Abstract
In the post-H. pylori eradication era, the clinical significance of gastric non-H. pylori helicobacters (NHPH; also referred to as H. heilmannii-like organisms and H. heilmannii sensu lato) is gradually increasing. This group of bacteria may inhabit the stomach of domestic and wild animals including cats, dogs, pigs, primates, rodents, cheetahs, and rabbits. NHPH are zoonotic microorganisms, meaning that they may transmit between animals and humans. They may be distinguished from H. pylori regarding their microbiology involving larger cells with more distinct spiral shape and bipolarity, localization in the stomach layer and regional distribution, urease activity and virulence factors, and relation to gastric diseases where gastric NHPH infection is often associated with milder gastritis than H. pylori but higher risk of gastric MALT lymphoma. At present, pure culture of NHPH species remains a challenge, but the full genome sequences of some of the species have been reported. Recent and ongoing prevalence studies indicate a higher clinical relevance of these bacteria than earlier impressions suggested. Current efforts in improving cultivation and detection methodology are contributing to an increased understanding of their microbiology, prevalence, and relevance to human diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Recently, many spiral bacteria belonging to the Helicobacter genus other than H. pylori have been described in many tissues, organs, and animals. They are generally longer than H. pylori with a size ranging from 4 to 10 μm, spiral shaped, highly motile with three to eight coils, up to 14 uni- or bipolar flagellae, and no periplasmic filaments [1]. These bacteria also differ from H. pylori when considering traits such as zoonosis, distribution/sublocations in the stomach, relation to gastric diseases, urease activity, and response to antibiotics. To date, 35 species belonging to the Helicobacter genus have been identified (Fig. 8.1) that may be divided into gastric helicobacters and enterohepatic helicobacters. In this chapter, we would like to focus on the gastric helicobacters and further aim at the gastric helicobacters other than H. pylori, the so-called non-H. pylori helicobacters (NHPH), and discuss these bacteria regarding their microbiology, pathogenesis, and significance for human health.
2 History
At first, we would like to introduce the reader to the somewhat complicated story of NHPH and nomenclature as this has been subject to some confusion in discussions and in published papers. In fact, the first discovery of spiral-shaped microorganism in the stomach from an animal dates back to 1881, a study conducted by Rappin using light microscopy [2]. Bizzozero and Salomon also showed similar studies [3, 4], and in 1906 the German scientist Krienitz reported spiral-shaped microorganisms in the human stomach [5]. In 1919, Kasai and Kobayashi were the first to achieve an interventional study by using salvarsan, an arsene compound, to treat infection [6]. In 1962, Weber et al. found spiral-shaped bacteria in the gastric mucosa of cats and dogs by using electron microscopy [7]. As 1984 will be remembered for the paramount discovery of gastric H. pylori by Warren and Marshall [8], 3 years later was marked by a German report by Heilmann who detected gastric spiral bacteria in 39 patients (0.25 %) with upper gastrointestinal symptoms. The report was later published in an English journal [9]. Among the patients examined 34 patients had chronic active gastritis and 4 had chronic gastritis, and they were all treated with the bismuth treatment. Following this report, Dent and McNulty found spiral-shaped bacteria in 6 human cases and further reported that they were incapable of cultivating these bacteria in vitro [10, 11]. They designated these spiral-shaped bacteria Gastrospirillum hominis. In 1988, they proposed that these spiral-shaped bacteria could transmit from cat to human [12]. Three years later, Ito and Takahashi found urease-positive gastric bacteria in a cynomolgus monkey [13], a strain that would turn out to be used for later experimentation [14]. In 1993, Solnick et al. analyzed the 16S rRNA of this bacterium and discovered that this bacterium was phylogenetically close to H. felis [15]. Gastrospirillum hominis was from that point renamed to H. heilmannii. Further phylogenetic analyses revealed that H. heilmannii did not only encompass one species, but rather a group of bacteria. Further renaming resulted in H. heilmannii type 1 and 2, of which type 1 was identical to the type found in pigs also called H. suis [16]. Type 2 represents a group of bacteria which includes, for instance, H. felis, H. bizzozeronii, and Candidatus H. heilmannii. Challenges in in vitro cultivation have hampered identification and phylogenetic analyses of these bacteria, but recent efforts have resolved many of the species that belong to the Helicobacter genus. In order to avoid further confusion, Haesebrouck proposed to use the terms H. heilmannii sensu stricto and sensu lato [17, 18]. Another commonly applied term is H. heilmannii-like organisms (HHLO), which may be further subdivided into types 1, 2, and 4 [19]. More recently, the term non-H. pylori helicobacters (NHPH) has been applied as it covers all “the other” helicobacters than H. pylori without manifesting to one such species of historical reason [20]. The term is per definition the same as H. heilmannii s.l. and will be used throughout this chapter.
