Abstract
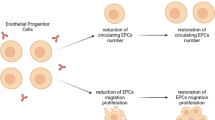
Systemic sclerosis (SSc) is characterized by excessive fibrosis and microvasculopathy with deficiency in vascular formation and repair. The postnatal vascular system is constantly maintained through angiogenesis and vasculogenesis. Endothelial progenitor cells (EPCs), major players of vasculogenesis, are heterogeneous cell population containing an extremely rare number of “true EPCs” and pro-angiogenic hematopoietic cells (PHCs) that promotes vascular formation through secretion of pro-angiogenic factors and differentiation into endothelial cells and mural cells with low efficiency. We have recently proposed a theory that defective vascular repair machinery is one of the important mechanisms contributing to SSc vasculopathy, based on reduced counts and impaired function of circulating CD34+CD133+CD309+CD45dimCD14− EPCs, which are now regarded as immature PHCs. In contrast, monocytic PHCs increased paradoxically in SSc patients and contribute to fibrotic aspects of the disease by differentiating into fibroblast-like cells. Understanding the roles of EPCs in pathogenic process of SSc may be key to dissecting its pathogenesis and to developing novel therapeutic strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Maintenance of the postnatal vascular system requires constant remodeling in response to injury and senescence. This occurs by collaborative effects of two distinct processes: (1) angiogenesis, which refers to the formation of new blood vessels by sprouting from preexisting vessels through proliferation and migration of mature endothelial cells, and (2) vasculogenesis, which refers to the de novo differentiation of mature endothelial cells through the recruitment and differentiation of endothelial progenitor cells (EPCs) [1]. Nascent vessels formed by angiogenesis and vasculogenesis are subsequently maturated via the recruitment of mesenchymal cells, such as pericytes and smooth muscle cells, through a process termed arteriogenesis. Since EPCs were first defined as a circulating primitive cell population that contributes to postnatal vasculogenesis [2], numerous studies have been conducted to evaluate the contribution of EPCs to the pathogenesis of a variety of vascular diseases, including atherosclerosis and connective tissue diseases.
Systemic sclerosis (SSc) is a multisystem connective tissue disease characterized by excessive fibrosis, microvasculopathy, and autoimmunity. Vascular involvement in patients with SSc mainly affects small arteries and causes reduced blood flow and tissue ischemia, leading to clinical manifestations such as digital ulcers, pulmonary arterial hypertension, and renal crisis. Two types of typical vascular pathology include progressive intimal proliferation and fibrosis in arterioles and the loss of capillaries. The mechanism of SSc vasculopathy is not fully understood, but increasing evidence indicates that endothelial injury is a primary event that triggers subsequent complex vascular pathophysiology [3]. The persistent increase in pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), observed in SSc patients indicates a strong pro-angiogenic response to vascular damage [4]. Nailfold capillaroscopic findings reveal loss of capillaries and formation of giant capillaries in the early phase of the disease and the vascular disorganization in the late phase [5]. The capillary loss may result from vascular damage, but there is almost no evidence of vascular recovery. In addition, the formation of abnormal blood vessels like bushy and leaky capillaries indicates an inadequate vascular repair process. These findings together suggest that, in patients with SSc, the vascular repair machinery does not work properly, and the disease progresses toward irreversible structural changes, despite the strong pro-angiogenic push. Thus, defective vascular repair process mediated through angiogenesis and vasculogenesis is an intriguing hypothesis to explain the pathogenesis of SSc vasculopathy. This chapter features current understandings of roles of EPCs in the vascular and fibrotic aspects of SSc.
3.2 Current Definition of EPCs
Since the first description of EPCs as circulating primitive cells that contribute to postnatal vasculogenesis, numerous studies have been carried out to clarify the mechanisms of postnatal vascular formation and repair, as well as the contribution of EPCs to the pathogenesis of various vascular diseases, and to develop potential therapeutic strategies that promote tissue regeneration or attenuate pathologic neovascularization. However, a great deal of controversy about EPCs and their roles in postnatal vascular formation has arisen because of discrepancies in the definition of EPCs [6]. The major problem in defining EPCs derives from the lack of specific markers. In the landmark paper by Asahara et al. [2], EPCs were characterized using endothelial cell marker-positive cells, which were selected as a cell fraction from peripheral blood mononuclear cells that was enriched in cells expressing CD34 or CD309, a VEGF receptor type 2. These cells were shown to contribute to revascularization and salvage of ischemic hind limbs in animal models.
