Abstract
The pathological mechanism of curve progression in idiopathic scoliosis is still obscure. In this study we investigated the pathological mechanism of idiopathic scoliosis in experimentally induced scoliosis in rats. A total 30 rats were divided into three groups: ten bipedal rats with a sham operation, which served as the control; ten quadrupedal rats with pinealectomy; and ten bipedal rats with pinealectomy. Scoliosis developed only in pinealectomized bipedal rats and not in pinealectomized quadrupedal rats. Cervicothoracic lordosis developed in bipedal rats with or without pinealectomy. These deformities of lordoscoliosis in pinealectomized bipedal rats were similar to human idiopathic scoliosis. Lordosis or lordotic tendency was sufficient to cause the spine to rotate to the side. Rotational instability of the spine with rotation of lordotic segment appears to produce a characteristic scoliotic deformity as a secondary phenomenon. Our findings suggest that lordosis may develop in bipedal rats, but pinealectomy is required for the development of lordoscoliosis. Balanced muscle tone controlled by the postural reflex is important to maintain normal posture with a straight spine in the bipedal condition. The disturbance of equilibrium and other postural mechanisms secondary to a deficiency of melatonin after pinealectomy may promote development of lordoscoliosis with vertebral rotation especially in the bipedal posture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The etiology and pathogenesis of idiopathic scoliosis are still obscure. The development of a pathologic curvature may be attributed to multifactorial abnormalities in genetics [6, 10, 39], skeletal development [8], and muscular dysfunction [9, 14, 29], metabolic and chemical issues [13, 30], or the central nervous system [2, 4, 5, 7, 28, 31, 38, 41–43]. Experiments on various quadruped animal models have suggested possible anatomic or functional influences for the etiology of idiopathic scoliosis [2, 4, 5, 8, 15, 17, 32, 34], but many of them may be phenomena rather than be caused etiologically.
We have shown that pinealectomy in chickens consistently produces scoliosis with similar anatomic characteristics to those of human idiopathic scoliosis [18–22, 25]. Serum melatonin levels in the pinealectomized chickens at 10:00 p.m. in complete darkness, except for a brief 4-s exposure to 0.2 foot candle of light from a 7.5-watt red lamp, was statistically significantly less compared with controls; 5.0±2.7 pg/ml in pinealectomized chickens, 2839.0±1989.1 pg/ml in controls (P<0.01) [23]. In addition, intramuscular implantation of the pineal gland [18] and intraperitoneal injection of melatonin [19] prevented the development of scoliosis in pinealectomized chickens. These findings suggest that a deficiency of melatonin may contribute to the development of experimental scoliosis [18, 19].
From a previous series of experimental studies, we have proposed that a deficiency of melatonin may contribute to the etiology of experimental scoliosis in chickens [20] and rats [24, 25] and the bipedal condition may also be an important factor for its development. O’Kelly et al. [27] tried to produce scoliosis following pinealectomy in quadrupedal models (rats and hamsters), but significant spinal curvature was not observed. Simultaneously, we demonstrated that experimentally induced scoliosis in pinealectomized rats occurred only in bipedal, not quadrupedal rats. Our results suggested a critical influence of a postural mechanism in scoliosis development [24]. While the mechanism underlying the production of scoliosis following pinealectomy is still unknown, it remains to be seen whether the methodology produces the same result in rats. The results would provide information as to the underlying etiologic mechanisms and also shed light on its applicability to the human situation.
Materials and methods
Experimental groups
All experiments were conducted according to the institutional guidelines, with the protocol approved by the Committee on the Use of Live Animals Research, Nihon University School of Medicine. Thirty male Sprague-Dawley rats, weighing between 45 and 50 g, were obtained from the Laboratory Animal Unit, Nihon University, where they were housed in separate cages in an air-conditioned room with controlled lighting (lights off from 7:00 p.m. to 7:00 a.m.). While bipedal rats, after removing forelimbs and tail, were typically housed in special high cages with raised food and water, so that they can keep a standing posture most of the time; both food and water were gradually elevated in the cage as the bipedal rats were growing. The quadrupedal rats with tails were housed in standard cages, which actually discouraged bipedal posture. They were fed laboratory food and given free access to water. Quadrupedal rats had food and water at a constant 7.0 cm from the floor, throughout the study. The rats were divided into three groups: ten bipedal rats received a sham operation (group 1), pinealectomy was performed on ten quadrupedal rats (group 2) and pinealectomy was performed on ten bipedal rats (group 3). They were euthanased at 3 months after surgery.
