structure of the rpoS mRNA [20, 33–36]. Binding of Hfq is thought to shift the equilibrium of the rpoS mRNA secondary structure, from a less active form, where translation is inefficient, to an active form that permits easy access to the ribosome. An alternate model suggests that Hfq does not affect the secondary structure of rpoS mRNA. This model describes Hfq as a ‘platform’ for binding of other regulatory molecules, which are involved in the translational control of rpoS. Consistent with this model, Hfq interacts directly with several small regulatory RNAs (DsrA, RprA, ArcZ and OxyS), which have been shown to regulate rpoS translation [2, 36]. Moreover, Hfq is able to stimulate base pairing, between the sRNA and the target mRNA to promote a specific response, which either inhibits or enhances translational initiation [37]. To date, a total of four sRNAs have been identified, which function to regulate σS translation. Three of which (DsrA, RprA, and ArcZ) positively regulate rpoS translation [38–40], while a single sRNA, OxyS, has a negative effect on rpoS translation [36, 41]. Each of these sRNAs is expressed in response to different stress conditions [24, 33, 42].
Although four different sRNAs have been shown to effect rpoS translation, a common model can be drawn from a single example and hence this section will focus on the most extensively studied – DsrA. DsrA (downstream from RcsA) was originally discovered in a study that examined capsule regulation in E. coli [43] and later shown to be required for translation of rpoS at low temperature [42, 44]. Biochemical analysis of DsrA has revealed that the mechanism that DsrA employs to promote translation of rpoS mRNA is via an interaction with the rpoS mRNA, which is facilitated by Hfq [45]. It is an 87 nucleotide RNA which folds into a stem loop structure and contains a small single-stranded region that is complementary to an element within the 5′ UTR of rpoS mRNA (Fig. 5.2). In vivo studies have shown that DsrA hybridises to the predicted rpoS mRNA duplex segment (self inhibitory stem) at a position that lies upstream of the start codon [39, 40, 46, 47]. This hybridisation induces a structural change in rpoS mRNA that permits accessibility to the RBS present on the other strand [45]. The binding of the sRNA is facilitated by formation of a ternary complex with Hfq, which results in the activation of translation. In order for sRNAs, such as DsrA to activate rpoS translation, Hfq requires a (AAN)4 repeat element located at the 5′ UTR of rpoS [26, 48]. Interestingly, both rpoS mRNA and DsrA appear to bind to the same binding site within the proximal RNA-binding domain of Hfq [49]. As such, the current model suggests that Hfq enhances the interaction of DsrA and the rpoS mRNA by (a) increasing the local concentration of both RNAs and (b) unwinding the inhibitory stem of the rpoS mRNA [49, 50]. Recently however, the role of DsrA and its involvement in stimulating rpoS translation has also expanded to include stabilisation of rpoS mRNA by base pairing to these sRNAs, potentially preventing the RNase E-dependent degradation of the target mRNA [51].
Activation of σS translation by sRNAs (e.g. DsrA). Under non-stressed conditions, σS translation is low, due largely to a stem loop structure in the rpoS mRNA, which occludes the RBS and initiating AUG. In the presence of the RNA chaperone, sRNAs such as DsrA hybridise to the mRNA exposing the RBS and the initiating AUG
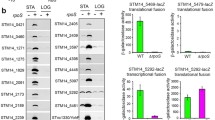
Consistent with the findings for DsrA; RprA and ArcZ also regulate translation of σS by base pairing to the 5′ UTR of rpoS [40, 47]. All three of these sRNAs are expressed in response to different stress conditions [24, 33, 42]. RprA (RpoS regulator), a 105 nucleotide RNA, was identified during a screening of a multi-copy suppressor library that increased the translation of rpoS-lacZ (translational fusion) in the absence of dsrA [33]. RprA in contrast to DsrA has been found to stimulate σS synthesis in response to cell envelope stress, and a modest effect has been observed in response to osmotic stress [33, 40]. ArcZ functions to positively regulate σS translation [21, 24]. Processing of ArcZ from a 121 nucleotide RNA to a stable 56 nucleotide species is required for the formation of a strong Hfq-dependent ternary complex with the 5′ UTR of rpoS mRNA [21, 47, 52]. Although the sequences of these regulatory RNAs differ, the mechanistic details appear to be conserved [47, 53]. Common to all of the sRNAs, they each interact with Hfq and activate translation by opening the stem-loop structure of the rpoS 5′ UTR, allowing access to the RBS [39, 40, 53]. Apart from binding to rpoS mRNA to promote translation, it’s also postulated that hybridisation of sRNAs to target mRNA promotes stabilisation of the target mRNA, in turn protecting it from degradation [51]. In contrast to the other sRNAs, OxyS is a negative regulator of rpoS translation. Encoded by the oxyS gene, this regulatory sRNA is induced upon exposure to hydrogen peroxide (oxidative stress) [36, 41]. Consistent with the positively regulating sRNAs, OxyS also associates with Hfq [36], however the mechanism of action of OxyS, is currently poorly understood. Nonetheless, based on secondary structure predictions, OxyS seems to share structural similarities with DsrA [19]. However, in contrast to DsrA, a linker region in OxyS seems to align with the RBS of the rpoS mRNA, suggesting that repression of σS translation may occur through the occlusion of the RBS by OxyS base pairing [32]. However, evidence for a direct interaction with rpoS mRNA is currently lacking. On the other hand, co-immunoprecipitation experiments, which confirm an interaction with Hfq, suggest an alternative model for the regulation of rpoS translation by OxyS [36]. This model proposes that the negative regulation of rpoS, by OxyS, is achieved through competitive binding to the Hfq-sRNAs binding site. For instance, competitive binding to Hfq may hinder the binding of positive regulatory sRNAs, which in turn inhibits rpoS translation [36].
Regulated Degradation of σS
Although the transcriptional and translational regulation of σS is rather extensive, this only accounts for a fraction of the overall regulation. The turnover of σS plays a major role in controlling the cellular levels of σS during the various growth phases. As mentioned previously, the relative amounts of σS present during exponential growth are extremely low, which is largely a result of degradation by the energy-dependent AAA+ protease, ClpXP [54], and is dependent on the two-component response regulator RssB [55–58] (see Fig. 5.3). In the absence of RssB, the ClpXP protease is unable to recognise σS, and hence RssB is required for the successful removal of σS from the cell. Importantly, RssB itself is not degraded in the process of substrate delivery and therefore is able to perform numerous cycles of substrate binding and delivery [58]. As a consequence, the limiting factor for σS degradation is RssB, which is present within the cell at very low levels (approximately 1 molecule of RssB for every 25 molecules of σS) [59]. Independent of the levels of RssB, the interaction with σS is modulated by phosphorylation [55, 58, 60, 61]. Although the phosphate donor (acetyl phosphate, AcP) and the two-component system ArcA/B have been shown to trigger RssB phosphorylation [61], a dedicated phosphatase or histidine kinase has yet to be identified. Regardless of how RssB is phosphorylated, the mechanistic effect of phosphorylation on RssB and the relevance it plays with respect to the regulation of σS remains unclear and currently several models exist to describe the contribution of phosphorylation.