Currently, it is widely accepted that there are at least two types of EPCs that can be discriminated based on their surface antigen expression, proliferation potential, and time of emergence in the cell culture system [7] (Table 3.1). Endothelial colony-forming cells (ECFCs) or late-outgrowth EPCs are lineage-restricted progenitor cells that only give rise to endothelium. These cells are regarded as “true EPCs,” based on their potential for clonogenic expansion in vitro and their ability to form vessels in vitro and in vivo [7]. Circulating precursors of ECFCs have not been identified yet, but they express CD34 and CD31 and completely lack expression of hematopoietic markers including CD45 [8]. Whether rare ECFCs are derived from hemangioblasts in the bone marrow or from endothelial stem cells that reside in the endothelium remains still undetermined [9].
On the other hand, it has been shown that the vast majority of the cells originally identified as EPCs in various assays are in fact hematopoietic lineage cells that display pro-angiogenic properties and are now termed pro-angiogenic hematopoietic cells (PHCs) [7]. PHCs include several different circulating cell types that are identified in the literature as circulating angiogenic cells (CACs), circulating endothelial precursors, monocytic EPCs, early-outgrowth EPCs, and colony-forming unit (CFU)-ECs. In fact, they fall into at least two distinct major subsets: CD14+ monocytic origin (monocytic PHCs) and CD14− cells positive for CD34, CD133, and CD309 (immature PHCs), initially termed circulating endothelial progenitors (CEPs) [10]. Currently, it is generally accepted that PHCs do not give rise to endothelial cells efficiently but rather work as pro-angiogenic supporting cells [11]. Monocytic PHCs cannot proliferate or form tubular structures in vitro in the absence of mature endothelial cells, but several studies reported that monocytic PHCs are capable of integrating into tubular structures and differentiate into endothelial cell-like cells in vivo [12, 13]. However, it is uncertain whether these endothelial cell-like cells are able to exert the full range of endothelial functioning. On the other hand, immature PHCs derived from stem cells in the bone marrow express immature hematopoietic markers CD34 and CD133 as well as a mature hematopoietic marker CD45 with low intensity, but lack expression of a monocytic marker CD14. Immature PHCs also represent expression of endothelial markers CD309 and Tie-2. These cells have typical features of progenitors, including capacity to proliferate and to differentiate into endothelial cells, but the efficiency is much lower in comparison with ECFCs. Nevertheless, PHCs, either in monocytic or immature subset, are capable of promoting blood vessel formation through multiple mechanisms, including secretion of a series of pro-angiogenic factors, including VEGF, hepatocyte growth factor, granulocyte colony-stimulating factor (G-CSF), and stromal cell-derived factor-1 (SDF-1) [14, 15], and differentiation into other elements of the vasculature, such as pericytes and smooth muscle cells. Essentially, PHCs are involved in the very early phase of vascular repair through attaching to the injured vascular lumen immediately after injury.
There is huge difference in circulating quantities among three EPC subsets. ECFCs are extremely rare cell population and presumed to present at less than 1 out of 106 circulating mononuclear cells [7], which can be detectable only in long-term cultures. Immature PHCs are also rare at a frequency of 10–100 cells in 1 mL of peripheral blood in healthy individuals. In contrast, monocytic PHCs comprise approximately 0.1–2 % of peripheral blood mononuclear cells [7, 12]. Monocytic PHCs’ capacity to enhance blood vessel formation is apparently inferior to immature PHCs, although they clearly predominate over immature PHCs and ECFCs in the absolute number in circulation [16]. In the neovascular microenvironment, ECFCs and monocytic and immature PHCs work in concert with platelets and residential endothelial cells to form new blood vessels [17].
Because of rarity of EPCs in circulation, cultivation of circulating mononuclear cells in medium favoring endothelial differentiation has been used to identify and expand these cells. In these cultures, it is difficult to determine which precursor cells give rise to EPCs, because the starting cell population is heterogeneous, and cellular phenotypes change over time in culture. In the original protocol by Asahara et al., peripheral blood mononuclear cells were cultured on fibronectin for 7 days [2]. These cells typically do not form colonies in culture but have endothelial features, including the ability to bind Ulex europeus agglutinin-1, to take up acetylated low-density lipoprotein, and to express CD31, CD105, CD309, and von Willebrand factor (vWF). The vast majority of the cells recovered in these cultures express both CD45 and CD14, indicating their monocytic origin. In contrast, Hill et al. developed a semisolid clonogenic assay, in which peripheral blood mononuclear cells that did not adhere to fibronectin within 48 h were reseeded on fibronectin, and formed cell clusters [18]. These cells express endothelial cell markers as well as CD45 and CD14, but only a tiny fraction of the cells express CD34 [19]. The cells recovered from different culture protocols contain varied cell populations, but mainly consist of monocytic PHCs, since the depletion of CD14+ monocytes from the mononuclear cells before seeding effectively prevents colony formation. ECFCs are apparently distinct from PHCs since they appear 10–21 days after culturing mononuclear cells in medium favoring endothelial differentiation and thus termed late-outgrowth EPCs [20, 21]. These cultured cells display cobblestone morphology and express endothelial markers but completely lack expression of hematopoietic markers. In addition, circulating precursors that give rise to ECFCs display clonal proliferative potential, self-renewal, and the ability to form vessels in vivo, compatible with features of traditional EPCs.
