Abstract
Since the first description of the human pathogen Helicobacter pylori in the early 1980s, the number of known species in the genus Helicobacter has increased largely. Currently, 45 different Helicobacter species have been identified. Bacteria belonging to this genus can roughly be divided into two major groups, gastric and enterohepatic species. Gastric helicobacters express urease at a high level which helps them to survive in the acidic environment of the stomach, whereas most enterohepatic helicobacters do not. The best-known gastric Helicobacter species is H. pylori. This chapter, however, deals with non-H. pylori helicobacters (NHPH). Most NHPH are animal-associated bacteria, but some of them are of zoonotic significance. First, gastric infections with these bacteria in humans are considered. Thereafter, an overview of natural and experimental gastric infections in animal hosts is given, with emphasis on the gastric helicobacters that are mainly associated with dogs, cats, and pigs. Finally, enterohepatic Helicobacter species are briefly discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastric non-Helicobacter pylori helicobacters
- Enterohepatic helicobacters
- Zoonotic significance
- Gastric disease
- Enterohepatic disease
- Animal models
1 Introduction
Since the original description of the human pathogen H. pylori in 1983, the number of known species in the genus Helicobacter has increased largely (Warren and Marshall 1983). Currently this genus includes 45 identified species. An overview is shown in Fig. 10.1. The Helicobacter bacteria can roughly be divided into gastric and enterohepatic species. Gastric Helicobacter species are able to survive the acidic environment in the stomach by expressing high levels of urease (Pot et al. 2007). Enterohepatic species do not normally colonize the gastric mucosa . Instead, they thrive at the mucosal surface of the intestinal tract and/or the liver (Sterzenbach et al. 2007). To date, Helicobacter spp. have been detected in nearly 150 vertebrate species, including animals from every continent and all four non-fish vertebrate taxonomic classes (Schrenzel et al. 2010). Animal-associated helicobacters, and especially the gastric species, are characterized by their extremely fastidious nature, which to date has resulted in a low number of in vitro isolates available worldwide (Haesebrouck et al. 2009). Several of these species have a pathogenic potential in different animal hosts, and some are capable of causing disease in humans (Table 10.1). The presence and diversity of helicobacters in the vertebrate fauna and their transfer possibilities between hosts are critical factors on how fast the Helicobacter ecology will evolve and what their impact is on animal and human health (Schrenzel et al. 2010). This chapter mainly focuses on the biology and pathogenesis of animal-associated gastric Helicobacter infections in humans and animals. An overview of the significance of enterohepatic Helicobacter spp. in human and animal disease is summarized at the end of this chapter.
Phylogenetic tree based on the near-complete 16s rRNA gene sequences from all gastric and enterohepatic Helicobacter species described so far. The alignment of the sequences and the construction of the phylogenetic tree were performed as described before (Smet et al. 2012)
2 Gastric Helicobacter Infections in Humans
In the early years of H. pylori research, pathologists examining human gastric biopsies already reported the presence of bacteria with a long spiral-shaped morphology. These microorganisms were similar to bacteria reported in the stomach of pigs, cats, dogs and nonhuman primates and were originally referred to as Gastrospirillum hominis (McNulty et al. 1989). Later on, they were renamed to H. heilmannii (Heilmann and Borchard 1991). Although at that time the name H. heilmannii had no official standing in nomenclature, it was used for many years to refer to the group of long spiral-shaped bacteria in the human stomach, which actually comprises several different Helicobacter species. Later on these microorganisms were reclassified into H. heilmannii type 1, representing H. suis from pigs, and H. heilmannii type 2, comprising a group of canine and feline Helicobacter spp. (Haesebrouck et al. 2009). The valid description of H. heilmannii further added some confusion on the nomenclature of this complex and expanding group of microorganisms (Smet et al. 2012). Therefore, the terms H. heilmannii (sensu lato), referring to the group of gastric non-H. pylori Helicobacter spp. (NHPH), and H. heilmannii (sensu stricto), referring to the species, have been proposed (Haesebrouck et al. 2011).
To date, these microorganisms have been associated with gastritis, gastric and duodenal ulcers, and low-grade mucosa-associated lymphoid tissue (MALT ) lymphoma in humans. Gastric NHPH have microscopically been detected in 0.2–6 % of humans with severe gastric complaints undergoing a gastroscopy, but this is most probably an underestimation of their true prevalence (Haesebrouck et al. 2009). It cannot be excluded that infections with these bacteria sometimes remain unapparent or cause mild disease signs which are often not thoroughly examined.
Clinical symptoms associated with gastric NHPH infections include acute or chronic epigastric pain, nausea, dyspepsia, reflux esophagitis , heartburn, vomiting, hematemesis, abdominal pain, irregular defecation frequency and consistency, and dysphagia, often accompanied by a decreased appetite (Haesebrouck et al. 2009).
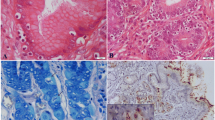
In patients undergoing an endoscopy, a variety of lesions can be observed ranging from a normal to slightly hyperemic mucosa, mucosal edema, nodular inflammation, and the presence of ulcerations in the antrum of the stomach or in the duodenum (Haesebrouck et al. 2009; Sykora et al. 2003). Histopathological examination of gastric biopsies reveals infiltration with lymphocytes and plasma cells, sometimes organized in lymphocytic aggregates (Joosten et al. 2013b). Other lesions, such as intestinal metaplasia , have occasionally been described in patients infected with NHPH, and some of these patients were also infected with H. pylori (Stolte et al. 1997; Yakoob et al. 2012). Compared to an H. pylori-associated gastritis, gastritis associated with NHPH is often less active and less severe. On the other hand, the risk of developing MALT lymphoma is higher with NHPH than with H. pylori (Haesebrouck et al. 2009).
Tests to rapidly diagnose infection with gastric NHPH are currently unavailable. Urea breath tests, used to diagnose infection with H. pylori , are often negative in patients infected with animal-associated Helicobacter spp. (Matsumoto et al. 2014). This can be explained by the fact that infections with these bacteria are, in contrast to H. pylori infections, more often focal and predominantly found in the antrum of the stomach (Stolte et al. 1997). Due to the fastidious nature of gastric NHPH, isolation of these bacteria is not an option for routine diagnostic purposes. Until now, only H. bizzozeronii (Andersen et al. 1999; Kivisto et al. 2010) and H. felis (Wüppenhorst et al. 2013) have been cultured from gastric biopsies. Therefore, analysis of gastric biopsies by molecular microbiological methods and histology is so far the only way to determine infections with these microorganisms. A German study analyzed 89 human gastric biopsies, previously shown to be NHPH positive (Trebesius et al. 2001). Eighty percent of these samples were positive for H. suis, 17 (19 %) samples were positive for H. heilmannii, and 5 (6 %) hybridized with a probe specific for H. felis, H. bizzozeronii, and H. salomonis. De Groote and co-workers (2005) screened paraffin-embedded gastric biopsies from 101 patients with confirmed gastric NHPH infection. Fourteen samples were positive for H. suis, whereas 49 were infected with helicobacters from cats and dogs. Another Belgian study showed similar results. H. suis was the most prevalent species (37 %), followed by H. salomonis (21 %), H. felis (15 %), H. heilmannii (8 %), and H. bizzozeronii (4 %) (Van Den Bulk et al. 2005b). A Polish study evaluated the incidence of gastric NHPH infection in dyspeptic children at the age of 4–18 years and found a prevalence of 0.2 % (Iwanczak et al. 2012). Another study focused on the association between coinfection with canine and feline NHPH and H. pylori and gastric pathology in patients with dyspepsia. H. pylori was found in 67 % of the patients, and only 6 % and 4 % of them were coinfected with H. heilmannii and H. felis, respectively (Yakoob et al. 2012). Recently, a remarkably high prevalence (27 %) of H. suis DNA was found in gastric biopsies from human patients with idiopathic parkinsonism. The putative significance of this bacterium in Parkinson’s disease requires further investigation (Blaecher et al. 2013).
Evidence is accumulating that pigs, cats, and dogs constitute reservoir hosts for gastric Helicobacter spp. with zoonotic potential (Haesebrouck et al. 2009). Helicobacter DNA has been detected in saliva from cats, dogs, and pigs, indicating that the oral cavity of these animals may act as source of NHPH infection for humans (Ekman et al. 2013; Casagrande Proieti et al. 2010; Shojaee Tabrizi et al. 2010). Fecal-oral transmission has also been suggested as a possible route for infection in cats (Ghil et al. 2009). Besides direct contact with animals, other routes of transmission of NHPH should not be neglected. It has been shown that H. felis is able to survive in water for several days, highlighting the possible role for water in the transmission of this species (Azevedo et al. 2008). Recently, De Cooman and co-workers (2013) demonstrated that H. suis can be present on and survive in minced pork. This indicates that raw or undercooked pork may also constitute a source of H. suis infection for humans. Nowadays, the prevalence of H. pylori in humans from the Western world is decreasing from generation to generation, leaving a niche for possible infection with these animal-associated gastric Helicobacter spp.
For patients with severe clinical symptoms and pathology, treatment is necessary. There is, however, a lack of clinical trials, and only a few reports deal with antimicrobial susceptibility and acquired resistance of gastric NHPH (Vermoote et al. 2011b; Van den Bulck et al. 2005a). Triple therapy using the combination of a proton-pump inhibitor and two antimicrobial agents, like clarithromycin , metronidazole , amoxicillin, or tetracycline, may be effective in most cases but not always. A Finnish patient infected with H. bizzozeronii received a triple therapy of tetracyclines, metronidazole, and lansoprazole for 1 week. The symptoms subsided, but the infection was not cleared and the patient continued to suffer from mild nausea. H. bizzozeronii was isolated from the stomach, and determination of its antimicrobial susceptibility showed resistance against tetracycline and metronidazole (Schott et al. 2012). Furthermore, it was demonstrated that acquired resistance to metronidazole in H. bizzozeronii was due to the contingency nature of an oxygen-insensitive NAD(P )H-nitroreductase. This phenomenon was also described for H. heilmannii (Kondadi et al. 2013).
3 Natural and Experimental Gastric Helicobacter Infections in Animal Hosts
3.1 Gastric Non- H. pylori Helicobacter spp. Associated with Dogs and Cats
3.1.1 Prevalence in Dogs and Cats
In pet animals, gastric Helicobacter spp. have been frequently described with a prevalence of 67–86 % in clinically healthy dogs, 61–100 % in dogs presenting chronic vomiting, and 41–100 % in healthy cats as well as cats showing chronic vomiting (Haesebrouck et al. 2009; Shojaee Tabrizi et al. 2010). Ghil and colleagues (2009) reported that the prevalence of Helicobacter spp. in feral cats was approximately twofold higher than in domestic cats. Often cats and dogs are naturally infected with multiple gastric Helicobacter spp. (Haesebrouck et al. 2009). The first Helicobacter species isolated from the stomach of cats and dogs was H. felis (Lee et al. 1988). Later on, H. bizzozeronii, H. salomonis, and H. cynogastricus were isolated from the canine gastric mucosa , whereas H. baculiformis, H. heilmannii and H. ailurogastricus isolates were obtained from the stomach of cats (Haesebrouck et al. 2009; Smet et al. 2012; Joosten et al. 2015). It has been shown that H. bizzozeronii is the most predominant species in the canine stomach, whereas H. felis and H. heilmannii are the predominant Helicobacter spp. in cats (Priestnall et al. 2004; Svec et al. 2000; Wiinberg et al. 2005). The prevalence of H. cynogastricus and H. baculiformis in pet animals as well as their zoonotic potential is so far unknown. Only few data is available on the transmission of NHPH infections in dogs and cats. Transmission of H. salomonis from a dam to her puppies, as well as between infected and noninfected pups, has been described (Hänninen et al. 1998). It has been suggested that in this case, transmission occurred through oral-oral or gastric-oral contact, as nursing dogs have very close contact with their offspring and puppies eat material vomited by the dam (Hänninen et al. 1998).
3.1.2 Role of Helicobacter spp. in the Development of Gastric Pathologies in Dogs and Cats
In general, canine and feline Helicobacter spp. have been associated with chronic active gastritis (Haesebrouck et al. 2009). Histological changes in the lamina propria include mild mononuclear inflammatory infiltration, the presence of lymphoid follicles, fibrosis, and glandular degeneration in the stomach of cats naturally infected with H. heilmannii (Takemura et al. 2009). One study reported a correlation between Helicobacter infection and the presence of feline lymphoma (Bridgeford et al. 2008). Gastric and duodenal ulcers have been rarely reported among cats and dogs, and an association with Helicobacter infections has not been made (Haesebrouck et al. 2009). To study the pathogenesis of NHPH infections in dogs and cats, several experimental infection studies have been performed. A mononuclear infiltration throughout the gastric mucosa , with follicular organization of the inflammatory cells, has been demonstrated in the stomach of H. felis-infected-specific pathogen-free (SPF) cats (Scanziani et al. 2001). Another experimental infection study with H. felis in young gnotobiotic dogs described lymphoid hyperplasia in the fundus and body of the stomach (Diker et al. 2002). On the contrary, Simpson and co-workers (1999) found a similar degree of inflammation in both H. felis-infected SPF dogs and uninfected control dogs. These contradictory results may be explained by differences in virulence between H. felis strains. The review by Haesebrouck and colleagues (2009) suggested that the pathogenic significance of gastric helicobacters in cats and dogs may be related to (1) the species expressing its own virulence which may be increased in cases of mixed infections due to synergistic effects, (2) differences within strains from the same species, or (3) host differences. An Italian study investigated the localization of Helicobacter spp. in the fundic mucosa of laboratory beagle dogs. They demonstrated that H. bizzozeronii was present in the superficial and basal portions of the fundic glands, while H. felis was only detected in the superficial portions of the glands. Additionally, these helicobacters were also located free in the cytoplasm or within lysosomes of parietal cells (Lanzoni et al. 2011). The urea breath test, widely used for rapid and noninvasive detection of H. pylori infection in humans, has recently been applied on laboratory beagle dogs. A sensitivity and specificity of 89 % was reported suggesting the usefulness of this technique to monitor gastric Helicobacter infections in dogs (Kubota et al. 2013).
3.1.3 Genomics , Genetics, and Experimental Studies in Rodents on the Pathogenesis of Canine and Feline Helicobacter Infection
Recently the genomes of H. felis, H. bizzozeronii, and H. heilmannii have been sequenced (Arnold et al. 2011; Schott et al. 2011; Smet et al. 2013). These genomes contain genes encoding homologues of known H. pylori virulence factors discussed in Chaps. 3, 4, 5, 6, and 7. These genomes possess a complete comB system conferring natural competence, but they all lack the cytotoxin-associated gene pathogenicity island (cagPAI), a functional vacuolating cytotoxin A (VacA ), and the H. pylori adhesins identified so far. Amorim and colleagues (2014) showed that several canine and feline helicobacters, like H. felis and H. heilmannii, are able to adhere to the canine gastric mucosa . Which adhesins are involved is so far unknown.
