Abstract
Adolescent idiopathic scoliosis (AIS) occurs in children during their pubertal growth spurt. Rapid skeletal growth and abnormal anthropometric parameters are associated with the development and progression of scoliotic curves, with the curves stabilized when skeletal maturity is reached. Systemic osteopenia affecting multiple skeletal sites, defined as bone mineral density (BMD) of Z-score ≤ −1 with reference to the age and ethnic-matched population, was found by dual-energy X-ray absorptiometry (DXA) in over 30% of the AIS patients. The osteopenia could persist into adulthood, thus predisposing the AIS patients to osteoporosis and other related complications in later life. Osteopenia in AIS was shown to be an important prognostic factor for curve progression. Recent studies with advanced high-resolution peripheral quantitative computed tomography (HR-pQCT) revealed significant alterations in systemic bone geometry, micro-architecture, volumetric BMD, and mechanical bone strength (finite element analysis) in addition to osteopenia in AIS. Serological and cellular functional studies supported the presence of abnormal bone formation and resorption that might have contributed to the abnormal bone qualities and bone strength which could be linked to the etiopathogenesis of AIS. Physical activities, calcium and vitamin D intake, and genetics are important factors affecting bone mass in AIS. Recent RCT studies on whole-body vibration therapy and supplement therapy with calcium and vitamin D have shown to improve low bone mass in AIS significantly. A better understanding on the possible association between bone qualities and curve progression will shed light on future development of novel prognostic biomarkers and therapeutic strategies in AIS.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Skeletal Growth and Bone Metabolism
6.1.1 Overview of Adolescent Skeletal Growth
Adolescence is a period of rapid skeletal growth; the skeletal mass nearly doubles at the end of adolescence [1]. During puberty the process of bone formation predominates, resulting in a steady increase of bone mass. This life stage represents an important opportunity for influencing peak bone mass and thus reducing the risk of osteoporotic fractures occurring later in life. Peak bone mass occurs from ages as early as 16–18 years at the lumbar spine, femoral neck, and midshaft, to as late as 35 years at the radius, skull, and whole body [2]. During puberty, a transient period of bone fragility at metaphysis was reported [3]. Previous studies on growth pattern showed asynchrony in bone mass accretion and growth, resulting in a transient decline in bone mineral density (BMD) and cortical weakness [4, 5].
Bone mass is the net result of the bone modeling and remodeling processes which are affected by complex hormonal changes that are programmed by genetics and also interact with nutritional and environmental factors. Endocrine factors that may influence bone mass during adolescence include insulin-like growth factor-1 (IGF-1), which stimulates systemic body growth; growth hormone, which promotes growth and cell reproduction and regeneration; gonadotropic hormones, which promote epiphyseal maturation; and sex hormones, which mediate calcium accretion, decrease bone resorption with estrogen, and increase bone thickness and periosteal bone formation with testosterone [6]. The gender differences in bone mass and geometry are noted during puberty. In general, boys have larger cortical cross-sectional areas and higher trabecular bone volume ratios, while girls have higher cortical density and less cortical porosity [7].
6.1.2 Bone Modeling and Remodeling
Bone is a metabolically active tissue undergoing modeling and remodeling which are highly coordinated by osteoblasts, osteoclasts, and osteocytes. Bone modeling is bone gain without previous bone resorption resulting in changes in size and shape of the bone. Bone remodeling is a structural replacement or repair to maintain bone integrity and strength in response to mechanical stimulation and damage. Bone remodeling takes place in basic multicellular unit (BMU) encased with a canopy of cells within an anatomical structure known as bone remodeling compartment (BRC) [8]. In normal bone remodeling, the amount of resorbed bone is often completely replaced by newly formed bone. Osteopenia and osteoporosis are the result of net bone loss, while in osteopetrosis, bone formation dominates.
Osteoblasts arise from the differentiation of mesenchymal stem cells. Runt-related transcription factor 2 (Runx2) is a critical transcription factor regulating osteogenic differentiation and encoding genes for the synthesis of bone matrix and mineralization [9]. Mature osteoblasts commit its function by laying down organic osteoid followed by mineralization and extracellular matrix formation. Along this process, some osteoblasts further differentiate into osteocytes or become lining cells or undergo apoptotic cell death. Osteoclasts are terminally differentiated myeloid cells from either adjacent bone marrow in trabecular bone or vasculature in cortical bone. The RANKL/RANK/NF-κB pathway is the main signaling pathway regulating osteoclasts differentiation. RANKL (receptor activator of NF-κB ligand) from osteoblasts binds to RANK expressing osteoclast precursor cells. Osteoprotegerin (OPG), released by osteoblasts, is a soluble decoy receptor for RANKL which plays as a physiological negative feedback regulation of bone resorption [10]. The RANKL/OPG ratio has been suggested to indicate the extent of osteoclasts differentiation and activation [11]. Osteocytes are the most abundant cell type (over 90%) in bone, and its death is suggested to initiate the formation of BRC wherein resorption and formation are activated [12]. Recent evidence suggests the modulatory roles of osteocytes in bone remodeling through the release of sclerostin, RANKL, and OPG. Sclerostin is a potent inhibitor of bone formation through the binding to low-density lipoprotein receptor-related protein 5/6 (LRP5/LRP6) and thus abolishing canonical Wnt/β-catenin signaling [13]. The Wnt signaling is the prime target for many bone anabolic drugs through the inhibition of Wnt antagonists such as sclerostin and dickkopf WNT signaling pathway inhibitor 1 (Dkk1).
6.1.3 Bone Mineralization
Bone is a bioceramic composite consisting of intimate organization of organic substances (collagenous proteins and non-collagenous proteins), minerals (mainly calcium phosphate of apatite structure), and water. The complex bone structure can be divided into seven hierarchical levels of organization [14] which is speculated to provide optimal strength and toughness [15]. Bone formation is composed of two main stages known as primary and secondary osteogenesis resulting in the formation of woven and lamellar bone, respectively [16]. The mineralization in woven bone is relatively rapid and unorganized and serves as a transient stage during endochondral ossification. The woven bone is remodeled into lamellar bone which in human is organized into osteons for the construction of cortical bone or cancellous bone. During lamellar bone formation, osteoblasts secrete collagen fibrils assembled in a highly organized, close-packed lamellar structure. The organization of crystals is directed along the collagen fibrils leading to intrafibrillar crystallization. Osteoblasts also secrete non-collagenous proteins, such as osteonectin, osteopontin, osteocalcin, and bone sialoprotein, which are enriched with acidic amino acids and promote mineral nucleation in collagen fibrils [17].
6.2 Abnormal Skeletal Growth and Maturation in AIS
AIS occurs in children during their pubertal growth spurt. Rapid growth is associated with the development and progression of scoliotic curves, with the curves stabilized at skeletal maturity [18]. These observations have led researchers to investigate growth and growth-related endocrine factors and their possible contribution to the etiopathogenesis of AIS.
6.2.1 Body Height and Proportion
Patients with AIS were found to be taller and leaner [19, 20]. Some studies reported that the tall stature returns to normal by skeletal maturity [21], while others have shown it to persist into adulthood [22]. Cheung et al. reported that girls with AIS were shorter before menarche but caught up and became taller, with higher sitting height and longer arm span and leg length during growth spurt when compared with control subjects [20]. To calculate the height loss due to the spinal deformity, different equations have been suggested, and it appeared that no formula can fit every curve of different severities [23]. In another study, linear correlation between arm span and standing height in healthy children and adolescents was found to be very high (r 2 = 0.99) [24]. Therefore, the use of arm span as a surrogate for body height might be a better solution in the estimation of height loss due to the scoliosis.
In addition to abnormal lengths, asymmetries in the limb length and segmental length were also reported [25, 26]. While the asymmetry in arm length was not found in patients with lumbar curves, asymmetry in iliac height and leg length inequality were reported instead [27, 28]. Researchers have interpreted these findings to be (a) secondary to the scoliosis curve, (b) nonspecific manifestations of developmental instability due to natural left-right asymmetry in humans [29], and (c) sentinels in paired bones of vertebral growth plate asymmetries implying putative pathogenic significance [30].