General surgical procedure
During the daytime, the bipedal condition was achieved by resection of the forelimbs and the tail at 3 weeks of age and pinealectomy was performed at 3 weeks of age under general anesthesia using sodium pentobarbital intraperitoneal injections (40 mg per kilogram of body weight). Before 3 weeks of age, deaths in pinealectomized or bipedal rats were the result of mother’s cannibalism; therefore, the surgeries were carried out after the rats were weaned. The forelimbs were tied with cotton transfixation ligatures at the humeroscapular level and the tail at its base. The upper extremities below the shoulder and tail were then removed.
A head holder with ear bars and an adjustable tooth bar were used to immobilize the rats in a prone position during pinealectomy. Under the microscope, a sagittal incision was made from between the eyes to the base of the skull and the skin flaps were reflected by retractors. The underlying fascia and muscles over the bone were mobilized. The parietal and innerparietal bones were drilled with a dental burr to make an oval hole approximately 3.5×2.5 mm. After removal of the bone, the confluence of the superior sagittal and transverse sinuses could be visualized. A specially constructed needle (22 gauge) was inserted through the venous sinuses. Then the pineal gland was gently removed via suction. Bleeding was controlled using Gelfoam. The sham operation consisted of insertion and withdrawal of the needle without application of suction. At the termination of the experiment, microscopical visual inspection verified the complete removal of both the superficial part and the deep part of the pineal gland. The skin flaps were pulled together and sutured.
Measurement of scoliosis and lordosis or kyphosis
Three months after surgery, anteroposterior and lateral views of spinal radiographs were taken with the rats under ether anesthesia and any scoliosis and lordosis or kyphosis from the second cervical to seventh thoracic spine were measured using Cobb’s method and recorded. In taking the radiographs in the anteroposterior view, the rats were placed in a head-up position, with the head stabilized by the head holder and lying on a support inclined 60° from the horizontal plane of the table to prevent functional lateral scoliosis; this method has produced the most consistent and reproducible measurement. The lateral view of spinal radiographs in the rats was taken in the neutral position without traction or correction.
Data analysis
Data are presented as mean ± standard error of mean (SEM). Two-factor analysis of variance (ANOVA) was used to examine the individual effects and interaction between interventions. These statistical analyses were done using the Stat View II program run on a MacIntosh computer.
Results
The bipedal rats without tails were encouraged to stand and to walk in an upright manner, which they learned rapidly. Rats, nocturnal in their activities, were best observed in the twilight. During these hours, the bipedal rats spent nearly all of their time walking in an erect position. The absence of forelimbs and tails did not appear to impair their activities and even when resting, they squatted with chest and abdomen off the floor. Sometimes the bipedal rats rested against the wall during sleeping. Thus, they were compelled to bear full weight on their hind limbs and this is a clear difference in the function of the spinal muscles between bipedal and quadrupedal rats. Adaptations followed this demand and true bipedalism became a habit. The bipedal rats walked nearly erect, spreading their feet and toes apart. Their trunks were flexed slightly forward in the midthoracic region, but readily straightened when stretching for high-placed food or when they sniffed their surroundings. No difficulties in drinking, feeding and defending themselves were observed. On the contrary, the quadrupedal rats with their tail were able to stand with hindlimbs but could not walk with erect posture. Their tails interfered with walking with erect posture. The bipedal rats spent more time than quadrupedal rats in an upright position during feeding, walking, and sleeping.