3.3 Circulating Counts of Immature PHCs in SSc Patients
Two approaches have been conducted to evaluate potential involvement of impaired vasculogenesis in the development of SSc vasculopathy. One utilizes EPC colonies obtained by short-term or long-term cultures of circulating mononuclear cells in medium favoring endothelial differentiation. This strategy is able to analyze function and gene expression of early-outgrowth EPCs, which correspond mainly to monocytic PHCs, and late-outgrowth EPCs or ECFCs, although direct quantification of EPCs in circulation is not feasible in these cultures. Another approach is an ex vivo analysis of circulating EPCs using peripheral blood, but this method is applicable only for immature PHCs, which can be identified by multicolor flow cytometry using freshly prepared peripheral blood. All markers currently used for identification of immature PHCs have considerable overlap with circulating endothelial cells, hematopoietic stem cells, and precursors. Nevertheless, in many studies, immature PHCs are identified as circulating cells expressing CD34, CD133, and CD309. We first reported that there is a reduced number of CD34+CD133+CD309+ immature PHCs in SSc patients, compared with age- and sex-matched patients with rheumatoid arthritis or healthy individuals [22]. In subsequent analyses by other groups, some confirmed our finding [23–25], but others even showed an increase in immature PHCs in SSc patients [26–30] (Table 3.2). These contradictory results may result from differences in protocols used for quantifying immature PHCs. In fact, quantification of circulating immature PHCs is a complex and difficult task from technical viewpoint. Flow cytometry using freshly prepared peripheral blood is used to quantify these cells, but accurate quantification is technically difficult due to extreme rarity of this population, even a large number of events were acquired. To overcome this limitation, flow cytometry is often combined with procedures that enrich immature PHCs, such as sorting of CD34+ cells and lineage (Lin)-negative cells. But, variable recovery rates among samples in the enrichment procedure significantly affect subsequent counts by flow cytometry. Furthermore, a large quantity of peripheral blood, usually at least 20 mL, is required as a starting material to obtain reliable and reproducible counts of immature PHCs.
In an effort to standardize the research on EPCs, the European League Against Rheumatism (EULAR) Scleroderma Trials and Research (EUSTAR) proposed recommendations on EPC researches, including both colony-forming cultures and direct detection by flow cytometry [31]. To verify these recommendations, we have directly compared several different protocols for quantifying immature PHCs [32]. In previous studies, immature PHCs were quantified using a variety of strategies and were expressed as a proportion (%) in mononuclear cells or the absolute number in peripheral blood or in 106 Lin− cells, but we have found that the quantification strategy strongly impacts the consistency of the results. Especially, the inter-method concordance was very poor when CD34+ or Lin− cell counts, based on acquisitioned events by a flow cytometer, were used as a reference. This is probably because CD34+ or Lin− cells are rare cell populations that comprise less than 1 % of circulating mononuclear cells. The purity of the enriched fractions varied greatly among samples even when an intensive gating protocol was applied to eliminate dead cells or irrelevant cells. As a result, the EUSTAR recommendations are valid when combined with an accurate quantification technique, such as the use of fluorospheres as an internal calibrator, which substantially improved the reproducibility of the results. Using standardized protocols, we have shown that circulating immature PHCs are reduced in SSc patients in comparison with healthy controls, although large-scale studies that conform to EUSTAR recommendations and use an accurate calibration protocol are definitely necessary to answer the central question if immature PHCs are increased or reduced in circulation of SSc patients.
Clinical associations of immature PHC counts have been examined in SSc patients. Most studies focused on vascular manifestations, including digital ulcer, but one cross-sectional study showed that lower immature PHC counts were associated with the higher Medsger’s severity score [29]. All studies evaluated found an association between the presence of digital ulcer and low immature PHC counts [24, 25, 27, 29]. A recent prospective study involving 100 patients with SSc revealed that low EPC counts in addition to high placental growth factor levels were identified as independent predictors of the occurrence of at least one new digital ulcer during follow-up [33]. A recent study by Avouac and colleagues has revealed that decreased immature PHC counts are associated with the late pattern of nailfold capillaroscopic findings [34]. On the other hand, associations of immature PHC counts with other manifestations of SSc have not been reported, but low immature PHC counts are shown to be associated with patients with pulmonary arterial hypertension [35] or those with idiopathic pulmonary fibrosis [36]. These findings together suggest that insufficient property exerted by immature PHCs leads to the formation of digital ulcers and probably other vascular manifestations of SSc. In addition, it has been reproducibly shown that disease duration of SSc is negatively correlated with circulating counts of immature PHCs [25, 27, 28, 30]. This correlation is confirmed in our series of Japanese patients with SSc as well (Fig. 3.1).