6.2.2 Growth Pattern and Skeletal Maturity
In a large cross-sectional study with 598 AIS girls and 307 healthy control girls stratified by chronological age and pubertal stage, abnormal growth was observed in scoliotic girls from ages 12 to 15 years or older and in all pubertal stages [20]. At prepubertal spurt, AIS girls were significantly shorter and leaner when compared with maturity-matched normal controls. After the onset of puberty, corrected height and sitting height were significantly greater in scoliotic girls than in controls. Segmental lengths, namely, arm span and leg length, were also significantly longer in AIS girls. These observations indicated that the abnormal development in the pattern of growth and anthropometric parameters coincided with the onset and progress of pubertal development during adolescence.
The age at onset of menarche is a widely used maturity indicator reflecting the growth potential in girls and is closely related to curve progression. Previous studies reported that risk of curve progression was markedly higher before the onset of menarche than after menarche has already started [31]. In addition, the age at onset of menarche was also reported to be associated with the incidence of AIS [32]. The age at the onset of menarche has been reported to be earlier, normal, and delayed in AIS girls [21, 33,34,35]. A large cross-sectional study conducted by Mao et al. reported delayed onset of menarche occurred more frequently in AIS girls with Chinese ethnicity [34], while Grivas et al. and Goldberg et al. reported no difference and earlier age at onset of menarche in Mediterranean and Irish girls with AIS, respectively [21, 35].
6.2.3 Body Composition
Apart from abnormal skeletal growth, many‑ studies have also reported lower body weight and lower body mass index (BMI) in patients with AIS [20,21,22]. The differences in height, weight, and BMI were found to be correlated with the curve severity [19]. The earlier studies on the body composition of AIS patients reported conflicting findings and were limited by their small sample sizes [36, 37]. Studies from different centers have all shown that the lower body weight and BMI in AIS girls were attributed to decrease in both body fat and fat-free mass [36,37,38]. The association of altered body composition with the occurrence of AIS was substantiated by a large population-based prospective cohort study which utilized the subjects recruited in the Avon Longitudinal Study of Parents and Children (ALSPAC) [39]. This important study have investigated the association between fat and lean mass at age 10 years as assessed by DXA, with the presence of scoliosis at age 15 years. 5299 children were included, of which 184 had developed scoliosis (Cobb angle ≥10°) at age 15. The study demonstrated that after adjustment for confounders, per SD decrease in lean mass at age 10 was associated with a 20% higher risk of scoliosis and per SD decrease in fat mass with a 13% higher risk.
Muscle mass was found to be closely and linearly correlated with bone mass particularly during growth and development [40]. In addition, muscle strength as assessed by grip strength was found to be strongly correlated with vBMD, cortical area, cortical thickness, and bone strength index assessed using pQCT [41,42,43]. Mechanical stimuli linked to body weight have been thought to underlie differences in bone mass [44]. The hypothesis that the bone adapts to mechanical forces was first postulated by Wolff [45]. Experiments have demonstrated that dynamic loads resulting from the use of the muscle could promote bone formation and that the response of the bone is governed by the amplitude and frequency of these stimuli which can greatly exceed the static gravitational loads resulting from body weight [44, 46]. In AIS, the reduced lean and fat mass might affect the mechanical stimuli and result in reduced bone formation and lower bone mass.
6.3 Abnormal Bone Mineral Density in AIS
The general goals of bone densitometry in adults are to identify patients at greatest risk of skeletal fragility fractures, to guide decisions regarding treatment, and to monitor responses to therapy. The bone health of an individual could be assessed quantitatively by the following approaches.
6.3.1 Bone Densitometry in Pediatric Patients
6.3.1.1 Dual-Energy X-Ray Absorptiometry (DXA)
DXA uses low-dose X-ray source with two different energy peaks. One energy peak is absorbed more by the soft tissue, while the other is absorbed more by the bone, and then the soft tissue component is subtracted to determine the BMD. DXA can be used to scan both central and peripheral skeletal sites. Due to its low radiation, high precision, and accessibility, DXA is widely used for BMD assessment nowadays and is regarded as the gold standard for clinical assessment of osteoporosis by World Health Organization, and for the evaluation and management of adult bone diseases [47]. Bone densitometry assessments have been recommended for children with recurrent fractures, bone pain, bone deformities, osteopenia on standard radiographs, or to monitor therapy [48]. Examples of whole-body less head and hip DXA scans of an AIS patient are shown in Fig. 6.1. The projectional nature of the areal BMD (aBMD) measurement by DXA was confounded by bone sizes and bone geometry [49]; when DXA is being used for the study of pediatric bone health, adjustments for the smaller size of children must be made, and caution must be taken as the bones of the children change markedly in size and shape as they grow, especially during puberty. Researchers have attempted to account for the effect of bone size on the DXA result and minimize the effect of growing skeleton on the BMD value using various methods of size adjustment [50]. On the other hand, because of its reproducibility and lack of areal density-related errors, the total body bone mineral content (BMC) is sometimes preferred for the assessment of bone mineral status by some researchers [51].
6.3.1.2 Quantitative Computed Tomography (QCT)
Although not as widely utilized as DXA, QCT allows separate measurements of cortical and trabecular bone compartments; provides volumetric, as opposed to areal BMD measurement, thus avoiding the problems associated with changes in bone size; and allows measurement of geometric and structural parameters which contribute to bone strength [52]. The term central QCT is used when the technique is being applied to the spine and proximal femur, while pQCT is the application of QCT to peripheral skeletal sites. High-resolution peripheral quantitative computed tomography (HR-pQCT) is also a pQCT method but has resolution high enough to allow the quantification of trabecular micro-architecture and cortical porosity [53, 54]. Central QCT can be used in children, however, it is largely limited to research use because of limited reference databases being available for the spine and femur and higher dose of ionizing radiation involved [52].
6.3.1.3 Peripheral Quantitative Computed Tomography (pQCT)
pQCT evaluates the bone geometry and true volumetric BMD (vBMD) for the cortical and trabecular bone compartments separately in peripheral sites such as radius and tibia. It is used more widely than central QCT in growing children and adolescents, largely because of the significantly lower dose of ionizing radiation, and availability and easy access of pQCT scanners in centers dedicated to research and clinical care of children with bone disorders [52, 53]. However, pQCT is not as widely available compared with DXA and is used primarily for research purposes only. It was noted that meaningful and effective use of pQCT for assessing BMD and overall bone health in all age groups will require better defined normative data derived with common measuring techniques, equipment, and analytical approaches.
6.3.1.4 High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT)
The resolution of HR-pQCT is high enough that when computer-based finite element analysis (FEA) is being used to assess bone strength, the structure can be represented directly by the elements in the model. FE models based on HR-pQCT images have been validated against micro-CT models [55] and mechanical testing [56]. It is important to recognize that HR-pQCT only assesses the distal radius and distal tibia typically, and there is a concern whether measurements at these sites could reflect the bone mineral status and bone strength at the hip and spine. The few studies that have examined the relationship between HR-pQCT measurements of the peripheral skeleton have shown a moderate correlation (r = 0.56–0.70) to the axial skeleton [57, 58].
6.3.2 Low Bone Mass (Osteopenia) in AIS
Special attention is required when performing DXA scan on patients with scoliosis, because the measured aBMD in the spine is likely to be affected by any deformity or axial rotation of the vertebrae, which commonly occurs in scoliosis subjects [59]. In addition, patients with scoliosis cannot be positioned with the spine straight on the DXA table, and in those with severe scoliosis, degenerative changes can lead to invalid spine measurement [60].
Many previous studies had reported the association between AIS and low BMD. Burner et al. was the first to report the relationship between osteoporosis and acquired back deformity in 1982 [61]. Healey and Lane reported a higher prevalence of scoliosis in biopsy-proven osteoporotic women (48%) [62]. Cook et al. noted that AIS subjects had significantly lower lumbar spine and proximal femur BMD when compared with age-matched control subjects [63]. Cheng et al. investigated a large cohort of AIS and reported 36–38% of cases had generalized osteopenia (Z-score < −1) [59]. In a cross-sectional study on 919 girls with AIS, Lee et al. reported an inverse relationship between curve severity and BMD [64]. Lower vBMD has also been reported in AIS patients with pQCT study [65]. In general, the average BMD value of AIS girls was 4.5% lower than the age- and sex-matched controls [59, 66]. Previous studies have reported that osteopenia in AIS girls is systemic in nature and could affect the whole body including the spine, hip, distal radius, distal tibia, and calcaneus [22, 59, 63, 67]. This systemic low bone mass is also likely to associate with lower bone strength and peak bone mass [63], which might contribute to osteoporosis, osteoporotic fracture, progression of scoliosis, and other associated complications in late adulthood.