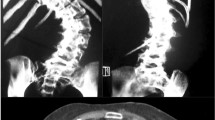
The weight of rats at 3 months after surgery averaged 270.5 g (range 230–350 g). We detected no significant differences in the mean weight of rats in any of the groups; 271.8±15.3 g for quadrupedal rats, 268.5±26.1 g for bipedal rats. This slight difference may be explained by the fact that the forelimbs amputation was done at 3 weeks old. Scoliosis did not develop in any of the bipedal rats that underwent the sham operation (Fig. 1), nor in any of the quadrupedal rats (Fig. 2). However, scoliosis developed in all of the pinealectomized bipedal rats (Fig. 3). There were two types of scoliosis, thoracic and thoracolumbar scoliosis, which were similar to those found in human idiopathic scoliosis, particularly the vertebral rotation and associated rib hump. The scoliosis curvature in bipedal rats after pinealectomy ranged from 25 to 47° (37.5±6.5° on average).
Cervicothoracic lordosis developed in all of the bipedal rats with (Fig. 3) or without pinealectomy (Fig. 1). The cervicothoracic lordosis measured between the second cervical to seventh thoracic spine in bipedal rats with pinealectomy was −51.8±15° (range −35 to −77°) and −45.9±6° (range −33 to −43°) without pinealectomy. In contrast, the lordosis in quadrupedal pinealectomized rats was −25±6.4° (range −18 to −35°). When the mean levels were analyzed by ANOVA, the mean levels differed significantly between bipedal rats and quadrupedal rats. Sagittal radiography revealed hyperlordosis at the cervicothoracic spine in bipedal rats, whereas the spines in the quadrupedal rats showed physiological cervicothoracic lordosis and thoracolumbar kyphosis (Table 1).
In the axial plane, spinous processes were deviated toward the concave side and vertebral bodies were rotated toward the convex side. These deformities of lordosis at the thoracic and thoracolumbar spine levels with vertebral rotation in rats were similar to human idiopathic scoliosis. The convexity of the curve was directed to either side with no consistent preference. No rat developed limb paralysis or showed difficulty in walking or running. Aside from the forelimb amputations, growth appeared to be otherwise normal.
Discussion
Despite the existence of numerous animal models of idiopathic scoliosis, its cause in humans remains obscure. Idiopathic scoliosis is a three-dimensional deformity of the spine combining lateral curvature with vertebral body rotation according to Adams [1]. The essential lesion, however, lies in the sagittal plane in the nature of the lordosis. Heuer and Sommerville [35] extended this idea and proposed that the lordosis resulted from overgrowth of the anterior vertebral column relative to the posterior elements. Roaf [29] describing the anatomy of the scoliotic deformity, emphasized that a lordotic region lies at the apex of the curvature.
We have found that pinealectomy in chickens consistently produces scoliosis, which has anatomic characteristics similar to that of human idiopathic scoliosis [18–20, 22]. The scoliosis produced has considerable deformation within the body and neural arch of a vertebra, most pronounced at the apex of the curve. The body was compressed from above downward on the concave side leading to a wedge-shaped deformity. The posterior elements of a scoliotic vertebra showed much more complicated deformations. On the concave side of the curve, these deformations were marked at the apex and the peri-apical segments: the pedicles and laminas were thick and wide and the spinous processes were deviated toward the concavity. On the convex side of the apex the pedicles were thin and the laminas and articular processes were small and highly compressed [21]. Here there was also clear evidence of asymmetric vertebral growth with anterior body height being greater than posteriorly. This anatomy supported a lordosis or lordotic tendency. Eventually the spinal deformity in pinealectomized chickens developed rotational lordoscoliosis, similar to human idiopathic scoliosis.