3 Characteristics of NHPH
3.1 Zoonosis
Unlike H. pylori, NHPH show a zoonotic infection pattern. Reported animal hosts for gastric NHPH include dogs, cats, primates, pigs, cheetahs, rodents, and rabbits [21]. Stolte et al. conducted a study in which the relationship between infection in humans and contact with domestic animals was made. In NHPH-positive cases, 70 % of the patients had a history of contact with pet animals, while in the general population, 37 % has a history of contact with pet animals [22]. In addition, the rate of coinfection with NHPH and H. pylori was found to be very low, suggesting that NHPH infection might conflict with and somehow prevent an H. pylori infection (although opposing reports do exist as mentioned later in this chapter). Another report demonstrated the transmission of NHPH from a cat to a veterinarian who was treating the cat [23]. The relationship between pet keeping and risk of infection for NHPH has also been demonstrated in a prevalence study conducted in Korea by Chung and colleagues [24]. Although transmission from animals to humans is a commonly accepted concept, studies that raise important questions also exist. For example, Priestnall et al. have reported that the NHPH most transmitted to human is HHLO type 1, or H. suis, which is a pig-specific species of NHPH. The type of NHPH predominant in cats and dogs are presumably HHLO type 2 and 4, suggesting that pet keeping is not necessarily the main transmission route of NHPH from animal to human [18]. A prevalence study conducted in China of more than 1500 patients all positive for H. pylori showed that of those who were coinfected with NHPH, about half was infected with H. suis [25]. An ongoing prevalence study in Japan by Øverby and Nakamura of gastric disease patients negative for H. pylori also suggests the pig-specific species H. suis to be the predominant type found in humans. Considering the number of people who are in contact with pet animals compared to the number of people who are in contact with live pigs, it is likely that there is a missing piece to this controversial puzzle of how humans are infected with NHPH. Flahou and Haesebrouck have shown that NHPH may be detected in minced meat from pork from the supermarket, suggesting that transmission through diet may also be a possibility [26].
3.2 Distribution in the Stomach
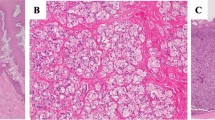
As for the localization in the gastric mucosa, it has been reported that the majority of the bacteria inhabit the mucus layer like H. pylori but that they also may reside in the fundic glandular tissue as shown, which is clearly detected in our histochemical observation in Fig. 8.2 [27]. A more precise pathological observation revealed that most of the cells residing in the fundic gland were actually in the intracellular canaliculi in the parietal cells [28]. However, by using electron microscopy, some of the bacteria were found to be localized in the lamina propria mucosae as well as in the intracellular canaliculus. This resulted in the adjacent parietal cells to become apoptotic suggesting a relation to gastric MALT lymphoma formation [23].