3.4 Mechanisms for Immature PHC Deficiency in SSc Patients
There are several mechanisms responsible for reduced counts of immature PHCs in SSc patients (Fig. 3.2). Tissue hypoxia caused by vascular damage and spasm induces upregulation of pro-angiogenic mediators, such as VEGF and SDF-1, which rapidly mobilize immature PHCs from the bone marrow and guide them into ischemic tissues. This response may be impaired in SSc patients potentially through mechanisms as described below. First, it is possible that persistent vascular damage eventually leads to depletion of immature PHCs. This theory is reported in patients with long-standing atherosclerosis [37]. In fact, a negative correlation between disease duration of SSc and circulating counts of immature PHCs has been reproducibly reported in several studies [25, 27, 28, 30]. This may explain reduced immature PHC counts in the later phase of the disease and may cause development of digital ulcer in clinical setting, but is irrelevant to the formation of vascular changes observed in the early SSc. Another mechanism includes impaired bone marrow microenvironment for immature PHCs despite upregulation of a series of pro-angiogenic factors. This dysregulated microenvironment within the bone marrow may suppress development and mobilization of immature PHCs. In this regard, we have recently found that immature PHC counts are inversely correlated with the level of circulating pentraxin 3, a multifunctional pattern recognition protein with capacity to inhibit angiogenesis through suppression of fibroblast growth factor-2 (FGF2) [38]. Pentraxin 3 is capable of inhibiting differentiation of bone marrow stem cells into immature PHCs in in vitro cultures with FGF2, indicating that exposure to a high concentration of pentraxin 3 suppresses the FGF2-mediated immature PHC differentiation in the bone marrow. Finally, immature PHCs in circulation may be destroyed though autoimmune mechanisms. Zhu and colleagues found that sera from SSc patients were able to induce apoptosis of immature PHCs, which was mediated through the Akt-FOXO3a-Bim pathway [23]. Moreover, George and co-workers recently reported the presence of IgG autoantibodies reactive with late-outgrowth EPCs in patients with a graduated atherosclerotic risk profile, using a cell-based enzyme-linked immunosorbent assay [39]. Interestingly, antigenic specificity of anti-late-outgrowth EPC antibodies was distinct from that of antibodies to mature endothelial cells (anti-endothelial cell antibodies, AECAs), whereas other studies found that AECAs induce apoptosis of bone marrow immature PHCs in SSc patients [40].
Mechanisms responsible for reduced counts of immature PHCs in SSc patients. Tissue hypoxia caused by vascular damage induces upregulation of pro-angiogenic mediators, such as VEGF and SDF-1, which rapidly mobilize immature PHCs from the bone marrow and guide them into ischemic tissues. This response is impaired in SSc patients through mechanisms including depletion by long-standing stimulation, defective supply and/or mobilization in the bone marrow, and destruction in circulation by autoantibodies
3.5 Altered Function in Immature PHCs in SSc Patients
Only few studies have evaluated the functional properties of EPCs in SSc patients, primarily because of difficulty of preparing sufficient numbers of these populations for functional assays. Nevertheless, we previously reported that the potential of SSc-derived immature PHCs to differentiate into mature endothelial cells was impaired in in vitro cultures of circulating CD133+ cells containing immature PHCs [22]. Another study utilizing late-outgrowth EPCs showed impaired differentiation potential to endothelial cells in cultured cells derived from SSc patients, compared with those from healthy controls [41], although their functional properties may be altered by repeated passages and exposure to high concentrations of pro-angiogenic factors in a long-term culture. We have recently established a system for evaluating the in vivo function of circulating immature PHCs, using a murine tumor neovascularization model, in which freshly isolated human CD133+ cells are transplanted into the back of mice in conjunction with syngeneic mouse tumor cells [42]. Using this assay system, we have successfully demonstrated that neovascularization capacity of circulating immature PHCs in SSc patients is impaired, partly due to deficiency in their vasculogenesis ability. Therefore, defects in vasculogenesis observed in SSc patients are likely to be mediated through impaired immature PHC function, irrespective of its quantity.
Currently, little is known about the mechanisms behind functional aberrations in circulating immature PHCs in SSc patients. In this regard, Del Papa and colleagues reported that immature PHCs in the bone marrow from SSc patients were defective in their ability to proliferate in long-term culture with pro-angiogenic factors [27], suggesting that immature PHCs were functionally altered before their release into circulation. A recent study of the bone marrow of patients with diffuse cutaneous SSc showed markedly reduced microvascular density and increased fibrosis [43]. This dysregulated microenvironment within the bone marrow may alter the developmental process of immature PHCs.