Besides SPF pets, rodent models have also been shown to be useful experimental infection models to obtain insights into the pathogenesis of gastric NHPH infections in animals and humans (O’Rourke et al. 2004a). H. felis infection in mice is often used as an animal model to study H. pylori -related gastric pathology in humans. Due to the lack of several important H. pylori virulence factors, researchers must be aware that H. felis might not always have similar outcomes in pathogenicity compared to H. pylori. Extrapolation of data obtained in an H. felis infection mouse model to H. pylori infections in humans should therefore be done with caution. To date, many reports studied the pathogenesis of canine and feline Helicobacter infection in rodent models. One of the very first steps in the pathogenesis of gastric infections caused by these microorganisms is colonization of the gastric mucosa in which binding to mucins (MUC) plays an important role. MUC1, MUC5AC, and MUC6 are the major mucins covering the gastric mucosa. Liu and colleagues (2014) investigated the gastric mucin expression pattern in the stomach of H. heilmannii-infected BALB/c mice . They showed a remarkable increased expression of Muc6 and Muc13 in the first 9 weeks postinfection. Since Muc6 is expressed by the gastric glands and, unlike H. pylori, H. heilmannii was mainly localized in the deep glands of the gastric mucosa, the potential role of Muc6 in H. heilmannii colonization was suggested. The MUC13 mucin is normally not expressed in a healthy stomach and has so far only been described as a marker for gastric cancer in humans. The increased expression already in the early stages of infection highlights its role in H. heilmannii colonization (Liu et al. 2014). The mucin Muc1 is constitutively expressed by the gastric mucosa and is likely the first point of contact between the host stomach and adherent pathogens. It has been shown that Muc1 limits H. felis binding to gastric epithelial cells. However, it does not limit colonization and gastric pathology following infection (Every et al. 2008). The pathological changes in the mouse stomach infected with H. salomonis, H. bizzozeronii, or H. felis have been evaluated as a means of distinguishing different NHPH species in terms of virulence. H. salomonis was not able to colonize the murine stomach. H. bizzozeronii showed moderate pathological changes, while H. felis induced the most severe inflammatory changes (De Bock et al. 2005). Another study by the same research group demonstrated that H. felis and H. bizzozeronii induce gastric parietal cell loss in Mongolian gerbil s , highlighting the preference of these bacteria for parietal cells as was also demonstrated in their natural host (De Bock et al. 2006). Takaishi and co-workers (2009) studied the effect of gastrin on H. felis-associated gastric carcinogenesis using hypergastrinemic, gastrin-deficient, and wild-type C57BL/6 mouse models. Gastrin is released by G cells mainly in the antrum of the stomach in response to food intake and stimulates the secretion of gastric acid by parietal cells. Severe corpus dysplasia with mild gastric atrophy was noted in H. felis-infected hypergastrinemic (INS-GAS) mice, while mild to moderate antral dysplasia was seen in the gastrin-deficient and wild-type mice. Gastrin deficiency did not result in an alteration of H. felis colonization, but it was shown that gastrin is an essential cofactor for the development of gastric dysplasia in H. felis-infected C57BL/6 mice. Joosten et al. (2013a) demonstrated that chronic H. heilmannii infection in Mongolian gerbils was associated with decreased gastric acid secretion and increased gastrin mRNA levels stimulated by interleukin- 1 beta (IL -1β). This latter finding could be considered as a reaction to the H. heilmannii-induced hypochlorhydria. Another study investigated the role of H. felis infection in the etiology of iron deficiency in INS-GAS C57BL/6 mice. Decreased serum iron concentrations were associated with a concomitant reduction in the number of parietal cells, strengthening the association between hypochlorhydria and gastric Helicobacter-induced iron deficiency. Additionally, the marked changes in gastric iron concentrations and the reduced number of parietal cells following Helicobacter infection may be relevant to the more rapid development of carcinogenesis in H. felis-infected INS-GAS mice (Thomson et al. 2012). The loss of parietal cells can lead to two distinct types of mucous metaplasia: intestinal metaplasia and spasmolytic polypeptide-expressing metaplasia (SPEM ). It has been suggested that intestinal metaplasia induced by Helicobacter infection develops in the presence of preexisting SPEM, supporting the role of SPEM as a neoplastic precursor in the carcinogenesis cascade (Liu et al. 2014; El-Zaatari et al. 2013). Several reports uniformly showed that H. felis predominantly causes a T helper (Th )1/Th17 immune response in mice, as has also been described for H. pylori (Ding et al. 2013; Obonyo et al. 2011; Sayi et al. 2011; Stoicov et al. 2009, Hitzler et al. 2012). Other gastric NHPH, like H. heilmannii, have been shown to cause a predominant Th2/Th17 host immune response in BALB/c mice which clearly differs from what is observed during H. felis infection (Liu et al. 2014). In BALB/c mice chronically infected with H. heilmannii, MALT lymphoma -like lesions were observed (Liu et al. 2014; O’Rourke et al. 2004a). Similar pathological findings have also been described in BALB/c mice chronically infected with H. felis suggesting that this mouse model can be seen as a critical model to study gastric MALT lymphoma (Stolte et al. 2002).
3.2 Gastric Non- H. pylori Helicobacter spp. Associated with Large Felines
Besides domesticated cats, Helicobacter spp. like H. felis and H. heilmannii have occasionally been found in the stomach of wild felines, such as lynx, leopards, pumas, bobcats, tigers, and cheetahs (Hamir et al. 2004; Mörner et al. 2008; O’Rourke et al. 2004b; Luiz de Camargo et al. 2011). In cheetahs, gastritis caused by these bacteria is characterized by the infiltration of lymphocytes and plasma cells in the epithelium and lamina propria with gland destruction and parietal cell loss. In some cases lymphoid follicles were observed, especially in captive animals and less frequently in wild animals (Terio et al. 2005; Munson et al. 2005). Terio and colleagues (2012) further investigated the local immune response in cheetahs with varying degree of Helicobacter-associated gastritis. The type of cells involved was similar among all types of gastritis with the exception that a large number of lamina proprial activated CD79a+CD21-B cells and plasma cells were only seen in cheetahs with severe gastritis (Terio et al. 2012). Another and more frequently found Helicobacter species in big cat predators is H. acinonychis. This species has been associated with severe chronic gastritis in cheetahs, tigers, and lions (Tegtmeyer et al. 2013). Cattoli and co-workers (2000) demonstrated that eradication of this bacterium from the stomach using antimicrobial treatment resulted in the resolution of gastric lesions in tigers. H. acinonychis has been described as the most closely related species to H. pylori (Tegtmeyer et al. 2013). Eppinger and colleagues (2006) sequenced the H. acinonychis genome and showed that this species arose after a single host jump of H. pylori from humans to large felines approximately 43,000–56,000 years ago. Comparison between the H. acinonychis and H. pylori genomes revealed that both species share a high number of core genes (Tegtmeyer et al. 2013). The H. acinonychis genome also possesses unique features that confirmed the direction of the host jump from humans to large felines. Interestingly, H. acinonychis lacks a cagPAI and a functional VacA as has been described for other animal-associated gastric helicobacters as well. Additionally, the H. acinonychis genome contains a high number of fragmented genes due to frameshifts and/or stop codons which are probably caused by niche changes and host specialization (Gressmann et al. 2005). So far, only little information is available about the interaction of H. acinonychis with its host. Dailidiene and co-workers (2004) showed that H. acinonychis was able to infect mice and to coexist and recombine with H. pylori in the stomach.
3.3 Gastric Helicobacter Infection in Pigs
In 1990, large spiral-shaped bacteria were described for the first time in the gastric mucus layer and on the mucosal surface of pig stomachs (Mendes et al. 1990; Queiroz et al. 1990). Initially, Gastrospirillum suis was proposed as a name, but subsequent characterization showed that this organism in fact belonged to the genus Helicobacter (De Groote et al. 1999b). A new name, ‘Candidatus Helicobacter suis’, was then proposed, and despite numerous attempts worldwide, the first successful in vitro isolate was only obtained in 2007, by using a new biphasic culture method, which finally led to the description of H. suis as a new species (Baele et al. 2008). Sequence analysis of 16S rRNA, 23S rRNA, partial hsp60, and partial ureAB gene sequences confirmed that H. suis is identical to the previously called H. heilmannii type 1. Besides pigs and humans, this species also colonizes the stomach of nonhuman primates, such as mandrills, macaques, and baboons (O’Rourke et al. 2004b).
3.3.1 Prevalence of Helicobacter suis in Pigs
The reported prevalence of H. suis infection in pigs depends on the study. In general, this bacterium is detected in more than 60 % of the European, Asian, and South and North American pigs at slaughter age (Barbosa et al. 1995; Grasso et al. 1996; Cantet et al. 1999; Park et al. 2004; Hellemans et al. 2007b; Kopta et al. 2010). Most likely, H. suis is transmitted via the oral-oral route through saliva or via the gastric-oral route through vomiting or regurgitation, but this remains to be investigated. Despite a very high prevalence in adult pigs, far lower degrees of infection have been shown in younger animals. Hellemans and co-workers (2007b) detected a prevalence of only 2 % in suckling piglets, which however increased rapidly after weaning.
So far, H. suis has not been detected in the stomach of wild boars. In a Polish study, the authors did find gastric Helicobacter spp., but interestingly, these bacteria were shown not to be H. suis (Fabisiak et al. 2010). More worldwide studies are, however, required to draw firm conclusions on the presence of H. suis in these wild ancestors of domesticated pigs.
3.3.2 Helicobacter suis and Its Role in the Development of Gastric Pathology in Pigs
H. suis has been shown to cause gastritis in experimentally and naturally infected pigs, mainly in the antrum (Mendes et al. 1991; Grasso et al. 1996; Queiroz et al. 1996; Park et al. 2000; Hellemans et al. 2007a; De Bruyne et al. 2012). Besides an occasional neutrophilic infiltrate, the inflammation mainly composes of a diffuse lymphocytic/plasmacytic infiltration as well as lymphocytic aggregates and lymphoid follicles. In experimentally infected pigs, this gastritis shows a spatial association with the main sites of colonization: the antrum and fundus (Hellemans et al. 2007a; De Bruyne et al. 2012). Interestingly, diffuse lymphocytic infiltration and lymphoid follicles have been shown to be present in the stomach, and mainly in the cardiac region, of newborn piglets without Helicobacter infection (Driessen et al. 2002; Mazzoni et al. 2011). Most likely, germinal centers in lymphoid follicles are formed in the presence of an antigenic stimulus, for instance, during H. suis infection.
Besides the strong association with gastritis, H. suis infection has also been associated with ulceration of nonglandular stratified squamous epithelium of the pars esophagea of the stomach, although H. suis bacteria probably do not colonize this specific stomach region (Barbosa et al. 1995; Queiroz et al. 1996; Choi et al. 2001; Roosendaal et al. 2000; Hellemans et al. 2007b; De Bruyne et al. 2012). Other research groups did not find this association (Grasso et al. 1996; Cantet et al. 1999; Melnichouk et al. 1999; Park et al. 2000; Szeredi et al. 2005), so the exact role of H. suis in the development of these lesions remains to be elucidated. Sapierzyński and colleagues (2007) demonstrated that H. suis infection in pigs results in an increased number of gastrin-producing cells and a decreased number of somatostatin-producing cells in the antrum. Since gastrin stimulates and somatostatin inhibits the secretion of hydrochloric acid by parietal cells , this may influence gastric acid production, which may also be altered due to the tropism of this bacterium for parietal cells (Hellemans et al. 2007a). An altered gastric acid secretion could in turn be involved in the development of ulceration of the pars esophagea. The discrepancies found in literature may be due to differences in laboratory techniques, different sampling practices, or differences in virulence between H. suis strains. In any case, hyperkeratosis and ulceration of the nonglandular part of the stomach have been reported in many countries. Up to 80 % of the market pigs in Australia (Robertson et al. 2002) and 60 % of the sows (Hessing et al. 1992) in the Netherlands have been described to be affected.
The development of lesions in the pars esophagea is most likely a process involving different factors, including stress, transport , the presence in the stomach of short-chain fatty acids, and pelleting and fine grinding of the feed (Haesebrouck et al. 2009; Argenzio and Eisemann 1996). The latter factor may have an influence on the fluidity of gastric contents (Elbers and Dirkzwager 1994), leading to an increased contact of the stratified squamous epithelium of the nonglandular region with the luminal content of the distal part with acid, bile (refluxed from the duodenum), and pepsin.
Ulceration of the porcine gastric nonglandular mucosa may result in decreased feed intake, a decrease in daily weight gain, and even sudden death due to fatal hemorrhage (Ayles et al. 1996; Haesebrouck et al. 2009), thus leading to significant economic losses. There is also little doubt that this disease can cause pain and discomfort. Interestingly, a decrease in daily weight gain of up to 10 % has been observed in pigs experimentally infected with H. suis, however, without a clear association with the development of lesions in the nonglandular part (De Bruyne et al. 2012).
Besides H. suis, other Helicobacter species have been described in the stomach of pigs, including an H. pylori -like bacterium, responsible for ulceration of the pars esophagea in gnotobiotic piglets (Krakowka and Ellis 2006), H. bilis and H. trogontum (Hänninen et al. 2003, 2005). The main site of colonization of the latter two is most probably the lower intestinal tract, and it remains to be determined whether these bacteria are able to colonize the porcine stomach and cause gastric pathology.
3.3.3 Genomics , Genetics, and Experimental Studies on the Pathogenesis of H. suis Infection
Genome sequencing of H. suis (Vermoote et al. 2011a) revealed the presence of homologues of H. pylori genes involved in acid acclimation, motility , chemotaxis, oxidative stress resistance , and adhesion to gastric epithelial cells. These include genes encoding urease A and B subunits and urease accessory proteins, genes encoding different components of the flagellar apparatus, several che and tlp genes, katA, sodB, genes encoding several members of the major H. pylori outer membrane families, and hpaA. In addition, H. suis harbors a near complete comB type IV secretion system and homologues of the H. pylori neutrophil-activating protein and γ-glutamyl transpeptidase, but it lacks most members of the cagPAI as well as a functional VacA .
In literature, several studies describe experimental infection of mice with H. heilmannii. Sometimes, however, this name might be misleading, as several of these studies have in fact used H. suis bacteria, obtained from mucus, or homogenized gastric tissue of infected mice, pigs, or nonhuman primates, because of the lack of in vitro isolated strains. Infection of mice with these inocula induces inflammation, already 7 days after inoculation (Moura et al. 1993). During long-term infection studies of up to 2 years, infiltration of the gastric mucosa with lymphocytes and plasma cells with subsequent development of lymphoid follicles has been observed, both in studies using mucosal homogenates and pure in vitro isolated H. suis strains (Park et al. 2008; Flahou et al. 2010). Conflicting reports have been published regarding the immune response underlying H. suis-induced gastritis. Several authors have described the inflammation and formation of gastric lymphoid follicles to be mainly driven by a Th1 response (Cinque et al. 2006; Mimura et al. 2011). Others have described a mixed Th1/Th2 response (Park et al. 2008), whereas studies using pure in vitro isolated H. suis strains revealed that experimental H. suis infection causes an upregulation of IL -17, IL-10, and IL-4 expression, in the absence of interferon (IFN)-γ upregulation (Flahou et al. 2012), which also clearly contrasts to the immune response elicited by H. pylori in this same animal model. Recently, however, Liang and colleagues (2015) described a strong upregulation of IFN-γ expression in H. suis-infected Mongolian gerbil s .