Immunohistochemical observation of Helicobacter suis in the human fundic mucosa. (a, b) Helicobacter suis are localized in the base of the fundic mucosa as well as in the mucus layer above the surface epithelial cells. (c, d) In the base of the fundic glands, Helicobacter suis are localized mainly in the actin-rich parietal cells. (a, c) Stained with Helicobacter antibody to the Helicobacter suis-positive human gastric mucosa. (b, d) stained with FITC-phalloidin showing the actin-rich erythrocytes in the tip portion and parietal cells in the base of the fundic mucosa. (a, b) x200, (c, d) x800
3.3 Prevalence of NHPH
Up until 2001, O’Rourke performed meta-analysis of 500 NHPH-positive cases [29–31], and she found that the infection rate is very diverse. In Western countries, the prevalence of NHPH in the general population was less than 0.5 %, while in East Europe and Asia, it varied from 1.2 % to 6.2 %. In a prevalence study conducted in China mentioned above with H. pylori-positive patients with symptoms of a gastric disease, close to 12 % was shown to coinhabit an NHPH infection [32]. In an ongoing study in Japan based on patients with a gastric disease but negative for H. pylori, more than half appear to be infected with NHPH (Øverby and Nakamura, unpublished data).
3.4 NHPH and Human Diseases
Stolte et al. compared the disease formation by H. pylori and NHPH and found that NHPH induced a milder gastritis than H. pylori but that NHPH was a stronger inducer of gastric MALT lymphoma than H. pylori [33, 34]. They used histochemistry, specific immunoabsorbent method, and PCR analysis using 16S rDNA, and five MALT lymphoma cases were H. pylori negative and NHPH positive. From the survey of the patients from 1988 to 1998, 1745 out of 263, 680 cases were H. pylori positive (0.66 %), while 8 out of 543 cases were NHPH positive (1.47 %) and confirmed the stronger relation of NHPH to the gastric MALT lymphoma formation. Okiyama et al. found 15 NHPH-positive cases out of 4074 serial cases, among these 11 patients constituted chronic gastritis cases, and 4 patients were diagnosed with gastric MALT lymphoma [35]. The relationship between NHPH infection and gastric cancer is still a controversional topic. Foschini et al. have reported that all NHPH-positive cases had gastritis, and 1 gastric cancer case was coinfected with NHPH and H. pylori [36]. The relation to the gastric dysplasia to NHPH infection was suggested in cases in Thailand [31]. Sasaki has recently reported a patient case positive for NHPH infection and with nodular gastritis, which is thought to be one of the precancerous lesions of the gastric cancer [37].
3.5 Diagnosis, Culture, and Genome Sequence Studies
Detection of NHPH may be performed with different approaches. However, PCR analysis of the 16S rDNA is the most reliable method but is also time-consuming. The pathological identification of gastric spiral bacteria may sometimes be inaccurate, as under certain conditions H. pylori, for instance, may display a different morphology than typically seen including a longer shape [38]. At present, serum serological test and fecal bacterial detection tests are not available for NHPH, somewhat limiting the detection methodology in patients. Difficulties in cultivation NHPH have hampered the identification of these microorganisms. Lee et al. reported culture of a spiral bacterium isolated from the antrum area of the stomach from a cat [39]. In 1996, Andersen et al. reported a presumably successful cultivation of H. heilmannii (s. s.) but later turned out to be identified as H. bizzozeronii [40, 41]. As a new diagnostic method, Trebesius et al. used fluorescent in situ hybridization of the 16S rDNA and reported five species of NHPH from human cases, most of which coincided with H. suis [42]. In 2003, Chisholm et al. invented a novel PCR method for NHPH detection from biopsy specimen and found 2.3 % positive cases from dyspeptic cases in New England, which was quite higher compared with their former report highlighting the importance of appropriate detection methodology [43]. In 2004, O’Rourke et al. analyzed 26 human and animal samples positive for NHPH infection and showed that 15 (28 %) of these were very infected with H. suis. The rest were identified as H. felis, H. bizzozeronii, H. salomonis, and Candidatus H. heilmannii [44]. In 2008, Baele et al. reported the first pure culture of H. suis isolated from pig stomach [45]. In 2011, the whole genome sequences of H. suis and H. felis were reported [46, 47]. When compared with H. pylori, the genome sequence of H. suis was shown to lack cagA but did contain hpaA and horH; comB, related to type IV secretory system and similar genes to H. pylori neutrophil-activating protein; γ-glutamyl transpeptidase (GGT); and flavodoxin vacuolating cytotoxin A gene. In H. felis, the genome sequence was shown to lack cagPAI and vacA but contain comB, GGT-encoding gene, immunomodulator (napA), collagenase and secretory serine protease htrA, and several chemotaxis sensors and restriction/modification system [48]. The sequence of H. bizzozeronii isolated from a gastritis patient [49] and the sequence of H. heilmannii isolated from cat [50] were subsequently reported.
