Abstract
O-linked β-N-acetylglucosamine (O-GlcNAc) is a ubiquitous and dynamic posttranslational modification that occurs on serine/threonine residues of nuclear and cytoplasmic proteins. This modification is regulated by O-GlcNAc transferase (OGT), which attaches O-GlcNAc to proteins and O-GlcNAcase (OGA), which removes O-GlcNAc. O-GlcNAc serves as a nutrient sensor to regulate virtually all cellular processes, as well as playing roles in various diseases, including Alzheimer’s disease, diabetes, and cancer. In this chapter, we present an overview of O-GlcNAcylation in different kinds of cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 O-GlcNAc and Enzymes Controlling Its Cycling
1.1 O-GlcNAc
O-GlcNAc is distinct from the other common forms of protein glycosylation in several major respects (Torres and Hart 1984). It occurs both on nuclear and cytoplasmic proteins of the cell (Hart 1997). The GlcNAc is generally not modified to form more complex structures (Comer and Hart 2000). It is attached and removed multiple times in the life of a polypeptide. In terms of its dynamics and functions, O-GlcNAcylation is more similar to protein phosphorylation than it is to classical protein glycosylation.
O-GlcNAc, first characterized in 1983 (Torres and Hart 1984), is an O-linked β-N-acetylglucosamine moiety attached to the side chain hydroxyl of a serine or threonine residue. O-GlcNAc has thus far been reported on over 3000 cytoplasmic and nuclear proteins. The addition of O-GlcNAc to proteins is catalyzed by O-GlcNAc transferase (OGT) (Haltiwanger et al. 1992; Kreppel and Hart 1999; Lubas and Hanover 2000), while the saccharide’s removal is catalyzed by O-linked N-acetyl-β-D-glucosaminidase (O-GlcNAcase, OGA (Comtesse et al. 2001; Gao et al. 2001). This dynamic and reversible modification is emerging as a key regulator of various cellular processes, such as signal transduction (Wells et al. 2001), transcription (Ozcan et al. 2010), cell cycle progression (Drougat et al. 2012), and protein-protein interaction (Lim and Chang 2010), documenting its importance in many basic cellular and disease processes.
It has been demonstrated that O-GlcNAc plays important roles in some human diseases, such as cancer (Caldwell et al. 2010; Gu et al. 2010; Slawson et al. 2010; Lynch et al. 2012; Fardini et al. 2013), diabetes (Akimoto et al. 2005; Dias and Hart 2007; Slawson et al. 2010), and neurological disorders (Lefebvre et al. 2003). Several key oncogene and tumor suppressor proteins involved in tumorigenesis and cancer progression have been identified to be O-GlcNAcylated, such as p53(Shaw et al. 1996) and c-Myc (Chou et al. 1995).
1.2 O-GlcNAc Transferase (OGT)
OGT catalyzes the addition of a single GlcNAc moiety to serine or threonine residues on proteins (Haltiwanger et al. 1990). In mammals, OGT is expressed in all cell types, with the highest level of expression in the pancreas followed by the brain (Lubas et al. 1997; Hanover et al. 1999). OGT itself is modified by O-GlcNAc and phosphorylation. OGT exists in three forms: two nucleocytoplasmic forms and one mitochondrial form (Love et al. 2003). In many tissues, OGT is composed of two 110 kDa subunits and one single 78 kDa subunit. However, the ratios of each type of subunit appear to vary depending upon the tissue.
Until now, only one single OGT gene has been identified (Shafi et al. 2000) in mammals, which is highly conserved through evolution. The 110 kDa OGT protein can be divided into two distinct domains, the amino-terminal half of the protein containing multiple tetratricopeptide repeats (TPRs) and the carboxyl-terminal half of the protein containing the catalytic domain of the enzyme. TPRs are found in a large number of proteins of diverse functions, where they serve as protein interaction sites to play a role in modulating a variety of cellular processes, including cell cycle (Hirano et al. 1990; Lamb et al. 1994; Tugendreich et al. 1995), transcription regulation (Schultz et al. 1990; Rameau et al. 1994; Tzamarias and Struhl 1995), and protein transport (Haucke et al. 1996; Goebl and Yanagida 1991).
