Abstract
Amongst the sap-sucking hemipteran scale insects, the pseudococcids (mealybugs) deserve a special place in pursuance of implying a peculiar situation in which sex determining mechanism and of the prevalence of puzzling meiotic chromosome behaviour. Pseudococcids represent sex-specific heterochromatization of the entire set of chromosome and transcriptional silencing of all male-oriented chromosome systems. In recent years, there have been significant contributions made in towards current understandings of mealybug chromosome systems essentially oriented upon molecular level progression of “genomic imprinting” phenomenon. This report will present available information pertaining to the types of cytological changes that occur at the molecular level organization and how such kind of heterochromatin status might be maintained. As this apparent from the foregoings based on the mealybug chromosomes, the role of constitutively heterochromatic zones in the genome has been defined facilitatively by means of specialized classical staining protocol. It seems evident enough to point out that the explicit nature of facultative heterochromatinization programme (formulated by three-pronged approach: the DNA sequences, the biochemical milieu and the chromatin remodeling) for chromatin-based differences that prevail in the maternal and paternal genomes. It is also apparent that the mealybug system may offer providing as a robust genetic example for the stable maintenance of chromatin code through to mitosis and meiosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The coccoids which include mealybugs are a relatively small group of highly specialized hemipteran insects. They are parasitic on plants and quite sedentary in behavior (Miller and Kosztarab 1979; Gullan and Kosztarab 1997; Mani 1989; Kondo et al. 2008). The chromosome system of coccoids is of special interest because it is characterized by chromosomal heterochromatization or elimination of the paternal endowment of chromosomes during early embryogeny of the male in the majority of scale insects. The first cytological insight into the nature of this remarkable system came from the pioneering cytology described by Schrader (1921, 1923a). Subsequent studies by Hughes-Schrader (1948) have provided insightful thoughts into the explanation of the genetic and evolutionary implications of “paternal heterochromatization” that could serve as an intermediate stage between regular diploidy and true male-haploidy.
Schrader’s interpretation was later confirmed experimentally with a mealybug, for example, Pseudococcus obscurus and/or Planococcus citri by Brown and his associates (Brown 1958, 1959, 1963, 1964, 1965, 1969; Brown and Nelson-Rees 1961; Chandra 1962, 1963a, b; Brown and Nur 1964; Baer 1965; Nur 1963, 1966a, b, 1967; Brown and Weigmann 1969). Earlier cytological scrutiny had been reviewed by White (1973) and certain aspects of coccoid chromosome systems especially their possible role in the involvement in the chromosome imprinting processes, have been aptly dealt with by Brown (1977) and Brown and Chandra (1977), about certain unusual features by Nur (1980, 1990), and about recent achievements made with respect to biochemical-based cytology by Prantera and Bongiorni (2012). Enormous and extensive cytological and genetic studies of mealybugs belonging to worldwide fauna are available (Little 1957; Carter 1962). However, the efforts on the systematic and cytogenetic aspects of Indian coccoids are very limited. There have been sporadic reports that provide incomplete and contradictory information pertaining to Indian fauna for their chromosome systems (Tulsyan 1963; Dikshith 1964, 1966; Chauhan 1970, 1977).
2 Mealybug Chromosomes
All coccoids possess holocentric chromosomes, that is, diffuse centromeres (Hughes-Schrader and Ris 1941). Inverse meiosis is a second ancestral condition manifested in coccoids that is also shared with the other closely allied aphids (Ris 1942; Hughes-Schrader 1944, 1948).
Although the cell cycle sequence is different from that of typical meiosis, results are the same; each of the four chromatids of meiotic bivalents reaches one of the four nuclei produced by meiosis. Coccoids are also manifested by those systems in which at least some of the females are produced parthenogenetically, in addition to the usual bisexual mode of reproduction. They are considered to have unique chromosome systems and they offer enormous potential in our understanding of problems such as chromosome imprinting and differential regulation of homologous chromosome sets (Chandra 1971; Chandra and Brown 1973). Chandra (1971) suggested for the first time that there are some similarities and also contrasts between mammalian X-chromosome inactivation and the inactivation of paternal chromosomes in mealybugs. These include genomic imprinting, facultative heterochromatization, and differential regulation of homologous chromosomes. Subsequently, Brown and Chandra (1977) have drawn attention to emphasize that coccoids are at the pinnacle of an evolutionary pyramid of cytogenetic variants and complexity. In order to understand these variations in chromosome mechanics, it becomes essential briefly to review pseudococcid chromosomes.
3 Chromosome Numbers and Chromosome Forms
Coccoid chromosomes lack specified centromeric regions. It appears obvious to point out that chromosome fragments perpetuate themselves during successive divisions. In the absence of kinetochore-based cell divisions that prevail in coccoid chromosome systems, and also, in order to accommodate the occurrence of karyotypic changes, Brown (1961) assays chromosome fracture and fusions in the place of the prevalent nature of chromosomal rearrangements. It was also envisaged that simple breakage can determine increase or decrease in chromosome numbers unless a breakage–fusion–bridge cycle intervenes to eliminate the breakage points.
Species relationships can be explained by citing chromosome variability occurring with respect to either chromosome numbers or morphology. Brown (1961) insists upon spontaneous occurrence of chromosome breakage resulting in abundant availability of ruptured chromosomes for increase in the diploid numbers either by chance or incurred by selection. There are an abundant number of cases dealing with karyotypic changes incurring based on chromosome fragmentation in mealybug genomes (Nur et al. 1987; Cook 2000).
Changes in chromosome numbers with respect to pseudococcid species have been reported to be in the range of 8 to 64 and that ranges within coccoids are rather small compared to other insect groups (Hughes-Schrader 1948; Nur et al. 1987). Until now, 115 cytogenetically studied species of mealybugs belonging to 44 genera have been made known (Gavrilov 2007; Gavrilov and Trapeznikova 2007, 2010). Eventhough, the diploid number of chromosomes ranges from 8 to 64, the modal number seem to fall on 10 (Plate 3.6 Fig. 4, 6, 8). Few mealybugs showed intrageneric variation in their chromosome numbers; for example, in genera such as Antonina (2n = 12, 16, 24 + Bs), Nesopedronia (2n = 18, 14, 10) and Trionymus (2n = 16, 10, 8), such instances can be cited as useful in taxonomic and phylogenetic considerations of the genus. Accessory chromosomal elements (B-chromosomes) were found in several species of mealybugs (Nur et al. 1987; Gavrilov 2004). But, the detailed investigation of B-chromosomes has been done only in Pseudococcus viburni (Signoret; Nur 1962a, 1966a, b). The majority of pseudococcids possess 2n = 10 (Nur et al. 1987; Moharana 1990; Nur 1990; Gavrilov and Trapeznikova 2007, 2010). Excepting Planococcus citri and a few other species, the number of species studied based on employing recently evolved cytogenetic techniques is very low. One of the reasons cited was the difficulties incurred in procuring enough cells for the preparation of chromosomes and of understanding of chromosome basics for detailed cytological analyses. For cytological investigations of Indian mealybug taxa, Parida and Moharana (1982) and Moharana (1990) attempted to enumerate chromosome numbers based on conventional cytological techniques and they were also able to present preliminary assessments of karyomorphological features for more than 20 different species. Based upon female pachytene chromomeric sequences, Raju (1994) made an initial attempt to describe karyotype and comparison of three species of the Indian genus Planococcus (viz. P. citri, P. lilacinus and P. pacificus) essentially based on differential banding patterns, but was unable to identify individualistic karyotypes because of lack of discriminating cytogenetic features (Plates 1–5). Gavrilov (2004a, 2007) and Gavrilov and Trapeznikova (2007, 2010) have made elaborate studies resulting in the elucidation of the karyotype for more than 25 species of Russian mealybugs based on squashing techniques for chromosomal preparations. Nur et al. (1987) were able to describe the karyotype of about 80 different species of mealybugs that were collected from various parts of Africa, America, and a few from South Asia. Tremblay et al. 1977 (Italy), Mckenzie 1967 (California), Drozdovskiy 1966 (Russia), Brown 1961 and Hughes-Schrader 1935 (USA), and Schrader 1923a (USA) have contributed enormously to the field of mealybug cytogenetics in the form of karyological studies. In an attempt to analyse mealybug chromosome morphology, chromosome preparations were studied through the fluorescent microscopy using appropriate dyes (e.g., Quinacrine Mustard (QM)/QM dihydrochloride), and it was found that these chromosomal complements did not provide any discriminative cytological signatures other than suggesting that they belong to and qualify themselves as belonging to the “Lecanoid type” of chromosome system (Jaipuriar et al. 1985; Venkatachalaiah and Chowdaiah 1987; Venkatachalaiah 1989).
4 Telomeres and C- Bands
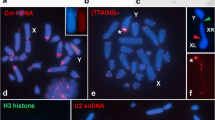
It is of interest to note that with particular importance to the diffuse nature of centromeric systems manifested by coccoid chromosome morphology it was expected to display discriminative C-staining profiles along the length of each chromosomal fragment in the complement. Employing classical C-staining protocol upon Planococcus citri metaphase chromosomal preparations, it was expected to highlight constitutively heterochromatic sites in the complement. But the cursory observations led in demonstrating that the C-specific bands were found specifically identifying the telomeric region specificity in the metaphasic chromosomal complement and this situation was ascribed as T-bands (Venkatachalaiah 1989; Raju 1994). However, the results obtained by Venkatachalaiah (1989) and Raju (1994) pertaining to C-banded stainings at telomeric ends of each chromosome in the complement were irrespective of a particular chromosome type (whether of mitotic, meiotic, or polyploid nuclei) or sexes (males or females), and thus, they contend that these cytological markers could be representing a particular type of constitutive heterochromatic component. The intense stainability at the telomeric regions in the chromosomal content allows one to assay that this chromosomal component may offer conveying information about its cytogenetic context. The situation acquires a genetic signature due to its co-orientation pairings during late meiotic (male or female) chromosome synaptic processes (Plate 3.7 Fig. 6, 7, 8, 9).
Ferraro et al. (1998), in their attempt to localize C-banded regions at P. citri chromosomal preparations, found evidence regarding C-positive bands localizing at the telomeric regions of all chromosomes in the complement. When they further insisted upon prior exposure to CMA3 (chromomycin A3) -methyl green and subsequent exposure to C-staining protocols, the implicit C-bands were found correspond to telomeric region-specific areas. This has led them to infer that these results could, however, representing GC-rich specific spots on chromosomes. However, when they insisted further upon H33258 fluorochrome to live cells prior to C-staining, they recovered images almost imitating C-banded telomeric-specific regions. Thus they were able to interpret the failure to find a dull appearance, instead of bright bands at the specified locales, and those of brighter and intense bandings could represent condensed constitutive heterochromatic regions, leading them to insist that there could be more DNA congregated per unit length per chromosome than in the euchromatic zones. Moreover, some of the telomeric regions being positive to DAPI stainings, it was inferred that the presence of AT-rich sequences were embedded within the predominantly GC-rich regions of individual chromosomes.
From the point of view of cytology, telomeres are marked by specialized DNA and protein components that usually decorate the chromosome ends or other specific loci. In several eukaryotes, their occurrence and prevalence has been tested, wherein they have been found composed of simple tandem pentameric (TTAGG) repeats localizable at specific chromosome loci accompanied by complex subtelomeric structures in close apposition (D’Aiuto et al. 2003; De Lange 2005). A large number of molecular cytological studies have led to the implication that telomeres of eukaryotes are usually composed of conserved short tandemly repeated GC-rich sequences. This kind of sequence conservation is reflected as a common mechanism for telomere region biosynthesis. This mechanism specifically dictates and involves the activity pattern of a telomerase, a ribonucleoprotein DNA polymerase enzyme that compensates any further loss of terminal sequences at every replication round by adding short tandem GC-rich sequences onto the chromosome ends (Greider 1995).
Studies in various insect species demonstrated characteristic presence towards the notion that TTAGG repeats are an ancient motif traceable in Arthropoda and that those pentameric TTAGG repeats that could have been originated from the vertebrate TTAGGG hexamers (Frydrychová et al. 2004; Vitkova et al. 2005).
Cytogenetic scrutiny undertaken by Mohan et al. (2011) with regard to Planococcus citri chromosomes have enabled them to delineate the presence of a characteristic pattern of telomeric sequences and also some of their placements upon the respective interstitial loci and the constitutive presence of active telomerases was detected and this was achieved by introducing single primer PCR and Southern hybridization protocols upon cytological preparations. The results so obtained suggest that in particular, P. citri chromosome complement seemed to provide as an efficient chromosome marker to demarcate the chromosomal loci at the site of the mechanism of formulation of TTAGG repeats at their respective chromosome ends. In addition, this study was also aided in identifying and thus disclosing whether some unrelated low copy repeats, called Intercept TTAGG Sequences (ITS) were displaying identifiable spots based on their presence, thereby intercepting the repetitive elements. It is well known that P. citri genomes are bestowed with diffuse centromere (holocentric) activity and as a consequence of this nature there could be an obvious presence of multiple centromeric zones occupying the length along individual chromosomes. Utilizing this extraordinary condition, in view of these genetic peculiarities persisted with elegant DNA repair machinery that ensures the protection of additional chromosomal elements localizing at interstitial zones; and thus they aptly recognize these sites as putative zones. Surprisingly, following X-ray irradiation upon these broken chromosomal ends it was disclosed that some loci were characteristic and were tagged with the association of TTAGG repeats decorating at chromosomal interstitial regions. Because of their resistance to higher doses of ionizing radiation, a unique feature characterizing the mealybug genome and this extraordinary chromosomal phenomena could as well serve as an asset towards relegating them to be considered as a “radiation-resistant coccid.”
Mohan et al. (2012) further attempted to test responses with still higher doses of ionizing radiation exposure on P. citri genomes and were thus able to utilize this opportunity to suggest that mealybug genome may well serve as a unique genetic system. The results of their explorations revealed that especially pounding concentration on the centromeric property that was eventually recognized as sites of activity sporadically spreading over the length, and in spite of this enormity there is no significant loss of the genetic material. Furthermore, with respect to the mealybug genome, it was considered to contain highly tolerable radiation doses as high as 1100 Gy. Presently, it is apparent that mealybug genomes may serve as very efficient agents of the DNA repair machinery system that ensures proper healing of double-strand breaks (dsb) invaded by ionizing radiation. Despite several special qualities,proclaimed as containing, for example, of telomeric repeats along with interstitial sites of chromosomes and with respect to maintenance and sustainability of telomeres to higher radiation effects, some authors believe regardless of the vulnerability of the telomeric-independent mechanism it could also be operating in a P. citri genetic system.