4 Conclusion
Infection with the zoonotic NHPH is becoming an increasing issue in the clinic and eventually in the general population. Recent studies have shown NHPH to be linked to human gastric diseases especially gastric MALT lymphoma, require up-to-date sensitive methodology in order to be detected, and be more prevalent than what was previously thought. In the post-H. pylori eradication era, gastric infection with NHPH is likely to become a significant burden and should be subject to further investigation in order to resolve its issues linked with gastric MALT lymphoma and gastric cancer.
References
Stoffel MH, Friess AE, Burnens A, Schmassmann A, Neiger R. Distinction of gastric Helicobacter spp. in humans and domestic pets by scanning electron microscopy. Helicobacter. 2000;5:232–9.
Rappin J. Contre a l’etude de bacteri de la bouche a l’etat normal.1881;68. In: Breed RS, Murray EGD, Hitchens AP, editors. Bergey’s manual of determinative bacteriology. 6th ed. Baltimore: Williams and Wilkins Co; 1948. p. 217.
Bizzozero G. Ueber die schlauchformigen drusen desmagendarmkanals und die beziehungen ihres epithels zu demoberflachenepithel der schleimhaut. Archivfur MikrofkopischeAnatomie Entwickiungs mechanik. 1893;42:82.
Salomon H. Ueber das spirillum des saugetiermagens und seinverhalten zu den belegzellen. Centralblatt fur Bakteriologie, Parasitenkunde V Infektionskrankheiten. 1896;XIX:433–43.
Krienitz W. Ueber das Auftreten von Spirochaten verscheidener Form im Mageninhalt bei Carcinoma ventriculi. Dtsch Med Wocknenschr. 1906;32:872.
Kasai K, Kobayashi R. The stomach spirochete occurring in mammals. J Parasitol. 1919;6:1–11.
Weber AF, Schmittdiel EF. Electron microscopic and bacteriologic studies of spirilla isolated from the fundic stomachs of cats and dogs. Am J Vet Res. 1962;23:422–7.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–5.
Heilmann KL, Bochard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–40.
Dent JC, McNulty CA, Uff JC, Wilkinson SP, Gear MW. Spiral organisms in the gastric antrum. Lancet. 1987;11:96.
McNulty CA, Dent JC, Curry A, Uff JS, Ford GA, Gear MW, Wilkinson SP. New spiral bacterium in gastric mucosa. J Clin Pathol. 1989;42:585–91.
Dye KR, Marshall BJ, Fnerson HF, Onerrant RT, McCall RW. Gastritis in a human due to infection with an organism resembling the cat gastric spirillum. Gastroenterology. 1988;94:A108.
Itoh T, Yanagawa M, Singaki N, Masubuchi N, Takahashi S, Saito S. Isolation of Helicobacter heilmannii like organism from the stomachs of cynomolgus monkey and colonization of them in mice. Gastroenterology. 1994;106:A99.
Nakamura M, Murayama SY, Serizawa H, Sekiya Y, Eguchi M, Takahashi S, Nishikawa K, Takahashi T, Matsumoto T, Yamada H, Hibi T, Tsuchimoto K, Matsui H. “Candidatus Helicobacter heilmannii” from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75:1214–22.
Solnick JV, O’Rourke J, Lee A, Paster BJ, Dewhirst FE, Tompkins LS. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–85.
Mendes EN, Queiroz DM, Rocha GA, Moura SB, Leite VH, Fonseca ME. Ultrastructure of a spiral micro-organism from pig gastric mucosa (“Gastrospirillum suis”). J Med Microbiol. 1990;33:61–6.