The mechanism of how OGT recognizes and glycosylates its protein substrates remains largely unknown. However, over the past few years, there are numbers of advances in the study of its structural and kinetic properties that may yield some ideas to us. Two crystal structures of human OGT have been reported, one is a binary complex with UDP (2.8A° resolution) and the other is a ternary complex with UDP and a peptide substrate (1.95A°) which indicated that OGT employs an ordered bi-bi kinetic mechanism where UDP-GlcNAc might bind first followed by the substrate (Lazarus et al. 2011). Posttranslational modifications involving tyrosine kinases, nitrosylation of cysteine residues, and O-GlcNAc modification may also regulate OGT activity (Shen et al. 2012). Most recently, it has been found out that host cell factor-1 is cleaved by OGT when the TPR domain of OGT binds to the carboxyl-terminal portion of an HCF-1 proteolytic repeat (Lazarus et al. 2013).
Ac4-S-GlcNAc (Fig. 6.1), which can penetrate into and be converted to its active form UDP-S-GlcNAc via the GlcNAc pathway, can be used as an OGT inhibitor (Dorfmueller et al. 2011; Gloster et al. 2011).
1.3 O-Linked N-Acetyl-β-D-Glucosaminidase (O-GlcNAcase, OGA)
OGA catalyzes the removal of O-GlcNAc from proteins. It is localized mainly to the cytoplasm but is also found within nuclei and mitochondria (Gao et al. 2001; Wells et al. 2002). OGA consists of two main domains: an N-terminal domain with glycoside hydrolase activity and a C-terminal histone acetyltransferase (HAT) domain. These domains flank a region containing a caspase-3 cleavage site (Butkinaree et al. 2008). Analogous to OGT, the highest expression OGA occurs in the pancreas and brain (Dong and Hart 1994; Gao et al. 2001; Whelan and Hart 2003). The HAT domain of OGA likely serves to target the enzyme to transcriptional machinery, but does not appear to have HAT enzymatic activity.
Several OGA inhibitors have been developed to study the biological roles of O-GlcNAc (Fig. 6.1). O-(2-acetamido-2-deoxy-D-glucopyranoseylidene) amino N-phenyl carbamate (PUGNAc), GlcNAcstatin, and Thiamet G are three inhibitors found to effectively limit OGA activity (Banerjee et al. 2013a).
2 O-GlcNAc: A Nutrient Sensor
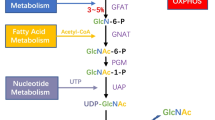
The well-known “Warburg effect” or aerobic glycolysis in which a cancer cell relies mainly on glycolysis instead of oxidative phosphorylation, even when there is high oxygen tension, was first described in 1956 by Otto Warburg (1956a, b). It is now well known that glycolysis is much less efficient in producing energy compared to oxidative phosphorylation. Since cancer cells often have a higher proliferation rate and higher needs for metabolic precursors, the uptake of glucose has to be increased in order to meet the basic needs of the cancer cell.
In most cells, about 2–5 % of glucose is used in the hexosamine biosynthetic pathway (HBP). The end product of the HBP is UDP-GlcNAc, the donor substrate used by OGT in the enzymatic addition of O-GlcNAc. Increased cancer cell glucose uptake likely also drives increased HBP flux that leads to hyper-O-GlcNAcylation. Indeed, increased protein O-GlcNAcylation has been observed in all types of cancer thus far (Shi et al. 2010; Li et al. 2011; Krzeslak 2012a, b; Lynch et al. 2012; Rozanski et al. 2012; Zhu et al. 2012).