Thus, the occurrence of C-heterochromatin occupying telomeric regions of chromosomes deserves some comments. In its usual courses of other cases, incidentally pertaining to holocentric chromosome systems, it was possible to ascribe that C-heterochromatin is preferentially located at or near telomeres (Muramoto 1980; Camacho et al. 1985; Papeschi 1998; Panzera et al. 1992). According to Heitz’s (1933) “equilocal heterochromatin distribution” hypothesis, it was inferred that the C-banding material in both homologous and nonhomologous chromosomal sets tends to congregate at homogeneous and homologous regions, thereby occupying similar kinds of cytological sites, and thus probably represented by either telomeric and/or centromeric sequences. Schweizer and Loidl (1987) have proposed a model that explains how C-heterochromatin enhances and leads to adherence of such chromosomal zones confining and/or inducing towards effecting interchanges of heterochromatic material between nonhomologous and homologous chromosomes in the complement and thus leading towards annealing into a common platform resulting in such situation that they belong to as though in a monocentric type of chromosome system; that also insists upon those of chromosomal regions with holokinetic activity that do not fit into this model. In view of the limited information gathered from other homopteran examples, an effort was made to define that the nature and kind of telomeric components that were found enhanced to establish as a C-banded heterochromatin. Moreover, Panzera et al. (1992) and Pérez et al. (1997) based on their limited experience offer the opinion, especially of Triatoma meiotic systems, that the tendency of the heterochromatin component inferring to change in accordance with from one chromosome to another or from proximal to distal sites of the same chromosomes within a complement. However, this characteristic cytological feature was found on preferential localization of telomeric heterochromatic content of some instance cases alone probably thereby reflecting upon C-banded components. These proposals are in congruence with those of the Schweizer and Loidl (1987) hypothesis, but this type of chromosomal behavior is not in any way agreeable to certain terms with other instance cases analyzed from other homopteran examples for the said purposes including the coccoid chromosome systems.
Ferraro et al. (1998) had undertaken an eloborate proceedings in view of eliciting and appropriating the preponderance of the ribosomal cistrons and upon highlighting of their cytological localization based on the mealybug chromosomal preparations. This analysis had led to the results so obtained by means of the FISH technique and of subsequently staining the same with silver nitrate solution for localizing NOR (Nucleolar Organizer Region) specificities on metaphase chromosomes. These results point to have driven them to ascribe that the FISH technique might help in identify with P. citri chromosomes at specific zones on all chromosomes except at one pair in the complement. But silver nitrate staining specificity had enabled in specifying at a single pair in the complement but characteristically demonstrating the site at which bearing very prominent macer-shaped, silver nitrate stainable entities, irrespective of their origin whether of euchromatic or heterochromatic chromosomal pair (Plate 3.7 Fig. 1, 2, 3, 4, 5).
5 B- Chromosomes
During the courses of systematic cytological study in the case of Pseudococcus affinis chromosomal complement that possesses supernumerary B chromosomes which were transmitted without the reduction during spermatogenetic courses that were found exhibiting a strong “meiotic drive”, in such processes (Nur 1962a, b, 1969). Prior to spermatogenesis, the B chromosome was heterochromatic, but during prophase I of spermatogenesis it became evident that even less condensed than the euchromatic set (i.e., negatively heteropycnotic) and this change in condensation property apparently makes this situation possible for the Bs to segregate with the euchromatic set and be transmitted over to 90 % of the offspring. Nur and Brett (1985, 1987, 1988) have presented subjective data supporting that acquisition of the condensation property of As and Bs during spermatogenesis seemed to infer that this situation is due to the presence of genotype that affects the rate of transmission of the Bs in males. However, it is somewhat clear that this situation became evident because of the influence of this genotype which has affected the condensation property of B, but not the property of heterochromatization. However, Klein and Eckhart (1976) theorized that difference between Bs and regular chromosomes of Pseudococcus affinis could be due to changes occurring at the DNA sequences level. Another probable reason sighted was the differences observed between the A and B sets that could be due to the occurrence of DNA of the two types of heterochromatin that being methylated. Thus, the percentage of 5-methylcytosine in the DNA of P. affinis was found to be higher in males than in females, and higher in females without Bs than in females with Bs (Scarbrough et al. 1984).
6 Polyploidy and Endosymbionts
In most species of mealybugs the polar bodies re-enter the egg and contribute to or give rise to large polyploid cells (mycetocytes) that house intracellular bacterial symbionts (Brown 1965). In some mealybugs, cells formed by polar bodies 1 and 2 are known to be totipotent. In male mealybugs and in other coccoid families, one portion of the genome becomes heterochromatic and the other becomes euchromatic (genetically active) in several tissues or organs (Tremblay and Caltagirone 1973). These include the midgut, the Malpighian tubules, the salivary glands, oenocytes, and serosa (Nur 1967, 1972). One characteristic of most of these tissues is that their nuclei later become polyploid as a result of endoreduplication or endomitosis (Plate 3.6 Fig. 3, 5, 9). During oogenesis, polar bodies do not degenerate; instead they re-enter the egg cell, and fuse with each other and also with some of the cleavage nuclei and form polyploid cells called mycetocytes. These mycetocytes are invaded by certain maternally transmitted microorganisms generally referred as symbionts. Mycetocytes harboring such symbionts form an organ called mycetomes whose function is not known (Brown 1965; Nur 1977). Such symbionts are transovarially transmitted to the next generation and thus show maternal inheritance (Buchner 1965). Euchromatization, however, is apparently not an essential step in the development of these tissues, because these types of tissues involved may vary between congeneric species. Moreover, the frequency of cells in which euchromatization occurs sometimes varies between individuals. However, in those nuclei in which the paternal genome remained heterochromatic, it usually did not replicate or having replicated once, the euchromatic sets replicated several times (Lorick 1970; Nur 1966c, 1970, 1972).
The sex-specific association of the microorganisms has led to the suggestion that they may have a role in sex determination (Buchner 1965). However, the precise nature and role of endosymbionts in normal development has not been clearly assessed. Biochemical and morphological analyses of isolated endosymbionts have established their prokaryotic characteristics (Houk and Griffiths 1980; Ishikawa 1989). The 16 s rRNA gene sequences of several homopteran insect endosymbionts including those of certain species of mealybugs and aphids, have been considered for their role in the prevalence of phylogenetic relationships among those species probed for those purposes (Munson et al. 1991, 1992; Kantheti 1994). However, the nature and extent of type of expression of the concerned gene inquisition during the course of insect development are not clearly explained. Buchner (1965) reported that extracellular symbionts are present in the females of Stictococcus but absent in the males. Most coccoids contain intracellular bacteria or yeastlike symbionts present in the cytoplasm of special cells, the mycetocytes (Tremblay 1977, 1989). The origin of the mycetocytes is of interest because it may vary between, as well as within, families. Therefore, it appears probable that the origin of mycetocytes may have an important bearing on the pseudococcid genetic system (Hughes-Schrader 1948).
Interestingly, Kantheti et al. (1996) reported an isolation of the 16S rRNA gene sequenced segment, designated as P7 from an embryonic cDNA library of Planococcus lilacinus, which was found to be an encouraging attempt and by hybridizing to the genomic DNA of females to the assay, but not to that of males. Interestingly P7 showed no hybridization to nuclei of either sex, raising the possibility that it was extrachromosomal in origin. Using electron microscopic images, especially of P7 clones but not of P3, annealing was found to the adult female abdominal organ called mycetomes. Electron microscopy has disclosed the presence of symbionts within the mycetocytes. Sequence analysis showed that P7 is a 16 s rRNA gene confirming its prokaryotic origin. P7 expression is detectable in young embryos of both sexes but the absence of P7 in third instar and adult males suggests that the designated gene containing isolated gene sequences assay and hence, consideration of provisional endosymbionts are the subject and object of sex-specific elimination/acquisition type of operating processes.
7 Mechanism of Sex Determination
In many species, sex determination is associated with the inheritance of a heteromorphic chromosome pair in one sex. However, not all species have evolved from a common ancestor that possessed such an heteromorphic sex chromosome set. Rather, XX–XY sex determination appears to have arisen independently many times in evolution from the XX–XO type form. The XX–XY sex chromosomes of flies and mammals also arose independently, but, the underlying mechanisms of sex determination are quite different and difficult to predict except in molecular terms.
Coccoids are a unique and very peculiar group of insects in view of their possessing a highly variable mode of sex-determining mechanisms. This situation becomes evident through the course of studying complex meiotic processes incurred in a few select examples analyzed thus far. Thus, this situation has led to the creation of some academic interest by some earlier cytologists to pursue further upon attempting understand the intricacies of meiosis and mitosis. Interestingly, White (1973, 1978) took special interest in accommodating this opportunistic situation prevailing in mealybugs (scale insects) summarily termed as “aberrant genetic systems,” and Nur (1980) proclaimed “unusual chromosome systems” but recent views indict them either as the “more diverse” or “asymmetric genetic system”. Serendipity, as applied to these scale insects, which are characterized by possession of a peculiar genetic system, was not found in any other animal system of comparable nature.
These bizarre genetic systems are of immense help in our attempt to understand further upon the occurrence of a variety of sex-determining mechanisms prevailing in scale insects in the light of their inherent property of inverse meiosis effectively driving them through the efforts of holokinetic chromosome mechanics. Most species of coccoids are bisexuals with extreme sexual dimorphism but due to precariousness of male populations at times, some of them have become parthenogenetic. These complex genetic systems appear invigorating due to the involvement of both the bisexual as well as the parthenogenetic mode of reproduction. Another noteworthy feature is inflicted on them due to the deliverance of quadrinucleate spermatid formation in many mealybug (bisexual) chromosome systems. It is thus possible to surmise that the various types of meiosis that were confronted within the scale insect examples could have arisen in a derivative form or in a succeeding form from that of primitive homopteran (aphid–coccid line) examples including aphid chromosome systems (XX–XO system). It is thus possible to note that during the derivation processes it became inevitable in view of the penchant situation prevailing with those participants driving in through to the equatorial orientation of meiotic bivalents at first meiosis and of the preponderance of prereduction at chiasma.
8 XX–XO System
Sex determination in primitive coccoids could have taken its initiation based on the XX (♀)–XO (♂) type of sex-determination mechanism. Consequent upon this exigency, oogenesis is of conventional type progressing through inverse meiotic pathways, whereas spermatogenesis is highly modified in most coccoids, mealybugs in particular. In view of this unique situation, variable modes of expression pathways become imminent as represented among analyzed primitive margarodid examples. Currently, cytological records have become known from margarodid assemblage of species that include taxa belonging to Margarodidae, Ortheziidae, and Putoidae. The cytological descriptions of these primitive groups of species characteristically reveal that the sex of progeny is predetermined prior to and at spermatogenesis; spermatozoa with the X-chromosome produce females and spermatozoa lacking it produce males (Nur 1980). In this extent, Brown (1977) placed primary emphasis upon the coccoid chromosome system imparting the implicit nature of acquiring adaptive specialization and the same was found reflected in the progression of meiotic processes which in turn enabled categorizing into three types: (1) Margarodid assemblage, (2) Lecanoid types, and (3) Diaspidid systems (Plate 3.8). In some Margarodid examples studied, spermatogenesis resembles that of conventional oogenesis, as is especially evident in the case of Puto. Some species of Puto demonstrated conventional meiotic chromosomal features. These species follow a typical heterogametic mode of sex-determination mechanisms, in which the males usually possess one chromosome less than that of females; characteristically the example includes Puto species, 2n = 14♀–13♂; Puto albicans, 2n = 20♀–19♂; and Callipappus rubigonosus, 2n = 14♀–13♂ (Brown and Cleveland 1968; Hughes-Schrader 1944). There are other taxa in which spermatogenesis is highly modified as was shown in meiosis of Protortonia and Matsucoccus gallicola defining multiple sex chromosome systems. Surprisingly, the only other report in which no morphologically identifiable sex chromosomes were shown is represented by an example showing cytological features for the whole Ortheziidae family, comprising 2n = 16 in both sexes (Brown 1958).
9 Lecanoid System
In the Lecanoid chromosome type of coccoids that exhibit a peculiar situation among coccoid chromosome systems is one in which one “haploid” set of chromosomes acquires heteropycnosis during the developmental course and also in the germline cells. Those cells destined to become males acquire this status probably from mid-blastula onwards and persist through to adult life. In the females, both sets of chromosomes in the cell remain in the euchromatic state throughout the developmental course (Schrader 1921, 1923b; Schrader and Hughes-Schrader 1926). The basis for such an extraordinary situation in the mealybug chromosome is in the procession of acquisition of the mechanism of heteropycnosis in male embryos and this involvement facilitates functional inequalities with respect to males that may point towards proclaiming them as physiological haploids, even though they have a duplex set in their nuclei (Plate 3.6). Brown and his group have ventured into delineating the processes and involvement of genetic mechanics of genomic inactivation and of cytogenetics of heterochromatinization processes in the genome (examples include Planococcus, Phenacoccus, Maconellicoccus, etc. of the family Pseudococcidae and Laccifer of Kerridae). It is of interest to learn more about and probe further the processes of heteropycnosis of the paternal composition of the Lecanoid chromosomes and as such it becomes imperative to note that this set passed through the male phase but expressivity was confined only to genetic male zygotes. Brown and Nur (1964) demonstrated earlier through their hybridization experiments that in hybrid male embryos the mechanisms of heterochromatinization of the paternal set can occur in the cytoplasm of the foreign cell and thus this mechanism is not neccessarily a species-specific characteristic, because heterochromatinization progression processes occur after several divisions of cleavage, and the paternal set must somehow seemed to have been marked (or learned) which could have been done or did prior to the entry of sperm into the egg (Chandra and Brown 1975). Of the two processes, the marking (i.e., imprinting) of and the activity status (of heterochromatization), of which the earlier process seems likely to be an effective one at the ancestral stock and this attribute might reflect in bringing about differential condensation activity of the concerned chromosomes. At this juncture, Brown (1977) felt that this situation appeared premature to theorize about or make any generalization unless substantial molecular data were made available.