De Groote D, Ducatelle R, van Doorn LJ, Tilmant K, Quint WGV, Verschuurn A, Haesebrouck F. Detection of “Candidatus Helicobacter suis” in gastric samples of pig by PCR: comparison with other invasive diagnostic techniques. J Clin Microbiol. 2000;38:1131–5.
Haesebrouck F, Pasmans F, Flahou B, Smet A, Vandamme P, Ducatelle R. Non-Helicobacter pylori Helicobacter species in the human gastric mucosa: a proposal to introduce the terms H. heilmannii sensu lato and sensu stricto. Helicobacter. 2011;16:339–40.
Priestnall SL, Wiinberg B, Spohr A, Neuhaus B, Kuffer M, Wiedmann M, Simpson W. Evaluation of “Helicobacter heilmannii” subtypes in the gastric mucosas of cats and dogs. J Clin Microbiol. 2004;42:2144–51.
Fox JG. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50:273–83.
Van den Bulck K, Baele M, Hermans K, Ducatelle R, Haesebrouck F, Decostere A. First report on the occurrence of “Helicobacter heilmannii” in the stomach of rabbits. Vet Res Commun. 2005;29:271–27.
Stolte M, Wellens E, Bethke B, Ritter M, Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994;29:1061–4.
Lavelle JP, Landas S, Mitros FA, Conklin JL. Acute gastritis associated with spiral organisms from cats. Dig Dis Sci. 1994;39:744–50.
Chung T-H, Kim H-D, Lee Y-S, Hwang C-Y. Determination of the prevalence of Helicobacter heilmannii-like organisms type 2 (HHLO-2) infection in humans and dogs using non-invasive genus/species-specific PCR in Korea. J Vet Med Sci. 2014;76:73–9.
Liu J, He L, Haesebrouck F, Gong Y, Flahou B, Cao Q, Zhang J. Prevalence of coinfection with gastric Non-Helicobacter pylori Helicobacter (NHPH) species in Helicobacter pylori-infected patients suffering from gastric disease in Beijing, China. Helicobacter. 2015;20:284–90. doi:10.1111/hel.12201.
De Cooman L, Flahou B, Houf K, Smet A, Ducatelle R, Pasmans F, Haesebrougk F. Survival of Helicobacter suis bacteria in retail pig meat. Int J Food Microbiol. 2013;166(1):164–7. doi:10.1016/j.ijfoodmicro.2013.05.020.
Carnot P, Lelievre A. Morphologie du product d’excretion des cellules bordants. Comptes Rendus Soc Biol. 1909;66:311–3.
Regard C. Sur une curieuse lacalisation de spirilles parasites dans les canalisations glandulaires de la gastrique normale, chez le chien et le chat. Soc Biol. 1909;66:229–31.
Kubonova K, Trupl J, Jancula L, Polák E, Vráblik V. Presence of spiral bacteria (‘Gastrospirillum hominis’) in the gastric mucosa. Eur J Clin Microbial Infect Dis. 1991;10:459–60.
Yang HT, Goliger JA, Song M, Zhou D. High prevalence of Helicobacter heilmannii infection in China. Dig Dis Sci. 1998;43:1493.
Yali Z, Yamada N, Wen M, Matsuhisa T, Miki M. Gastrospirillum hominis and Helicobacter pylori infection in Thai individuals – comparison of histopathological changes of gastric mucosa. Pathol Int. 1998;48:507–11.
Kato S, Ozawa K, Sekine H, Ohyauchi M, Shimosegawa T, Minoura T, Iinuma K. Helicobacter heilmannii infection in a child after successful eradication of Helicobacter pylori: case report and review of literature. J Gastroenterol. 2005;40:94–7.
Stolte M, Kroher G, Meining A, Morgner A, Bayerdörffer E, Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997;32:28–33.
Morgner A, Lehn N, Andersen LPP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–8.
Okiyama Y, Matsuzawa K, Hidaka E, Sano K, Akamatsu T, Ota H. Helicobacter heilmannii infection: clinical, endoscopic and histopathological features in Japanese patients. Pathol Int. 2005;55:398–404.