3 O-GlcNAc and Cancer
Increased O-GlcNAcylation and changes in OGT/OGA expression have been described in many different cancer types including breast, prostate, liver, pancreatic, colorectal, bladder, lung, colon, ovarian, and chronic lymphocytic leukemia (Slawson and Hart 2011; Fardini et al. 2013; Ma and Vosseller 2013) (Table 6.1).
3.1 Breast Cancer
Breast cancer remains a major clinical problem worldwide. Most patients succumb to the disease as a result of the metastatic spread of their primary tumor (Chambers et al. 2001; Steeg 2006). Early in the disease process many of these tumors are fueled by estrogen. Estrogen receptors are dynamically modified by O-GlcNAc (Jiang and Hart 1997). Early studies by Slawson et al. documented increased OGA activity in primary breast tumors as compared to matched adjacent breast tissues (Slawson et al. 2001). Donadio et al. showed that glutaminase inhibition greatly reduces glucose:fructose amidotransferase (GFAT) activity, the first committed step in the HBP, and changes the O-GlcNAc pattern of key proteins that control cell proliferation and differentiation (Donadio et al. 2008).
Caldwell et al. showed that OGT and O-GlcNAc levels are elevated in breast cancer cells and that reducing high O-GlcNAcylation inhibits cancer cell growth in vitro and in vivo and also reduces breast cancer cell invasion. They further found that targeted deletion of OGT inhibited the growth of tumor cells and was associated with reduction in the FoxM1 transcriptional target MMP-2 (Caldwell et al. 2010). Additional studies, using immunohistochemistry analysis, observed that the global O-GlcNAcylation levels in breast tumor tissues were significantly elevated compared to the corresponding adjacent normal tissue (Gu et al. 2010). Krzeslak and coworkers showed that the expression of MGEA5 (O-GlcNAcase; OGA) decreased while the expression of OGT increased in higher-grade tumors, suggesting that increased O-GlcNAcylation might be implicated in breast tumor progression and metastasis (Krzeslak et al. 2012a).
By using 2D O-GlcNAc immunoblotting and LC-MS/MS analysis, Champattanachai and colleagues identified 29 proteins (Champattanachai et al. 2013), seven of which are O-GlcNAcylated or associated with O-GlcNAcylation in cancer. Moreover, OGT knockdown revealed that decreasing O-GlcNAcylation was related to inhibition of anchorage-independent growth in vitro. Altogether the results indicate that aberrant protein O-GlcNAcylation is associated with breast cancer. Huang et al. identified that the actin-binding protein cofilin is O-GlcNAcylated at Ser108 and further showed that during three-dimensional invasion, O-GlcNAcylation of cofilin is required for its localization to invadopodia (Huang et al. 2013).
Most recently, Kanwal et al. noticed that increased O-GlcNAcylation protected MCF-7 cells from death induced by tamoxifen; in contrast, inhibition of OGT expression enhanced the ability of tamoxifen to induce cell death. The results indicate that the inhibition of O-GlcNAcylation may improve the sensitivity of some breast cancers to tamoxifen therapy (Kanwal et al. 2013).
3.2 Prostate Cancer
Prostate cancer is the most common type of non-cutaneous cancer found in American men and the second leading cause of cancer death behind lung cancer. One in six men will get prostate cancer during his lifetime and one man in 36 will die of this disease. Despite the enormity of these statistics, prostate cancer remains a relatively understudied disease with respect to its biology and molecular mechanisms of action (Chunthapong et al. 2004).
It has been found that OGT is overexpressed in prostate cancer tissue compared to normal prostate epithelium and the expression of OGT and levels of O-GlcNAc modifications are elevated in prostate cancer cell lines compared to non-transformed prostate cells. In addition, reducing OGT levels inhibits VEGF expression and the angiogenic potential of PC3-ML cells, which is dependent on the transcription factor FoxM1. Finally, reducing OGT expression in human prostate cancer cells inhibited metastasis to bone. Thus, OGT is positioned as a novel target for therapeutic intervention in the treatment of human prostate cancer (Lynch et al. 2012).