The resulting chronic gastritis has been shown not to depend upon the presence of Peyer’s patches in the small intestine, in contrast to what has been described for H. pylori (Nobutani et al. 2010). Often, this chronic gastritis evolves to more severe histopathological lesions. In BALB/c mice experimentally infected with different H. heilmannii bacteria, “isolated” in vivo in mice and originating both from humans and animals, gastric MALT lymphoma has been shown to develop starting from 18 months postinfection (O’Rourke et al. 2004a). In this study, the most severe pathological changes were seen in mice infected with in vivo “isolates” from a human patient, a bobcat, a crab-eating macaque, and mandrill monkeys. Except for the strain from the bobcat, these strains were in fact shown to be H. suis. Similar lesions were observed in C57BL/6 mice infected for at least 6 months with an in vivo “isolate” of ‘Candidatus H. heilmannii’, which in fact was shown to belong to the species H. suis (Nakamura et al. 2007; Haesebrouck et al. 2009), as well as in Mongolian gerbil s infected with an in vitro isolated strain of H. suis (Flahou et al. 2010). These histopathological changes resemble inflammation-related changes in the stomach of humans infected with NHPH, including H. suis.
In addition to the involvement of several cytokines, including IL -4 and chemokine (C-X-C motif) ligand 13 (CXCL-13) (Flahou et al. 2012; Yamamoto et al. 2014), several mechanisms have been described to stimulate lymphomagenesis in this setting. An overexpression of miR- 142-5p and miR-155, as well as increased lymphangiogenesis and angiogenesis, has been shown to be involved in H. suis-induced gastric MALT lymphoma (Saito et al. 2012; Nakamura et al. 2008). The latter changes are accompanied by an increased expression of vascular endothelial growth factors (VEGF), such as VEGF-A and VEGF-C, and some of its receptors, including Flt-1 and Flt-4 (Nishikawa et al. 2007; Nakamura et al. 2007, 2008, 2010). In addition, the formation of peripheral lymph node addressin (PNAd)- and mucosal addressin cell adhesion molecule 1 (MadCAM-1)-expressing high endothelial venule-like vessels plays a role (Suzuki et al. 2010). These alterations may cause a sustained “homing” of lymphocytes to the gastric mucosa . Interestingly, the administration of antibodies raised against certain of the abovementioned factors, including CXCL-13, Flt-1, and Flt-4, induces a marked reduction of the size of the region affected by gastric MALT lymphoma, which may have implications for the future treatment guidelines of Helicobacter-induced gastric MALT lymphoma (Nakamura et al. 2010, 2014; Yamamoto et al. 2014).
Besides inflammation-related pathological changes, H. suis also interacts with the mucosal epithelium. In infected humans, the majority of H. pylori bacteria remain in the mucus layer, whereas a limited number adhere to gastric epithelial (mucus-secreting) cells (Lindén et al. 2008). H. suis, on the other hand, is most often observed in the vicinity of or inside the canaliculi of acid-producing parietal cells in pigs, humans, and experimentally infected mice and Mongolian gerbil s (Hellemans et al. 2007a; Joo et al. 2007; Flahou et al. 2010). Regularly, these parietal cells show signs of degeneration or oncosis. Many questions remain unanswered with regard to the mechanisms underlying the epithelium-related changes observed during H. suis infection. As described above, several important H. pylori virulence factors, such as the cagPAI and VacA , are absent or nonfunctional in H. suis (Vermoote et al. 2011a). So far, the H. suis γ-glutamyl transpeptidase (GGT) is the only virulence factor from H. suis with a confirmed role in death of gastric epithelial cells. In vitro research using a human gastric epithelial cell line (AGS ) has shown that the enzyme causes apoptosis or necrosis, depending in part on the amount of reactive oxygen species (ROS ) generated through degradation of reduced glutathione (GSH), an important antioxidant (Flahou et al. 2011). Besides its role in death of epithelial cells, the same enzyme has been shown to impair lymphocyte function, which is in line to what has been published for H. pylori (Zhang et al. 2013). Supplementation of GGT -treated cells with known substrates of the enzyme was shown to modulate the observed effects: glutamine restored normal proliferation of lymphocytes , whereas supplementation with reduced glutathione strengthened H. suis GGT-mediated inhibition of proliferation. Most likely, depletion of glutamine and the generation of reactive oxygen species through degradation of GSH are involved. In this same study, H. suis outer membrane vesicles were identified as a possible delivery route of H. suis GGT to lymphocytes residing in the deeper mucosal layers.
Parallel to the cell death it induces, H. suis infection also causes an increased proliferation of progenitor cells in the isthmus of gastric glands (Flahou et al. 2010). A direct effect of the bacteria may be involved (Fast et al. 2011), or alternatively, this may reflect a compensatory hyperproliferation due to increased epithelial cell loss (Shirin and Moss 1998).
3.4 Gastric Helicobacter Infection in Nonhuman Primates
Captive rhesus macaques are commonly infected with H. pylori (Drazek et al. 1994). The anatomy and physiology of the stomach resemble that of humans, which suggests that the rhesus monkey model can serve as a good experimental model to study H. pylori infection. For instance, it has been shown that socially housed rhesus monkeys rapidly acquire H. pylori infection, particularly during the peripartum period (Solnick et al. 2003, 2006). Once acquired, infection is associated with chronic gastritis resembling that seen in humans.
Besides H. pylori , nonhuman primates can be naturally infected with gastric NHPH. Although an exact species designation of the colonizing Helicobacter species is sometimes lacking, H. suis seems to be the species involved (O’Rourke et al. 2004b; Martin et al. 2013; Nakamura et al. 2007; Matsui et al. 2014). In the stomachs of rhesus monkeys, gastric NHPH which were not identified to the species level have been observed in the mucus covering the surface epithelial cells, as well as in the lumina of gastric glands. These microorganisms were shown to be able to invade and on occasion damage parietal cells , which was however often accompanied by an apparent hyperchlorhydria. This contrasted with H. pylori infection in this model, which appeared to cause a more pronounced gastritis, while apparently not modifying the acid output (Dubois et al. 1991). In another study, H. suis was shown to be present in the stomach of all rhesus monkeys that were used for determining the effects of an experimental H. pylori infection on the gastric microbiota . Interestingly, the populations of H. suis and H. pylori were shown to be highly dynamic, and a potential competitive inhibition/exclusion was observed between both species (Martin et al. 2013).
NHPH infection in baboons has been associated with the development of gastritis by Mackie and O’Rourke (2003) but not by others (Curry et al. 1989). NHPH have been described to be naturally present in the stomachs of cynomolgus monkeys from different geographic regions (Reindel et al. 1999; Drevon-Gaillot et al. 2006). Again, H. suis seems to be the species involved (O’Rourke et al. 2004b), and these microorganisms can be found in the gastric pits, in the superficial glands, or on the surface epithelium. In one study, no correlation was observed between these bacteria and the infiltration of lymphoplasmacytic cells and inflammatory lesions in these gastric tissues (Drevon-Gaillot et al. 2006).
Recently, a putative new gastric Helicobacter species was detected in wild chimpanzees and gorillas, which was provisionally named ‘Candidatus H. homininae’ (Flahou et al. 2014).
3.5 Gastric Helicobacters Associated with Ruminants and Horses
‘Candidatus Helicobacter bovis’ has been demonstrated in the pyloric part of the abomasum of calves and adult cattle. So far, this bacterium has not been cultivated in vitro (De Groote et al. 1999a), and its involvement in bovine gastric disease is unknown. Although gastric ulcers regularly occur in calves and adult cattle (Haringsma and Mouwen 1992; Jelinski et al. 1995; Ok et al. 2001), no association between gastric ulceration and Helicobacter colonization was observed in a recent study, since no Helicobacter bacteria could be detected in the animals that were sampled (Valgaeren et al. 2013). H. pylori has been demonstrated in the stomach of sheep (Dore et al. 2001), and so far, no helicobacters have been demonstrated in the stomach of goats (Gueneau et al. 2002; Momtaz et al. 2014). Interestingly, H. pylori has also been detected in milk from cows, sheep, and other ruminants (Quaglia et al. 2008; Angelidis et al. 2011; Rahimi and Kheirabadi 2012). Although Rahimi and colleagues claim to have isolated H. pylori bacteria, most studies used polymerase chain reaction or fluorescence in situ hybridization to detect H. pylori. Further studies are therefore needed to assess whether H. pylori or H. pylori-like bacteria are involed. In addition, it needs to be confirmed whether milk consumption can serve as a route of transmission for H. pylori.
Although some studies describe the absence of Helicobacter spp. in the stomach of horses (Husted et al. 2010; Perkins et al. 2012), others describe the presence of Helicobacter-like organisms or their DNA in this niche. So far, however, helicobacters have not yet been cultivated from the equine stomach (Scott et al. 2001; Contreras et al. 2007), so their possible role in the development of gastric ulcers, which are common in horses (Haesebrouck et al. 2009), remains speculative.
3.6 Gastric Helicobacters Associated with Other Animal Species
3.6.1 Marine Mammals
Urease-positive helicobacters have been isolated from the main stomach or feces of various cetaceans, including stranded wild Atlantic white-sided dolphins and captive Pacific white-sided dolphins, Atlantic bottlenose dolphins, and a beluga whale (Harper et al. 2002, 2003a). In 2002, these bacteria were characterized and described as H. cetorum (Harper et al. 2002). The results of a health study, in which 20 wild Atlantic bottlenose dolphins were sampled, showed that the prevalence in these animals was at least 50 % (Harper et al. 2003a). In addition to the studies described above, helicobacters with a high homology to H. cetorum have been isolated from or detected in fecal samples from captive seals and sea lions from Australia, South American fur seals, the stomach of an Atlantic spotted dolphin, gastric fluids, dental plaques, saliva and gastric tissue of captive dolphins and a killer whale from Argentina, fecal material from wild and captive Yangtze finless porpoises, the stomach of common dolphins, an Atlantic white-sided dolphin and a striped dolphin from European waters, and the aquatic environment of captive dolphins (Oxley and McKay 2005; Goldman et al. 2009a, b, 2011; Suárez et al. 2010; McLaughlin et al. 2011; Davison et al. 2014).
Some of the captive animals from which H. cetorum was recovered showed clinical signs, such as intermittent inappetence, lethargy, or chronic regurgitation. Endoscopic or gross examination revealed the presence of esophageal and forestomach ulcers, as well as gastric mucosa l hemorrhages (Harper et al. 2002; Davison et al. 2014). Cytological examination of gastric fluid of some animals indicated the presence of inflammation in the stomach (Harper et al. 2002). This was confirmed by histopathological analysis, revealing Helicobacter colonization of the epithelial surface accompanied by a diffuse lymphoplasmacytic gastritis in the main stomach and to a lesser extent in the pyloric stomach, and a mild distortion of the adjacent glands (Harper et al. 2002; Suárez et al. 2010).
In a recent study, two genomes of H. cetorum were sequenced: one strain originated from a dolphin and one strain isolated from a captive Beluga whale (Kersulyte et al. 2013). Although these genomes, differing markedly from one another in gene content, appeared to be larger than H. pylori genomes, the strains were shown to be more closely related to H. pylori and H. acinonychis than to other known species. They lack the cagPAI but do possess novel alleles of the vacA gene. In addition, they reveal an extra triplet of vacA genes, metabolic genes distinct from H. pylori, as well as genes encoding both an iron- and nickel -cofactored urease .
Besides H. cetorum, other putative novel Helicobacter spp., distinct from H. cetorum, have been detected in the gastric fluids, gastric mucosa , or dental plaque from dolphins, harp seals, and a sea lion with chronic gastritis (Harper et al. 2003b; Oxley et al. 2004, 2005; Goldman et al. 2011).
3.6.2 Ferrets
Not so long after the discovery and description of H. pylori in humans, spiral organisms were isolated from a gastric ulcer of a ferret and from the gastric mucosa of two healthy ferrets (Fox et al. 1986). In 1989, these organisms were named Helicobacter mustelae (Goodwin et al. 1989). Only a minority of ferrets younger than 6 weeks are colonized by this bacterium, in contrast to the vast majority of adult ferrets (Fox et al. 1988), indicating that widespread colonization and persistence occur after weaning (Fox et al. 1991a; Forester et al. 2000). Keeping in mind the ease by which ferrets vomit, oral-oral and gastric-oral contact most likely play a role in transmission of H. mustelae (Fox et al. 1991a). Fecal-oral transmission has, however, also been suggested. H. mustelae has indeed been isolated successfully from feces of ferrets, and successful isolation correlated with periods of transient hypochlorhydria, which may allow larger numbers of bacteria to exit the stomach (Fox et al. 1992).
H. mustelae colonizes the mucosal surface in the corpus region, which often induces only a superficial gastritis (Marini and Fox 1999). In the antrum, however, H. mustelae colonizes the surface, gastric pits, and superficial portion of the glands, leading to the development of a diffuse mononuclear gastritis with inflammatory cells often occupying the full thickness of the mucosa (Fox et al. 1991b).
The incidence of gastric ulceration in ferrets varies between 1.4 and 35 % (Andrews et al. 1979). Both gastric and duodenal ulcerations have been reported in ferrets infected with H. mustelae (Fox et al. 1986, 1990). Given the high prevalence of H. mustelae in adult ferrets, long-term observations of experimentally infected pathogen-free ferrets are needed to elucidate the exact role of H. mustelae infection in the development of peptic ulcer disease . An increased epithelial cell proliferation has been detected in the gastric mucosa of ferrets infected with H. mustelae, which may play a role in the development of gastric tumors (Yu et al. 1995). Indeed, gastric adenocarcinoma has been described in the pyloric mucosa of two ferrets infected with H. mustelae (Fox et al. 1997). In both cases, the invasion of neoplastic tubules into the deep submucosa was described. Gastric MALT lymphoma , accompanied by destruction of the gastric glands, has also been described in the antrum of ferrets infected with H. mustelae (Erdman et al. 1997). For both tumor types, however, evidence remains so far circumstantial (Solnick and Schauer 2001).