9.1 Parthenogenesis (Unisexual Reproduction)
While further pursuing the nature of the evolutionary trend involved during the course of sexuality of scale insects, Hughes-Schrader (1948) asserted that the prevalence of the parthenogenetic mode of reproduction could be due to concordance with a higher incidence of disparity in their reproductive potential. Thus, Hughes-Schrader (1948) was able to discriminate bisexuals from those parthenogenetic ones and further suggested the appraisal of three fundamental types of parthenogenetic products in them. Since then, there have been considerable amounts of coccoid cytogenetic information procured by Brown and his associates that also affirmed that this situation based on cytogenetic surveillance which acquired an innovative stimulus and prospects including overall frequency of both bisexual and parthenogenetic life cycle analysis from among the select taxa of Coccoidea (Schrader 1923b, 1931; Brown and Bennett 1957; Brown 1963, 1964, 1965, 1966; Nur 1963, 1967, 1969; Hartl and Brown 1970). Subsequently, White (1973), who placed greater emphasis upon the parthenogenetic mode of reproduction and furthermore, on the implication and validity of heterosis during the courses of haplo-diploidy to diplo-diploidy, was a matter of great antiquity. This and other cumulative studies (White 1978) have moved towards arriving at a conceptualization relating to efficiency of homozygosity that would more likely to have an effective impact upon haplo-diploids rather than diplo-diploidy. Brown (1977), Hughes-Schrader (1948), and Nur (1971) have drawn inclinations towards suggesting that it was at the cost of fragility and precariousness of males and of their implicit nature of effectiveness upon population measures, thereby imposing greater inconvenience on the part of life-cycle strategies. Consequently, this kind of adaptiveness could have been driven towards an alternative mode of reproduction. Thus, the parthenogenetic modes could be initiated by means of adapting and involving either of Arrhenotoky or Deuterotoky or Thelytoky. But it was at the greater behest of Nur’s (1969, 1970) concerted efforts that had enabled him in eliciting and categorizing parthenogenomes each exhibiting distinct types of expression pattern. Subsequently, Nur (1980) was also instrumental in documenting a revised format for parthenogenetic modes of expression based on the following strategies: whether the unfertilized eggs develop into males (Arrhenotoky); or females (Thelytoky) or both (Deuterotoky); whether the males are haploid or diploid; and whether first meiotic products between bivalents and oogonia remain the same (Gonoid thelytoky) or different (agonoid thelytoky).
10 Arrhenotoky
Arrhenotoky is also called as haplo-diploid or haploid parthenogenesis in which males arise from unfertilized eggs. Males of haplo-diploids may be referred to as impaternate because they have no fathers. From the classical genetic point of view, haplo-diploid species may have been involved in recombinational processes (and hence, Mendelian in character) inasmuch as they behave much to the same extent as those of sex-limited characteristics, but possess no Y-chromosome. This, in a way, projects as a sort of male heterogamety in genetic characteristics; on the other hand, Thelytoky offers a non-Mendelian material thereby propelling it as a reproductive devise. Haplo-diploidy (Arrhenotoky), on the other hand, is a method of sex determination as well as a reproductive system that involves replacement of an original sex-determining mechanism by an entirely new one under extraordinary circumstances. Hence, it could have occurred rarely in nature and was estimated to have actively participated about eight times during the course of evolutionary history (Brown 1965; White 1973). It was also felt that the frequency of males (through Arrhenotoky) in such populations was determined by the frequency of haplo-diploids that arose in such considerations. It is thus characteristic of any group with haplo-diploidy becoming much more inclined towards responding to the oppressive impact of environmental factors that accrued in which sex ratio potential was deemed to have been highly variable and it was also found to differ from species to species and even to the extent of genetic strains or of population level extremes.
11 Sex-Ratio Potential
In a majority of animal systems studied for the prevalence of genetic-based sex-determining mechanisms the extent was revealed of separate sexes that direct each whether to become male or female, whereas in some taxa, hermaphroditism may serve the primary mode of reproduction. Whether a homomorphic (XX–XX) or heteromorphic (XX–XO) mode of sex determination prevails in them, the sex ratio proportion seems to be maintained in a harmonious manner (in a 1:1 ratio). In such cases, where conventional diploidy exists, fathers and mothers often obtain equal fitness potential through to their sons and daughters and hence, no sexual conflict. But in the case of alternative genetic systems, it becomes essential to involve Trivers and Hare’s hypothesis (1976) that advocates the probabilities prevailing for reproductive success of sons and daughters that can differ markedly from parents. To that extent, they made a proposal in which males and females were drawn into evolutionary forces over the aspects of sex-allocation theory that depends on the inclusion and involvement of a particular genetic system (e.g., haplo-diploidy or paternal genome elimination). In such instances, a different set of reproductive trends seemed to follow in compliance with any biased genetic transmission event that ensues which in turn can offer scope for the eventuality of sexual conflict. With particular reference to mealybug examples, it becomes imperative to address the Dusing–Fischer formula (1976) that insists upon fitness consequences of male and female offspring that can vary with respect to the direct influence or under duress of genetic and/or environmental factors, even though selection prefers operating in such a way as to bring each into an equilibrium state. These generalizations can evidently be tested upon certain cases wherein genes in fathers are only transmitted through daughters, with sons being of no reproductive potential to males. On the other hand, females gain fitness benefits through both sons and daughters thereby on demand for a possible expression of conflicts over sex ratio. Orienting primarily towards genetic consequences, the conventional diplo-diploidy (♀XX–♂XX system), becomes apparent, in which case they still attempt to maintain a rigid sex ratio in the form of 1:1 because mothers and fathers do not vary through to the contributions of daughters and sons and hence no sexual conflict. However, in unusual situations pertaining to scale insects either haplo-diploidy or paternal genome elimination (PGE) are offered as interesting but in extremity for an eventuality (as the case may be).
On the other side, scale insects exhibit a different array of genetic systems, including haplo-diploidy as well as PGE offering as extremities. The case of the mealybug (example Planococcus citri), provides an ideal system to pursue and probe the kind of involvement and promotion of sexual conflict that it exhibits. It has PGE wherein the male component is in possession of haploid nuclei, is (either it heterochromatized or) eliminated from meiotic cell lineage, and it is present in somatic cells but untranscribed (Nur 1980). In terms of genetic mechanisms, the role of genomic imprinting may be crucial. Scale insects are known to represent the case in point of genomic imprinting and imprinting of a paternal chromosomal set alone is affected and also acts as a marker system for sex-determination mechanisms. In the case of mealybug paternal chromosomes especially of Lecanoids it is essential to point out that they remain in a latent state during the course of cell lineages. However, they get transmitted at the cost of a selective advantage. Intriguingly, in P. citri, the site of genomic imprinting of the paternal set of chromosomes lies in the female germline tissues, suggesting that paternally inherited genes may still have the ability to influence the fate of paternal chromosomes in the germline but not in soma.
Scale insects consequently exhibit considerable variations in their expression patterns based on genetic and sex-determining mechanisms, in spite of exhibiting similarity in their life-style strategies (Gullan and Kosztarab 1997; Nur 1980; Ross et al. 2011). Due to compulsions of their adaptive specializations imposed upon the morphology of male and female coccoids, however, they differ enormously in terms of certain other anatomical features. As is well-known among scale insects, males are winged hence motile, but with fragile stature although they have a short life span based on acquisition of no feeding habituation, which may eventually succumb to shortage of male populations. In contrast, females are robust, ornamental, and sedentary in habituation but have a longer life span and are engrossed with gluttonous feeding habits that might propel them to do better in controlling sex-ratio propensities (Bull 1983). Earlier, Hughes-Schrader (1948) predicted that even though scale insects are besieged with variable life-cycle strategies leading towards variable modes of genetic schemes, impulsively adaptive specialization could have driven them to acquiring Thelytoky and hermaphroditism. In fact imposition of such kind of dwellings could have been forced to serve as a clever device to dispense with the shortage of males. However, Brown (1977) contends that in spite of the complexities of several chromosome systems within the scale insects genetic systems, he contemplates acquisition of adaptive specialization, in turn expanding towards acquiring exponential taxonomic diversifications. However, Nur (1980) asserts that the fragility of the males may serve as a primary instrument in an easing-out progression during the courses of acquisition of a particular mode of life-style activities or by adapting to a particular chromosome system in succession. The results obtained based on P. citri, have driven James (1937, 1938) and Nelson-Rees (1960) to address that in Lecanoids, it appears possible to ascribe that females might play an impressive role in selection and maintenance of sex-ratio variability by their so adapting towards changes and at times realigning to overcome any shortage of males. Changes in the sex ratio result in reinforcement of certain changes in developmental phases in the females which probably would make arrangements towards shifting in the proportion of procuring the requisite number of eggs and further upon imposing and resetting the paternal component onto the gambit of heterochromatinization drive and in addition in an effort to furthering towards a certain proportion of eggs to be obtained profusely or curtailment. All these adjustments mean amending changes in contemplation within the scope of remodeling themselves towards acquiring and procuring a sufficient number of males.
Consideration of imparting environmental forces upon such forces is based on the population structure of offspring in responding to fitness potential which would otherwise be driven towards differential expression patterns based on the part of engrossing of established conflict drawn between parents and offspring. It is known that natural selection operates on those ratios in which propelling forces rest on whether the parents or offspring are in driving mode (Shuker et al. 2009). It is also suspected that the offspring can manipulate the sex-ratio potential thereby affecting the sex allocation pattern.
Trivers and Willard (1973) have conceived of a pertinent opinion that environmental factors could present oppressive effects on parents with the possibilities of parental interference in an effort to adjust sex allocation efficacy in the sex-ratio potential. Environmental conditions experienced by parents can have direct interference during the course of sex allocation decisions possibly in one of two ways: either directly influencing parental conditions, or indirectly maintaining environmental factors as a cue to offspring fitness. In P. citri, several environmental factors have been explored especially pertaining to embryogeny and other biological features of female reproduction. These measures include the role of population density (Varndell and Godfrey 1996; Ross et al. 2010a, b), impact of temperature (James 1937; Nelson-Rees 1960), and age of females prior to mating (James 1938; Ross et al. 2010a). An increased understanding of sex allocation theory in mealybugs might therefore yield insightful opportunities to probe further into the potential ramifications that drive in eliciting evolutionary advantages operating in proceeding towards extraordinary modes of sex-determination mechanisms. The results obtained by Ross et al. (2011) upon P. citri experimentations seem to point out appropriate levels that were maintainable based on the role of high temperature, older age at matings, and the starvation level, all of which seem to impress during the course of the consideration of sex-allocation theories. These results may have influenced changes of expression patterns of female-biased sex ratios. But, they also propose that the effect of temperature seemed rather weak and upon the influences of food restriction could have strongly implicated in reduced longevity and a transaction of the unusual schedule of male and offspring production across a female reproductive lifetime.
12 Recent Innovation Made in Mealybug Genomes
12.1 Some Molecular Features
Heitz’s (1928, 1929) unleashing of the operational definition of the term “heterochromatin” in terms of its role in cell-cycle progression has triggered momentum in cell biology to roll on towards its own toll. This situation came as a natural ingredient for Brown (1966) to elicit succinctly the ubiquitous nature of heterochromatinization serving as a pillar to the cytogenetic conundrum. While nurturing functional strategies, he succumbed to subdivide heterochromatin into two types: constitutive heterochromatin (CH) and facultative heterochromatin (FH).
As a constituent of chromosomal architecture, constitutive heterochromatin comprises considerable portions of the genome in higher eukaryotes that include specialized chromosomal domains that are endowed with repetitive DNA sequence specificities (e.g., centromeres, telomeres, and nucleolar organizer regions). CH at the molecular level is marked by distinctive structural changes incurred at the level of DNA sequences, and with active participation of constituent histones, and consequently upon its chromatin remodeling. In addition, recruitment of HP1 (heterochromatin protein 1) at times serves as an essential ingredient of heterochromatin structure. The interactions and dimerization activities of HP1 with that of DNA sequences, RNA, and histone moieties using appropriate combinations bring about repressive chromosomal complexes. This situation is thought to be widespread in many eukaryotic genomes and in some instances, this appears to be a conserved genome component lending its role appropriately from yeast to mammals (Nokayama et al. 2001; Nielsen et al. 2001). The CH could also be diagnosed by highly methylated DNA sequences, and/or histone modifications that are enriched with, for example, methylated lysines (H3K9Me3) and yet in depleted form in the case of both H3K4Me3 and acetylated H4 (acH4).
As the name implies, facultative heterochromatin comes into force or effectiveness upon their need to undertake any exigency purposes (such as gene regulatory activities). FH is a euchromatic component but upon developmental cues acquires a highly compacted chromatin structure to transform itself into an heterochromatic comportment. In its native state, FH is devoid of repetitive DNA sequences. Facultative heterochromatin differs from constitutive heterochromatin with respect to DNA sequence rearrangements but not at the nucleosomal level. At the nucleosomal level, FH has many molecular features similar to CH. From the pointing of its impaction among higher organisms and in cytogenetic context, FH affects only one of two homologous loci or homologous chromosomes, or homologous chromosomal set.
Genomic imprinting is defined as a parent-of-origin specific expression of selected or affected gene(s), and has generally been associated with specific changes in DNA methylation profiles and in histone modification processes. Even though there are numerous examples available for the study of genomic imprinting, operating at the level of a gene and/or at a single chromosome or a whole chromosome set, wherein inactivation of (1) one of the two X chromosomes in female mammals and (2) a male haploid set of mealybug chromosomes in a complement, serve as a unique example for the consideration of epigenetic phenomena.
There is good evidence that the control of transcription involves active participation of various proteins which bind specifically to methylated DNA, wring in histone modification complexes, and eventually in local chromatin remodeling processes.
Considering these features, the differential chromatin formation during the course of chromosome inactivation processes in the case of mammalian females and in the case of the paternal chromosomal set in male mealybugs represents a very clear case and an outstanding genetic manifestation offered in the studies pertaining to an effort to understand the modes and methodologies involved during such processes (FH formation).