3.6 Roles of Monocytic PHCs in SSc Pathogenesis
In contrast to immature PHCs, information on the roles of monocytic PHCs in SSc vasculopathy is limited. We recently evaluated the number of monocytic PHCs in SSc patients using a culture system developed to enrich this cell population [44]. Unexpectedly, we observed a paradoxical increase in circulating monocytic PHCs in SSc patients compared with age- and sex-matched healthy controls. Intriguingly, monocytic PHCs derived from SSc patients showed enhanced in vitro tubular structure formation compared with those from healthy controls. Furthermore, in a murine tumor neovascularization model, the transplantation of SSc-derived monocytic PHCs dramatically promoted tumor growth and tumor vessel formation in vivo, indicating that monocytic PHCs derived from SSc patients have enhanced angiogenic activity. On the other hand, vasculogenic capacity of SSc-derived monocytic PHCs was impaired, same as immature PHCs.
The increased number and enhanced pro-angiogenic potency of monocytic PHCs might be a compensatory response to impaired vasculogenesis mediated through immature PHCs. Circulating monocytic PHCs are mobilized from the bone marrow and recruited to SSc-induced lesions in response to chemokines such as MCP-1 and SDF-1, which are upregulated in the affected skin of SSc patients [45, 46]. In addition, it has been reported that hypoxic condition of the affected tissues of SSc patients stimulates the differentiation of circulating monocytic PHCs [47]. Taken together, these stimulations may promote accumulation of functionally altered monocytic PHCs into affected lesions of SSc. Since monocytic PHCs are oligopotent in terms of their capacity to differentiate into mesenchymal lineage cells [48–50], they may differentiate into fibroblast-like cells, produce collagens and other extracellular matrix proteins, and participate in the fibrotic process of SSc. In this regard, fibrocytes derived from CD14+ monocytes home to the site of tissue injury and contribute to tissue repair and fibrosis by differentiating into myofibroblasts [51]. In addition, circulating CD14+ monocytes acquire the ability to produce extracellular matrix components in an MCP-1/CCR2-dependent amplification loop [52]. Our recent data on gene expression profiling of circulating CD14+ monocytes identified MCP-1 and versican as genes upregulated in SSc monocytes [53]. Since versican is a chondroitin sulfate proteoglycan with capacity to bind a variety of growth factors and chemokines, including MCP-1, and works as their reservoir, increased expression of versican by SSc monocytes may amplify MCP-1-/CCR2-dependent fibrogenic process and contribute to the fibrotic aspects of SSc. The enhanced profibrotic phenotype of circulating CD14+ monocytes was also reported in SSc patients with interstitial lung disease [54]. Another report described a correlation between fibrotic clinical features and the increased proportion of CXCR4+ circulating cells with monocytic and endothelial markers, which correspond to monocytic PHCs, in SSc patients [55]. Therefore, monocytic PHCs accumulated at affected sites of SSc may acquire profibrotic characteristics and contribute to the promotion of fibrosis.
3.7 Development of SSc Vasculopathy Mediated Through Defective Angiogenesis and Vasculogenesis
Despite the robust pro-angiogenic responses, appropriate blood vessel formation apparently does not occur in patients with SSc. Thus, impaired angiogenesis and vasculogenesis play a critical role in the pathogenic process of SSc vasculopathy. In the early phase of SSc, a variety of processes continuously damage the endothelium, leading to subsequent expression of a series of angiogenic factors, growth factors, and chemokines, including VEGF, MCP-1, and SDF-1. Normally, the denuded vessels would be rapidly fixed by a highly regulated process of vasculogenesis through recruitment of various EPCs, but, in SSc patients, vasculogenesis mediated by immature PHCs is impaired because of their reduced counts in circulation and dysfunctional maturation potential. In compensation for defect in immature PHC-mediated vasculogenesis, monocytic PHCs are recruited into circulation and function to enhance angiogenesis. But, this mechanism eventually fails to repair vessels because of local mechanisms inhibiting angiogenesis. In this regard, in microvascular endothelial cells isolated from the skin of SSc patients, matrix metalloproteinase (MMP)-12 is overexpressed and cleaves urokinase-type plasminogen activator receptor, causing inhibition of the invasion/migration capacities of endothelial cells [56, 57]. The reduction of tissue kallikreins 9, 11, and 12, which exert a mitogenic effect on endothelial cells, and the upregulation of anti-angiogenic kallikrein 3 were reported in the SSc skin [58]. In SSc skin lesions, endothelial cells lose their expression of VE-cadherin, which is required for vascular tube formation [59]. Furthermore, selective upregulation of the anti-angiogenic VEGF-b isoform was observed in the circulation and skin of SSc patients, indicating a switch from the pro-angiogenic to the anti-angiogenic VEGF isoform [60]. Finally, loss of epidermal growth factor-like domain 7 expression in endothelial cells and EPCs plays a role in the defective vascular repair process in patients with SSc [61]. These dysregulated endothelial features at the affected site of SSc are responsible for the disease-related defects in angiogenesis and prevent vascular repair. In addition, monocytic PHCs accumulated at affected sites acquire profibrotic characteristics and contribute to the promotion of fibrosis under strong anti-angiogenic and profibrotic environment.