Degradation of σS by ClpXP requires the adaptor protein, RssB. The adaptor protein, RssB (green) is phosphorylated by either the phosphordonor, acetyl phosphate (AcP) or the response regulator, ArcB. The phosphorylated form of RssB binds to σS (grey) with high affinity. Binding of RssB to σS, triggers a conformational change in σS, exposing a ClpX-recognition motif. The RssB/σS complex, then docks to ClpX by an unknown mechanism, and delivers the substrate to the ClpXP protease for ATP-dependent degradation. The degradation of σS can be inhibited by specific anti-adaptors (i.e. IraP, IraD and IraM), which are induced by phosphate starvation, DNA damage or Mg starvation, respectively
Moreover, it has been recently shown that the stability of σS is also controlled by another group of proteins termed Ira (Inhibitor of RssB activity). Currently, three Ira proteins have been identified: IraP, IraD and IraM, which stabilise σs in response to phosphate starvation, DNA damage and magnesium starvation, respectively [62, 63]. However, to date little is known about the mechanism by which these anti-adaptors inhibit the RssB-mediated delivery of σS to ClpXP. The following section will focus on our current understanding of the components involved in the regulated turnover of σS.
The Protease – ClpXP
In the cytosol of E. coli there are five ATP-dependent proteases (ClpXP, ClpAP, HslUV, Lon and FtsH). Each protease is composed of two components; a peptidase component and an AAA+ (ATPase associated with a variety of cellular activities) unfoldase component and as such they are commonly referred to as AAA+ proteases [64]. A unifying feature of the AAA+ protein superfamily is the presence of an AAA+ domain spanning 200–250 amino acids. This domain is composed of two subdomains – the small and large subdomain. The nucleotide is bound, in a cleft created by the large and small subdomains of a single subunit and the large subdomain of the adjacent subunit [65, 66]. Each domain contains several highly conserved sequence motifs required for the binding and hydrolysis of ATP (e.g. Walker-A and Walker-B), the binding and translocation of substrates (e.g. pore-1 and pore-2) and the binding of the peptidase, ClpP (e.g. IGF loop) [67–70]. For a detailed description of the different AAA+ proteases in E. coli, refer to [71].
In E. coli, a single protease (ClpXP) is responsible for the turnover of σS. Like other AAA+ proteases, ClpXP is composed of two components. The peptidase component (ClpP) is composed of two heptameric rings that stack back-to-back to form a barrel-shaped oligomer. The catalytic residues of ClpP are sequestered away from cytosolic proteins, within an aqueous chamber. Access to this chamber is limited by a narrow axial portal (∼10 Å in diameter), which only allows entry of short peptides and unfolded proteins [72, 73]. As a consequence of this narrow entry portal, the degradation of folded proteins requires an additional component – the unfoldase (ClpX), which is responsible, not only for the recognition of the substrate but also for its unfolding and translocation into ClpP. Complexes of ClpXP, as illustrated by electron micrographs, can be either single- or double-headed [74–76]. Single-headed ClpXP complexes contain a single hexamer of ClpX stacked onto one end of the ClpP dodecamer, while double-headed complexes of ClpXP contain a ClpX hexamer bound to both ends of ClpP [76]. The interaction between ClpX and ClpP is mediated by two structural elements. The primary interaction occurs between the IGF loop (located on ClpX) and a hydrophobic pocket (composed of Tyr60 and Tyr62 from one subunit and Phe82 from the adjacent subunit) located on the apical surface of ClpP [68, 73]. Interestingly, these loops are disordered in the crystal structure of ClpX, hence they are likely to exhibit a high degree of flexibility required to facilitate the asymmetric connection between the ClpX hexamer and the heptameric ring of ClpP [65, 66, 68]. Consistent with the role of these loops in docking to ClpP, mutation of specific residues within the IGF motif, inhibits ClpP binding without affecting the ClpP-independent activity of ClpX [68, 77]. Indeed binding of the IGF loop to the hydrophobic pocket on ClpP is believed to open the axial channel of the protease [78]. Likewise, several recently identified antibiotics (acyldepsipeptides (ADEPs) and activators of self-compartmentalising proteases (ACPs)) that activate ClpP for unregulated degradation, have also been shown to open the axial pore of ClpP through binding to the hydrophobic pocket [79–83]. The second interaction site, between ClpX and ClpP, is mediated by the N-terminal β-hairpin loop (∼20 residues) of ClpP [84, 85] and the pore-2 loop of ClpX. This interaction is dynamic and sensitive to the nucleotide-bound state of ClpX [78] and mutation of either region results in the destabilisation of the ClpXP complex [84, 86–88].
Protein degradation by ClpXP can be divided into four fundamental steps, (substrate recognition, unfolding and translocation), which are performed by the unfoldase ClpX, and hydrolysis of the protein into short peptide fragments which is performed by the associated peptidase. In general, the first step (substrate recognition) requires nucleotide binding by the unfoldase, but not its hydrolysis. In this state, ClpX is able to recognise a wide variety of different protein substrate, largely through short sequence motifs (commonly referred to as tags or degrons). These tags are often located at the N- or C-terminus of the substrate protein [89]. While, the majority of these motifs are intrinsic to the protein, some proteins require processing or modification (e.g. attachment of a tag such as the SsrA tag) for ClpX recognition to occur. For a more detailed description of the SsrA tagging system, refer to [71]. Following recognition, the substrate is unfolded by ClpX and translocated into ClpP, in an ATP-dependent fashion. Finally, the translocated polypeptide is degraded into small peptides, by ClpP [76, 90–92]. In the case of ClpX, many of the molecular details of substrate recognition and translocation have been defined. In general, substrates are recognised by a conserved aromatic-hydrophobic motif (GYVG) located on the pore-1 loop [87]. These loops protrude from each subunit into the central cavity of the hexamer [93–96]. Mutations in the highly conserved aromatic residue of the pore-1 loop have been shown to impair substrate binding and processing, with little to no effect on oligomerisation or ATPase activity of the AAA+ protein [93, 94, 96, 97]. Cycles of ATP binding and hydrolysis, drive rigid-body movements in ClpX, which translate to a pulling force on the substrate, resulting in unfolding and translocation of the substrate [87, 88, 94]. For a detailed analysis of the mechanism of action of AAA+ proteases refer to [71].