This exceptional situation offers immense academic help in eliciting more on these topics; Lakhotia (2004) made efforts to shortlist achievements dwelling upon ongoing excitements that prevail in the arena of epigenetical phenomena contributing towards the current phase of knowledge available regarding heterochromatinization progression. Several recent reviews have been forwarded detailing the prospects of mechanisms and functioning of various epigenetical programs that incorporate during gene regulatory activities (Surani 1991; Li 2002; Cairns 2007; Kouzarides 2007; Skiniotis et al. 2007; Bell and Spector 2011). Of particular significant and recent progress are achievements heralded in the case of epigenetic regulatory activities during genomic imprinting programming of the mammalian X-chromosome (Sado et al. 2005). On the other hand, the present review focuses on recent achievements made in our current understanding of the role of DNA methylation, histone modifications, and some points upon the chromatin remodeling processes pertaining to genomic analysis (e.g., Planococcus citri /P. lilacinus) essentially based on chromosome organization have been targeted to serve as a model genetic system.
12.2 On Biochemical Paradigm
The regulation of gene expression plays a pivotal role in expediting complex phenotypes and in differential expression patterns of epigenetic mechanisms, in which the role of DNA methylation has been considered as playing an essential role in depiction of variable modes of operation elicited during the course of chromosomal mechanics. In order to understand better the functioning of DNA methylation processes is to learn more about its modes and methods and that reflect upon an operative course during distribution patterns in the genome of interest. Cytosine DNA methylation has been demonstrated in several eukaryotic organisms and has been demonstrated to play inquisitive roles in various developmental activities. Variable portions of the genomes are being subjected to the operative part of methylation with the help of 5-mehtylCytosine (5mC) along the lengths of DNA sequence moiety. DNA methylation has been cited in numerous physiological functions depending on the kind of model organisms utilized for said purpose and upon redesigning particular experimental protocols. Presence of DNA methylation in and around promoter regions is generally been thought to be associated with gene silencing processes and the loss of such kind of methylation processes is reported to be accompanied by virtual transcriptional activation.
Several ideal examples can be cited inciting activities based on a methyl-transferase enzyme conglomerate that operates during such instances that have been profusely documented in several vertebrate and plant examples. In animals, the spectrum of methylation levels and patterns is projected to reflect upon a broader range and also indicate a highly variable mode of expression. Excepting cases such as Caenorhabditis elegans and Drosophila melanogaster, most invertebrate examples are reported in specificities reflected in indicating possession of a low to moderate level of DNA methylation patterns. Vertebrate examples, on the other hand, have been shown as demonstrating having acquired in the range of higher levels of 5mC activities and were evidently documented, especially from among several higher animal examples. However, Bird (2002) is of the opinion that it was not possible to corroborate this situation to the same level of methylation processes prevailing by 5Me between the vertebrate level to that of an insect system. The available data indicate varying levels of methylation processes that do not seem to point out any conserved function. For example, the role of CpG methylation as an epigenetic mark responsible for genomic imprinting has been clearly established in some mammalian examples (Feil and Khosla 1999). Evidently, the case of human inactive X-chromosome in the female somatic chromosomal complement serves as an ideal one for such kind of enquiry.
On the other hand, the role of DNA methylation in insects is still in its infancy. Thus, this situation could reflect upon their leading a high diversity of life-cycle strategies prevailing from among individual cases pursued in each instance for said purposes. The familiar one is the case of Drosophila melanogaster, in which DNA methylation seems to be representing in an elusive way, because overall mechanisms prevail upon developmental phases and more non-CpG methylation processes controlled by the role of Dnmt2. In contrast, the case of Mamestra brassicae, a cabbage moth, based on HPLC analysis demonstrates the higher level of DNA methylation, which appears considerably closer to the standard level cited with respect to certain vertebrate examples. Methylation experiments including restriction enzymes as a parameter showed that CpG sites were more spread out in the genome, dispensing more towards the outer C of the 5’-CCGG-3’sequences. However, results based on transposons are intriguing because mobile elements are harboring and/ or congregating at or proximal to repetitive sequences that seem heavily methylated, as was shown effectively in some vertebrate and plant examples. However, a very interesting case was that of Myzus persicae, a peach-potato aphid, wherein the enzyme systems have been amplified drastically due to spurious developmental activities of insecticide (E4 & FE4) resistance genes and thus, forcing upon detoxifying esterases that have been spurred up due to spurt in DNA methylation processes (Hick et al. 1996; Field et al. 2004). Overt expression patterns of CpG methylation in these cases might have reflected upon the amplification events of the concerned genes, the situation of such kind may be considered reflecting upon the mechanics of methylation processes associated with the copious presence of DNA transposons as was found necessary in the cases of several vertebrates and in transgene experiments carried out in plants (Feil and Khosla 1999; Field 2000).
The historic findings of Schrader (1921, 1923b) and Hughes-Schrader (1948) in which male chromosomes were found to be characterized by the presence of a haploid chromosomal set acquiring precocious condensation property and thus, becoming inactive ones (in Lecanoids) or put into an ordeal of genomic elimination (in Diaspidids) during the course of embryogenesis. Brown and Nelson-Rees (1961) described such an event occurring by elaborating on chromosomal mechanics imposed upon heterochromatic components by means of undergoing a facultatively heterochromatinization program.
The condensation property of the paternal chromosomal set of mealybug chromosomes is correlated in parallel with the expression for maleness. In mealybugs and other coccoids, radiation-induced chromosomal fragments are not lost during mitosis but persist as stable entities in nuclei of both sexes, demonstrating that the centromere is diffuse and that freshly broken chromosomal ends can still form telomeres or telomerelike structures and regulate associated functions (Brown and Nelson-Rees 1961; Chandra 1963a). When broken chromosomes were transmitted by fathers to their sons, each chromosomal fragment underwent heterochromatization progression suggesting the presence of multiple centers of chromosome inactivation. This situation contrasts with the condensation property exhibited in mammalian females, wherein the inactive X-chromosome is identifiable with a single center of activity and is thought to control the whole of the inactivation program (Cattanach 1974; Lyon 1999; Brown et al. 1991). Characteristically, the mammalian inactive X-chromosome shows a typical characteristic organization as scored by micrococceal endonuclease treatment, because transcriptional factors do (or can) not bind to its condensed domains (Pfeifer and Riggs 1991). On the other hand, chromosomes play a different role in view of the situation that coccoid genomes have offered as a readily packed and amenable material of chromosome research in any cytogenetic and/or biochemical exploration activities.
One of the unique features while characterizing genomes is to introduce an enhancing mechanistic driving so as to yield differential organization of homologous chromosomal sets dwelling in one point of reference which allows one to pursue gratuitously such as, for example, to pursue more upon the mechanisms of sex-determination, genomic imprinting processes, and into inactivation progression (Hughes-Schrader 1948; Chandra and Brown 1975; Peterson and Sapienza 1993). For example, the mealybug genome is unique because it is in possession of unusual chromosomal characteristics, involving diffuse centromeric organization (holokinetic activity) that encompasses inverse meiotic processes, leading to a signaling of an unorthodox mode of cell-cycle manipulation in males (Hughes-Schrader and Ris 1941; Brown and Nur 1964; Nur 1990). Thus, some of these unusual genetic bounties could have driven Chandra and his collaborators in attempting and exploring further these genomic contents (e.g., P. lilacinus or P. citri) at the DNA sequence level and of modified version of bases in the DNA sequence organization.
Employing appropriate but standardized biochemical protocols (Jamaluddin et al. 1979; Achwal and Chandra 1982; Achwal et al. 1983, 1984; Karnik 1983; Deobagkar et al. 1982, 1986) have enabled their fruitful extraction of total nuclear DNA content based on an Indian Planococcus genome. These assays were utilized for the purposes of studying the primary nature of methylation status by means of HPLC and chromatography which enabled disclosing the presence of significantly higher amounts of 5-methyl cytosines in some portions of the genome. This was verified by dinucleotide analysis in which 5-mC seemed over represented with respect to other sequences (viz., CpA, CpT, CpC). Unusually higher amounts of 6-mAdinosine (6-mA), 7-mGuanosine (7-mG) were also encountered (Deobagkar et al. 1982). Achwal et al. (1983) reported a new protocol to isolate and characterize antibodies raised specifically to 5-mC, 6-mA, and 7-mG, a situation rarely found in higher eukaryotes at that time. With the use of immunobiochemical approaches they were able to evaluate the samples to the same level of contention to that of higher eukaryotic samples (viz., Drosophila, Human, etc.).
Devajyothi and Brahmachari (1989, 1992) present evidence of obtaining homogeneous extraction of DNA-methyl transferase enzyme that were found specific to the test material (Planococcus citri/ P.lilacinus). The enzyme extracts exhibited a proactive mode of action and found preference for salt extraction techniques, because that appeared equivalent to routine extraction protocols utilized in the case of mammalian methylase assays. These results demonstrate that the enzyme assays have had high specificities for denatured DNA substrates. Mohan et al. (2002) using random stretches of P. lilacinus DNA sequences, the technique of which was found to be helpful in delineating repetitive sequence analyses that were inferred as higher than those of other conventional sequences and were also found much higher than those of Drosophila samples scrutinized and compared wherein GCs were found less frequent. Thus, they infer based on this situation that seemed promising for the considerations upon influencing on CpG dinucleotide sequence frequencies which was found exclusively in those genomic samples. Methylation specific arbitararily primed (MS-AP), polymerase chain reaction (PCR), and subtraction hybridization protocols were found helpful to Mohan and Chandra (2005) and thus to describe the isolation and sequencing of sex-specific CpG methylation sequences that were prevalent in genomic DNA samples of P. lilacinus. These sequences showed male specific methylation processes and were found to occur about 2.5 times more frequently than those showing female specific methylation sequences. Bisulphite modified DNA samples revealed an interspersion of CpG and non-CpG methylation among sex-specific methylated sequences. This study also pointed out that there were more non-CpG methylates and/or at least twice as many sex-specific methylated sequences found in males than in females. They thus based on those sequences that there could be offering a closer association between sex-specific methylated sequences located in transcriptionally silent chromatin zones and those assays resistant to DNase I zones.
Scarbrough et al. (1984) studies were based on the differential levels of 5-mC in the males and females of Pseudococcus calceolariae and P. obscurus and thus they were able to relate their findings and that these results driving them to arrive at conclusions that males display higher incidences of methylated sequences than those of female samples. Kantheti (1994) describes, with the help of specific antibodies raised against 5-mC, that there were more 5-mC localization spots identifiable on male cells than on female ones in the case of P. lilacinus. There were also two more studies reported on Planococcus citri (Bongiorni et al. 1999; and Buglia et al. 1999) whose genomic exploration of P. citri samples related to the prevalence of sex-specific cytosine specificities but arrived at conflicting inferences.
Khosla et al. (1996) present evidence suggesting existence of specific DNA fragments that were perhaps offering to serve as a primary signal during the elaborate mechanism as a contributing factor towards chromosomal imprinting activities. Chromatin organization of Planococcus lilacinus was chosen for the purpose of extrapolating rather than to consider offering as contributory factors to their functional spectrum. Digestion of P. lilacinus samples with micrococcal nucleases showed 3–5 % of the male genome samples were different and the same were assayed and found to be more resistant to the introduction of enzymatic activities; as such these samples were designated nuclease resistant chromatins (NRCs) fractions. This component was present invariably in both sexes and throughout the genome. However, cloned NRC DNA contained A + T rich sequences that were found revealing some homology towards that of samples of mouse α- satellites. Salt fractionation techniques revealed that these sequences were found to be matrix-associated. Based on these experiments, they were tempted to offer some solutions in the form of those DNA sequences present explicitly in NRC fractions and it was possible to infer that this sample would serve as a resource material for a future course of genetic studies. Thus, Khosla et al. (1996) findings thus are directed towards offering these parameters that could as well be serving as a mode of strategy and further to consider them as putative centers for initiation of facultative heterochromatization processes. However, they also cautioned that there are other contributory factors that they might interact with this grand executive operation. In the meanwhile a thorough scrutinization is necessary and required in an extensive way prior to arriving at any kind of generalization in this regard.
With the help of southern hybridization and FISH techniques, Khosla et al. (1999) provide results proclaiming the extrapolation of NRCs and further about prevalence of subdivisions of these fractions in the form of two middle repetitive sequences, designated as nrc50 and nrc51 samples. It was also found that they were differentially organized within NRC composition and more interestingly they have enabled distinguishing the sexes based on the placement of differential proximity. The NRCs were also found resistant to both MNase and DNAase I treatment and thereby enable exhibiting indistinct patterns that may help in identifying two sexes. Their enrichment in NRC accounted to contain 50 and 83 % for nrc50 and nrc51 type, respectively. Thus, 25–30 % of samples remain resistant in males but none in females. It has been shown consistently that NRC is associated with the nuclear matrix. On a nuclear matrix isolation platform regarding male and female sample nuclei, it was found evident that the NRC fractions were present only in males but not in females. They further imply that it is the paternally derived hypomethylation set that drives towards processing of the heterochromatization program. It was also felt that some nrc51 fractions were not accessible to MNases even in euchromatic chromosomes. For the same they offer the suggestion that these sequences might have been inferred to contribute towards centromeric-type activity; instead, they were found to be dispensed with all along the length of the chromosomes. It was well known that a single inactivation center exists in the case of the mammalian inactive X-chromosome, in contrast to the situation prevailing in the mealybug chromosomes exhibiting multiple centers along the length of individual chromosomes that serve as a model system for the chromosomal inactivation program. In the light of these findings, these are the distribution specificities for nrc50 and nrc51fractions over the mealybug chromosome samples and considering them for their presence in the form of several heterogeneous NRC–DNA fragments and of enrichment within the unusually organized chromatins of the male would raise the possibility of examining them and perhaps serving as putative nuclear sequence loci in the form of expression of multiple inactivation centers.
Extending these experiments as an extrapolation undertaken by Khosla et al. (1999), they provide descriptions based on their explicit pattern of expression of this unusual chromatin organization designated as NRC fractions during the course of cytologically identifiable regions and during spermatogenesis and especially over sperm nuclei even though their expression was on a maternal background. Furthermore, it was made possible for them to infer that this component can perpetuate through mitotic and meiotic progression.
