Abstract
The aim of the present investigation was isolation and characterization of active components derived from whole plant of Mucuna pruriens. The plant were extracted with various solvents (pet. ether, ethyl acetate and methanol), methanol was found to be more active among them. The preliminary phytochemical results revealed that phytosterols, flavonoids and amino acids as active constituents in methanolic extract of Mucuna pruriens. The methanolic extract of Mucuna pruriens was undergone column chromatography with different solvent fractions. Despite, three compounds were isolated from methanolic extract of Mucuna pruriens with the compound 1 was eluted with benzene: Chloroform 90:10, v/v and compound 2 were eluted with eluted with ethyl acetate: methanol 80:20, v/v and then compound 3 were eluted with ethyl acetate: methanol, 70:30, v/v. The structures of the two isolated compounds were characterized by using FT-IR, NMR and Mass spectrophotometric methods. Thus, the compound 1 was characterized as acetate of 3β-Hydroxy-5α-Cholanic acid (C26H38O3), the compound 2 was characterized as 3, 5, 7, 4′-Tetrahydroxy-6-methoxyflavone (C16H12O7) and the compound 3 was characterized as Ethyl 2-amino-5-hydroxy-3, 6, 6-trimethyl heptonate (C12H25NO3). Furthermore, pharmacological studies required for the isolated compounds.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
38.1 Introduction
Mucuna pruriens Linn belongs to the family fabaceae, commonly known as cowhage plant or kapikacho or kevach in Hindi, is the most popular drug in Ayurvedic system of medicine [1]. Traditionally, in India, the seeds of Mucuna pruriens are used as a tonic and aphrodisiac for male virility. It has been reported to be antidiabetic [2]. Its different preparations (from seeds) are used for the management of several free radical-mediated diseases such as ageing, rheumatoid arthritis, diabetes, atherosclerosis, male infertility and nervous disorders. It is also used as an aphrodisiac and in the management of Parkinsonism, as it is a good source of L-dopa [3]. The anti-epileptic and anti-neoplastic activity of methanol extract of Mucuna pruriens has been reported [4]. It had been reported analgesic and anti-inflammatory [5]. It is also used as a fertility agent in men [6]. The plant is rich in alkaloids such as prurienine, prurieninine and prurienidine [7]. Triterpenes and sterols (β-sitosterol, ursolic acid, etc.,) were found in the root and seeds of Mucuna pruriens. The seeds contain proteins, amino acids such as L-DOPA [8]. Therefore, the objective of the present investigation was isolation and characterization of active components derived from whole plant of Mucuna pruriens by using FT-IR, NMR and mass spectrophotometric methods.
38.2 Experimental Section
38.2.1 Plant Material
The whole plant of Mucuna pruriens (Linn), were collected from Neiyur dam, Kanyakumari District of Tamil Nadu, India. Taxonomic identification was made from Botanical Survey of Medicinal Plants Unit Siddha, Government of India, Palayamkottai. The whole plant of Mucuna pruriens (Linn), were dried under shade, segregated, pulverized by a mechanical grinder and passed through a 40 mesh sieve.
38.2.2 Extraction
The powdered plant materials were successively extracted with Petroleum ether (40–60 °C) by hot continuous percolation method in Soxhlet apparatus [9] for 24 h. Then the marc was subjected to Ethyl acetate (76–78 °C) for 24 h and then marc was subjected to Methanol for 24 h. The extracts were concentrated by using a rotary evaporator and subjected to freeze drying in a lyophilizer till dry powder was obtained. All the three extract were stored in screw cap vial at 4 °C until further use.
38.2.3 Preliminary Phytochemical Screening
The extract was subjected to preliminary phytochemical screening for the detection of various plant constituents present. The various extracts of Mucuna pruriens was subjected to the following chemical tests such as tests for Alkaloids [10], test for Carbohydrates [10], tests of Glycosides [10], tests for Phytosterol [11], test for Coumarins [11], test for Flavonoids [12, 13], test for Tannins and Phenolic compounds [14], tests for Proteins and Amino Acids [10], test for Saponins [10], test for Fixed Oils [10].
38.2.4 TLC Characterization of Methanolic Extract of Mucuna pruriens
The principle of separation is either partition or adsorption. The constituent which is having more affinity for mobile phase moves with it, while the constituent which is having more affinity for stationary phase gets adsorbed on it. This way various compounds appear as a band on the TLC plate, having different Rf values. The methanolic extract of Mucuna pruriens was subjected to thin layer and high performance thin layer chromatographic studies for the separation and identification of their components.