6.3.3 Is Osteopenia a Transient or Persistent Problem?
A few researchers have continued further and questioned whether this osteopenic status could persist into skeletal maturity, thus affecting the acquisition of peak bone mass. Thomas et al. have conducted a follow-up study of the BMD of 22 AIS girls with an average age of 11.5 years for an average follow-up period of 30.8 months [68]. Compared to the initial scans, at the follow-up evaluation, the prevalence of BMD below two SDs in the AIS group increased from 38% to 60% for the BMD measured from different standard sites. Cheng et al. have conducted a longitudinal follow-up study on the aBMD of bilateral proximal femurs in 14 AIS girls with significant osteopenia with more than two SDs below the mean normal value and 70 healthy control subjects using DXA for an average follow-up period of 29 months [66]. The study has found that the follow-up aBMD decreased from the initial evaluation of −2.96 to −3.84 SD in the follow-up visit. The same group of researchers have subsequently conducted a larger longitudinal 2-year follow-up study on the aBMD of proximal neck of femur and lumbar spinal BMC using DXA, and the vBMD of distal tibia using pQCT in 196 AIS girls and 122 healthy girls aged 12–15 years [33]. The vBMD of distal tibia in AIS group was persistently and significantly lower than the controls from 13 to 16 years (Fig. 6.2). Similarly, lumbar spinal BMC and femoral neck aBMD were persistently and significantly lower among AIS (moderate and severe severity) than the controls from age 13 to 17 years. This study indicated that both axial and peripheral BMD of AIS were persistently lower than the healthy girls throughout age 12–17 years.
Mean volumetric BMD of distal tibia of AIS (moderate and severe curve severity) and controls by age. *p < 0.05 comparison between AIS and control by t-test; ^p < 0.05 comparison among AIS moderate, severe, and control by one-way ANOVA; post hoc Bonferroni multiple comparison: a p < 0.05 (control vs. moderate), b p < 0.05 (control vs. severe), c p < 0.05 (moderate vs. severe) [33]
Could the lower rate of increase of BMD in the AIS group a result of bracing during the follow-up period? Snyder et al. have conducted a follow-up study on 52 AIS girls with brace treatment and found the annual rate of bone density accumulation was similar to the reported normal values [69]. Summarizing the evidence, the osteopenic condition found in AIS girls appeared to be a persistent rather than a transient phenomenon. It is likely that the persistent osteopenia in AIS girls could lead to significantly lower peak bone mass and manifest with complications of osteoporosis in adult life.
6.3.4 Clinical Relevance: BMD as a Prognostic Factor of Curve Progression in AIS
One recurrent important clinical question in the management of AIS is whether one can predict and prognosticate which curves will deteriorate so that appropriate and timely treatment can be started [18]. The widely accepted prognostic factors include chronologic age, menarchal status, Risser sign, degree of curvatures at presentation, and the curve pattern [18, 70]. The widely reported association between osteopenia and AIS has led researchers to investigate the prognostic value of BMD and bone quality in the prediction of curve progression in girls with AIS.
Hung et al. followed a cohort of 324 AIS girls until skeletal maturity or until the curve had progressed ≥6°. Osteopenia with a Z-score ≤ −1 in the femoral neck of the hip on the side of the concavity was identified as a significant prognostic factor for curve progression with an adjusted odds ratio of 2.3 [71]. A predictive model was established in the study, and the area under the receiver operating characteristic (ROC) curve of the model was 0.80. Subsequently, Lam et al. have conducted a prospective cohort study with 294 AIS girls being followed beyond skeletal maturity in order to investigate the use of quantitative ultrasound in predicting curve progression. The study has reported stiffness index (SI) was a significant and independent prognostic factor for curve progression with an odds ratio of 2.0 after adjustment for age, puberty, and curve severity [72]. The area under the ROC curve was reported to be 0.831. Recently, the same group has conducted a longitudinal cohort study of 513 newly diagnosed AIS girls to validate the prognostic value of osteopenia on the risk of curve progression to surgical threshold defined as Cobb angle ≥45° and/or undergone surgery. The results showed that osteopenic patients had significantly higher risk of surgery with a hazard ratio of 2.25 after adjustment for confounders [73]. Despite the fact that osteopenia represents a promising new prognostic factor for curve progression in clinical management, its use has not been popularized, probably due to the lack of multicenters validation study, availability and cost of DXA, and other bone densitometry machines in scoliosis clinics.
6.3.5 Factors Contributing to Low BMD in AIS
6.3.5.1 Environmental Lifestyle: Physical Activities
The importance of physical activity and mechanical loading on bone mass accrual has been well documented, particularly in children and adolescents. Slemenda et al. found that the weight bearing activity level was positively correlated to BMD of the radius and hip in children and adolescents between 5 and 14 years old [74]. Besides, Rubin et al. found a positive correlation between physical activity and lumbar spine BMD [75]. Lee et al. in a large-scale cross-sectional study have found lower physical activity level in AIS during the pubertal period, which was also significantly correlated with both the aBMD and vBMD at various sites as measured by DXA and pQCT, respectively [76]. Yu et al. studied 214 AIS girls and 187 healthy girls aged 11–13 years old and have also found lower physical activity level in the AIS group [77]. Physical activity level was found to be positively and independently associated with greater cortical area and total vBMD with multivariate analysis.
6.3.5.2 Calcium and Vitamin D
Calcium is required for normal growth and development as well as maintenance of the skeleton. During adolescence, there is nearly a doubling of body mineral stores due to increase in the size of the skeleton, with minor changes in volumetric BMD [78]. This increase in demand must be met through dietary intake for the optimum bone mineral accretion. Vitamin D is essential for intestinal calcium absorption and plays a central role in maintaining calcium homeostasis and skeletal integrity. Vitamin D insufficiency and deficiency are highly prevalent among children and adolescents worldwide, and calcium intake often falls below recommended levels [48].
It was suggested that the osteopenia in AIS could be a result of suboptimal bone mineralization both qualitatively and quantitatively, and thus fails to catch up with abnormally escalated bone growth during the peri-pubertal period. Lee et al. studied 596 AIS girls and 302 healthy control girls aged 11–16 years old and found that the mean calcium intake of both AIS and control groups have reached only 36% and 32% of the Chinese calcium Dietary Reference Intake (DRI) of 1000 mg/day, respectively [79], and there was no difference between the AIS and controls [76]. Yu et al. have reported slightly higher calcium intake in a case-control study of 214 AIS and 187 healthy girls aged 11–13 years old [77]. The median calcium intake was found to be 571.9 mg/day in the AIS group and 587.9 mg/day in the control group, with no difference between the two groups.
Despite the importance of vitamin D in bone mineralization, there was only one study reporting the dietary vitamin D intake in AIS patients and with small sample size. Akseer et al. have studied the daily dietary vitamin D intake including supplement in 15 women with AIS who had no treatment, 15 women with AIS who had brace treatment, and 19 healthy controls [80], and reported no differences between the three groups. Our recent longitudinal study revealed a significant improvement of BMD in AIS with supplementary calcium and vitamin D. The result is discussed further in Sect. 6. With the rising interest in the involvement of vitamin D deficiency in various diseases, researchers have also looked into the serum levels of 25-hydroxyvitamin D (25(OH)Vit-D, the main circulating form of Vit-D) in patients with AIS [81, 82]. A study from Poland by Gozdzialska et al. have compared the serum 25(OH)Vit-D levels in four groups of 50 girls aged 11–14. The groups were premenarchal and postmenarchal girls with AIS vs. matched controls. The study reported significantly lower serum 25(OH)Vit-D levels in both groups of AIS patients when compared with controls of matched menarchal status, with levels reaching the status of deficiency [81]. Balioglu et al. have compared the serum 25(OH)Vit-D levels in 229 AIS patients aged 10–22 years old and 389 age-matched comparison group of athletes without scoliosis. The results indicated significantly lower serum 25(OH)Vit-D levels in AIS patients, and the vitamin D level was correlated negatively (R = −0.147) with Cobb angle, which suggested a possible role of vitamin D in the etiopathogenesis of AIS [82].