It also appeared interesting that differential chromatin organization forms procured from the samples of the mealybug (Planococcus lilacinus) provide an important biochemical tool in consideration of assessing and identifying maleness or femaleness based on the presence or absence of NRCs from the total genomic organization. Thus, based on this important biochemical discovery, it was made possible for Khosla et al. (2006) to hypothesize and suggest a biochemical model that may be able to answer some of the vexing problems confronted by geneticists included during the course of understanding genomic imprinting mechanics. They are of the opinion that by regulating NRC as a discriminating organization in the paternal/maternal genome, it becomes possible to discriminate male- oriented cells from those of females while attempting to recognize facultatively heterochromatinized chromatin organization in one or the other sex. At this juncture, their inference was to ascribe that in the preceding zygote formation, the zygote is in possession of the paternal genome in the form of the NRC positive state and as such, the status of heterochromatin is in the form of negative effect. Subsequent to sixth cleavage divisions, the said NRC-positive paternal genome acquires heterochromatization status based on the developmental decision made at some point in the ooplasm, in order to acquire a decision either to procure or lose heterochromatin mediating proteins, thereby acquiring a specific functional role based on a NRC-positive or negative fraction.
Subsequently, Mathur et al. (2010) present a genomic organization of another pseudococcid, Maconellicoccus hirsutus, thereby evaluating the obvious presence of the effective NRC fraction and its mode of association with that of nuclear histone matrix content. They insist based on previous experience that the affinity patterns between NRC and histone matrix form an important binding property for a meaningful differential expression especially eliciting developmental courses promoting the paternal mode of inheritance. The exhaustive study revealed by means of extraction and the identification of H3K27Me3, H4K20Me3, and H3K9Me3 proteins in both in male- and in female-based samples and with a significant enrichment of H3K27M3 in the nuclear matrix of males compared to that of females form an important and critical contribution. This particular biochemical component seems pointing towards and directing a cell-based signal for a male sex-specific discriminating factor. Furthermore, the analysis of cytologically sorted nuclei indicates the presence of NRC in nuclei with different DNA content including the haploid nuclei from males, is another interesting phenomenon disclosed in this genome.
12.3 Molecular Cytogenetics
HP-1 (Heterochromatin Protein-1) is a nonhistone chromosomal protein with two highly conserved domains. The amino terminal “chromodomain” (CD) has the capacity to bind either mono-, di-, or tri-methylated histone moiety (e.g., lysines) of H3 or H4 or others. The carboxy terminal “chromoshadow” (CS) domains are involved in mediating protein– protein interactions (Eissenberg and Elgin 2000; Lachner et al. 2001). Historically, HP-1 was identified and isolated originally based on Drosophila melanogaster polytene chromosome heterochromatin regions and subsequently, were procured from several other sources and from several other organisms, considering these format posed us as the basis for isolation and they were acquainted through to the cloning experiments. By raising antibody (CIA 9) against those subdivisions of several homologues were procured. HP-1 are highly conserved and play a role in gene silencing efforts in a diverse range of organisms (Singh and Georgatos, 2002). There appear to have been instances wherein euchromatic zones require HP-1 s for stabilization of their elongating transcripts (Vakoc et al. 2005).
Epstein et al. (1992) were keen on extrapolating the molecular biology of HP-1 and their efficiency towards cloning and thus isolated several patterns of expression from Drosophila HP-1 homologues and the same were used to compare with samples drawn from several other sources wherein their genomes were known towards exhibiting heterochromatin programs in which the role of HP-1 takes dominance. Because they knew that the degree of similarity between chromodomains (of polycomb) and HP-1 at the nucleic acid level it was found sufficient to detect and isolate other genes from other organisms using low-stringency nucleic acid hybridization (Singh et al. 1991). Epstein et al. (1992) were exploring the possibilities of procuring HP-1 homologues from several other sources; however, they preferred to examine HP-1 s from mealybug genomes because it was well-known that these scale insect provide a robust example for such kind of consideration and thus may serve as a suitable target (Hughes-Schrader 1948; Nur 1990). Thus, the coccoid genetic system is well recognized as one of the first examples to pursue for examining parent-of-origin (parental imprinting) specific effects; subsequently, other examples were perused for said purposes including humans (Solter 1998). But Epstein et al. (1992) were able to describe their attempts by means of molecular characterization of two chromodomain-containing proteins called PCHET-1 and PCHET-2 (for putative coccid heterochromatin proteins 1 and 2), from the mealybug genome, Planococcus citri. They were able to prepare cDNA encoding these proteins realized in cloning and in which it was shown that PCHET-1 seemed to have more potential than that of PCHET-2. This fusion product was later utilized for exploring the expression patterns of PCHET-1 in other mealybug tissues and it was confirmed that it assisted in a male tissue-specific manner. However,, the specificities of tissue distribution of this protein may suggest the most sought after gene, but it was not at the level of correlating to the extent of identifying the male-specific heterochromatic chromosomal set. Moreover, PCHET-1 was not found traceable on female cells. Thus, they opine that PCHET-1 in combination with other factors may help in providing a role for the sex-determination device.
Many decades of concentrated work on heterochromatization in terms of cytological and molecular characterization reveal that this chromosomal component (whether constitutive or facultative) consists based on a macromolecular mould in the form of a repressive chromatin complex (Spofford 1976). It is well known that methylation of lysine 9 of H3 by Suv (3)9 methyl transferase creates a binding site for HP-1 (CD) resulting in the formation of a repressive protein complex; since it was considered the most robust histone modifications known.
While attempting to elicit mutual relationships existing between heterochromatin, HP-1, and trimethylated lysine 9 of H3 (Me(3)K9H3) as a requirement in analyzing X-chromosome inactivation program is resolvable us in the mammalian examples including humans, Cowell et al. (2002) observed that there were elevated levels of trimethylation at the notified sites resulting in chromatin suppression. An extension of such kind of exploration made on the mealybug genome (P. citri) was represented and shown by intense staining of DAPI; but male cells were highlighted by discrete staining localization rather than that of interphase nuclei. Only flecks of stainability marks were found over the euchromatic portions, but the representation at the male prometaphase stage was by and large very clear (Cowell et al. 2002). Thus, they made an assertion towards this effect that the role played by the HP-1 protein in silencing of concerned genes is thought to be a conserved function (Nokayama et al. 2001; Nielsen et al. 2001).
Recent studies on methylated histones have revealed that the level of methylation of the specific lysines may have an important functional consequence for the assembly of heterochromatin formation. Acetylation and methylation are the two types of post-transcriptional modifications known that have been identified in histones (Wu et al. 1986). The histone “code” is a suggestion made in which covalent modifications may be brought about by the kind and mode of the participation of chromosomal proteins and as such, a modification will have effects on driving towards tissue-specific expression patterns. Kourmouli et al. (2004) have made observations that on the N-terminal tails of lysine 20 of H4, it is trimethylation of this lysine that occurs; but if it is dimethylation of lysines it was shown to be associated with euchromatic portions of the genomes (Fang et al. 2002; Kourmouli et al. 2004). Furthermore, Kourmouli et al. (2004) have reported that in the murine examples, the trimethylated lysine 20 of H4 (but not the Me(2)K20H4) establish specific relationships in the presence of Suv(3)9 histone methyl transferase activity, with that of Me(3)K9H3 thereby accounting for epigenetic crosstalk between H3 and H4. Extension of such kind of study revealed that in the coccoid examples analyzed as a target for action it was expounded that its expressivity was observed on the facultative heterochromatized paternal chromosomal set. They made a detailed assessment of this situation by means of DAPI stainings where the heterochromatic component forms a brightly stained property (Epstein et al. 1992; Bongiorni et al. 2001; Kourmouli et al. 2004). In the female mealybug cells, Me(3)K20H4 is found scattered uniformly throughout the chromosomal set.
Most imprinted loci may have key regulatory elements that are methylated on one of the parental chromosomes. For several of these differentially methylated regions, recent studies establish that the unmethylated chromosome has a specialized chromatin organization that is characterized by nuclease hypersensitivity. In such a situation, the question is raised as to whether associated chromatin features regulate the allele specificity of DNA methylation at those imprinting control regions.
Taking cognizance of a lead from the biochemical front that was well demonstrated from the reports of Scarbrough et al. (1984) and Devajyothi and Brahmachari (1992) and its relevance to the possibility of establishing prevalence of relationships between two states, DNA methylation processes and chromosome imprinting phenomena in the coccoid genetic system is a jerk in our understanding of chromosome imprinting phenomena and is considered monumental in coccoid genetic research. In order to probe further this important component of scale insects, Prantera and his team (2012) have initiated unearthing several molecular cytogenetic complexities. Following is a descriptive account of their research accomplishments.
In order to probe and enlighten based upon implications of molecular and chromosomal level investigations undertaken by Bongiorni et al. (1999) who made a beginning towards prevalence of procuring knowledge of the P. citri genome of Italian origin. They utilized the RE/NT technique (restriction enzyme directed in situ nick translation) upon exploring of DNA sequence-level organization, thereby extrapolating the P. citri chromosome (Ferraro et al. 2001). Concentrating specifically based on MSPI and its methyl-sensitive isoschizomer HPa II when used as nicking agents, led them to make incisions into the genome by exposing organizational differences prevailing between homologous chromosomes and subchromosomal regions (Prantera and Ferraro 1990). The P. citri genome was targeted for such an exploration in order to delineate chromosomal differences, especially pointing out DNA sequences pertaining to differences occurring at the organizational level, to the extent of identifying methylated and nonmethylated chromosomes.
Bongiorni et al. (1999) have made a detailed account of the structural organization in respect to both males and females, and the paternal derived haploid set was found to be hypomehtylated to that of the maternally derived chromosome. In males it is the paternally derived hypomethylated haploid set that is heterochromatized. To their surprise, in female embryos, half of the chromosomal complement was undermethylated and thus, they inferred that the undermethylated chromosomal set in females represented was of paternal origin, emphasizing that DNA methylation could be at the basis of imprinting phenomena at the chromosomal level. Thus they suggest that the two haploid sets are imprinted by parental-of-origin-specific DNA methylation with no correlation with the known gene silencing properties of the base modification.
In their next venture (Bongiorni et al. 2001), they carried out experiments based on western blotting and immunolocalization with fluorescent microscope-level observations upon mealybug genome P. citri. Their intuition was to identify a cross-reactive protein epilope whose properties suggest that of a homologue of Drosophila HP-1, present in this species. By analyzing the distribution patterns upon immunofluorescence spottings they could infer the distribution of this HP-1-like protein in male and female cells during the cell cycle and in the early embryogenesis. It was evident to point out this (HP-1-like) protein colocalizes with male-specific heterochromatin, thereby implying that this protein plays a role in the process of facultative heterochromatization.
However, they allay some doubts as to the nature of the presence of P. citri HP-1-like protein in embryos of both sexes which had led them to infer a protein factor was involved in the recognition of the imprint signal, suggesting that at least there could be another factor provision which was found to be involved in the induction of facultative heterochromatization and thus this factor should be male-limited in characteristics. Moreover, as to the nature of C-banding staining, it was not a strict cytological correlative measure to assign any heterochromatic role. It is well known that C-bands always coincide with constitutive heterochromatic composition.
In their next exploration of coccoid chromosome systems, Bongiorni et al. (2004) concentrated on detailing the inverted meiotic cycle established by means of indirect immune-fluorescent tapping. This issue drew special features because P. citri genetics revolves around diffuse centromeres and inverted meiosis. This study also focused specifically on second meiotic division in which the male cell-cycle was manicured by monopolar spindle activities and as a part of this special system, they dwelled more on the mode of meiotic drive enforced upon this genetic system. They were more interested and engrossed on interpretation of meiotic spindle activity in which the cytological preparations made were based on the use of an antibody that was directed against insect α–tubulin.
Earlier, Hughes-Schrader (1948) suggested the prevalence of monopolar spindle during male meiosis and interpreted that heterochromatic chromosomes are the ones participating in such kind of activity. However, based on the introduction of recent protocols (Bongiorni et al. 2004) upon P. citri meiosis revealed that the spindle is associated with the euchromatic set facilitated by enhanced staining by DAPI that distinguishes each set by differential fluorescent stainability.
The monopolar spindle could originate either from a lack of centromeric duplication or from the lack of separation of duplicated centrosomes. These authors were of the view that the formation of a monopolar spindle and the lack of microtubule binding by heterochromatic chromosomes are a necessary condition to ensure the nonindependent segregation of homologous chromosomal sets at the second meiotic division. The nonindependent assortment at the reductional division together with the degeneration of the heterochromatic spermatid nuclei formulate a basis of the strong meiotic drive that leads to exclusion of the heterochromatic chromosomes from genetic continuum.
Earlier experience was driven to understand that the HP-2 protein, a homologous HP-1 partner acquired from the D. melanogaster genome, acts as a dominant suppressor of PEV, therefore demonstrating a role involved in the structure and maintenance of heterochromatin structural integrity. Implying the foregoing concept, Volpi et al. (2007) wanted to probe more of its effectiveness upon the mealybug (P. citri) genome. With the help of an antibody raised against Drosophila HP homologue epilope samples, they acquired the set that was able to present cross-reactive epilope and thus they designated the product as an Hp-2-like protein. Following the life-cycle patterns through to the male phase of the mealybugs revealed that they became with acquainted with a heterochromatinized chromosome set containing the requisite amount of antibody deposition that was estimated by immunofluorescent scanning. During the observations of the euchromatic chromosomes, HP-2-like impressions were sometimes traceable over the telomeric regions. The interplay between HP-2-like and HP-1 was critically examined based on the introduction of ds RNAi experiments. Knocking out HP-1-like protein expression with the introduction of the RNAi method did not prevent the association of HP-2-like with facultative heterochromatization, thereby endorsing that the latter and its presence by binding to chromatin is independent. They also utilized that this property extended to the processes of condensation or decondensation upon other cell types.