The Adaptor Protein–RssB
Although the vast majority of substrates are recognised directly by the unfoldase, some substrates require the assistance of an adaptor protein for their recognition and hence their degradation by the protease. In the case of ClpXP, three adaptor proteins have been identified – SspB, UmuD and RssB. SspB is the best characterised of these adaptor proteins and is required for the enhanced delivery and degradation of SsrA-tagged proteins as well as the delivery of a fragment of the anti-sigma factor (RseA). In contrast to SspB, both UmuD and RssB are essential for the delivery of their respective substrates [58, 98]. UmuD is essential for the in trans delivery of UmuD′ [98], while RssB (also referred to as SprE) is essential for the recognition and delivery of σS [54, 56, 57]. Interestingly, despite little overall homology between the three adaptor proteins, all appear to contain a short sequence motif in common. This motif is located at the C-terminus of SspB and RssB, and near the N-terminus of UmuD. In SspB, this motif was termed the ClpX binding region (XBR) as it was shown to be critical for binding to ClpX and hence delivery of its cargo to the protease [99–101]. Specifically, the XBR of SspB docks onto the N-terminal domain of ClpX, placing the adaptor protein SspB in an ideal position to deliver its bound substrate [99, 101]. The increased local concentration of the substrate (tethered to ClpX, by the adaptor protein) enhances its recognition by the pore residues of ClpX, where it is unfolded and translocated into ClpP. Although RssB was identified over 15 years ago, the mechanism by which it binds to and delivers its substrate (σS) to ClpXP for degradation still remains elusive. However, based on the sequence similarity of the XBR region of SspB and RssB, a model for the RssB-mediated delivery of σS has been proposed (Fig. 5.3).
Although RssB shares little-to-no sequence similarity with other adaptor proteins, it does share considerable homology with a family of proteins known as two-component response regulators (RRs). These proteins are generally composed of two domains, an N-terminal receiver domain and a C-terminal output (or effector) domain. In contrast to the majority of RRs (which contain a C-terminal DNA-binding domain and serve as transcriptional regulators) the C-terminal effector domain of RssB is a PP2C-type Ser/Thr phosphatase [102]. Interestingly, this region in RssB lacks the critical residues required for phosphatase activity and hence the precise role of this domain remains unclear [102]. The receiver domain on the other hand, is highly conserved amongst all RRs, both in sequence and structure. In the case of RssB, this domain is phosphorylated at a highly conserved aspartic acid residue (Asp58), which is proposed to trigger a conformational change in RssB resulting in an improved interaction with σS [55, 58, 59, 61]. Consistent with this idea, mutation of Asp58 prevents RssB phosphorylation and reduces the rate of σS turnover in vivo [55, 59, 103]. However, the role of RssB phosphorylation remains controversial, as σS is still degraded in an E. coli strain containing a non-phosphorylatable mutant of RssB [103]. Similarly, given that phosphorylation of Asp is transient, any structural changes that occur to RssB remain undefined. Nevertheless, the effect of phosphorylation has been examined at the molecular level for some RRs [104, 105]. Indeed in these cases, phosphorylation has been shown to trigger both local changes to the N-terminal receiver domain, as well as long-range changes to the RR [106–110]. From these data several models have been proposed. One possibility is that the receiver domain exists in an equilibrium, between two-states (an active and an inactive state) that is influenced by phosphorylation. In most cases, phosphorylation of the RR is linked to activation of the protein, while in a handful of cases phosphorylation appears to inhibit the activity of the RR.
Regardless of the role of RssB phosphorylation, the molecular details of substrate interaction and delivery to ClpXP are also poorly defined. Currently, two different models of substrate delivery have been proposed. The first model implies a direct interaction between the adaptor and ClpX, for delivery of the substrate, while the second model suggests that RssB is only required to “activate” the substrate for binding to ClpX and does not, itself, dock to ClpX. Both models nevertheless, converge to suggest that binding of RssB to σS, triggers a conformational change in σS that exposes a concealed “low affinity” ClpX binding site on the substrate. Exposure of this site then permits the downstream recognition of σS by ClpX, when presented by RssB (Fig. 5.3). Consistent with this idea, the N-terminus of σS does not contribute to RssB binding, but is predicted to contain a ClpX binding motif [89, 111]. In addition to the predicted ClpX binding site, located on the N-terminus of σS, a “turnover element” in σS, located downstream of the promoter recognising region 2.4, is also required for its degradation [60]. Mutations introduced into the “turnover element” of σS, in particular Lys173, have been shown to inhibit the turnover of σS in growing cells [60]. Consistently, mutation of Lys173 also inhibited the binding of σS in vitro [60]. Collectively these data suggest that the “turnover element” in σS is an important region for interaction with RssB. Hence, in the absence of RssB, the N-terminal region of σS is occluded, possibly by the C-terminal region of σS which upon binding of RssB becomes exposed for recognition by ClpX [111].
Anti-adaptors (Inhibitors of RssB Activity, Ira)
Interestingly, in the last 5 years another level of σS regulation was discovered (see below). In this case, a group of unrelated proteins were shown to inhibit the ClpXP-mediated turnover of σS. These proteins were termed anti-adaptors, and as the name suggests they inhibit or antagonise the activity of the adaptor protein, RssB. The first anti-adaptor to be characterised was identified as a regulator of competence development in B. subtilis [112–114]. In non-competent cells, the adaptor protein MecA, recognises the competence transcription factor ComK, and delivers it to the ClpCP protease for degradation [115, 116]. When competence development is initiated, by a quorum sensing mechanism, the levels of ComS increase [112, 117]. ComS then acts as a “suicide” anti-adaptor binding to MecA and thereby preventing the turnover of ComK [114, 116, 117]. More recently however, similar proteins were also identified in E. coli. Consistent with the regulatory role of ComS in the development of competence, these novel E. coli anti-adaptor proteins function to regulate the stationary-phase stress response. As such, the following section will describe recent insights into these small, yet interesting proteins that work to stabilise σS.
Using an E. coli genomic DNA library, Gottesman and colleagues identified three different genes of unknown function that specifically affected the activity of an rpoS-lacZ translational fusion [62, 63]. Through a series of elegant genetic and biochemical experiments, the proteins encoded by these genes were shown to act as specific inhibitors of RssB activity and hence were collectively termed anti-adaptors. Interestingly, deletion of each gene did not affect the stability of σS under all starvation conditions; rather stabilisation of σS was limited to a specific condition. The first identified anti-adaptor, YiaB renamed IraP (Inhibitor of RssB activity during phosphate starvation) is a small 86 amino acid protein, which is transcribed in response to phosphate starvation, and mediated by ppGpp [63, 118]. A multi-copy plasmid carrying the iraP gene demonstrated that expression of IraP, driven from the plasmid, resulted in an approximately three-fold increase in σS stability in the exponential-phase in comparison to a seven-fold increase in the stationary phase [63]. These results, in conjunction with in vitro ‘pull-down’ experiments confirmed IraP as a bona fide regulator of σS, which prevents σS turnover through direct interaction with RssB [63].
Following the identification of the first anti-adaptor (i.e. IraP), two additional genes (yjiD and ycgW) were also shown to stabilise σS. These gene products were renamed IraD and IraM respectively, because of their ability to stabilise σS in response to DNA damage (IraD) and during magnesium starvation (IraM) [62]. Consistently, both IraD and IraM were able to inhibit the RssB-mediated degradation of σS in vitro [62]. Although all three anti-adaptors seem to perform the same role, their interaction with RssB and/or σS seems to vary [62]. Current data suggests that IraP functions by binding directly to RssB (forming an RssB-IraP complex) which sequesters RssB from σS, thereby preventing its turnover [63]. In vitro ‘pull-down’ experiments using IraD and IraM suggest that IraD, like IraP, interacts directly with RssB, whilst the mode of action of IraM remains unclear [62] (Fig. 5.3). Interestingly, the transcriptional regulator AppY, was also able to stabilise σS in a mutant strain lacking all three anti-adaptors, which suggests a putative role for AppY in activating the transcription of a yet to be identified anti-adaptor [62]. This has physiological importance when considering the action of multiple stresses on the cell. Based on our current understanding of IraP, the presence of multiple stresses may induce the expression of multiple anti-adaptors. Given that each anti-adaptor may exhibit a different mechanistic approach to inhibit σS degradation, this may cause an avidity effect, which could culminate in rapid stabilisation of σS. As such, this provides an efficient way of coupling external stress stimuli to a rapid survival response.