6.3.5.3 Genetics
Twin and family studies have consistently shown that peak bone mass, ultrasound properties of the bone, skeletal geometry, bone turnover, and fracture are heritable [83]. Candidate gene association studies have been widely used in investigating the genetic factors associated with variations in BMD and osteoporosis. About 150 candidate genes related to osteoporosis have currently been identified [84]. The most widely studied among these genes include type I collagen, vitamin D receptor, estrogen receptor, androgen receptor, aromatase, LRP5 and LRP6, sclerostin, transforming growth factor β1, interleukin-6, and insulin growth factor-1. These factors act by inhibiting osteoblast activation and/or increasing osteoclast function, leading to osteoporosis. However, findings from a large consortium of five population-based studies involving 19,195 participants suggested that most of the previously studied candidate genes were not replicated in a well-powered study with standardized phenotyping and genotyping [85, 86]. Subsequent genome-wide association studies (GWASs) have identified 62 loci that are genome-wide significant for BMD at either the lumbar spine or the femoral neck [86].
6.4 Abnormal Bone Quality and Bone Strength in AIS
The critical role of bone quality in determination of bone strength has been well recognized, and bone quality has been taken as one of the key elements in the clinical definition of osteoporosis. Since then a large number of evidence have been published, supporting the theory that bone quality is of paramount importance for bone strength. Previous investigations with detailed bone geometry and micro-architecture parameters had enhanced predictive power for osteoporotic fracture and could better explain the mechanism underlying fragility fractures [87]. Bone geometry such as cortical thickness has been established as crucial factors for determining bone strength [88]. Recent studies have also suggested the importance of trabecular bone micro-architecture in the determination of bone quality and bone strength [87, 89]. In 2001, the NIH reinforced the theory that bone strength reflects both bone quality and BMD by defining osteoporosis as “A skeletal disorder characterized by compromised bone strength predisposing to an increased rate of fracture. Bone strength reflects the integration of two main features: bone density and bone quality” [90].
6.4.1 Studies on Bone Quality in AIS
At the time of writing, there were only a few studies which have reported the bone quality of AIS patients. It was speculated that the low bone mass and deranged bone quality could lead to mechanically weakened spinal column in the osteopenic patients, who might be more susceptible to the development of scoliosis and curve progression during the rapid growth in peri-pubertal period.
6.4.1.1 Bone Geometry
Yu et al. performed HR-pQCT assessment in the non-dominant distal radius of 214 newly diagnosed AIS girls between the age of 11 and 13 and 187 healthy age- and gender-matched controls [77]. The trabecular area, cortical area, cortical perimeter, and mean cortical thickness were measured. AIS girls were found to have lower cortical area (% difference = −8.88%) and cortical thickness (% difference = −8.53%) (Table 6.1). Cortical area remained significantly lower after adjustment for age, but not after adjustment for age, calcium intake, and physical activity level (Table 6.2).
6.4.1.2 Abnormal Bone Mineralization
Yu et al. have also assessed the total vBMD, cortical bone vBMD, and trabecular bone vBMD in their HR-pQCT study of 214 AIS girls and 187 healthy age- and gender-matched controls [77]. Total vBMD and cortical bone vBMD were significantly lower in the AIS group with mean difference of −3.97% and −2.65%, respectively (Table 6.1). No significant difference was found in the trabecular vBMD between AIS and control groups. Multivariate regression analysis indicated that AIS was associated with lower total vBMD and cortical bone vBMD after adjustment for age; cortical bone vBMD remained significantly lower after adjustment for age, calcium intake, and physical activity level (Table 6.2).
Due to the lack of good animal model that could mimic the 3D pathoanatomy of the spinal deformity in AIS, the mechanisms underlying abnormal bone mineralization remain unclear. A recent study by Wang et al. reported for the first time a comprehensive comparative study using SEM/EDX on iliac crest bone tissues collected from AIS and non-AIS controls undergoing bony fusion surgery [91]. Scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM/EDX) is a sensitive tool to detect small difference in calcium content which is not distinguishable with conventional DXA and micro-CT. Previous studies suggested that the amount of carbon could be regarded as organic components and the ratio of calcium to carbon (R Ca/C) was proportional to the BMD [92], while the ratio of carbon to phosphorus (R Ca/P) could reflect the status of bone mineralization [92, 93]. The lower R Ca/C in AIS suggested decreased mineralization in AIS which was in agreement with the increased osteoid volume and width in the same study (Table 6.3).
6.4.1.3 Bone Micro-architecture
Quantitative ultrasound (QUS) offers a radiation-free and portable modality of investigation and can provide indirect assessment on material properties and micro-architecture of bone. Using QUS, Lam et al. reported significantly lower broadband ultrasound attenuation (BUA) and stiffness index (SI) at the non-dominant calcaneus of 635 AIS girls when compared to 269 controls [67], suggesting possibility of altered bone micro-architecture in AIS. The latest HR-pQCT can offer noninvasive measurement of trabecular bone micro-architecture, without being confounded by bone size. Using this technique, Yu et al. assessed the trabecular bone micro-architecture in the non-dominant distal radius of 214 young AIS girls between the age of 11 and 13 and 187 healthy age- and gender-matched controls and have found lower trabecular number and higher trabecular separation in the AIS group after adjustment for age, calcium intake, and physical activity level (Table 6.2) [77]. In another study by Yu et al., where the bone qualities of osteopenic AIS girls, non-osteopenic AIS girls, osteopenic controls, and non-osteopenic controls were assessed with HR-pQCT, the osteopenic AIS girls had additional abnormal trabecular vBMD and micro-architecture that were not found in the osteopenic control girls [94]. This finding suggested the osteopenia is different between osteopenic AIS and osteopenic control girls, and that predominant changes of osteopenia in AIS girls occurred in the trabecular bone compartment (Fig. 6.3).
The measurements with HR-pQCT on distal radius dominated by cortical bone might underestimate particularly the changes in trabecular bone compartment. The micro-structure of plate and rod trabeculae is also critical in determining the bone strength and is associated with changes in BMD, which can be delineated with individual trabeculae segmentation (ITS) [95, 96]. Our current study on iliac crest bone biopsies with micro-CT40 and ITS analysis showed that AIS had significantly lower rod thickness (rTbTh) and number (rTbN) when compared with controls. Subsequent FEA also revealed significantly lower bone strength than controls (Table 6.4, [91]).
6.5 Abnormal Bone Turnover and Hormonal Changes
6.5.1 Bone Turnover Markers
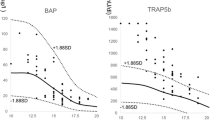
A previous study has shown serum bone-specific alkaline phosphatase (bALP) in AIS was 38.6% higher than that of controls [97]. Additionally, Suh et al. [98] reported higher serum-soluble RANKL levels and RANKL-to-OPG ratios in AIS. A recent study by Ishida et al. assessed the BMD and TRAP5b in 49 AIS girls aged 10–19. Sixty percent of AIS girls had osteopenia or osteoporosis at the femoral site, and 59% of AIS girls had high values of TRAP5b with >1.88 SDs. The AIS girls with high values of TRAP5b had lower Z scores, younger age, and lower BMI than those with normal values of TRAP5b. TRAP5b was also found to be positively correlated with Cobb angle. Apart from serological study, our recent study on iliac crest bone biopsies showed higher expression level of genes regulating bone formation markers, including collagen type I (COL1), osteocalcin (BGLAP) and osteopontin (SPP1), and bone resorption markers, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K (CTSK), in AIS (Fig. 6.4). Taking these together, concurrent abnormal bone turnover in AIS might play a role in the etiopathogenesis of AIS.
6.5.2 Abnormal Hormonal Changes and Abnormal Responses in Primary Culture
6.5.2.1 Melatonin
Melatonin is a secretory hormone mainly synthesized by pinealocytes in the pineal gland [99]. Melatonin plays an essential role in the physical health and skeletal development of the human body. Several animal studies shown that pinealectomy in chickens [100] and bipedal rats [101] lead to scoliosis, and this has led to the postulation that disturbance of melatonin production can be a potential cause of AIS [102]. In cellular models, Moreau et al. reported impaired melatonin signaling transduction in osteoblasts, myoblasts, and lymphocytes of progressive AIS and have linked the findings to the inactivation of Gi proteins [103, 104]. Man et al. reported a lack of response to the melatonin’s stimulating effects on proliferation and differentiation in osteoblast cultures prepared from bone biopsies obtained intraoperatively during spine surgeries from girls with AIS [105], which could be partly explained by a low melatonin receptor 1B expression [106, 107]. Melatonin receptor 1B gene polymorphism was also reported to be associated with AIS [108]. Yim et al. have further linked the abnormal skeletal growth in AIS girls to abnormal quantitative expression of melatonin receptor 1B [107]. However, it remains unclear whether melatonin has a definite role in the etiopathogenesis of AIS.