3.8 Potential Therapeutic Strategies Targeting EPCs
There has been minimal success in treating the vascular manifestations of SSc with nonselective vasodilators. Given a critical role of impaired vasculogenesis in the pathogenic process of SSc vasculopathy, agents that modify counts and/or function of EPCs may be promising treatment modalities in patients with SSc. Table 3.3 lists currently available drugs that have been shown to have ability to increase EPC counts and/or improve EPC function [62–65]. One set of candidates is the statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors [66]. Besides their well-established lipid-lowering effect, statins have cholesterol-independent “pleiotropic” effects, which can restore or improve endothelial functions [67]. In a randomized, double-blind, placebo-controlled trial of atorvastatin on vascular manifestations of SSc, significant reductions in the onset and overall number of digital ulcers were observed in the atorvastatin group, compared with the placebo group [68]. In addition, in animal models of ischemia, statins were shown to induce recruitment of immature PHCs into the periphery and repair of injured endothelium in vivo [69–72]. Therefore, we conducted an open-label, prospective study of atorvastatin in patients with SSc [73]. Atorvastatin treatment resulted in an increase in immature PHCs in circulation, and the number returned to baseline after discontinuation of the drug. During the treatment, Raynaud’s condition score was improved, and soluble VCAM-1, an endothelial injury marker, was gradually reduced. On the other hand, simvastatin was shown to increase immature PHC counts in patients with hypercholesterolemia, but fails to increase immature PHCs in SSc patients [74], suggesting varied effects on immature PHCs among statins. We further evaluated the long-term effect of atorvastatin by an open-label study for 24 months [75]. Interestingly, immature PHC counts peaked at 1 month and gradually decreased thereafter. Nevertheless, Raynaud’s condition score and soluble VCAM-1 level remained low. These long-term effects of statins on peripheral vascular disease cannot be explained by mobilization of immature PHCs alone, and it is likely that other pleiotropic effects of statins, such as improvement of endothelial function, play a role. Other small molecules and recombinant growth factors, including G-CSF and erythropoietin, may provide favorable effects on vascular manifestations in SSc patients, although there is no data supporting their efficacy to date. In addition, continuous exercise program is also shown to promote mobilization of immature PHCs [76].
We have recently found that cyclophosphamide, which is an immunosuppressant proved to be effective for treating interstitial lung disease associated with SSc, also has capacity to increase circulating immature PHCs. Cyclophosphamide is capable of mobilizing hematopoietic stem cells/progenitors from the bone marrow when a high dose was administered intravenously, but it remains unknown if low-dose regimen used for SSc patients mobilized immature PHCs as well. To evaluate the effects of intravenous cyclophosphamide (IVCY) on immature PHC counts, we performed an open-label, prospective study involving SSc patients treated with six courses of IVCY in combination with low-dose prednisolone [77]. As a result, IVCY plus corticosteroids, but not corticosteroids alone, increased the circulating counts of immature PHCs in nearly half of the patients. Immature PHC responders showed trends toward reduced levels of circulating vascular injury markers and a low probability of developing end-stage lung disease, while nonresponders did not. These findings suggest that mobilization of immature PHCs may contribute to the efficacy of IVCY for treating SSc-associated interstitial lung disease.
3.9 Conclusion
Immature and monocytic PHCs contribute to postnatal blood vessel formation and vascular repair, mainly through their immediate recruitment to the site of vascular injury, their secretion of a variety of pro-angiogenic factors, and their differentiation into endothelial cell-like cells. These cells are also oligopotent and can differentiate into fibroblast-like cells. These unique features raise the intriguing hypothesis that EPCs are involved in the pathogenesis of SSc by participating in two major pathological aspects of the disease, microvasculopathy and excessive fibrosis. Understanding the roles of EPCs in disease process of SSc may be key to dissecting its pathogenesis and to developing novel therapeutic strategies.
References
Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol. 2006;176:157–212.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
Guiducci S, Giacomelli R, Matucci-Cerinic M. Vascular complications of scleroderma. Autoimmun Rev. 2007;6:520–3.
Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;110:590–4.
Herrick A, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 2010;62:2595–604.
Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface. 2010;7:S731–51.
Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9.
Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom. 2010;52:9.33.1–11.
Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–103.
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8.
Richardson MR, Yoder MC. Endothelial progenitor cells: Quo Vadis? J Mol Cell Cardiol. 2011;50:266–72.
Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–55.
Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–43.