H. mustelae adheres firmly to the gastric epithelium, and only a few bacteria are seen lying in the mucus (O’Rourke et al. 1992). In H. mustelae infected ferrets, the gastric mucosa l hydrophobicity is reduced, which correlates with the degree of mucosal inflammation (Gold et al. 1996). This may promote the attachment of H. mustelae, which is thought to be mainly hydrophilic. H. mustelae binds to the same receptor lipids as H. pylori , in particular phosphatidylethanolamine (Gold et al. 1995). Like other gastric helicobacters, H. mustelae possesses a urease enzyme and flagella, consisting of a body, hook, and flagellar filament composed of FlaA and FlaB subunits. Clyne and coworkers (2000) showed that these flagella do not play a direct role in promoting adherence of H. mustelae to gastric epithelial cells. Double mutants of H. mustelae in flaA and flaB genes were shown to be completely nonmotile and unable to colonize the ferret, whereas single-gene flaA and flaB mutants have a decreased motility but are still able to colonize the ferret’s stomach (Andrutis et al. 1997; Josenhans et al. 1995). An isogenic urease-negative mutant of H. mustelae was shown to fail to colonize the ferret stomach (Andrutis et al. 1995; Solnick et al. 1995). In addition to the standard nickel -dependent UreAB, H. mustelae has been shown to possess a second, nickel-independent urease (Stoof et al. 2008). Instead, this UreA2B2 is activated with ferrous ions in the absence of auxiliary proteins. This unique metalloprotein is sufficient for the bacteria to survive an acid shock in the presence of urea, and it is thought to play a role in survival of the bacteria in the stomach of carnivores, with a diet rich in iron and low in nickel (Stoof et al. 2008; Carter et al. 2011, 2012).
H. mustelae produces an array of surface rings, which seem to be unique to this Helicobacter species. They are composed of the Helicobacter surface ring (Hsr) protein, comprising approximately 25 % of the total envelope protein of H. mustelae (O’Toole et al. 1994). These surface rings have been shown to play a role in bacterial colonization and the pathogenesis of H. mustelae infection, as shown by reduced numbers of bacteria recovered from mutant-dosed ferrets (Patterson et al. 2003). Moreover, animals inoculated with the Hsr-negative strain show less inflammation compared to ferrets infected with the wild-type strain. Like other animal-associated gastric Helicobacter species, H. mustelae lacks a cagPAI and VacA .
3.6.3 Hamsters and Mice
H. aurati has been isolated from the inflamed stomachs and ceca of adult Syrian hamsters (Patterson et al. 2000a). Certain features, including the fusiform shape and the presence of periplasmic fibrils, distinguish it from other enterohepatic Helicobacter species detected in hamsters, such as H. cholecystus, H. mesocricetorum, and H. cinaedi (Solnick and Schauer 2001; Whary and Fox 2004; Ceelen et al. 2007a). Although H. aurati possesses urease activity, the fact that bacteria were recovered from cecal samples more often than from antral samples indicates that the preferential colonization site of H. aurati in hamsters is probably the intestinal tract (cecum) with subsequent spreading of this bacterial agent to the stomach in some animals, possibly due to the coprophagic behavior of hamsters (Patterson et al. 2000b). The precise role of H. aurati in gastric diseases in hamsters has not yet been fully elucidated, although the organism has been identified in hamsters suffering from chronic gastritis and intestinal metaplasia (Patterson et al. 2000a, b). In the stomach of these same hamsters, the authors reported the presence of another helical, urease-negative Helicobacter species, as well as a smaller, urease-negative Campylobacter species. Likewise, natural infection with different Helicobacter species, including H. aurati, was reported in a Syrian hamster with gastritis-associated adenocarcinoma (Nambiar et al. 2005). There are no indications that H. aurati is of zoonotic significance.
Also in mice, urease -positive helicobacters have been described in the stomach. In line with what has been described for H. aurati infection in Syrian hamsters, H. muridarum has occasionally been detected in the stomach, with or without concurrent inflammation, although these bacteria are found more frequently in the ileal and cecal mucosa of the animals (Lee et al. 1992). H. suncus has been isolated from the stomach of house musk shrews with chronic gastritis (Goto et al. 1998).
3.6.4 Rabbits
Only two reports describe the presence of H. felis and H. salomonis DNA in the stomach of rabbits. So far these bacteria have not been cultivated from rabbits, and their pathogenicity toward these animals is unknown (Haesebrouck et al. 2009; Van den Bulck et al. 2006).
4 Enterohepatic Helicobacter Species
The NHPH species described above are mainly found colonizing the stomach of their hosts. A large number of Helicobacter species, however, prefer to settle in the lower intestinal tract or the liver of a wide variety of mammalian, reptilian, avian, or amphibian host species as well as humans (Schrenzel et al. 2010; Hansen et al. 2011).
Several enterohepatic Helicobacter species are found in rodents with or without signs of intestinal or hepatic disease, including H. bilis, H. hepaticus, H. cholecystus, H. muridarum, H. mastomyrinus, H. typhlonius, H. rodentium, H. mesocricetorum, H. magdeburgensis, H. aurati, H. cinaedi, H. ganmani, H. trogontum, and H. muricola (Lee et al. 1992; Fox et al. 1994, 1995; Patterson et al. 2000a; Vandamme et al. 2000; Robertson et al. 2001; Solnick and Schauer 2001; Won et al. 2002; Shen et al. 2005; Traverso et al. 2010). Several models of experimental enterohepatic Helicobacter infection, including H. hepaticus infection in immunodeficient IL -10-/- knockout mice, are used to model the development of colitis/inflammatory bowel disease in humans (Solnick and Schauer 2001; Hansen et al. 2011).
H. canis has been detected in or isolated from healthy dogs and cats as well as dogs suffering from endemic diarrhea or necrotic hepatitis (Solnick and Schauer 2001; Shen et al. 2001). Several other enterohepatic Helicobacter species have been demonstrated in dogs and cats, including H. bilis, H. cinaedi, ‘Candidatus H. colifelis’, H. marmotae, and H. fennelliae (Solnick and Schauer, 2001; Fox et al. 2002; Misawa et al. 2002; Hänninen et al. 2005; Rossi et al. 2008; Otte et al. 2012; Castiglioni et al. 2012). The pathogenic significance of these microorganisms for dogs and cats remains uncertain.
H. equorum has been isolated from the feces of healthy horses (Moyaert et al. 2007a, b). The prevalence of this microorganism in different adult horse populations varies between 0.8 % and 7.9 % but is much higher (66 %) in 1- to 6-month-old foals (Moyaert et al. 2009). Experimental infections revealed that this microorganism colonizes the cecum, colon, and rectum of adult horses without causing apparent pathological changes (Moyaert et al. 2007c).
Several Helicobacter spp., including H. trogontum, H. bilis, and H. canis, have been identified or isolated from sheep, and infection with the first two species has been associated with abortion and the presence of hepatic necrosis in aborted lambs (Dewhirst et al. 2000; Solnick and Schauer 2001; Hänninen et al. 2003, 2005; Swennes et al. 2014). Enterohepatic Helicobacter species have not been described in other ruminants, including cattle and goats.
H. pamatensis (Seymour et al. 1994), H. trogontum (Hänninen et al. 2003), H. bilis (Hänninen et al. 2005), and atypical H. canadensis strains (Inglis et al. 2006) have been isolated from the gastrointestinal tract or feces of pigs, but their pathogenic significance for this animal host is not known.
H. pullorum has been isolated from the ceca and feces of apparently healthy chickens, as well as from laying hens with vibrionic hepatitis (Stanley et al. 1994). In two in vivo studies, experimentally infected chickens remained clinically healthy although mild lesions were observed in the ceca (Neubauer and Hess 2006; Ceelen et al. 2007b). H. pullorum or its DNA have also been detected in other bird species, including turkeys (Zanoni et al. 2011) and a parakeet suffering from diarrhea (Ceelen et al. 2006b). Other Helicobacter species detected in birds include H. canadensis, which has mainly been associated with geese but also with chickens, guinea fowl, and pheasants (Fox et al. 2000; Nebbia et al. 2007; Robino et al. 2010). H. anseris and H. brantae have been isolated from the feces of resident Canada geese (Fox et al. 2006), and still now, new species are being discovered, including H. valdiviensis, isolated from wild bird fecal samples (Collado et al. 2014).
Also in monkeys, enterohepatic helicobacters have been detected, including H. callitrichis in common marmosets, H. cinaedi and H. macacae in rhesus macaques and baboons, and several enterohepatic Helicobacter spp. in gorillas and chimpanzees (Fernandez et al. 2002; García et al. 2006; Fox et al. 2007; Won et al. 2007; Flahou et al. 2014). It has been suggested that infection with H. macacae plays a role in the development of chronic idiopathic colitis and intestinal adenocarcinoma in rhesus macaques (Lertpiriyapong et al. 2014).
Several enterohepatic Helicobacter species have been associated with disease in humans. These reports include associations between H. canis/H. winghamensis and gastroenteritis; H. hepaticus and cholecystitis, liver carcinogenesis, or chronic pancreatitis; H. bilis and chronic cholecystitis, biliary duct, and gallbladder cancer; H. ganmani and liver disorders; H. pullorum and enteritis or diarrhea; H. canadensis and enteritis; and H. cinaedi/H. fennelliae and chronic diarrhea, enteritis, proctitis, or proctocolitis in homosexual men (Totten et al. 1985; Stanley et al. 1994; Steinbrueckner et al. 1997; Fox et al. 2000; Melito et al. 2001; Solnick and Schauer 2001; Matsukura et al. 2002; Murata et al. 2004; Tolia et al. 2004; Kobayashi et al. 2005; Apostolov et al. 2005; Nilsson et al. 2006; Zhang et al. 2006; Hamada et al., 2009). Enterohepatic Helicobacter species have also been associated with the various forms of inflammatory bowel disease (Hansen et al. 2011), and several species, including H. cinaedi, H. fennelliae, H. canadensis, H. canis, H. westmaedii, and H. rappini, have been isolated from (immunosuppressed) patients with bacteremia (Solnick and Schauer 2001; Tee et al. 2001, Matsumoto et al. 2007; Prag et al. 2007; Abidi et al. 2013; Rimbara et al. 2013).
Most enterohepatic Helicobacter species do not contain an urease enzyme, although there are exceptions, including H. hepaticus and H. bilis. A large number of enterohepatic Helicobacter species contain a bacterial toxin called the cytolethal distending toxin (CDT). The toxic effects of this major virulence factor involve cellular distension, actin cytoskeleton remodeling, G2/M cell cycle arrest, and cytolethality (Ceelen et al. 2006a; Varon et al. 2014). CDT is typically composed of three subunits: CdtA, CdtB, and CdtC, which are all required for a maximal cytotoxic activity (Liyanage et al. 2013; Varon et al. 2014). Other factors involved in host-bacteria interactions include a type VI secretion system, which is expressed by several enterohepatic helicobacters, including H. hepaticus, H. pullorum, H. cinaedi, and H. trogontum (Chow and Mazmanian 2010; Goto et al. 2012; Kaakoush et al. 2013; Sirianni et al. 2013).
5 Conclusions and Outlook
There are clear indications that gastric NHPH species can cause disease in humans. Some distinct features, such as the association with gastric MALT lymphoma , indicate that these zoonotic bacteria should not just be considered as a “light” version of H. pylori . There are clear indications that domestic animals constitute reservoir hosts for these gastric Helicobacter species with zoonotic potential. A correct diagnosis to the species level remains sometimes problematic. Therefore, diagnostic methods enabling the correct identification of these bacteria are needed to help clarify the epidemiology and pathology of these infections in humans. The successful in vitro isolation of several of these species has opened new perspectives for understanding the pathogenesis of non-H. pylori Helicobacter associated gastric pathology in their natural hosts as well as humans. An increased knowledge may in the end contribute to the development of new treatment and prevention measures.