Itkonen et al. found that expression of c-MYC and OGT was tightly correlated in human prostate cancer samples. Moreover, they identified c-MYC as an upstream regulator of OGT target genes and OGT inhibition decreased the c-MYC protein level, which suggests that HBP acts as a modulator of prostate cancer growth and c-MYC as a key target of OGT function in prostate cancer cells (Itkonen et al. 2013).
Recently, our lab also found out that the level of O-GlcNAc and its enzymes is increased in prostate cancer cells compared to normal cells. Through in vitro assays, the results indicate that O-GlcNAc and its cycling might be an important factor during the progression of prostate cancer (Liu et al., unpublished).
3.3 Liver Cancer
In 2007, it was reported that in human hepatoma cells (HCC) protein O-GlcNAcylation modulates the promoter activities of the transcription factors CRE and activation protein-1 (AP-1) and enhances E-selectin protein expression (Azuma et al. 2007).
Guo et al. observed that O-GlcNAcylation of HSP27 in HCC cells might be a novel regulatory mode of HSP27 function, particularly for its entry into the nucleus. Crosstalk or interplay between glycosylation and phosphorylation of HSP27 could regulate its subcellular localization and biological functions in liver cancer (Guo et al. 2012). Zhu et al. found that global O-GlcNAcylation levels were significantly elevated in HCC tissues compared to that in healthy ones. Global O-GlcNAcylation was also enhanced in the tumor tissues of patients who had suffered from HCC recurrence after liver transplant compared with those who had not. Moreover, in vitro assays demonstrated that O-GlcNAcylation plays important roles in migration, invasion, and viability of HCC cells, partly through regulating E-cadherin, MMP1, MMP2, and MMP3 expression. Most importantly, a lower OGA expression level was a prognostic factor for predicting tumor recurrence in HCC (Zhu et al. 2012).
3.4 Pancreatic Cancer
Pancreatic cancer is the fourth most prevalent cancer-related cause of death in the United States. Most pancreatic cancer patients have glucose intolerance or diabetes. Interestingly, the pancreatic β-cells, which secrete insulin, have high levels of O-GlcNAc. The β-cell is unique in containing much more OGT than any other cell type (Konrad and Kudlow 2002). Park et al. revealed that increasing O-GlcNAcylation protein levels were accompanied by enhanced apoptosis in pancreatic β-cells, and they also identified ten new O-GlcNAcylated proteins (Park et al. 2007). By using mass spectrometry, Kang and coworkers found that Ser473 in Akt1 may be modified with O-GlcNAc, and that O-GlcNAc modification and phosphorylation of Ser473 are reciprocally regulated by hyperglycemic treatment in murine β-pancreatic cells (Kang et al. 2008).
Banerjee et al. partially elucidated the mechanism of action of triptolide, a bioactive ingredient in traditional Chinese medicine that has anticancer properties. They showed that triptolide-induced downregulation of HSP70, which leads to cell death, is mediated by impaired O-GlcNAc modification of Sp1 in pancreatic cancer. Triptolide decreases the expression and activity of OGT in these cells, resulting in reduced Sp1 translocation to the nucleus and reduced Sp1 activity. In turn, Sp1 leads to lower expression of HSF1 and other HSPs, finally resulting in tumor cell death (Banerjee et al. 2013b).
Increased HBP flux and hyper-O-GlcNAcylation has also been observed in pancreatic ductal adenocarcinoma cell (PDAC). Reducing O-GlcNAcylation inhibited PDAC cell growth and tumor formation, but did not affect the growth of non-transformed pancreatic epithelial cells. They also found that the NF-κB p65 subunit and kinases IKK α/IKK β were O-GlcNAc modified in PDAC. Reduction of PDAC hyper-O-GlcNAcylation inhibited constitutive NF-κB activity, while elevation of O-GlcNAc activated NF-κB and suppressed apoptosis (Ma et al. 2013).