The Heat-Shock Response
In contrast to the general stress response, the heat shock response (HSR) is a specific cellular response to a rapid, sub-lethal, increase in temperature. This response was first observed in the salivary glands of Drosophila melanogaster, where the synthesis of a small group of proteins (termed heat-shock proteins (HSPs)) increased in response to a temperature upshift [119] and was later shown to be a universal response. In E. coli, the HSR is controlled by a single transcription factor, σ32 (also known as σH) and results in the expression of HSPs (i.e. molecular chaperones and proteases). The molecular chaperones (e.g. DnaK/J, GroEL/S and the small HSPs – IbpA and IbpB) and the ATP-dependent proteases (e.g. Lon and FtsH), help to maintain a productive protein-folding environment in the cell by refolding or removing the misfolded proteins, thereby returning the cell to its pre-stressed state. The response is controlled not only by σ32 translation, but also through its turnover, and can be divided into three distinct phases; induction, adaptation and a final steady state phase.
Regulated Turnover of σ32
Similar to σS, the steady-state levels of σ32 are low under non-stressed conditions. In the absence of stress (i.e. at 30 °C), low levels of σ32 (∼50 molecules/cell) are maintained by (a) inefficient initiation of translation due to base pairing within the rpoH mRNA which occludes the Shine-Dalgarno sequence and (b) rapid degradation of σ32 primarily by FtsH (half-life of ∼1 min.), which is mediated by both the DnaK and GroE chaperone systems [120, 121]. Upon temperature upshift, the inhibitory structure of the rpoH mRNA is opened and translation of σ32 increases. Simultaneously, the accumulation of unfolded proteins in the cell sequesters chaperones and proteases, albeit transiently (∼5–10 min.) thereby stabilising σ32 [122] (see Fig. 5.4). As a result, there is a rapid increase in the levels of σ32 and hence HSPs during the induction phase of this response. During the next phase – adaptation – the synthesis of HSPs is blocked, as the activity of σ32 becomes inhibited through (a) the presence of high levels of chaperones and proteases and (b) the accelerated turnover of σ32 at higher temperatures, resulting in a new steady-state level of σ32 (and HSPs) during the final “steady-state” phase. As such, the cellular levels of both chaperones and proteases, not only control induction of the HSR, but also the shutdown of this response.
The heat-shock response is controlled by the transcription factor, Sigma32 (σ32) . (a) Under non-stressed conditions the RBS of rpoH is occluded and hence translation of σ32 is low. In the absence of unfolded proteins chaperones and proteases are free to binding to σ32 mediating its rapid degradation and inhibiting its binding to RNAP. (b) Under heat shock conditions, the secondary structure of rpoH is melted and transcription increases. The accumulation of unfolded proteins, sequesters the chaperones and proteases from σ32, which results in an increase in the half-life of σ32. The increased cellular concentration of σ32 allows binding to RNAP and hence transcription of the h.s. genes
Although several cytoplasmic proteases including HslUV (also known as ClpYQ) contribute to the turnover of σ32, the metabolic stability of σ32 in vivo is primarily controlled by the membrane bound protease FtsH [123–125]. Both proteases (HslUV and FtsH) belong to the AAA+ protein superfamily [69]. Each protease is composed of two components (an unfoldase and a peptidase). In the case of HslUV, the two components are located on separate polypeptides, while in the case of FtsH both components are located on a single polypeptide. While both machines exhibit a six fold symmetry, the HslUV complex is formed by one or two ring-shaped unfoldase components (composed of six subunits of HslU), that stack onto either or both ends of the peptidase component (i.e. HslV), which is composed of two hexameric rings stacked back-to-back [126]. By contrast, FtsH forms a homohexameric ring-shaped complex that is embedded in the periplasmic membrane with its active sites exposed to the cytoplasm. For a detailed description of FtsH structure and function refer to the accompanying review by Okuno and Ogura [127].
Interestingly, the FtsH-mediated degradation of σ32 is accelerated, not only by increased temperature [128], but also by the presence of molecular chaperones such as the GroEL-GroES (ELS) chaperone system and the DnaK-DnaJ-GrpE (KJE) chaperone system [125]. Although the exact role of these chaperone systems in promoting the FtsH-mediated degradation of σ32 is yet to be determined, hydrogen-deuterium exchange (HDX) experiments have shown that both DnaK and DnaJ are able to promote unfolding of σ32 [129]. Binding of DnaJ to region 2.1 triggers a conformational change in σ32, which facilitates DnaK binding to region 3.2 (Fig. 5.5) and further unfolding of σ32, which is believed to mediate delivery to, and degradation by, FtsH. Interestingly, in contrast to most other bacterial proteases (e.g. ClpXP or ClpAP) in which substrate recognition involves a single N- or C-terminal motif (see [71] for further details), the FtsH-mediated degradation of σ32 appears to require two distinct regions; region 2.1 (Leu47, Ala50 and Ile54) and region C (Ala131 and Lys134) [130, 131], both of which are located internally (Fig. 5.5). Given that (a) these “turnover elements” are located within the middle of the polypeptide, and (b) that FtsH lacks a robust unfoldase activity [132], it is likely that molecular chaperones facilitate the FtsH-mediated degradation of σ32, by either triggering a local conformational change in the substrate or by “unfolding” it. Consistently, the binding sites for both DnaJ and DnaK are located on, or adjacent to, the FtsH turnover elements on the substrate. Interestingly, although still somewhat speculative, molecular modelling of σ32 using known sigma factor structures has revealed that the residues implicated in the two turnover elements may form a single discontinuous “motif” for recognition by FtsH [133, 134].