References
Abidi MZ, Wilhelm MP, Neff JL, Hughes JG, Cunningham SA, Patel R (2013) Helicobacter canis bacteremia in a patient with fever of unknown origin. J Clin Microbiol 51:1046–1048
Amorim and colleagues I, Freitas DP, Magalhaes A, Faria F, Lopes C, Faustino AM, Smet A, Haesebrouck F, Reis CA, Gärtner F (2014) A comparison of Helicobacter pylori and non-Helicobacter pylori Helicobacter spp. binding to canine gastric mucosa with defined gastric glycophenotype. Helicobacter 19:249–259. doi:10.1111/hel.12125
Andersen LP, Boye K, Blom J, Holck S, Norgaard A, Elsborg L (1999) Characterization of a culturable “Gastrospirillum hominis” (Helicobacter heilmannii) strain isolated from human gastric mucosa. J Clin Microbiol 37:1069–1076
Andrews PL, Illman O, Mellersh A (1979) Some observations of anatomical abnormalities and disease states in a population of 350 ferrets (Mustela furo L.). Z Verstierkd 21:346–353
Andrutis KA, Fox JG, Schauer DB, Marini RP, Murphy JC, Yan L, Solnick JV (1995) Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun 63:3722–3725
Andrutis KA, Fox JG, Schauer DB, Marini RP, Li X, Yan L, Josenhans C, Suerbaum S (1997) Infection of the ferret stomach by isogenic flagellar mutant strains of Helicobacter mustelae. Infect Immun 65:1962–1966
Angelidis AS, Tirodimos I, Bobos M, Kalamaki MS, Papageorgiou DK, Arvanitidou M (2011) Detection of Helicobacter pylori in raw bovine milk by fluorescence in situ hybridization (FISH). Int J Food Microbiol 151:252–256
Apostolov E, Al-Soud WA, Nilsson I, Kornilovska I, Usenko V, Lyzogubov V, Gaydar Y, Wadström T, Ljungh A (2005) Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol 40:96–102
Argenzio RA, Eisemann J (1996) Mechanisms of acid injury in porcine gastroesophageal mucosa. Am J Vet Res 57:564–573
Arnold I, Zigova Z, Holden M, Lawley T, Rad R, Dougan G, Falkow S, Bentley SD, Müller A (2011) Comparative whole genome sequence analysis of the carcinogenic model pathogen Helicobacter felis. Genome Biol Evol 3:302–308
Ayles HL, Friendship RM, Ball EO (1996) Effect of dietary particle size on gastric ulcers, assessed by endoscopic examination, and relationship between ulcer severity and growth performance of individually fed pigs. Swine Health Prod 4:211–216
Azevedo NF, Almeida C, Fernendes I, Cerqueira L, Dias S, Keevil CW, Vieira MJ (2008) Survival of gastric and enterohepatic Helicobacter spp. in water: implications for transmission. Appl Environ Microbiol 74:1805–1811
Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Chiers K, Ducatelle R, Haesebrouck F (2008) Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol 58:1350–1358
Barbosa AJA, Silva JCP, Nogueira AMMF, Paulino E, Miranda CR (1995) Higher incidence of Gastrospirillum sp. in swine with gastric ulcer of the pars oesophagea. Vet Pathol 32:134–139
Blaecher C, Smet A, Flahou B, Pasmans F, Ducatelle R, Taylor D, Weller C, Bjarnason I, Charlett A, Lawson AJ, Dobbs RJ, Dobbs SM, Haesebrouck F (2013) Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther 38:1347–1353
Bridgeford EC, Marini RP, Feng Y, Parry NM, Rickman B, Fox JG (2008) Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol 123:106–113
Cantet F, Magras C, Marais A, Federighi M, Mégraud F (1999) Helicobacter species colonizing pig stomach: molecular characterization and determination of prevalence. Appl Environ Microbiol 65:4672–4676
Carter EL, Tronrud DE, Taber SR, Karplus PA, Hausinger RP (2011) Iron-containing urease in a pathogenic bacterium. Proc Natl Acad Sci U S A 108:13095–13099
Carter EL, Proshlyakov DA, Hausinger RP (2012) Apoprotein isolation and activation, and vibrational structure of the Helicobacter mustelae iron urease. J Inorg Biochem 111:195–202
Casagrande Proietti P, Bietta A, Brachelente C, Lepri E, Davidson I, Franciosini MP (2010) Detection of Helicobacter spp. in gastric fecal and saliva samples from swine affected by gastric ulceration. J Vet Sci 11:221–225
Castiglioni V, Vailati Facchini R, Mattiello S, Luini M, Gualdi V, Scanziani E, Recordati C (2012) Enterohepatic Helicobacter spp. in colonic biopsies of dogs: molecular, histopathological and immunohistochemical investigations. Vet Microbiol 159:107–114
Cattoli G, Bart A, Klaver PS, Robijn RJ, Beumer HJ, van Vugt R, Pot RG, van der Gaag I, Vandenbroucke-Grauls CM, Kuipers EJ, Kusters JG (2000) Helicobacter acinonychis eradication leading to the resolution of gastric lesions in tigers. Vet Rec 147:164–165
Ceelen L, Decostere A, Ducatelle R, Haesebrouck F (2006a) Cytolethal distending toxin generates cell death by inducing a bottleneck in the cell cycle. Microbiol Res 161:109–120
Ceelen L, Decostere A, Martel A, Pasmans F, Haesebrouck F (2006b) First report of Helicobacter pullorum in the faeces of a diarrhoeic psittacine bird (Psephotus haematogaster). Vet Rec 159:389–390
Ceelen L, Haesebrouck F, Ducatelle R, Decostere A (2007a) The occurrence and clinical significance of enterohepatic Helicobacter species in laboratory rodents. Vlaams Diergen Tijds 76:103–116
Ceelen L, Decostere A, Chiers K, Ducatelle R, Maes D, Haesebrouck F (2007b) Pathogenesis of Helicobacter pullorum infections in broilers. Int J Food Microbiol 116:207–213
Choi YK, Han JH, Joo HS (2001) Identification of novel Helicobacter species in pig stomachs by PCR and partial sequencing. J Clin Microbiol 39:3311–3315
Chow J, Mazmanian SK (2010) A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 7:265–276
Cinque SMS, Rocha GA, Correa-Oliveira R, Soares TF, Moura SB, Rocha AMC, Nogueira AM, Cabral MM, Vieira LQ, Martins-Filho OA, Queiroz DM (2006) The role of IFN-γ and IL-4 in gastric mucosa inflammation associated with Helicobacter heilmannii type 1 infection. Braz J Med Biol Res 39:253–261
Clyne M, Cróinín TO, Suerbaum S, Josenhans C, Drumm B (2000) Adherence of isogenic flagellum-negative mutants of Helicobacter pylori and Helicobacter mustelae to human and ferret gastric epithelial cells. Infect Immun 68:4335–4339
Collado L, Jara R, Gonzalez S (2014) Description of Helicobacter valdiviensis sp. nov., a novel Epsilonproteobacteria isolated from wild bird faecal samples in Southern Chile. Int J Syst Evol Microbiol 64:1913–1919. doi:10.1099/ijs.0.057141-0
Contreras M, Morales A, Garcia-Amado MA, De Vera M, Bermúdez V, Gueneau P (2007) Detection of Helicobacter-like DNA in the gastric mucosa of thoroughbred horses. Lett Appl Microbiol 45:553–557
Curry A, Jones DM, Skelton-Stroud P (1989) Novel ultrastructural findings in a helical bacterium found in the baboon (Papio anubis) stomach. J Gen Microbiol 135:2223–2231
Daidiliene D, Dailide G, Ogura K, Zhang M, Mukhopadhyay AK, Eaton KA, Cattoli G, Kusters JG, Berg DE (2004) Helicobacter acinonychis: genetic and rodent infection studies of a Helicobacter pylori-like gastric pathogen of cheetahs and other big cats. J Bacteriol 186:356–365
Davison NJ, Barnett JE, Koylass M, Whatmore AM, Perkins MW, Deaville RC, Jepson PD (2014) Helicobacter cetorum infection in striped dolphin (Stenella coeruleoalba), Atlantic white-sided dolphin (Lagenorhynchus acutus), and short-beaked common dolphin (Delphinus delphus) from the Southwest Coast of England. J Wildl Dis. doi:10.7589/2013-02-047
De Bock M, Decostere A, Van den Bulck K, Baele M, Duchateau L, Haesebrouck F, Ducatelle R (2005) The inflammatory response in the mouse stomach to Helicobacter bizzozeronii, Helicobacter salomonis and two Helicobacter felis strains. J Comp Pathol 133:83–91
De Bock M, Decostere A, Hellemans A, Haesebrouck F, Ducatelle R (2006) Helicobacter felis and Helicobacter bizzozeronii induce gastric parietal cell loss in Mongolian gerbils. Microbes Infect 8:503–510
De Bruyne E, Flahou B, Chiers K, Meyns T, Kumar S, Vermoote M, Pasmans F, Millet S, Dewulf J, Haesebrouck F, Ducatelle R (2012) An experimental Helicobacter suis infection causes gastritis and reduced daily weight gain in pigs. Vet Microbiol 160:449–454
De Cooman L, Fahou B, Houf K, Smet A, Ducatelle R, Pasmans F, Haesebrouck F (2013) Survival of Helicobacter suis bacteria in retail pig meat. Int J Food Microbiol 166:164–167
De Groote D, Van Doorn LJ, Ducatelle R, Verschuuren A, Tilmant K, Quint WGV, Haesebrouck F, Vandamme P (1999a) Phylogenetic characterization of ‘Candidatus Helicobacter bovis’, a new gastric Helicobacter in cattle. Int J Syst Bacteriol 49:1707–1715
De Groote D, van Doorn LJ, Ducatelle R, Verschuuren A, Haesebrouck F, Quint WGV, Jalava K, Vandamme P (1999b) ‘Candidatus Helicobacter suis’, a gastric Helicobacter from pigs, and its phylogenetic relatedness to other gastrospirilla. Int J Syst Bacteriol 49:1769–1777
De Groote D, Van Doorn LJ, Van den Bulck K, Vandamme P, Vieth M, Stolte M, Debongnie JC, Burette A, Haesebrouck F, Ducatelle R (2005) Detection of non-pylori Helicobacter species in “Helicobacter heilmannii”-infected humans. Helicobacter 10:398–406
Dewhirst FE, Fox JG, Mendes EN, Paster BJ, Gates CE, Kirkbride CA, Eaton KA (2000) ‘Flexispira rappini’ strains represent at least 10 Helicobacter taxa. Int J Syst Evol Microbiol 50:1781–1787
Diker KS, Haziroglu R, Akan M, Celik S, Kabakci N (2002) The prevalence colonization sites and pathological effects of gastric helicobacters in dogs. Turk J Vet Anim Sci 26:345–351
Ding H, Nedrud JG, Blanchard TG, Zagorski BM, Li G, Shiu J, Xu J, Czinn SJ (2013) Th1-mediated immunity against Helicobacter pylori can compensate for lack of Th17 cells and can protect mice in the absence of immunization. PLoS ONE 8:e69384
Dore MP, Sepulveda AR, El-Zimaity H, Yamaoka Y, Osato MS, Mototsugu K, Nieddu AM, Realdi G, Graham DY (2001) Isolation of Helicobacter pylori from sheep-implications for transmission to humans. Am J Gastroenterol 96:1396–1401
Drazek ES, Dubois A, Holmes RK (1994) Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol 32:1799–1804
Drevon-Gaillot E, Perron-Lepage MF, Clement C, Burnett R (2006) A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol 58:77–88
Driessen A, Van Ginneken C, Creemers J, Lambrichts I, Weyns A, Geboes K, Ectors N (2002) Histological and immunohistochemical study of the lymphoid tissue in the normal stomach of the gnotobiotic pig. Virchows Arch 441:589–598
Dubois A, Tarnawski A, Newell DG, Fiala N, Dabros W, Stachura J, Krivan H, Heman-Ackah LM (1991) Gastric injury and invasion of parietal cells by spiral bacteria in rhesus monkeys. Are gastritis and hyperchlorhydria infectious diseases? Gastroenterology 100:884–891
Ekman E, Fredriksson M, Trowald-Wigh G (2013) Helicobacter spp. in the saliva, stomach, duodenum and faeces of colony dogs. Vet J 195:127–129
Elbers ARW, Dirkzwager A (1994) Changes in stomach mucosa in swine: a literature review. Tijdschr Diergeneeskd 119:669–674
El-Zaatari M, Kao JY, Tessier A, Bai L, Hayes MM, Fontaine C, Eaton KA, Merchant JL (2013) Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS ONE 8:e58935
Eppinger M, Baar C, Linz B, Raddatz G, Lanz C, Keller H, Morelli G, Gressmann H, Achtman M, Schuster SC (2006) Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet 7:e120
Erdman SE, Correa P, Coleman LA, Schrenzel MD, Li X, Fox JG (1997) Helicobacter mustelae-associated gastric MALT lymphoma in ferrets. Am J Pathol 151:273–280
Every AL, Chionh YT, Skene CD, McGuckin MA, Sutton P (2008) Muc1 limits Helicobacter felis binding to gastric epithelial cells but does not limit colonization and gastric pathology following infection. Helicobacter 13:489–493
Fabisiak M, Sapierzyński R, Salamaszyńska-Guz A, Kizerwetter-Swida M (2010) The first description of gastric Helicobacter in free-ranging wild boar (Sus scrofa) from Poland. Pol J Vet Sci 13:171–174
Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, Frydman HM (2011) Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 334:990–992
Fernandez KR, Hansen LM, Vandamme P, Beaman BL, Solnick JV (2002) Captive rhesus monkeys (Macaca mulatta) are commonly infected with Helicobacter cinaedi. J Clin Microbiol 40:1908–1912
Flahou B, Haesebrouck F, Pasmans F, D’Herde K, Driessen A, Van Deun K, Smet A, Duchateau L, Chiers K, Ducatelle R (2010) Helicobacter suis causes severe gastric pathology in mouse and mongolian gerbil models of human gastric disease. PLoS ONE 5(11):e14083. doi:10.1371/journal.pone.0014083
Flahou B, Haesebrouck F, Chiers K, Van Deun K, De Smet L, Devreese B, Vandenberghe I, Favoreel H, Smet A, Pasmans F, D’Herde K, Ducatelle R (2011) Gastric epithelial cell death caused by Helicobacter suis and Helicobacter pylori γ-glutamyl transpeptidase is mainly glutathione degradation-dependent. Cell Microbiol 13:1933–1955
Flahou B, Van Deun K, Pasmans F, Smet A, Volf J, Rychlik I, Ducatelle R, Haesebrouck F (2012) The local immune response of mice after Helicobacter suis infection: strain differences and distinction with Helicobacter pylori. Vet Res 43:75
Flahou B, Modrý D, Pomajbíková K, Petrželková KJ, Smet A, Ducatelle R, Pasmans F, Sá RM, Todd A, Hashimoto C, Mulama M, Kiang J, Rossi M, Haesebrouck F (2014) Diversity of zoonotic enterohepatic Helicobacter species and detection of a putative novel gastric Helicobacter species in wild and wild-born captive chimpanzees and western lowland gorillas. Vet Microbiol 174:186–194
Forester NT, Parton K, Lumsden JS, O’Toole PW (2000) Isolation of Helicobacter mustelae from ferrets in New Zealand. N Z Vet J 48:65–69
Fox JG, Edrise BM, Cabot EB, Beaucage C, Murphy JC, Prostak KS (1986) Campylobacter-like organisms isolated from gastric mucosa of ferrets. Am J Vet Res 47:236–239
Fox JG, Cabot EB, Taylor NS, Laraway R (1988) Gastric colonization by Campylobacter pylori subsp. mustelae in ferrets. Infect Immun 56:2994–2996