6.5.2.2 Leptin
Leptin is a relatively new topic in AIS research, the first study related to leptin in AIS by Qiu et al. showed that the circulating leptin levels were significantly lower in AIS girls, even after adjustment for age and menstrual status [109]. Liu et al. in a study of 95 AIS girls and 46 healthy matched controls aged 11–16 years found abnormal leptin bioavailability with increased levels of soluble leptin receptor (sOB-R) and lower free leptin index (FLI) in AIS girls after adjusting for age and body weight [110]. Significant correlations were also found between sOB-R, FLI, and curve severity in AIS girls. Subsequently, Tam et al. showed that this abnormal leptin bioavailability was also associated with deranged bone quality and lower muscle and fat mass in AIS girls [38, 111]. Burwell et al. formulated an etiologic theory of autonomic nervous system and leptin-sympathetic nervous system (SNS) concept for AIS and hypothesized an altered sensitivity to leptin in the hypothalamus of AIS girls, resulted in increased SNS activity, which contributed to skeletal overgrowth, generalized osteopenia, lower BMI, asymmetric spinal growth, and other phenotypes of AIS [112].
6.5.2.3 Estrogen and Its Receptor
Several studies have suggested that estrogen and/or estrogen receptors could play an important role in the pathogenesis and progression of AIS [113]. This hypothesis is reasonable and appealing as it attempts to explain the increased occurrence of scoliosis in girls and also the manifestation of osteopenia in scoliotic individuals. Studies on the circulating estrogen levels in AIS girls have reported conflicting findings. In in vitro study involving human osteoblasts isolated from AIS and control patients, Letellier et al. have shown that the increased cAMP levels induced by melatonin can be corrected by the treatment of the cells with 17-β-estradiol, which suggested an interaction between 17-β-estradiol and the already defective melatonin signaling pathway in human AIS osteoblasts. It was also suggested that the melatonin receptor MT2, which is normally physiologically coupled with the Gi protein, could switch to the Gs protein in the osteoblasts of a specific group of AIS patients when their cells are exposed to 17-β-estradiol [104].
6.6 Potential Clinical Interventions
When managing patients with idiopathic scoliosis, apart from focusing on curve control, therapeutic measures for achieving good bone health should be considered. Despite the lack of high level evidence in favor of any particular regimen for treating low bone mass in AIS, the general principles of physical stimulation and nutritional measures for enhancing positive balance in bone metabolism should be followed. Physical stimulation refers to weight bearing exercise and, more recently, vibration treatment; and nutritional measures include vitamin D and calcium supplementation.
6.6.1 Whole-Body Vibration Therapy
While weight bearing exercise can be prescribed and recommended as an integral part of healthy lifestyles for good bone health, compliance with the advice and high variability in exercise characteristics can be an issue. On the other hand, a non-pharmacological and extracorporeal modality recently receiving attention is the low-magnitude high-frequency whole-body vibration therapy (WBV). Rubin et al. reported mechanical vibration at a magnitude of 0.3 g and a frequency of 30 Hz could lead to increased bone mineral content and improved bone quality when applied at the hind limb of sheep [114]. The effect of WBV in increasing aBMD of the femur [115, 116] and the spine [115, 116] without adverse outcomes [117] has been reported. Lee et al. reported low bone mass in AIS was related to inadequate weight bearing physical activity [76]. Given that mechanical loading is osteogenic especially for children [118], Lam et al. thus carried out a randomized controlled trial to evaluate whether WBV, as a form of mechanical loading simulating weight bearing physical activity [114], could improve low BMD and bone quality for osteopenia associated with AIS. AIS subjects between 15 and 25 years old and with BMD Z-score < −1 were recruited. The treatment group received low-magnitude (acceleration = 0.3 g) high-frequency (32–37 Hz) vibration therapy by standing on the WBV platform 20 min/day and 5 days/week for 12 months. The control group received observation alone. Bone parameters were measured at baseline and at the 12-month time-point using DXA at bilateral femoral necks and the lumbar spine (L2–L4), and HR-pQCT at bilateral distal tibiae and the non-dominant distal radius. Results showed that at the 12-month time-point, while there was no statistically significant difference on the changes in HR-pQCT parameters between the treatment and the control group, WBV was noted to have positive effects on increasing femoral neck aBMD at the convex leg but less obviously at the concave side [119]. To evaluate if there is other factor that modulates and enhances the therapeutic effect of WBV, a nested study was carried out to analyze the percentage changes in aBMD across the 1-year period for the treatment and control group through subgroup analysis according to 25-hydroxyvitamin D (25(OH)Vit-D) levels. Results indicated the presence of factor interaction between WBV and 25(OH)Vit-D on increment of aBMD with statistical significance noted at the concave side (p = 0.027). For the subgroup with 25(OH)Vit-D > 40 nmol/L, not only were the positive effects of WBV greater at both sides, the treatment effect was also more obviously seen at the concave side indicating interaction between mechanical loading and Vit-D on bone metabolism in alignment with results obtained from previous animal and in vitro studies.
6.6.2 Supplement Therapy
Apart from physical stimulation, vitamin D physiology in AIS deserves further attention. It has been reported that the prevalence of vitamin D insufficiency is higher in areas at high latitude [120]. This latitude dependence in prevalence is also found in AIS [121]. Lam et al. have reported a case-control study showing that both the AIS and control group had mean 25(OH)Vit-D levels at the insufficient range. The findings did not suggest AIS was associated with lower 25(OH)Vit-D level when compared with controls. On the other hand, the positive correlation between 25(OH)Vit-D and aBMD that was seen in normal controls was not present in AIS subjects, spelling out the possibility of certain degree of abnormal physiology with vitamin D being present in AIS [122].
Apart from having synergistic effects with WBV on bone anabolism mentioned earlier, vitamin D itself may play an important role in optimizing bone status in AIS. As reported at the 2016 Scoliosis Research Society Annual Meeting, Lam et al. carried out the first randomized controlled trial evaluating the therapeutic effects of vitamin D and calcium supplementation on improving bone health and controlling curve progression in AIS. Three hundred and thirty AIS girls (mean age: 12.9 ± 0.9 years) with femoral neck aBMD Z-score < 0 and radiological Cobb angle ≥15° were randomized to Group 1 (N = 110, placebo), Group 2 (N = 110, 600 mg Ca + 400 IU Vit-D3/day), and Group 3 (N = 110, 600 mg Ca + 800 IU Vit-D3/day). At baseline and at end of 2-year treatment, serum 25(OH)Vit-D, DXA of femoral necks, and HR-pQCT at distal radius were performed. Curve progression was defined as increase in Cobb angle ≥6°. Results showed that 270 (81.8%) subjects completed the 2-year treatment (91 in Group 1 and 2, 88 in Group 3). At baseline, mean serum 25(OH)Vit-D was 41.2 ± 14.7 nmol/L, and mean Cobb angle was 25.9 ± 8.4°. Mean increase in serum 25(OH)Vit-D at 2-year was 6.3 ± 15.3, 20.4 ± 19.6, and 28.0 ± 23.3 nmol/L for Group 1, 2, and 3, respectively (p < 0.001). Changes in aBMD, average and trabecular vBMD, and trabecular HR-pQCT parameters at the end of 2-year treatment showed improvement in bone health with Ca + Vit-D supplement (p < 0.05). At the latest follow-up for (1) those with initial Cobb angle ≤40° and (2) those who were not on brace treatment or never been braced (N = 132), 21.7% in Group 3 and 24.4% in Group 2 had curve progression as compared with 46.7% in Group 1 (p < 0.05). For those with baseline serum 25(OH)Vit-D ≤ 50 nmol/L (N = 103), 16.2% had curve progression in Group 3 as compared with 48.6% in Group 1 (p = 0.003). The study provided evidences that daily 600 mg Ca + 400/800 IU Vit-D3 can improve low bone mass and prevent curve progression in AIS. Vit-D status and bone density and quality should be assessed for AIS subjects and be followed with Ca + Vit-D supplementation as appropriate. This paper was well received during the conference and was granted the Russell A. Hibbs Clinical Research Award (http://www.srs.org/about-srs/news-and-announcements/winners-of-the-russell-a-hibbs-john-h-moe-louis-a-goldstein-awards).