Domain organisation of σ32. Sigma factors are divided into five functional regions (region 1, 2, 3, 4 and H/region C). These regions can be further divided into subregions (e.g. 2.1, 2.2, 2.3 and 2.4). The RNAP core enzyme binds to regions 2.2 and H/region C. This partially overlaps with the FtsH turnover element, which has be mapped to region 2.1 and H/region C. DnaJ and DnaK bind to regions 2.1 and 3.2, respectively
Removal of Misfolded and/or Aggregated Proteins – Degradation by AAA+ Proteases as a Last Resort
As mentioned above, heat shock results in the accumulation of unfolded proteins, which may be detrimental to the viability of the cell. As such, the primary aim of the heat-shock response is to maintain cell viability by restoring the protein-folding environment of the cell. This is achieved, through the expression of chaperones and proteases, which either refold or remove the misfolded proteins. Interestingly, in contrast to oxidative stress, which results in the irreversible damage to proteins, heat stress largely results in a “reversible” damage to proteins. Moreover, given that it is generally more energetically favourable to refold a protein than to degrade and resynthesize it, the primary strategy of the heat-shock response is to refold the misfolded proteins. As such, it is important for the cell to discriminate between unfolded proteins that can be refolded by chaperones, and terminally damaged proteins that must be removed from the cell by proteases. One possibility is that the final fate of a misfolded (or aggregated) protein is controlled by the kinetics of chaperone and protease binding [135, 136]. Consistent with this view, chaperones such as DnaK and DnaJ recognize largely hydrophobic residues, which are commonly exposed in unfolded proteins, while the proteases such as Lon, bind primarily to sequences rich in aromatic residues, which are less common in unfolded proteins [137]. Importantly, chaperones such as DnaK and DnaJ are significantly more abundant than proteases, especially under heat-shock conditions [138–140] and hence refolding is generally favoured over degradation. Therefore not surprisingly, following heat-shock most unfolded proteins are refolded directly by “folder” chaperones (i.e. KJE or ELS) before they can be captured by proteases for degradation. Interestingly, and somewhat contrary to this view, the degradation of some protein substrates is promoted by chaperones, possibly by altering the confirmation of the substrate, exposing a protease binding site [129]. However in most cases, chaperones and proteases appear to compete for binding to the misfolded substrate to deliver their specific activities (refolding versus degradation, respectively).
Interestingly, even during conditions of prolonged stress when protein folding chaperones are sequestered, the refolding arm of protein quality control network is favoured. Under these conditions, the accumulation of misfolded proteins results in their aggregation. However these aggregated proteins can be refolded by a specialised bi-chaperone system, which combines the “disaggregation-power” of the AAA+ unfoldase ClpB, with the refolding activity of the KJE chaperone system [140, 141]. Interestingly, despite the fact that some proteases can degrade aggregated proteins in vitro [138, 142, 143] and that several B. subtilis Clp components (ClpC, ClpE and ClpP) have been implicated in protein disaggregation [144, 145], there is currently little evidence in E. coli to suggest that aggregated proteins are degraded in vivo. This is, partly due to binding of “folder” chaperones (i.e. DnaK) to protein aggregates, which restricts the binding of proteases [138, 146], and partly due to the action of “holder” chaperones (i.e. inclusion body proteins A and B, IbpA and IbpB, respectively), which trap the substrates in a “folding” competent state. Indeed, the “holder” chaperones that bind to misfolded and aggregated proteins, appear to facilitate their subsequent reactivation and refolding by the ClpB/KJE bi-chaperone system [147, 148]. It is likely that the competitive edge, of chaperones over proteases, in the recognition of most misfolded and/or aggregated proteins is energetically advantageous to bacteria.
Interestingly, both “holder” chaperones (IbpA and IbpB) are degraded by Lon, [149], and despite the high overall sequence similarity of both proteins, IbpB is degraded significantly faster by Lon, than IbpA is [149]. Surprisingly however, the rate of IbpA degradation, by Lon, was substantially increased under heat-shock conditions suggesting an intriguing link between protein aggregation and degradation. Therefore from these data, Baker and colleagues have proposed several interesting models whereby (a) free inclusion bodies are degraded (b) both the aggregated proteins and the Ibp’s are degraded or alternatively (c) only the Ibp’s are degraded by Lon (Fig. 5.6).
Misfolded and aggregated proteins are refolded by chaperones and/or removed by proteases. Following thermal stress, native proteins unfold. Unfolded proteins are preferentially refolded by chaperones, however in order to maintain a productive folding environment in the cell, some unfolded proteins may be degraded by AAA+ proteases. If stress is prolonged, misfolded proteins tend to aggregate, either in the absence or presence of small heat-shock proteins (i.e. IbpA and IbpB). These aggregated proteins can be refolded by a specialised bi-chaperone system; ClpB together with the DnaK chaperone system (ClpB/KJE). ClpB/KJE-mediated reactivation of the aggregate can be accelerated by the presence of IbpA and IbpB. Recently, it was proposed that these Ibp’s are degraded by the AAA+ protease Lon, either (a) together with the aggregated protein, (b) to release the aggregated protein and further accelerate the ClpB/KJE-reactivation or (c) to free proteins
Conclusion
It has been long known, that proteases play a crucial role in the removal of unwanted proteins from the cell under a variety of different cellular conditions. However, recent findings have highlighted that these cellular machines also play an important role in controlling several different stress response pathways. These studies have illustrated that the proteases responsible for the degradation of these transcription factors, are not only highly regulated, but also exhibit an exquisite specificity. Despite these advances many questions remain unanswered, currently little is known regarding the regulation or removal of the adaptor or anti-adaptor proteins that regulate the turnover of these transcription factors. Similarly at a structural level, our current understanding of how each component interacts with one another, remains limited. As such many important challenges, for current and future researchers, still remain in this field.
References
Barchinger SE, Ades SE (2013) Regulated proteolysis: control of the Escherichia coli σE-dependent cell envelope stress response. In: Dougan DA (ed) Regulated proteolysis in microorganisms. Springer, Subcell Biochem 66:129–160
Sledjeski DD, Gupta A, Gottesman S (1996) The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J 15(15):3993–4000
Muffler A, Traulsen DD, Lange R, Hengge-Aronis R (1996) Posttranscriptional osmotic regulation of the sigma(s) subunit of RNA polymerase in Escherichia coli. J Bacteriol 178(6):1607–1613