3.5 Colorectal Cancer
Yehezkel and colleagues noticed that the metastatic colorectal cancer cell line, SW620, exhibited higher levels of O-GlcNAcylation and lower levels of OGA expression compared with its parent line, SW480. Elevating O-GlcNAcylation levels through RNA interference of OGA resulted in phenotypic alterations that included acquisition of a fibroblast-like morphology. Microarray analysis revealed that OGA silencing altered the expression of about 1300 genes, most of which are involved in cell movement and growth and specifically affected metabolic pathways of lipids and carbohydrates (Yehezkel et al. 2012).
Very recent studies have documented that O-GlcNAcylation and OGT levels are increased in primary colorectal cancer tissues. Using immunoblotting and LC-MS/MS analysis, 16 proteins were successfully identified and eight proteins showed an increase in O-GlcNAcylation. Among all the identified proteins, annexin A2 was further confirmed to show increased O-GlcNAcylation in all cancer samples. The results indicate that aberrant O-GlcNAcylation of proteins is associated with colorectal cancer and O-GlcNAc-modified proteins may provide novel biomarkers for cancer.
3.6 Bladder Cancer (BC)
Cyclophosphamide-induced cystitis is an established model for the study of bladder injury and wound healing. In 2000, the first study was reported on the alterations in O-GlcNAcylation in bladders with cyclophosphamide-induced cystitis. They concluded that O-GlcNAcylation may have a significant role in the bladder wound healing process (Chung et al. 2010). Rozanski and colleagues analyzed mRNA expression of genes encoding enzymes involved in O-GlcNAcylation using samples in urine obtained from 176 bladder cancer (BC) patients and 143 healthy persons. OGT expression was not detected in the urine of healthy persons but it was found in 51.7 % of BC samples. Positive expression of the MGEA5 gene, encoding OGA, was found in urine of both healthy persons (47.1 %) and BC patients (52.3 %). Poorly differentiated BC (grade III) showed significantly lower MGEA5 expression than grade I tumors. On the contrary, OGT transcript levels were significantly higher in grade II and III in comparison to grade I BC. Moreover, there were significant differences in OGT expression between early bladder cancers and invasive or advanced bladder cancers. These results suggest that analysis of urinary content of OGA and OGT may be useful for bladder cancer diagnostics (Rozanski et al. 2012).
3.7 Other Cancers
Changes in O-GlcNAc levels or expression of O-GlcNAc-cycling enzymes have also been described in leukemia and ovarian and lung cancers.
Shi et al. found that chronic lymphocytic leukemia (CLL) cells expressed high levels of O-GlcNAcylated proteins, including p53, c-myc, and Akt compared to normal circulating and tonsillar B cells. Also, high baseline O-GlcNAc levels associated with impaired signaling responses to TLR agonists, chemotherapeutic agents, B-cell receptor cross-linking, and mitogens were observed (Shi et al. 2010). Interestingly, while all CLL cells had higher O-GlcNAcylation, those patients with levels at the lower end of the scale had a poor prognosis, while those with the highest levels of O-GlcNAcylation had a better prognosis because their CLL cells became more indolent.
Recently Jin and coworkers found that O-GlcNAcylation was enhanced in HO-8910PM cells, which is a more metastatic human ovarian cancer cell line compared to OVCAR3 cells. Additionally, the migration of OVCAR3 cells was dramatically enhanced by OGA inhibition, and the migration ability of HO-8910PM cells was significantly inhibited by OGT silencing. Moreover, E-cadherin, an O-GlcNAcylated protein in ovarian cancer cells, was reduced by OGA inhibition in OVCAR3 cells and elevated by OGT silencing in HO-8910PM cells (Jin et al. 2013).
O-GlcNAcylation levels and the expressions of OGT and OGA in human lung and colon cancer tissues were examined by immunohistochemistry. O-GlcNAcylation as well as OGT expression were significantly elevated in cancer tissues compared with that in the corresponding adjacent tissues. Additionally, the roles of O-GlcNAcylation in the malignancy of lung and colon cancer were investigated in vitro. The results showed that O-GlcNAcylation dramatically enhanced the anchorage-independent growth of lung and colon cancer cells and could also enhance lung and colon cancer invasion. All together, this study suggests that O-GlcNAcylation might play important roles in lung and colon cancer formation and progression and may be a valuable target for diagnosis and therapy of cancer (Li et al. 2011).