Fox JG, Correa P, Taylor NS, Lee A, Otto G, Murphy JC, Rose R (1990) Helicobacter mustelae-associated gastritis in ferrets. Gastroenterology 99:352–361
Fox JG, Otto G, Murphy JC, Taylor NS, Lee A (1991a) Gastric colonization of the ferret with Helicobacter species: natural and experimental infections. Rev Infect Dis 13(Suppl 8):S671–S680
Fox JG, Otto G, Taylor NS, Rosenblad W, Murphy JC (1991b) Helicobacter mustelae-induced gastritis and elevated gastric pH in the ferret (Mustela putorius furo). Infect Immun 59:1875–1880
Fox JG, Paster BJ, Dewhirst FE, Taylor NS, Yan LL, Macuch PJ, Chmura LM (1992) Helicobacter mustelae isolation from feces of ferrets: evidence to support fecal-oral transmission of a gastric Helicobacter. Infect Immun 60:606–611
Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ Jr, Gorelick PL, Ward JM (1994) Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol 32(5):1238–1245
Fox JG, Yan LL, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN (1995) Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol 33(2):445–454
Fox JG, Dangler CA, Sager W, Borkowski R, Gliatto JM (1997) Helicobacter mustelae-associated gastric adenocarcinoma in ferrets (Mustela putorius furo). Vet Pathol 34:225–229
Fox JG, Chien CC, Dewhirst FE, Paster BJ, Shen Z, Melito PL, Woodward DL, Rodgers FG (2000) Helicobacter canadensis sp. nov. isolated from humans with diarrhea as an example of an emerging pathogen. J Clin Microbiol 38:2546–2549
Fox JG, Shen Z, Xu S, Feng Y, Dangler CA, Dewhirst FE, Paster BJ, Cullen JM (2002) Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J Clin Microbiol 40:2513–2519
Fox JG, Taylor NS, Howe S, Tidd M, Xu S, Paster BJ, Dewhirst FE (2006) Helicobacter anseris sp. nov. and Helicobacter brantae sp. nov., isolated from feces of resident Canada geese in the greater Boston area. Appl Environ Microbiol 72:4633–4637
Fox JG, Boutin SR, Handt LK, Taylor NS, Xu S, Rickman B, Marini RP, Dewhirst FE, Paster BJ, Motzel S, Klein HJ (2007) Isolation and characterization of a novel Helicobacter species, “Helicobacter macacae,” from rhesus monkeys with and without chronic idiopathic colitis. J Clin Microbiol 45:4061–4063
García A, Xu S, Dewhirst FE, Nambiar PR, Fox JG (2006) Enterohepatic Helicobacter species isolated from the ileum, liver and colon of a baboon with pancreatic islet amyloidosis. J Med Microbiol 55:1591–1595
Ghil HM, Yoo JH, Jung WS, Chung TH, Youn HY, Hwang CY (2009) Survey of Helicobacter infection in domestic and feral cats in Korea. J Vet Sci 10:67–72
Gold BD, Dytoc M, Huesca M, Philpott D, Kuksis A, Czinn S, Lingwood CA, Sherman PM (1995) Comparison of Helicobacter mustelae and Helicobacter pylori adhesion to eukaryotic cells in vitro. Gastroenterology 109:692–700
Gold BD, Islur P, Policova Z, Czinn S, Neumann AW, Sherman PM (1996) Surface properties of Helicobacter mustelae and ferret gastrointestinal mucosa. Clin Investig Med 19:92–100
Goldman CG, Loureiro JD, Matteo MJ, Catalano M, Gonzalez AB, Heredia SR, Zubillaga MB, Solnick JV, Cremaschi GA (2009a) Helicobacter spp. from gastric biopsies of stranded South American fur seals (Arctocephalus australis). Res Vet Sci 86:18–21
Goldman CG, Matteo MJ, Loureiro JD, Degrossi J, Teves S, Heredia SR, Alvarez K, González AB, Catalano M, Boccio J, Cremaschi G, Solnick JV, Zubillaga MB (2009b) Detection of Helicobacter and Campylobacter spp. from the aquatic environment of marine mammals. Vet Microbiol 133:287–291
Goldman CG, Matteo MJ, Loureiro JD, Almuzara M, Barberis C, Vay C, Catalano M, Heredia SR, Mantero P, Boccio JR, Zubillaga MB, Cremaschi GA, Solnick JV, Perez-Perez GI, Blaser MJ (2011) Novel gastric helicobacters and oral campylobacters are present in captive and wild cetaceans. Vet Microbiol 152:138–145
Goodwin CS, Armstrong JA, Chilvers T, Peters M, Colins MD, Sly L, Mcconnell W, Harper WES (1989) Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int J Syst Bacteriol 39:397–405
Goto K, Ohashi H, Ebukuro S, Itoh K, Tohma Y, Takakura A, Wakana S, Ito M, Itoh T (1998) Isolation and characterization of Helicobacter species from the stomach of the house musk shrew (Suncus murinus) with chronic gastritis. Curr Microbiol 37:44–51
Goto T, Ogura Y, Hirakawa H, Tomida J, Morita Y, Akaike T, Hayashi T, Kawamura Y (2012) Complete genome sequence of Helicobacter cinaedi strain PAGU611, isolated in a case of human bacteremia. J Bacteriol 194:3744–3745
Grasso GM, Ripabelli G, Sammarco ML, Ruberto A, Iannitto G (1996) Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis 19:213–217
Gressmann H, Linz B, Ghai R, Pleissner KP, Schlapbach R, Yamaoka Y, Kraft C, Suerbaum S, Meyer TF, Achtman M (2005) Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet 4:e43
Gueneau P, Fuenmayor J, Aristimuno OC, Cedeno S, Baez E, Reyes N, Michelangeli F, Domínguez-Bello MG (2002) Are goats naturally resistant to gastric Helicobacter infection? Vet Microbiol 84:115–121
Haesebrouck F, Pasmans F, Flahou B, Chiers K, Baele M, Meyns T, Decostere A, Ducatelle R (2009) Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev 22:202–223
Haesebrouck F, Pasmans F, Flahou B, Smet A, Vandamme P, Ducatelle R (2011) Non-Helicobacter pylori Helicobacter species in the human gastric mucosa: a proposal to introduce the terms H. heilmannii sensu lato and sensu stricto. Helicobacter 16:339–340
Hamada T, Yokota K, Ayada K, Hirai K, Kamada T, Haruma K, Chayama K, Oguma K (2009) Detection of Helicobacter hepaticus in human bile samples of patients with biliary disease. Helicobacter 14:545–551
Hamir AN, Stasko J, Rupprecht CE (2004) Observation of Helicobacter-like organisms in gastric mucosa of grey foxes (Urocyon cinereoargenteus) and bobcats (Lynx rufus). Can J Vet Res 68:154–156
Hänninen ML, Happonen I, Jalava K (1998) Transmission of canine gastric Helicobacter salomonis infection from dam to offspring and between puppies. Vet Microbiol 62:47–58
Hänninen ML, Utriainen M, Happonen I, Dewhirst FE (2003) Helicobacter sp. flexispira 16S rDNA taxa 1, 4 and 5 and Finnish porcine Helicobacter isolates are members of the species Helicobacter trogontum (taxon 6). Int J Syst Evol Microbiol 53:425–433
Hänninen ML, Kärenlampi RI, Koort JM, Mikkonen T, Björkroth KJ (2005) Extension of the species Helicobacter bilis to include the reference strains of Helicobacter sp. flexispira taxa 2, 3 and 8 and Finnish canine and feline flexispira strains. Int J Syst Evol Microbiol 55:891–898
Hansen R, Thomson JM, Fox JG, El-Omar EM, Hold GL (2011) Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol 61(1):1–14
Haringsma PC, Mouwen JMVM (1992) Mogelijke betekenis van spirilvormige bacteriën bij het ontstaan van lebmaagzweren bij het volwassen rund. Tijdschr Diergeneeskd 117:485–486
Harper CG, Feng Y, Xu S, Taylor NS, Kinsel M, Dewhirst FE, Paster BJ, Greenwell M, Levine G, Rogers A, Fox JG (2002) Helicobacter cetorum sp. nov., a urease-positive Helicobacter species isolated from dolphins and whales. J Clin Microbiol 40:4536–4543
Harper CG, Whary MT, Feng Y, Rhinehart HL, Wells RS, Xu S, Taylor NS, Fox JG (2003a) Comparison of diagnostic techniques for Helicobacter cetorum infection in wild Atlantic bottlenose dolphins (Tursiops truncatus). J Clin Microbiol 41:2842–2848
Harper CG, Xu S, Rogers AB, Feng Y, Shen Z, Taylor NS, Dewhirst FE, Paster BJ, Miller M, Hurley J, Fox JG (2003b) Isolation and characterization of novel Helicobacter spp. from the gastric mucosa of harp seals Phoca groenlandica. Dis Aquat Organ 57:1–9
Heilmann KL, Borchard F (1991) Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut 32:137–140
Hellemans A, Chiers K, Decostere A, De Bock M, Haesebrouck F, Ducatelle R (2007a) Experimental infection of pigs with “Candidatus Helicobacter suis”. Vet Res Commun 31:385–395
Hellemans A, Chiers K, Maes D, De Bock M, Decostere A, Haesebrouck F, Ducatelle R (2007b) Prevalence of “Candidatus Helicobacter suis” in pigs of different ages. Vet Rec 161:189–192
Hessing MJC, Geudeke MJ, Scheepens CJM, Tielen MJM, Schouten WGP, Wiepkema PR (1992) Mucosal lesions in the pars oesophagea in pigs: prevalence and influence of stress. Tijdschr Diergeneeskd 117:445–450
Hitzler I, Kohler E, Engler DB, Yazgan AS, Müller A (2012) The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front Immunol 3:142
Husted L, Jensen TK, Olsen SN, Mølbak L (2010) Examination of equine glandular stomach lesions for bacteria, including Helicobacter spp. by fluorescence in situ hybridisation. BMC Microbiol 10:84
Inglis GD, McConville M, de Jong A (2006) Atypical Helicobacter canadensis strains associated with swine. Appl Environ Microbiol 72:4464–4471
Iwanczak B, Biernat M, Iwanczak F, Grabinska J, Matusiewicz K, Gosciniak G (2012) The clinical aspects of Helicobacter heilmannii infection in children with dyspeptic symptoms. J Physiol Pharmacol 63:133–136
Jelinski MD, Ribble CS, Chirino-Trejo M, Clark EG, Janzen ED (1995) The relationship between the presence of Helicobacter pylori, Clostridium perfringens type A, Campylobacter spp, or fungi and fatal abomasal ulcers in unweaned beef calves. Can Vet J 36:379–382
Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Kim KA, Yang JH, Lee JS, Moon YS, Kim KM (2007) Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci 22:63–69
Joosten M, Blaecher C, Flahou B, Ducatelle R, Haesebrouck F, Smet A (2013a) Diversity in bacterium-host interactions within the species Helicobacter heilmannii sensu stricto. Vet Res 44:65
Joosten M, Flahou B, Meyns T, Smet A, Arts J, De Cooman L, Pasmans F, Ducatelle R, Haesebrouck F (2013b) Case report: Helicobacter suis infection in a pig veterinarian. Helicobacter 18:392–396
Joosten M, Lindén S, Rossi M, Tay A, Skoog E, Padra M, Thirriot F, Perkins T, Vandamme P, Van Nieuwerburg F, Flahou B, Deforce D, Ducatelle R, Haesebrouck F, Smet A (2015) Genomic divergence and differences in adhesion to the gastric mucosa between Helicobacter heilmannii and its closest relative Helicobacter ailurogastricus sp. nov. Infect Immun 84:293–306
Josenhans C, Labigne A, Suerbaum S (1995) Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol 177:3010–3020
Kaakoush NO, Sirianni A, Raftery MJ, Mitchell HM (2013) The secretome of Helicobacter trogontum. Helicobacter 18:316–320
Kersulyte D, Rossi M, Berg DE (2013) Sequence divergence and conservation in genomes of Helicobacter cetorum strains from a dolphin and a whale. PLoS ONE 8:e83177
Kivistö R, Linros J, Rossi M, Rautelin H, Hänninen ML (2010) Characterization of multiple Helicobacter bizzeronii isolates from a Finish patient with severe dyspeptic symptoms and chronic active gastritis. Helicobacter 15:58–66
Kobayashi T, Harada K, Miwa K, Nakanuma Y (2005) Helicobacter genus DNA fragments are commonly detectable in bile from patients with extrahepatic biliary diseases and associated with their pathogenesis. Dig Dis Sci 50:862–867
Kondadi PK, Pacini C, Revez J, Hänninen ML, Rossi M (2013) Contingency nature of Helicobacter bizzozeronii oxygen-insensitive NAD(P)H-nitroreductase (HBZC1 00960) and its role in metronidazole resistance. Vet Res 44:56
Kopta LA, Paquette JA, Bowersock TL, Choromanski LJ, Godbee TK, Galvin JE, Foss DL (2010) Information of Helicobacter suis in pig-producing regions of North America. Conference of Research Workers in Animal Diseases, Chicago, 5–7 December 1998
Krakowka S, Ellis J (2006) Reproduction of severe gastroesophageal ulcers (GEU) in gnotobiotic swine infected with porcine Helicobacter pylori-like bacteria. Vet Pathol 43:956–962
Kubota S, Ohno K, Tsukamoto A, Maeda S, Murata Y, Nakashima K, Fukushima K, Uchida K, Fujino Y, Tsujimoto H (2013) Value of the 13C urea breath test for the detection of gastric Helicobacter spp. infection in dogs undergoing endoscopic examination. J Vet Med Sci 75:1049–1054
Lanzoni A, Faustinelli I, Cristofori P, Luini M, Simpson KW, Scanziani E, Recordati C (2011) Localization of Helicobacter spp. in the fundic mucosa of laboratory beagle dogs: an ultrastructural study. Vet Res 42:42
Lee A, Hazell SL, O’Rourke J, Kouprach S (1988) Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun 56:2843–2850
Lee A, Phillips MW, O’Rourke JL, Paster BJ, Dewhirst FE, Fraser GJ, Fox JG, Sly LI, Romaniuk PJ, Trust TJ, Kouprach S (1992) Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int J Syst Bacteriol 42:27–36
Lertpiriyapong K, Handt L, Feng Y, Mitchell TW, Lodge KE, Shen Z, Dewhirst FE, Muthupalani S, Fox JG (2014) Pathogenic properties of enterohepatic Helicobacter spp. isolated from Rhesus macaques with intestinal adenocarcinoma. J Med Microbiol 63:1004–1016
Liang J, De Bruyne E, Ducatelle R, Smet A, Haesebrouck F, Flahou B (2015) Purification of Helicobacter suis strains from biphasic cultures by single colony isolation: influence on strain characteristics. Helicobacter 20:206–216. doi:10.1111/hel.12192
Lindén SK, Wickström C, Lindell G, Gilshenan K, Carlstedt I (2008) Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter 13:81–93
Liu C, Smet A, Blaecher C, Flahou B, Ducatelle R, Linden S, Haesebrouck F (2014) Gastric de novo Muc13 expression and spasmolytic polypeptide-expressing metaplasia during Helicobacter heilmannii infection. Infect Immun 82:3227–3239
Liyanage NP, Dassanayake RP, Kuszynski CA, Duhamel GE (2013) Contribution of Helicobacter hepaticus cytolethal distending toxin subunits to human epithelial cell cycle arrest and apoptotic death in vitro. Helicobacter 18:433–443
Luiz de Camargo P, Akemi Uenaka S, Bette Motta M, Harumi Adania C, Yamasaki L, Alfieri AA, Bracarense AP (2011) Gastric helicobacter spp. Infection in captive neotropical brazilian feline. Braz J Microbiol 42:290–297