It is now certain that the HP-2-like protein binding to chromatin is a perquisite for facultative heterochromatization assembly and it indeed poses an interesting possibility that this component must be tested by inactivation of HP-2-like. Hp-2 antibody signals aggregate over distinct chromatin areas, which identify the future chromocenters after they have already been bound by HP-1-like. This suggests that the recruitment of HP-2-like to the potential heterochromatic domains depends on the presence of HP-1-like. In adult tissues, where the heterochromatization reversal occurs, the HP-2-like epitope is lost by the chromocenter remnants before the HP-1-like, which thus seems to be insufficient to anchor HP-2-like to chromatin. It has also become evident that the strict colocalization of HP-2-like with the chromocenter is not abolished in HP-1-like knockout embryos.
Molecular results based on some mammalian examples, also including the mealybug genome, were obtained independently by Kourmouli et al. (2004) and Schotta et al. (2004) in an experiment to certify the effect that Me(3)K9H3 employing Me(3)K20H4 through the participation of HP-1 promoting heterochromatin formation appears to be a global-level event. But what was not clear about this was how HP-1 modulation is involved during gene activation processes in the case of the mealybug genome (P. citri; Bongiorni and Prantera 2003; Bongiorni et al. 2007; Kourmouli et al. 2004). In contrast, acetylation of histone H4 (AcH4) was found to be absent on the male-specific heterochromatization processes (Ferraro et al. 2001), whereas the depleted level of activation of AcH4 was observed in the case of human X-chromosome inactivation (Jeppesen and Turner 1993). The foregoing issues have driven to an understanding with a suggestion that Me(3)K9H3 via HP-1 to the Me(3)K20H4 pathway in an evolutionarily conserved mechanism of action for an epigenetic route to silencing large chromosomal domains by facultative heterochromatization (Chadwick and Willard 2004).
While establishing the prevalence of Me(3)K9H3 to HP-1to Me(3)K20H4 relationships in the case of P. citri genomes, Bongiorni et al. (2007) proceeded further to interrelate the position of the HP-2-like protein (PCHET-2) based on RNAi experiments. With the intermediation of ds RNAi (Fire et al. 1998) and by interference of knocking down PCHET-2 in P. citri embryos, it was resolved that the consequential depletion of the heterochromatization pathway resulted in deheterochromatization with respect to gut cells and Malpighian tubules, whereas Hp-1 and Me(3)K20H4 in the same nuclei are either dispersed or absent. Embryos treated with ds RNAi (double-stranded RNA interference) targeting PCHET-2 also exhibit chromosomal abnormalities (such as chromosome lagging, abnormal condensation, segregation defects), more so on structural maintenance components (SMCs).
12.4 Chromatin Remodeling
In many diverse organisms, gamete formation originates in a cytoplasmic, but highly conserved structure, known as germ-line cysts. Germ-line cysts (or saclike structures) are composed of a group of cells; it is apparent that they took their initiation from a single cell that underwent synchronous cell divisions followed by incomplete cytokinesis. Modification of the chromatin structure is one of the main epigenetic regulations conceived to carry out its operation in order to undertake unique gene expression modalities. The male germ-line cyst is the organ that facilitates executing the meiotic and/or post-meiotic mode of gene regulation activity sharing during gametogenesis. The germ-line cyst morphogenesis acquires the responsibility of delivering the respective genomic content to their destined sites.
Male meiosis of scale insects is interesting because meiotic sequence progressions proceed in accordance with those of inverse meiosis. Thus, during male meiosis each spermatogonial precursor cell nucleus produces a bunch of synchronously dividing spermatogonia in a cytoplasmic cyst. Each spermatogonium divides four times to produce a cyst of 16 primary spermatocytes which then undergo two meiotic divisions. Subsequently, each spermatogonium undergoes the first equational and then the second reduction division, which is characterized by specialized movements directed and dictated by some unknown sources. But recent studies undertaken by Buglia and Ferraro (2004), Buglia et al. (2009), and Bongiorni et al. (2009) have provided some clues to learn more about the extent and nature of expression, wherein these chromosomal movements were maintained and manipulated by the monopolar spindle in which microtubules make physical connection with the euchromosomal set, rather than with the heterochromatic component as was contended earlier by Hughes-Schrader (1948). Even though Sciara chromosomes practice monopolar spindle activities, it seemed to be maintained through the occurrence of monokinetic activity wherein meiotic products were manicured by sister-chromatid cohesion (Esteban et al. 1997).
By utilizing antibody-specific tracings, Buglia and Ferraro (2004) describe an immunofluorescent staining protocol backed by enhanced active participation of fusomal elements, such as F-actin, included in the elaborate descriptions of factors that demarcate local morphogenesis essentially demarcating the cytoplasmic composition of the male germ-line cysts. The co-localization of all these factors is an indication of the triggering action that could be measured by densitometric profiles which further enable in providing descriptions about the prevalence of two kinds of sperms emerging but equipped with variable loads with respect to individual sperm content.
A continuing search by Bongiorni et al. (2009) proceeded towards extrapolating procurement of the resources to be used during the gametogenetic processes and seemed to be in possession until early embryonic development. Immunolabeling of such components in order to probe has enabled identifying the presence of protein components such as H3K9Me2 & 3, H4K20Me3, HP-2, and PCHET-2-like, that were concentrated in the paternal part of the meiotic stages throughout, but not in the female line to the extent of oocyte formation. On the other hand, there were no traces of these modifiers in the female gametogenesis. The redistribution of epigenetic signaling marks in spermatids might be related in the tracings of the processes concerned with the establishment of parental imprinting. Bongiorni et al. (2009) narrate the modes of operation through to the entry of sperm into the oocyte environment, where they are in possession of distinct H3K9Me2 and 3 methylation marks that were found in the early pronucleus. Observations were made of such kind of effect during the course of spermatogenesis indicating the presence in the form of heterochromatic components decorated by H3K9Me2 & 3 and PCHET-2. Regarding the euchromatic component, it was shown containing HP-2-like and H4K20Me3. This was found to be a consistent expression pattern until the spermatid formation, thereby demonstrating the supremacy of histone modifications throughout the male part of the meiosis. This situation is in congruence with that of Khosla et al. (1999, 2006) observations and of their proposals advocating the presence of NRCs on paternal cell lineage until sperm maturation. By now, it seems evident by pointing out that by the end of spermatogenesis PCHET-2 may be losing its grip. Bongiorni et al. (2009) contend that the presence and supremacy of H3K9Me2 & 3 methylation processes dominate throughout the course of gametogenesis, and with respect to the content of these proteins, they are in disagreement with the contention of Buglia and Ferraro’s (2004) observations. This pertains to the quantum of differential distributions regarding euchromatic spermatids, because these products take their origin from a single meiotic event. However, Buglia and Ferraro (2004) strongly define that values they procured were essentially based on densitometric tracings, citing differing values with respect to H3K9Me2 & 3 and of CIA9.
In their subsequent study, Buglia et al. (2009) have elaborated mustering of resources pertaining to the development of female phases of gametogenesis of P. citri. Their results provide the presence of a proteic component; this time the presence of HP1 and Su (var) 3–9 (a different chromosomal protein), makes all the more important contributions occurring during female gamete formation. Pertaining to the deposition of variable contents of eggs it was found to contain two different kinds of cell inclusions, deposited in eggs, thereby categorizing in such a way as to act differently upon different ages of females. Based on these biochemical characteristics, females with 40-days older age were considered as a younger group and those of 80-days old as an older (aged) group. The findings of larger amounts of epigenetic factors accumulated in the group of aged females in comparison to the younger ones was found to be an important deciding factor. These studies have led to the supposition of playing as a primary role based on differential maternal contribution.
It was observed that the concept of genomic imprinting phenomena seemed to be lending effective support in the cases of both Sciara and coccoids, that the primary sex determination mechanism relied upon considering consequences of occurrence of chromosome imprinting (Chandra and Brown 1975; Brown and Chandra 1977). It also appears obvious in the case of mealybugs, that possibly it was at the instance of mothers that enable directing and discriminating the sex of her offspring. In addition, the extension of this provision should yield mechanistic support to the concept that maternally controlled sex determination could also give way or leverage for control of the progeny sex ratio. Earlier, Nelson-Rees (1960) had contended that the sex ratio in mealybugs fluctuates among females and is markedly influenced by mother’s age at conception towards the brood. In both sexually reproducing and in parthenogenetic mode of reproduction, the imprinting process initiates at and in the egg cytoplasm at the time of the fertilization program.
Parental genomic influences on the fate of offspring development are evident in both invertebrate and vertebrates. Maternal effects are commonly mediated through deposition of the cytoplasmic transcripts essaying protein products in oocytes during oogenesis in the female germ-line. These then exert their effects on the fertilized eggs and drive impulses upon early embryonic developmental processes (De Robertis et al. 2000; Gosden 2002), unlike some mammalian examples that may provide guidelines for any kind of eventuality (Li et al. 2008). However, there are no specific studies undertaken pertaining to the operating mechanisms responsible for maintenance of genomic methylation imprints, even though the P. citri genome may serve as very good material for such kind of expeditions.
In view of this trepidation, it is possible to infer that the mother can embark upon an initiation or directing a particular path towards the choice of her offspring and its bestowing effectiveness on the sex-ratio potential. In promulgating the imprinting phenomenon, in terms of evolutionary consequences with reference to a choice-based progeny sex ratio, it was also postulated that the role of the mother’s cytoplasmic environment might have been inflicted by imposition of environmental disturbances. Thus, during routine life-style courses, a one-to-one ratio in the case of sexually reproducing and one-to-none in the case of parthenogenetic system, the sex ratio will operate in an expected line. However, if any change is incurred with respect to the sex ratio it could possibly be envisioned and perceived as operating under constraints due to the external forces that thrust upon maternal environmental cues.
Currently, the mechanism of the genomic imprinting phenomenon is still unclear although the role of PCHET-2 and histone modifications seems evidently involved in effecting the facultative heterochromatization process in the case of the mealybug genome (P. citri). Females may volunteer and might offer to alter the concentration of those proteins in their eggs to their contention so as to modulate the sex ratio of their broods. Along this line, Buglia and Ferraro (2004) and Buglia et al. (2009) observations point towards the situation that under the varied concentration of CIA9-based positively stained protein and of those of observations pertaining to the eggs of females possessing variable amounts of proteins and at variable ages prior to mating should bring forth more differences in the egg chamber. They also apprise that females would produce male-biased offspring whereas the opposite effect of maternal aging prior to mating was also observed in other studies.
Prantera and Bongiorni (2012) postulated that the embryonic cytoplasm at the blastoderm stage determines whether the paternal chromosomes, which are marked by DNA hypomethylation and H3K9me3 methylation marks, could be able to drive towards undergoing heterochromatization processes or not, and thereby giving rise to a male or female embryo, respectively. Given the causative role and presence of PCHET-2 on male-specific heterochromatin formation and also based on the amount of PCHET-2 in the developing embryo, may prefer it as a crucial factor to drive the embryo either towards maleness or femaleness. It is already envisioned that the effectiveness of facultative heterochromatinization makes its presence in the seventh cleavage division onwards regarding male embryos and moving in the form of a wave from one pole towards the other, suggesting a graded distribution on the part of PCHET-2. Inasmuch as PCHET-2 could not possibly be observed either in sperm or in ooplasm, its presence only in the embryo should be at the courtesy of an early de novo synthesis under the control of above-said maternal factors.
Investigations pertaining to finding answers to several questions have been raised and are still pending for clarity with respect to our current understanding of mealybug genomes and of their possibly related roles in expression patterns of chromosomal facultative heterochromatization (inactivation) processes. However, the molecular and cytogenetic data acquired by both the Indian and the Italian investigators’ offer us highly commendable efforts since these contributions have driven towards arriving at a mutual interest in the form of a common platform of subjective comprehension.
As part of a supposition made by Prantera and Bongiorni (2012) and with those of the Khosla et al. (2006) and Mathur et al. (2010) opinion that NRC composition may have been influenced by DNA hypomethylation and histone H3K9Me3 methylation mark and furthermore, upon such a drive seemed to have made markings and then spread over the whole of paternal but not maternal chromosomes. Then, in the cleavage embryos, some maternal factor(s) present in the ooplasm might be able to regulate the imprinting process by means of having acquired the requisite amount of PCHET-2 that gradually spreads from one pole towards the other end of the developing embryo. A critical amount of regulated PCHET-2 will then determine whether the paternal imprinted chromosomes will become heterochromatic, thus picking up the path leading towards male embryonic development or will remain euchromatic, thereby losing repressive histone modifications and NRCs, and that eventually by not acquiring the requisite amount of markers, hence rely on the path leading towards female embryonic development.