38.2.5 Preparation of Plates
100 g of silica gel G was weighed and made into a homogenous suspension with 200 ml of distilled water to form slurry. The slurry was poured into a TLC applicator, which was adjusted to 0.25 mm thickness on flat glass plate of different dimensions (10 × 2, 10 × 5, 30 × 5, 20 × 10 cm etc.,). The coated plates were allowed to dry in air, followed by heating at 100–105 °C for 1 h, cooled and protected from moisture. Before using, the plates were activated at 110 °C for 10 min.
38.2.6 Separation of Components
The methanolic extracts of Mucuna pruriens was dissolved in methanol separately and spotted using a capillary tube on TLC plates 2 cm above from the bottom of the plate. The selection of solvent systems was based on increasing the order of polarity. The different spots developed in each system were detected by means of iodine staining.
38.2.7 Isolation of Methanolic Extract of Mucuna Pruriens by Using Column Chromatography
The 20 g of methanolic extract of Mucuna pruriens was admixed with 20 g silica gel (60/120 meshes) to get uniform mixing. 200 g of silica gel (70/325 meshes) was taken in a suitable column and packed very carefully without air bubbles using hexane as filling solvent. The column was kept aside for 1 h and allowed for close packing. Admixture was then added at the top of the stationary phase and started separation of compounds by the eluting with various solvent mixtures with increasing order of polarity. All the column fractions were collected separately and concentrated under reduced pressure. Finally the column was washed with ethyl acetate and methanol.
38.2.8 Characterization of Isolated Compounds
38.2.8.1 FT-IR
IR spectra of the compounds isolated from the methanolic extracts of Mucuna pruriens were recorded using a Nicolet 170SX. The spectral resolution for the Nicolet 170SX was 0.25 cm−1, and the spectral data were stored in the database at intervals of 0.5 cm−1 at 4000–2000 cm−1, and of 0.25 cm−1 at 2000–400 cm−1. Liquid samples were measured with liquid film method, and solid samples were measured by using KBr disc methods.
38.2.8.2 1HNMR
1HNMR spectra of the compounds isolated from the methanolic extracts of Mucuna pruriens was recorded using a JEOL AL-400 (399.65 MHz). The measuring conditions for the most of the spectra were as follows: flip angle of 22.5–30.0°, pulse repetition time of 30 s. The long pulse repetition time and small flip angle is used to ensure precise relative intensities. The 1HNMR chemical shifts were referred to TMS in organic solvents and TSP in D2O.
38.2.8.3 13CNMR
13CNMR spectra of the compounds isolated from the methanolic extracts of Mucuna pruriens was recorded with a Bruker AC-200 (50.323 MHz). The measuring conditions for the most of the spectra were as follows: a pulse flips angle of 22.45–45°, a pulse repetition time of 4–7 s, and a resolution of 0.025–0.045 ppm. The spectra whose spectral codes started with “CDS” were reconstructed from peak positions, intensities, and line widths by assuming all resonance peaks were Lorenz lines. The chemical shift was referred to a TMS for all solvents.
38.2.8.4 Mass Spectrum
Mass spectra of the compounds isolated from the methanolic extracts of Mucuna pruriens was recorded with JEOL JMS-700 by the electron impact method where an electron is accelerating voltage 75 eV and an ion accelerating voltage of 8–10 nV. The reservoir inlet systems were used. The dynamic range for the peak intensities were three digits and the accuracy of the mass number was 0.5.
38.3 Results and Discussion
The various extracts of Mucuna pruriens (Linn.) were subjected to screening for its phytochemical constituents. The phytochemical screening results are shown in Table 38.1. The petroleum ether extract of Mucuna pruriens was contains phytosterols and fixed oils and fats. Ethyl acetate extracts containing Alkaloids, Carbohydrates, protein and amino acid compounds and fixed oils and fats. The Methanolic extracts containing Alkaloids, Carbohydrates, glycoside, Phenolic compounds, Saponins, Tannins, Protein and amino acids, coumarins and flavonoids.