Lange R, Hengge-Aronis R (1991) Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol 173(14):4474–4481
Hengge-Aronis R (1993) Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72(2):165–168
McCann MP, Kidwell JP, Matin A (1991) The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol 173(13):4188–4194
Farewell A, Kvint K, Nystrom T (1998) Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol 29(4):1039–1051
Lange R, Hengge-Aronis R (1994) The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev 8(13):1600–1612
Atlung T, Brondsted L (1994) Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. J Bacteriol 176(17):5414–5422
Altuvia S, Almiron M, Huisman G, Kolter R et al (1994) The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol 13(2):265–272
Dong T, Schellhorn HE (2009) Global effect of RpoS on gene expression in pathogenic Escherichia coli O157:H7 strain EDL933. BMC Genomics 10:349
Weber H, Polen T, Heuveling J, Wendisch VF et al (2005) Genome-wide analysis of the general stress response network in Escherichia coli: sigmaS-dependent genes, promoters, and sigma factor selectivity. J Bacteriol 187(5):1591–1603
Lacour S, Landini P (2004) SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J Bacteriol 186(21):7186–7195
Patten CL, Kirchhof MG, Schertzberg MR, Morton RA et al (2004) Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol Genet Genomics 272(5):580–591
Bishop RE, Leskiw BK, Hodges RS, Kay CM et al (1998) The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J Mol Biol 280(4):583–596
Dong T, Schellhorn HE (2010) Role of RpoS in virulence of pathogens. Infect Immun 78(3):887–897
Lange R, Fischer D, Hengge-Aronis R (1995) Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J Bacteriol 177(16):4676–4680
Lange R, Hengge-Aronis R (1994) The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol Microbiol 13(4):733–743
Hengge-Aronis R (2002) Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66(3):373–395
Muffler A, Fischer D, Hengge-Aronis R (1996) The RNA-binding protein HF-I, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev 10(9):1143–1151
Argaman L, Hershberg R, Vogel J, Bejerano G et al (2001) Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol 11(12):941–950
Eddy SR (2002) Computational genomics of noncoding RNA genes. Cell 109(2):137–140
Storz G (2002) An expanding universe of noncoding RNAs. Science 296(5571):1260–1263
Wassarman KM, Repoila F, Rosenow C, Storz G et al (2001) Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev 15(13):1637–1651
Storz G, Opdyke JA, Zhang A (2004) Controlling mRNA stability and translation with small, noncoding RNAs. Curr Opin Microbiol 7(2):140–144
Updegrove T, Wilf N, Sun X, Wartell RM (2008) Effect of Hfq on RprA-rpoS mRNA pairing: Hfq-RNA binding and the influence of the 5′ rpoS mRNA leader region. Biochemistry 47(43):11184–11195
Waters LS, Storz G (2009) Regulatory RNAs in bacteria. Cell 136(4):615–628
Zhang A, Wassarman KM, Rosenow C, Tjaden BC et al (2003) Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol 50(4):1111–1124
Brown L, Elliott T (1996) Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol 178(13):3763–3770
Moller T, Franch T, Hojrup P, Keene DR et al (2002) Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell 9(1):23–30
Muffler A, Traulsen DD, Fischer D, Lange R et al (1997) The RNA-binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the sigmaS subunit of RNA polymerase in Escherichia coli. J Bacteriol 179(1):297–300
Zhang A, Wassarman KM, Ortega J, Steven AC et al (2002) The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol Cell 9(1):11–22
Majdalani N, Chen S, Murrow J, St John K et al (2001) Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol Microbiol 39(5):1382–1394
Sledjeski DD, Whitman C, Zhang A (2001) Hfq is necessary for regulation by the untranslated RNA DsrA. J Bacteriol 183(6):1997–2005
Ueguchi C, Misonou N, Mizuno T (2001) Negative control of rpoS expression by phosphoenolpyruvate: carbohydrate phosphotransferase system in Escherichia coli. J Bacteriol 183(2):520–527
Zhang A, Altuvia S, Tiwari A, Argaman L et al (1998) The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J 17(20):6061–6068
Kawamoto H, Koide Y, Morita T, Aiba H (2006) Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol Microbiol 61(4):1013–1022
Lease RA, Cusick ME, Belfort M (1998) Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc Natl Acad Sci U S A 95(21):12456–12461
Majdalani N, Cunning C, Sledjeski D, Elliott T et al (1998) DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci U S A 95(21):12462–12467
Majdalani N, Hernandez D, Gottesman S (2002) Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol Microbiol 46(3):813–826
Altuvia S, Weinstein-Fischer D, Zhang A, Postow L et al (1997) A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90(1):43–53
Repoila F, Gottesman S (2001) Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. J Bacteriol 183(13):4012–4023
Sledjeski D, Gottesman S (1995) A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc Natl Acad Sci U S A 92(6):2003–2007
Repoila F, Gottesman S (2003) Temperature sensing by the dsrA promoter. J Bacteriol 185(22):6609–6614
Lease RA, Woodson SA (2004) Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J Mol Biol 344(5):1211–1223
Cunning C, Brown L, Elliott T (1998) Promoter substitution and deletion analysis of upstream region required for rpoS translational regulation. J Bacteriol 180(17):4564–4570
Soper T, Mandin P, Majdalani N, Gottesman S et al (2010) Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A 107(21):9602–9607
Soper TJ, Woodson SA (2008) The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA 14(9):1907–1917
Hwang W, Arluison V, Hohng S (2011) Dynamic competition of DsrA and rpoS fragments for the proximal binding site of Hfq as a means for efficient annealing. Nucleic Acids Res 39(12):5131–5139
Arluison V, Mutyam SK, Mura C, Marco S et al (2007) Sm-like protein Hfq: location of the ATP-binding site and the effect of ATP on Hfq– RNA complexes. Protein Sci 16(9):1830–1841
McCullen CA, Benhammou JN, Majdalani N, Gottesman S (2010) Mechanism of positive regulation by DsrA and RprA small noncoding RNAs: pairing increases translation and protects rpoS mRNA from degradation. J Bacteriol 192(21):5559–5571
Papenfort K, Said N, Welsink T, Lucchini S et al (2009) Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved, Hfq-dependent small RNA. Mol Microbiol 74(1):139–158
Mandin P, Gottesman S (2010) Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J 29(18):3094–3107
Schweder T, Lee KH, Lomovskaya O, Matin A (1996) Regulation of Escherichia coli starvation sigma factor (sigma s) by ClpXP protease. J Bacteriol 178(2):470–476
Klauck E, Lingnau M, Hengge-Aronis R (2001) Role of the response regulator RssB in sigma recognition and initiation of sigma proteolysis in Escherichia coli. Mol Microbiol 40(6):1381–1390
Muffler A, Fischer D, Altuvia S, Storz G et al (1996) The response regulator RssB controls stability of the sigma(S) subunit of RNA polymerase in Escherichia coli. EMBO J 15(6):1333–1339
Pratt LA, Silhavy TJ (1996) The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci U S A 93(6):2488–2492
Zhou Y, Gottesman S, Hoskins JR, Maurizi MR et al (2001) The RssB response regulator directly targets sigma(S) for degradation by ClpXP. Genes Dev 15(5):627–637
Becker G, Klauck E, Hengge-Aronis R (2000) The response regulator RssB, a recognition factor for sigmaS proteolysis in Escherichia coli, can act like an anti-sigmaS factor. Mol Microbiol 35(3):657–666