References
Achwal CW, Chandra HS (1982) A sensitive immunochemical method for detection of 5mC in DNA fragments. FEBS Lett 150:469–472
Achwal CW, Iyer CA, Chandra HS (1983) Immunochemical evidence for the presence of 5mC, 6mC and 7mG in human, Drosophila and mealybug DNA. FEBS Lett 158:353–358
Achwal CW, Ganguly P, Chandra HS (1984) Estimation of the amount of 5 –methylcytosine in Drosophila melanogaster by photoacoustic spectroscopy. EMBO J 3(2):263–266
Baer D (1965) Asynchronous replication of DNA in a heterochromatic set of chromosomes in Pseudococcus obscurus. Genetics 52:275–285
Bell JT, Spector TD (2011) A twin approach to unraveling epigenetic. Trends Genet 27(3):116–125
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Bongiorni S, Prantera G (2003) Imprinted facultative heterochromatization in mealybugs. Genetica 117:271–279
Bongiorni S, Cintio O, Prantera G (1999) The relationship between DNA methylation and chromosome imprinting in the Coccid Planococcus citri. Genetics 151:1471–1478
Bongiorni S, Pasqualini B, Taranta M, Singh B, Prantera G (2007) Epigenetic regulation of facultative heterochromatinization in Planococcus citri via the Me(3)K9H3-HP1-Me(3)K20H4 pathway. J Cell Sci 120:1072–1080
Bongiorni S, Mazzuoli M, Masci S, Prantera G (2001) Facultative heterochromatization in parahaploid male mealybugs: involvement of a heterochromatin-associated protein. Development 128:3809–3817
Bongiorni S, Fiorenzo P, Pippoletti D, Prantera G (2004) Inverted meiosis and meiotic drive in mealybugs. Chromosoma 112:331–341
Bongiorni S, Pugnali M, Volpi S, Bizzaro D, Singh B, Prantera G (2009) Epigenetic marks for chromosome imprinting during spermatogenesis in coccoids. Chromosoma 118:501–512
Brown SW (1958) The chromosomes of an Orthezia species (Coccoidea- Homoptera). Cytologia 23:429–434
Brown SW (1959) Lecanoid chromosome behavior in three more families of the Coccoidea (Homoptera). Chromosoma 10:278–300
Brown SW (1961) Fracture and fusion of coccid chromosomes. Nature 191:1419–1420
Brown SW (1963) The Comstockiella system of chromosome behavior in the armored scale insects (Coccoidea: Diaspididae). Chromosoma 14:360–406
Brown SW (1964) Automatic frequency response in evolution of male haploidy and other coccid chromosome systems. Genetics 49:797–817
Brown SW (1965) Chromosomal survey of the armored and palm scale insects (Coccoidea: Diaspididae and Phoenicococcidae). Hilgardia 36:189–294
Brown SW (1966) Heterochromatin. Science 151:417–425
Brown SW (1969) Developmental control of heterochromatization in coccoids. Genetics 61(No. 1, part 2, Suppl):191–198
Brown SW (1977) Adaptive status and genetic regulation in major evolutionary changes of coccid chromosome systems. Nucleus 20:145–157
Brown SW, Bennett FD (1957) On sex determination in the Diaspine scale Pseudaulacaspis pentagona (Targ) (Coccoidea). Genetics 42:510–523
Brown SW, Chandra HS (1977) Chromosome imprinting and the differential regulation of homologous chromosomes. In: Goldstein L, Prescott DM (ed) Cell biology a comprehensive treatise, Academic press, New York, vol 1, pp 109–189
Brown SW, Cleveland C (1968) Meiosis in the male of Puto albicans (Coccoidea-Homoptera). Chromosoma 24:210–232
Brown SW, Nelson-Rees WA (1961) Radiation analysis of a lecanoid genetic system. Genetics 46:983–1007
Brown SW, Nur U (1964) Heterochromatic chromosomes in Coccoids. Science 145:130–136
Brown SW, Weigmann LI (1969) Cytogenetics of the mealybug Planococcus citri (Risso). Chromosoma 28:255–279
Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Balabio A, Pettigrew AL, Ledbetter DH, Levy E, Craig IW, Willard HF (1991) Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 349:82–84
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Inter science, New York
Buglia GL, Ferraro M (2004) Germline cyst development and imprinting in male mealybug Planococcus citri. Chromosoma 113(6):284–294
Buglia GL, Predazzi V, Ferraro M (1999) Cytosine methylation is not involved in the heterochromatization of the paternal genome of mealybug Planococcus citri. Chromosom Res 6:1–3
Buglia GL, Dionisi D, Ferraro M (2009) The amount of heterochromatic proteins in the egg is correlated with sex-determination in Planococcus citri (Homoptera: Coccoidea). Chromosoma 118(6):737–746
Bull JJ (1983) The evolution of sex determining mechanisms. Benjamin Cummings, Menlo Park
Cairns BR (2007) Chromatin remodeling! Insights and intrigue from single molecule studies. Nat Struct Mol Biol 14(1):1989–1996
Camacho JPM, Belda J, Cabrero J (1985) Meiotic behaviour of the holocentric chromosomes of Nezara viridula (Insecta: Heteroptera) analyzed by C-banding and silver impregnation. Can J Genet Cytol 27:490–497
Carter W (1962) Insects in relation to plant diseases. Interscience Publishers/Willey, NewYork, pp 247–265
Cattanach BM (1974) Position effect variegation in the mouse. Genet Res 23:291–306
Chadwick BP, Willard HF (2004) Multiple spatially distinct types of facultative heterochromatin of the human inactive X-chromosome. PNAS 101:17450–17455
Chandra HS (1962) Inverse meiosis in triploid females of the mealybug, Planococcus citri. Genetics 47:1441–1454
Chandra HS (1963a) Cytogenetic studies following high dosage paternal irradiation in the mealybug. Planococcus citri I. Cytology of X1 females and the problem of lecanoid sex determination. Chromosoma 14:310–329
Chandra HS (1963b) Cytogenetic studies following high dosage paternal irradiation in the mealybug. Planococcus citri II. Cytology of X1 females and the problem of lecanoid sex determination. Chromosoma 14:330–346
Chandra HS (1971) Inactivation of whole chromosomes in mammalian X-chromosomes. Nature 253:165–168
Chandra HS, Brown SW (1973) Regulation of X-chromosome inactivation in mammals. Genetics 78:342–349
Chandra HS, Brown SW (1975) Chromosome imprinting and the mammalian X chromosome. Nature 253:165–168
Chauhan NS (1970) Genetic evidence of an unorthodox chromosomal system in the lac insect, Kerria lacca (Kerr). Genet Res 16:341–344
Chauhan NS (1977) Gene expression and transmission in Kerria lacca (Kerr). Heredity 38:155–159
Cook LG (2000) Extraordinary and extensive karyotypic variation: A 48-fold range in chromosome number in the gall-inducing scale insect Apiomorpha (Hemiptera: Eriococcidae). Genome 43:255–263
Cowell IG, Aucott R, Mahadevaiah SK, Burgoyne PS, Huskisson N, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Wu R, Gilbert DM, Shi W, Fundele R, Morrison H, Jeppesen P, Singh PB (2002) Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 111:22–36
D’Aiuto L, de las Heras JI, Ross A, Shen MH, Cooke H (2003) Generation of a telomere-based episomal vector. Biotechnol Prog 19:1775–1780
De Lange T (2005) The protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110
De Robertis EM, Larrain J, Oelgeschlager M, Wessely O (2000) The establishment of Spemann’s organizer and patterning of vertebrate embryo. Nat Rev Genet 1(3):171–181
Deobagkar DN, Muralidharan K, Devare SG, Kalghatgi K, Chandra HS (1982) The mealybug chromosome system I: unusual methylated bases and dinucleotides in DNA of a Planococcus species. J Biosci 4:513–526
Deobagkar DN, Shankar V, Deobagkar DD (1986) Separation of 5-methylcytosine-rich DNA using immobilized antibody. Enzyme Microb Technol 8:97–100
Devajyothi C, Brahmachari V (1989) Modulation of DNA methyl transferase during the life cycle of a Planococcus lilacinus. FEBS Lett 250:134–138
Devajyothi C, Brahmachari V (1992) Detection of CpA methylase in an insect system: characterisation and substrate specificity. Mol Cell Biochem 110:103–111
Dikshith TSS (1964) Chromosome behaviour in Laccifer lacca (Kerr) Lacciferidae-Coccoidea. Cytologia 29:337–345
Dikshith TSS (1966) Spermiogenesis in Laccifer lacca (Kerr) (Lacciferidae-Coccoidea). Cytologia 31:302–308
Drozdovsky EM (1966) On chromosomal sets in some coccoids (Homoptera: Coccoidea). Entomologicheskoe Obozrenie 45(4):712–714 [In Russian]
Eissenberg JC, Elgin SC (2000) The HP-1 protein family: getting a grip on chromatin current opinion. Genet Dev 10:204–210
Epstein H, James TC, Singh PB (1992) Cloning and expression of Drosophila, HP-1 homologs from a mealybug, Planococcus citri. J Cell Sci 101:463–474
Esteban MR, Campos MC, Perondini AL, Goday C (1997) Role of microtubules and microtubule organizing centers on meiotic chromosome elimination in Sciara ocellaris. J Cell Sci 110:721–730
Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L, Erdjudumat-Bromage H, Tempst P, Simon JS, Zhong Y (2002) Purification and functional heterochromatin of SETs a nucleosomal histone & lysine −20- specific methyl transferase. Curr Biol 12:1086–1099
Feil R, Khosla S (1999) Genomic imprinting in mammals: interplay between chromatin and DNA methylation. Trends Genet 15:431–435
Ferraro M, Buglia GL, Romano F (2001) Involvement of histone H4 acetylation in the epigenetic inheritance of different activity states of maternally and paternally derived genomes in the Planococcus citri. Chromosoma 110(2):93–101
Ferraro M, Epifani C, Bongiorni S, Nardone AM, Parodi-Delfino S, Prantera G (1998) Cytogenetic characterization of the genome of mealybug Planococcus citri (Homoptera, Coccoidea). Caryologia 51(1):37–49
Field LM (2000) Methylation and expression of amplified esterase genes in the aphid Myzus persicae (S). Biochem J 349:863–868
Field LM, Lyko F, Mandrioli M, Prantera G (2004) DNA methylation in insects. Insect Mol Biol 13(2):109–115
Fire A, Xa S, Montgomery MK, Kostos SA, Driver SE, Nelin CC (1998) Potent and sporadic genetic interference by double stranded RNA in C. elegans. Nature 391:306–311
Frydrychová R, Grossmann P, Trubac P, Vítková M, Marec F (2004) Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 47(1):163–178
Gavrilov IA (2004) Taxonomic and cytogenetic studies of scale insects (Homoptera: Coccinea) of European Russia. Proc Zool Inst RAS 300:77–82
Gavrilov IA (2007) A catalog of chromosome numbers and genetic systems of scale insects (Homoptera: Coccinea) of the world. Isr J Entomol 37:1–45
Gavrilov IA, Trapeznikova IV (2007) Karyotypes and reproductive biology of some mealybugs (Insecta: Coccinea: Pseudococcidae). Comp Cytogenet 1(2):139–148
Gavrilov IA, Trapeznikova IV (2010) Karyotypes of six previously unstudied European mealybugs (Homoptera: Pseudococcidae). Comp Cytogenet 4(2):203–205
Gosden RG (2002) Oogenesis as a foundation of embryogenesis. Mol Cell Endocrinol 186:149–153
Greider CW (1995) Telomerase biochemistry and regulation. Cold Spring, NewYork, (CSH Laboratory Press), pp 35–68
Gullan PJ, Kosztarab M (1997) Adaptations in scale insects. Annu Rev Entomol 42:23–50
Hartl DL, Brown SW (1970) The origin of male haploid genetic systems and their expected sex ratio. Theor Pop Biol 1:165–190
Heitz E (1928) Das Heterochromatin der Moose I. Jahrb Wiss Bot 69:762–818
Heitz E (1929) Heterochromatin, chromocenter, chromomere. Ber Deutsch Bot Ges 47:274
Heitz E (1933) Die Herkunft der chromocentren. Planta 18:571–636
Hick CA, Field LM, Devousluire AL (1996) Changes in the methylation of amplied Esterase DNA during loss and reselection of insecticide resistance in Peach-Potato aphids, Myzus Persicae. Insect Biochem Mol Biol 26:41–47
Houk EJ, Griffiths GW (1980) Intracellular symbionts of the Homoptera. Annu Rev Entomol 25:161–187
Hughes-Schrader S (1935) The chromosome cycle of Phenacoccus (Coccidae). Biol Bull 69(3):62–468
Hughes-Schrader S (1944) A primitive coccid chromosome cycle in Puto sp. Biol Bull 87:167–176
Hughes-Schrader S (1948) Cytology of coccoids (Coccoidea: Homoptera). Adv Genet 2:127–203
Hughes-Schrader S, Ris H (1941) The diffuse spindle attachment of coccoids, verified by the mitotic behavior of induced chromosome fragments. J Exp Zool 87:29–456
Ishikawa H (1989) Biochemical and molecular aspects of endosymbionts in insects. Int Rev Cytol 116:1–45
Jaipuriar SK, Teotia TPS, Lakhotia SC, Chauhan NS (1985) A reinvestigation of the lecanoid chromosome system in Kerria lacca (Kerr). Cytobios 42:263–270
Jamaluddin M, Philip M, Chandra HS (1979) A rapid and gentle method for the salt extraction of Chromatin. J Biosci 1:49–59
James TC (1937) Sex ratios and the status of the male in Pseudococcinae (Hemiptera: Coccidae). Bul Entomol Res 28:429–461
James TC (1938) The effect of the humidity of the environment on sex ratios from over-aged ova of Pseudococcus citri (Risso) (Hemiptera: Coccidae). Proc R Entomol Soc Lond: Ser A Gen Entomol 13:73–79
Jeppesen P, Turner BM (1993) The inactive X-chromosome in female mammals is distinguished by a lack of histons H4 acetylation, a cytogenetic marker for gene expression. Cell 74:281–289
Kantheti P (1994) Studies on a female-specific cDNA clone and chromatin organization in a, Planococcus lilacinus. Ph.D thesis. IISc, Bangalore
Kantheti P, Jayarama KS, Chandra HS (1996) Developmental analysis of a female-specific 16S rRNA gene from Mycetome associated endosymbionts of a mealybug, Planococcus lilacinus. Insect Biochem Mol Biol 26:997–1009
Karnik PS (1983) Correlation between phosphorylated H 1 histone and condensed chromatin in Planococcus citri. FEBS 163(1):128–130
Khosla S, Kantheti P, Brahmachari V, Chandra HS (1996) A male-specific nuclease- resistant chromatin fraction in the Planococcus lilacinus. Chromosoma 104(5):386–392
Khosla S, Augustus M, Brahmachari V (1999) Sex-specific organization of middle repetitive DNA sequences in the mealybug Planococcus lilacinus. Nucleic Acids Res 27(18):3745–3751
Khosla S, Mendiratta G, Brahmachari V (2006) Genomic imprinting in the mealybugs. Cytogenet Genome Res 113:41–52