In conclusion, it is now clear that altered O-GlcNAcylation occurs in most, if not all, types of cancer. However, very little is known with respect to how O-GlcNAc contributes to the oncogenic phenotype at a mechanistic level. The possible numbers of mechanisms affected by altered O-GlcNAcylation are enormous, including altering signaling cascades, modulation of gene expression at both the transcriptional and translational levels, and by regulation of cytoskeletal dynamics, including mechanisms regulating cell adhesion and epithelial-mesenchymal transitions. Similar to phosphorylation’s roles in cancer, elucidation of O-GlcNAc’s roles will require focused work of many laboratories, but also these studies will undoubtedly lead to novel and powerful therapeutics which were previously unimagined.
References
Akimoto Y, Hart GW, Hirano H, Kawakami H (2005) O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med Mol Morphol 38(2):84–91
Azuma Y, Miura K, Higai K, Matsumoto K (2007) Protein O-N-acetylglucosaminylation modulates promoter activities of cyclic AMP response element and activator protein 1 and enhances E-selectin expression on HuH-7 human hepatoma cells. Biol Pharm Bull 30(12):2284–2289
Banerjee PS, Hart GW, Cho JW (2013a) Chemical approaches to study O-GlcNAcylation. Chem Soc Rev 42(10):4345–4357
Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, Saluja AK (2013b) Triptolide-induced cell death in Pancreatic cancer is mediated by O-GlcNAc modification of transcription factor sp1. J Biol Chem 288(47):33927–33938
Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW (2008) Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem 283(35):23557–23566
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ (2010) Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29(19):2831–2842
Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC (2001) Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am 10(2):243–255, vii
Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J (2013) Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics 13(14):2088–2099
Chou TY, Dang CV, Hart GW (1995) Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci USA 92(10):4417–4421
Chung S, Kang DO, Yamzon J, Warburton D, Koh CJ (2010) O-GlcNAc mediated glycosylation down-regulation in mice with cyclophosphamide induced cystitis. J Urol 183(1):351–356
Chunthapong J, Seftor EA, Khalkhali-Ellis Z, Seftor RE, Amir S, Lubaroff DM, Heidger PM Jr, Hendrix MJ (2004) Dual roles of E-cadherin in prostate cancer invasion. J Cell Biochem 91(4):649–661
Comer FI, Hart GW (2000) O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem 275(38):29179–29182
Comtesse N, Maldener E, Meese E (2001) Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun 283(3):634–640
Dias WB, Hart GW (2007) O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst 3(11):766–772
Donadio AC, Lobo C, Tosina M, de la Rosa V, Martin-Rufian M, Campos-Sandoval JA, Mates JM, Marquez J, Alonso FJ, Segura JA (2008) Antisense glutaminase inhibition modifies the O-GlcNAc pattern and flux through the hexosamine pathway in breast cancer cells. J Cell Biochem 103(3):800–811
Dong DL, Hart GW (1994) Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem 269(30):19321–19330
Dorfmueller HC, Borodkin VS, Blair DE, Pathak S, Navratilova I, van Aalten DM (2011) Substrate and product analogues as human O-GlcNAc transferase inhibitors. Amino Acids 40(3):781–792
Drougat L, Olivier-Van Stichelen S, Mortuaire M, Foulquier F, Lacoste AS, Michalski JC, Lefebvre T, Vercoutter-Edouart AS (2012) Characterization of O-GlcNAc cycling and proteomic identification of differentially O-GlcNAcylated proteins during G1/S transition. Biochim Biophys Acta 1820(12):1839–1848
Fardini Y, Dehennaut V, Lefebvre T, Issad T (2013) O-GlcNAcylation: a new cancer hallmark? Front Endocrinol (Lausanne) 4:99
Gao Y, Wells L, Comer FI, Parker GJ, Hart GW (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem 276(13):9838–9845
Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ (2011) Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol 7(3):174–181