Becker G, Klauck E, Hengge-Aronis R (1999) Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc Natl Acad Sci U S A 96(11):6439–6444
Bouche S, Klauck E, Fischer D, Lucassen M et al (1998) Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol Microbiol 27(4):787–795
Bougdour A, Cunning C, Baptiste PJ, Elliott T et al (2008) Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol Microbiol 68(2):298–313
Bougdour A, Wickner S, Gottesman S (2006) Modulating RssB activity: IraP, a novel regulator of sigma(S) stability in Escherichia coli. Genes Dev 20(7):884–897
Sauer RT, Baker TA (2011) AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80:587–612
Glynn SE, Martin A, Nager AR, Baker TA et al (2009) Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell 139(4):744–756
Kim DY, Kim KK (2003) Crystal structure of ClpX molecular chaperone from Helicobacter pylori. J Biol Chem 278(50):50664–50670
Iyer LM, Leipe DD, Koonin EV, Aravind L (2004) Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol 146(1–2):11–31
Kim YI, Levchenko I, Fraczkowska K, Woodruff RV et al (2001) Molecular determinants of complex formation between Clp/Hsp100 ATPases and the ClpP peptidase. Nat Struct Biol 8(3):230–233
Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9(1):27–43
Walker JE, Saraste M, Gay NJ (1982) E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature 298(5877):867–869
Gur E, Ottofuelling R, Dougan DA (2013) Machines of destruction – AAA+ proteases and the adaptors that control them. In: Dougan DA (ed) Regulated proteolysis in microorganisms. Springer, Subcell Biochem 66:3–33
Jennings LD, Lun DS, Medard M, Licht S (2008) ClpP hydrolyzes a protein substrate processively in the absence of the ClpA ATPase: mechanistic studies of ATP-independent proteolysis. Biochemistry 47(44):11536–11546
Wang J, Hartling JA, Flanagan JM (1997) The structure of ClpP at 2.3 A resolution suggests a model for ATP-dependent proteolysis. Cell 91(4):447–456
Grimaud R, Kessel M, Beuron F, Steven AC et al (1998) Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J Biol Chem 273(20):12476–12481
Maglica Z, Kolygo K, Weber-Ban E (2009) Optimal efficiency of ClpAP and ClpXP chaperone-proteases is achieved by architectural symmetry. Structure 17(4):508–516
Ortega J, Lee HS, Maurizi MR, Steven AC (2002) Alternating translocation of protein substrates from both ends of ClpXP protease. EMBO J 21(18):4938–4949
Joshi SA, Hersch GL, Baker TA, Sauer RT (2004) Communication between ClpX and ClpP during substrate processing and degradation. Nat Struct Mol Biol 11(5):404–411
Martin A, Baker TA, Sauer RT (2007) Distinct static and dynamic interactions control ATPase-peptidase communication in a AAA+ protease. Mol Cell 27(1):41–52
Dougan DA (2011) Chemical activators of ClpP: turning Jekyll into Hyde. Chem Biol 18(9):1072–1074
Kirstein J, Hoffmann A, Lilie H, Schmidt R et al (2009) The antibiotic ADEP reprogrammes ClpP, switching it from a regulated to an uncontrolled protease. EMBO Mol Med 1(1):37–49
Lee BG, Park EY, Lee KE, Jeon H et al (2010) Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat Struct Mol Biol 17(4):471–478
Leung E, Datti A, Cossette M, Goodreid J et al (2011) Activators of cylindrical proteases as antimicrobials: identification and development of small molecule activators of ClpP protease. Chem Biol 18(9):1167–1178
Li DH, Chung YS, Gloyd M, Joseph E et al (2010) Acyldepsipeptide antibiotics induce the formation of a structured axial channel in ClpP: A model for the ClpX/ClpA-bound state of ClpP. Chem Biol 17(9):959–969
Gribun A, Kimber MS, Ching R, Sprangers R et al (2005) The ClpP double ring tetradecameric protease exhibits plastic ring-ring interactions, and the N termini of its subunits form flexible loops that are essential for ClpXP and ClpAP complex formation. J Biol Chem 280(16):16185–16196
Kang SG, Maurizi MR, Thompson M, Mueser T et al (2004) Crystallography and mutagenesis point to an essential role for the N-terminus of human mitochondrial ClpP. J Struct Biol 148(3):338–352
Jennings LD, Bohon J, Chance MR, Licht S (2008) The ClpP N-terminus coordinates substrate access with protease active site reactivity. Biochemistry 47(42):11031–11040
Martin A, Baker TA, Sauer RT (2008) Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat Struct Mol Biol 15(11):1147–1151
Martin A, Baker TA, Sauer RT (2008) Diverse pore loops of the AAA+ ClpX machine mediate unassisted and adaptor-dependent recognition of ssrA-tagged substrates. Mol Cell 29(4):441–450
Flynn JM, Neher SB, Kim YI, Sauer RT et al (2003) Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell 11(3):671–683
Kim YI, Burton RE, Burton BM, Sauer RT et al (2000) Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol Cell 5(4):639–648
Ortega J, Singh SK, Ishikawa T, Maurizi MR et al (2000) Visualization of substrate binding and translocation by the ATP-dependent protease, ClpXP. Mol Cell 6(6):1515–1521
Thompson MW, Singh SK, Maurizi MR (1994) Processive degradation of proteins by the ATP-dependent Clp protease from Escherichia coli. Requirement for the multiple array of active sites in ClpP but not ATP hydrolysis. J Biol Chem 269(27):18209–18215
Schlieker C, Weibezahn J, Patzelt H, Tessarz P et al (2004) Substrate recognition by the AAA+ chaperone ClpB. Nat Struct Mol Biol 11(7):607–615
Siddiqui SM, Sauer RT, Baker TA (2004) Role of the processing pore of the ClpX AAA+ ATPase in the recognition and engagement of specific protein substrates. Genes Dev 18(4):369–374
Wang J, Song JJ, Franklin MC, Kamtekar S et al (2001) Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9(2):177–184
Yamada-Inagawa T, Okuno T, Karata K, Yamanaka K et al (2003) Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J Biol Chem 278(50):50182–50187
Lum R, Tkach JM, Vierling E, Glover JR (2004) Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J Biol Chem 279(28):29139–29146
Gonzalez M, Rasulova F, Maurizi MR, Woodgate R (2000) Subunit-specific degradation of the UmuD/D′ heterodimer by the ClpXP protease: the role of trans recognition in UmuD′ stability. EMBO J 19(19):5251–5258
Dougan DA, Weber-Ban E, Bukau B (2003) Targeted delivery of an ssrA-tagged substrate by the adaptor protein SspB to its cognate AAA+ protein ClpX. Mol Cell 12(2):373–380
Neher SB, Sauer RT, Baker TA (2003) Distinct peptide signals in the UmuD and UmuD′ subunits of UmuD/D′ mediate tethering and substrate processing by the ClpXP protease. Proc Natl Acad Sci U S A 100(23):13219–13224
Wah DA, Levchenko I, Rieckhof GE, Bolon DN et al (2003) Flexible linkers leash the substrate binding domain of SspB to a peptide module that stabilizes delivery complexes with the AAA+ ClpXP protease. Mol Cell 12(2):355–363
Galperin MY (2006) Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol 188(12):4169–4182
Peterson CN, Ruiz N, Silhavy TJ (2004) RpoS proteolysis is regulated by a mechanism that does not require the SprE (RssB) response regulator phosphorylation site. J Bacteriol 186(21):7403–7410
Bren A, Welch M, Blat Y, Eisenbach M (1996) Signal termination in bacterial chemotaxis: CheZ mediates dephosphorylation of free rather than switch-bound CheY. Proc Natl Acad Sci U S A 93(19):10090–10093
Hess JF, Bourret RB, Simon MI (1988) Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature 336(6195):139–143