Petroleum ether, ethyl acetate and methanol were used individually as solvent for the extraction of Mucuna pruriens. The methanolic extract of Mucuna pruriens was found more active among them. Therefore, the methanolic extract of Mucuna pruriens was subjected to the TLC chromatographic profile and column chromatographic separation. The methanolic extract of Mucuna pruriens dissolved in their mother solvent was taken in a capillary tube and spotted on TLC plates 2 cm above its bottom. Most of the sample for application were between 0.1 and 1 %. The applied spots were of equal size as far as possible and diameter ranging from 2 to 3 mm. The solvent system for methanolic extracts was developed by trial and error method using various solvents which were differing in polarities (Table 38.2).
The methanolic extract of Mucuna pruriens was subjected to column chromatographic separation using normal phase silica gel column. The dark brown solid (20 g methanolic extract of Mucuna pruriens) was adsorbed on silica gel (20 g) and transferred to a column of silica gel (200 g equilibrated with benzene). Despite, three compounds were isolated in column chromatography with different solvents. Obviously, the compound 1 (140 mg) was eluted with benzene: Chloroform 90:10, v/v, compound 2 (165 mg) was eluted with ethyl acetate: methanol, 80:20 v/v and compound 3 was eluted with ethyl acetate: methanol, 70:30, v/v. This active fraction was used to identify the chemical tests showed the presence of phytosterols, flavonoids and amino acid as active compounds. The actual compounds were isolated from column chromatography as mentioned in the experimental section. The spectra (IR, 1H and 13CNMR and Mass) of these compounds as mentioned in the experimental section.
38.3.1 Characterization of Compound 1
The spectral data IR, 1HNMR and 13CNMR and Mass of the compound 1 are good in agreement with the structure proposed for the compound. The melting point of the compound 1 was found as 149 °C. The IR spectrum of the compound 1 was analysed from the IR data. The absorption at 3,427 cm−1 indicates the presence of −OH group. The presence of −C=O group in this compound is revealed by the strong absorption at 1692 cm−1. The unsaturation such as −C=C is known from the absorption at 1458 cm−1. The strong absorption at 1027 cm−1 indicates the presence of –C–O-C link.
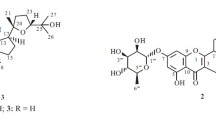
The 1HNMR chemical shift values the chemical shift values at δ, 11.94 and 2.50 ppm show the presence of –OH group (–COOH). The singlet at δ, 5.13 and 4.29 ppm are due to proton at C6 and C22 carbon atom respectively. The chemical shifts as multiplets at δ, 2.11–0.86 ppm are due to the presence of methylene and methyl groups. The 13CNMR chemical shift at δ, 178.58 and 178.28 ppm are due to the carbonyl carbon of acid group (–COOH) and acetyl group(–CO–O-CH3). The chemical shift at δ, 138, 124 and 121 ppm are due to C22, C6 and C3 respectively. The mass spectral analysis of compound 1 led to the molecular peak m/z 404(M + 4), which indicated the molecular formula C26H38O3. Thus, the compound 1 was characterized as acetate of 3β-Hydroxy-5α-Cholanic acid was given in Fig. 38.1. The Molecular Formula of the compound 1 was deduced as C26H38O3.
38.3.2 Characterization of Compound 2
The spectral data IR, 1HNMR and 13CNMR and Mass of the compound 2 are good in agreement with the structure proposed for the compound. The melting point of the compound 2 was found as 235 °C. The IR spectrum of the compound 2 was analysed from the IR data. The presence of –OH group known from the absorption at the range 3330–3463 cm−1. A strong band at the range 1655–1617 cm−1 is due to the presence of –C=O group. The presence of –C–O–C– indicates in the absorption at 1060 cm−1. The 1HNMR and 13CNMR chemical shift values of the compound 2 was found to be 3, 5, 7, 4′-Tetrahydroxy-6-methoxyflavone (Figs. 38.2 and 38.3). The mass spectral analysis of compound 2 led to the molecular peak m/z 316, which indicated the molecular formula C16H12O7. Thus, the compound 2 was characterized as 3, 5, 7, 4′-Tetrahydroxy-6-methoxyflavone was given in Fig. 38.4. The Molecular Formula of the compound 2 was deduced as C16H12O7. This is the first report of occurrence of this compound in nature as well as the alkaloids in this plant.