Foschini MP, Pieri F, Cerasoli S, Accardo P, Formica G, Biasiucci A, Donzelli C, et al. Helicobacter heilmannii: anatomo-clinical study of 14 new cases. Pathologica. 1999;91:18–24.
Sasaki M, Goji S, Tamura Y, Nakamura M, Matsui H, Murayama SY, Ebi M, Ogasawara N, Funai Y, Kasugai K. Helicobacter suis-infected nodular gastritis and a review of diagnostic sensitivity for Helicobacter heilmannii-like organisms. Case Rep Gastroenterol. 2015;9:179–87.
Vinette KM, Gibney KM, Proujansky R, Fawcett PT. Growth of Helicobacter pylori in a long spiral form does not alter expression of immunodominant proteins. BMC Microbiol. 2002;2:24.
Lee A, Dent J, Hazell S, McNulty C. Origin of spiral organisms in human gastric antrum. Lancet. 1988;1(8580):300–1.
Andersen LP, Norgaard A, Holck S, Blom J, Elsborg L. Isolation of a Helicobacter heilmannii-like organism from the human stomach. Eur J Clin Microbiol Infect Dis. 1996;15:95–6.
Jalava K, On SLW, Harrington CS, Andersen LP, Hanninen ML, Vandamme P. A cultured strain of “Helicobacter heilmannii”, a human gastric pathogen, identified as H. bizzozeronii: evidence for zoonotic potential of Helicobacter. Emerg Infect Dis. 2001;7:1036–8.
Trebesius K, Adler K, Vieth M, Stolte M, Haas R. Specific detection and prevalence of Helicobacter heilmannii-like organisms in the human gastric mucosa by fluorescent in situ hybridization and partial 16S ribosomal DNA sequencing. J Clin Microbiol. 2001;39:1510–6. doi:10.1128/JCM.39.4.1510-1516.2001.
Chisholm SA, Owen RJ. Development and application of a novel screening PCR assay for direct detection of ‘Helicobacter heilmannii’-like organisms in human gastric biopsies in Southeast England. Diagn Microbiol Infect Dis. 2003;46(1):1–7.
O’Rourke JL, Solnick JV, Neilan BA, Seidel K, Hayter R, Hansen LM, Lee A. Description of “Candidatus Helicobacter heilmannii” based on DNA sequence analysis of 16S rRNA and urease genes. Int J Syst Evol Microbiol. 2004;54:2203–11.
Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Chiers K, Ducatelle R, Haesebrouck F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58:1350–8.
Vermoote M, Vandekerckhove TTM, Flahou B, Pasmans F, Smet A, De Groote D, Van Criekinge W, et al. Genome sequence of Helicobacter suis supports its role in gastric pathology. Vet Res. 2011;42:51.
Arnold IC, Zigova Z, Holden M, Lawley TD, Rad R, Dougan G, Falkow S, et al. Comparative whole genome sequence analysis of the carcinogenic bacterial model pathogen Helicobacter felis. Genome Biol Evol. 2011;3:302–8.
Schott T, Rossi M, Hänninen M-L. Genome sequence of Helicobacter bizzozeronii strain CIII-1, an isolate from human gastric mucosa. J Bacteriol. 2011;193:4565–6.
Smet A, Van Nieuwerburgh F, Ledesma J, Flahou B, Deforce D, Ducatelle R, Haesebrouck F. Genome sequence of Helicobacter heilmannii sensu stricto ASB1 isolated from the gastric mucosa of a kitten with severe gastritis. Genome Announc. 2013;1:e00033–12.
Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457–66.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Nakamura, M. et al. (2016). Gastric Non-Helicobacter pylori Helicobacter: Its Significance in Human Gastric Diseases. In: Suzuki, H., Warren, R., Marshall, B. (eds) Helicobacter pylori. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55705-0_8
Download citation
DOI: https://doi.org/10.1007/978-4-431-55705-0_8
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55704-3
Online ISBN: 978-4-431-55705-0
eBook Packages: MedicineMedicine (R0)