Bachhawat P, Stock AM (2007) Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride. J Bacteriol 189(16):5987–5995
Bachhawat P, Swapna GV, Montelione GT, Stock AM (2005) Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure 13(9):1353–1363
Lee SY, Cho HS, Pelton JG, Yan D et al (2001) Crystal structure of activated CheY. Comparison with other activated receiver domains. J Biol Chem 276(19):16425–16431
Toro-Roman A, Mack TR, Stock AM (2005) Structural analysis and solution studies of the activated regulatory domain of the response regulator ArcA: a symmetric dimer mediated by the alpha4-beta5-alpha5 face. J Mol Biol 349(1):11–26
Toro-Roman A, Wu T, Stock AM (2005) A common dimerization interface in bacterial response regulators KdpE and TorR. Protein Sci 14(12):3077–3088
Studemann A, Noirclerc-Savoye M, Klauck E, Becker G et al (2003) Sequential recognition of two distinct sites in sigma(S) by the proteolytic targeting factor RssB and ClpX. EMBO J 22(16):4111–4120
D’Souza C, Nakano MM, Zuber P (1994) Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc Natl Acad Sci U S A 91(20):9397–9401
Hamoen LW, Eshuis H, Jongbloed J, Venema G et al (1995) A small gene, designated comS, located within the coding region of the fourth amino acid-activation domain of srfA, is required for competence development in Bacillus subtilis. Mol Microbiol 15(1):55–63
Turgay K, Hamoen LW, Venema G, Dubnau D (1997) Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev 11(1):119–128
Persuh M, Turgay K, Mandic-Mulec I, Dubnau D (1999) The N- and C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol Microbiol 33(4):886–894
Turgay K, Hahn J, Burghoorn J, Dubnau D (1998) Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J 17(22):6730–6738
Ogura M, Liu L, Lacelle M, Nakano MM et al (1999) Mutational analysis of ComS: evidence for the interaction of ComS and MecA in the regulation of competence development in Bacillus subtilis. Mol Microbiol 32(4):799–812
Bougdour A, Gottesman S (2007) ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc Natl Acad Sci U S A 104(31):12896–12901
Tissieres A, Mitchell HK, Tracy UM (1974) Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol 84(3):389–398
Gamer J, Bujard H, Bukau B (1992) Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell 69(5):833–842
Guisbert E, Herman C, Lu CZ, Gross CA (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev 18(22):2812–2821
Straus DB, Walter WA, Gross CA (1987) The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature 329(6137):348–351
Herman C, Thevenet D, D’Ari R, Bouloc P (1995) Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci U S A 92(8):3516–3520
Kanemori M, Nishihara K, Yanagi H, Yura T (1997) Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of sigma32 and abnormal proteins in Escherichia coli. J Bacteriol 179(23):7219–7225
Tomoyasu T, Gamer J, Bukau B, Kanemori M et al (1995) Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor sigma 32. EMBO J 14(11):2551–2560
Sousa MC, Trame CB, Tsuruta H, Wilbanks SM et al (2000) Crystal and solution structures of an HslUV protease-chaperone complex. Cell 103(4):633–643
Okuno T, Ogura T (2013) FtsH protease-mediated regulation of various cellular functions. In: Dougan DA (ed) Regulated proteolysis in microorganisms. Springer, Subcell Biochem 66:53–69
Kanemori M, Yanagi H, Yura T (1999) Marked instability of the sigma(32) heat shock transcription factor at high temperature. Implications for heat shock regulation. J Biol Chem 274(31):22002–22007
Rodriguez F, Arsene-Ploetze F, Rist W, Rudiger S et al (2008) Molecular basis for regulation of the heat shock transcription factor sigma32 by the DnaK and DnaJ chaperones. Mol Cell 32(3):347–358
Horikoshi M, Yura T, Tsuchimoto S, Fukumori Y et al (2004) Conserved region 2.1 of Escherichia coli heat shock transcription factor sigma32 is required for modulating both metabolic stability and transcriptional activity. J Bacteriol 186(22):7474–7480
Obrist M, Milek S, Klauck E, Hengge R et al (2007) Region 2.1 of the Escherichia coli heat-shock sigma factor RpoH (sigma32) is necessary but not sufficient for degradation by the FtsH protease. Microbiology 153(Pt 8):2560–2571
Herman C, Prakash S, Lu CZ, Matouschek A et al (2003) Lack of a robust unfoldase activity confers a unique level of substrate specificity to the universal AAA protease FtsH. Mol Cell 11(3):659–669
Langklotz S, Baumann U, Narberhaus F (2012) Structure and function of the bacterial AAA protease FtsH. Biochim Biophys Acta 1823(1):40–48
Obrist M, Langklotz S, Milek S, Fuhrer F et al (2009) Region C of the Escherichia coli heat shock sigma factor RpoH (sigma 32) contains a turnover element for proteolysis by the FtsH protease. FEMS Microbiol Lett 290(2):199–208
Tomoyasu T, Arsene F, Ogura T, Bukau B (2001) The C terminus of sigma(32) is not essential for degradation by FtsH. J Bacteriol 183(20):5911–5917
Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286(5446):1888–1893
Gur E, Sauer RT (2008) Recognition of misfolded proteins by Lon, a AAA(+) protease. Genes Dev 22(16):2267–2277
Dougan DA, Reid BG, Horwich AL, Bukau B (2002) ClpS, a substrate modulator of the ClpAP machine. Mol Cell 9(3):673–683
Farrell CM, Grossman AD, Sauer RT (2005) Cytoplasmic degradation of ssrA-tagged proteins. Mol Microbiol 57(6):1750–1761
Mogk A, Tomoyasu T, Goloubinoff P, Rudiger S et al (1999) Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J 18(24):6934–6949
Goloubinoff P, Mogk A, Zvi AP, Tomoyasu T et al (1999) Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci U S A 96(24):13732–13737
Schlothauer T, Mogk A, Dougan DA, Bukau B et al (2003) MecA, an adaptor protein necessary for ClpC chaperone activity. Proc Natl Acad Sci U S A 100(5):2306–2311
Wawrzynow A, Wojtkowiak D, Marszalek J, Banecki B et al (1995) The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J 14(9):1867–1877
Kock H, Gerth U, Hecker M (2004) The ClpP peptidase is the major determinant of bulk protein turnover in Bacillus subtilis. J Bacteriol 186(17):5856–5864
Miethke M, Hecker M, Gerth U (2006) Involvement of Bacillus subtilis ClpE in CtsR degradation and protein quality control. J Bacteriol 188(13):4610–4619
Dougan DA, Mogk A, Bukau B (2002) Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. Cell Mol Life Sci 59(10):1607–1616
Matuszewska M, Kuczynska-Wisnik D, Laskowska E, Liberek K (2005) The small heat shock protein IbpA of Escherichia coli cooperates with IbpB in stabilization of thermally aggregated proteins in a disaggregation competent state. J Biol Chem 280(13):12292–12298
Mogk A, Deuerling E, Vorderwulbecke S, Vierling E et al (2003) Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol 50(2):585–595
Bissonnette SA, Rivera-Rivera I, Sauer RT, Baker TA (2010) The IbpA and IbpB small heat-shock proteins are substrates of the AAA+ Lon protease. Mol Microbiol 75(6):1539–1549
Acknowledgments
Work in the DAD laboratory is funded by the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Micevski, D., Dougan, D.A. (2013). Proteolytic Regulation of Stress Response Pathways in Escherichia coli . In: Dougan, D. (eds) Regulated Proteolysis in Microorganisms. Subcellular Biochemistry, vol 66. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5940-4_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-5940-4_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5939-8
Online ISBN: 978-94-007-5940-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)