Bipedal rats were first produced by Goff [12] and followed by others [24, 30, 33, 40, 42, 43]. According to their reports the bipedal rats spent nearly all of their time walking in an erect position, spreading their feet and toes apart. Their bodies were flexed forward in the mid-thoracic region but readily straightened when they stretched for high-placed food or when they sniffed their surroundings, a characteristic behavior. They showed anatomical and behavioral changes in accordance with Wolff’s law. Goff and Landmesser [12] explained that this condition was produced by the bending of the proximal portion of the tibia and fibula and that the femur was proportionately longer in relation to the tibia. In our previous study, we found that the bipedal’s bones were larger and heavier than normal relative to animal weight. Regarding the effect of bipedalism, Smith and Saville [33] have reported that at comparable body weight bipedal rats have a denser femur with a greater breaking force than quadrupedal rats, and also that the weight of muscle associated with the femur is greater in bipedal rats. In this study there was no significant difference in body weight between bipedal and quadrupedal rats.
Recently, Bailey et al. [3] reported that bipedal rats with their tail do not assume a more erect posture and spend no more time in an upright position compared with quadrupedal rats. Their tails interfered with walking with erect posture. In their study bipedal rats were not raised in an environment that encouraged bipedal activity. The diet was kept at floor level, and water was delivered through drip bottle placed 2 inches above the floor of the cage. It is common practice in bipedal studies to encourage bipedal activity by progressively raising the food and water as bipedal rats grow. This encouragement may be necessary cofactor in establishing bipedal behavior in bipedal rats. It seems that the new posture of standing and walking upright position plays the major role for development of lordosis.
Scoliosis developed only in pinealectomized rats with bipedal gait, not in quadrupedal animals. We postulate that the bipedal condition, as in chickens or humans, plays an important role in the development of scoliosis [24]. Rodriguez et al. [30] have shown that spinal development in bipedal rats results in a prominent thoracolumbar kyphosis. In this study, cervicothoracic lordosis developed in bipedal rats without pinealectomy. This sagittal curve is the same as Yamada et al. [42] reported, wherein there is a tendency for a gradual increase in cervicothoracic lordosis and thoracic kyphosis with decreased lumbar kyphosis in bipedal rats. The presence of a lordotic region in the thoracic spine may well be essential for the development of idiopathic scoliosis. The spine in rabbits is generally kyphotic in the sagittal plane, but if it is tethered into lordosis, there develops the typical lordoscoliosis with growth [8]. Lawton et al. [16] also reported that scoliosis regressed when release of the lordotic segment was performed deliberately in rabbits. Also the scoliotic curvature resolved with growth in the manner of the resolving infantile idiopathic cases.
The spine’s sagittal profile changes during growth and in normal children the thoracic kyphosis reduces in size from the age of 8, reaching a minimum at about the age of 12 [8, 26]. That this occurs at the same time in both boys and girls suggests its independence from growth velocity, because boys on average mature about 2 years later than girls. This age range coincides with the age when the incidence of scoliosis in the community rises sharply in both boys and girls. Also, this age coincides with the age when the melatonin level precipitously drops by 80% from the highest nocturnal level at the ages of 1–3 years [36].
Normal spine growth requires a precise and delicate mechanical balance of equilibrium and postural tone. Disturbances in primary structuring, support structures, growth centers, position of the spine, and related neural or muscular components could result in the development of scoliosis. Our previous study suggested that a deficiency of melatonin may be important for the symmetric development of paraspinal muscles and straight spine growth [18, 24]. Recently, Wan and Pang [37] demonstrated, using autoradiography studies, that melatonin binding sites in chickens were localized in the dorsal gray matter of the spinal cord . They concluded that melatonin plays a role in regulating the spinal cord function. Also, Fraschini et al. [11] reported that the role of melatonin in the control of sensorimotor performances, and the cerebellar receptors might be correlated with the control of human balance. Our theory is that asymmetrical dysfunction of the paraspinal muscles may be due to loss of proprioceptive innervation. This concept is further supported by experiments in which injections of 5-hydroxytryptophan, a precursor of serotonin, which acts as a modulator for postural muscle tone, appeared to halt the progression of scoliosis in pinealectomized chickens [22]. The question is whether thoracic scoliosis in pinealectomized bipedal rats develop a right or left convex curve. This may be different sites or volumes of melatonin receptors in thalamus, brainstem, cerebellum, and spinal cord between in rats and humans.