Klein AS, Echardt RA (1976) The DNA’s of the A and B chromosomes of the Pseudococcus obscurus. Chromosoma 57:333–340
Kondo T, Gullan PJ, Williams DJ (2008) Coccidology. The study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Revista Corpoica – Ciencia y Tecnología Agropecuaria 9(2):55–61
Kourmouli N, Jeppesen P, Mahadevhaiah S, Burgoyne P, Wu R, Gilbert DM, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Shi W, Fundele R, Singh PB (2004) Heterochromatin and trimethylated lysine 20 of histone H4 in animals. J Cell Sci 117:2491–2501
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705
Lachner M, O’Caroll D, Rea S, Mechtler K, Jenuwein T (2001) Methylation of histone H3 lysine 9 creatsa binding site for HP-1, proteins. Nature 410:116–120
Lakhotia SC (2004) Epigenetics of heterochromatin. J Biosci 29(3):219–224
Li E (2002) Chromatin modifications and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673
Li X, Ito M, Zhon F, Youngson N, Zuo X, Leder P, Ferguson Smith AC (2008) A maternal zygotic effect, Zfp57, maintains both maternal and paternal imprints. Dev Cell 15:547–557
Little BA (1957) General and applied entomology, 3rd edn. Edition Harper and Row Publishers, New York, pp 165–173
Lorick G (1970) Differential DNA synthesis in heterochromatic and euchromatic chromosome sets of Planococcus citri. Chromosoma 31:11–30
Lyon MF (1999) Imprinting and X-chromosome inactivation. Results Prob Cell Different 25:73–90
Mani MS (1989) Indian insects, 1st edn. Satish Book Enterprises, Agra, pp 103–105
Mathur V, Mendiratta G, Ganapathi M, Kennady PK, Dwarkanath BS, Pande G, Brahmachari V (2010) An analysis of histone modifications in relation to sex-specific chromatin organization in the mealybug Maconellicoccus hirsutus. Cytogenet Genome Res 129(4):323–331
Mckenzie HL (1967) Mealybugs of California with taxonomy, biology and control of North American species ( Homoptera: Coccoidea: Pseudococcidae). University of California Press, Berkeley/Los Angeles, p 525
Miller DR, Kosztarab M (1979) Recent advances in the study of scale insects. Annu Rev Entomol 24:1–27
Mohan KN, Chandra HS (2005) Isolation and analysis of sequences showing sex-specific cytosine methylation in the Planococcus lilacinus. Mol Gen Genomics 274(6):557–568
Mohan KN, Ray P, Chandra HS (2002) Characterization of the Planococcus lilacinus, a model organism for studying the whole chromosome imprinting and inactivation. Genet Res 79(2):111–118
Mohan KN, SandhyaRani B, Kulashrestha PS, Kadandale JS (2011) Characterisation of TTAGG telomeric repeats, their interstitial occurrence and constitutively active telomerase in the Planococcus lilacinus (Homoptera; Coccoidea). Chromosoma 120:165–175
Mohan KN, Jun G, Kadandale JS (2012) Mealybug as a model for studying responses to high doses of ionizing radiation. Curr Topics Ionizing Rad Res 6:101–116
Moharana S (1990) Cytotaxonomy of Coccoids (Coccidea: Homoptera). In: Sixth international symposium of scale insect studies, Part II, Cracow, Poland, August 6–12. Agricultural University Press, Cracow, pp 47–54
Munson MA, Baumann P, Clark MA, Baumann L, Moran A, Vogtlin DJ, Campbell BC (1991) Evidence for the establishment of aphid eubacterial endosymbionts in an ancestor of four aphid families. J Bacteriol 173:6321–6324
Munson MA, Baumann P, Moran A (1992) Phylogenetic relationships of the endosymbionts of mealybugs (Homoptera: Pseudococcidae) based on the 16s rRNA sequences. Mol. Phylogenetics and Evolution 1:26–30
Muramoto N (1980) A study of the C-banded chromosomes in some species of heteropteran insects. Proc Japan Acad Scien phys and BiolScien 56:126–130
Nielsen SJ, Oulad-Abdelghani M, Oritz JA, Remboutsika E, Chambon P, Lesson R (2001) Heterochromatin formation in mammalian cells. Interactions between histones and HP1 proteins. Mol. Cell 7:729–731
Nelson-Rees WA (1960) A study of sex predetermination in the mealybug Planococcus citri (Risso). J Exp Zool 144:111–137
Nokayama J, Rice JC, Strahl BD, Allis CD, Grewal SI (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110–113
Nur U (1962a) A supernumerary chromosome with an accumulation mechanism in the lecanoid genetic system. Chromosoma 13:249–271
Nur U (1962b) Sperms, sperm bundles and fertilization in a mealybug, Pseudococcus obscurus Essig – (Homoptera: Coccoidea). J Morphol 111:173–199
Nur U (1963) Meiotic parthenogenesis and heterochromatization in a soft scale, Pulvinaria hydrangeae (Coccoidea: Homoptera). Chromosoma 14:123–139
Nur U (1966a) Harmful supernumerary chromosomes in a mealybug population. Genetics 54:1225–1238
Nur U (1966b) The effect of supernumerary chromosomes on the development of mealybugs. Genetics 54:1239–1249
Nur U (1966c) Non replication of heterochromatic chromosomes in a mealybug Planococcus citri (Coccoidea-Homoptera). Chromosoma 19:439–448
Nur U (1967) Reversal of heterochromatization and the activity of paternal chromosome set in male mealybug. Genetics 56:375–389
Nur U (1969) Harmful B-chromosome in a mealybug. Chromosoma 28:280–297
Nur U (1970) Translocations between euchromatic and heterochromatic chromosomes and spermatocytes lacking a heterochromatic set in male mealybugs. Chromosoma 29:42–61
Nur U (1971) Parthenogenesis in Coccoids (Homoptera). Am Zool 11:301–308
Nur U (1972) Diploid arrhenotoky and automictic thelytoky in soft scale insects (Lecaniidae: Coccoidea: Homoptera). Chromosoma 39:381–401
Nur U (1977) Maternal inheritance of enzymes in the, Pseudococcus obscurus (Homoptera). Genetics 86:149–160
Nur U (1980) Evolution of unusual chromosome systems in scale insects (Coccoidea: Homoptera). In: Blackman RL, Hewitt GM, Ashburner M (eds) Insect cytogenetics. Royal Entomological Society, London, pp 97–117, 278
Nur U (1990) Heterochromatization and euchromatization of whole genome in scale insects (Coccoidea: Homoptera). Development supplement:29–34
Nur U, Brett BLH (1985) Genotypes suppressing meiotic drive of a B-chromosome in the mealybug Planococcus obscurus. Genetics 110:73–92
Nur U, Brett BLH (1987) Control of meiotic drive of B-chromosomes in the mealybug, Planococcus affinis. Genetics 115:499–510
Nur U, Brett BLH (1988) Genotypes affecting the condensation and transmission of heterochromatic B-chromosomes in the mealybug, Planococcus affinis. Chromosoma 96:201–212
Nur U, Brown SW, Beardsley JW (1987) Evolution of chromosome number in mealybugs (Pseudococcidae: Homoptera). Genetica 74:53–60
Panzera F, Alvarez F, Sanchez-Rufas J, Pérez R, Suja JA, Scvortzoff E, Dujardin JP, Estramil E, Salvatella R (1992) C-heterochromatin polymorphism in holocentric chromosomes of Triatoma infestans (Hemiptera: Reduviidae). Genome 35(6):1068–1074
Papeschi AG (1998) C-banding and DNA content in these species of Belastoma (Heteroptera) with large differences in chromosome size and number. Genetica 76:43–51
Parida BB, Moharana S (1982) Studies on the chromosome constitution in 42 species of scale insects (Coccoidea: Homoptera) from India. Chromosome Information Service 32:18–20
Pérez R, Panzera F, Page J, Suja JA, Rufas JS (1997) Meiotic behaviour of holocentric chromosomes: Orientation and segregation of autosomes in Triatoma infestans (Heteroptera). Chromosom Res 5:47–56
Peterson K, Sapienza C (1993) Imprinting the genome: imprinted genes, imprinting genes and an hypothesis for their interaction. Annu Rev Genet 27:7–31
Pfeifer GP, Riggs AD (1991) Chromatin differences between active and inactive X chromosomes revealed by genomic foot printing of permeabilized cells using DNase I and ligation mediated PCR. Genes Dev 5:1102–1113
Prantera G, Bongiorni S (2012) chromosome cycle as a paradigm of epigenetics. Genetics Research International ID : 867390:1–11
Prantera G, Ferraro M (1990) Analysis of methylation and distribution of CpG sequence in human active and inactive X-chromosome by in situ nick translation. Chromosoma 99:18–23
Raju NG (1994) A study of the chromosomes in three species of Indian. Dissertation, Bangalore University, Bangalore, Planococcus. M.Phil
Ris H (1942) A cytological and experimental analysis of the meiotic behavior of the univalent X-chromosome in the bearberry aphid Tamalia (d'hyllaphis) Coweni (Ckll.). J Exptl Zool 90:267–326
Ross L, Pen I, Shuker DM (2010a) Genomic conflict in scale insects: the causes and consequences of bizarre genetic systems. Biol Rev 85(4):807–828
Ross L, Langenhof MBW, Pen I, Beukeboom LW, West SA, Shuker DM (2010b) Sex allocation in a species with paternal genome elimination: clarifying the role of crowding and female age in the mealybug Planococcus citri. Evol Ecol Res 12:89–104
Ross L, Dealy EJ, Beukeboom LW, Shuker DM (2011) Temperature, age of mating and starvation determine the role of maternal effects on sex allocation in the mealybug Planococcus citri. Behav Ecol Sociobiol 65:909–919
Sado T, Hoki Y, Sasaki K (2005) Tsix silence xist through modification of chromatin structure. Dev Cell 9:159–165
Scarbrough K, Hattman S, Nur U (1984) Relationship of DNA methylation level to the presence of heterochromatin in mealybugs. Mol Cell Biol 4:599–603
Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T (2004) A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18:1251–1262
Schrader F (1921) The chromosomes of Pseudococcus nipae. Biol Bull 40:259–270
Schrader F (1923a) The origin of the mycetocytes in Pseudococcus. Biol Bull 45(6):279–302
Schrader F (1923b) A study of the chromosomes in three species of Pseudococcus. Archiv für Zellforschung 17:45–62
Schrader F (1931) The chromosome cycle of Protortonia primitiva (Coccidae) and considerationof the meiotic division apparatus in the male. Z Wiss Zool 138:386–408
Schrader F, Hughes-Schrader S (1926) Haploidy in Icerya purchasi. Z Wiss Zool 128:182–200
Schweizer D, Loidl J (1987) A model for heterochromatin dispersion and the evolution of C-band patterns. Chromos Today 9:61–74
Shuker DM, Moynihan AM, Ross L (2009) Sexual conflict, sex allocation and the genetic system. Biol Lett 5:682–685
Singh PB, Georgatos SD (2002) HP1: facts, open questions and speculation. J Struct Biol 140:10–16
Singh PB, Miller JR, Pearce J, Kothary R, Burton RD, Paro R, James TC, Gaunt SJ (1991) A sequence motif found in a Drosophila heterochromatin protein is conserved in animals and plants. Nucleic Acids Res 19:789–794
Skiniotis G, Moazed D, Waitz T (2007) Acetylated histone tail peptides induce structural rearrangements in the RJC chromatin remodeling complex. J. Biol Chem 282:20804–20808
Solter D (1998) Imprinting. Intl J Dev Biol 42:951–954
Spofford J (1976) In: Ashburmer & Novitski E (eds) Position effect variation in Drosophila. Academic Press, London, pp 955–1018
Surani MAH (1991) Genomic imprinting: developmental significance and molecular mechanism. Curr Opin Genes Dev 1:241–246
Tremblay E (1977) Advances in endosymbiotic studies in Coccoidea. Va. Polytech. Ins. State University. Res Div Bull 127:23–33
Tremblay E (1989) Coccoidea endocytobiosis. In: Insect endocytobiosis: Morphology, physiology, genetics, evolution (eds.W. Schwemmler and G. Gassner). CRC Press, Boca Raton, Florida, pp 145–173
Tremblay E, Caltagirone LE (1973) Fate of polar bodies in insects. Annu Rev Entomol 18:421–444
Tremblay E, Tranfaglia A, Rotundo G, Iccarino FM (1977) Osservazioni comparate su alcune specie di Pseudococcidi (Homoptera: Coccoidea). Bollettino del Laboratorio di Entomologia Agraria ‘Filippo Silvestri’. Portici 34:113–135
Trivers RL, Hare H (1976) Haplo-diploidy and the evolution of the social insect. Science 191:249–263
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex ratio of offspring. Science 179:90–92
Tulsyan GP (1963) Studies on chromosome number and spermatogenesis in the lac insect Laccifera lacca (Kerr). Curr Sci 32:374–375
Vakoc CR, Mandst SA, Okachak BA, Blobel GA (2005) Histone H3 lysine methylation and HP-1gamma are associated with transcription elongation through mammalian chromate. Mol Cell 19:381–391
Varndell NP, Godfray HCG (1996) Facultative adjustment of the sex-ratio in an insect (P. citri: Pseudococcidae) with paternal genome loss. Evolution 50(5):2100–2105
Venkatachalaiah G (1989) Characterization of heterochromatin in chromosomes of Planococcus citri. XIII All India Cell Biology Conference and Cell Biology Symposia. CCMB, Hyderabad
Venkatachalaiah G, Chowdaiah BN (1987) Air-drying technique for the preparation of mosquito chromosomes. Nucleus 30(1, 2): 44–46
Vitkova M, Karl J, Traut W, Zrzavy J, Marec F (2005) The evolutionary origin of insect telomeric repeats (TTAGG)n. Chromosomal Res 13:145–156
Volpi S, Bongiorni S, Prantera G (2007) HP2-like protein: a new piece of the facultative heterochromatin puzzle. Chromosoma 116(3):249–258
White MJD (1973) Animal cytology and evolution, 3rd edn. Cambridge University Press, Cambridge, p 961
White MJD (1978) Modes of speciation. W. H. Freeman, San Francisco
Wu RS, Penuaz HT, Hatch CI, Bonner WM (1986) Histones and their modifications. CRC Crit Ren Biochrem 20:201–263
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Sompalaym, R., Lingarajaiah, K.A., Narayanappa, R.G., Jayaprakash, Govindaiah, V. (2016). Cytogenetics. In: Mani, M., Shivaraju, C. (eds) Mealybugs and their Management in Agricultural and Horticultural crops . Springer, New Delhi. https://doi.org/10.1007/978-81-322-2677-2_3
Download citation
DOI: https://doi.org/10.1007/978-81-322-2677-2_3
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2675-8
Online ISBN: 978-81-322-2677-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)