Rehman J, Li JL, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9.
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42.
Yamaguchi Y, Kuwana M. Proangiogenic hematopoietic cells of monocytic origin: roles in vascular regeneration and pathogenic processes of systemic sclerosis. Histol Histopathol. 2013;28:175–83.
Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–7.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–67.
Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60.
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9.
Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364:603–10.
Zhu S, Evans S, Yan B, Povsic TJ, Tapson V, Goldschmidt-Clermont PJ, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118:2156–65.
Mok MY, Yiu KH, Wong CY, Qiuwaxi J, Lai WH, Wong WS, et al. Low circulating level of CD133+KDR+ cells in patients with systemic sclerosis. Clin Exp Rheumatol. 2010;28:S19–25.
Andrigueti FV, Arismendi MI, Ebbing PC, Kayser C. Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvasc Res. 2015;98:82–7.
Del Papa N, Colombo G, Fracchiolla N, Moronetti LM, Ingegnoli F, Maglione W, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50:1296–304.
Del Papa N, Quirici N, Soligo D, Scavullo C, Cortiana M, Borsotti C, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54:2605–15.
Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–6.
Avouac J, Juin F, Wipff J, Couraud P, Chiocchia G, Kahan A, et al. Circulating endothelial progenitor cells in systemic sclerosis: association with disease severity. Ann Rheum Dis. 2008;67:1455–60.
Nevskaya T, Bykovskaia S, Lyssuk E, Shakhov I, Zaprjagaeva M, Mach E, et al. Circulating endothelial progenitor cells in systemic sclerosis: relation to impaired angiogenesis and cardiovascular manifestations. Clin Exp Rheumatol. 2008;26:421–9.
Distler JH, Allanore Y, Avouac J, Giacomelli R, Guiducci S, Moritz F, et al. EULAR Scleroderma Trials and Research group statement and recommendations on endothelial precursor cells. Ann Rheum Dis. 2009;68:163–8.
Kuwana M, Okazaki Y. Quantification of circulating endothelial progenitor cells in systemic sclerosis: a direct comparison of protocols. Ann Rheum Dis. 2012;71:617–20.
Avouac J, Meune C, Ruiz B, Couraud PO, Uzan G, Boileau C, et al. Angiogenic biomarkers predict the occurrence of digital ulcers in systemic sclerosis. Ann Rheum Dis. 2012;71:394–9.
Avouac J, Vallucci M, Smith V, Senet P, Ruiz B, Sulli A, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther. 2013;15(2):R55.
Chen H, Strappe P, Chen S, Wang LX. Endothelial progenitor cells and pulmonary arterial hypertension. Heart Lung Circ. 2014;23:595–601.
Malli F, Koutsokera A, Paraskeva E, Zakynthinos E, Papagianni M, Makris D, et al. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: an evolving concept. PLoS One. 2013;8:e53658.
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7.
Shirai Y, Okazaki Y, Inoue Y, Tamura Y, Yasuoka H, Takeuchi T, et al. Elevated levels of pentraxin 3 in systemic sclerosis: associations with vascular manifestations and defective vasculogenesis. Arthritis Rheumatol. 2015;67:498–507.
George J, Matucci-Cerinic M, Bar I, Shimoni S. Circulating autoantibodies to endothelial progenitor cells: binding characteristics and association with risk factors for atherosclerosis. PLoS One. 2014;9:e97836.
Del Papa N, Quirici N, Scavullo C, Gianelli U, Corti L, Vitali C, et al. Antiendothelial cell antibodies induce apoptosis of bone marrow endothelial progenitors in systemic sclerosis. J Rheumatol. 2010;37:2053–63.
Avouac J, Wipff J, Goldman O, Ruiz B, Couraud PO, Chiocchia G, et al. Angiogenesis in systemic sclerosis: impaired expression of vascular endothelial growth factor receptor 1 in endothelial progenitor-derived cells under hypoxic conditions. Arthritis Rheum. 2008;58:3550–61.
Kuwana M, Okazaki Y. Impaired in vivo neovascularization capacity of endothelial progenitor cells in patients with systemic sclerosis. Arthritis Rheumatol. 2014;66:1300–5.
Carrai V, Miniati I, Guiducci S, Capaccioli G, Alterini R, Saccardi R, et al. Evidence for reduced angiogenesis in bone marrow in SSc: immunohistochemistry and multiparametric computerized imaging analysis. Rheumatology. 2012;51:1042–8.
Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z, et al. Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther. 2010;12:R205.
Distler O, Pap T, Kowal-Bielecka O, Meyringer R, Guiducci S, Landthaler M, et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum. 2001;44:2665–78.
Cipriani P, Franca Milia A, Liakouli V, Pacini A, Manetti M, Marrelli A, et al. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic implications. Arthritis Rheum. 2006;54:3022–33.