Idiopathic scoliosis is the result of either lateral asymmetry of the spine or a primary rotational phenomenon. The disturbances in equilibrium may ultimately lead to the rotation, shifting and bending of parts of the vertebrae. In lordoscoliosis, the primary underlying lesion appears to be a lordosis which rotates out to the side, producing the secondary deformity, scoliosis. Rotation of the vertebral bodies always occurs in the direction of the convexity of scoliosis, confirming that it is a lordosis which has rotated. A lordosis alone would still theoretically be in equilibrium. Rotational instability of the spine with rotation of the lordotic segment may produce a scoliotic deformity as a secondary phenomenon. The rotation deformity and reduced kyphosis are the basic elements in progressive idiopathic scoliosis. There is evidence that the production of scoliosis is in part mechanical in nature. Balance exercised to the thoracic column by the equal load brought from the two sides through the ribs may be disturbed by many factors.
We postulate that balanced muscle tone controlled by postural reflexes is important to maintain a straight spine in the bipedal condition. Disturbances of equilibrium and posture secondary to a defect of melatonin after pinealectomy may promote the development of rotational lordoscoliosis especially in the bipedal posture.
References
Adams W (1865) Lectures on the pathology and treatment of lateral and other forms of curvature of the spine. Churchill & Sons, London
Alexander MA, Bunch WH, Ebbesson SOE (1972) Can experimental dorsal rhizotomy produce scoliosis? J Bone Joint Surg Am 54:1509–1513
Bailey AS, Alder FA, Lai SM, Asher MA (2001) A comparison between bipedal and quadrupedal rats; do bipedal rats actually assume an upright posture? Spine 26:E308–E313
Barrios C, Tunon MT, DeSalis JA, Beguiristain JL, Canadell J (1987) Scoliosis induced by medullary damage: an experimental study in rabbits. Spine 12:433–439
Barrios C, Arrotegui JI (1992) Experimental kyphoscoliosis induced in rats by selective brain stem damage. Int Orthop 16:146–151
Cowell HR, Hall JN, MacEwen GD (1972) Genetic aspects of idiopathic scoliosis. Clin Orthop 86:121–131
DeSalis J, Beguiristain JL, Canadell J (1980) The production of experimental scoliosis by selective arterial ablation. Int Orthop 3:311–315
Dickson RA, Lawton JO, Archer IA, Butt WP (1984) The pathogenesis of idiopathic scoliosis: biplanar spinal asymmetry. J Bone Joint Surg Br 66:8–15
Fidler MW, Jowett RL (1976) Muscle imbalance in the aetiology of scoliosis. J Bone Joint Surg Br 58:200–201
Fischer RL, DeGeorge FV (1976) Idiopathic scoliosis: an investigation of genetic and environmental factors. J Bone Joint Surg Am 49:1005–1006
Fraschini F, Cesarani A, Alpini D, Esposti D, Stanker BM (1999) Melatonin influences human balance. Biol Signals Recept 8:111–119
Goff CW, Landmesser W (1975) Bipedal rats and mice. J Bone Joint Surg Am 39:616–622
Kazmin AI, Merkorieva RV (1971) Role of disturbance of glucosaminoglycaine metabolism in the pathogenesis of scoliosis. Ortop Travmatol Protez 32:87–91
Kindsfater K, Lowe T, Lawellin D, Weinsein D, Akmakjian J (1994) Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg Am 76:1186–1192
Lagenskiold A, Michelsson JE (1961) Experimental progressive scoliosis in the rabbit. J Bone Joint Surg Br 43:116–120
Lawton JO, Dickson RA (1986) The experimental basis of idiopathic scoliosis. Clin Orthop 210:9–17
Liszka O (1961) Spinal cord mechanisms leading to scoliosis in animal experiments. Acta Med Pol 2:45–63
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J (1993) An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine 18:1609–1615
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J (1995) Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br 77:134–138