Goebl M, Yanagida M (1991) The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci 16(5):173–177
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W (2010) GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 70(15):6344–6351
Guo K, Gan L, Zhang S, Cui FJ, Cun W, Li Y, Kang NX, Gao MD, Liu KY (2012) Translocation of HSP27 into liver cancer cell nucleus may be associated with phosphorylation and O-GlcNAc glycosylation. Oncol Rep 28(2):494–500
Haltiwanger RS, Holt GD, Hart GW (1990) Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem 265(5):2563–2568
Haltiwanger RS, Blomberg MA, Hart GW (1992) Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem 267(13):9005–9013
Hanover JA, Lai Z, Lee G, Lubas WA, Sato SM (1999) Elevated O-linked N-acetylglucosamine metabolism in pancreatic beta-cells. Arch Biochem Biophys 362(1):38–45
Hart GW (1997) Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 66:315–335
Haucke V, Horst M, Schatz G, Lithgow T (1996) The Mas20p and Mas70p subunits of the protein import receptor of yeast mitochondria interact via the tetratricopeptide repeat motif in Mas20p: evidence for a single hetero-oligomeric receptor. EMBO J 15(6):1231–1237
Hirano T, Kinoshita N, Morikawa K, Yanagida M (1990) Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell 60(2):319–328
Huang X, Pan Q, Sun D, Chen W, Shen A, Huang M, Ding J, Geng M (2013) O-GlcNAcylation of cofilin promotes breast cancer cell invasion. J Biol Chem 288(51):36418–25
Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG (2013) O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res 73(16):5277–5287
Jiang MS, Hart GW (1997) A subpopulation of estrogen receptors are modified by O-linked N-acetylglucosamine. J Biol Chem 272(4):2421–2428
Jin FZ, Yu C, Zhao DZ, Wu MJ, Yang Z (2013) A correlation between altered O-GlcNAcylation, migration and with changes in E-cadherin levels in ovarian cancer cells. Exp Cell Res 319(10):1482–1490
Kang ES, Han D, Park J, Kwak TK, Oh MA, Lee SA, Choi S, Park ZY, Kim Y, Lee JW (2008) O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp Cell Res 314(11–12):2238–2248
Kanwal S, Fardini Y, Pagesy P, N’Tumba-Byn T, Pierre-Eugene C, Masson E, Hampe C, Issad T (2013) O-GlcNAcylation-inducing treatments inhibit estrogen receptor alpha expression and confer resistance to 4-OH-Tamoxifen in human breast cancer-derived MCF-7 cells. PLoS One 8(7):e69150
Konrad RJ, Kudlow JE (2002) The role of O-linked protein glycosylation in beta-cell dysfunction. Int J Mol Med 10(5):535–539
Kreppel LK, Hart GW (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem 274(45):32015–32022
Krzeslak A, Forma E, Bernaciak M, Romanowicz H, Brys M (2012a) Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med 12(1):61–65
Krzeslak A, Wojcik-Krowiranda K, Forma E, Bienkiewicz A, Brys M (2012b) Expression of genes encoding for enzymes associated with O-GlcNAcylation in endometrial carcinomas: clinicopathologic correlations. Ginekol Pol 83(1):22–26
Lamb JR, Michaud WA, Sikorski RS, Hieter PA (1994) Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J 13(18):4321–4328
Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S (2011) Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469(7331):564–567
Lazarus MB, Jiang J, Kapuria V, Bhuiyan T, Janetzko J, Zandberg WF, Vocadlo DJ, Herr W, Walker S (2013) HCF-1 is cleaved in the active site of O-GlcNAc transferase. Science 342(6163):1235–1239
Lefebvre T, Caillet-Boudin ML, Buee L, Delacourte A, Michalski JC (2003) O-GlcNAc glycosylation and neurological disorders. Adv Exp Med Biol 535:189–202
Li Y, Zeng Y, Mooney SM, Yin B, Mizokami A, Namiki M, Getzenberg RH (2011) Resistance to paclitaxel increases the sensitivity to other microenvironmental stresses in prostate cancer cells. J Cell Biochem 112(8):2125–2137
Lim K, Chang HI (2010) O-GlcNAc inhibits interaction between Sp1 and sterol regulatory element binding protein 2. Biochem Biophys Res Commun 393(2):314–318
Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA (2003) Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci 116(Pt 4):647–654