38.3.3 Characterization of Compound 3
The spectral data IR, 1HNMR and 13CNMR and Mass of the compound 3 are good in agreement with the structure proposed for the compound. The melting point of the compound 3 was found as 235 °C. The IR spectrum of the compound 3 was analysed from the IR data. Absorption at 3412 cm−1 shows the presence of –OH group, whereas the strong band at 1734 cm−1 indicates the presence of carbonyl group of aliphatic compound. The absorption at 1173 cm−1 reveals the presence of –C–N stretching. The 1HNMR and 13CNMR chemical shift values of the compound 2 was found to be ethyl 2-amino-5-hydroxy-3, 6, 6-trimethyl heptonate (Figs. 38.5 and 38.6). The mass spectral analysis of compound 3 led to the molecular peak m/z 434, which indicated the molecular formula C16H12O7. Thus, the compound 2 was characterized as ethyl 2-amino-5-hydroxy-3,6,6-trimethyl heptonate was given in Fig. 38.7. The Molecular Formula of the compound 3 was deduced as C12H25O3.
38.4 Conclusion
From the above reports, three compounds were isolated from methanolic extract of Mucuna pruriens (Linn.) such as 3β-Hydroxy-5α-Cholanic acid (C26H38O3), 3, 5, 7, 4′-Tetrahydroxy-6-methoxyflavone (C16H12O7) and ethyl 2-amino-5-hydroxy-3,6,6-trimethyl heptonate (C12H25NO3). However, this is the first report of occurrence of 3, 5, 7, 4′-Tetrahydroxy-6-methoxyflavone (C16H12O7) in nature as well as the alkaloid in this plant. Therefore, further in-depth biological investigations need for the isolated compounds.
References
Chopra RN, Nayar SL, Chopra IC (1956) Glossary of Indian medicinal plants. CSIR, New Delhi
Dhawan BN, Dubey MP, Mehrotra BN, Rastogi RP, Tandon JS (1980) Screening of Indian plants for biological activity. Ind J Expt Biol 18:594–606
Vaidya RA, Allorkar SD, Seth AR, Panday SK (1978) Activity of bromoergocryptine, Mucuna pruriens and L-Dopa in the control of hyperprolactinemia. Neurology 26:179–186
Gupta M, Mazumder UK, Chakraborti S, Rath N, Bhawal SR (1997) Antiepileptic and anticancer activity of some indigenous plants. Indian J Physiol Allied Sci 51(2):53–56
Hishika R, Shastry S, Shinde S, Guptal SS (1981) Prliminary, phytochemical and anti-inflammatory activity of seeds of Mucuna pruriens. Indian J Pharmacol 13(1):97–98
Buckles D (1995) Velvet bean: a ‘new’ plant with a history. Econ Bot 40:13–25
Misra L, Wagner H (2004) Alkaloidal constituents of mucuna pruriens seeds. Phytochemistry 65:2565–2567
Siddhuraju P, Vijayakumari K, Janardhanan K (1996) Chemical composition and protein quality of the little known legume, velvet bean (Mucuna pruriens (L.) DC.). J Agric Food Chem 44:2541–2636
Harborne JB (1984) Phytochemical methods 11th edn. In: Chapman & Hall, New York, pp 4–5
Evans WC (1997) An index of medicinal plants. A text book of pharmacognosy, vol 7(5), 14th edn, pp 12–14
Finar G (1986) Plants of economic importance. Medicinal plants and medicine in Africa, vol 78. Spectrum books Ltd, Ibadan, pp 150–153
Dey PM, Harborne JB (1987) Methods in plant biochemistry. Academic Press, London
Evans WC (1989) Pharmacognosy, 13th edn. Balliere-Tindall, London
Mace Gorbach SL (1963) Anaerobic bacteriology for clinical laboratories. Pharmacognosy 23:89–91
Acknowledgments
The authors are grateful to University Grants Commission, Govt. of India, New Delhi for providing financial assistance and motivation for the present investigation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this paper
Cite this paper
Satheesh Kumar, D., Kottai Muthu, A., Kannan, K. (2013). Isolation and Characterization of Active Components Derived from Whole Plant of Mucuna pruriens (Linn.). In: Malathi, R., Krishnan, J. (eds) Recent Advancements in System Modelling Applications. Lecture Notes in Electrical Engineering, vol 188. Springer, India. https://doi.org/10.1007/978-81-322-1035-1_38
Download citation
DOI: https://doi.org/10.1007/978-81-322-1035-1_38
Published:
Publisher Name: Springer, India
Print ISBN: 978-81-322-1034-4
Online ISBN: 978-81-322-1035-1
eBook Packages: EngineeringEngineering (R0)