Mackie JT, O’Rourke JL (2003) Gastritis associated with Helicobacter-like organisms in baboons. Vet Pathol 40:563–566
Marini RP, Fox JG (1999) Animal models of Helicobacter (ferrets). In: Zak O, Sande MA (eds) Handbook of animal models of infection. Academic, London, pp 273–284
Martin ME, Bhatnagar S, George MD, Paster BJ, Canfield DR, Eisen JA, Solnick JV (2013) The impact of Helicobacter pylori infection on the gastric microbiota of the rhesus macaque. PLoS ONE 8:e76375
Matsui H, Takahashi T, Murayama SY, Uchiyama I, Yamaguchi K, Shigenobu S, Matsumoto T, Kawakubo M, Horiuchi K, Ota H, Osaki T, Kamiya S, Smet A, Flahou B, Ducatelle R, Haesebrouck F, Takahashi S, Nakamura S, Nakamura M (2014) Development of new PCR primers by comparative genomics for the detection of Helicobacter suis in gastric biopsy specimens. Helicobacter 19:260–271. doi:10.1111/hel.12127
Matsukura N, Yokomuro S, Yamada S, Tajiri T, Sundo T, Hadama T, Kamiya S, Naito Z, Fox JG (2002) Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Vet Res 93:842–847
Matsumoto T, Goto M, Murakami H, Tanaka T, Nishiyama H, Ono E, Okada C, Sawabe E, Yagoshi M, Yoneyama A, Okuzumi K, Tateda K, Misawa N, Yamaguchi K (2007) Multicenter study to evaluate bloodstream infection by Helicobacter cinaedi in Japan. J Clin Microbiol 45:2853–2857
Matsumoto T, Masatomo K, Akamatsu T, Koide N, Ogiwara N, Kubota S, Sugano M, Kawakami Y, Katsuyama T, Ota H (2014) Helicobacter heilmannii sensu stricto-related gastric ulcers: a case report. World J Gastroenterol 20:3376–3382
Mazzoni M, Bosi P, De Sordi N, Lalatta-Costerbosa G (2011) Distribution, organization and innervation of gastric MALT in conventional piglet. J Anat 219:611–621
McLaughlin RW, Zheng JS, Chen MM, Zhao QZ, Wang D (2011) Detection of Helicobacter in the fecal material of the endangered Yangtze finless porpoise Neophocaena phocaenoides asiaeorientalis. Dis Aquat Organ 95:241–245
McNulty CA, Dent JC, Curry A, Uff JS, Ford GA, Gear MW, Wilkinson SP (1989) New spiral bacterium in gastric mucosa. J Clin Pathol 42:585–591
Melito PL, Munro C, Chipman PR, Woodward DL, Booth TF, Rodgers FG (2001) Helicobacter winghamensis sp. nov., a novel Helicobacter sp. isolated from patients with gastroenteritis. J Clin Microbiol 39:2412–2417
Melnichouk SI, Friendship RM, Dewey CE, Bildfell RJ, Smart NL (1999) Helicobacter-like organisms in the stomach of pigs with and without gastric ulceration. Swine Health Prod 7:201–205
Mendes EN, Queiroz DMM, Rocha GA, Moura SB, Leite VHR, Fonseca MEF (1990) Ultrastructure of a spiral micro-organism from pig gastric mucosa (“Gastrospirillum suis”). J Med Microbiol 33:61–66
Mendes EN, Queiroz DMM, Rocha GA, Nogueira AMMF, Carvalho ACT, Lage AP, Barbosa AJ (1991) Histopathological study of porcine gastric mucosa with and without a spiral bacterium (“Gastrospirillum suis”). J Med Microbiol 35:345–348
Mimura T, Yoshida M, Nishiumi S, Tanaka H, Nobutani K, Takenaka M, Suleiman YB, Yamamoto K, Ota H, Takahashi S, Matsui H, Nakamura M, Miki I, Azuma T (2011) IFN-γ plays an essential role in the pathogenesis of gastric lymphoid follicles formation caused by Helicobacter suis infection. FEMS Immunol Med Microbiol 63(1):25–34
Misawa N, Kawashima K, Kondo F, Kushima E, Kushima K, Vandamme P (2002) Isolation and characterization of Campylobacter, Helicobacter and Anaerobiospirillum strains from a puppy with bloody diarrhea. Vet Microbiol 87:353–364
Momtaz H, Dabiri H, Souod N, Gholami M (2014) Study of Helicobacter pylori genotype status in cows, sheep, goats and human beings. BMC Gastroenterol 14:61
Mörner T, Bröjer C, Ryser-Degiorgis MP, Gavier-Widén D, Nilsson HO, Wadström T (2008) Detection of gastric Helicobacter species in free-ranging lynx (Lynx lynx) and red foxes (Vulpes vulpes) in Sweden. J Wildl Dis 44:697–700
Moura SB, Queiroz DMM, Mendes EN, Nogueira AMMF, Rocha GA (1993) The inflammatory response of the gastric mucosa of mice experimentally infected with “Gastrospirillum suis”. J Med Microbiol 39:64–68
Moyaert H, Decostere A, Vandamme P, Debruyne L, Mast J, Baele M, Ceelen L, Ducatelle R, Haesebrouck F (2007a) Helicobacter equorum sp. nov., a urease-negative Helicobacter species isolated from horse faeces. Int J Syst Evol Microbiol 57:213–218
Moyaert H, Haesebrouck F, Baele M, Picavet T, Ducatelle R, Chiers K, Ceelen L, Decostere A (2007b) Prevalence of Helicobacter equorum in faecal samples from horses and humans. Vet Microbiol 121:378–383
Moyaert H, Decostere A, Pasmans F, Baele M, Ceelen L, Smits K, Ducatelle R, Haesebrouck F (2007c) Acute in vivo interactions of Helicobacter equorum with its equine host. Equine Vet J 39:370–372
Moyaert H, Haesebrouck F, Ducatelle R, Pasmans F (2009) Helicobacter equorum is highly prevalent in foals. Vet Microbiol 133:190–192
Munson L, Terio KA, Worley M, Jago M, Bagot-Smith A, Marker L (2005) Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J Wildl Dis 41:542–548
Murata H, Tsuji S, Tjusii M, Fu HY, Tanimura H, Tjujimoto M, Matsuura N, Kawano S, Hori M (2004) Helicobacter bilis infection in biliary tract cancer. Aliment Pharmacol Ther 20:90–94
Nakamura M, Murayama SY, Serizawa H, Sekiya Y, Eguchi M, Takahashi S, Nishikawa K, Takahashi T, Matsumoto T, Yamada H, Hibi T, Tsuchimoto K, Matsui H (2007) “Candidatus Helicobacter heilmannii” from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun 75:1214–1222
Nakamura M, Takahashi S, Matsui H, Murayama SY, Aikawa C, Sekiya Y, Nishikawa K, Matsumoto T, Yamada H, Tsuchimoto K (2008) Microcirculatory alteration in low-grade gastric mucosa-associated lymphoma by Helicobacter heilmannii infection: its relation to vascular endothelial growth factor and cyclooxygenase-2. J Gastroenterol Hepatol 23(Suppl 2):S157–S160
Nakamura M, Matsui H, Takahashi T, Ogawa S, Tamura R, Murayama SY, Takahashi S, Tsuchimoto K (2010) Suppression of lymphangiogenesis induced by Flt-4 antibody in gastric low-grade mucosa-associated lymphoid tissue lymphoma by Helicobacter heilmannii infection. J Gastroenterol Hepatol 25(Suppl 1):S1–S6
Nakamura M, Takahashi T, Matsui H, Takahashi S, Murayama SY, Suzuki H, Tsuchimoto K (2014) New pharmaceutical treatment of gastric MALT lymphoma: anti-angiogenesis treatment using VEGF receptor antibodies and celecoxib. Curr Pharm Des 20:1097–1103
Nambiar PR, Kirchain S, Fox JG (2005) Gastritis-associated adenocarcinoma and intestinal metaplasia in a Syrian hamster naturally infected with Helicobacter species. Vet Pathol 42:386–390
Nebbia P, Tramuta C, Ortoffi M, Bert E, Cerruti Sola S, Robino P (2007) Identification of enteric Helicobacter in avian species. Schweiz Arch Tierheilkd 149:403–407
Neubauer C, Hess M (2006) Tissue tropism of Helicobacter pullorum in specific pathogen-free chickens determined by culture and nucleic acid detection. Avian Dis 50:620–623
Nilsson HO, Stenram U, Ihse I, Wadström T (2006) Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. J Gastroenterol 12:3038–3043
Nishikawa K, Nakamura M, Takahashi S, Matsui H, Murayama SY, Matsumoto T, Yamada H, Tsuchimoto K (2007) Increased apoptosis and angiogenesis in gastric low-grade mucosa-associated lymphoid tissue-type lymphoma by Helicobacter heilmannii infection in C57BL/6 mice. FEMS Immunol Med Microbiol 50:268–272
Nobutani K, Yoshida M, Nishiumi S, Nishitani Y, Takagawa T, Tanaka H, Yamamoto K, Mimura T, Bensuleiman Y, Ota H, Takahashi S, Matsui H, Nakamura M, Azuma T (2010) Helicobacter heilmannii can induce gastric lymphoid follicles in mice via a Peyer’s patch-independent pathway. FEMS Immunol Med Microbiol 60:156–164
O’Rourke J, Lee A, Fox JG (1992) An ultrastructural study of Helicobacter mustelae and evidence of a specific association with gastric mucosa. J Med Microbiol 36:420–427
O’Rourke JL, Dixon MF, Jack A, Enno A, Lee A (2004a) Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J Pathol 203:896–903
O’Rourke JL, Solnick JV, Neilan BA, Seidel K, Hayter R, Hansen LM, Lee A (2004b) Description of “Candidatus Helicobacter heilmannii” based on DNA sequence analysis of 16S rRNA and urease genes. Int J Syst Evol Microbiol 54:2203–2211
O’Toole PW, Austin JW, Trust TJ (1994) Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol 11:349–361
Obonyo M, Rickman B, Guiney DG (2011) Effects of myeloid differentiation primary response gene 88 (MyD88) activation on Helicobacter infection in vivo and induction of a Th17 response. Helicobacter 16:398–404
Ok M, Sen I, Turgut K, Irmak K (2001) Plasma gastrin activity and the diagnosis of bleeding abomasal ulcers in cattle. J Vet Med A 48:563–568
Otte CM, Gutiérrez OP, Favier RP, Rothuizen J, Penning LC (2012) Detection of bacterial DNA in bile of cats with lymphocytic cholangitis. Vet Microbiol 156:217–221
Oxley AP, McKay DB (2005) Comparison of Helicobacter spp. genetic sequences in wild and captive seals, and gulls. Dis Aquat Organ 65:99–105
Oxley AP, Powell M, McKay DB (2004) Species of the family Helicobacteraceae detected in an Australian sea lion (Neophoca cinerea) with chronic gastritis. J Clin Microbiol 42:3505–3512
Oxley AP, Argo JA, McKay DB (2005) Helicobacter spp. from captive bottlenose dolphins (Tursiops spp.) and polar bears (Ursus maritimus). Vet J 170:377–380
Park JH, Lee BJ, Lee YS, Park JH (2000) Association of tightly spiraled bacterial infection and gastritis in pigs. J Vet Med Sci 62:725–729
Park JH, Seok SH, Cho SA, Baek MW, Lee HY, Kim DJ, Park JH (2004) The high prevalence of Helicobacter sp. in porcine pyloric mucosa and its histopathological and molecular characteristics. Vet Microbiol 104:219–225
Park JH, Seok SH, Baek MW, Lee HY, Kim DJ, Park JH (2008) Gastric lesions and immune responses caused by long-term infection with Helicobacter heilmannii in C57BL/6 mice. J Comp Pathol 139:208–217
Patterson MM, Schrenzel MD, Feng Y, Fox JG (2000a) Gastritis and intestinal metaplasia in Syrian hamsters infected with Helicobacter aurati and two other microaerobes. Vet Pathol 37:589–596
Patterson MM, Schrenzel MD, Feng Y, Xu S, Dewhirst FE, Paster BJ, Thibodeau SA, Versalovic J, Fox JG (2000b) Helicobacter aurati sp. nov., a urease-positive Helicobacter species cultured from gastrointestinal tissues of Syrian hamsters. J Clin Microbiol 38:3722–3728
Patterson MM, O’Toole PW, Forester NT, Noonan B, Trust TJ, Xu S, Taylor NS, Marini RP, Ihrig MM, Fox JG (2003) Failure of surface ring mutant strains of Helicobacter mustalae to persistently infect the ferret stomach. Infect Immun 71:2350–2355
Perkins GA, den Bakker HC, Burton AJ, Erb HN, McDonough SP, McDonough PL, Parker J, Rosenthal RL, Wiedmann M, Dowd SE, Simpson KW (2012) Equine stomachs harbor an abundant and diverse mucosal microbiota. Appl Environ Microbiol 78:2522–2532
Pot RGJ, Stoof J, Nuijten PJM, De Haan LAM, Loeffen P, Kuipers EJ, van Vliet AH, Kusters JG (2007) UreA2B2: a second urease system in the gastric pathogen Helicobacter felis. FEMS Immunol Med Microbiol 50:273–279
Prag J, Blom J, Krogfelt K (2007) Helicobacter canis bacteraemia in a 7-month-old child. FEMS Immunol Med Microbiol 50:264–267
Priestnall SL, Wiinberg B, Spohr A, Neuhaus B, Kuffer M, Wiedmann M, Simpson KW (2004) Evaluation of Helicobacter heilmannii subtypes in the gastric mucosa of cats and dogs. J Clin Microbiol 42:2144–2151
Quaglia NC, Dambrosio A, Normanno G, Parisi A, Patrono R, Ranieri G, Rella A, Celano GV (2008) High occurrence of Helicobacter pylori in raw goat, sheep and cow milk inferred by glmM gene: a risk of food-borne infection? Int J Food Microbiol 124:43–47
Queiroz DMM, Rocha GA, Mendes EN, Lage AP, Carvalho ACT, Barbosa AJA (1990) A spiral microorganism in the stomach of pigs. Vet Microbiol 24:199–204
Queiroz DMM, Rocha GA, Mendes EN, Moura SB, Rocha De Oliveira AM, Miranda D (1996) Association between Helicobacter and gastric ulcer disease of the pars oesophagea in swine. Gastroenterology 111:19–27
Rahimi E, Kheirabadi EK (2012) Detection of Helicobacter pylori in bovine, buffalo, camel, ovine, and caprine milk in Iran. Foodborne Pathog Dis 9:453–456
Reindel JF, Fitzgerald AL, Breider MA, Gough AW, Yan C, Mysore JV, Dubois A (1999) An epizootic of lymphoplasmacytic gastritis attributed to Helicobacter pylori infection in Cynomolgus Monkeys (Macaca fascicularis). Vet Pathol 36:1–13
Rimbara E, Mori S, Kim H, Matsui M, Suzuki S, Takahashi S, Yamamoto S, Mukai M, Shibayama K (2013) Helicobacter cinaedi and Helicobacter fennelliae transmission in a hospital from 2008 to 2012. J Clin Microbiol 51:2439–2442
Robertson BR, O’Rourke JL, Vandamme P, On SL, Lee A (2001) Helicobacter ganmani sp. nov., a urease-negative anaerobe isolated from the intestines of laboratory mice. Int J Syst Evol Microbiol 51(Pt 5):1881–1889
Robertson LD, Accioly JM, Moore KM, Driesen SJ, Pethick DW, Hampson DJ (2002) Risk factors for gastric ulcers in Australian pigs at slaughter. Prev Vet Med 53:293–303
Robino P, Tomassone L, Tramuta C, Rodo M, Giammarino M, Vaschetti G, Nebbia P (2010) Prevalence of Campylobacter jejuni, Campylobacter coli and enteric Helicobacter in domestic and free living birds in North-Western Italy. Schweiz Arch Tierheilkd 152:425–431
Roosendaal R, Vos JH, Roumen T, van Vugt R, Cattoli G, Bart A, Klaasen HL, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG (2000) Slaughter pigs are commonly infected by closely related but distinct gastric ulcerative lesion-inducing gastrospirilla. J Clin Microbiol 38:2661–2664
Rossi M, Hänninen ML, Revez J, Hannula M, Zanoni RG (2008) Occurrence and species level diagnostics of Campylobacter spp., enteric Helicobacter spp. and Anaerobiospirillum spp. in healthy and diarrheic dogs and cats. Vet Microbiol 129:304–314