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S (1994) Pathogenesis of idiopathic scoliosis: SEPs in chickens with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop 14:329–335
Machida M, Dubousset J, Satoh T, Murai I, Wood KB, Ryu J (2001) Pathological mechanism of experimental scoliosis in pinealectomized chickens. Spine 26:E385–E391
Machida M, Miyashita Y, Murai I, Dubousset J, Yamada T, Kimura J (1997) Role of serotonin for scoliotic deformity in pinealectomized chicken. Spine 22:297–301
Machida M, Miyashita Y, Yamada H, Imamura Y, Murai I (1998) Pathogenesis in idiopathic scoliosis: serum melatonin and scoliotic deformity in chickens in continuous darkness. Spine Spinal Cord 11:135–138
Machida M, Murai I, Miyashita Y, Dubousset J, Yamada T, Kimura J (1999) Pathogenesis of idiopathic scoliosis: experimental study in rats. Spine 24:1985–1989
Machida M (1999) Cause of idiopathic scoliosis. Spine 24:2576–2583
Ohlen G, Aaro S, Byland P (1988) The sagittal configuration and mobility of the spine in idiopathic scoliosis. Spine 13:413–416
O’Kelly C, Wang X, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K (1999) The production of scoliosis following pinealectomy in young chickens, rats and hamsters. Spine 24:35–43
Pincott JR, Davies JS, Taffs LF (1984) Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg Br 66:27–29
Roaf R (1966) The basic anatomy of scoliosis. J Bone Joint Surg Br 48:786–792
Rodriguez RM, Bailey RW, Rodriguez RP (1965) Skeletal lesions of lathyrism effects of bipedalism on spine development. Clin Orthop 41:189–197
Sahlstrand T, Ortengren R, Nachemson A (1978) Postural equilibrium in patients in adolescent idiopathic scoliosis. Acta Orthop Scand 49:354–365
Sarwark JF, Dabney KW, Salzman SK, Wakabayashi T, Kitadai HK, Beachamp JT, Beckman AL, Bunnel WP (1988) Experimental scoliosis in rat. 1. Methodology, anatomic features and neurologic characterization. Spine 13:466–471
Smith RE, Saville PD (1965) Bone breaking stress as a function of weight bearing in bipedal rats. Am J Phys Anthropol 25:159–164
Smith RM, Dickson RA (1987) Experimental structural scoliosis. J Bone Joint Surg Br 69:576–581
Somerville EW (1952) Rotational lordosis: the development of single curve. J Bone Joint Surg Br 34:421–427
Waldhauser F, Weiszenbacher G, Tatzer E, Gisinger B, Waldhauser M, Schemper M, Frisch H (1988) Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab 66:648–652
Wan Q, Pang SF (1994) Segmental, coronal, and subcellular distribution of 2-[125I] iodomelatonin binding sites in the chicken spinal cord. Neurosci Lett 180:253–256
Wyatt MP, Barrack RL, Mubarak SJ, Whitecloud TS, Burke SE (1986) Vibratory response in idiopathic scoliosis. J Bone Joint Surg Br 68:714–718
Wyanne-Davies R (1968) Familial (idiopathic) scoliosis. A family survey. J Bone Joint Surg Br 50:24–30
Yamada K (1962) The dynamics of experimental posture: experimental study of intervertebral disc herniation in bipedal animals. Clin Orthop 25:20–31
Yamada K, Ikata I, Yamamoto H, Nakagawa Y, Tanaka H, Tezuka A (1969) Equilibrium function in scoliosis and active corrective plaster jacket for the treatment. Tokushima J Exp Med 16:1–7
Yamada K, Yamamoto H, Nakagawa Y, Tezuka A, Tamura Y, Kawata S (1984) Etiology of idiopathic scoliosis. Clin Orthop 184:50–57
Yamamoto H (1966) Experimental scoliosis in rachitic bipedal rats. Tokushima J Exp Med 13:1–34
Acknowledgements
This study was conducted at the Department of Orthopaedic Surgery, Nihon University School of Medicine, Tokyo, Japan. None of the authors received financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machida, M., Saito, M., Dubousset, J. et al. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J 14, 843–848 (2005). https://doi.org/10.1007/s00586-004-0806-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-004-0806-1