In brief and as far as low bone mass in AIS is concerned, for those with low BMD, WBV and vitamin D and calcium supplementation can be recommended. Further studies are warranted to confirm the roles of vitamin D and calcium supplementation and WBV, and their mutual synergistic effect in treating low bone mass in AIS; and to evaluate whether this anabolic bone effects can help to control curve progression through its positive effect on BMD [123].
6.7 Future Research Direction
We hypothesize that dysfunctional interaction of specific genetic and multiple environmental factors acting through different biochemical pathways could lead to abnormal regulation and modulation of systemic bone metabolism and growth, which are phenotypically manifested as abnormal mineralization and structure affecting bone strength and contributing to the initiation/progression of AIS (Fig. 6.5). More in-depth studies in collaboration with multidisciplinary experts will help to understand the underlying mechanisms better.
6.7.1 Osteocytes: The Missing Link in AIS Bone Metabolism?
Osteocytes residing in mineralized matrix canals are interconnected with neighboring cells via multiple dendritic processes, resulting in a lacuno-canalicular network (LCN) [124]. Osteocytes regulate bone phosphate metabolism [125], and their viability is closely associated with bone quality [126]. Change in connectivity of the LCN is reported in diverse skeletal disorders such as osteoporosis, osteoarthritis, osteomalacia, and osteogenesis imperfecta [127, 128]. We have previously reported decreased osteocytes number in AIS trabecular bone [129] and have recently identified abnormal ultrastructure of osteocytes and LCN in severe AIS with SEM and FITC-Imaris technique [130]. Abnormal clusters of roundish irregular shape osteocytes with short and disorganized canaliculae were found in the AIS bone biopsies in contrast to the well-organized LCN and osteocytes with clearly spotted spindle-cell body and longer canaliculi protruded perpendicularly from the cell bodies in normal controls (Fig. 6.6). Abnormal morphology and function of the osteocyte and LCN could be a sign of impaired osteocyte functions. Recent evidence indicates that serum sclerostin level was correlated positively with BMD and micro-architecture [131]. It is of clinical interest to investigate if such change in serum sclerostin level is associated with curve severity and abnormal BMD in AIS, and could serve as a potential quantifiable prognostic factor for curve progression.
Representative acid etched SEM images (2000×) of osteocytes in non-AIS control (a) and AIS (b) iliac crest bone biopsies. Canaliculi are indicated by red arrows. Confocal images (600×) of LCN (green) and osteocytes (presented as lacunar volume in yellow) in non-AIS control (c) and AIS (d) biopsies stained with FITC. Canalicular length and lacunar volume analyzed with Imaris are presented as mean ± SD *p < 0.05 compared with control (e and f, N = 5)
6.7.2 Time-Lapse Monitoring of Bone Remodeling in AIS Patients
The impact of bone remodeling in the etiopathogenesis in AIS remains a debatable issue because HR-pQCT scan and other measurements like serological biomarkers and primary osteoblast culture studies are not able to delineate bone formation and resorption at bone sites directly to unveil how the remodeling is affected in AIS. Conventional HR-pQCT parameters only depict the overview of bone quality from three different aspects, including geometry, vBMD, and micro-architecture, which are the consequences of many complicated cellular events. Recently, a new approach in image analysis emerges as a feasible solution for time-lapse monitoring of bone remodeling in human beings [132]. In vivo bone remodeling site quantification is done with a series of HR-pQCT images registered to corresponding baseline images using rigid 3D registration. Bone formation and resorption are determined using image processing language (IPL, Scanco) after density-based characterization and noise removal (Fig. 6.7). With this, we started a preliminary study using previous data on the effect of whole-body vibration on BMD in osteopenic AIS girls [119]. Although we did not find significant difference in global bone remodeling between the five treated and three nontreated subjects, it was noted that there were more endocortical and periosteal apposition and resorption in treatment group, which might increase mechanical integrity without affecting global bone density or microstructural parameters. We admitted that this result should be interpreted with caution because of small sample size, but this advanced approach provides new research direction to elucidate how bone remodeling is affected in AIS.
References
Riggs BL, Khosla S, Melton LJ III. The assembly of the adult skeleton during growth and maturation: implications for senile osteoporosis. J Clin Invest. 1999;104(6):671–2.
Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75(4):1060–5.
Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J Bone Joint Surg Am. 1989;71(8):1225–31.
Wang Q, Alen M, Nicholson P, Lyytikainen A, Suuriniemi M, Helkala E, et al. Growth patterns at distal radius and tibial shaft in pubertal girls: a 2-year longitudinal study. J Bone Miner Res. 2005;20(6):954–61.
Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25(7):1521–6.
Weaver CM. Adolescence: the period of dramatic bone growth. Endocrine. 2002;17(1):43–8.
Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012;27(2):273–82.
Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16(9):1575–82.
Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–9.
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–46.
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15(1):2–12.
Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25–34.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7.
Weiner S, Wagner HD. The material bone: structure mechanical function relations. Annu Rev Mater Sci. 1998;28:271–98.
Zhang Z, Zhang YW, Gao H. On optimal hierarchy of load-bearing biological materials. Proc Biol Sci. 2011;278(1705):519–25.
Olszta MJ, Cheng XG, Jee SS, Kumar R, Kim YY, Kaufman MJ, et al. Bone structure and formation: a new perspective. Mat Sci Eng R. 2007;58(3–5):77–116.
Addadi L, Weiner S. Interactions between acidic proteins and crystals: stereochemical requirements in biomineralization. Proc Natl Acad Sci U S A. 1985;82(12):4110–4.
Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Ser A. 1984;66(7):1061–71.
Shohat M, Shohat T, Nitzan M, Mimouni M, Kedem R, Danon YL. Growth and ethnicity in scoliosis. Acta Orthop Scand. 1988;59(3):310–3.
Cheung CSK, Lee WTK, Tse YK, Tang SP, Lee KM, Guo X, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine. 2003;28(18):2152–7.
Goldberg CJ, Dowling FE, Fogarty EE. Adolescent idiopathic scoliosis – early menarche, normal growth. Spine. 1993;18(5):529–35.
Cheung CSK, Lee WTK, Tse YK, Guo X, Qin L, Cheng JCY. Generalized osteopenia in adolescent idiopathic scoliosis – association with abnormal pubertal growth, bone turnover, and calcium intake? Spine. 2006;31(3):330–8.
Ylikoski M. Height of girls with adolescent idiopathic scoliosis. Eur Spine J. 2003;12(3):288–91.
Cheng JCY, Leung SSF, Lau J. Anthropometric measurements and body proportions among Chinese children. Clin Orthop Relat Res. 1996;323:22–30.
Burwell RG, Freeman BJ, Dangerfield PH, Aujla RK, Cole AA, Kirby AS, et al. Left-right upper arm length asymmetry associated with apical vertebral rotation in subjects with thoracic scoliosis: anomaly of bilateral symmetry affecting vertebral, costal and upper arm physes? Stud Health Technol Inform. 2006;123:66–71.
Burwell RG, Aujla RK, Grevitt MP, Randell TL, Dangerfield PH, Cole AA, et al. Upper arm length model suggests transient bilateral asymmetry is associated with right thoracic adolescent idiopathic scoliosis (RT-AIS) with implications for pathogenesis and estimation of linear skeletal overgrowth. Stud Health Technol Inform. 2012;176:188–94.
Burwell RG, Aujla RK, Freeman BJ, Dangerfield PH, Cole AA, Kirby AS, et al. Patterns of extra-spinal left-right skeletal asymmetries in adolescent girls with lower spine scoliosis: relative lengthening of the ilium on the curve concavity & of right lower limb segments. Stud Health Technol Inform. 2006;123:57–65.
Schwender JD, Denis F. Coronal plane imbalance in adolescent idiopathic scoliosis with left lumbar curves exceeding 40°: the role of the lumbosacral hemicurve. Spine. 2000;25(18):2358–63.
Goldberg CJ, Fogarty EE, Moore DP, Dowling FE. Scoliosis and developmental theory: adolescent idiopathic scoliosis. Spine. 1997;22(19):2228–38.