Saito Y, Suzuki H, Tsugawa H, Imaeda H, Matsuzaki J, Hirata K, Hosoe N, Nakamura M, Mukai M, Saito H, Hibi T (2012) Overexpression of miR-142-5p and miR-155 in gastric mucosa-associated lymphoid tissue (MALT) lymphoma resistant to Helicobacter pylori eradication. PLoS ONE 7:e47396
Sapierzyński R, Fabisiak M, Kizerwetter-Swida M, Cywińska A (2007) Effect of Helicobacter sp. infection on the number of antral gastric endocrine cells in swine. Pol J Vet Sci 10:65–70
Sayi A, Kohler E, Toller IM, Flavell RA, Müller W, Roers A, Müller A (2011) TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol 186:878–890
Scanziani E, Simpson KW, Monestiroli S, Soldati S, Strauss-Ayali D, Del Pierro F (2001) Histological and immunohistochemical detection of different Helicobacter species in the gastric mucossa of cats. J Vet Diagn Invest 13:3–12
Schott T, Rossi M, Hänninen M (2011) Genome sequence of Helicobacter bizzozeronii strain CIII-1, an isolate from human gastric mucosa. J Bacteriol 193:4565–4566
Schott T, Kondadi PK, Hänninen ML, Rossi M (2012) Microevolution of a zoonotic Helicobacter population colonizing the stomach of a human host before and after failed treatment. Genome Biol Evol 4:1310–1315
Schrenzel MD, Witte CL, Bahl J, Tucker TA, Fabian N, Greger H, Hollis C, Hsia G, Siltamaki E, Rideout BA (2010) Genetic characterization and epidemiology of helicobacters in non-domestic animals. Helicobacter 15:126–142
Scott DR, Marcus EA, Shirazi-Beechey SSP (2001) Evidence of Helicobacter infection in the horse. Proceedings of the American Society for Microbiology, Washington, DC, p 287
Seymour C, Lewis RG, Kim M, Gagnon DF, Fox JG, Dewhirst FE, Paster BJ (1994) Isolation of Helicobacter strains from wild bird and swine feces. Appl Environ Microbiol 60:1025–1028
Shen Z, Feng Y, Dewhirst FE, Fox JG (2001) Coinfection of enteric Helicobacter spp. and Campylobacter spp. in cats. J Clin Microbiol 39:2166–2172
Shen Z, Xu S, Dewhirst FE, Paster BJ, Pena JA, Modlin IM, Kidd M, Fox JG (2005) A novel enterohepatic Helicobacter species ‘Helicobacter mastomyrinus’ isolated from the liver and intestine of rodents. Helicobacter 10:59–70
Shirin H, Moss SF (1998) Helicobacter pylori induced apoptosis. Gut 43:592–594
Shojaee Tabrizi A, Jamshidi S, Oghalaei A, Zahraei Salehi T, Bayati Eshkaftaki A, Mohammadi M (2010) Identification of Helicobacter spp. in oral secretions vs gastric mucosa of stray cats. Vet Microbiol 140:142–146
Simpson KW, McDonough PL, Strauss-Ayali D, Chang YF, Harpending P, Valentina BA (1999) Helicobacter felis infection in dogs: effect on gastric structure and function. Vet Pathol 36:237–248
Sirianni A, Kaakoush NO, Raftery MJ, Mitchell HM (2013) The pathogenic potential of Helicobacter pullorum: possible role for the type VI secretion system. Helicobacter 18:102–111
Smet A, Flahou B, D’Herde K, Vandamme P, Cleenwerck I, Ducatelle R, Pasmans F, Haesebrouck F (2012) Helicobacter heilmannii sp. nov., isolated from the feline gastric mucosa. Int J Syst Evol Microbiol 62:299–306
Smet A, Van Nieuwerburgh F, Ledesma J, Flahou B, Deforce D, Ducatelle R, Haesebrouck F (2013) Genome sequence of helicobacter heilmannii Sensu Stricto ASB1 isolated from the gastric mucosa of a kitten with severe gastritis. Genome Announc 1 pii:e00033-12
Solnick JV, Schauer DB (2001) Emergence of diverse Helicobacter species in the pathogenesis of gastric en enterohepatic diseases. Clin Microbiol Rev 14:59–97
Solnick JV, Josenhans C, Suerbaum S, Tompkins LS, Labigne A (1995) Construction and characterization of an isogenic urease-negative mutant of Helicobacter mustelae. Infect Immun 63:3718–3721
Solnick JV, Chang K, Canfield DR, Parsonnet J (2003) Natural acquisition of Helicobacter pylori infection in newborn rhesus macaques. J Clin Microbiol 41:5511–5516
Solnick JV, Fong J, Hansen LM, Chang K, Canfield DR, Parsonnet J (2006) Acquisition of Helicobacter pylori infection in Rhesus Macaques is most consistent with oral-oral transmission. J Clin Microbiol 44:3799–3803
Stanley J, Linton D, Burnens A, Dewhirst FE, On SLW, Porter A, Owen RJ, Costas M (1994) Helicobacter pullorum sp. nov. – genotype and phenotype of new species isolated from poultry and from humans patients with gastroenteritis. Microbiology 140:3441–3449
Steinbrueckner B, Haerter G, Pelz K, Weiner S, Rump JA, Deissler W, Bereswill S, Kist M (1997) Isolation of Helicobacter pullorum from patients with enteritis. Scand J Infect Dis 29:315–318
Sterzenbach T, Lee SK, Brenneke B, von Goetz F, Schauer DB, Fox JG, Suerbaum S, Josenhans C (2007) Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun 75:2717–2728
Stoicov C, Fan X, Liu JH, Bowen G, Whary M, Kurt-Jones E, Houghton J (2009) T-bet knockout prevents Helicobacter felis-induced gastric cancer. J Immunol 183:642–649
Stolte M, Kroger G, Meining A, Morgner A, Bayerdörffer E, Bethke B (1997) A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol 32:28–33
Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A (2002) Helicobacter and gastric MALT lymphoma. Gut 50:iii19–iii24
Stoof J, Breijer S, Pot RG, van der Neut D, Kuipers EJ, Kusters JG, van Vliet AH (2008) Inverse nickel-responsive regulation of two urease enzymes in the gastric pathogen Helicobacter mustelae. Environ Microbiol 10:2586–2597
Suárez P, Contreras M, Fernández-Delgado M, Salazar V, Peña R, Michelangeli F, García-Amado MA (2010) Detection of Helicobacter in the digestive tract of an Atlantic spotted dolphin (Stenella frontalis). J Wildl Dis 46:622–626
Suzuki A, Kobayashi M, Matsuda K, Matsumoto T, Kawakubo M, Kumazawa S, Koide N, Miyagawa S, Ota H (2010) Induction of high endothelial venule-like vessels expressing GlcNAc6ST-1-mediated L-selectin ligand carbohydrate and mucosal addressin cell adhesion molecule 1 (MAdCAM-1) in a mouse model of “Candidatus Helicobacter heilmannii”-induced gastritis and gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Helicobacter 15:538–548
Svec A, Kordas P, Pavliz Z, Novotný J (2000) High prevalence of Helicobacter heilmannii-associated gastritis in a small, predominantly rural area: further evidence in support of a zoonosis? Scand J Gastroenterol 35:925–928
Swennes AG, Turk ML, Trowel EM, Cullin C, Shen Z, Pang J, Petersson KH, Dewhirst FE, Fox JG (2014) Helicobacter canis colonization in sheep: a zoonotic link. Helicobacter 19:65–68
Sykora J, Hejda V, Varvarovska J, Stozicky F, Gottrand F, Siala K (2003) Helicobacter heilmannii related gastric ulcer in childhood. J Pediatr Gastroenterol Nutr 36:410–413
Szeredi L, Palkovics G, Solymosi N, Tekes L, Méhesfalvi J (2005) Study on the role of gastric Helicobacter infection in gross pathological and histological lesions of the stomach in finishing pigs. Acta Vet Hung 53:371–383
Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, Rogers AB, Lertkowit N, Varro A, Fox JG, Wang TC (2009) Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol 175:365–375
Takemura LS, Camargo PL, Alfieri AA, Bracarense AP (2009) Helicobacter spp. In cats: association between infecting species and epithelial proliferation within the gastric lamina propria. J Comp Pathol 141:127–134
Tee W, Montgomery J, Dyall-Smith M (2001) Bacteremia caused by a Helicobacter pullorum-like organism. J Infect Dis 188:1893–1898
Tegtmeyer N, Rivas Traverso F, Rohde M, Oyarzabal OA, Lehn N, Schneider-Brachert W, Ferrero RL, Fox JG, Berg DE, Backert S (2013) Electron microscopic, genetic and protein expression analyses of Helicobacter acinonychis strains from a Bengal tiger. PLoS ONE 8:e71220
Terio KA, Munson L, Marker L, Aldridge BM, Solnick JV (2005) Comparison of Helicobacter spp. in Cheetahs (Acinonyx jubatus) with and without gastritis. J Clin Microbiol 43:229–234
Terio KA, Munson L, Moore PF (2012) Characterization of the gastric immune response in cheetahs (Acinonyx jubatus) with Helicobacter-associated gastritis. Vet Pathol 49:824–833
Thomson MJ, Pritchard DM, Boxal SA, Abuderman AA, Williams JM, Varro A, Crabtree JE (2012) Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS ONE 7:e50194
Tolia V, Nilsson HO, Boyer K, Wuerth A, AL-Soud WA, Rabah R, Wadström T (2004) Detection of Helicobacter ganmani-like 16S rDNA in pediatric liver tissue. Helicobacter 9:460–468
Totten PA, Fennell CL, Tenover FC, Wezenberg JM, Perine PL, Stamm WE, Holmes KK (1985) Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis 151:131–139
Traverso FR, Bohr UR, Oyarzabal OA, Rohde M, Clarici A, Wex T, Kuester D, Malfertheiner P, Fox JG, Backert S (2010) Morphologic, genetic, and biochemical characterization of Helicobacter magdeburgensis, a novel species isolated from the intestine of laboratory mice. Helicobacter 15:403–415
Trebesius K, Adler K, Vieth M, Stolte M, Haas R (2001) Specific detection and prevalence of Helicobacter heilmannii-like organisms in the human gastric mucosa by fluorescent in situ hybridization and partial 16S ribosomal DNA sequencing. J Clin Microbiol 39:1510–1516
Valgaeren BR, Pardon B, Flahou B, Verherstraeten S, Goossens E, Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F, Deprez PR (2013) Prevalence and bacterial colonisation of fundic ulcerations in veal calves. Vet Rec 172:269
Van den Bulck K, Decostere A, Gruntar I, Baele M, Krt B, Ducatelle R, Haesbrouck F (2005a) In vitro susceptibility testing of Helicobacter felis, H. bizzozeronii and H. salomonis. Antimicrob Agents Chemother 49:2997–3000
Van den Bulck K, Decostere A, Baele M, Driessen A, Debongnie JC, Burette A, Stolte M, Ducatelle R, Haesebrouck F (2005b) Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs and cats. J Clin Microbiol 43:2256–2260
Van den Bulck K, Decostere A, Baele M, Marechal M, Ducatelle R, Haesebrouck (2006) Low frequency of Helicobacter species in the stomachs of experimental rabbits. Lab Anim 40:282–287
Vandamme P, Harrington CS, Jalava K, On SL (2000) Misidentifying helicobacters: the Helicobacter cinaedi example. J Clin Microbiol 38:2261–2266
Varon C, Mocan I, Mihi B, Péré-Védrenne C, Aboubacar A, Moraté C, Oleastro M, Doignon F, Laharie D, Mégraud F, Ménard A (2014) Helicobacter pullorum cytolethal distending toxin targets vinculin and cortactin and triggers formation of lamellipodia in intestinal epithelial cells. J Infect Dis 209:588–599
Vermoote M, Vandekerckhove TTM, Flahou B, Pasmans F, Smet A, De Groote D, Van Criekinge W, Ducatelle R, Haesebrouck F (2011a) Genome sequence of Helicobacter suis supports its role in gastric pathology. Vet Res 42:51
Vermoote M, Pasmans F, Flahou B, Van Deun K, Ducatelle R, Haesebrouck F (2011b) Antimicrobial susceptibility pattern of Helicobacter suis strains. Vet Microbiol 153:339–342
Warren JR, Marshal BJ (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1:1273–1275
Whary MT, Fox JG (2004) Natural and experimental Helicobacter infections. Comp Med 54:128–158
Wiinberg B, Spohr A, Dietz HH, Egelund T, Greiter-Wilke A, McDonough SP, Olsen J, Priestnall S, Chang YF, Simpson KW (2005) Quantitative analysis of inflammatory and immune responses in dogs with gastritis and their relationship to Helicobacter spp. infection. J Vet Intern Med 19:4–14
Won YS, Yoon JH, Lee CH, Kim BH, Hyun BH, Choi YK (2002) Helicobacter muricola sp. nov., a novel Helicobacter species isolated from the ceca and feces of Korean wild mouse (Mus musculus molossinus). FEMS Microbiol Lett 209:45–51
Won YS, Vandamme P, Yoon JH, Park YH, Hyun BH, Kim HC, Itoh T, Tanioka Y, Choi YK (2007) Helicobacter callitrichis sp. nov., a novel Helicobacter species isolated from the feces of the common marmoset (Callithrix jacchus). FEMS Microbiol Lett 271:239–244
Wüppenhorst N, von Loewenich F, Hobmaier B, Vetter-Knoll M, Mohadjer S, Kist M (2013) Culture of a gastric non-Helicobacter pylori Helicobacter from the stomach of a 14-year old girl. Helicobacter 18:1–5
Yakoob J, Abbas Z, Khan R, Ahmad Z, Islam M, Awan S, Jafri F, Jafri W (2012) Prevalence of non-Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol 12:3
Yamamoto K, Nishiumi S, Yang L, Klimatcheva E, Pandina T, Takahashi S, Matsui H, Nakamura M, Zauderer M, Yoshida M, Azuma T (2014) Anti-CXCL13 antibody can inhibit the formation of gastric lymphoid follicles induced by Helicobacter infection. Mucosal Immunol. doi:10.1038/mi.2014.14
Yu J, Russell RM, Salomon RN, Murphy JC, Palley LS, Fox JG (1995) Effect of Helicobacter mustelae infection on ferret gastric epithelial cell proliferation. Carcinogenesis 16:1927–1931
Zanoni RG, Piva S, Rossi M, Pasquali F, Lucchi A, De Cesare A, Manfreda G (2011) Occurrence of Helicobacter pullorum in turkeys. Vet Microbiol 149:492–496
Zhang L, Day A, McKenzie G, Mitchell H (2006) Nongastric Helicobacter species detected in the intestinal tract of children. J Clin Microbiol 44:2276–2279
Zhang G, Ducatelle R, Pasmans F, D’Herde K, Huang L, Smet A, Haesebrouck F, Flahou B (2013) Effects of Helicobacter suis γ-glutamyl transpeptidase on lymphocytes: modulation by glutamine and glutathione supplementation and outer membrane vesicles as a putative delivery route of the enzyme. PLoS ONE 8:e77966
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Flahou, B., Haesebrouck, F., Smet, A. (2016). Non-Helicobacter pylori Helicobacter Infections in Humans and Animals. In: Backert, S., Yamaoka, Y. (eds) Helicobacter pylori Research. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55936-8_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55936-8_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55934-4
Online ISBN: 978-4-431-55936-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)