Lubas WA, Hanover JA (2000) Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem 275(15):10983–10988
Lubas WA, Frank DW, Krause M, Hanover JA (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272(14):9316–9324
Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ (2012) Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem 287(14):11070–11081
Ma Z, Vosseller K (2013) O-GlcNAc in cancer biology. Amino Acids 45(4):719–733
Ma Z, Vocadlo DJ, Vosseller K (2013) Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem 288(21):15121–15130
Ozcan S, Andrali SS, Cantrell JE (2010) Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta 1799(5–6):353–364
Park J, Kwon H, Kang Y, Kim Y (2007) Proteomic analysis of O-GlcNAc modifications derived from streptozotocin and glucosamine induced beta-cell apoptosis. J Biochem Mol Biol 40(6):1058–1068
Rameau G, Puglia K, Crowe A, Sethy I, Willis I (1994) A mutation in the second largest subunit of TFIIIC increases a rate-limiting step in transcription by RNA polymerase III. Mol Cell Biol 14(1):822–830
Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P, Lipinski M (2012) Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin Lab 58(5–6):579–583
Schultz J, Marshall-Carlson L, Carlson M (1990) The N-terminal TPR region is the functional domain of SSN6, a nuclear phosphoprotein of Saccharomyces cerevisiae. Mol Cell Biol 10(9):4744–4756
Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, Hart GW, Marth JD (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97(11):5735–5739
Shaw P, Freeman J, Bovey R, Iggo R (1996) Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 12(4):921–930
Shen DL, Gloster TM, Yuzwa SA, Vocadlo DJ (2012) Insights into O-linked N-acetylglucosamine ([0–9]O-GlcNAc) processing and dynamics through kinetic analysis of O-GlcNAc transferase and O-GlcNAcase activity on protein substrates. J Biol Chem 287(19):15395–15408
Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE (2010) Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia 24(9):1588–1598
Slawson C, Hart GW (2011) O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 11(9):678–684
Slawson C, Pidala J, Potter R (2001) Increased N-acetyl-beta-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetylglucosamine containing proteins. Biochim Biophys Acta 1537(2):147–157
Slawson C, Copeland RJ, Hart GW (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35(10):547–555
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12(8):895–904
Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259(5):3308–3317
Tugendreich S, Tomkiel J, Earnshaw W, Hieter P (1995) CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81(2):261–268
Tzamarias D, Struhl K (1995) Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8-Tup1 corepressor complex to differentially regulated promoters. Genes Dev 9(7):821–831
Warburg O (1956a) On respiratory impairment in cancer cells. Science 124(3215):269–270
Warburg O (1956b) On the origin of cancer cells. Science 123(3191):309–314
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291(5512):2376–2378
Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW (2002) Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem 277(3):1755–1761
Whelan SA, Hart GW (2003) Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res 93(11):1047–1058
Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I (2012) O-linked beta-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem 287(34):28755–28769
Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S (2012) O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol 29(2):985–993
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Liu, X., Hart, G.W. (2016). Nutrient Regulation of Cancer Cells by O-GlcNAcylation. In: Furukawa, K., Fukuda, M. (eds) Glycosignals in Cancer: Mechanisms of Malignant Phenotypes . Springer, Tokyo. https://doi.org/10.1007/978-4-431-55939-9_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-55939-9_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55937-5
Online ISBN: 978-4-431-55939-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)