Burwell RG, Aujla RK, Grevitt MP, Dangerfield PH, Moulton A, Randell TL, et al. Pathogenesis of adolescent idiopathic scoliosis in girls – a double neuro-osseous theory involving disharmony between two nervous systems, somatic and autonomic expressed in the spine and trunk: possible dependency on sympathetic nervous system and hormones with implications for medical therapy. Scoliosis. 2009;4:24.
Weinstein SL. Natural history. Spine (Phila Pa 1976). 1999;24(24):2592–600.
Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. 2006;1(1):9.
Lee WTK, Cheung CSK, Tse YK, Chau WW, Qin L, Cheng JCY. Persistent osteopenia in adolescent idiopathic scoliosis (AIS) – factors predisposing to generalized osteopenia, a cross-sectional and longitudinal investigation. Int Congr Ser. 2007;1297:25–31.
Mao SH, Jiang J, Sun X, Zhao Q, Qian BP, Liu Z, et al. Timing of menarche in Chinese girls with and without adolescent idiopathic scoliosis: current results and review of the literature. Eur Spine J. 2011;20(2):260–5.
Grivas TB, Samelis P, Pappa AS, Stavlas P, Polyzois D. Menarche in scoliotic and nonscoliotic Mediterranean girls. Is there any relation between menarche and laterality of scoliotic curves? Stud Health Technol Inform. 2002;88:30–6.
Ramirez M, Martinez-Llorens J, Sanchez JF, Bago J, Molina A, Gea J, et al. Body composition in adolescent idiopathic scoliosis. Eur Spine J. 2013;22(2):324–9.
Barrios C, Cortes S, Perez-Encinas C, Escriva MD, Benet I, Burgos J, et al. Anthropometry and body composition profile of girls with nonsurgically treated adolescent idiopathic scoliosis. Spine. 2011;36(18):1470–7.
Tam EM, Liu Z, Lam TP, Ting T, Cheung G, Ng BK, et al. Lower muscle mass and body fat in adolescent idiopathic scoliosis are associated with abnormal leptin bioavailability. Spine (Phila Pa 1976). 2016;41(11):940–6.
Clark EM, Taylor HJ, Harding I, Hutchinson J, Nelson I, Deanfield JE, et al. Association between components of body composition and scoliosis: a prospective cohort study reporting differences identifiable before the onset of scoliosis. J Bone Miner Res. 2014;29(8):1729–36.
Witzke KA, Snow CM. Lean body mass and leg power best predict bone mineral density in adolescent girls. Med Sci Sports Exerc. 1999;31(11):1558–63.
Schonau E. The development of the skeletal system in children and the influence of muscular strength. Horm Res. 1998;49(1):27–31.
Kaji H, Kosaka R, Yamauchi M, Kuno K, Chihara K, Sugimoto T. Effects of age, grip strength and smoking on forearm volumetric bone mineral density and bone geometry by peripheral quantitative computed tomography: comparisons between female and male. Endocr J. 2005;52(6):659–66.
Hasegawa Y, Schneider P, Reiners C. Age, sex, and grip strength determine architectural bone parameters assessed by peripheral quantitative computed tomography (pQCT) at the human radius. J Biomech. 2001;34(4):497–503.
Faje A, Klibanski A. Body composition and skeletal health: too heavy? Too thin? Curr Osteoporos Rep. 2012;10(3):208–16.
Wolff J. The law of bone remodeling. New York, NY: Springer; 1986.
Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17(12):897–905.
World Health Organization W. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129.
Endocrinology TSo. Bone densitometry in children and adolescents. Pediatrics. 2011;127(1):189–94.
Bacchetta J, Boutroy S, Vilayphiou N, Ranchin B, Fouque-Aubert A, Basmaison O, et al. Bone assessment in children with chronic kidney disease: data from two new bone imaging techniques in a single-center pilot study. Pediatr Nephrol. 2011;26(4):587–95.
Fewtrell MS, Gordon I, Biassoni L, Cole TJ. Dual X-ray absorptiometry (DXA) of the lumbar spine in a clinical paediatric setting: does the method of size-adjustment matter? Bone. 2005;37(3):413–9.
Binkovitz LA, Henwood MJ. Pediatric DXA: technique and interpretation. Pediatr Radiol. 2007;37(1):21–31.
Adams JE. Quantitative computed tomography. Eur J Radiol. 2009;71(3):415–24.
Adams JE, Engelke K, Zemel BS, Ward KA. Quantitative computer tomography in children and adolescents: the 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17(2):258–74.
Nishiyama KK, Shane E. Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep. 2013;11(2):147–55.
Liu XS, Zhang XH, Sekhon KK, Adams MF, McMahon DJ, Bilezikian JP, et al. High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. 2010;25(4):746–56.
Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42(6):1203–13.
Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25(10):2229–38.
Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int. 2010;21(2):263–73.
Cheng JCY, Qin L, Cheung CSK, Sher AHL, Lee KM, Ng SWE, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15(8):1587–95.
El Maghraoui A, Roux C. DXA scanning in clinical practice. QJM. 2008;101(8):605–17.
Burner WL III, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop. 1982;2(4):383–5.
Healey JH, Lane JM. Structural scoliosis in osteoporotic women. Clin Orthop Relat Res. 1985;195:216–23.
Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, et al. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7(2):168–74.
Lee WTK, Cheung CSK, Tse YK, Guo X, Qin L, Lam TP, et al. Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int. 2005;16:1924–32.
Yeung HY, Qin L, Hung VW, Lee KM, Guo X, Ng BW, et al. Lower degree of mineralization found in cortical bone of adolescent idiopathic scoliosis (AIS). Stud Health Technol Inform. 2006;123:599–604.
Cheng JCY, Guo X, Sher AHL. Persistent osteopenia in adolescent idiopathic scoliosis – a longitudinal follow-up study. Spine. 1999;24(12):1218–22.
Lam TP, Hung VW, Yeung HY, Tse YK, Chu WC, Ng BK, et al. Abnormal bone quality in adolescent idiopathic scoliosis: a case-control study on 635 subjects and 269 normal controls with bone densitometry and quantitative ultrasound. Spine (Phila Pa 1976). 2011;36(15):1211–7.
Thomas KA, Cook SD, Skalley TC, Renshaw SV, Makuch RS, Gross M, et al. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow-up study. J Pediatr Orthop. 1992;12(2):235–40.
Snyder BD, Katz DA, Myers ER, Breitenbach MA, Emans JB. Bone density accumulation is not affected by brace treatment of idiopathic scoliosis in adolescent girls. J Pediatr Orthop. 2005;25(4):423–8.
Soucacos PN, Zacharis K, Soultanis K, Gelalis J, Xenakis T, Beris AE. Risk factors for idiopathic scoliosis: review of a 6-year prospective study. Orthopedics. 2000;23(8):833–8.
Hung VWY, Qin L, Cheung CSK, Lam TP, Ng BKW, Tse YK, et al. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg. 2005;87(12):2709–16.
Lam TP, Hung VW, Yeung HY, Chu WC, Ng BK, Lee KM, et al. Quantitative ultrasound for predicting curve progression in adolescent idiopathic scoliosis: a prospective cohort study of 294 cases followed-up beyond skeletal maturity. Ultrasound Med Biol. 2013;39(3):381–7.
Yip BH, Yu FW, Wang Z, Hung VW, Lam TP, Ng BK, et al. Prognostic value of bone mineral density on curve progression: a longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220.
Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC Jr. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6(11):1227–33.
Rubin K, Schirduan V, Gendreau P, Sarfarazi M, Mendola R, Dalsky G. Predictors of axial and peripheral bone mineral density in healthy children and adolescents, with special attention to the role of puberty. J Pediatr. 1993;123(6):863–70.
Lee WTK, Cheung CSK, Tse YK, Guo X, Qin L, Ho SC, et al. Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int. 2005;16:1024–35.
Yu WS, Chan KY, Yu FW, Ng BK, Lee KM, Qin L, et al. Bone structural and mechanical indices in adolescent idiopathic scoliosis evaluated by high-resolution peripheral quantitative computed tomography (HR-pQCT). Bone. 2014;61C:109–15.
Rizzoli R, Bonjour JP. Physiology of calcium and phosphate homeostases. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of bone and cartilage metabolism. London: Academic; 2006. p. 345–60.
Society CN. Chinese dietary reference intakes. Beijing: Chinese Light Industry Press; 2000.
Akseer N, Kish K, Rigby WA, Greenway M, Klentrou P, Wilson PM, et al. Does bracing affect bone health in women with adolescent idiopathic scoliosis? Scoliosis. 2015;10:5.