Bellik L, Musilli C, Vinci MC, Ledda F, Parenti A. Human mature endothelial cells modulate peripheral blood mononuclear cell differentiation toward an endothelial phenotype. Exp Cell Res. 2008;314:2965–74.
Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32.
Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45.
Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22.
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62.
Sakai N, Wada T, Fruichi K, Shimizu K, Kokubo S, Hara A, et al. MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14-positive monocytes. J Leukoc Biol. 2006;79:555–63.
Masuda A, Yasuoka H, Satoh T, Okazaki Y, Yamaguchi Y, Kuwana M. Versican is upregulated in circulating monocytes in patients with systemic sclerosis and amplifies a CCL2-mediated pathogenic loop. Arthritis Res Ther. 2013;15:R74.
Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–23.
Campioni D, Lo Monaco A, Lanza F, Moretti S, Ferrari L, Fotinidi M, et al. CXCR4+ circulating progenitor cells coexpressing monocytic and endothelial markers correlating with fibrotic clinical features are present in the peripheral blood of patients affected by systemic sclerosis. Haematologica. 2008;93:1233–7.
D’Alessio S, Fibbi G, Cinelli M, Guiducci S, Del Rosso A, Margheri F, et al. Matrix metalloproteinase 12-dependent cleavage of urokinase receptor in systemic sclerosis microvascular endothelial cells results in impaired angiogenesis. Arthritis Rheum. 2004;50:3275–85.
Margheri F, Manetti M, Serratì S, Nosi D, Pucci M, Matucci-Cerinic M, et al. Domain 1 of the urokinase-type plasminogen activator receptor is required for its morphologic and functional, β2 integrin-mediated connection with actin cytoskeleton in human microvascular endothelial cells: failure of association in systemic sclerosis endothelial cells. Arthritis Rheum. 2006;54:3926–38.
Giusti B, Serratì S, Margheri F, Papucci L, Rossi L, Poggi F, et al. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 2005;52:3618–28.
Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452.
Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res. 2011;109:e14–26.
Manetti M, Guiducci S, Romano E, Avouac J, Rosa I, Ruiz B, et al. Decreased expression of the endothelial cell-derived factor EGFL7 in systemic sclerosis: potential contribution to impaired angiogenesis and vasculogenesis. Arthritis Res Ther. 2013;15:R165.
Roks AJ, Rodgers K, Walther T. Effects of the renin angiotensin system on vasculogenesis-related progenitor cells. Curr Opin Pharmacol. 2011;11:162–74.
Schwartz BG, Jackson G, Stecher VJ, Campoli-Richards DM, Kloner RA. Phosphodiesterase type 5 inhibitors improve endothelial function and may benefit cardiovascular conditions. Am J Med. 2013;126:192–9.
Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complications. 2013;27:519–25.
Sanchis-Gomar F, Garcia-Gimenez JL, Pareja-Galeano H, Romagnoli M, Perez-Quilis C, Lippi G. Erythropoietin and the heart: physiological effects and the therapeutic perspective. Int J Cardiol. 2014;171:116–25.
Kuwana M. Potential benefit of statins for vascular disease in systemic sclerosis. Curr Opin Rheumatol. 2006;18:594–600.
Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15:467–78.
Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801–8.
Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–10.
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7.
Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405.
Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24.
Kuwana M, Kaburaki J, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Increase in circulating endothelial precursors by atorvastatin in patients with systemic sclerosis. Arthritis Rheum. 2006;54:1946–51.
Del Papa N, Cortiana M, Vitali C, Silvestris I, Maglione W, Comina DP, et al. Simvastatin reduces endothelial activation and damage but is partially ineffective in inducing endothelial repair in systemic sclerosis. J Rheumatol. 2008;35:1323–8.
Kuwana M, Okazaki Y, Kaburaki J. Long-term beneficial effects of statins on vascular manifestations in patients with systemic sclerosis. Mod Rheumatol. 2009;19:530–5.
Ribeiro F, Ribeiro IP, Alves AJ, do Céu Monteiro M, Oliveira NL, Oliveira J, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil. 2013;92:1020–30.
Furuya Y, Okazaki Y, Kaji K, Sato S, Takehara K, Kuwana M. Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis: potential association with efficacy for interstitial lung disease. Rheumatology. 2010;49:2375–80.
Acknowledgments
This work was supported by a research grant on intractable diseases from the Japanese Ministry of Health, Labour and Welfare and a grant from the Japanese Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Kuwana, M. (2016). Endothelial Progenitor Cells. In: Takehara, K., Fujimoto, M., Kuwana, M. (eds) Systemic Sclerosis. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55708-1_3
Download citation
DOI: https://doi.org/10.1007/978-4-431-55708-1_3
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55707-4
Online ISBN: 978-4-431-55708-1
eBook Packages: MedicineMedicine (R0)