Gozdzialska A, Jaskiewicz J, Knapik-Czajka M, Drag J, Gawlik M, Ciesla M, et al. Association of calcium and phosphate balance, vitamin D, PTH, and calcitonin in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2016;41(8):693–7.
Balioglu MB, Aydin C, Kargin D, Albayrak A, Atici Y, Tas SK, et al. Vitamin-D measurement in patients with adolescent idiopathic scoliosis. J Pediatr Orthop B. 2017;26(1):48–52.
Andrew T, Macgregor AJ. Genes and osteoporosis. Curr Osteoporos Rep. 2004;2(3):79–89.
Sobieszczanska M, Jonkisz J, Tabin M, Laszki-Szczachor K. Osteoporosis: genetic determinants and relationship with cardiovascular disease. Adv Clin Exp Med. 2013;22(1):119–24.
Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151(8):528–37.
Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13(8):576–88.
Pothuaud L, Van Rietbergen B, Mosekilde L, Beuf O, Levitz P, Benhamou CL, et al. Combination of topological parameters and bone volume fraction better predicts the mechanical properties of trabecular bone. J Biomech. 2002;35(8):1091–9.
Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–8.
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22(3):425–33.
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95.
Wang Z, Chen H, Yu YE, Zhang J, Cheuk KY, Ng BK, et al. Unique local bone tissue characteristics in iliac crest bone biopsy from adolescent idiopathic scoliosis with severe spinal deformity. Sci Rep. 2017;7:40265.
Okata H, Nakamura M, Henmi A, Yamaguchi S, Mikami Y, Shimauchi H, et al. Calcification during bone healing in a standardised rat calvarial defect assessed by micro-CT and SEM-EDX. Oral Dis. 2015;21(1):74–82.
Tzaphlidou M, Speller R, Royle G, Griffiths J, Olivo A, Pani S, et al. High resolution Ca/P maps of bone architecture in 3D synchrotron radiation microtomographic images. Appl Rad Isotopes. 2005;62(4):569–75.
Yu W, Chan Ky YFWP, Hy Y, Ng BKW, Lee K, et al. Abnormal bone quality versus low bone mineral density in adolescent idiopathic scoliosis: a case-control study with in vivo high-resolution peripheral quantitative computed tomography. Spine J. 2013;13(11):1493–9.
Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23(2):223–35.
Liu XS, Stein EM, Zhou B, Zhang CA, Nickolas TL, Cohen A, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27(2):263–72.
Cheung CS, Lee WT, Tse YK, Lee KM, Guo X, Qin L, et al. Generalized osteopenia in adolescent idiopathic scoliosis—association with abnormal pubertal growth, bone turnover, and calcium intake? Spine (Phila Pa 1976). 2006;31(3):330–8.
Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor-kappaB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2007;16(10):1563–9.
Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336(3):186–95.
O'Kelly C, Wang X, Raso J, Moreau M, Mahood J, Zhao J, et al. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine. 1999;24(1):35–43.
Machida M, Saito M, Dubousset J, Yamada T, Kimura J, Shibasaki K. Pathological mechanism of idiopathic scoliosis: Experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14(9):843–8.
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine. 1993;18(12):1609–15.
Moreau A, Wang DS, Forget S, Azeddine B, Angeloni D, Fraschini F, et al. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine. 2004;29(16):1772–81.
Moreau A, Akoumé Ndong MY, Azeddine B, Franco A, Rompré PH, Roy-Gagnon MH, et al. Molecular and genetic aspects of idiopathic scoliosis: Blood test for idiopathic scoliosis. Orthopade. 2009;38(2):114–21.
Man G, Wang W, Yeung B, Lee S, Ng B, Hung W-Y, et al. Abnormal proliferation and differentiation of osteoblasts from girls with adolescent idiopathic scoliosis to melatonin. J Pineal Res. 2010;49:69–77.
Man GCW, Wong JH, Wang WWJ, Sun GQ, Yeung BHY, Ng TB, et al. Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J Pineal Res. 2011;50(4):395–402.
Yim AP, Yeung HY, Sun G, Lee KM, Ng TB, Lam TP, et al. Abnormal skeletal growth in adolescent idiopathic scoliosis is associated with abnormal quantitative expression of melatonin receptor, MT2. Int J Mol Sci. 2013;14(3):6345–58.
Qiu XS, Tang NLS, Yeung HY, Lee KM, Hung VWY, Ng BKW, et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine. 2007;32(16):1748–53.
Qiu Y, Sun X, Qiu X, Li W, Zhu Z, Zhu F, et al. Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32(24):2703–10.
Liu Z, Tam EM, Sun GQ, Lam TP, Zhu ZZ, Sun X, et al. Abnormal leptin bioavailability in adolescent idiopathic scoliosis: an important new finding. Spine (Phila Pa 1976). 2012;37(7):599–604.
Tam EM, Yu FW, Hung VW, Liu Z, Liu KL, Ng BK, et al. Are volumetric bone mineral density and bone micro-architecture associated with leptin and soluble leptin receptor levels in adolescent idiopathic scoliosis? A case-control study. PLoS One. 2014;9(2):e87939.
Burwell RG, Dangerfield PH, Moulton A, Anderson SI. Etiologic theories of idiopathic scoliosis: autonomic nervous system and the leptin-sympathetic nervous system concept for the pathogenesis of adolescent idiopathic scoliosis. Stud Health Technol Inform. 2008;140:197–207.
Leboeuf D, Letellier K, Alos N, Edery P, Moldovan F. Do estrogens impact adolescent idiopathic scoliosis? Trends Endocrinol Metab. 2009;20(4):147–52.
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–4.
Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21(9):1464–74.
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–51.
Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010;21(12):1969–80.
Kohrt WM. Aging and the osteogenic response to mechanical loading. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S137–42.
Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–36.
Lee WT, Jiang J. The resurgence of the importance of vitamin D in bone health. Asia Pac J Clin Nutr. 2008;17(Suppl 1):138–42.
Grivas TB, Vasiliadis E, Savvidou O, Mouzakis V, Koufopoulos G. Geographic latitude and prevalence of adolescent idiopathic scoliosis. Stud Health Technol Inform. 2006;123:84–9.
Lam TP, Yu WS, Mak WY, Cheung TF, Lee KM, BKW N, et al., editors. Vitamin D insufficiency and its association with low bone mass in girls with adolescent idiopathic scoliosis (AIS). 48th Scoliosis Research Society (SRS) annual meeting, Sep 2013. Lyon: Scoliosis Research Society (SRS); 2013.
Hung VWY, Qin L, Cheung CSK, Lam TP, Ng BKW, Tse YK, et al. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87:2709–16.
Schneider P, Meier M, Wepf R, Muller R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone. 2010;47(5):848–58.
Ruchon AF, Tenenhouse HS, Marcinkiewicz M, Siegfried G, Aubin JE, DesGroseillers L, et al. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15(8):1440–50.
Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. Expression of leptin and its functional receptor on disc cells: contribution to cell proliferation. Spine (Phila Pa 1976). 2008;33(23):E858–64.
Jones SJ, Glorieux FH, Travers R, Boyde A. The microscopic structure of bone in normal children and patients with osteogenesis imperfecta: a survey using backscattered electron imaging. Calcif Tissue Int. 1999;64(1):8–17.
Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;36(1):1–8.
Cheng JC, Tang SP, Guo X, Chan CW, Qin L. Osteopenia in adolescent idiopathic scoliosis: a histomorphometric study. Spine (Phila Pa 1976). 2001;26(3):E19–23.
Ren Y, Lin S, Jing Y, Dechow PC, Feng JQ. A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect Tissue Res. 2014;55(Suppl 1):129–33.
Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant. 2012;27(1):226–30.
Christen P, Ito K, Ellouz R, Boutroy S, Sornay-Rendu E, Chapurlat RD, et al. Bone remodelling in humans is load-driven but not lazy. Nat Commun. 2014;5:4855.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK
About this chapter
Cite this chapter
Cheng, J.C.Y., Lee, W.Y.W., Tam, E.M.S., Lam, T.P. (2018). Bone Metabolism in AIS. In: Machida, M., Weinstein, S., Dubousset, J. (eds) Pathogenesis of Idiopathic Scoliosis. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56541-3_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-56541-3_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56539-0
Online ISBN: 978-4-431-56541-3
eBook Packages: MedicineMedicine (R0)